Abstract
Several decades of observational data have accumulated to implicate a potential role for melatonin in cancer prevention. Experimental studies suggest that the antineoplastic action of melatonin arises through many different mechanisms, including melatonin’s antioxidant, antimitotic, and antiangiogenic activity, as well as its ability to modulate the immune system and alter fat metabolism. Melatonin interacts with membrane and nuclear receptors, and may be linked to the regulation of tumor growth. Of particular relevance to breast cancer risk, melatonin may also block the estrogen receptor ERα and impact the enzyme aromatase, which produces estradiol. A growing number of epidemiologic studies have evaluated the relationship between night shift work as well as how varying duration of sleep affects peak melatonin secretion at night. While the studies demonstrate lower nightly melatonin levels in night workers, the evidence for an association between sleep duration and melatonin production is less clear. Similarly, both case-control and prospective cohort studies have consistently linked night shift work with breast cancer risk and, more recently, endometrial cancer—another tumor highly sensitive to estrogens. While, to date, the evidence for an association between sleep duration and breast cancer risk is less convincing, overall, there is increasing support for a potentially important link between melatonin and breast cancer risk and perhaps the risk of other tumors. As evidence increases, modifiable factors that have been shown to affect melatonin production, such as night shift work, are likely to gain increasing recognition as potential public health hazards. Additional studies are needed to delineate further the potential of melatonin in cancer prevention.
Keywords: cancer, treatment, melatonin
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), a hormone produced primarily by the pineal gland, appears to protect against cancer development. Melatonin biosynthesis depends on dietary intake of the essential amino acid tryptophan, which is converted to serotonin and subsequently metabolized to melatonin by the enzyme hydroxyindole-O-methyltransferase (HIOMT).1 Normal melatonin daytime serum levels range from 1.4–2.1 pg/ml.2 Melatonin exerts its actions by binding to nuclear receptors that belong to the RZR/ROR family and membrane receptors MT1, MT2, and MT3, the mRNA expression of which fluctuates based on the circadian rhythm, the melatonin plasma concentration and the amount of light.3–5
Several authors have proposed that nighttime shift workers have altered melatonin levels and are more likely to develop cancer, including breast and endometrial cancer in women.6–8 In 2007, a total of 178,480 cases of female breast cancer were diagnosed in the U.S., making it the most frequently diagnosed cancer among women.9 Known risk factors include age, family or personal history of breast cancer, high breast tissue density, atypical hyperplasia, a history of chest radiation, early menarche, nulliparity, recent use of oral contraceptives, age of birth of first child over 30, obesity after menopause, physical inactivity, and consumption of one or more alcoholic beverages a day.10 Endometrial cancer was the most common gynecologic malignancy in the U.S. in 2007, with over 40,000 new cases and approximately 7,000 deaths.11 Risk factors for endometrial cancer include factors that increase unopposed estrogen exposure, including obesity, postmenopausal hormone use, nulliparity, older age at first birth, early menarche, and late menopause.12 Smoking and oral contraceptive use decrease risk.13 This review will explore the current epidemiologic evidence on nighttime workers and the association between melatonin and breast and endometrial cancer risk in women, as well as discuss possible mechanisms by which melatonin may act specifically to reduce breast and endometrial cancer risk among women.
Melatonin: Possible Oncostatic Mechanisms
Experimental studies suggest that the antineoplastic action of melatonin arises through myriad possible routes, including antioxidant, antimitotic, and/or antiangiogenic activity, as well as its ability to modulate the immune system and alter fat metabolism. More generally, melatonin may have regulatory signaling functionality in that it appears to regulate initiation, promotion, and/or progression of cancer.14, 15 Thus, the decrease in serum melatonin seen in night workers may enhance tumor development, as suggested by numerous animal and in vitro studies. Apart from melatonin’s antimitotic and antioxidant activity,16 immune system modulation of both cellular and humoral responses by melatonin may also play a role in carcinogenesis.3 Melatonin is thought also to play a role in apoptosis and angiogenesis;17–20 it has been shown to inhibit endothelin-1 synthesis in blood vessels, by inhibiting the endothelin-converting enzyme-1, which subsequently affects the release of vascular endothelial growth factors, a primary stimulator of tumor angiogenesis.21
In vitro studies support an effect of melatonin particularly on breast cancer.22 Mechanistically, this appears plausible given the antiestrogenic activity of melatonin and altered estrogen production by melatonin’s ability to alter aromatase activity.23 Whether melatonin alters progesterone levels is unclear. Further, melatonin in the presence of serum estradiol reversibly inhibits cell proliferation, reduces the metastatic capacity of cancer cells, and counteracts the stimulation by estradiol on cancer cell invasiveness.24, 25 This change in metastatic capacity may be mediated by an increase in the expression of E-cadherin and β1-integrin. However, melatonin also interacts with membrane receptors MT1 and MT2 as well as the nuclear RZR/ROR α receptors4 and experimental evidence linking the MT1 melatonin receptor with growth inhibition of MCF-7 human breast cancer cells in vitro was shown by Ram et al. after MT1 receptor antagonism.26 Yuan et al. found that 1nM melatonin inhibited MCF-7 cell growth by over 20 percent.27 An MT1/MT2 G protein-coupled receptor antagonist abolished the growth-suppressive effects.27 The basal proliferation rate of ERα-positive MCF-7 cells was inhibited by the overexpression of the MT1 receptor.27 In addition, melatonin has been hypothesized to lower circulating estrogen levels through hypothalamic modulation, was described to block selectively the estrogen receptor ERα, and prohibits the estradiol-ER complex from binding to the estrogen response element in DNA.28–31
As a more indirect mechanistic pathway, an effect of melatonin on fat metabolism has been described in relation to its cancer protective effects.32 Melatonin causes a decrease in cAMP production, which results in decreases in the uptake of linoelic acid by fatty acid transporters.33 Inhibition of lineolic acid may be antiproliferative, as it may be an energy source for tumor growth.33 Animals exposed to short days gain more weight than those exposed to a normal day’s length.34 In humans, an association between night shift work and the metabolic syndrome is thought to exist.35 At least in one study, exogenous melatonin reduced weight gain, particularly among obese women,36 and melatonin may influence appetite.37
Epidemiologic Evidence
1. Sleep duration, night shift work, and association with melatonin production
In humans, melatonin secretion is regulated by the hypothalamus, which receives environmental light signals from the retina via the retino-hypothalamic tract. Artificial light alters the natural release of melatonin, with highest levels of melatonin physiologically occurring during the dark night phase and relatively low levels throughout the day.38
1.1. Sleep and melatonin
Three studies have explored the relationship between number of hours slept and production of melatonin, as measured by excretion of the primary urinary metabolite of melatonin, 6-sulfatoxymelatonin (aMT6S). Two of these studies did not find an association between number of hours slept,6, 7 whereas one did.39 In the most recent of these studies, Wu and colleagues examined the association between self-reported sleep duration and breast cancer risk in a cohort with 525 postmenopausal breast cancer cases.39 They found breast cancer risk to decrease with increasing sleep duration (9+ hours of sleep compared with <=6 hours of sleep, OR 0.67; 95% confidence interval (CI), 0.4–1.1). Those participants who slept 9 or more hours a night had a 42% higher mean level of urinary melatonin compared with those who slept 6 or fewer hours; the association was modified by body mass index such that lean women appeared to have the greatest benefit from longer sleep duration. In contrast, a Japanese study of 206 postmenopausal women found that urinary aMT6s levels were not significantly associated with the hours of sleep (p=0.08), nor were they associated with serum estradiol, estrone, testosterone, or DHEAS levels.40 However, serum concentrations of estradiol and estrone levels were higher for women awake at night versus those who were asleep by 1 AM, whereas testosterone and DHEAS levels were not. Results from the Nurses’ Health Study (NHS) did not support an important association between hours slept and urinary melatonin.41 Given the conflicting evidence, whether longer durations of sleep are linked to increased nightly melatonin production is unclear.
1.2. Night shift work and melatonin
Several studies confirm that night shift workers tend to have lower melatonin levels than those who work day shifts due to their exposure to light at night. One study identified a significant reduction of melatonin after approximately 2 weeks of intermittent night light exposure.38 An analysis of aMT6s in Danish nurses also found that those women working night shifts had a significantly lower urinary concentration than those working the day shift (p<0.01).42 All models were adjusted for age, BMI, smoking, number of children, age at birth of first child, and menopausal status. Similarly, another study confirmed that night shift nurses had lower levels of aMT6S during night work compared with either their days off or day shift work.43
2. Sleep duration and breast cancer risk
As suggested earlier, sleep duration may be a surrogate for time for, and therefore amount of, melatonin production overnight, though observational evidence for such an association is scant and inconsistent. Few prospective studies have evaluated the association between sleep duration and breast cancer risk, and results have also been inconsistent. McElroy et al. studied sleep duration in relation to breast cancer risk in 4,033 community-dwelling women with breast cancer and 5,314 controls.44 Women who slept 9 or more hours a night had a non-significant increased risk of breast cancer (RR 1.13, 95% CI 0.93–1.37). Similarly, data from the prospective Nurses’ Health study cohort also do not support an inverse association between longer sleep duration and breast cancer risk (OR, 0.95; 95% CI, 0.81–1.11 for those with 9+ hours of self-reported sleep duration).45 Conversely, Verkasalo and colleagues describe a modestly inverse association between longer sleep durations and breast cancer risk in a population-based study conducted in Finland. Among 242 women who developed breast cancer throughout follow-up of 12,222 women with sleep duration data, the risk of developing breast cancer was 0.69 (95% CI, 0.45–1.06) for those with 9+ hours of sleep, relative to those with 7–8 hours of sleep.46 The most recent prospective study of 23,995 Japanese women found a significantly higher risk of breast cancer (HR 1.62, 95% CI 1.05–2.50) for those who slept less than or equal to 6 hours a night.47 In sum, to date, evidence regarding an association between sleep duration and breast cancer risk is conflicting and more studies are needed to delineate fully a potential link between longer sleep durations and risk of cancer.
3. Night shift work and breast cancer risk
Evidence from ecologic studies suggesting a lower breast cancer rate in women living in (presumably darker) Arctic regions, these observations led to the hypothesis that environmental light is related to cancer risk.6 Though other factors like diet or vitamin D may in part explain geographic variation in breast cancer incidence,48, 49 one possible contributing factor to these variations is exposure to artificial light at night, as encountered by night shift workers.48 Another line of evidence in favor of an association between light at night and breast cancer risk is that blind women, who may be less receptive to light, might produce more melatonin at night and therefore may have a lower incidence of breast cancer.50 However, while several studies have found a non-significant inverse relationship of blindness with decreased risk, the two most thorough studies by Lee et al. and Klein et al. did not find an association.51–55
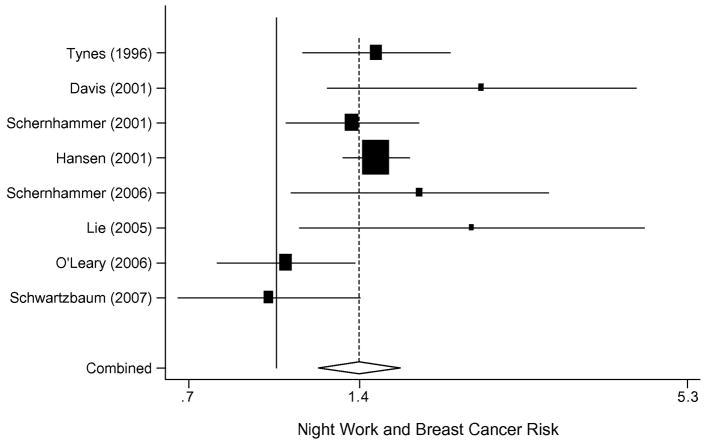
Compelling evidence for an association between night work and breast cancer risk stems from eight observational studies. More specifically, an increase in female breast cancer risk has been noted in all but two of the eight studies among night shift workers. Figure 1 displays the summary estimate from a meta-analysis of all eight studies, suggesting a 40% increased risk of breast cancer among women who work night shifts. The aggregate risk of breast cancer for all studies of night shift workers combined was 1.40 (95% CI, 1.19–1.65).
Figure 1.
Meta-analysis of the risk of night shift work and breast cancer risk.
Of all night shift work reports published to date, two are from prospective cohort data, one is retrospective, and five are matched case-control analyses, some of which are nested in larger cohorts. The two prospective cohort study reports both arise from the Nurses’ Health Study (NHS) cohorts. The NHS questionnaire asked women in 1988 how many years in total they had worked rotating night shifts with at least three nights per month in addition to days or evenings in that month. Information on lifetime years worked on rotating night shift was queried in eight categories: never, 1–2, 3–5, 6–9, 10–14, 15–19, 20–29, and 30 or more years. Although other covariates were updated every two years from the beginning of the NHS cohort in 1976 onwards, the night shift work question was assessed only once. Of 78,562 women followed for 10 years, 2,441 developed breast cancer. Women who worked 30 or more years of rotating night shifts had a relative risk (RR) of 1.36 (95%CI, 1.04–1.78).56 Among the Nurses’ Health Study II cohort of 115,022 predominantly premenopausal women at enrollment, 1,352 developed breast cancer. Those who worked 20 or more years of rotating night work had a RR of 1.79 (95% CI, 1.06–3.01).57 Both cohort analyses controlled thoroughly for breast cancer risk factors, including age and reproductive history, body mass index, family history of breast cancer, benign breast disease, the use of hormones, and smoking status.
Tynes et al. published the first retrospective assessment of shift work and breast cancer risk.58 In women aged 50 or above, they found a 4.3-fold increased risk of breast cancer after more than 3 years of shift work. Occupational information was derived from the Norwegian seaman registry in a cohort of 2,619 female radio and telegraph operators at sea. Hansen et al. conducted a population-based nested case-control study among 7,565 women with breast cancer as derived from the cancer registry, and their matched controls. Women in employments where at least 60% of women had nighttime schedules had a 30% increased risk, compared with women in occupations with less than 40% night work (OR, 1.3; 95% CI, 1.1–1.4).59 Davis et al. published in 2001 a retrospective case-control study in which they included 813 women with breast cancer and 793 controls. Women were interviewed with regards to their light exposure during the prior 10 years and lifetime occupational history. An increased risk of breast cancer (RR 2.3, 95% CI, 1.0–5.3) was seen for those who worked 5.7 or more hours per week of overnight shift, after controlling for parity, family history of breast cancer, oral contraceptive use, and recent discontinuation of hormone replacement therapy.60 Another population-based case-control study by Lie et al., conducted in Norway, showed an increased risk of breast cancer after controlling for duration of employment, age, social class, age at birth of first child, age at birth of last child, and number of children.61 In this study, women who worked 30 or more years had an increased breast cancer risk (RR 2.21, 95% CI, 1.10–4.45).
A register-based case-control study by O’Leary et al. showed no association between any shift work and breast cancer risk (OR, 1.21; 95% CI, 0.90–1.64) but has important limitations in their exposure definition.62 Further, another retrospective occupational cohort analysis of 2,102,126 male and 1,148,661 female workers in the Swedish population who worked at least 20 hours a week in 1970, and were followed from 1971 to 1989, was also negative.63 Information on shift work was obtained by personal interviews of 46,438 randomly selected people. Night shift workers were defined as those who reported that their workplace had a rotating schedule with three or more possible shifts per day or had work hours during the night at least 1 day during the week preceding the interview. Workers were categorized as either exposed or not exposed and by duration of shift work. Among 268 female night shift workers, the standardized incidence ratio (SIR) for all sites combined was 1.00 (95% CI, 0.89–1.13); 70 breast cancer cases occurred, resulting in an SIR of 0.94 (95% CI, 0.74–1.18). The authors raise the issue that there may be exposure misclassification; people assigned to night work may have worked only a few nighttime shifts and therefore were relatively unexposed. The duration of exposure may have also been fairly short, and other studies have found that the increased risk is often seen in those who have several decades of exposure.63 In addition to studies of night shift workers, seven flight attendant studies were published with the general population as their referent group. All but one of these seven studies indicated a higher incidence of breast cancer among the female flight attendants.64 The largest study reviewed 44,021 flight attendants and found 129 breast cancer cases from 1988 to 1995. The SIR for breast cancer was 1.42 (95% CI, 1.09–1.83).65 In a meta-analysis combining all seven studies, the SIR for breast cancer was 1.44 among all flight attendants (95% CI, 1.26–1.65).64
4. Circulating melatonin and breast cancer risk
4.1. Early evidence from retrospective studies of melatonin and breast cancer risk
Several authors have proposed that the reduction in melatonin seen in night shift workers may be related to an increased risk of breast cancer.48 The first report to evaluate an association between circulating melatonin levels and breast cancer risk in 10 women was conducted by Bartsch et al. in 1981. It found in a small sample of women with advanced breast cancer, when compared with healthy controls, that they had lower levels of urinary melatonin.66 Subsequently, Tamarkin et al. found that women with ER-positive breast cancer had a reduced nocturnal increase in melatonin, and observed an inverse correlation between ER levels and peak melatonin values.67 Several subsequent studies examined melatonin levels in cancer patients.66–77 Because blood samples for melatonin were typically collected after a diagnosis of cancer in these retrospective studies, they are limited in their ability to assess the hormone’s predictive value for breast cancer risk. However, data from untreated patients with localized breast cancer provide evidence for a nocturnal surge of melatonin that parallels an increase in tumor-size and the development of distant metastases.68–70 Together with evidence from untreated primary prostate cancer patients, where melatonin levels were particularly high if well-differentiated G1 (incidental) carcinomas were present,78 these observations suggest complex interactions between the pineal gland and tumor growth. Melatonin has a potential role in different phases of carcinogenesis such as initial activation, inhibition of tumor growth, and re-stimulation as cancer cells disseminate; this complexity may account for apparent inconsistencies found in prospective studies.
4.2. Prospective studies of melatonin and breast cancer risk
More recently, evidence from prospective case-control studies nested in larger cohorts has been published. An Italian case-control study nested within the ORDET cohort assessed the concentration of melatonin’s major metabolite, 6-sulfatoxymelatonin (aMT6s) in 178 postmenopausal women with incident invasive breast cancer and 710 matched controls. Matching factors were age, date of recruitment (+/− 180 days), and laboratory batch. The multivariate relative risk, reported as the odds ratio (OR), for women in the highest quartile of total overnight aMT6s output compared with the lowest, was 0.56 (95% CI, 0.33–0.97). Overnight urinary aMT6s level and breast cancer risk were more strongly associated in women who were diagnosed with invasive breast cancer more than 4 years after urine collection (OR 0.34 highest versus lowest quartile, 95% CI, 0.15–0.75).79 A second case-control study in postmenopausal women was conducted nested within the NHS cohort.80 In that study, aMT6s levels were available for 357 postmenopausal women who developed incident breast cancer along with 533 matched control subjects. An increased concentration of urinary aMT6s was statistically significantly associated with a lower risk of breast cancer with an odds ratio for the highest versus lowest quartile of morning urinary 6-sulfatoxymelatonin of 0.62 (95% CI, 0.41–0.95; p for trend = 0.004).
Evidence for an association between urinary melatonin and breast cancer risk among premenopausal women is also sparse and has been less consistent, perhaps in part due to varying urine sampling methods used in these studies. Only two prospective studies have evaluated the associations, one of which did not find an increased risk,81 whereas the other one described a significantly reduced risk of breast cancer risk in women with the highest melatonin levels. In the first study, a prospective study of urinary 6MTs in 127 cases and 353 controls matched for age, recruitment date, menopausal status, day of menstrual cycle for premenopausal women, or number of years postmenopausal for postmenopausal women, the OR for breast cancer was 0.99, (95% CI, 0.58–1.70), comparing the highest to the lowest category.81 This study utilized 24-hour urine collection, in contrast to the NHS II, which used first-morning urine samples.82 The use of 24-hour urine may decrease power to detect potential differences by case-control status, but the confidence limits of this study do not preclude an effect of melatonin on breast cancer risk. In the NHS II cohort, finally, aMT6s levels were measured in the first-morning urine of 147 women with invasive breast cancer and 291 matched control subjects. The OR for women in the highest quartile of urinary aMT6s was 0.59 compared with those in the lowest quartile, and remained unchanged after adjusting for several important confounding factors.57
In summary, though evidence for an association between melatonin and breast cancer risk continues to accumulate, further definitive studies are needed. These studies should address optimized sampling strategies (i.e., to obtain nocturnal or first morning urine instead of 24-hour samples) and the time between urine collection (as an apparently healthy person) and cancer diagnosis, as it is conceivable that melatonin secretion is stimulated during early sub-clinical stages of tumor development.
5. Endometrial cancer
Prior evidence indicates that women with endometrial cancer have lower melatonin levels.83 Whether or not an MT2 melatonin receptor subtype found in a human endometrial cancer cell line may mediate the cancer protective effect is not clear.84 In 2007, the first prospective cohort study of shift work and endometrial cancer was published, and demonstrated that the risk of endometrial cancer was significantly elevated among women with 20 or more years work on rotating night shifts, particularly obese women.85 No significant increase in endometrial cancer risk was noted among leaner women working rotating night shifts. In this analysis from the Nurses’ Health Study, 121,701 women enrolled in a prospective cohort study in 1976, and 53,487 women provided data on rotating night shift work in 1988 and were followed through June 1, 2004. A total of 515 women developed medical record–confirmed invasive endometrial cancer. Cox regression models were used to calculate multivariate relative risks (MV RR), controlling for endometrial cancer risk factors. Women who worked 20+ years of rotating night shifts had a significantly increased risk of endometrial cancer (multivariate relative risk (MV RR) 1.47, 95% CI, 1.03–1.14).
Body mass index (BMI) is a known risk factor for endometrial cancer, and when stratified by body mass index, the highest endometrial cancer risk was identified among night workers with a BMI>30. Obese women working rotating night shifts doubled their baseline risk of endometrial cancer (MV RR 2.09, 95% CI 1.24–3.52) compared with obese women who did no night work, whereas a non-significant increase was seen among non-obese women (MV RR 1.07, 95% CI 0.60–1.92). Therefore, evidence of effect modification by BMI was present; that is, the risk of endometrial cancer among night shift workers varied by whether the women are obese or not. On stratification for other known endometrial cancer risk factors, including postmenopausal hormone use, oral contraceptive use, or smoking, no significant interaction was noted. Confirmation by other prospective trials of these interesting findings in endometrial cancer will substantiate potential future public health recommendations
Conclusion
Melatonin exhibits several oncostatic actions, including effects on estrogen and fat metabolism, which may impact the risk of breast and endometrial cancer in women. Exposure to light at night, as it occurs in night shift workers, has been shown to reduce melatonin levels. Evidence for a relationship between melatonin production and cancer risk is accumulating from several more recent nested case-control studies and is further supported by indirect evidence from observational studies of night workers, in whom a higher breast and endometrial cancer risk has been described. Future studies in this area will help to clarify whether melatonin has a role in future cancer prevention strategies.
Acknowledgments
Dr. Viswanathan receives support from NIH Grant 5K07 CA117979-01. Dr. Schernhammer is funded through the NIH CA114534.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Axelrod J, Wurtman RJ. Photic and neural control of indoleamine metabolism in the rat pineal gland. Adv Pharmacol. 1968;6(Pt A):157–66. doi: 10.1016/s1054-3589(08)61169-2. [DOI] [PubMed] [Google Scholar]
- 2.Kerenyi NA, Pandula E, Feuer GM. Oncostatic effects of the pineal gland. Drug Metab Drug Interact. 1990;8(3–4):313–9. [PubMed] [Google Scholar]
- 3.Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–53. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan V, Spence DW, Pandi-Perumal SR, et al. Melatonin, environmental light, and breast cancer. Breast Cancer Res Treat. 2008;108(3):339–50. doi: 10.1007/s10549-007-9617-5. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther. 2008;7(3):189–203. doi: 10.1177/1534735408322846. [DOI] [PubMed] [Google Scholar]
- 6.Stevens RG. Electric power use and breast cancer: a hypothesis. Am J Epidemiol. 1987;125(4):556–61. doi: 10.1093/oxfordjournals.aje.a114569. [DOI] [PubMed] [Google Scholar]
- 7.Stevens RG, Blask DE, Brainard GC, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115(9):1357–62. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schernhammer ES, Schulmeister K. Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer. 2004;90(5):941–3. doi: 10.1038/sj.bjc.6601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer Facts and Figures. Atlanta, Georgia: 2007. [Google Scholar]
- 10.Willett WC, Rockhill B, Hankinson SE, Hunter D, Colditz GA. Nongenetic Factors in the Causation of Breast Cancer. 3. Philadelphia, PA: Lippincott, Williams and Wilkins; 2004. [Google Scholar]
- 11.ACS. Cancer Facts and Figures. Atlanta: American Cancer Society; 2007. [Google Scholar]
- 12.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–43. [PubMed] [Google Scholar]
- 13.Viswanathan AN, Feskanich D, De Vivo I, et al. Smoking and the risk of endometrial cancer: results from the Nurses’ Health Study. Int J Cancer. 2005;114(6):996–1001. doi: 10.1002/ijc.20821. [DOI] [PubMed] [Google Scholar]
- 14.Blask DE, Brainard GC, Dauchy RT, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65(23):11174–84. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 15.Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27(2):179–88. doi: 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- 16.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336(3):186–95. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 17.Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003;60(7):1407–26. doi: 10.1007/s00018-003-2319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blask DE, Sauer LA, Dauchy RT, Holowachuk EW, Ruhoff MS, Kopff HS. Melatonin inhibition of cancer growth in vivo involves supression of tumor fatty acid metabolism via melatonin receptor-mediated signal. Cancer Res. 1999;59:4693–701. [PubMed] [Google Scholar]
- 19.Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, Maestroni GJ. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol Lett. 2001;22(1):45–7. [PubMed] [Google Scholar]
- 20.Vijayalaxmi, Thomas CRJ, Reiter RJ, Herman TS. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol. 2002;20(10):2575–601. doi: 10.1200/JCO.2002.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Kilic E, Kilic U, Reiter RJ, Bassetti CL, Hermann DM. Prophylactic use of melatonin protects against focal cerebral ischemia in mice: role of endothelin converting enzyme-1. J Pineal Res. 2004;37(4):247–51. doi: 10.1111/j.1600-079X.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 22.Hill SM, Blask DE. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988;48(21):6121–6. [PubMed] [Google Scholar]
- 23.Cos S, Gonzalez A, Guezmes A, et al. Melatonin inhibits the growth of DMBA-induced mammary tumors by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int J Cancer. 2006;118(2):274–8. doi: 10.1002/ijc.21401. [DOI] [PubMed] [Google Scholar]
- 24.Cos S, Blask DE, Lemus-Wilson A, Hill AB. Effects of melatonin on the cell cycle kinetics and “estrogen-rescue” of MCF-7 human breast cancer cells in culture. J Pineal Res. 1991;10(1):36–42. doi: 10.1111/j.1600-079x.1991.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 25.Cos S, Fernandez R, Guezmes A, Sanchez-Barcelo EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58:4383–90. [PubMed] [Google Scholar]
- 26.Ram PT, Dai J, Yuan L, et al. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett. 2002;179(2):141–50. doi: 10.1016/s0304-3835(01)00873-4. [DOI] [PubMed] [Google Scholar]
- 27.Yuan L, Collins AR, Dai J, Dubocovich ML, Hill SM. MT(1) melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol. 2002;192(1–2):147–56. doi: 10.1016/s0303-7207(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 28.del Rio B, Garcia Pedrero JM, Martinez-Campa C, Zuazua P, Lazo PSRS. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004;279(37):38294–302. doi: 10.1074/jbc.M403140200. [DOI] [PubMed] [Google Scholar]
- 29.Lawson NO, Wee BE, Blask DE, Castles CG, Spriggs LL, Hill SM. Melatonin decreases estrogen receptor expression in the medial preoptic area of inbred (LSH/SsLak) golden hamsters. Biol Reprod. 1992;47(6):1082–90. doi: 10.1095/biolreprod47.6.1082. [DOI] [PubMed] [Google Scholar]
- 30.Molis TM, Spriggs LL, Hill SM. Modulation of estrogen receptor mRNA expression by melatonin in MCF-7 human breast cancer cells. Mol Endocrinol. 1994;8(12):1681–90. doi: 10.1210/mend.8.12.7708056. [DOI] [PubMed] [Google Scholar]
- 31.Rato AG, Pedrero JG, Martinez MA, del Rio B, Lazo PS, Ramos S. Melatonin blocks the activation of estrogen receptor for DNA binding. FASEB Journal. 1999;13(8):857–68. doi: 10.1096/fasebj.13.8.857. [DOI] [PubMed] [Google Scholar]
- 32.Barrenetxe J, Delagrange P, Martinez JA. Physiological and metabolic functions of melatonin. J Physiol Biochem. 2004;60(1):61–72. doi: 10.1007/BF03168221. [DOI] [PubMed] [Google Scholar]
- 33.Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Trop Med Chem. 2002;2(2):113–32. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 34.Delagrange P, Atkinson J, Boutin JA, et al. Therapeutic perspectives for melatonin agonists and antagonists. J Neuroendocrinol. 2003;15(4):442–8. doi: 10.1046/j.1365-2826.2003.01016.x. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58(11):747–52. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachtigal MC, Patterson RE, Stratton KL, Adams LA, Shattuck AL, White E. Dietary supplements and weight control in a middle-age population. J Altern Complement Med. 2005;11(5):909–15. doi: 10.1089/acm.2005.11.909. [DOI] [PubMed] [Google Scholar]
- 37.Alonso-Vale MI, Borges-Silva CN, Anhe GF, et al. Light/dark cycle-dependent metabolic changes in adipose tissue of pinealectomized rats. Horm Metab Res. 2004;36(7):474–9. doi: 10.1055/s-2004-825723. [DOI] [PubMed] [Google Scholar]
- 38.Reiter RJ, Tan DX, Korkmaz A, et al. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog. 2007;13(4):303–28. doi: 10.1615/critrevoncog.v13.i4.30. [DOI] [PubMed] [Google Scholar]
- 39.Wu AH, Wang R, Koh WP, Stanczyk FC, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata C, Nagao Y, Yamamoto S, Shibuya C, Kashiki Y, Shimizu H. Light exposure at night, urinary 6-sulfatoxymelatonin, and serum estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1418–23. doi: 10.1158/1055-9965.EPI-07-0656. [DOI] [PubMed] [Google Scholar]
- 41.Schernhammer ES, Kroenke CH, Dowsett M, Folkerd E, Hankinson SE. Urinary 6-sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res. 2006;40(2):116–24. doi: 10.1111/j.1600-079X.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 42.Hansen A, Grade A, Hansen J. Diurnal urinary 6-sulfatxoymelatonin levels among healthy Danis nurses during work and leisure time. Chronobiol Int. 2006;23(6):1203–15. doi: 10.1080/07420520601100955. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi H, Iwamoto M, Harada N. Physiological effects of shift work on hospital nurses. J Hum Ergol (Tokyo) 2001;30(1–2):251–4. [PubMed] [Google Scholar]
- 44.McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res. 2006;15(3):241–9. doi: 10.1111/j.1365-2869.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 45.Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66(10):5521–5. doi: 10.1158/0008-5472.CAN-05-4652. [DOI] [PubMed] [Google Scholar]
- 46.Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65(20):9595–600. doi: 10.1158/0008-5472.CAN-05-2138. [DOI] [PubMed] [Google Scholar]
- 47.Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008 doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens RG. Artificial lighting in the industrialized world: circadian disruption and breast cancer. Cancer Causes Control. 2006;17(4):501–7. doi: 10.1007/s10552-005-9001-x. [DOI] [PubMed] [Google Scholar]
- 49.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19(6):614–22. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 50.Hahn RA. Profound bilateral blindness and the incidence of breast cancer. Epidemiology. 1991;2(3):208–10. doi: 10.1097/00001648-199105000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Feychting M, Oesterlund B, Ahlbom A. Reduced cancer incidence among the blind. Epidemiology. 1998;9(5):490–4. [PubMed] [Google Scholar]
- 52.Pukkala E, Verkasalo PK, Ojamo M, Rudanko SL. Visual impairment and cancer: a population-based cohort study in Finland. Cancer Causes & Control. 1999;10(1):13–20. doi: 10.1023/a:1008897317401. [DOI] [PubMed] [Google Scholar]
- 53.Kliukiene J, Tynes T, Andersen A. Risk of breast cancer among Norwegian women with visual impairment. Br J Cancer. 2001;84(3):397–9. doi: 10.1054/bjoc.2000.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophtalmol. 2002;120(11):1544–50. doi: 10.1001/archopht.120.11.1544. [DOI] [PubMed] [Google Scholar]
- 55.Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophtalmol. 1999;117(11):1487–95. doi: 10.1001/archopht.117.11.1487. [DOI] [PubMed] [Google Scholar]
- 56.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst. 2001;93(20):1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 57.Schernhammer E, Kroenke C, Laden F, Hankinson S. Night Work and Melatonin Levels in Women Participating in the Nurses’ Health Study II: Associate with Breast Cancer Risk. 2nd Symposium of the Dana-Farber/Harvard Cancer Center Program in Breast Cancer; Boston. 2005. [Google Scholar]
- 58.Tynes T, Hannevik M, Andersen A, Vistnes A, Haldorsen T. Incidence of breast cancer in Norwegian female radio and telegraph operators. Cancer Causes Control. 1996;7(2):197–204. doi: 10.1007/BF00051295. [DOI] [PubMed] [Google Scholar]
- 59.Hansen J. Breast Cancer Among Women Who Work at Night. Epidemiology. 2001;12(5):588. doi: 10.1097/00001648-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 60.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 61.Lie J-AS, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes & Control. 2005 doi: 10.1007/s10552-005-3639-2. in press. [DOI] [PubMed] [Google Scholar]
- 62.O’Leary ES, Schoenfeld ER, Stevens RG, et al. Shift work, light at night, and breast cancer on Long Island, New York. Am J Epidemiol. 2006;164(4):358–66. doi: 10.1093/aje/kwj211. [DOI] [PubMed] [Google Scholar]
- 63.Schwartzbaum J, Albohm A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health. 2007 doi: 10.5271/sjweh.1150. in press. [DOI] [PubMed] [Google Scholar]
- 64.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41(13):2023–32. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. Cancer incidence in California flight attendants (United States) Cancer Causes & Control. 2002;13(4):317–24. doi: 10.1023/a:1015284014563. [DOI] [PubMed] [Google Scholar]
- 66.Bartsch C, Bartsch H, Jain AK, Laumas KR, Wetterberg L. Urinary melatonin levels in human breast cancer patients. J Neural Transm. 1981;52(4):281–94. doi: 10.1007/BF01256753. [DOI] [PubMed] [Google Scholar]
- 67.Tamarkin L, Danforth D, Lichter A, et al. Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science. 1982;216(4549):1003–5. doi: 10.1126/science.7079745. [DOI] [PubMed] [Google Scholar]
- 68.Bartsch C, Bartsch H, Bellmann O, Lippert TH. Depression of serum melatonin in patients with primary breast cancer is not due to an increased peripheral metabolism. Cancer. 1991;67(6):1681–4. doi: 10.1002/1097-0142(19910315)67:6<1681::aid-cncr2820670634>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 69.Bartsch C, Bartsch H, Fuchs U, Lippert TH, Bellmann O, Gupta D. Stage-dependent depression of melatonin in patients with primary breast cancer. Correlation with prolactin, thyroid stimulating hormone, and steroid receptors. Cancer. 1989;64(2):426–33. doi: 10.1002/1097-0142(19890715)64:2<426::aid-cncr2820640215>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 70.Bartsch C, Bartsch H, Karenovics A, Franz H, Peiker G, Mecke D. Nocturnal urinary 6-sulphatoxymelatonin excretion is decreased in primary breast cancer patients compared to age-matched controls and shows negative correlation with tumor-size. J Pineal Res. 1997;23(2):53–8. doi: 10.1111/j.1600-079x.1997.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 71.Cook MR, Graham C, Kavet R, Stevens RG, Davis S, Kheifets L. Morning urinary assessment of nocturnal melatonin secretion in older women. J Pineal Res. 2000;28(1):41–7. doi: 10.1034/j.1600-079x.2000.280106.x. [DOI] [PubMed] [Google Scholar]
- 72.Danforth DNJ, Tamarkin L, Mulvihill JJ, Bagley CS, Lippman ME. Plasma melatonin and the hormone-dependency of human breast cancer. J Clin Oncol. 1985;3(7):941–8. doi: 10.1200/JCO.1985.3.7.941. [DOI] [PubMed] [Google Scholar]
- 73.Falkson G, Falkson HC, Steyn ME, Rapoport BL, Meyer BJ. Plasma melatonin in patients with breast cancer. Oncology. 1990;47(5):401–5. doi: 10.1159/000226857. [DOI] [PubMed] [Google Scholar]
- 74.Graham C, Cook MR, Kavet R, Sastre A, Smith DK. Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res. 1998;24(4):230–8. doi: 10.1111/j.1600-079x.1998.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 75.Lissoni P, Bastone A, Sala R, et al. The clinical significance of melatonin serum determination in oncological patients and its correlations with GH and PRL blood levels. Eur J Cancer Clin Oncol. 1987;23(7):949–57. doi: 10.1016/0277-5379(87)90340-3. [DOI] [PubMed] [Google Scholar]
- 76.Lissoni P, Crispino S, Barni S, et al. Pineal gland and tumor cell kinetics: serum levels of melatonin in relation to Ki-67 labeling rate in breast cancer. Oncology. 1990;47(3):275–7. doi: 10.1159/000226831. [DOI] [PubMed] [Google Scholar]
- 77.Skene DJ, Bojkowski CJ, Currie JE, Wright J, Boulter PS, Arendt J. 6-sulphatoxymelatonin production in breast cancer patients. J Pineal Res. 1990;8(3):269–76. doi: 10.1111/j.1600-079x.1990.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 78.Bartsch C, Bartsch H, Fluchter SH, Attanasio A, Gupta D. Evidence for modulation of melatonin secretion in men with benign and malignant tumors of the prostate: relationship with the pituitary hormones. J Pineal Res. 1985;2(2):121–32. doi: 10.1111/j.1600-079x.1985.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 79.Schernhammer E, Berrino F, Krogh V, et al. Urinary 6-Sulphatoxymelatonin levels and risk of breast cancer in postmenopausal women: the ORDET cohort. 2008 doi: 10.1158/1055-9965.EPI-09-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk in the Nurses’ Health Study cohort. 2008 doi: 10.1158/1055-9965.EPI-08-0637. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Travis RC, Allen DS, Fentiman IS, Key TJ. Melatonin and breast cancer: a prospective study. J Natl Cancer Inst. 2004;96(6):475–82. doi: 10.1093/jnci/djh077. [DOI] [PubMed] [Google Scholar]
- 82.Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst. 2005;97(14):1084–7. doi: 10.1093/jnci/dji190. [DOI] [PubMed] [Google Scholar]
- 83.Grin WGW. A significant correlation between melatonin deficiency and endometrial cancer. Gynecol Obstet Invest. 1998;45:62–5. doi: 10.1159/000009926. [DOI] [PubMed] [Google Scholar]
- 84.Kobayashi Y, Itoh MT, Kondo H, et al. Melatonin binding sites in estrogen receptor-positive cells derived from human endometrial cancer. J Pineal Res. 2003;35(2):71–4. [PubMed] [Google Scholar]
- 85.Viswanathan A, Hankinson SE, Schernhammer ES. Night Shift Work and the Risk of Endometrial Cancer. Cancer Res. 2007;67(21):10618–22. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]