Abstract
Behavioral and brain imaging research indicates that human infants, humans adults, and many non-human animals represent large non-symbolic numbers approximately, discriminating between sets with a ratio limit on accuracy. Some behavioral evidence, especially with human infants, suggests that these representations differ from representations of small numbers of objects. To investigate neural signatures of this distinction, event-related potentials (ERPs) were recorded as adult humans passively viewed the sequential presentation of dot arrays in an adaptation paradigm. In two studies, subjects viewed successive arrays of a single number of dots interspersed with test arrays presenting the same or a different number; numerical range (small numerical quantities 1-3 vs. large numerical quantities 8-24) and ratio difference varied across blocks as continuous variables were controlled. An early-evoked component (N1), observed over widespread posterior scalp locations, was modulated by absolute number with small but not large number arrays. In contrast, a later component (P2p), observed over the same scalp locations, was modulated by the ratio difference between arrays for large but not small numbers. Despite many years of experience with symbolic systems that apply equally to all numbers, adults spontaneously process small and large numbers differently. They appear to treat small-number arrays as individual objects to be tracked through space and time, and large-number arrays as cardinal values to be compared and manipulated.
Keywords: event-related potentials, numerical cognition, parietal lobe, visual attention
Introduction
From an early age, humans and other animals represent the approximate numerical magnitude of large sets of objects, with a ratio limit on precision (see Feigenson, Dehaene, & Spelke, 2004 for a review). It is not clear, however, whether this ability extends to small sets of objects and allows representations of numerical magnitude in the small number range (3 or less). Behavioral studies suggest that sets of 1, 2, or 3 objects are represented differently from larger sets, both for human infants (e.g. Xu, 2005) and for adults (e.g. Mandler & Shebo, 1982). Nonetheless, the interpretation of these studies is controversial, as other evidence supports a common representation of small and large numerical sets in human infants (Brannon, 2002), human adults (e.g. Cordes, Gellman, & Gallistel, 2002), and non-human animals (e.g. Brannon & Terrace, 1998). The present experiments attempt to address this controversy through electrophysiological studies of human adults' spontaneous response to number.
Research in cognitive psychology and neuroscience has revealed a consistent signature of numerical cognition; the size of a detectable difference is a constant proportion of the original stimulus value (see Dehaene, 2007). For example, functional Magnetic Resonance Imaging (fMRI) experiments using an adaptation paradigm revealed a bilateral increase in BOLD signal of the intraparietal sulci in response to change in number, but not shape, that was proportional to the ratio of change between adaptation and test number (Piazza, Izard, Le Bihan, & Dehaene, 2004). Furthermore, the activation patterns were observed across the same ratio changes of different numbers providing neural evidence of ratio dependent numerical encoding in accord with Weber's law. Similar findings have been observed using a variety of behavioral and neuroimaging paradigms (Ansari, Dhital, & Siong, 2006; Cantlon, Brannon, Carter, & Pelphrey, 2006; Cordes, Gelman, & Gallistel, 2001; Whalen, Gallistel, & Gelman, 1999; although see Shuman & Kanwisher, 2004), but ratio dependency has yet to be tested in the small number range.
Although a variety of brain response measures have been used to investigate numerical cognition, event related potentials (ERPs) in response to numerical tasks are especially well established, allowing great sensitivity to processing over time (Dehaene, 1996). ERP studies have shown a second posterior positivity (P2p), peaking around 250 ms over posterior electrode sites within the parieto-occipito-temporal junction, associated with numerical representation, estimation, and comparison processes (Dehaene, 1996; Libertus, Woldorff, & Brannon, 2007; Pinel, Dehaene, Riviere, & Le Bihan, 2001; Temple & Posner, 1998). Nevertheless, a ratio effect similar to that observed with fMRI (Ansari et al., 2006; Piazza et al., 2004) has yet to be shown electrophysiologically. Following from the cited work, ratio dependency would most likely be observed as a systematic modulation of P2p amplitude in the large number range. If small and large non-symbolic numbers share a common representational format, moreover, then small-number changes of the same ratio should evoke similar patterns of P2p modulation.
Alternatively, it has been proposed that small numbers of objects are represented by parallel mechanisms of object-directed attention, such that spatial attention is recruited to the specific locations of the objects to be tracked (see Feigenson, Dehaene, & Spelke, 2004 for a review). On this view, arrays of three objects are not represented as a set with cardinal value three but rather as an object x and an object y and an object z, each in distinct locations. If this hypothesis is correct, therefore, the brain response to small numbers may not be modulated by differences in cardinal values across arrays, but rather by the absolute number of objects in each array. This prediction is supported by fMRI work showing that in multiple object tracking tasks the BOLD response in brain areas associated with spatial attention increases as the number of objects to be tracked increases (Culham, Brandt, Cavanagh, Kanwisher, Dale, & Tottell, 1998; Culham, Cavanagh, & Kanwisher, 2001).
Other research lends support for the differential role of attention in small number compared to large number processing. A recent experiment using fMRI found heightened BOLD signal in the temporal-parietal junction for small non-symbolic number comparisons and suppression of this region for large number comparisons (Ansari, Lyons, Eimeren, and Xu, 2007). The authors interpreted these results as evidence of stimulus-driven attention for small but not large number comparison tasks. Similarly, a recent ERP study found that the first negative component (N1) was reliably modulated by cardinal value for non-symbolic arrays containing 1-4 objects, but not for arrays containing 6-10 objects (Libertus, et al., 2007). An extensive literature suggests that N1 is modulated by the distribution of spatial attention (see Hillyard & Anllo-Vento, 1998; Hillyard, Teder-Sälejärvi, & Münte, 1998; or Luck, 2005b for a review), consistent with the thesis that arrays with small numbers of objects are processed by a distinct system of object-directed attention. Nevertheless, the authors attributed these differences to sensory processing differences between the arrays presented rather than to distinct systems of numerical processing. Moreover, although Ansari et al. (2007) employed proper controls to equate individual dot area, total cumulative area, and overall perimeter within blocks of small and within blocks of large number comparisons, overall area was many times larger in large-number conditions compared to small-number conditions, consistent with a sensory explanation for the differing findings with small and large numbers.
The present experiments test more focused predictions stemming from the two-systems of number hypothesis (Feigenson et al., 2004). First, if a small array of objects evokes spatial attention to individuals, the magnitude of the early attentional response (N1) to numerical arrays should scale with the absolute number of objects in the arrays, in the small but not the large number range. Second, if large number arrays spontaneously evoke summary representations of numerical magnitude to be compared and manipulated, the magnitude of the neural response to numerical changes, reflected in P2p amplitude, should scale with the ratio size of the change in the large but not the small number range. Two experiments tested these predictions by recording scalp potentials as subjects viewed a stream of dot arrays in an adaptation paradigm.
Experiment 1
Adult participants viewed a succession of arrays of dots while scalp potentials were measured continuously by means of a 128-channel geodesic sensor net. On separate blocks of trials, subjects were presented with either small or large numerical values (1, 2, and 3 or 8, 16, and 24). Within each block, one context number was presented on about 87% of the trials, interspersed with other numbers within the same range. Participants were asked to attentively view arrays on a computer screen in order to answer post-test questions; number was never mentioned as a dimension of interest. Analyses focused on the electrophysiological response to the infrequent test arrays, both in relation to and regardless of the numerical context in which they were presented.
Method
Participants
Fifty-seven adult subjects (18-29 years) were recruited from the Cambridge, Massachusetts community through posters and a web-based study pool. Participants were offered either class credit or paid 15 dollars for their participation. Data from 9 of the 57 subjects were rejected (7 for excessive artifacts (< 50 % artifact-free trials in at least one experimental condition; 1 as a result of equipment malfunction during recording; 1 as a result of experimenter error in set-up). Data from the remaining 48 participants were used for analysis.
Procedure
Participants viewed the sequential presentation of novel, non-symbolic numerical images (white dots on a gray background). A majority the images contained the same number of dots (base or adaptation number). Occasionally, test images were presented that contained either the same number of dots (no change test condition) or a different number of dots (number change test condition). Displays were presented for 250 ms1 and separated by an inter-stimulus-interval during which a blank gray screen appeared for a random duration between 900-1800 ms. This stimulus jitter was applied to reduce overlap in the ERP response to successive images (Luck, 2005a) and to reduce as much as possible the repetitive nature of the stimuli. A small white fixation cross was present during both the displays and the inter-stimulus intervals. This method of stimulus presentation is very similar to previously conducted adaptation studies of numerical cognition (Cantlon et al., 2006; Piazza et al., 2004; Izard, Dehaene-Lambertz, & Dehaene, 2008). No overt response was required of participants, who were not told the experiment focused on number. Participants simply were instructed to pay close attention to the displays in order to answer questions about them after the session. Breaks were given throughout the experiment and subjects were reminded to maintain close attention during stimulus presentation. Informal, open-ended post-test questioning probed participants' encoding of the displays and ideas about the purpose of the experiment.
Design
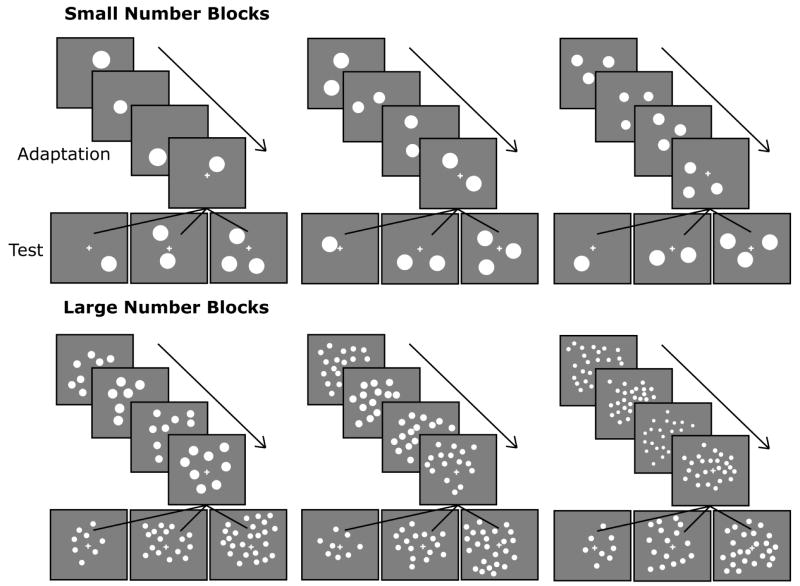
Each subject was randomly assigned to 1 of 3 possible conditions. Each condition contained a block of adaptation to a small number of dots and an adaptation block to a large number of dots with corresponding ratio changes. Order of presentation (small number block first or large number block first) was counterbalanced across subjects. In the large number blocks, participants were adapted to displays of 8, 16, or 24 dots with occasional test displays of 8, 16, and 24 dots. In the small number blocks, participants were adapted to 1, 2, or 3 with occasional test displays of 1, 2 and 3 dots (see Figure 1.). The order of test display presentations was random with the constraint that a test display appeared every 4th-6th trial.
Figure 1.
Schematic description of adaptation and test number pairs presented to subjects. Each subject was presented with a large number and corresponding small number condition containing the same ratio changes. Each array was presented for 250 ms followed by a random inter-stimulus interval of 900-1800 ms (or 750-1500 in experiment 2). In between stimuli, a small fixation cross was presented on the middle of a gray background. (Dot arrays pictured are not the actual images used in the experiment.)
A total of 450 trials was presented in each block for a total of 900 total trials per subject. Each of the three test conditions was presented 30 times for a total of 90 test trials per adaptation block, 180 test trials per subject. This means subjects viewed adaptation trials 80 percent of the time and test trials 20 percent of the time. Since one third of the test trials contained the same number of dots as the adaptation trials approximately 13 percent of all trials contained a deviant number of dots.
Displays
Stimuli were images of solid white dots on a gray background with a small centered fixation cross. All images were created using Adobe Illustrator software. The images of dot arrays were constructed so as to control for continuous parameters other than the number of dots eliciting systematic effects. The controls, described below, are based on those originally devised by Xu and Spelke (2000), and used in a variety of studies of numerical cognition (e.g. Brannon, Abbott, & Lutz, 2004; Wood & Spelke, 2005).
Adaptation images were individually created, containing 1, 2, 3, 8, 16 or 24 dots within a 12.25 × 12.25 centimeter envelope area. Element density, therefore, correlated positively with number of dots. Individual dot positions were selected pseudo-randomly for each display with the constraint that individual dots did not overlap. Individual dots were the same size within each array and varied across arrays. Five different dot sizes were used within each adaptation number condition varying from diameters of 2.34-5.24 cm for 1 dot arrays, 1.65-3.70 cm for 2 dot arrays, 1.35-3.03 cm for 3 dot arrays, .83-1.85 cm for 8 dot arrays, .59-1.31 cm for 16 dot arrays, and .48-1.07 cm for 24 dot arrays. On average, individual dot size was scaled across adaptation number conditions such that arrays with, say, 8 dots had average individual dot size that was twice the area of the average individual dot size of arrays with 16 dots. Therefore, on average the total summed area and brightness of displays between the adaptation numerosities were equated across the different numerical values and individual dot size varied inversely with number in the adaptation displays.
Those properties that varied in the adaptation displays (individual dot size and density) were equated in test displays and the properties that were equated in the adaptation displays varied in the test displays (total cumulative area and brightness). Small number test displays (arrays with 1,2, or 3 dots) consisted of individual dots 2.87 cm in diameter and densities of .013 dots/cm2. Large number test displays contained individual dots 1 cm in diameter and densities of .106 dots/cm2. Thus, total cumulative area and total image size/total envelope area, which were equated in the adaptation displays, scaled positively with number in the test arrays.
Controls were also implemented between small (1-3) and large (8-24) numbers where total cumulative dot area was equated between small and large number conditions, but individual dot size varied (average dot size was larger for small numbers) between small and large numbers.
Data Acquisition and Processing
An EGI system was used to record the ongoing EEG from 128 scalp locations using a geodesic sensor net (Electrical Geodesics, Eugene, OR.) as subjects passively viewed dot arrays. Signals were recorded 250 samples per second. Recordings were low pass filtered at 40 Hz. Test trials were segmented into experimental conditions based on 200 ms of recording before and 700 ms of recording after each stimulus presentation. Trials containing artifacts (eye blink, eye movement, head movement, or excessive noise) and/or more than 10 bad channels were detected and rejected by computer algorithm. The remaining artifact free trials were averaged for each of the experimental conditions for each subject. Finally, data were re-referenced and baseline corrected to 200 ms before stimulus onset. A grand average for each cardinal value as well as for each of the ratio conditions (no change, small change, medium change, and large change) within the small and large number ranges was created for visualization and inspection purposes.
Data Analysis
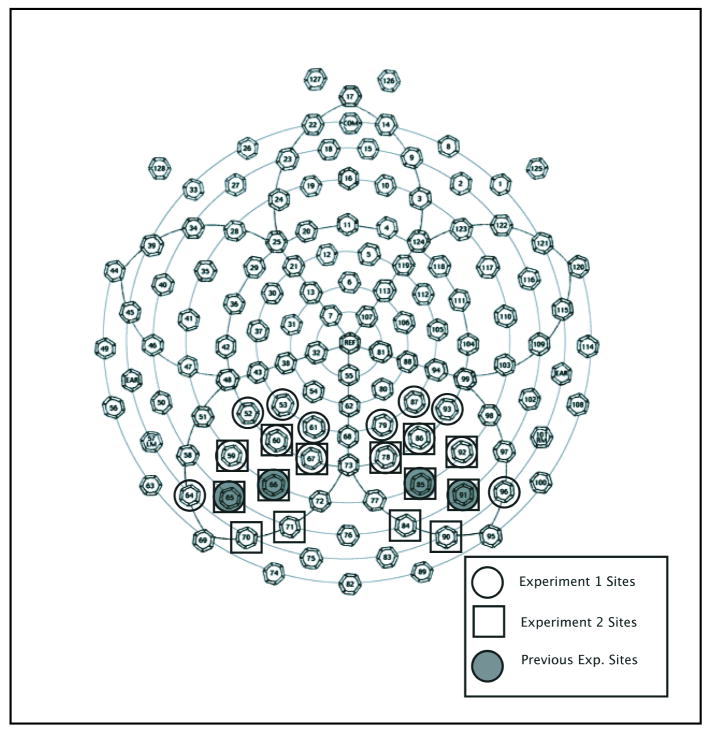
Through visual inspection of the average waveform, guided by previous studies of numerical cognition (Temple and Posner, 1998, Dehaene, 1996; Pinel, Dehaene, Riviere, & LeBihan, 2001), we identified three time windows of interest corresponding to the major observed ERP components: P1 (75-139 ms), N1 (139-199 ms), and P2p (175-250 ms). Given the established role of the parietal lobe in both tasks of numerical cognition and spatial attention (Culham, Brandt, Cavanagh, Kanwisher, Dale, & Tottell, 1998; Culham, Cavanagh, & Kanwisher, 2001; Dehaene, Piazza, Pinel, & Cohen, 2003; Piazza et al., 2004) and our inspection of the electrophysiological scalp topography, we focused our analyses over posterior regions. To characterize the components conservatively, we averaged across an electrode group that included pairs of bilateral scalp sites over widespread parietal-occipito-temporal regions (POT): left parieto-occipito-temporal junction electrodes 59, 60, 65, 66, 67, 70, 71; and right parieto-occipito-temporal junction electrodes, 78, 84,85,86, 90, 91, 92. As seen in Figure 2, these scalp site groupings closely overlapped with parieto-occipito-temporal groupings from previous ERP studies of number processing (Dehaene, 1996; Pinel et al., 2001; Temple and Posner, 1998).
Figure 2.
Map of 128 channel geodesic sensor net. Electrode groupings used for averaging and analysis reported here and those groupings used in previous experiments are identified (Dehaene, 1996; Temple and Posner, 1998). (Original figure modified with permission from www.egi.com)
First, we examined the effects of cardinal value on both the mean amplitude and peak latency of the ERP response to small and large test numbers on two early posterior components (P1, N1 defined above) for the average response over POT region (defined above). Test trials2 of each cardinal value (1, 2, 3, 8, 16, 24) were averaged across all subjects, creating experimental conditions that included test trials of each cardinal value in every context (e.g. 1 in the adaptation context of 1, 2, and 3). Responses to different numbers were compared using a repeated measures ANOVA with number range (large and small) and cardinal value (lowest value, middle value, highest value) as within-subject factors.
Next, we tested the ratio dependency of large and small number representations by comparing average P2p amplitudes over the same POT region across the same ratio change conditions for both the small and large number ranges. In order to control for the possible effects of the direction of change (increasing or decreasing) from adaptation to test number and for cardinal value of the test images (1,2, 3, 8, 16, 24), we averaged ERPs to the same ratio changes involving different numbers between subjects. This created the following 8 experimental conditions: small number-no change (S-NC); small number-small change (S-SC); small number-medium change (S-MC); small number-large change (S-LC); large number- no change (L-NC); large number –small change (L-SC); large number-medium change (L-MC); large number-large change (L-LC) (content of each condition is displayed in Table 1). Organized in this way, every subject received 6 of the 8 experimental conditions and all subjects received a no-change test condition for both large and small numbers. Thus, 48 subjects contributed to the no change test conditions, whereas 32 subjects contributed to each of the other test conditions.
Table 1.
Specific adaptation number to test number pairs used for each experimental ratio change condition (no ratio change, small ratio change, medium ratio change, large ratio change) for each number range (small and large). The first number represents the adaptation context and the second number represents the test number presented.
Ratio Changes Included In Each Experimental Condition
| Small Number Changes | Large Number Changes | ||||||
|---|---|---|---|---|---|---|---|
| S-NC | S-SC | S-MC | S-LC | L-NC | L-SC | L-MC | L-LC |
| 1-1 | --- | 1-2 | 1-3 | 8-8 | --- | 8-16 | 8-24 |
| 2-2 | 2-3 | 2-1 | --- | 16-16 | 16-24 | 16-8 | --- |
| 3-3 | 3-2 | --- | 3-1 | 24-24 | 24-16 | --- | 24-8 |
Given the unbalanced nature of the design, along with the unequal samples between experimental conditions, we employed a Linear Mixed Model Analysis to the average P2p amplitude between 175-250 ms post stimulus onset averaged over bilateral posterior parietal sites3. The Mixed Model Analysis (Cnaan, Laird, & Slasor, 1997) could most efficiently take into account between subject and between block variability as random effects, while testing our manipulations of the repeated within subject fixed effects of number range (small or large) and ratio change (no change, small change, medium change, large change).
Results
Early processing (P1)
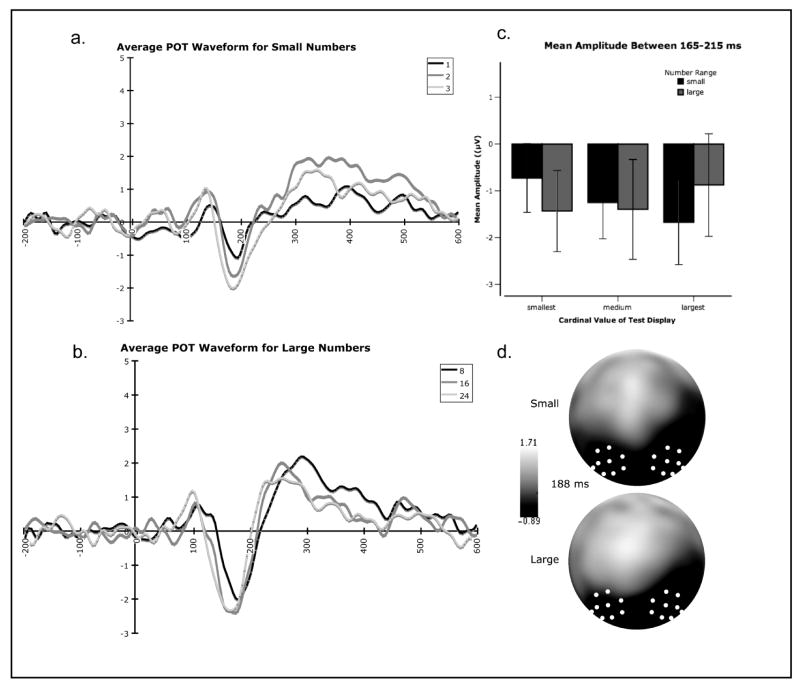
The ERP response revealed a significant main effect of numerical range on mean amplitude of the P1 (75-139 ms) with small numbers eliciting greater positive amplitudes than large numbers (F (1, 47) = 13.18, p = 0.001). In addition, a main effect of number range on latency was observed such that P1 peaked earlier for large numbers than for small numbers (F (1,47) = 24.08, p < 0.001). A main effect of cardinal value on latency was also observed within each range (F (2, 94) = 21.72, p < 0.001), with P1 peak latency decreasing as number increased for both small and large numbers.
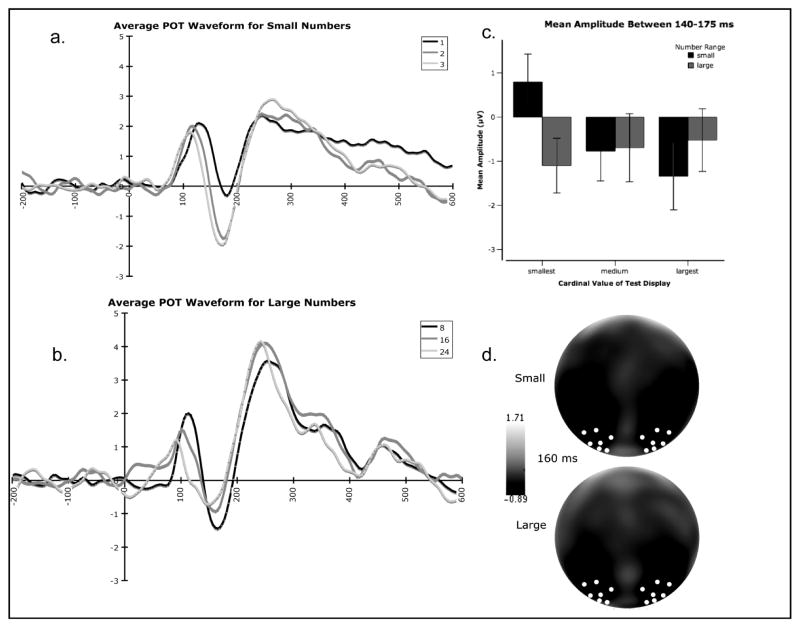
Effects of Cardinal Value on N1
The analysis of N1 revealed a different pattern. A significant main effect of cardinal value (F (2, 94) = 8.86, p < 0.001) and a significant number range by cardinal value interaction (F (2, 94) = 29.40, p < 0.001) were observed in mean amplitude of the N1 (140-175 ms). Post-hoc analysis revealed a significant linear contrast for small numbers (F (1, 47) = 49.54, p < 0.001) with mean N1 amplitude becoming more negative as cardinal value increased. In contrast, a significant linear contrast in the opposite direction for large numbers (F (1, 47) = 4.78, p = 0.033) was observed with amplitude becoming more positive as cardinal value increased for large numbers (see Figure 3). On the latency measure, main effects of number range (F (1, 47) = 55.18, p < 0.001) and cardinal value (F (2, 94) = 10.07, p < 0.001) were observed with small numbers eliciting an N1 that peaked later compared to large numbers and with both small and large numbers showing a decrease in latency with an increase in number.
Figure 3.
Summary of the effects of cardinal value of test displays on event-related potentials in Experiment 1. (a.) Average waveform over parieto-occipito-temporal sites in response to test displays of each cardinal value in the small number range collapsed across adaptation contexts. (b.) Average waveform over parieto-occipito-temporal sites in response to test displays of each cardinal value in the large number range collapsed across adaptation contexts. (c.) Mean amplitude averaged over parieto-occipito-temporal sites between 140-175 ms post stimulus onset characterizing the N1 for each number range (small and large) and each cardinal value in that range (smallest, medium, and largest). (d.) Scalp topography at 160 ms characterizing the N1 for both small and large numbers. The white dots represent the electrode grouping used for analyses.
Effects of Ratio Change on P2p amplitude
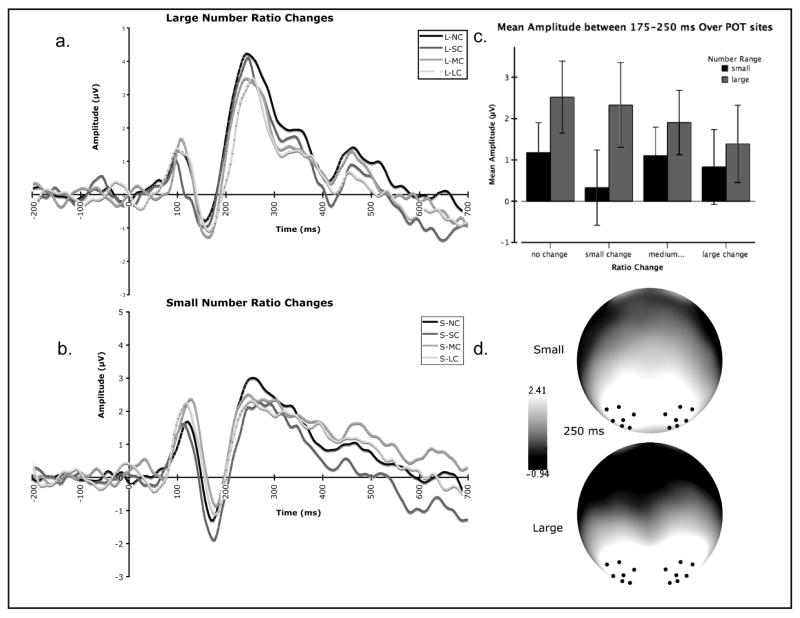
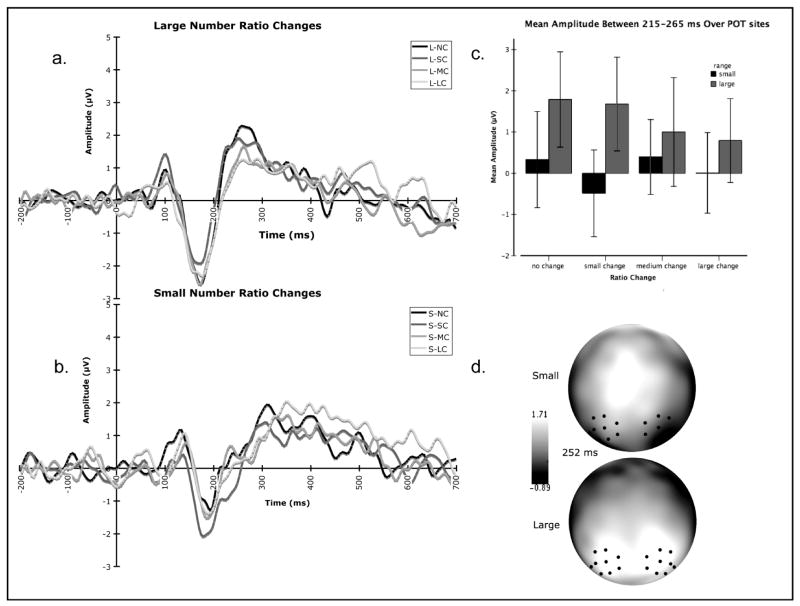
Although the early components (P1, N1) revealed no effects of ratio, the P2p response revealed significant main effects for number range (F (1, 227.994) = 43.857, p < .001), ratio change (F (3, 232.686) = 2.841, p = .039) and a significant number range by ratio change interaction (F (3, 227.994) = 2.939, p = .034). In the large number range, no- change test conditions elicited the highest P2p amplitude and P2p amplitude became more negative as change ratio between adaptation and test number increased regardless of the specific adaptation and test numbers themselves. Importantly, P2p amplitude was not modulated by ratio change for the small number conditions (see Figure 4).4
Figure 4.
Summary of the effects of ratio change between adaptation context and test number on event-related potentials in Experiment 1. (a.) Average waveform over POT sites in response to the four ratio change conditions for large numbers (no change, small change, medium change, and large change). (b). Average waveform over POT sites in response to the four ratio change conditions for small numbers (no change, small change, medium change, and large change). (c.) Mean amplitude between 175-250 ms post stimulus onset over POT sites for each ratio change condition (no change, small change, medium change, large change) for each number range (large and small). (d.) Scalp topography at 250 ms characterizing the P2p for both large and small numbers. The black dots represent electrode grouping used for analyses.
Discussion
Experiment 1 yielded two main findings. First, early negative scalp potentials (N1) over posterior cortex revealed effects of numerical range and cardinal value, regardless of context. Specifically, the magnitude of the N1 increased as cardinal value increased for small, but not large, sets of objects. This finding accords with previous studies suggesting processing of small numbers differentially recruits attention compared to processing of large numbers (Ansari et al., 2007; Libertus et al., 2007).
Second, the later rising P2p (175-250 ms) revealed an effect of numerical ratio change for large but not for small numbers. Specifically, P2p amplitude decreased as the ratio of change between adaptation and test number increased in the large number range, but not in the small number range. This finding with large numbers accords with previous research using fMRI (Ansari et al., 2006). The contrasting findings with small numbers provide the first neurophysiological evidence for separate processing mechanisms in the small and large number ranges.
Nevertheless, we cannot rule out an alternative interpretation of this experiment: the observed processing differences may be due to differences in the individual dot sizes presented in the numerical displays, rather than differences in numerical range. In Experiment 1, as in Ansari et al. (2007), larger dots were presented in the small number range so as to control for a host of continuous quantities in the test arrays. Thus, differences in dot size, rather than in set size, may account for the processing and/or representational differences observed in Experiment 1. Experiment 2 tested this possibility.
Experiment 2
Experiment 2 replicated Experiment 1 using a different design and stimuli (after Piazza et al., 2004), both to test the generality of the effects observed with large numbers and to rule out the above alternative explanations for the effects observed with small numbers. Specifically, we equated individual dot size across small and large numerical displays and then tested for the specific effects of cardinal value and ratio observed in Experiment 1.
Method
Participants
Eighteen adult subjects were recruited from the Cambridge, Massachusetts community via posters and a web-based psychology study pool. Data from 2 of the participants were eliminated because of excessive artifacts (< 50% artifact free trials in at least one experimental condition). Sixteen participants made up the final data set.
Procedure
The procedure was identical to Experiment 1 except that the inter-stimulus interval was shortened and varied randomly from 750-1500 ms. Given the components of interest all occurred well before the end of the inter-stimulus-interval in Experiment 1, the interval was shortened in an effort to reduce the total duration of the experiment while maintaining a reasonable number of test trials per condition for each subject.
Design
In contrast to the mixed design of Experiment 1, Experiment 2 used a 2 × 4 within-subjects experimental design with the factors of number range (small numbers and large numbers) and ratio change (no change, small change, medium change, and large change). The levels of ratio change were created by grouping test trials according to ratio of change between the number of dots in the adaptation displays to the number of dots in the specific test display as in Experiment 1 (see Table 1).
In Experiment 2, each subject completed all 6 blocks that involved adaptation to the numbers 1, 2, 3, 8, 16, and 24. Blocks were divided into small number blocks (1-3) and large number blocks (8-24). All small number or all large number blocks were presented first and order of presentation was counterbalanced across participants.
The 3 small number blocks and the 3 large number blocks presented the same context (1, 2, 3, 8, 16, 24) and test numbers (1, 2, 3, 8, 16, 24) as in Experiment 1. A total of 1800 images were presented to each subject. In an effort to reduce the length of the experiment, fewer test trials per condition were presented. Each test number was presented 20 times per block (30 in Experiment 1). A total of 360 test trials were presented to each subject: 120 no-change, 80 small change, 80 medium change, and 80 large change test conditions. In an effort equate the number of trials potentially contributing to the average, the last 5 no-change test trials in each of the 6 blocks were dropped from the analysis.
Displays
Displays again were images (650 × 650 pixels) of white dots on a gray background, constructed as to control for continuous parameters other than the number using a method and automated program developed by Dehaene and colleagues (see Dehaene, Izard, and Piazza, 2005 for documentation; Piazza et al., 2004). Specifically, we equated the intensive parameters (individual item size and inter-item spacing) of the arrays across the test stimuli and varied the extensive parameters (total occupied area and total luminance) of the test arrays randomly so that these variables were equated, on average, across adaptation stimuli with the constraint that the values for the extensive parameters were drawn randomly from fixed distributions that spanned the range of values used for test stimuli. This design resulted in test stimuli that were equally familiar with regard to the continuous parameters other than number, because these values had already been presented equally often in the adaptation images. This method of controlling for intensive and extensive parameters has been employed in several recent neuroimaging studies of numerical cognition (see Piazza et al., 2004; Piazza et al., 2007; Izard et al., 2008). Critically, these controls allowed us to equate individual item size within and between small and large number test trials, although total summed area necessarily scaled positively with number across test arrays.
Data Acquisition and Processing
Our protocol for EEG recording and data processing was identical to that used in Experiment 1.
Analysis
We focused our analyses to test for the two signature phenomena observed in Experiment 1: increasing magnitude of the early N1 component based on cardinal value of displays in the small number range and modulation of P2p based on ratio change for large numbers. In addition, we also tested for earlier processing differences (P1). The effects of cardinal value and number range were statistically assessed by comparing mean P1 (85-155 ms) and N1 (167-215 ms) amplitude and peak latency for the average response over the POT region with a repeated measures ANOVA as in Experiment 1. And, we assessed the effects of ratio change on the time window characterizing the rising P2p (215-265 milliseconds) by using a 2 ×4 within-subjects repeated measures ANOVA with the factors of number range (small or large) and ratio change (no change, small change, medium change, large change).
Time windows best characterizing the major components (defined above) showed high overlap with those identified in Experiment 1. Slight differences in component latency were observed and most likely due to the changes in inter-stimulus timing between experiments. Scalp topography was also highly similar to that observed in Experiment 1 and in previous research (Dehaene, 1996; Pinel et al., 2001; Libertus et al., 2007; Temple & Posner, 1998). However, we did notice a slightly dorsal-lateral shift in the scalp topography of the posterior components. To best characterize the observed components in this group of subjects, we defined the clusters comprising posterior activation slightly differently for Experiment 2, although they overlap highly with those of Experiment 15 (see Figure 2).
Results
Early Processing (P1)
Analysis of the first positivity revealed a significant main effect of number range (F (1, 15) = 4.76, p = 0.045), with small numbers showing a greater P1 amplitude than large numbers. A significant interaction between number range and cardinal value (F (2,30) = 3.41, p = 0.046), with amplitude increasing as cardinal value increased for small numbers and decreasing as cardinal value increased for large numbers was also observed. However, post hoc testing revealed neither the small number nor the large number trend to show a significant linear contrast. An analysis of P1 latency again revealed a main effect for number range (F (1, 15) = 42.29, p < 0.001) with small number test trials eliciting slower positive peaks than large number test trials during this time window.
Effects of Cardinal Value on N1
We again observed a significant interaction between number range and cardinal value on mean N1 amplitude (F (2, 30) = 3.38, p = 0.048). Post hoc tests revealed a significant linear contrast with increasing component magnitude (more negative mean amplitude) with increasing cardinal value for small numbers (F (1, 15) = 6.52, p = 0.022), but not large numbers (see Figure 5). And, again we observed a main effect of number range on N1 latency, with small number trials eliciting negativities slower to peak than large number trials (F (1, 15) = 23.92, p < 0.001).
Figure 5.
Summary of the effects of cardinal value of test displays on the event-related potentials in Experiment 2. (a.) Average waveform over POT sites in response to test displays of each cardinal value in the small number range collapsed across adaptation contexts. (b.) Average waveform over POT sites in response to test displays of each cardinal value in the large number range collapsed across adaptation contexts. (c.) Mean amplitude averaged over POT sites between 165-215 ms post stimulus onset characterizing the N1 for each number range (small and large) and each cardinal value in that range (smallest, medium, and largest). (d.) Scalp topography at 188 ms characterizing the N1 for both small and large numbers.
Effects of Ratio Change on P2p amplitude
The analysis of the later evoked response (P2p) revealed a significant main effect for number range (F (1, 15) = 16.962, p = 0.001) and a significant number range by ratio change interaction (F (3, 45) = 3.365, p = 0.027). Examination of the mean P2p amplitudes for each of the ratio change conditions revealed a significant linear contrast (F (1,15) = 7.64, p = 0.014) within the large number block where no-change test conditions elicited the greatest positivity and mean P2p amplitude systematically decreased as ratio of adaptation to test number increased (see Figure 6). In contrast, this pattern of attenuation according to ratio was not observed with matched ratio changes within the small number range (F (1, 15) = 0.002, p = 0.962). Instead, we observed medium change test conditions to elicit the greatest positivity during this time frame, closely followed by the no-change test condition. The small change test condition elicited the lowest average amplitude over this time window (see Figure 6).
Figure 6.
Summary of the effects of ratio change between adaptation context and test number on event-related potentials in Experiment 2. (a.) Average waveform over POT sites in response to the four ratio change conditions for large numbers (no change, small change, medium change, and large change). (b.). Average waveform over POT sites in response to the four ratio change conditions for small numbers (no change, small change, medium change, and large change). (c.) Mean amplitude between 215-265 ms post stimulus onset over POT sites for each ratio change condition (no change, small change, medium change, large change) for each number range (large and small). (d.) Scalp topography at 252 ms characterizing the P2p for both large and small numbers.
Discussion
The results of Experiment 2 replicate the two principal findings of Experiment 1. First, after equating individual item size across large and small test images, we again observed an increase in the amplitude of the early N1 with an increase in cardinal value for small numbers, but not for large numbers. Moreover, small numbers elicited slower peaking P1 and N1 components compared to large numbers.
Second, we found a systematic modulation of P2p amplitude by ratio change for large numbers, but not for small numbers. This finding supports the conclusion that the numerical representations evoked by a larger quantity of items are fundamentally different from those representations evoked by a small number of items. It seems that while large non-symbolic number representations are ratio dependent, representations of a small number of objects are not.
General Discussion
Neural Responses to Changes in Number
These data replicate previous fMRI findings of a parietal response to numerical changes, with a greater response to changes at narrower numerical ratios (Ansari et al., 2006). Furthermore, the findings extend previous findings in three respects. First, the present studies used passive viewing of non-symbolic numerical displays whose numerical content was never described or highlighted. Despite the absence of any behavioral task or overt response, our results are nearly identical to those involving voluntary comparison of symbolic and non-symbolic quantities (Dehaene, 1996; Libertus et al., 2007; Temple & Posner, 1998). In all cases, small ratio changes elicit higher P2p amplitudes than do large ratio changes. This finding suggests that adults automatically engage in spontaneous processes of representing and comparing numerosities in the large number range, without being instructed to do so. In fact, in almost all cases, subjects were not even aware the studies were about number and reported attending to other visual features of the displays such as spatial arrangement. This observation suggests that distance/ratio dependency effects are truly a result of numerical computations, not task difficulty or decision related factors, since there was no task presented and no decision to be made.
Second, the scaling of the electrophysiological response to ratio extended to the no-change test condition, with a ratio difference of zero. This finding contrasts with the findings of experiments using symbolic stimuli (Naccache & Dehaene, 2001) probably because the exact equality of two symbolic large-number arrays can easily be determined, whereas the exact equality of two non-symbolic numbers cannot. It is possible that each successive numerical presentation generated an automatic and spontaneous comparison process in the mind: a search for numerical differences that terminated quickly and easily when the differences were large but required more processing when the differences were small or nonexistent.
Participants may not have been actively engaged in numerical comparisons, as they did not report number as a variable of interest when probed as to the purpose of the experiment. But, their scalp potentials, nevertheless, revealed that numerical comparison was occurring. This finding accords with neuropsychological research of patients with impairments to language and symbolic numerical processing such as L.E.C. (Lemer, Dehaene, Spelke, & Cohen, 2003). When presented with an approximate number task L.E.C. claimed to have lost all ability to process number and predicted that he would fail at tasks of numerical comparison with arrays containing large numbers of dots. When given such tasks and asked to guess, however, he performed well above chance and comparable to healthy controls suggesting awareness need not be requisite for approximate numerical comparisons to occur (Lemer et al., 2003).
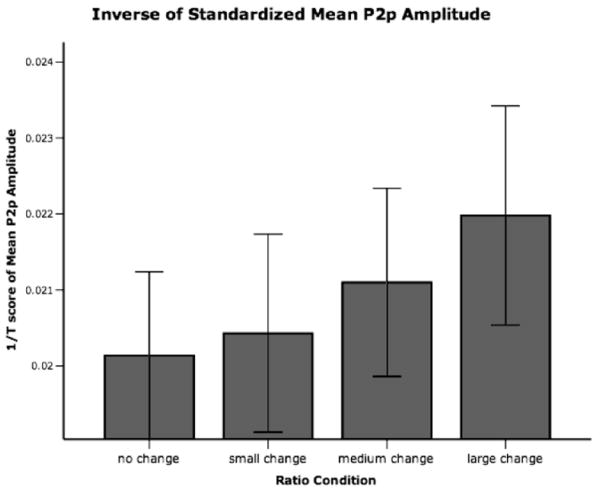
Whatever its explanation, the ratio-dependent pattern of results obtained in these experiments is very similar both to the tuning curves observed by fMRI researchers using a similar passive task (Piazza et al., 2004) and recordings from individual neurons in response to number in non-human primates (Nieder & Miller, 2003). The similarity can be more easily seen by reversing the actual direction of change (increasingly more negative as ratio increased) and graphing the inverse of the mean P2p amplitude across both experiments (Figure 7)6. Just as observed by Piazza et al. (2004) there is greater change from baseline (no-change) as the numerical ratio of change increases. Thought of in this way, differences in the nature of the signal of ERP and fMRI results in response to number can be reconciled. However, further spatial analyses and source localization with ERP possibly combined with other techniques such as fMRI are needed to understand the nature of the component referred to as the second posterior positivity (P2p).
Figure 7.
Inverse of ratio change results reported in Experiments 1 and 2. The values were derived by standardizing the mean P2p amplitudes (T scores) for each subject and then computing the inverse of each value.
Third and most important, our findings provide evidence that the numerical representation processes, engendered spontaneously for large numbers, are not engaged in the same way when adults view small numbers7. The neural response to test displays of small numbers is not modulated by the relationship of the cardinal value of a test display to that of the context display. Thus, our experiments provide the first evidence that the ratio dependence of large number comparisons is not observed spontaneously for small-number comparisons.
Why do small number comparisons fail to show a ratio effect as observed with large numbers in our data? Two potential explanations for this failure can be rejected based on our design and findings. First, the difference between the ratio effect for large and small numbers is not attributable to other, non-numerical differences between the large and small number displays. All quantitative properties of the displays were equated across the small and large number displays in Experiment 1 except for item size. In Experiment 2, item size was equated and the contrasting effects of ratio were still found. Moreover, similar findings were observed across both experiments that varied in the methods employed to control for continuous variables. The set-size (large or small) modulation of the ratio effect therefore is robust over a host of stimulus and factors.
Second, the difference is not attributable to a floor effect in the neural response to small numbers. Although responses to small numbers were not modulated by ratio, they were modulated by a different numerical property: cardinal value independent of numerical context. The neural response to number scaled positively with cardinal value during early time windows characterizing the N1 for small numbers, but not for large numbers.
The double dissociation between the numerical information modulating electrophysiological responses to large versus small numbers supports the theory, heretofore based primarily on behavioral evidence with infants and non-human primates, that small number arrays evoke parallel representations of individual objects, whereas large number arrays evoke summary representations of approximate cardinal values. Just as brain activity increases with increases in the number of objects that must be tracked (Culham et al., 2001) or individuated (Xu, in press), brain activity in the presence of small numbers may spontaneously engage these tracking mechanisms and serve as the basis of representations of small numbers.
Nevertheless, this conclusion must be qualified. Although small numbers did not engage ratio dependent summary representations in the present passive viewing studies, it remains possible that they would engage such processes if participants were primed with large numbers and then asked to make comparisons between large and small numbers or explicitly told to compare numerosity across sets8. To be sure, these adult subjects are capable of forming summary representations of small cardinal values, and of comparing one cardinal value to another. Our findings suggest only that such processes are spontaneously engaged for large numbers and not for small numbers.
Implications for a Theory of Number Representation
Based on the present results, it is possible that early spatial attention is deployed differentially based on the number of objects in the set. This explanation fits nicely with the idea that a limited number of items can be individuated or tagged as what has been termed “object files” (Sears & Pylyshyn, 2000; Scholl and Pylyshyn, 1999). From the object files perspective, the small number system is not a “number system” per se, but a system for keeping track and encoding features of individuals in parallel (Feigenson & Carey, 2003).
Both our results and others (e.g. Culham et al., 1998; Culham et al., 2001) raise the question why activation scales with the number of objects presented in the small number range. It is important to note that our data within the small number range do not show a positive relationship between N1 latency and cardinal value indicative of a serial process, but an amplitude increase without latency differences that may reflect the degree to which we must split our attention in parallel (Pylyshyn & Storm, 1988). We suggest the N1 amplitude effect most likely reflects subprocesses within the system of object representation and attentive object tracking. Recent neuroimaging research suggests multiple and distinct parietal processes are at work in the representation of a small number of objects (Xu, in press), including a process of individuation that scales with the number of objects in the array and a process of identification that does not scale with number (see also Pylyshyn, 2003). The N1 effect observed in the small number range might reflect this individuation process.
Further, our results provide evidence against views that propose one system of representations spanning the all numbers (Cordes et al., 2002; Gallistel & Gelman, 2000). First, early component amplitude increased with cardinal value for small sets and not for large sets. In some cases the opposite pattern of modulation was observed for large sets. Furthermore, small sets were found to evoke slower peaks over a variety of components compared to large sets of objects. An accumulator or iterative model of numerical representation (e.g. Gallistel, 1990) would predict greater amplitude and/or latency with increases in cardinal value, but this was not the case. In fact, no case was observed in which amplitude or latency was increased as cardinal value increased for arrays containing large numbers.
Large numbers did, however, elicit greater P2p amplitudes than small numbers, although amplitude was not modulated by cardinal value but by ratio of change. The results of Piazza, Giacomini, LeBihan, and Dehaene, (2003) indirectly support this finding. In a counting task, no brain region was observed to show greater activation for enumeration of the subitizing range (1-3) compared to conditions of 4 or greater. Widespread brain regions, however, showed greater activation for numbers 4-7 compared to 1-3. Our finding of greater positive amplitude over posterior sites for large compared to small numbers is congruent with these findings.
It is important to note that the actual nature and origin of the latent component being labeled P2p in the small number range cannot be fully characterized by these data. For purposes of analysis it was used in comparison to the P2p component in the large number range. In our analysis we assumed it to be the same underlying component as observed in the large number range, but the spatial resolution of the current method does not allow a distinction between spatially adjacent cortical regions. It is just as plausible to assume the underlying neural mechanism driving small number representations is distinct from that driving the positivity in the large number range. While further investigation combined with source localization techniques is needed to find the neural locus of these components, either explanation supports the proposed idea that a small number of items are represented differently than a large number of items.
Experiments 1 and 2 used different methods to control for continuous parameters other than number. In particular, the small-number arrays in Experiment 1 (but not Experiment 2) consisted of larger objects, and the successive arrays presented changes in object size that were more predictable in Experiment 1 than in Experiment 29. These differences were associated with some differences in the ERP response. In Experiment 1 we observed a significant effect of cardinal value on latency of P1 and N1 for both large and small numbers, such that peak latency decreased as number increased. We also observed a significant effect of cardinal value on N1 amplitude, such that amplitude increased with cardinal value for small numbers and significantly decreased for cardinal value with large numbers. In Experiment 2, in contrast, there was no effect of cardinal value on P1 or N1 latency and no effect of cardinal value on N1 amplitude in the large number range. These findings suggest that early processing is not driven completely by the number of objects in an array, but is also influenced by other continuous parameters and presentation sequences9.
Despite these differences, the present experiments show the same two distinct signatures of small and large number processing: signatures that are not tied to other continuous stimulus parameters. When presented with small number arrays, adults appear to spontaneously focus on individual objects, with neural responses that scale with the number of objects to be attended. Faced with large number arrays, in contrast, adults appear to focus spontaneously on cardinal values and to compare successive values over time. Thus, the automatic, foundational systems of numerical cognition observed in young infants and other non-human primates appear to be present in human adults despite years of formal numerical and mathematical training.
Acknowledgments
We would like to thank Susan Carey, Veronique Izard, and Charles Nelson for their helpful comments and feedback at various stages of this project. We would also like to thank Ana Franco, Jeff Reardon, and Amy Yoshitsu for their help with data collection.
Footnotes
Other experiments using methods similar to ours suggest that 250 ms is more than sufficient to encode both large and small numbers (e.g. Piazza et al., 2004 used 150 ms).
We also conducted the same comparisons of cardinal value using an average of adaptation trials instead of test trials and found similar results.
No hemispheric differences were observed
To investigate whether a ratio dependent magnitude representation occurs later for small numbers we conducted a second analysis on a later time window (250-350 ms) for just the small number ratio changes. This analysis also failed to show a main effect of ratio change (F (3, 83.772) = .852, p = 0.469).
Left POT electrodes: 52, 53, 59, 60, 61, 64, 65, 66, 67; Right POT electrodes: 78, 79, 85, 86, 87, 91, 92, 93, 96
Mean P2p amplitudes for all subjects in both experiments were first standardized by converting them to T scores which essentially sets the mean to 50 and the standard deviation to 10 (T = 50 + (z score * standard deviation)). T scores were used so that the range of values would all be positive. Then the inverse of the T scores was computed (1/x) for visualization as displayed in Figure 7.
Interestingly, our results contrast with the observations of a recent ERP experiment suggesting similar signatures of small number and large number change detection in infants (Izard et al., 2008). However, comparisons between Izard et al. (2008) and our experiments are difficult for three reasons. First, the between subjects design used in their experiment did not allow them to test for the effects of different ratio changes of the same number range nor for the effects of the same ratio changes in different number ranges. Second, the comparisons of large and small numbers were made over a later time window than one might expect given our data on the early effects of small numbers. Third, they only tested for number change (averaged over increasing and decreasing cardinal values) and not for the effects of cardinal value alone.
While sufficient priming in one domain could transfer to representing say a small number using the system of large number representation, we did not find evidence of this in our study. The same general N1 and P2p trends were observed when only the first block (large or small) was analyzed.
Although individual dot size varied in adaptation images and was equated in test images in both experiments, subjects in Experiment 1 were only exposed to 5 object sizes per adaptation block, whereas those in Experiment 2 were exposed to a continuum of many more dot sizes. As a result, subjects in Experiment 1 might have encoded all the item sizes within a block and tracked this over the experiment, rendering item size a relevant dimension of the images. In contrast, subjects in experiment 2, exposed to many more item sizes, may not have focused on item size.
References
- Ansari D, Dhital, Siong Parametric effects of numerical distance on the intraparietal sulcus during passive viewing of rapid numerosity changes. Brain Research. 2006;1067:181–188. doi: 10.1016/j.brainres.2005.10.083. [DOI] [PubMed] [Google Scholar]
- Ansari D, Lyons IM, van Eimeren L, Xu F. Linking visual attention and number processing in the brain: The role of the right temporal-parietal junction in the small and large non-symbolic number comparison. Journal of Cognitive Neuroscience. 2007;19:1845–1853. doi: 10.1162/jocn.2007.19.11.1845. [DOI] [PubMed] [Google Scholar]
- Brannon EM. The development of ordinal numerical knowledge in infancy. Cognition. 2002;83:223–240. doi: 10.1016/s0010-0277(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Brannon EM, Abbott S, Lutz D. Number bias for the discrimination of large visual sets in infancy. Cognition. 2004;93:B59–B68. doi: 10.1016/j.cognition.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brannon EM, Terrace HS. Ordering of the numerosities 1-9 by monkeys. Science. 1998;282:746–749. doi: 10.1126/science.282.5389.746. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and 4-y-old children. PLOS Biology. 2006;4:e125, 1–11. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyze unbalanced repeated measures and longitudinal data. Statistics in Medicine. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cordes S, Gelman R, Gallistel CR. Variability signatures distinguish verbal from nonverbal counting in both large and small numbers. Psychological Bulletin and Review. 2001;8:698–707. doi: 10.3758/bf03196206. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RBH. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;88:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG. Attention response functions: Characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32:737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Dehaene S. The organization of brain activations in number comparison: Event-related potentials and the additive-factors method. Journal of Cognitive Neuroscience. 1996;8:47–68. doi: 10.1162/jocn.1996.8.1.47. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Symbols and quantities in parietal cortex: Elements of a mathematical theory of number representation and manipulation. In: Haggard P, Rossetti Y, editors. Attention and Performance XXII. Sensori-motor foundations of higher cognition. Cambridge, MA: Harvard University Press; 2007. [Google Scholar]
- Dehaene S, Izard V, Piazza M. Control over non-numerical parameters in numerosity experiments. 2005 Unpublished manuscript (available on www.unicog.org)
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Feigenson L, Carey S. Tracking individuals via object-files: Evidence from infants' manual search. Developmental Science. 2003;6:568–584. [Google Scholar]
- Feigenson L, Dehaene S, Spelke ES. Core systems of number. Trends in Cognitive Sciences. 2004;8:307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge, MA: Bradford Books/MIT Press; 1990. [Google Scholar]
- Gallistel CR, Gelman R. Non-verbal numerical cognition: From reals to integers. Trends in Cognitive Sciences. 2000;4:59–65. doi: 10.1016/s1364-6613(99)01424-2. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences, USA. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Teder-Sälejärvi WA, Münte TF. Temporal dynamics of early perceptual processing. Current Opinion in Neurobiology. 1998;8:202–210. doi: 10.1016/s0959-4388(98)80141-4. [DOI] [PubMed] [Google Scholar]
- Izard V, Dehaene-Lambertz G, Dehaene S. Distinct cerebral pathways for object identity and number in human infants. PLOS Biology. 2008;6:e11, 1–11. doi: 10.1371/journal.pbio.0060011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemer C, Dehaene S, Spelke ES, Cohen L. Approximate quantities and exact number words: Dissociable systems. Neuropsychologia. 2003;41:1942–1958. doi: 10.1016/s0028-3932(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Libertus ME, Woldorff MG, Brannon EM. Electrophysiological evidence for notation intendance in numerical processing. Behavioral and Brain Functions. 2007;3(1) doi: 10.1186/1744-9081-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005a. [Google Scholar]
- Luck SJ. The operation of attention-millisecond-by-millisecond-over the first half second. In: Ogmen H, Breitmeyer BG, editors. The first half-second: The microgenesis and temporal dynamics of unconscious and conscious visual processes. Cambridge, MA: MIT Press; 2005b. pp. 187–206. [Google Scholar]
- Mandler G, Shebo BJ. Subitizing: An analysis of its component processes. Journal of Experimental Psychology: General. 1982;111:1–21. doi: 10.1037//0096-3445.111.1.1. [DOI] [PubMed] [Google Scholar]
- Naccache L, Dehaene S. The priming method: Imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cerebral Cortex. 2001;11:966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- Nieder A, Miller EK. Coding of cognitive magnitude: Compressed scaling of numerical information in the primate prefrontal cortex. Neuron. 2003;37:149–157. doi: 10.1016/s0896-6273(02)01144-3. [DOI] [PubMed] [Google Scholar]
- Piazza M, Giacomini E, Le Bihan D, Dehaene S. Single-trial classification of parallel pre-attentive and serial attentive processes using functional magnetic resonance imaging. Proceeding of the Royal Society Biological Sciences. 2003;270:1237–1245. doi: 10.1098/rspb.2003.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53(2):293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Pinel P, Dehaene S, Riviere D, LeBihan D. Modulation of parietal activation of semantic distance in a number comparison task. Neuroimage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW. Seeing and visualizing: It's not what you think. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: Evidente for a parallel tracking mechanism. Spatial Vision. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- Scholl BJ, Pylyshyn ZW. Tracking multiple items through occlusion: Clues to visual objecthood. Cognitive Psychology. 1999;38:259–290. doi: 10.1006/cogp.1998.0698. [DOI] [PubMed] [Google Scholar]
- Sears CR, Pylyshyn ZW. Multiple object tracking and attentional processing. Canadian Journal of Experimental Psychology. 2000;54:1–14. doi: 10.1037/h0087326. [DOI] [PubMed] [Google Scholar]
- Shuman M, Kanwisher N. Numerical magnitude in the human pairetal lobe: tests of representational generality and domain specificity. Neuron. 2004;44:557–569. doi: 10.1016/j.neuron.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Temple E, Posner MI. Brain mechanisms of quantity are similar in 5-year-old children and adults. Proceedings of the National Academy of Sciences, USA. 1998;95:7836–7841. doi: 10.1073/pnas.95.13.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen J, Gallistel CR, Gelman R. Nonverbal counting in humans: The psychophysics of number representation. Psychological Science. 1999;10:130–137. [Google Scholar]
- Wood JN, Spelke ES. Chronometric studies of numerical cognition in five-month-old infants. Cognition. 2005;97(1):23–39. doi: 10.1016/j.cognition.2004.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. Categories, kinds, and object individuation in infancy. In: Gershkoff-Stowe L, Rakison D, editors. Building object categories in developmental time; Papers from the 32nd Carnegie Symposium on Cognition; New Jersey: Lawrence Erlbaum; 2005. pp. 63–89. [Google Scholar]
- Xu F, Spelke ES. Large number discrimination in 6-month-old infants. Cognition. 2000;74:B1–B11. doi: 10.1016/s0010-0277(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Xu Y. Distinctive neural mechanisms supporting visual object individuation and identification. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.21024. in press. [DOI] [PubMed] [Google Scholar]