Abstract
Background
Cruciferous vegetables, rich in isothiocyanates, may protect against lung cancer. Glutathione S-transferases are important in metabolizing isothiocyanates; hence, variants in GST genes may modify the association between cruciferous vegetable intake and lung cancer. We carried out a systematic review to characterize the association between cruciferous vegetable intake and lung cancer risk, with an emphasis on the potential interaction between cruciferous vegetables and GST gene variants.
Methods
A search of the epidemiological literature through December, 2007 was conducted using 15 bibliographic databases without language restrictions. Thirty-one studies on the association between lung cancer and either total cruciferous vegetable consumption (6 cohort and 13 case-control studies) or specific cruciferous vegetables (1 cohort and 11 case-control studies) were included.
Results
We ascertained 31 studies of either total cruciferous vegetable consumption (6 cohort,13 case-control studies) or specific cruciferous vegetables (1 cohort, 11 case-control studies) in relation to lung cancer risk. The risk of lung cancer among those in the highest category of total cruciferous vegetable intake was 23% lower in case-control studies (random-effects pooled odds ratio 0.77; 95% CI 0.68-0.88) and 17% lower in cohort studies (pooled relative risk 0.83; 95% CI 0.62-1.08) compared to those in the lowest category of intake. The strongest inverse association of total cruciferous vegetable intake with lung cancer risk was seen among individuals with GSTM1 and GSTT1 double null genotypes (OR 0.41; 95% CI 0.26-0.65).
Conclusions
Epidemiologic evidence suggests that cruciferous vegetable intake may be weakly and inversely associated with lung cancer risk. Due to a gene-diet interaction, the strongest inverse association was among those with homozygous deletion for GSTM1 and GSTT1.
INTRODUCTION
Lung cancer is the leading worldwide cause of cancer death (1). Cigarette smoking accounts for approximately 85% of the population burden of lung cancer in developed countries such as the United States, but selected dietary factors may modulate lung cancer risk (2). A major report of the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) concluded that the evidence was “limited—suggestive” that vegetable intake is inversely associated with lung cancer (3). However, the associations with lung cancer may vary according to the specific class of vegetable considered.
In particular, cruciferous vegetables (broccoli, cabbage, cauliflower, Brussels sprouts, kale) have been hypothesized to have anti-cancer properties that may contribute to reduced risk of lung cancer. Cruciferous vegetables are a rich source of isothiocyanates. Isothiocyanates may inhibit the bioactivation of procarcinogens found in tobacco smoke such as polycyclic aromatic hydrocarbons. Isothiocyanates may also enhance excretion of carcinogens before they can damage DNA (4, 5). Sulforaphane, a major isothiocyanate found in broccoli, can induce cell cycle arrest and apoptosis (5, 6).
GSTM1 and GSTT1, two Phase II enzymes encoded by two genes belonging to the Glutathione S-transferase (GST) family (6) play an important role in isothiocyanate metabolism (7). A common polymorphism in both the GSTM1 and GSTT1 genes results in gene deletion, and individuals with homozygous deletions are devoid of the respective enzyme activity (6). Individuals with homozygous deletion of GSTM1, GSTT1, or both, may metabolize isothiocyanates less efficiently and may be more intensely exposed to them after consumption of cruciferous vegetables. For this reason, individuals with the null GSTM1or GSTT1genotypes may have a lower risk of lung cancer when exposed to isothiocyanates (8-10).
Several epidemiological studies have assessed the association between cruciferous vegetable intake and lung cancer, but no systematic reviews are available to thoroughly unify this information. To address this information gap, we performed a meta-analysis of the evidence on this topic, including the potential gene-dietary interaction between cruciferous vegetable intake and GST genotypes.
METHODS
This systematic review stemmed from a project funded by the WCRF/AICR to develop a report entitled `Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective' (3). All work adhered to a standardized protocol developed by WCRF (http://www.wcrf.org/research/second_wcrf_aicr_report.lasso) (2). The WCRF report did not specifically consider the topic of cruciferous vegetables and lung cancer risk.
Search strategy
For the WCRF report, we sought all evidence on the associations between dietary intake, physical activity, or anthropometric measures and lung cancer that were reported in randomized clinical trials, cohort studies, and case-control studies. We used the search strategy for dietary factors as previously in (11), adapted for the outcome of lung carcinoma as described in (12). The following electronic databases were searched: PubMed, Embase, Pascal, ISI Web of Science, the Cochrane Library, Biological Abstracts, Cumulative Index to Nursing and Allied Health Literature, National Institute on Alcohol Abuse and Alcoholism (NIAAA)-Alcohol and Alcohol Problems Science Database, Agricola, CINAHL-EBSCOhost, Index Medicus for WHO Eastern Mediterranean Region, Index Medicus for South East Asian Region, and Latin American and Caribbean Center on Health Sciences Information. The search included all studies published up to April 2006. We also hand-searched references in the relevant review articles from the bibliographic database search and those cited in the 1997 WCRF report (2) or chosen for data abstraction. After the original WCRF search, we extended the PubMed search through December 2007. There were no language restrictions. If a published article was in a language that was beyond the expertise of our research team, WCRF had the article (13) translated into English.
Study Selection
The following exclusion criteria were applied to the screening of articles for the WCRF report: 1) no original data (reviews, editorials, meta-analyses); 2) studies not addressing the association between dietary intake, physical activity, or anthropometric measures and lung cancer; 3) studies not in humans; and 4) case reports and case series. The eligibility of each abstract or full-text article was assessed independently by two reviewers.
For the present report, we further limited the studies to those that reported on the association between cruciferous vegetable intake and lung cancer. Cruciferous vegetables were measured in different ways, including: 1) total cruciferous vegetable intake, 2) total isothiocyanate intake, and 3) intake of specific individual cruciferous vegetables (e.g. broccoli, cabbage, or cauliflower). When measures of association or variability were not reported or could not be calculated using the data provided, we excluded the papers from the formal meta-analysis but discussed the findings of the paper qualitatively. We made no systematic effort to contact authors. If separate reports from the same study were published, the report with the most updated data was selected for inclusion.
Data abstraction
For each eligible article, two reviewers abstracted the data into an electronic database created by WCRF. The data abstraction was performed serially, with any disagreements between reviewers resolved by consensus. Each reviewer classified the vegetables studied in each paper into classes (e.g. cruciferous vegetables, allium vegetables) according to the WCRF protocol (2). If a specific vegetable was not listed in the protocol, a nutritionist [LEC] assigned the appropriate vegetable subgroup. In the WCRF protocol, broccoli, cabbage, turnip/mustard greens, kale, sauerkraut, and cauliflower were classified as cruciferous vegetables. To assess study quality, we adapted the criteria used by Longnecker et al(14) for observational studies.
Statistical analysis
The primary quantitative analyses focused on total dietary intake of cruciferous vegetables. Total cruciferous vegetable consumption was typically defined as a combination of at least 3 cruciferous vegetables (15), which usually included broccoli and cabbage plus other cruciferous vegetables. We also analysed the associations between specific cruciferous vegetables and lung cancer risk. Separate meta-analyses were conducted for case-control and prospective cohort studies, by smoking status (never smokers or ever smokers) and by ethnicity (Western or Asian).
For all studies, odds ratios (OR) or relative risks (RR) and their respective 95% confidence intervals (95% CI) were abstracted. When a study reported several relative risk estimates, we abstracted the one adjusted for the most covariates. For studies that did not report estimates (16-18), we calculated the unadjusted ORs and 95% CIs based on published data. Pooled OR and RR estimates were obtained using inverse-variance weights in random effects models. Statistical heterogeneity was assessed using the DerSimonian and Laird's Q statistic and the I2 statistic.
Sensitivity analyses to examine the influence of each individual study were conducted by excluding each study from the meta-analysis and comparing the point estimates including and excluding the study. Meta-regression was used to explore for sources of heterogeneity. Publication bias was examined using funnel plots.
When sufficient data were presented in the original publication (≥3 exposure categories of intake frequency along with the numbers of cases and controls within each category), we assessed for the presence of a dose-response trend (19), To assess for interaction between GST genotypes and cruciferous vegetable intake on the risk of lung cancer, we estimated the association between total cruciferous vegetable intake and lung cancer stratified by GSTM1 and GSTT1 status. With the exception of one cohort study (20) which used a genotyping assay that could differentiate between three GSTM1 and GSTT1 genotypes of, two possible genotypes (present and null) were reported for GSTM1 and GSTT1 in all case-control studies. Data analyses were thus stratified as follows: GSTM1 present or null, GSTT1 present or null, and GSTM1/GSTT1 double present or double null. We tested for within-study effect modification by GST genotype by calculating the difference in (log) odds ratios between GST subgroups within each study (21, 22). We then obtained a summary interaction ORs by pooling these differences across studies.
RESULTS
Search results
We identified 37 studies that quantified the association between cruciferous vegetable consumption and lung cancer risk (Figure 1). Of these, we excluded two early reports from studies that subsequently published updated data (23, 24), two that reported on individual cruciferous vegetables but did not report the total number of cases (25, 26), one that reported on interaction between cruciferous vegetables and GST genes reported genotyping data that were not comparable and did not provide sufficient data to calculate standard errors to be included in the meta-analysis (20), and one that reported only on a biomarker (rather than dietary intake) of isothiocyanates (8). Of the 31 studies included in the meta-analyses, 19 studies (6 cohort (15, 27-30) and 13 case-control (13, 16-18, 31-39)) reported on total cruciferous vegetable intake. One cohort study (40) and 11 case-control studies (41-51) reported on intake of individual cruciferous vegetables. Quality assessment for the studies reported on total cruciferous consumption and lung cancer is summarized in Appendix 1.
Figure 1.
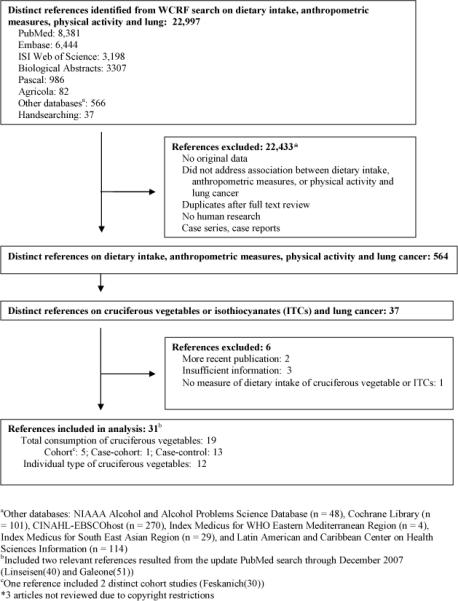
Study selection process
Appendix 1.
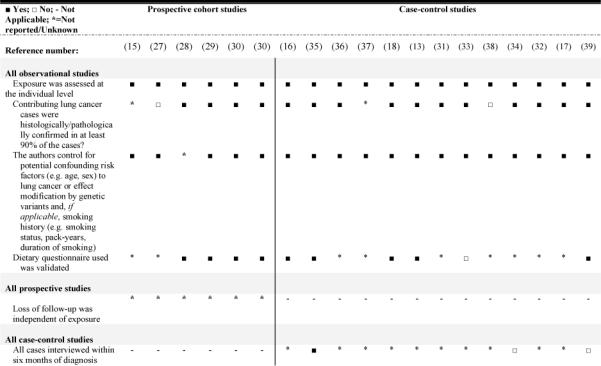

Quality criteriaa for evaluating the design and data analysis of observation studies on total cruciferous vegetable/ITC consumption and incident lung cancer.
Total cruciferous vegetables
Six prospective cohort studies (Table 1) and 13 case-control studies (Table 2), representing a total of 8227 lung cancer cases, reported associations between total cruciferous vegetable intake and lung cancer risk. The studies were carried out in Europe (8 studies (13, 27, 28, 32, 34, 36-38)), the United States (6 studies (15, 16, 18, 29, 30)), Asia (3 studies (17, 31, 35)), Canada (1 study (39)), and Australia (1 study (33)). The duration of follow-up ranged from 4-12 years in the six prospective cohort studies (15, 27-30). All prospective studies adjusted for smoking status. Of the 13 case-control studies, five were confined to never smokers (31, 34, 36, 38, 39) Of the eight studies that included ever smokers, five reported ORs adjusted for smoking (13, 32, 33, 35, 37).
Table 1.
Characteristics of cohort studies reporting relative risks (RR) and 95% confidence intervals (95% CI) for the association between total cruciferous vegetable consumption (highest versus lowest category) and lung cancer incidence
| Reference (Study name, Year) |
Country Study |
Follow-up (years) |
Sex | Age (at recruitment) |
No. of cases |
Size of cohort |
Case ascertainment |
Type of Dietary Questionnaire |
Total cruciferous vegetables measured as cabbage, cauliflower, and Brussels sprouts plus: |
|---|---|---|---|---|---|---|---|---|---|
| (15)Chow (LBIS, 1992) | US | 11.5 | M | 35+ | 219 | 17,633 | Death certificates | FFQ | Not specified |
| (30)Feskanich (NHS, 2000) | US | 12 | F | 30-55 | 519 | 121,700 | Pathology | FFQ | Broccoli, cole slaw/sauerkraut |
| (30)Freskanich (HPFS, 2000) | US | 10 | M | 40-75 | 274 | 51,529 | Medical records | FFQ | Broccoli, cole slaw/sauerkraut |
| (28)Voornips (NCS,2000)c | Netherlands | 6 | F/M | N/A | 1010 | 3500 | Pathology and cancer registries | FFQ | Kale |
| (29)Neuhouser (CARET, 2003) | US (Heavy smokers) | 8 | F/M | N/A | 326 | 7048 | Pathology and clinical records | FFQ | Broccoli, cole slaw, sauerkraut, mustard greens, turnip greens, and collards |
| (27)Miller (EPIC, 2004) | Europe | 4 | F/M | 25-70 | 860 | 482,924 | Histology, pathology, and cancer registries | FFQ | Broccoli |
Sex & centre stratified though author did not specified which sex
RR & 95% CI of placebo arm
Case cohort study (N of subcohort = 3500)
FFQ: Food frequency questionnaire; N/A: Unknown or not reported
Study's acronym: LBIS: Lutheran Brotherhood Insurance Society; NHS: Nurse's Health Study; HPFS: Health Professional Follow-up Study; NCS: Netherlands Cohort Study on Diet and Cancer; EPIC: European Prospective Investigation into Cancer and Nutrition; CARET: β-Carotene and Retinol Efficacy Trial
Table 2.
Characteristics of case-control studies reporting odd ratios (OR) and 95% confidence intervals (95% CI) for the association between total cruciferous vegetable consumption (highest versus lowest category) and lung cancer risk, without stratification by GST status
| Reference (Year) |
Country Study |
Source of Controls | Cases/ Ctrls | Sex | Age (Mean) |
Case ascertainment |
Type of Dietary Questionnaire |
Total cruciferous vegetables measured as broccoli, cabbage, and plus: |
|---|---|---|---|---|---|---|---|---|
| (31)Koo (1988) | Hong Kong | Unknown (Never smokers) | 88/137 | F | N/A (58) | Histology | FFQ | Non-specific |
| (33)Pierce (1989) | Australia: Melbourne | Hospital | 71/71 | M | N/A (67) | Histology & cytology | Dietary questions | Brussels sprouts |
| (32)Agudo (1997) | Spain | Hospital | 103/206 | F/M | 32-88 (63) | Histology | FFQ | Cauliflower |
| (34)Nyberg (1998) | Sweden | Hospital (Never smokers) | 124/235 | F/M | 30-80 (N/A) | Histology & cytology | FFQ | Cauliflower |
| (38)Brennan (2000) | Europe: Sweden, Germany, France, Spain, UK, and Italy | Hospital (Never smokers) | 506/1045 | F/M | N/A | Histology | FFQ | Kale, cauliflower |
| (16)Spitz (2000)b | US | Insurance registry (Ever smokers) | 503/465 | F/M | N/A (61) | Histology | FFQ | Cauliflower, Brussels sprouts, kale, sauerkraut, mustard greens, turnip greens, collard greens |
| (35)Zhao (2001)b | Asia: Singapore | Hospital | 233/187 | F | N/A (64) | Pathology | FFQ | Cauliflower, Chinese white/flowering cabbage, Chinese mustard, watercress, Chinese kale |
| (17)Seow (2001) | Asia: Singapore | Hospital | 54/174 | F | N/A (62) | Histology & pathology | FFQ | Total CV: Non-specific |
| (36)Lewis (2002)b | Europe and S.America | N/A (Never-smokers) | 122/123 | F/M | 18-85 (59) | Histology | FFQ | Cauliflower |
| (13)Caicoya (2002) | Europe: Spain | Hospital | 197/196 | F/M | N/A | Pathology & cytology | FFQ | Brussels sprouts |
| (43)Hu (2002) | Canada | Population (Never smokers) | 161/483 | F | 20-70+(N/R) | Histology | FFQ | |
| (18)Wang (2003)b | US | Hospital; Friends and non-blood related family members | 716/939 | F/M | 18-85 (59) | Histology | FFQ | Coleslaw/sauerkraut, cauliflower, Brussels sprouts, kale/mustard greens |
| (37)Brenna n (2005)b | Europe: Poland, Slovakia, Czech Republic, Romania, Russia, and Hungary | Hospital & Population | 2141/2168 | F/M | N/A | NA | FFQ | Brussels sprouts |
OR & 95% CI calculated from published data using EpiCalc 2000
Bolded studies reported ORs and 95% CIs stratified by GST status
FFQ: Food frequency questionnaire; N/A or N/R: Unknown or not reported
Compared to those in the lowest categories of total cruciferous vegetable intake, the risk of lung cancer among those in the highest consumption categories was 23% lower (random-effects pooled odds ratio 0.77; 95% CI 0.68-0.88; p heterogeneity = 0.31; I2=13.8%) in case- control studies and 17% lower in cohort studies (pooled relative risk 0.83; 95% CI: 0.62-1.08; p heterogeneity = 0.02; I2 = 62.8%) (Figure 2). For case-control studies, the results did not substantially differ when the meta-analysis was restricted to studies from never smokers (31, 32, 34-39) (pooled OR: 0.79; 95% CI: 0.64-0.97; p heterogeneity 0.48; I2=0%).
Figure 2.
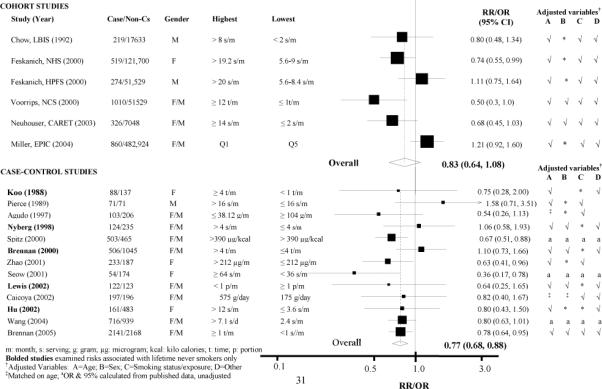
Forest plot of highest-versus-lowest category of total cruciferous vegetable/ITC consumption and lung cancer risk in cohort and case-control studies
All prospective cohort and 10 case-control studies reported at least three categories of total cruciferous vegetable intake (Table 3). Of the 6 prospective cohort studies, 3 studies were compatible with an inverse dose-response association between cruciferous vegetable intake and lung cancer risk (28-30), whereas three others showed no evidence of a dose-response trend (15, 27, 30). Eight case-control studies provided dose-response data in sufficient detail to be included in dose-response meta-analysis. The pooled OR for lung cancer associated with an increase of one cruciferous vegetable serving per day was 0.74 (95% CI: 0.73-0.75). Two additional case-control studies (18, 32) reported frequency of intake (i.e. low, medium, high) that could not be combined with the other studies. Of the two studies, one (32) was compatible with a decrease in lung cancer risk with increasing consumption of cruciferous vegetables whereas the other (18) showed no apparent trend.
Table 3.
Results of studies examining the association between total cruciferous vegetable intake and lung cancer using more than 2 categories of vegetable intake (dose-response analyses)
| Reference (Year) | Types of cruciferous vegetables (CV) examined | Intake frequency | Cases/Controlsa | OR(95% CI) | Pfor trend | Matched/Adjusted Variables |
|||
|---|---|---|---|---|---|---|---|---|---|
| A | B | c | o | ||||||
| Cohort studies | |||||||||
| (15)Chow (LBIS, 1992) | Not specified | < 2 time/month | 43/58,455a | 1.0 (ref) | NR | √ | * | √ | √ |
| 2-4 time/month | 112/146,730a | 0.9(0.6-1.3) | |||||||
| 5-8 time/month | 46/56,035a | 1.0(0.7-1.5) | |||||||
| >8 time/month | 18/25,861a | 0.8 (0.5-1.4) | |||||||
| (30)Feskanich (NHS, 2000) | Cabbage, cauliflower, and Brussels sprouts, broccoli, cole slaw/sauerkraut | 0-1.3 servings/week | NR | 1.0 (ref) | NR | √ | * | √ | √ |
| 1.4-2.2 servings/week | 0.80 (0.62-1.05) | ||||||||
| 2.3-3.2 servings/week | 0.85 (0.65-1.12) | ||||||||
| 3.3-4.8 servings/week | 0.76(0.57-1.01) | ||||||||
| >4.8 servings/week | 0.74 (0.55-0.99) | ||||||||
| (30)Feskanich (HPFS, 2000) | Cabbage, cauliflower, and Brussels sprouts, broccoli, cole slaw/sauerkraut | 0-1.3 servings/week | NR | 1.0 (ref) | NR | √ | * | √ | √ |
| 1.4-2.1 servings/week | 1.12(0.76-1.64) | ||||||||
| 2.2-3.3. servings/week | 1.05 (0.72-1.53) | ||||||||
| 3.4-5.0 servings/week | 0.86(0.57-1.30) | ||||||||
| >5.0 servings/week | 1.11(0.76-1.64) | ||||||||
| (28)Voornips (NCS,2000) | Cabbage, cauliflower, and Brussels sprouts, kale | ≥ 1 time/month | 52/598a | 1.0 (ref) | 0.003 | √ | √ | √ | √ |
| 2-3 time/month | 150/2713a | 0.7(0.4-1.1) | |||||||
| 4 time/month | 347/6676a | 0.6 (0.4-0.9) | |||||||
| 8 time/month | 308/6085a | 0.5 (0.4-0.8) | |||||||
| > 12 time/month | 53/988a | 0.5 (0.3-0.9) | |||||||
| (29)Neuhouser (CARET,2003) | Cabbage, cauliflower, and Brussels sprouts, broccoli, cole slaw, sauerkraut, mustard greens, turnip greens, and collards | ≥0.5 servings/week | NR | 1.0 (ref) | 0.01 | √ | √ | √ | √ |
| 0.6-1.2 servings/week | 1.36(0.98-1.88) | ||||||||
| 1.3-1.9 servings/week | 0.89(0.62-1.27) | ||||||||
| 2.0-3.4 servings/week | 0.96(0.67-1.39) | ||||||||
| ≥3.5 servings/week | 0.68 (0.45-1.04) | ||||||||
| (27)Miller (EPIC, 2004) | Cabbage, cauliflower, and Brussels sprouts, and broccoli | Q1 | NR | 1.0 (ref) | 0.25 | √ | √ | ||
| Q2 | 1.3 (0.89-1.43) | ||||||||
| Q3 | 1.21 (0.94-1.55) | ||||||||
| Q4 | 1.11 (0.87-1.43) | ||||||||
| Q5 | 1.21 (0.92-1.60) | ||||||||
| Case-control studies | |||||||||
| (31)Koo (1988) | Total CV: Non-specific | Never to < 1 time/month | 88/137 | 1.0 (ref) | 0.36 | √ | b | * | b |
| 1-3 times/month | 1.3 (0.64-1.99)b | ||||||||
| > 4 time/month | 0.75 (0.28-2.00)b | ||||||||
| (33)Pierce (1989) | Total CV: Broccoli, cabbage, and Brussels sprouts | Never to 1 time/month | 7/5 | 1.0 (ref) | 0.23 | b | b | b | b |
| 1-5 times/week | 21/43 | 0.35(0.1-1.23)b | |||||||
| > 7 times/week | 43/23 | 1.34(0.38-4.68)b | |||||||
| (32)Agudo (1997) | Total CV: Broccoli, cabbage, and cauliflower | Low | NR | 1.0 (ref) | 0.13 | √ | √ | √ | |
| Medium | NR | 0.93 (0.52-1.66) | |||||||
| High | NR | 0.54(0.26-1.13) | |||||||
| (34)Nyberg (1998) | Total CV: Broccoli, cabbage, and cauliflower | < Weekly | 49/81 | 1.0 (ref) | 0.33 | √ | √ | * | √ |
| Once weekly | 28/66 | 0.79 (0.42-1.52) | |||||||
| > Once weekly | 47/88 | 1.06(0.58-1.92) | |||||||
| (38)Brennan (2000) | Total CV: Broccoli, cabbage/kale, and cauliflower | Never to < Weekly | 200/382 | 1.0 (ref) | 0.76 | √ | √ | * | √ |
| < Weekly to Weekly | 111/234 | 1.0(0.7-1.3) | |||||||
| > Once weekly to Daily | 113/254 | 1.1 (0.7-1.6) | |||||||
| (17)Seow (2001) | Total CV: Non-specific | < 9 servings/week | 30/59 | 1.0 (ref) | NR | b | b | b | b |
| 9-15 servings /week | 13/55 | 0.46 (0.22-0.98)b | |||||||
| ≥16 servings/week | 11/60 | 0.36 (0.17-0.79)b | |||||||
| (36)Lewis (2002)c | Total CV: Broccoli, cabbage, and cauliflower | < 0.9 portion/month | 54/51 | 1.0 (ref) | 0.35 | √ | √ | * | √ |
| 1-4 portions/month | 37/53 | 0.58 (0.26-1.32) | |||||||
| > 4 portion/month | 31/19 | 0.64(0.25-1.67) | |||||||
| (39)Hu (2002) | Total CV: Broccoli and cabbage | ≤0.9 servings/week | 44/110 | 1.0 (ref) | 0.43 | √ | * | √ | |
| 1-2 servings/week | 50/155 | 0.7(0.4-1.3) | |||||||
| 2.1-6.0 servings/week | 32/101 | 0.7(0.4-1.4) | |||||||
| >6 servings/week | 33/112 | 0.8 (0.4-1.4) | |||||||
| (18)Wang (2003)c | Total CV: Broccoli, cabbage/coleslaw/sauerkraut, cauliflower, Brussel sprouts, kale/mustard greens | Low | 294/329 | 1.0 (ref) | NR | b | b | * | b |
| Medium | 198/296 | 0.75 (0.59-0.95)b | |||||||
| High | 224/314 | 0.80(0.63-1.01)b | |||||||
| (37)Brennan (2005)c | Total CV: Broccoli, cabbage, and Brussel sprouts | < Once monthly | 327/250 | 1(ref) | NR | √ | √ | √ | √ |
| Once weekly | 677/754 | 0.77 (0.62-0.95) | |||||||
| > Once weekly | 1137/1164 | 0.78 (0.64-0.96) | |||||||
Person-years for cohort studies
OR & 95% CI calculated from published data using EpiCalc 2000
Bolded studies reported ORs and 95% CIs stratified by GST status
Never smokers only; NR: Not reported
Study's acronym: LBIS: Lutheran Brotherhood Insurance Society;
NHS: Nurse's Health Study; HPFS: Health Professional Follow-up Study
EPIC: European Prospective Investigation into Cancer and Nutrition
CARET:β-Carotene and Retinol Efficacy Trial
NCS: Netherlands Cohort Study on Diet and Cancer
FFQ: Food frequency questionnaire; N/A
NR: Unknown or not reported
CV: Cruciferous vegetables
Matched/Adjusted Variables: A=Age; B=Sex; C=Smoking status/exposure; O=Other
Broccoli and cabbage as individual cruciferous vegetables were also inversely associated with lung cancer risk. In data generated from case-control studies, the pooled odds ratios for lung cancer risk comparing the highest-versus-lowest categories of intake were 0.53 (95% CI: 0.34-0.83; p heterogeneity = 0.14; I2 = 43.0%) for broccoli (39, 42, 44-46) and 0.70 (95% CI: 0.54-0.91; p heterogeneity = 0.02; I2=54.9%) for cabbage (37, 39-41, 43, 48-51).
Results stratified by GST genotypes
Five case-control studies (16, 35-37, 52) (N=3,715 lung cancer cases) reported on the association between cruciferous vegetable consumption and lung cancer risk stratified by GSTM1 and/or GSTT1 genotypes (Table 4). Of these, one reported only on GSTM1 (36), whereas four assessed both GSTM1 and GSTT1 (16, 18, 35, 37). Cruciferous vegetable consumption was measured by food frequency questionnaire in all 5 studies, and exposure was quantified either as intake of isothiocyanates (n=2 studies (16, 36)) or of total cruciferous vegetables (n=3 studies (18, 35, 37)).
In these 5 studies, the pooled OR of lung cancer for the highest-versus-lowest category of cruciferous vegetable intake was 0.74 (95% CI: 0.66-0.84) (Figure 3). When stratified by genotype, the inverse association between cruciferous vegetable intake and lung cancer was stronger in those who were null for both GSTM1 and GSTT1 (pooled OR: 0.41; 95% CI: 0.26-0.65; p heterogeneity=0.64; I2= 0) than in those with the GSTM1 and GSTT1 present genotype (OR: 0.75; 95% CI: 0.62-0.91; p heterogeneity=0.43; I2= 0). This gene-diet interaction was statistically significant (OR 0.48; 95% CI: 0.28-0.84).
Figure 3.
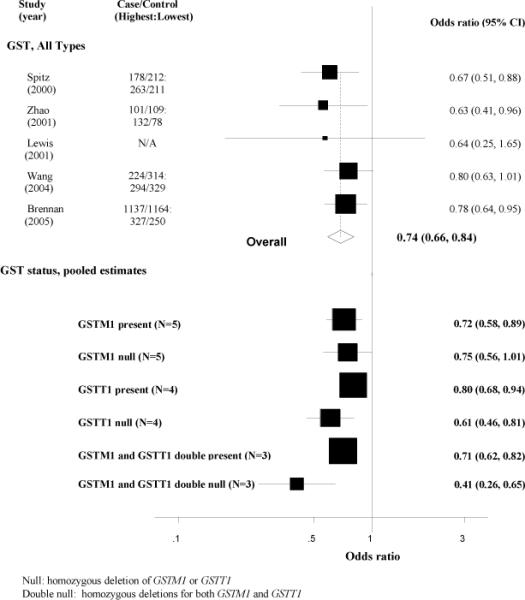
Forest plot of highest-versus-lowest category of total cruciferous vegetable/ITC consumption and lung cancer risk in case-control studies, stratified by GSTM1 and GSTT1 genotypes.
Heterogeneity and publication bias
Meta-regression results showed that study size, geographic location, or gender could not explain the observed heterogeneity for cohort studies. Sensitivity analysis results showed that the exclusion of individual studies did not substantially alter the pooled relative risks, which ranged from 0.75 to 0.91. All funnel plots to assess for possible indication of publication bias for meta-analyses of cohort and case-control studies, in addition to subgroup analyses by GST status, appear symmetrical (data not shown).
DISCUSSION
In our systematic review, which included 19 studies of the association between total cruciferous vegetable intake and lung cancer, we found a modest inverse association between cruciferous vegetable intake and lung cancer risk. Compared to those who consumed the least amount of cruciferous vegetables, the risk of lung cancer among those who consumed the most cruciferous vegetables was 23% lower in case-control studies (statistically significant) and 17% lower in prospective cohort studies (not statistically significant). Furthermore, case-control studies showed a significant inverse dose-response trend, although cohort studies provided only equivocal support for the presence of a dose-response trend. Intake of individual cruciferous vegetables (12 studies), such as broccoli and cabbage, was also inversely associated with lung cancer risk. These associations could not be explained by lack of adjustment for smoking in the original studies.
The summary estimates for case-control and cohort studies both provide evidence of an inverse association between cruciferous vegetable intake and lung cancer risk, but the association seen in cohort studies was slightly weaker and was not statistically significant. This may in part be due to the heterogeneity observed among the prospective cohort studies. A possible source of the heterogeneity in cohort studies may be the diverse populations studied. Of the six cohort studies, four were from the United States, and many of these were of unique study populations, such as white male life-insurance holders (15), health professionals (30), and asbestos-exposed, heavy smokers (29). The levels of cruciferous vegetable intake, along with the prevalence of lung cancer risk factors, would be expected to range widely across these study populations. This could have introduced heterogeneity and attenuated the summary relative risk.
A unique characteristic of cruciferous vegetables is that they are a rich source of glucosinolates (53). The anti-carcinogenic properties of cruciferous vegetables may be attributable to isothiocyanates derived specifically from glucosinolates (53, 54). Several experimental and mechanistic studies support a potential anti-cancer role of isothiocyanates (55, 56). Sulforaphane, an isothiocyanate found in broccoli, is involved in several pathways including induction of detoxifying genes, cell cycle control, and apoptosis; acting as an antioxidant (56), and inhibiting histone deacetylase (57). These experimental findings buttress the biologic plausibility of the association between cruciferous vegetable intake and lung cancer risk. However, the epidemiologic evidence considered in this systematic review does not allow inferences to pinpoint isothiocyanates as the key protective constituent of cruciferous vegetables as other nutrients and phytochemicals (e.g. folate, flavonols, and carotenoids) found in cruciferous vegetables may also be responsible for the protection against lung cancer.
Genetic factors related to isothiocyanate metabolism have been hypothesized to contribute to inter-individual differences in the degree of protection conferred by cruciferous vegetable consumption (58). Specifically, individuals with GSTM1 and GSTT1 null genotypes metabolize isothiocyanates less efficiently, permitting isothiocyanates to remain biologically active for a longer period (58, 59). In our meta-analysis, a gene-diet interaction was present; when stratified by GSTM1 and GSTT1 variants, the inverse associations between cruciferous vegetable intake and lung cancer risk were more marked in those with the double null genotype. Corroborative findings were also reported in a nested case-control study carried out in China, in which the significant inverse association between urinary isothiocyanate levels and lung cancer risk was stronger among men with the GSTM1 and GSTT1 double null genotype (8). The only cohort study (20) to report on the potential interaction between cruciferous vegetables and GSTM1 on lung cancer risk only presented partial results in the manuscript text, so could not be included in the formal meta-analyses, found no statistically significant interaction not for individuals with one functional allele or homozygous deletion (i.e. null). The presence of a potential gene-diet interaction adds internal consistency to the overall body of evidence on the association between cruciferous vegetables and lung cancer, and takes a step toward addressing the causal criteria of biologic plausibility and coherence. The genotype prevalences for GSTM1 and GSTT1 homozygous deletion vary by race/ethnicity but range from 42-60% and 24-51%, respectively (60), making this an important question to resolve due its public health relevance.
Any consideration of a dietary factor in relation to lung cancer needs to carefully evaluate the potential confounding role of cigarette smoking. Cigarette smoking is the principal cause of lung cancer and cigarette smokers tend to eat less healthful diets than nonsmokers (61). Thus, even studies that statistically adjusted for cigarette smoking may show associations due to residual confounding (62). The inverse association between cruciferous vegetable intake and lung cancer, however, was similar in studies limited to never smokers. Furthermore, residual confounding by smoking is unlikely to explain the interaction between cruciferous vegetable intake and GST genotypes. A weakness of the evidence that comprises this systematic review is the measurement error inherent in the use of dietary questionnaires; in the retrospective case-control studies, the potential for recall bias due to cases and controls differential recall of dietary habits is a particular concern (63). Furthermore, the association between cruciferous vegetables and lung cancer may differ depending on whether the vegetables are consumed raw or cooked, because this influences the bioavailability of isothiocyanates (64). The lack of information about cooking methods is thus a potential source of heterogeneity in the results.
In this systematic review, higher intake of cruciferous vegetables was modestly inversely associated with lung cancer risk. The inverse association was stronger among individuals with the null genotype for both GSTM1 and GSTT1. Compared to case-control studies, the associations observed in cohort studies were weaker, were not statistically significant, and had more heterogeneity. Furthermore, the evidence for the gene-diet interaction is based only on case-control data. Consequently, additional cohort data are needed to help understand the lack of consistency in prospective studies and to provide a more precise estimate of the interaction between cruciferous vegetable intake and GST genotype.
Table 4.
Evidence table of case-control studies reporting odds ratios (OR) and 95% confidence limits (95% CL) for the association between total cruciferous vegetable/ITC consumption (lowest versus highest category) and lung cancer risk, stratified by GST status
| Study | Cases/ Controls |
Exposure | GSTM1 null | GSTT1 null | Intake Frequency |
GST status | OR(95%CI)a |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Ctrls | Cases | Ctrls | Present | Null | |||||
| (16)Spitz,2000 | ||||||||||
| 503/465 | Dietary ITC intake | 246 (49.4) | 226 (48.8) | 132 (27.3) | 104 (22.7) | ≤ median > median | GSTM1 | 0.56 (0.38-0.82) | 0.81 (0.55-1.19) | |
| GSTT1 | 0.69 (0.50-0.94) | 0.53 (0.31-0.91) | ||||||||
| GSTM1&T1* | 0.67 (0.50-0.89) | 0.46 (0.20-1.04) | ||||||||
| (35)Zhao, 2001 | ||||||||||
| 233/187 | Dietary ITC intake | 146 (62.7) | 119 (63.6) | 132 (56.7) | 102 (54.5) | ≤ median > median | GSTM1 | 0.78 (0.39-1.59) | 0.55 (0.33-0.93) | |
| GSTT1 | 0.75 (0.40-1.40) | 0.54(0.31-0.95) | ||||||||
| GSTM1/T1* | 0.69(0.41-1.17) | 0.47 (0.23-0.95) | ||||||||
| (36)Lewis, 2002 | ||||||||||
| 122/123 | Dietary cruciferous vegetable intake | 65 | 53 | N/A | N/A | < 1 per month | GSTM1 | 0.65 (0.16-2.66) | 0.27 (0.06-1.33) | |
| (53.3) | (43.1) | > 4 per month | ||||||||
| (18)Wang,2003 | ||||||||||
| 716/939 | Dietary cruciferous vegetables intake | 404 (56.4) | 516 (55.0) | 138 (19.4) | 185 (19.8) | GSTM1 | 0.61 (0.39-0.95) | 1.15 (0.78-1.68) | ||
| GSTT1 | 0.87 (0.63-1.21) | 0.81 (0.42-1.51) | ||||||||
| GSTM1&T1* | 0.67 (0.54-0.82)b | 0.70 (0.39-1.26)b | ||||||||
| (37)Brennan, 2005 | ||||||||||
| 2141/2168 | Dietary cruciferous vegetable intake | 1022 | 986 | 340 | 344 | < once/ month | GSTM1 | 0.89(0.67-1.18) | 0.67 (0.49-0.91) | |
| (47.7) | (45.4) | (15.8) | (15.8) | ≥ 4 times/month | ||||||
| GSTT1 | 0.83 (0.66-1.03) | 0.63 (0.37-1.07) | ||||||||
| GSTM1&T1* | 0.88 (0.65-1.21) | 0.28(0.11-0.67) | ||||||||
Lowest (reference group) versus highest
OR & 95% CI calculated from published data using EpiCalc 2000
ITC: Isothiocyanates
PCR:Polymerase Chain Reaction
GSTM1&T1: GSTM1 and GSTT1; GSTM1/T1: GSTM1 or GSTT1
Acknowledgments
Funding: This research was primarily funded by the World Cancer Research Fund. This report was reviewed by the funding source prior to submission (with no substantive changes), but is independent of the funding source.
REFERENCES
- (1).Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARCPress; Lyon: 2004. [Google Scholar]
- (2).Food, nutrition, and the prevention of cancer: a global perspective. World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR); Washington D.C: 1997. [DOI] [PubMed] [Google Scholar]
- (3).World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- (4).Gasper A, Al-janobi A, Smith J, Bacon J, P F, C A, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–91. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- (5).Seow A, Vainio H, Yu MC. Effect of glutathione-S-transferase polymorphisms on the cancer preventive potential of isothiocyanates: An epidemiological perspective. Mutat Res. 2005;592(12):58–67. doi: 10.1016/j.mrfmmm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- (6).Ketterer B. A bird's eye view of the glutathione transferase field. Chem Biol Interact. 2001;138(1):27–42. doi: 10.1016/s0009-2797(01)00277-0. [DOI] [PubMed] [Google Scholar]
- (7).Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995;206(2):748–55. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- (8).London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, et al. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356(9231):724–9. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- (9).Hirvonen A. Polymorphisms of xenobiotic-metabolizing enzymes and susceptibility to cancer. Environ Health Perspect. 1999;107(Suppl 1):37–47. doi: 10.1289/ehp.99107s137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555(12):191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- (11).Gallicchio L, Matanoski G, Tao XG, Chen L, Lam TK, Boyd K, et al. Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. Int J Cancer. 2006;119(5):1125–35. doi: 10.1002/ijc.21946. [DOI] [PubMed] [Google Scholar]
- (12).Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, et al. Arsenic in drinking water and lung cancer: A systematic review. Environ Res. 2008 doi: 10.1016/j.envres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- (13).Caicoya M. Lung cancer and vegetable consumption in Asturias, Spain. A case control study. Med Clin (Barc) 2002;119(6):206–10. [PubMed] [Google Scholar]
- (14).Longnecker M, Berlin J, Orza M, Chalmers T. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA. 1988;260:652–6. [PubMed] [Google Scholar]
- (15).Chow WH, Schuman LM, McLaughlin JK, Bjelke E, Gridley G, Wacholder S, et al. A cohort study of tobacco use, diet, occupation, and lung cancer mortality. Cancer Causes Control. 1992;3(3):247–54. doi: 10.1007/BF00124258. [DOI] [PubMed] [Google Scholar]
- (16).Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L, et al. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9(10):1017–20. [PubMed] [Google Scholar]
- (17).Seow A, Zhao B, Lee EJ, Poh WT, Teh M, Eng P, et al. Cytochrome P4501A2 (CYP1A2) activity and lung cancer risk: a preliminary study among Chinese women in Singapore. Carcinogenesis. 2001;22(4):673–7. doi: 10.1093/carcin/22.4.673. [DOI] [PubMed] [Google Scholar]
- (18).Wang Y, Spitz MR, Schabath MB, Ali-Osman F, Mata H, Wu X. Association between glutathione S-transferase p1 polymorphisms and lung cancer risk in Caucasians: a case-control study. Lung Cancer. 2003;40(1):25–32. doi: 10.1016/s0169-5002(02)00537-8. [DOI] [PubMed] [Google Scholar]
- (19).Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- (20).Sorensen M, Raaschou-Nielsen O, Brasch-Andersen C, Tjonneland A, Overvad K, Autrup H. Interactions between GSTM1, GSTT1 and GSTP1 polymorphisms and smoking and intake of fruit and vegetables in relation to lung cancer. Lung Cancer. 2007;55(2):137–44. doi: 10.1016/j.lungcan.2006.10.010. Epub 2006 Nov 22. [DOI] [PubMed] [Google Scholar]
- (21).Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit? Lancet. 2005;365(9456):341–6. doi: 10.1016/S0140-6736(05)17790-3. [DOI] [PubMed] [Google Scholar]
- (23).Miller AB. Vegetables and fruits and lung cancer. IARC Sci Publ. 2002;156:85–7. [PubMed] [Google Scholar]
- (24).Agudo A, Slimani N, Ocke MC, Naska A, Miller AB, Kroke A, et al. Consumption of vegetables, fruit and other plant foods in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Public Health Nutr. 2002;5(6B):1179–96. doi: 10.1079/PHN2002398. [DOI] [PubMed] [Google Scholar]
- (25).Steinmetz KA, Potter JD, Folsom AR. Vegetables, fruit, and lung cancer in the Iowa Women's Health Study. Cancer Res. 1993;53(3):536–43. [PubMed] [Google Scholar]
- (26).Speizer FE, Colditz GA, Hunter DJ, Rosner B, Hennekens C. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA) Cancer Causes Control. 1999;10(5):475–82. doi: 10.1023/a:1008931526525. [DOI] [PubMed] [Google Scholar]
- (27).Miller AB, Altenburg HP, Bueno-de-Mesquita B, Boshuizen HC, Agudo A, Berrino F, et al. Fruits and vegetables and lung cancer: Findings from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2004;108(2):269–76. doi: 10.1002/ijc.11559. [DOI] [PubMed] [Google Scholar]
- (28).Voorrips LE, Goldbohm RA, Verhoeven DT, van Poppel GA, Sturmans F, Hermus RJ, et al. Vegetable and fruit consumption and lung cancer risk in the Netherlands Cohort Study on diet and cancer. Cancer Causes Control. 2000;11(2):101–15. doi: 10.1023/a:1008906706084. [DOI] [PubMed] [Google Scholar]
- (29).Neuhouser ML, Patterson RE, Thornquist MD, Omenn GS, King IB, Goodman GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET) Cancer Epidemiol Biomarkers Prev. 2003;12(4):350–8. [PubMed] [Google Scholar]
- (30).Feskanich D, Ziegler RG, Michaud DS, Giovannucci EL, Speizer FE, Willett WC, et al. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst. 2000;92(22):1812–23. doi: 10.1093/jnci/92.22.1812. [DOI] [PubMed] [Google Scholar]
- (31).Koo LC. Dietary habits and lung cancer risk among Chinese females in Hong Kong who never smoked. Nutr Cancer. 1988;11(3):155–72. doi: 10.1080/01635588809513983. [DOI] [PubMed] [Google Scholar]
- (32).Agudo A, Esteve MG, Pallares C, Martinez-Ballarin I, Fabregat X, Malats N, et al. Vegetable and fruit intake and the risk of lung cancer in women in Barcelona, Spain. Eur J Cancer. 1997;33(8):1256–61. doi: 10.1016/s0959-8049(97)00050-6. [DOI] [PubMed] [Google Scholar]
- (33).Pierce RJ, Kune GA, Kune S, Watson LF, Field B, Merenstein D, et al. Dietary and alcohol intake, smoking pattern, occupational risk, and family history in lung cancer patients: results of a case-control study in males. Nutr Cancer. 1989;12(3):237–48. doi: 10.1080/01635588909514023. [DOI] [PubMed] [Google Scholar]
- (34).Nyberg F, Agrenius V, Svartengren K, Svensson C, Pershagen G. Dietary factors and risk of lung cancer in never-smokers. Int J Cancer. 1998;78(4):430–6. doi: 10.1002/(sici)1097-0215(19981109)78:4<430::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- (35).Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, et al. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1063–7. [PubMed] [Google Scholar]
- (36).Lewis S, Brennan P, Nyberg F, Ahrens W, Constantinescu V, Mukeria A, et al. Cruciferous vegetable intake, GSTM1 genotype and lung cancer risk in a non-smoking population. IARC Sci Publ. 2002;156:507–8. [PubMed] [Google Scholar]
- (37).Brennan P, Hsu CC, Moullan N, Szeszenia-Dabrowska N, Lissowska J, Zaridze D, et al. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomisation approach. Lancet. 2005;366(9496):1558–60. doi: 10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- (38).Brennan P, Fortes C, Butler J, Agudo A, Benhamou S, Darby S, et al. A multicenter case-control study of diet and lung cancer among non-smokers. Cancer Causes Control. 2000;11(1):49–58. doi: 10.1023/a:1008909519435. [DOI] [PubMed] [Google Scholar]
- (39).Hu J, Mao Y, Dryer D, White K. Risk factors for lung cancer among Canadian women who have never smoked. Cancer Detect Prev. 2002;26(2):129–38. doi: 10.1016/s0361-090x(02)00038-7. [DOI] [PubMed] [Google Scholar]
- (40).Linseisen J, Rohrmann S, Miller AB, Bueno-de-Mesquita HB, Buchner FL, Vineis P, et al. Fruit and vegetable consumption and lung cancer risk: updated information from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2007;121(5):1103–14. doi: 10.1002/ijc.22807. [DOI] [PubMed] [Google Scholar]
- (41).Axelsson G, Rylander R. Diet as risk for lung cancer: a Swedish case-control study. Nutr Cancer. 2002;44(2):145–51. doi: 10.1207/S15327914NC4402_04. [DOI] [PubMed] [Google Scholar]
- (42).Bond GG, Thompson FE, Cook RR. Dietary vitamin A and lung cancer: results of a case-control study among chemical workers. Nutr Cancer. 1987;9109(23) doi: 10.1080/01635588709513918. [DOI] [PubMed] [Google Scholar]
- (43).Hu J, Johnson K, Mao Yea. A case-control study of diet and lung cancer in northeast China. Int J Cancer. 1997;71:924–31. doi: 10.1002/(sici)1097-0215(19970611)71:6<924::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- (44).Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92(2):154–60. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- (45).Mettlin C. Milk drinking, other beverage habits, and lung cancer risk. Int J Cancer. 1989;43(4):608–12. doi: 10.1002/ijc.2910430412. [DOI] [PubMed] [Google Scholar]
- (46).Mohr DL, Blot WJ, Tousey PM, Van Doren ML, Wolfe KW. Southern cooking and lung cancer. Nutr Cancer. 1999;35(1):34–43. doi: 10.1207/S1532791434-43. [DOI] [PubMed] [Google Scholar]
- (47).Kvale G, Bjelke E, Gart JJ. Dietary habits and lung cancer risk. Int J Cancer. 1983;31(4):397–405. doi: 10.1002/ijc.2910310402. [DOI] [PubMed] [Google Scholar]
- (48).Sankaranarayanan R, Varghese C, Duffy SW, Padmakumary G, Day NE, Nair MK. A case-control study of diet and lung cancer in Kerala, south India. Int J Cancer. 1994;58(5):644–9. doi: 10.1002/ijc.2910580505. [DOI] [PubMed] [Google Scholar]
- (49).Ruano-Ravina A, Figueiras A, Dosil-Diaz O, Barreiro-Carracedo A, Barros-Dios JM. A population-based case-control study on fruit and vegetable intake and lung cancer: a paradox effect? Nutr Cancer. 2002;43(1):47–51. doi: 10.1207/S15327914NC431_5. [DOI] [PubMed] [Google Scholar]
- (50).Gao CM, Tajima K, Kuroishi T, Hirose K, Inoue M. Protective effects of raw vegetables and fruit against lung cancer among smokers and ex-smokers: a case-control study in the Tokai area of Japan. Jpn J Cancer Res. 1993;84(6):594–600. doi: 10.1111/j.1349-7006.1993.tb02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Galeone C, Negri E, Pelucchi C, La Vecchia C, Bosetti C, Hu J. Dietary intake of fruit and vegetable and lung cancer risk: a case-control study in Harbin, northeast China. Ann Oncol. 2007;18(2):388–92. doi: 10.1093/annonc/mdl387. [DOI] [PubMed] [Google Scholar]
- (52).Wang J, Deng Y, Cheng J, Ding J, Tokudome S. GST genetic polymorphisms and lung adenocarcinoma susceptibility in a Chinese population. Cancer Lett. 2003;201(2):185–93. doi: 10.1016/s0304-3835(03)00480-4. [DOI] [PubMed] [Google Scholar]
- (53).Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–36. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Fahey JW, Zalcmann P, Talalay P. The chemistry diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- (55).Myzak MC, Dashwood RH. Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 2006;233(2):208–18. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64(9):1105–27. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Experimental biology and medicine (Maywood, NJ. 2007;232(2):227–34. [PMC free article] [PubMed] [Google Scholar]
- (58).Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132(10):2991–4. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- (59).Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: structure-activity studies. Biochem J. 1995;311:453–9. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ye Z, Song H, Higgins JP, Pharoah P, Danesh J. Five glutathione s-transferase gene variants in 23,452 cases of lung cancer and 30,397 controls: meta-analysis of 130 studies. PLoS Med. 2006;3(4):e91. doi: 10.1371/journal.pmed.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180(2):121–37. doi: 10.1016/s0300-483x(02)00386-4. [DOI] [PubMed] [Google Scholar]
- (62).Stram DO, Huberman M, Wu AH. Is residual confounding a reasonable explanation for the apparent protective effects of beta-carotene found in epidemiologic studies of lung cancer in smokers? Am J Epidemiol. 2002;155(7):622–8. doi: 10.1093/aje/155.7.622. [DOI] [PubMed] [Google Scholar]
- (63).Natarajan L, Flatt SW, Sun X, Gamst AC, Major JM, Rock CL, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol. 2006;163(8)(8):770. doi: 10.1093/aje/kwj082. Epub 2006 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Vermeulen M, van den Berg R, Freidig AP, van Bladeren PJ, Vaes WH. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. Journal of agricultural and food chemistry. 2006;54(15):5350–8. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]


