Abstract
Circadian transcriptional rhythms in confluent NIH 3T3 fibroblast cultures become more coherent as they mature, as defined by reduced relative amplitude error. This increased coherence is unlikely to be mediated by GAP junction or NO-mediated signalling, but it is correlated with increased culture density. Coherence in immature cultures can be increased by inhibition of the cell cycle, indicating that mature cultures likely desynchronise more slowly due to reduced cell division, which might otherwise perturb circadian phase. We conclude that mature fibroblast cultures will be of great utility for high-throughput pharmacological and mutational circadian screening.
Keywords: Fibroblast, circadian rhythms, cell culture, coherence, synchrony, techniques
INTRODUCTION
Immortalised cell lines, in particular Rat 1 and NIH 3T3 fibroblasts are a valuable platform for studying mammalian circadian pacemaking in vitro due to their self-sustained circadian rhythms in gene expression (Akashi et al. 2000; Balsalobre et al. 1998) and because they are readily cultured and manipulated. Although circadian cycles of gene expression have been observed using techniques such as northern blotting (Balsalobre et al. 1998), protein activity assays (Iitaka et al. 2005) and transcriptomic microarrays (Duffield et al. 2002), more recently real-time bioluminescence recordings using both primary and immortalised cell lines expressing luciferase-based reporters have become the method of choice. Such studies routinely employ apparatus based around photomultiplier tubes (PMTs) to monitor light emission (Izumo et al. 2003), in some cases to undertake large-scale, high-throughput chemical genetics or RNAi-mediated screening in order to further characterise known, or identify novel, components of the cellular oscillator (Sato et al. 2006). A limitation of this approach has been the tendency for bioluminescence rhythms to damp after a few days in culture, which stands in marked contrast to the ability of suprachiasmatic nucleus (SCN) explants to sustain bioluminescence rhythms almost indefinitely. Damping arises from progressive phase desynchonisation between cells (Izumo et al. 2003; Nagoshi et al. 2004; Welsh et al. 2004). Single-cell luciferase emission has confirmed that whereas the transcriptional amplitude of individual fibroblasts remains high throughout, relative cellular phases across the culture drift due to stochastic effects intrinsic to uncoupled cells (Welsh et al. 2004). Damping is prevented in the SCN because of interneuronal synchrony, maintained by mechanisms including GAP junctions and chemical synapse and electrical synapses which facilitate accurate, phase-locked time-keeping from cycle to cycle. When any of these systems are pharmacologically disrupted SCN neurons desynchronise from one another, and a loss in overall amplitude and precision of time-keeping results (Colwell 2000; Maywood et al. 2006; Yamaguchi et al. 2003).
The apparent absence of such coupling factors in peripheral tissues (Allen et al. 2001; Balsalobre et al. 1998) introduces significant problems when interpreting experimental fibroblast data. To collect useful information on oscillator properties e.g. period, phase etc it is often necessary to overcome the observed reduction in amplitude by employing base-line subtraction or through single-cell imaging. Whilst the latter approach is extremely powerful, it is highly time-consuming and with limited experimental capacity, whilst 24-hour moving average base-line subtraction can be problematic as the period of plated fibroblasts has been shown to vary away from 24 hours with serum concentration (Nagoshi et al. 2004), and the age of the culture (vide infra), as well as obscuring more subtle effects such as differences between cultures in magnitude or acute changes in phase.
We observed that under long-term confluent culture conditions (> 3-4 weeks), 3T3 transcriptional rhythms continue to increase in coherence to the point where they approach SCN-like precision i.e. the quality of rhythms is sufficient that raw data can be used for analysis and publication, obviating the need for any background subtraction or similar post-hoc data processing. The aim of this paper is to highlight the experimental advantages of working with such mature cultures.
MATERIALS AND METHODS
Cells were cultured as described by Kume et al (Kume et al. 1999). NIH 3T3 fibroblasts (passage no. < 40) or PER2-LUC primary fibroblasts (passage no. < 3) (Yoo et al. 2004) were plated in 35 mm dishes. 3T3 cells were transfected with bmal1::luc reporter (Sato et al. 2006) using Gene Juice (Novagen), according to the manufacturer’s protocol. The following day, cultures were refreshed with new media, either Hepes-buffered “Air Medium” (Hastings et al. 2005) for immediate recording, or else DMEM (GIBCO) supplemented with 10% serum. Mature cultures received media changes every 3-4 days, at approximately the same time of day, for up to 6 weeks before luciferase activity was recorded by PMTs. Luciferase activity was recorded continuously in 6 minute bins at 37°C. Phase images were taken on a Leica inverted microscope using a Hamamatsu Orca II CCD camera running “Wasabi” software (Hamamatsu UK). All drugs were purchased from Sigma-Aldrich (UK), and were made up as 1000x stock in Air Medium or DMSO. SCN organotypic slices and Air Media were prepared as Hastings et al (Hastings et al. 2005) using 10% Bovine Foetal Calf Serum.
Period and relative amplitude error (RAE, a measure of goodness-of-fit to a theoretical sine wave) of bioluminescence rhythms were determined with BRASS 3 software (www.amillar.org) using FFT-NLLS (Fast Fourier Transform – Nonlinear Least Squares) (Plautz et al. 1997) with a time window of t = 5 – 80 hrs where t = 0 was the start of data collection. Statistical analysis was performed using Graphpad Prism.
RESULTS
Mature NIH 3T3 cultures display increased coherence of circadian rhythms
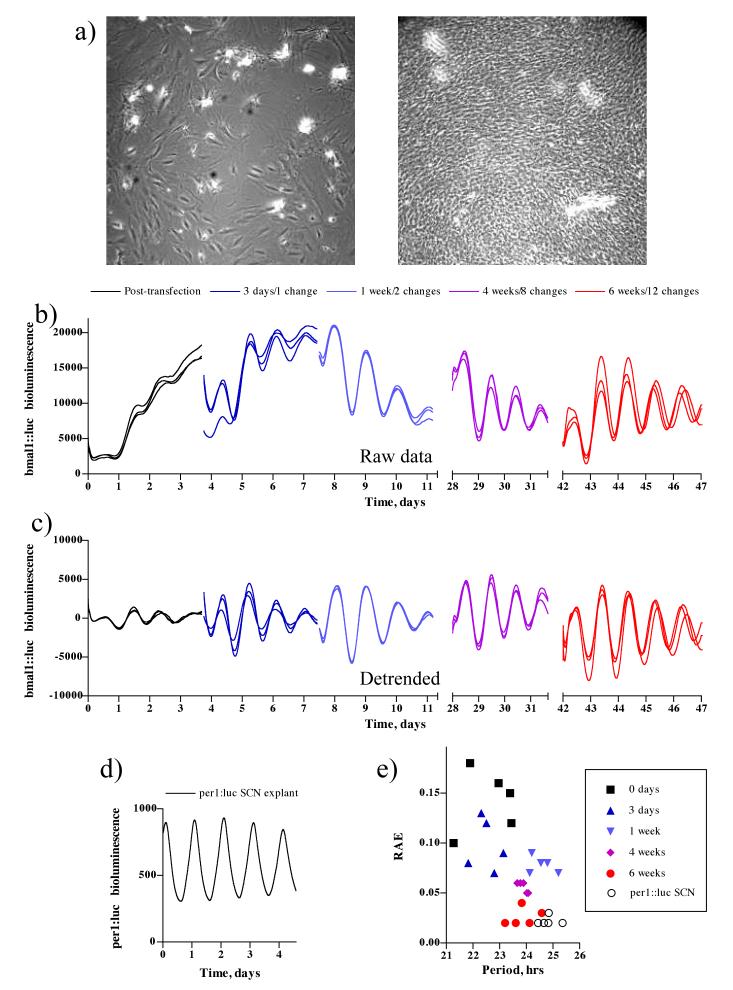
5 sets of 5 replicate NIH 3T3 cultures were simultaneously transfected with the bmal1::luc reporter and cultured for various durations prior to recording. Although nascently transfected cells were apparently confluent (~ 1×106 cells/dish), cells in mature cultures were of higher density increasing to ~ 4×106 cells/dish (figure 1 a).
Figure 1. Greater coherence of mature fibroblast cultures.
(a) Phase image of a nascently transfected confluent culture (left panel) compared with a mature culture (right panel). (b) Bioluminescent activity from NIH 3T3 fibroblasts transfected with bmal1::luc and cultured for varying intervals before recording, 3 replicates are shown. (c) Detrended data from (b) with a 24 hour moving average subtracted. (d) Example of bioluminescent activity from per1::luc SCN explant. (e) FFT-NLLS analysis of data from (b) shown as RAE vs period plot.
In those cultures where reporter activity was immediately recorded, baseline subtraction was necessary to be able to observe overt circadian rhythms, as oscillations in the raw data rapidly damped and were subject to a changing baseline (figure 1b, c). Over time, however, the rhythms recorded from cultures that had been allowed to age for several weeks stabilised to the point that the oscillation was evident without such manipulation (figure 1 b). After 6 weeks, culture profiles were comparable to SCN explants (figure 1 d, e), and this effect could be demonstrated formally through FFT-NLLS analysis using RAE as a reliable proxy for rhythmic coherence. RAE of six week old cultures and per1::luc SCN explants were not significantly different (paired t-test for RAE, p = 0.749, n = 5). Similar data were obtained using PER2::LUCIFERASE embryonic fibroblasts (data not shown).
RAE of nascently transfected cultures was high (> 0.05) for the first week and declined significantly as the time cultures had been allowed to mature, prior to recording, increased (RAE, one-way ANOVA, p = 0.0022, n = 5). Interestingly, period also significantly lengthened with time from a mean of 22.59 ± 0.43 hrs to 23.87 ± 0.23 hrs (period, one-way ANOVA, p = 0.0002), and there was a strong negative correlation between RAE and period (Pearson, r = -0.497, p = 0.012). The dynamics of cellular timekeeping were therefore subject to regulation by the length of time in culture conditions i.e. the maturity of the culture.
Inhibitors of GAP Junctions and NO Signalling do not affect Coherence of Mature Cultures
It is plausible that the observed increase in rhythmic coherence of mature cultures is facilitated by some capacity for intercellular communication that develops in the culture over time. It has been proposed, separately, that both nitric oxide (NO) and GAP junction-mediated connexin-dependent signalling play roles in coupling neighbouring cells in the SCN (Colwell 2000; Menger et al. 2007). As both connexin and nitric oxide synthase are expressed in this cell line (Dermietzel et al. 1990; Kelner et al. 1995; Menger et al. 2007; Wei et al. 2005), their potential relevance was investigated by applying well characterized inhibitors of the two signaling systems to mature cultures at concentrations reported to effect maximal inhibition without being cytotoxic. 18ß-glycyrrhetinic acid (ß-GA), 18α-glycyrrhetinic acid (α-GA), and halothane were used to inhibit GAP junction communication (Burt et al. 1989; Davidson et al. 1988; Le et al. 1998), and carboxy-PTIO (CPTIO, a NO scavenger) was used to disrupt NO signaling (Hu et al. 2005). The prediction being that, if either system were necessary for coupling, a loss of coherence would result from their inhibition, as determined by increasing RAE, compared with vehicle treated cultures. There were, however, no significant effects, of any of these agents on RAE, nor on circadian period of the cultures (Figure 2, paired t-test vs. vehicle, RAE and period, p > 0.1, n = 5), which both remained in the ranges characteristic of mature cultures.
Figure 2. Coherence of mature cultures is not GAP Junction or NO-mediated.
(a) Representative plot showing mature cultures treated with 50 μM CPTIO vs. vehicle. (b) Representative plot showing mature cultures treated with 25 μM α-GA, 25 μM β-GA, 4 mM halothane or vehicle. (c) FFT-NLLS analysis shown as RAE vs period plot.
Inhibition of DNA Polymerase Increases Coherence in Immature Cultures
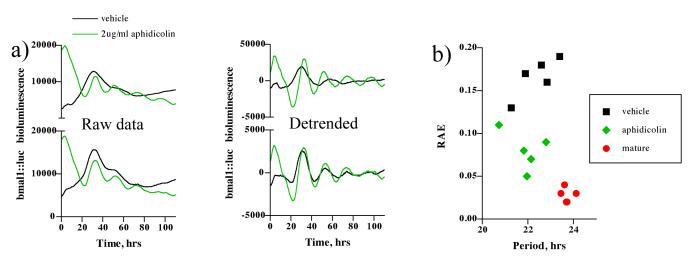
It has previously been reported that although dividing cells conserve circadian phase, the phase of daughter cells is perturbed relative to that observed before mitosis (Nagoshi et al. 2004). Because mature cultures were observed to have higher cell density than those nascently transfected but nevertheless 100% confluent, it is plausible that continued cell division in the latter perturbs the phase of dividing cells leading to an increased rate of desynchronisation, and thus damping, compared to cultures where the cell cycle is terminally arrested. To test this hypothesis, we applied an inhibitor of DNA polymerase, aphidicolin, to nascently transfected fibroblast cultures. Aphidicolin is reported to arrest fibroblasts in G1, at the concentration used (David-Pfeuty et al. 1997; Nuckolls et al. 1998), as they cannot progress through S-phase. From the raw data (figure 3 a) and detrended data (figure 3 b) it is clear that drug treated cultures sustained rhythmicity better than those treated with vehicle, moreover, a significant decrease in RAE was observed in drug-treated cultures compared to vehicle (paired t-test, p < 0.001, n = 5), vehicle mean RAE = 0.166 ± 0.010; aphidicolin mean RAE = 0.080 ± 0.010. This implies that cell division contributes to the damping, by desynchronisation, observed in immature cultures. Importantly, circadian period, which lengthens with maturation (figure 1 d) was not affected by this treatment (p = 0.46), vehicle mean = 22.4 ± 0.37 hrs; aphidicolin mean = 21.89 ± 0.34 hrs.
Figure 3. Nascently transfected fibroblast cultures become more coherent during inhibition of the cell cycle.
(a) Representative plots showing nascently transfected cultures treated with 2 μg/ml aphidicolin vs. vehicle. Left panel – raw data, right panel - 24 hour detrended (c) FFT-NLLS analysis shown as RAE vs period plot.
DISCUSSION
We have shown that circadian transcriptional rhythms in fibroblast cultures become more coherent as they mature. This finding is likely to be helpful for any investigation using cell lines as a platform for assaying cellular rhythms, as data from mature cultures are evident over longer time courses and are of sufficient quality as to obviate the need for post-hoc data processing prior to analysis and publication. These findings will be of particular relevance for high throughput chemical genetics and other screening technologies, that many in the field are moving towards, for identifying novel circadian-relevant targets and testing system dynamics.
For analysis of raw data we employed FFT-NLLS, a tool that has been widely used to compare oscillatory properties by the plant circadian community; we find it is also of great utility for quickly and objectively describing the rhythmic properties of fibroblast cultures.
The observed loss of amplitude in nascently plated or transfected cultures has been reported previously (Nagoshi et al. 2004), and in these conditions cells in under-confluent cultures continued to divide with circadian phase maintained yet perturbed. It has further been shown that oscillations in single fibroblasts are robust, independent and undamped but with variable cycle-to-cycle period (Welsh et al. 2004) leading to the reasonable conclusion that overall damping results from intercellular desynchronisation (Rougemont et al. 2007) with weak or no coupling between cells (in contrast to the SCN). The observation that rhythmic activity, in hyper-confluent mature cultures, becomes significantly more coherent therefore invites two obvious explanations: that either i) a capacity for intercellular coupling develops as cultures age which contributes to synchronisation or ii) mature cultures become fully quiescent such that the phase perturbation introduced by cell division is no longer present and thus cells desynchronise less quickly.
To test the first possibility we disrupted NO and GAP junction-mediated signaling in mature cultures, but this had no effect on their coherence. Although we cannot discount that another cellular signalling mechanism may contribute to this phenomenon, without any more obvious candidates we proceeded to test the second possibility, that continued cell division in apparently confluent cultures might contribute to the observed damping. Inhibition of the cell cycle significantly decreased the RAE of nascently transfected cultures, i.e. lead to greater coherence, and we thus conclude it is likely that mature cultures maintain coherence better because they are a more homogeneous population, without the noise introduced to the oscillator by cell division
Finally, we conclude that mature fibroblast cultures will be of great utility for high-throughput pharmacological and mutational circadian screening due to their reproducibility and because they allow longer recordings to be made, without significant loss of amplitude.
ACKNOWLEDGMENTS
Thanks to Hiroki Ueda (Kobe, Japan) for the bmal1::luc reporter plasmid, and to Andrew Millar for the BRASS 3 software (freely available at www.amillar.org). Kind thanks to Liz Maywood, Gabriel Wong and Rachel Edgar for valuable advice and discussion. This research was supported by the Medical Research Council, as well as the BBSRC, and the EUCLOCK FP6 programme. The Centre for Systems Biology at Edinburgh is a Centre for Integrative Systems Biology (CISB) funded by BBSRC and EPSRC, reference BB/D019621/1.
REFERENCES
- Akashi M, Nishida E. Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev. 2000;14:645–649. [PMC free article] [PubMed] [Google Scholar]
- Allen G, Rappe J, Earnest DJ, Cassone VM. Oscillating on borrowed time: diffusible signals from immortalized suprachiasmatic nucleus cells regulate circadian rhythmicity in cultured fibroblasts. J Neurosci. 2001;21:7937–7943. doi: 10.1523/JNEUROSCI.21-20-07937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Burt JM, Spray DC. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ Res. 1989;65:829–837. doi: 10.1161/01.res.65.3.829. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. J Neurobiol. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::aid-neu6>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T, Nouvian-Dooghe Y, Rouillard D. Delaying the onset of M phase in NIH 3T3 cells blocked in early S phase occurs via accumulating cyclin B1 and tyrosine-phosphorylated p34cdc2 in the nucleus. Biol Cell. 1997;89:179–197. [PubMed] [Google Scholar]
- Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- Dermietzel R, Hwang TK, Spray DS. The gap junction family: structure, function and chemistry. Anat Embryol (Berl) 1990;182:517–528. doi: 10.1007/BF00186458. [DOI] [PubMed] [Google Scholar]
- Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–557. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, McMahon DG, Maywood ES. Analysis of circadian mechanisms in the suprachiasmatic nucleus by transgenesis and biolistic transfection. Methods Enzymol. 2005;393:579–592. doi: 10.1016/S0076-6879(05)93030-9. [DOI] [PubMed] [Google Scholar]
- Hu RG, Sheng J, Qi X, Xu Z, Takahashi TT, Varshavsky A. The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature. 2005;437:981–986. doi: 10.1038/nature04027. [DOI] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A. 2003;100:16089–16094. doi: 10.1073/pnas.2536313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner MJ, Uglik SF. Superoxide dismutase abolishes the platelet-derived growth factor-induced release of prostaglandin E2 by blocking induction of nitric oxide synthase: role of superoxide. Arch Biochem Biophys. 1995;322:31–38. doi: 10.1006/abbi.1995.1432. [DOI] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev Biol. 1998;204:80–96. doi: 10.1006/dbio.1998.9030. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Menger GJ, Allen GC, Neuendorff N, Nahm SS, Thomas TL, Cassone VM, Earnest DJ. Circadian profiling of the transcriptome in NIH/3T3 fibroblasts: comparison with rhythmic gene expression in SCN2.2 cells and the rat SCN. Physiol Genomics. 2007;29:280–289. doi: 10.1152/physiolgenomics.00199.2006. [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Nuckolls FJ, Khan AS, Butler R, Katula KS. Differential response of the human cyclin B1 promoter to inhibitors of the cell cycle in NIH3T3 cells. Biochem Biophys Res Commun. 1998;244:280–284. doi: 10.1006/bbrc.1998.8205. [DOI] [PubMed] [Google Scholar]
- Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA. Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms. 1997;12:204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
- Rougemont J, Naef F. Dynamical signatures of cellular fluctuations and oscillator stability in peripheral circadian clocks. Mol Syst Biol. 2007;3:93. doi: 10.1038/msb4100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]