Abstract
Reward is crucial for survival of animals and influences animal behaviors. For example, an approaching behavior to reward is more frequently and quickly elicited when big reward is expected than when small reward is expected. Midbrain dopamine neurons are thought to be crucial for such reward-based control of motor behavior. Indeed, dopamine neurons are excited by cues predicting reward and inhibited by cues predicting no-reward. These excitatory and inhibitory signals would then be used for enhancing and depressing sensorimotor processing, respectively, in the brain areas targeted by dopamine neurons (e.g., striatum). However, it was unknown which parts of the brain provide dopamine neurons with reward-related signals necessary for their responses. We recently showed evidence that the lateral habenula transmits reward-related signals to dopamine neurons, especially to inhibit dopamine neurons. This recent study suggested that the lateral habenula suppresses less rewarding saccadic eye movements by inhibiting dopamine neurons. In the present review, we first summarize anatomical and functional aspects of the lateral habenula. We will then describe our own study. Finally, we will discuss how the lateral habenula, as well as dopamine neurons, contributes to the reward-based control of saccadic eye movements.
Keywords: reward, lateral habenula, dopamine neuron, saccade, monkey
What is the lateral habenula?
The lateral habenula, part of the structure called the epithalamus, is a good candidate for a source of reward-related signals in dopamine neurons. It is considered to carry information from the forebrain limbic system down to midbrain structures (Fig. 1A). The origins of its afferents are the medial prefrontal cortex, basal forebrain regions, lateral hypothalamus, and globus pallidus internal segment (Herkenham and Nauta, 1977; Lecourtier and Kelly, 2007). Its main targets are the ventral tegmental area and substantia nigra pars compacta containing dopamine neurons, and raphe nuclei containing serotonin neurons (Herkenham and Nauta, 1979; Lecourtier and Kelly, 2007). Thus, the lateral habenula is in a good position to regulate the brain's monoaminergic (dopaminergic and serotonergic) systems. Indeed, electrical stimulation of the lateral habenula inhibits dopamine neurons (Christoph et al., 1986) and serotonin neurons (Wang and Aghajanian, 1977).
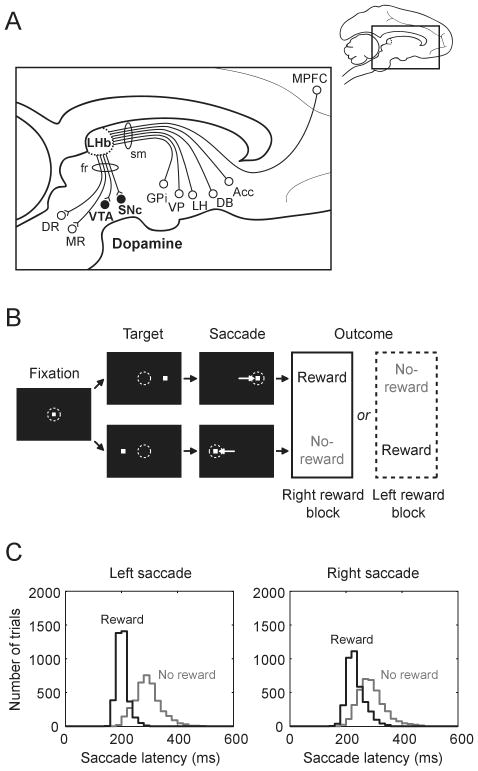
Figure 1.
(A) Scheme showing the location of the lateral habenula and its afferent and efferent connections in a parasagittal section of the macaque brain. Acc, nucleus accumbens; DB, diagonal band of Broca; DR, dorsal raphe; fr, fasciculus retroflexus; GPi, globus pallidus internal segment; LH, lateral hypothalamus; LHb, lateral habenula; MPFC, medial prefrontal cortex; MR, medial raphe; sm, stria medullaris; SNc, substantia nigra pars compacta; VP, ventral pallidum; VTA, ventral tegmental area. (B) Sequence of events in the visually guided saccade task. (C) Distribution of saccade latencies in reward trials (black) and in no-reward trials (gray) in one example monkey. Modified from Nature (Matsumoto and Hikosaka, 2007).
Consistent with the view from the anatomy, the lateral habenula has been implicated in many emotional and cognitive functions. These include anxiety, stress, pain, learning, attention, and reward processing (Lecourtier and Kelly, 2007; Sutherland, 1982). However, the physiological properties of the lateral habenula remained poorly understood. In the next section, we summarize our recent study which provided physiological data suggesting that the lateral habenula transmits reward-related signals to dopamine neurons (Matsumoto and Hikosaka, 2007).
Lateral habenula as a source of negative reward signals in dopamine neurons
To test the hypothesis that the lateral habenula transmits reward-related signals to dopamine neurons, we compared the activity of lateral habenula neurons and dopamine neurons while monkeys were performing a visually guided saccade task with positional reward bias (Fig. 1B). The target was presented randomly on the right or left and the monkey had to make a saccade to it immediately. In one block of trials (24 trials) saccades to one fixed direction were rewarded while saccades to the other direction were not rewarded. The rewarded direction was reversed in the next block with no external instruction. Saccade latencies were much shorter when saccades were followed by reward than when they were followed by no-reward (Fig. 1C).
We found that a majority of lateral habenula neurons were excited by targets predicting no-reward and inhibited by targets predicting reward (Fig. 2A). The excitation and inhibition depended on the reward contingency (reward or no-reward), regardless of target position. In contrast, dopamine neurons were excited by targets predicting reward and inhibited by targets predicting no-reward (Fig. 2B). In addition, lateral habenula neurons and dopamine neurons also responded to the delivery and omission of reward in the opposite manner, especially at the first trials after the reversal of rewarded direction (dotted lines in Fig. 2A and B).
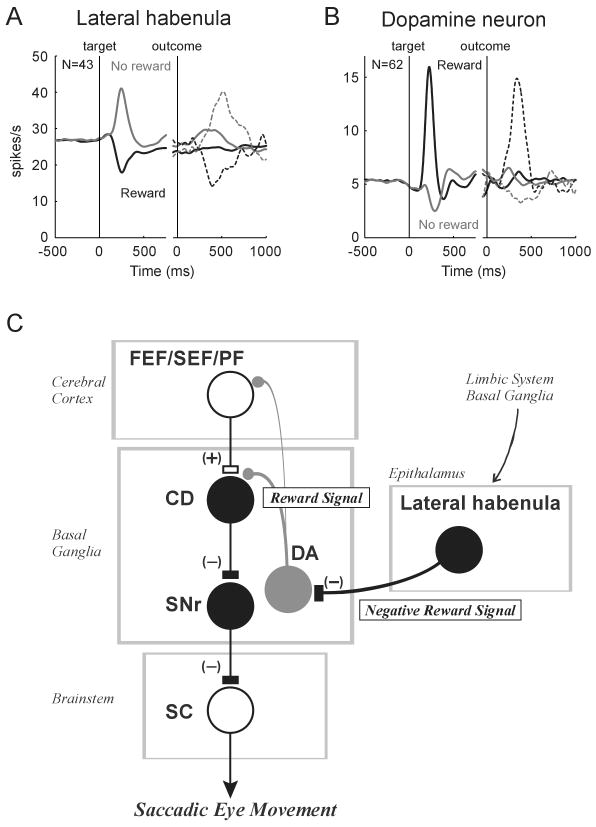
Figure 2.
(A) and (B) Averaged activity of lateral habenula neurons (A, n=43) and dopamine neurons (B, n=62) during the visually guided saccade task with positional reward bias. Spike density functions (SDFs) are aligned at the onset of target (left) and at the onset of outcome (right). The SDFs are shown for reward trials (black) and no-reward trials (gray). Continuous curves indicate activity in trials excluding the first trials after the reversal of rewarded direction. Dotted curves indicate activity in the first trials. The data from ipsilateral and contralateral saccades are combined. (C) A hypothetical scheme showing the role of the lateral habenula in reward-based control of saccadic eye movements. CD, caudate nucleus; DA, dopamine neurons; FEF, frontal eye field; PF, prefrontal cortex; SC, superior colliculus; SEF, supplementary eye field; SNr, substantia nigra pars reticulata. Excitatory and inhibitory connections are indicated by (+) and (-), respectively. It is unknown whether lateral habenula neurons themselves are inhibitory. Modified from Nature (Matsumoto and Hikosaka, 2007).
In no-reward trials, the excitatory response of lateral habenula neurons started earlier than the inhibitory response of dopamine neurons. In reward trials, however, the inhibitory response of lateral habenula neurons started later than the excitatory response of dopamine neurons. Furthermore, electrical stimulation of the lateral habenula inhibited dopamine neurons. These results suggest that the lateral habenula transmits negative reward signals to dopamine neurons, especially to inhibit dopamine neurons.
Discussion
Recent studies from our laboratory have suggested that the reward-modulated activity of dopamine neurons plays a key role in the reward-based control of saccadic eye movements (Hikosaka et al., 2006). These studies proposed that the efficacy of cortico-caudate synapses carrying visuo-saccadic signals is enhanced or depressed depending on the concurrent increase or decrease, respectively, in dopaminergic inputs. The activity of the superior colliculus would then be enhanced or depressed through the caudate nucleus and substantia nigra pars reticulata (Fig. 2C). Indeed, neurons in the superior colliculus are more excited by saccadic targets predicting reward than targets predicting no-reward (Ikeda and Hikosaka, 2003). By transmitting negative reward signals to dopamine neurons, the lateral habenula would suppress less rewarding saccadic eye movements.
References
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700. doi: 10.1016/s0896-6273(03)00464-1. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience and biobehavioral reviews. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neuroscience and biobehavioral reviews. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197:89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]