Abstract
The lambda holin, or S105, is a small cytoplasmic membrane protein that controls the timing of host lysis. Using thiol-specific reagents, we determined that the single cysteine residue within S105 was heterogeneously modified during membrane extraction and subsequent immobilized metal ion chromatography. Here we describe the use of a specific and reversible thiol reagent, 2,2′ dithiodipyridine, to generate purified protein with its cysteine residues in the native thiol state. The 2,2′ dithiodipyridine-protection protocol was also successfully used for another, unrelated holin, S2168, and should be generally useful for the purification of membrane proteins.
Keywords: holin; thiol modification; cysteine sulfhydryl; 2,2′ dithiodipyridine
Holins are bacteriophage-encoded membrane proteins that terminate the infection cycle by permeabilizing the host membrane at a precisely determined, allele-specific time. The best-characterized holin is S105, so designated because it is a 105 amino acid product of the S gene of bacteriophage lambda [1]; it is a class I holin, with three transmembrane domains, or TMDs (Fig. 1A). The extensive genetic and molecular data available for S105 made it an attractive target for biochemical and structural characterization. However, holins are not only small membrane proteins, which are always toxic in terms of over-expression and difficult to purify, but also have evolved to be lethal to bacterial cells at very low expression levels. Typically, detergent extraction from the membrane has to be assiduous and on the time scale of hours, and the only practical chromatographic option is immobilized metal ion chromatography (IMAC), which is incompatible with traditional thiol protection reagents like dithiothreitol (DTT). Here, we report evidence that during extraction and purification, the S105 protein is damaged by covalent modification of its single cysteine sulfhydryl group Cys51 and document an approach for protection of this thiol using a reversible thiol-specific reagent.
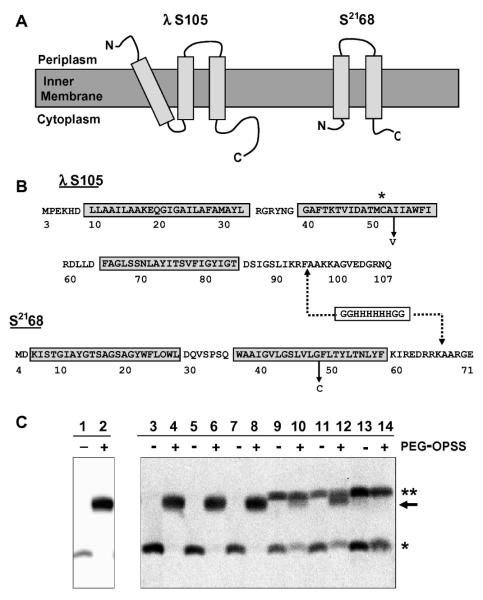
Figure 1.
(A) Topologies of holin proteins. S105 and S2168 are inner membrane proteins with three or two TMDs, respectively. (B) Sequences of holin proteins. S105 begins with the Met residue from codon 3 of the S gene [15]. S2168 begins with Met at position 4 of S21 [9]. The TMDs are indicated by boxes, and the relevant mutations A52V and G48C, for S105 and S2168, respectively, are labeled. The asterisk highlights the position of the Cys residue in S105. The dashed lines indicate the position of the oligohistidine tag inserted in each sequence: between positions 94 and 95 in S105 [2] and between positions 66 and 67 in S2168 [9]. For simplicity, the his-tagged lambda holin is called S105 in the text, although it actually corresponds to the protein S105τ94 [2] where τ94 refers to the position at which the oligo-his tag was inserted (C) Western blot for samples from PEGylation of S105A52V purification, detected with antibodies against peptides corresponding to the C-terminus of S105 [7]. Lanes 1 and 2, TCA-precipitated whole cells expressing S105A52V; lanes 3 and 4, cell suspension; lanes 5 and 6, French pressate; lanes 7 and 8, clearing spin supernatant; lanes 9 and 10, resuspended membranes; lanes 11 and 12, membrane extraction spin supernatant; lanes 13 and 14, purified S105A52V (* indicates the S monomer band, ** indicates the S dimer band, and → indicates the PEGylation product).
For the purposes of obtaining purified holin for studying hole formation in vitro, T7-promoter-based over-expression clones of oligohistidine-tagged versions of S105 and a non-functional missense mutant protein, S105A52V [2] (Fig. 1B) were induced, resulting in the accumulation of 0.5 - 1 mg purified protein per liter for the parental, and 1 - 2 mg per liter for the A52V mutant. The over-expression and protein purification procedures have been previously described [3].
The state of the Cys51 sulfhydryls in the purified preparations of S105 and S105A52V was assessed with Ellman’s Reagent, also known as 5,5′-dithiobis (2-nitrobenzoic acid), or DTNB (Sigma) [4]. Solutions of reduced glutathione, cystine, and purified S105 and S105A52V were prepared at final concentrations of 25 μM in a volume of 95 μl. Five microliters of 10 mM DTNB (dissolved in ethanol) was added to the above solutions. The samples were mixed by pipetting, incubated in the dark at room temperature for 30 min, and assayed for the presence of the anionic byproduct 2-nitro-5-thiobenzoate, or TNB (ε = 14,150 M-1cm-1 at 412 nm [5]). Surprisingly, the purified S105 and S105A52V exhibited ∼94% and ∼95% non-reactive sulfhydryls, respectively, indicating significant thiol modification during purification.
It was important to determine when the sulfhydryl modification was occurring, so a protocol was adapted from published PEGylation procedures [6] that allowed assessment of the state of the thiol group at each stage of holin purification, using orthopyridyl disulfide functionalized polyethylene glycol, or PEG-OPSS (Nektar). A 200 ml culture expressing S105A52V was induced at A550 = 0.5 for 50 min. An aliquot of the culture was removed at 25 min for a whole cell sample, and the remaining culture was harvested for purification. For the whole cell sample, 5 mL of the induced culture was added to 5 mL of cold 11.1% TCA, vortexed, incubated on ice for 30 min, then centrifuged at maximum speed in a tabletop IEC clinical centrifuge at 4 °C for 15 min. The pellet was washed with 5 mL of cold acetone, vortexed, and centrifuged (see above). The pelleted protein was air-dried and resuspended in 1 mL of PEGylation buffer (0.5 M Tris HCl, pH 7.0, 1% sodium dodecyl sulfate (SDS), 1 mM EDTA). Two hundred microliters of the resuspended protein was combined with 10 λL of 60 mM PEG-OPSS, incubated at room temperature for 30 min, treated with 1.4 mL of cold acetone for 10 min at -20 °C, and centrifuged at 14,000 rpm in a tabletop microcentrifuge for 15 min at 4 °C. The supernatant was carefully removed and the dried pellet was resuspended in non-reducing 2X protein sample buffer (0.5 mg/mL bromophenol blue in 0.25 M Tris, pH 6.8). The harvested cells from the original culture were used for protein purification, and aliquots, which contained ∼0.01 μg of purified protein, were diluted by 10-fold into PEGylation buffer. Ten microliters of 60 mM PEG-OPSS were added to each of the samples, and incubated at room temperature for 30 min. The samples were combined with an equivalent volume of non-reducing 2X protein sample buffer and analyzed by SDS-PAGE, then immunoblotted with an antibody directed against a peptide which corresponds to the C-terminus of S105 [7].
The results clearly show that purified S105 protein from the whole cell, cell suspension, and French press-disruption steps was fully PEGylated by PEG-OPSS (Fig. 1C). This indicates that the Cys51 sulfhydryl group is accessible and unmodified through these steps. Modification was first apparent in the concentrated membrane material, which had been collected by high-speed centrifugation, resuspended in membrane extraction buffer containing detergent, and incubated overnight with agitation. Quantification of the Western blot bands revealed that more than half of the S105 had been oxidized to intermolecular disulfides (double asterisk, Fig. 1C), and about half of the remaining monomeric S105 was unable to react with the thiol-specific reagent PEG-OPSS at this stage of the purification (Fig. 1C, lanes 9 and 10). The degree of modification increased in the final steps of IMAC purification, such that most, if not all, of the purified protein was modified (Fig. 1C, lanes 13 and 14).
To prevent non-specific modification of the Cys51 sulfhydryl group and also artifactual intermolecular disulfide bond formation, we selected another thiol reagent, 2,2′ dithiodipyridine, or DTDP (Sigma) [8]. This reagent is small, making it less likely to be sterically restricted from access to thiol groups and fully reversible with Tris (2-carboxyethyl) phosphine hydrochloride, or TCEP (Sigma) (Fig. 2A). S105 was purified as described [3], except a saturating concentration of 20 mM DTDP was included in the membrane extraction buffer. DTDP dissolved in the buffer only after the solution was incubated for a few minutes in a boiling waterbath, and the reagent remained in solution after the buffer was returned to room temperature. Membrane extraction was carried out overnight at room temperature instead of at 4 °C, to ensure that DTDP did not precipitate. After elution from the Talon metal affinity column and concentration determination by A280, the protein was treated with 10 mM TCEP for 5 min to reduce the bond between DTDP and the S105 Cys51 sulfhydryl.
Figure 2.
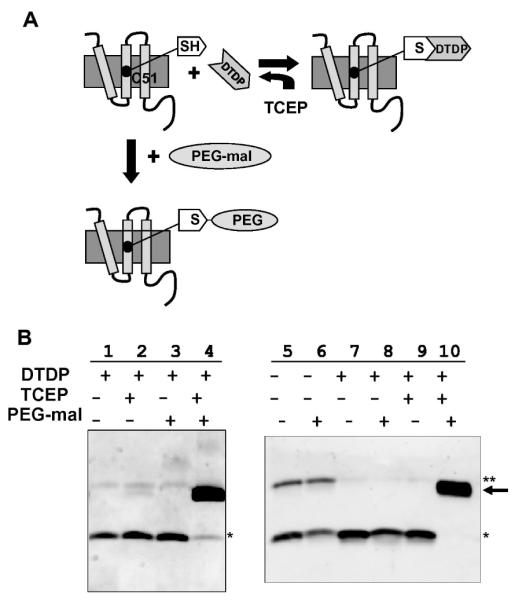
(A) Schematic of DTDP-protection of S105 Cys51 sulfhydryls. DTDP was included in the membrane extraction buffer prior to purification, and the thiol-specific reagent was removed from the purified protein with the reducing agent, TCEP. Cys51 was fully accessible to PEG-mal once the DTDP had been removed. (B) Western blots of PEGylation samples from S105 and S68 purifications, detected with antibodies directed against peptides corresponding to the C-termini of S105 [7] or S68 [9]. Lanes 1-4, DTDP-protection of S105 thiols during membrane extraction and protein purification. Lanes 5 and 6, purified S2168G48Chis without DTDP included in the membrane extraction buffer; lanes 7-10, purified S2168G48Chis with DTDP included in the membrane extraction buffer (* indicate the monomer bands, ** indicate the dimer bands, and → indicate the PEGylation products).
The DTDP-protection results were assessed by PEGylation [6] followed by SDS-PAGE and immunoblotting [3]. Specifically, 14 μL of 10 mM PEG-maleimide, or PEG-mal (Creative Biochem Laboratories), was added to ∼50-150 ng of reduced S105 protein in PEGylation buffer, then incubated for 30 min at room temperature. Aliquots of the samples were diluted at least 20-fold with 2X protein sample buffer containing β-mercaptoethanol for immunoblot detection with the Supersignal West Femto Maximum Sensitivity Substrate (Pierce) and to reduce the high reaction concentrations of PEG-mal, which may affect the mobility of the proteins during electrophoresis. The results show clearly that the purified S105 that had been DTDP-protected during extraction and subsequent purification can be PEGylated to completion (Fig. 2B, lanes 1-4).
To demonstrate that this method may be applicable to other proteins with reactive thiol groups, DTDP-protection and PEGylation were carried out with a second protein, S2168G48Chis (Fig. 1B). S2168, the holin of the lambdoid phage 21 [9], is an unrelated class II holin with two TMDs (Fig. 1A). S2168G48C is a non-lethal Cys-substitution mutant of S21 (T. Pang et al., unpublished) (Fig. 1B). For over-expression of an oligohistidine-tagged version of S2168G48C, the plasmid pETS2168G48Chis, was constructed from the previously-described pETS2168his [9], by site-directed mutagenesis, changing codon Gly48 to a Cys residue. The purification of S2168G48C was carried out the same way as for the lambda holin and the A52V derivative. Again, PEG-mal assays revealed that the single thiol was chemically modified during purification, only part of which could be explained by formation of disulfide-linked dimers (Fig. 2B, lanes 5 and 6). When DTDP was incorporated in the membrane extraction buffer during S2168G48Chis purification, it protected the sulfhydryl side chain of Cys48 quantitatively and could be removed efficiently by TCEP (Fig. 2B, lanes 7-10).
Currently, there are a variety of thiol-specific reagents commercially available for modification of free sulfhydryl groups within proteins. Some of these reagents have been used for thiol modification during protein purification, in an effort to maintain free sulfhydryls [10; 11; 12; 13]. However, thiol protection during the rigors of detergent extraction and IMAC purification of membrane protein has not been specifically addressed. We have shown here that inclusion of DTDP (also known as 2,2′-dipyridyldisulfide (PDS) or 2-PD [14]) in the membrane extraction buffer results in complete modification and, thus protection, of the thiols during the purification of two different holin proteins, and that it can be removed quantitatively after purification. DTDP is small, inexpensive, stable, and has a broad pH range of 3.4 – 8.1 [8]. It is our expectation that it may be generally useful for application to cysteine-containing membrane proteins.
Acknowledgements
We thank the members of the Young lab, past and present, for their support, especially Ting Pang who generously provided the plasmid pETS2168G48Chis, and Daisy Wilbert for her clerical assistance. This work was supported by PHS grant GM27099 to R.Y. and the Program for Membrane Structure and Function, a Program of Excellence grant from the Office of the Vice President for Research at Texas A&M University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wang IN, Smith DL, Young R. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol. 2000;54:799–825. doi: 10.1146/annurev.micro.54.1.799. [DOI] [PubMed] [Google Scholar]
- [2].Smith DL, Young R. Oligohistidine tag mutagenesis of the lambda holin gene. J Bacteriol. 1998;180:4199–211. doi: 10.1128/jb.180.16.4199-4211.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Savva CG, Dewey JS, Deaton J, White RL, Struck DK, Holzenburg A, Young R. The holin of bacteriophage lambda forms rings with large diameter. Mol Microbiol. 2008;69:784–793. doi: 10.1111/j.1365-2958.2008.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ellman GL. Tissue Sulfhydryl Groups. Archives of Biochemistry and Biophysics. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- [5].Riddles PW, Blakeley RL, Zerner B. Ellman’s reagent: 5,5′-dithiobis(2-nitrobenzoic acid)--a reexamination. Anal Biochem. 1979;94:75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- [6].Lu J, Deutsch C. Pegylation: a method for assessing topological accessibilities in Kv1.3. Biochemistry. 2001;40:13288–301. doi: 10.1021/bi0107647. [DOI] [PubMed] [Google Scholar]
- [7].Chang CY, Nam K, Young R. S gene expression and the timing of lysis by bacteriophage lambda. J Bacteriol. 1995;177:3283–94. doi: 10.1128/jb.177.11.3283-3294.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grassetti DR, Murray JF., Jr. Determination of sulfhydryl groups with 2,2′- or 4,4′-dithiodipyridine. Arch Biochem Biophys. 1967;119:41–9. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- [9].Park T, Struck DK, Deaton JF, Young R. Topological dynamics of holins in programmed bacterial lysis. Proc Natl Acad Sci U S A. 2006;103:19713–8. doi: 10.1073/pnas.0600943103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Faulstich H, Tews P, Heintz D. Determination and derivatization of protein thiols by n-octyldithionitrobenzoic acid. Anal Biochem. 1993;208:357–62. doi: 10.1006/abio.1993.1061. [DOI] [PubMed] [Google Scholar]
- [11].Cartwright T, Senussi O, Grady MD. Reagents which Inhibit Disulphide Bond Formation Stabilize Human Fibroblast Interferon. J. Gen. Virol. 1977;36:323–327. doi: 10.1099/0022-1317-36-2-323. [DOI] [PubMed] [Google Scholar]
- [12].Murphy ME, Scholich H, Sies H. Protection by glutathione and other thiol compounds against the loss of protein thiols and tocopherol homologs during microsomal lipid peroxidation. Eur J Biochem. 1992;210:139–46. doi: 10.1111/j.1432-1033.1992.tb17401.x. [DOI] [PubMed] [Google Scholar]
- [13].Hochgrafe F, Mostertz J, Pother DC, Becher D, Helmann JD, Hecker M. S-cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress. J. Biol. Chem. 2007;282:25981–25985. doi: 10.1074/jbc.C700105200. [DOI] [PubMed] [Google Scholar]
- [14].Britto PJ, Knipling L, McPhie P, Wolff J. Thiol-disulphide interchange in tubulin: kinetics and the effect on polymerization. Biochem J. 2005;389:549–58. doi: 10.1042/BJ20042118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blasi U, Nam K, Hartz D, Gold L, Young R. Dual translational initiation sites control function of the lambda S gene. EMBO J. 1989;8:3501–10. doi: 10.1002/j.1460-2075.1989.tb08515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]