Abstract
The genes encoding prokineticin 2 polypeptide (Prok2) and its cognate receptor (Prokr2/Gpcr73l1) are widely expressed in both the suprachiasmatic nucleus (SCN) and its hypothalamic targets, and this signaling pathway has been implicated in the circadian regulation of behavior and physiology. We have previously observed that the targeted null mutation of Prokr2 disrupts circadian co-ordination of cycles of locomotor activity and thermoregulation. We have now observed spontaneous but sporadic bouts of torpor in the majority of these transgenic mice lacking Prokr2 signaling. During these torpor bouts, which lasted for up to 8h, body temperature and locomotor activity decreased markedly. Oxygen consumption and carbon dioxide production also decreased, and there was a decrease in RQ. These spontaneous torpor bouts generally began towards the end of the dark phase or in the early light phase when the mice were maintained on a 12:12 light-dark cycle, and persisted when mice were exposed to continuous darkness. Periods of food deprivation (16-24h) induced a substantial decrease in body temperature in all mice, but the duration and depth of hypothermia was significantly greater in mice lacking Prokr2 signaling compared to heterozygous and wild-type litter mates. Likewise, when tested in metabolic cages, food deprivation produced greater decreases in oxygen consumption and carbon dioxide production in the transgenic mice than the controls. We conclude that Prokr2 signaling plays a role in the hypothalamic regulation of energy balance, and loss of this pathway results in physiological and behavioral responses normally only detected when mice are in negative energy balance.
Keywords: prokineticin, torpor, metabolic rate, thermoregulation, CLAMS, radiotelemetry
INTRODUCTION
Prokineticins are small secreted proteins (Prok1 and Prok2) which share approximately 44% amino acid sequence identity, with a highly conserved N-terminal (15). These prokineticins are thought to influence a wide variety of physiological processes both in the central nervous system and in peripheral tissues, including intestinal contraction, hyperalgesia, spermatogenesis, neuronal survival, circadian rhythmicity, angiogensis, ingestive behavior and hematopoesis (for reviews 17, 31). These proteins are the cognate ligands for two closely related G-protein-coupled receptors (Prokr1 and Prokr2) which share 87% amino acid sequence identity and exhibit the greatest differences in the N-terminal sequences (16, 25). There are differences in the distribution of these receptors: Prokr1 is mainly located in peripheral tissues, while Prokr2 is predominantly expressed in the brain (16), particularly in the suprachiasmatic nucleus (19), but also in many other hypothalamic regions (6). The expression pattern of Prok2 in the SCN is highly circadian, and intracerebroventricular treatment of rats with Prok2 inhibits nocturnal locomotor activity (4, 5). It has therefore been proposed that Prok2 functions as a humoral output signal communicating circadian information from the SCN to regions of the brain controlling motor output (4-6).
In support of this hypothesized role for Prok2 as a circadian output signal from the SCN, we and others have generated mice in which the genes encoding either Prok2 (14) or Prokr2 (20, 23) are mutated and rendered dysfunctional. The common phenotypic feature of targeted ablation of either the ligand or receptor was that the amplitude of circadian rhythms of body temperature and locomotor activity was decreased (14, 23), and there were also changes in the profile of the rhythm of body temperature such that the duration of the nocturnal elevation in body temperature was shorter in mice lacking Prok2 signaling as compared to control mice (23). In mice expressing the mutated Prokr2 gene we also occasionally observed very low light phase body temperatures that appeared to be bouts of torpor. Torpor bouts are not routinely observed in laboratory strains of mice maintained on ad libitum feed at constant temperature, so this phenotypic trait is indicative of altered energy metabolism.. The purpose of the present study was therefore to carry out observations for an extended period of time to characterize further these torpor bouts, and to determine if changes in energy intake or expenditure might contribute to the occurrence of the periods of hypothermia.
MATERIALS AND METHODS
Animals
All in vivo experimental procedures were approved by the University of Nottingham Local Ethical Review Committee and were carried out in accordance with the Animals Scientific Procedures Act (UK) 1986. Male and female transgenic mice were generated as previously described (23), thus carried functionally-null alleles for the Prokr2 gene (prokr2Brdm1) henceforth abbreviated as m. Homozygous mutant mice (Prokr2m/m) and their litter mates (Prokr2+/+ and Prokr2+/m) were obtained as adults from The Wellcome Trust Sanger Institute (Hinxton, UK), and studies were carried out in two separate batches starting 8 months apart. All studies were carried out on both batches of mice using the same protocols. The first batch consisted of 4 males (2 Prokr2m/m mice and 2 littter mates) and 4 females (also 2 Prokr2m/m and 2 litter mates). Studies 1 to 4 (see below) were carried out sequentially in these mice. The second batch consisted of 5 males (2 Prokr2m/m mice and 3 litter mates) and 3 females (2 Prokr2m/m and 1 littter mate), but one Prokr2m/m female died before the various studies could be completed so data from this mouse have been excluded from analysis. Studies 3 and 4 were carried out initially in the second batch of mice, and then studies 1 and 2. The mice were housed in individual cages under controlled temperature (21 ± 1 C) and on a photocycle of 12 h light / 12 h dark cycle; lights off at 17:00 h, with ad libitum access to food intake and water, unless stated otherwise. A dim red light (<10lux) was present continuously during the dark phase. Studies did not commence until two weeks after transport from Hinxton to allow a habituation period, at which point the mice were aged between approximately 4 and 8 months (Table 1).
C) and on a photocycle of 12 h light / 12 h dark cycle; lights off at 17:00 h, with ad libitum access to food intake and water, unless stated otherwise. A dim red light (<10lux) was present continuously during the dark phase. Studies did not commence until two weeks after transport from Hinxton to allow a habituation period, at which point the mice were aged between approximately 4 and 8 months (Table 1).
Table 1.
Age, body weight and tissues weight of the study population
| male | female | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| +/+ or +/m | m/m | +/+ or +/m | m/m | genotype | gender | interaction | |
| n | 5 | 4 | 3 | 3 | |||
| age at start of the study (months) |
5.1±0.3 | 4.9±0.1 | 5.2±0.3 | 6.7±1.5 | F=1.00 P=0.34 |
F=2.47 P=0.14 |
F=1.92 P=0.19 |
| body weight at start (g) |
34.8±3.0 | 23.5±1.6 | 24.7±0.8 | 28.8±4.1 | F=1.62 P=0.23 |
F=0.72 P=0.41 |
F=7.26 P<0.05 |
| body weight at autopsy (g) |
39.1±3.8 | 29.0±2.2 | 30.2±1.1 | 31.4±3.0 | F=1.64 P=0.22 |
F=0.80 P=0.39 |
F=2.85 P=0.12 |
| paired testes (mg) | 210±14 | 30±23 | t=7.0 P<0.001 |
||||
| EPIFAT (mg) | 1188±471 | 1691±408 | t=0.78 P=0.46 |
||||
| uterus (mg) | 185±72 | 16±2 | |||||
| PARAFAT (mg) | 952±166 | 1570±322 | |||||
Values are group mean±SEM. EPIFAT = paired epididymal fat pads, PARAFAT = parametrial fat pad.
Radiotelemetry
Mice were anesthetized with a mixture of ketamine (Vetalar 100 mg/kg ip, Forte Dodge Animal Health, Southampton, UK) and medetomidine (Dormitor 1 mg/kg ip, Pfizer, Kent, UK) in a ratio of 1:4. Analgesia was maintained via subcutaneous (sc) injection of carprofen (50 mg/kg Rimadyl, Pfizer, Kent, UK) and administered before surgery. Telemetry devices (TA10TA-F20, Data Sciences International, St Paul, MN) were placed in the peritoneal cavity of the mice. Following surgery, the animals were given the anesthetic reversal atipamezole (Antisedan 1 mg/kg ip, Pfizer, Kent, UK) and also 0.9% saline (10 μl/g body weight, sc). Signals from the radiotelemetry implants were detected using RPC-1 receivers and processed using ARTv3.1 silver software (Data Sciences International, St Paul, MN).
Study 1: Effects of Prokr2 gene mutation on body temperature rhythms in entrained and free-running conditions
Body temperature was monitored in all ad libitum fed Prokr2+/+, Prokr2+/m and Prokr2m/m mice for 10 seconds in every two minutes over a period of approximately 4 weeks in a 12L:12D light dark cycle. The photoperiod was then switched to DD, ie continual dim red light at the same intensity as during the dark phase of the 12L:12D photocycle, and mice monitored for a further 3 weeks.
Study 2: Effects of food deprivation on body temperature in Prokr2m/m mice
After the evaluations of body temperature rhythms in 12L:12D and in DD, the mice bearing radiotelemetry implants were returned to a 12L:12D photocycle for 14 days and maintained on ad libitum feed prior to starting this study. Access to food but not water was then completely removed from mice just before lights out, and replaced 16 h later. Body temperature was monitored throughout this study as described in Study 1. After a further 7 days on ad libitum feed, access to food but not water was completely removed from mice 6h before lights out, and replaced 24 h later, and body temperature monitored for a further 7 days.
Study 3: Feeding behavior and metabolic rate in Prokr2m/m mice
VO2, VCO2, locomotor activity and various parameters of eating behavior (frequency and duration of feeding bouts, food consumption per bout and total food intake) were measured using a Columbus Instruments Comprehensive Lab Animal Monitoring System (CLAMS: Linton Instrumentation, Linton, UK / Columbus Instruments, Columbus, OH) as previously described (11). This is a modified open circuit calorimeter, and the configuration used consisted of 8 chambers in which mice were studied individually. The chambers had feeders positioned in the middle of the cages, and the weight of each removal of food was recorded along with the timing and duration of this feeding bout. For subsequent analysis a “meal” was defined as a bout of food intake of greater than 0.02g. We operated the system with an air intake of 0.6L per minute per chamber, and an extracted outflow of 0.4L per minute. The chambers have a volume of ~2.7L, thus get approximately 14 air changes per hour. A sample of air was extracted from each chamber every 9 minutes for sequential analysis of CO2 then O2, the room air (ie input air to the chambers) was similarly analysed every 9 minutes. Water was provided by dropper bottles, but intake was not recorded. Activity was recorded when two or more consecutive infra red beams positioned approximately 2 cm apart were broken. All measurements were taken at an ambient temperature of 21-22 C. Metabolic gas values and feeding behaviors were monitored over a 96h period in ad libitum fed mice. For the purposes of data analysis and presentation the first 12 h of data were discarded as the high activity levels and high VO2 indicated that the mice were incompletely habituated to the metabolic cages.
C. Metabolic gas values and feeding behaviors were monitored over a 96h period in ad libitum fed mice. For the purposes of data analysis and presentation the first 12 h of data were discarded as the high activity levels and high VO2 indicated that the mice were incompletely habituated to the metabolic cages.
Study 4: Effects of food deprivation on the metabolic rate of Prokr2m/m mice
Mice were placed in the CLAMS apparatus (as above: Study 3), but after a habituation period of at least 24h, beginning an hour before lights out, food was withdrawn for 16 h. Drinking water was available at all times. Metabolic gases and activity were monitored over this period and for a further 24 hours. Food intake was also assessed before and after the period of deprivation so that the effects of the loss of Prokr2 signaling on compensatory hyperphagia could be determined.
Autopsy
After the completion of studies all mice were autopsied, for the first batch of animals this was 7 days after the last period of food deprivation, and for the mice in the second batch this was 28 days after the last period of food deprivation. Following euthanasia with pentobarbitone sodium (Euthatal, Rhone Merieux, Harlow, UK) the reproductive tissues and surrounding fat depots were dissected and their wet weight recorded.
Data analysis
In all analyses of body temperature, a moving average was calculated in 1h epochs based upon the values for 10 second intervals sampled every 2 minutes using the ARTv3.1 silver software (Data Sciences International, St Paul, MN). These data are generally presented in figures as representative individuals or as group mean ± standard error of the mean (sem). The analysis of mean nocturnal (i.e mean for the 12h dark phase) and mean diurnal (i.e mean for the 12h light phase) body temperatures was based on 28 days of data whilst mice were on ad libitum feed and entrained to 12L:12D; data for the first week after surgery were disregarded. Values were calculated for individual mice, and these were compared using a two-way ANOVA (effect of genotype vs. effect of gender; Prism 4.0, San Diego, CA). The nocturnal maximum and diurnal minimum values for each mouse over the 28 day period were compared using the same ANOVA model. The minimum values for the hourly temperature epochs during the periods of 16 or 24 h food deprivation were also compared using two-way ANOVA.
For analysis of data obtained from the CLAMS apparatus, mean values for VO2 and VCO2, RQ, locomotor activity and parameters of food intake were calculated for the 12h light phase and the 12h dark phase, then analysed using two-way ANOVA with genotype and gender as the main effects. For the analysis of the effects of food deprivation, the physiological parameters were calculated for 1h time bins, and two-factor ANOVAs carried out with genotype and time (repeated measure) as the main factors because data from one female was lost due to technical failure leaving data from just two female Prokr2m/m mice. Where a significant time × genotype interaction was identified the effects of genotype at specific time points was revealed by post hoc Bonferroni tests (Prism 4.0, San Diego, CA).
A torpor bout was defined as such if the hourly mean body temperature fell below 33.0°C for two or more consecutive hours. The rationale for this is that the group mean±SD minimum diurnal body temperature in both genders in wild-type mice was 34.8±0.4 °C (Table 2), thus 33.0°C represents a cut-off 4SD below the minimum temperature that would be expected in an ad libitum fed wild-type mouse. The criterion of 33.0°C for the onset of a torpor bout is more stringent than that which would be predicted by the model recently developed by Willis (29). The incidence of torpor bouts was compared between genotypes by a Fisher’s Exact Probability Test (Prism 4.0, San Diego, CA). For all analyses, data from Prokr2+/+ and Prokr2+/m mice were considered as a single control group because preliminary analyses revealed no differences in any parameters between these genotypes. P<0.05 was considered statistically significant in all analyses.
Table 2.
Analysis of core body temperature and incidence of torpor bouts
| male | female | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| +/+ or +/m | m/m | +/+ or +/m | m/m | genotype | gender | interaction | |
| n | 5 | 4 | 3 | 3 | |||
| 12L:12D ad lib nocturnal max (°C) |
38.2±0.3 | 37.4±0.1 | 38.9±0.2 | 37.4±0.2 | F=24.2 P<0.001 |
F=1.47 P=0.23 |
F=2.66 F=0.13 |
| nocturnal mean (°C) |
36.9±0.1 | 36.3±0.1 | 37.1±0.2 | 36.1±0.2 | F=33.6 P<0.0001 |
F=0.00 P=0.98 |
F=1.81 P=0.21 |
| diurnal mean (°C) |
35.9±0.1 | 35.7±0.1 | 36.3±0.1 | 35.2±0.6 | F=7.00 P=0.02 |
F=0.06 P=0.81 |
F=2.91 F=0.12 |
| diurnal minimum (°C) |
34.8±0.2 | 29.3±2.9 | 34.8±0.2 | 27.8±3.4 | F=9.08 P=0.01 |
F=0.13 P=0.73 |
F=0.13 P=0.72 |
| 16h food restrict diurnal minimum (°C) |
31.9±1.0 | 22.4±0.3 | 29.5±1.0 | 23.0±0.8 | F=77.2 P<0.0001 |
F=1.04 P=0.33 |
F=2.74 P=0.12 |
| 24h food restrict diurnal minimum (°C) |
34.0±0.9 | 30.3±3.0 | 32.9±1.6 | 25.7±2.0 | F=7.05 P=0.02 |
F=2.01 P=0.18 |
F=0.76 P=0.40 |
| Incidence of torpor (on ad lib feed) |
0/5 (0%) | 3/4 (75%) | 0/3 (0%) | 2/3 (67%) | P=0.007 Fisher’s Exact Test |
||
| Mean interval between torpor bouts (days)a |
n/a | 6.1, 8.6, 10.4 |
n/a | 1.4, 20.0 | |||
Values are group mean±SEM. n/a not applicable.
values for individual mice which had two or more bouts of torpor.
Results
Study 1: Effects of Prokr2 gene mutation on body temperature rhythms in entrained and free-running conditions
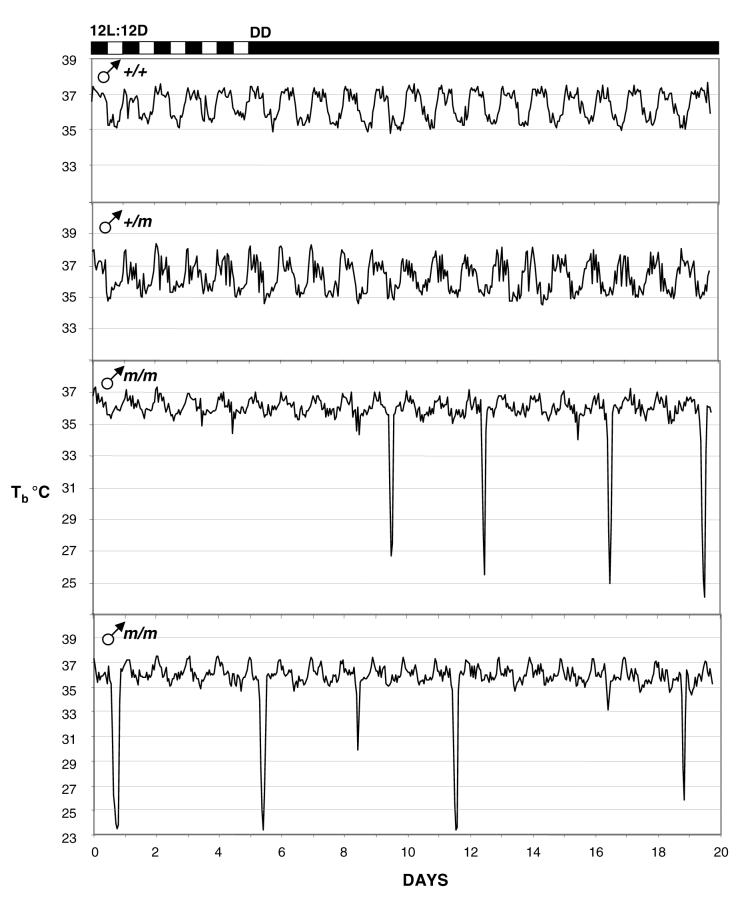
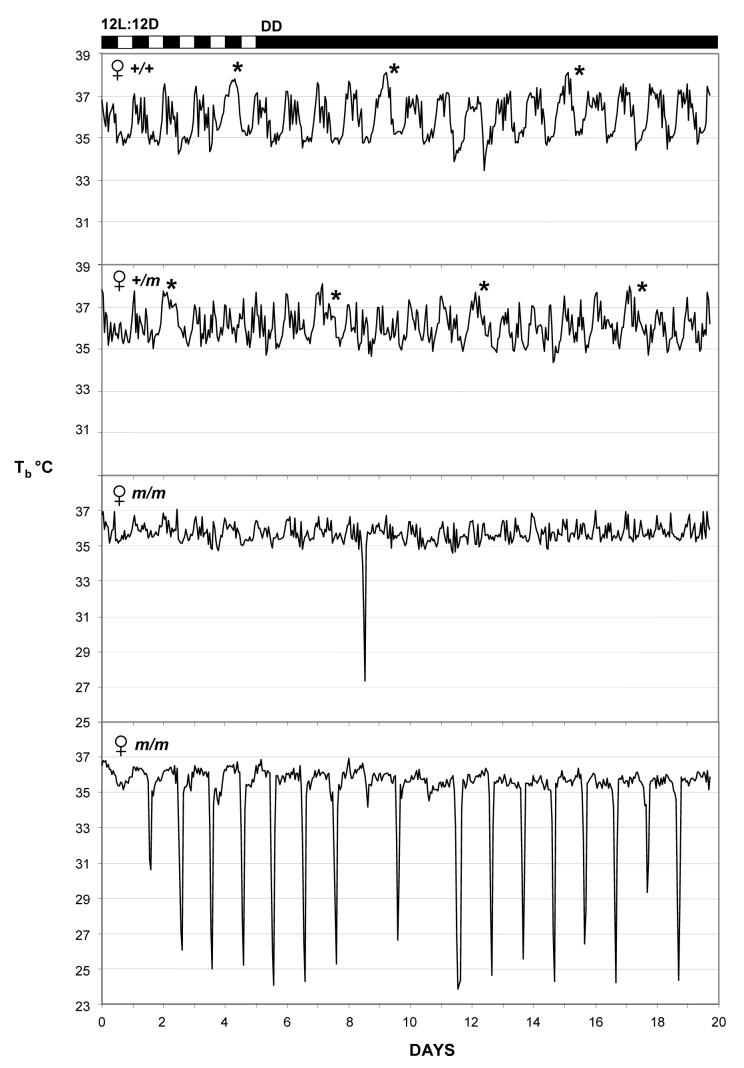
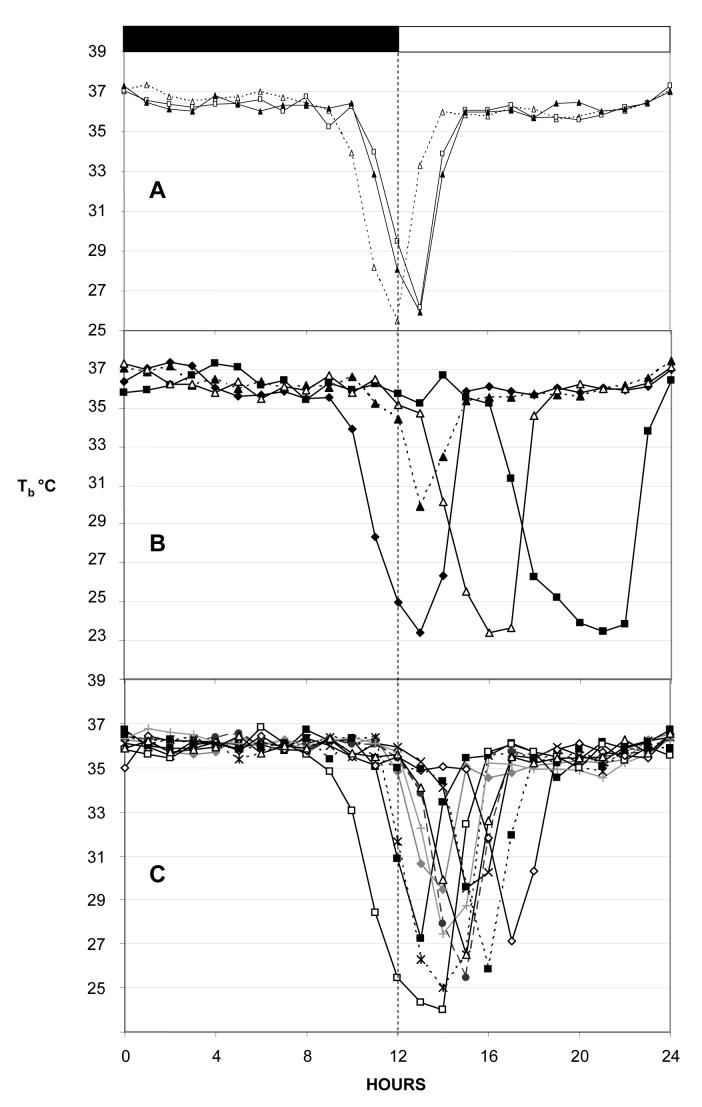
The control mice (Prokr2+/+ and Prokr2+/m) all displayed robust daily rhythms in body temperature while entrained to the 12L:12D photocycle, and clear circadian rhythms which free-ran when the mice were exposed to DD (Figs. 1 and Fig. 2, upper panels). The range in hourly mean body temperature varied by over 2°C (Figs. 1 and 2), and in females there was evidence of raised nocturnal temperatures at regular 5 or 6 day intervals, presumably reflecting cyclical estrus activity (Fig. 2, upper panels). Maximum and mean nocturnal body temperatures were significantly lower in Prokr2m/m mice of both sexes (Table 2), and correspondingly minimum and mean diurnal body temperatures were also significantly lower in Prokr2m/m mice (Table 2). The circadian organization of the temperature rhythms in Prokr2m/m mice was less defined, in general a nocturnal increase in temperature occurred in such mice on 12L:12D, but the rise was truncated ie returned to diurnal values long before the onset of the light phase. Strikingly, 5 of 7 Prokr2m/m mice displayed bouts of torpor, that is, decreases in body temperature below 33°C lasting two or more hours (Figs. 1 and 2, lower panels). Such torpor bouts were never observed in control mice (P<0.005). These torpor bouts were sporadic in appearance, varied greatly in frequency between individual Prokr2m/m mice, and were not synchronized between individuals (Figs. 1 and 2). Three of 4 Prokr2m/m males displayed torpor bouts (eg Fig. 1), the bouts occurring in these on average 6-10 days apart (Table 2), and 2 of 3 Prokr2m/m females displayed torpor bouts (Fig. 2), one at a very high frequency (Fig. 2 bottom), the other very infrequently (Fig. 2 middle panel). The propensity to display torpor did not correlate with the body weight of the individual mice, nor did it correlate with weight gain over the experimental period (data not shown). In general the timing of the onset of individual torpor bouts was restricted to the late dark phase/early light phase (Figure 3), though in some individuals the timing was far more precise (Fig. 3A) than in others (Fig. 3B). The duration of individual torpor bouts also varied within individuals (eg Fig. 3C) and between individuals (eg Fig. 3A vs Fig. 3B), but in no individuals did the bouts last longer than 7h (Fig. 3).
Figure 1.
Representative body temperature traces in male mice for 5 days at the end of a period of entrainment to a 12L:12D photocycle and then for 15 days on continual dim red light DD. Traces are shown for a Prokr2+/+ (top) and a Prokr2+/m mouse (second panel), and for two Prokr2m/m littermates (lower panels). Note the sporadic bouts of torpor in the Prokr2m/m males.
Figure 2.
Representative body temperature traces in female mice for 5 days at the end of a period of entrainment to a 12L:12D photocycle and then for 15 days on continual dim red light DD. Traces are shown for a Prokr2+/+ (top) and a Prokr2+/m mouse (second panel) and for two Prokr2m/m littermates (lower panels). * indicates likely proestrous days. Note the single bout of torpor but disorganized circadian temperature profile in one Prokr2m/m female (third panel), and the almost daily occurrence of torpor in the other Prokr2m/m female (bottom).
Figure 3.
Examples of torpor bouts in individual Prokr2m/m mice on a 12L:12D photocycle. Note the similarity of profile and timing of the initiation of the torpor bouts in the late dark phase in mouse A (top) compared to the more variable timing in mouse B (middle). In mouse C the onset is restricted to the late dark phase/early light phase, but the duration and depth of the torpor bout varies considerably.
Study 2: Effects of food deprivation on body temperature in Prokr2m/m mice
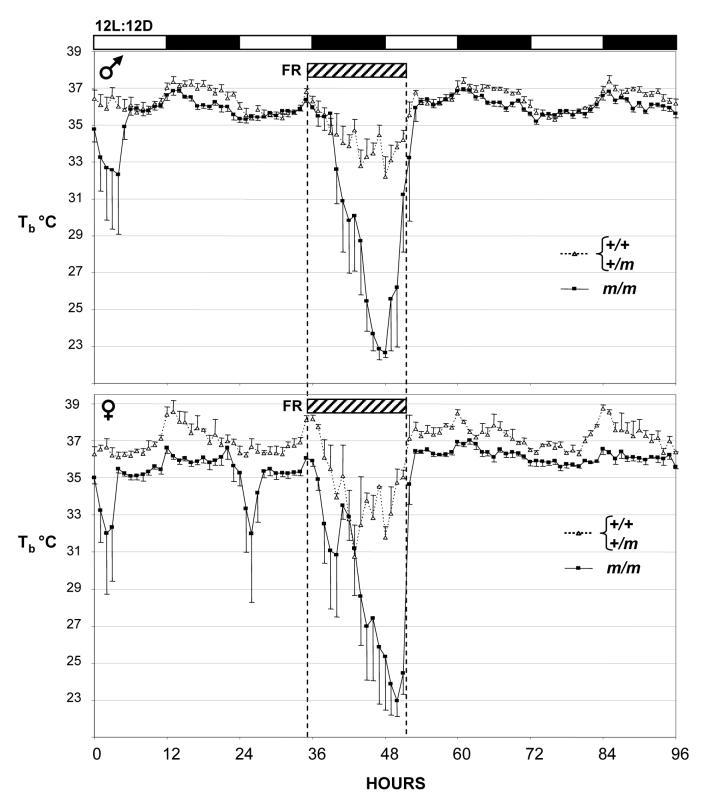
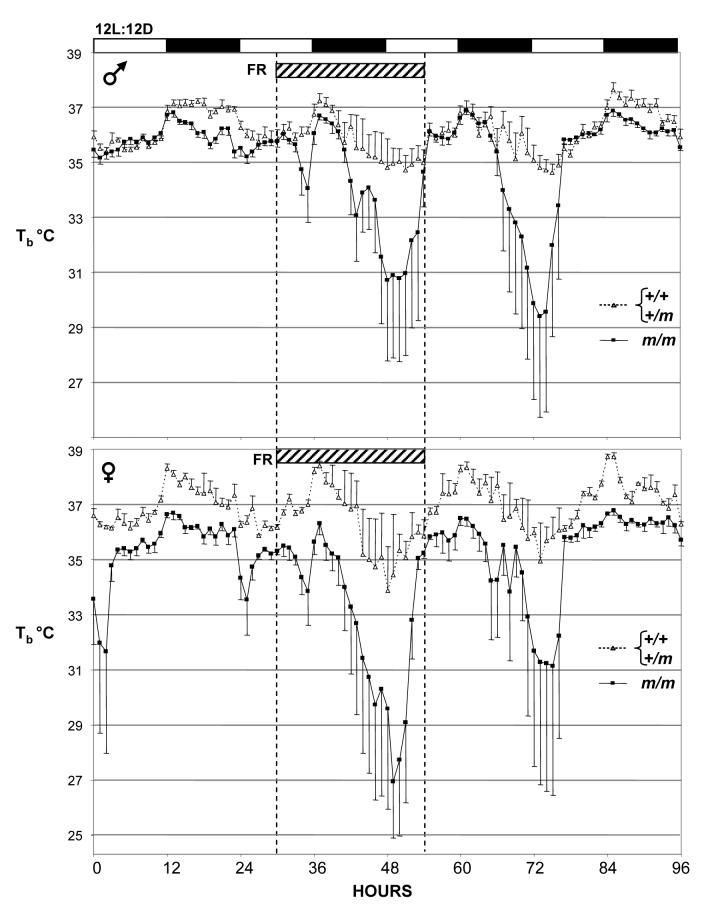
Removal of food for 16h (Fig 4) or 24h (Fig. 5) produced a marked decrease in diurnal body temperature in both the control and Prokr2m/m mice, but the magnitude of the hypothermia was significantly greater in the latter group (Table 2). In the study where 16h food deprivation was initiated shortly before lights off, mean body temperature in the control mice during the period of food deprivation descended to 31.9°C (males) and 29.5°C (females) compared to a mean of 34.8°C during the light phase whilst on ad libitum feed (Fig. 4, Table 2). In contrast, all Prokr2m/m mice entered torpor during the light phase when food-deprived; mean body temperature fell to 22.4°C and 23.0°C for males and females respectively (Fig. 4, Table 2). A similar outcome was observed in the study where 24h food deprivation was initiated midway through the light phase (Fig. 5). Although the magnitude of hypothermia was somewhat less than in the 16h study in both the control and Prokr2m/m mice (Table 2), there was an extended effect of the 24h food deprivation such that despite being returned to ad libitum feed, the majority of both the male and female Prokr2m/m mice showed a second bout of hypothermia 24h after the initial response (Fig. 5).
Figure 4.
Body temperature in control (Prokr2+/+ / Prokr2+/m) and Prokr2m/m mice on a 12L:12D photocycle subject to a 16h period of food deprivation (FR) beginning at hour 35 (hatched bar). Values are group mean±SEM, n=5 control and n=4 Prokr2m/m males (top), and n=3 for both control and Prokr2m/m females (bottom). Note the lower body temperature in the Prokr2m/m mice compared to the controls both before and after the food deprivation, and the far greater hypothermic response to food deprivation.
Figure 5.
Body temperature in control (Prokr2+/+ / Prokr2+/m) and Prokr2m/m mice on a 12L:12D photocycle subject to a 24h period of food deprivation (FR) beginning at hour 30 (hatched bar). Values are group mean±SEM, n=5 control and n=4 Prokr2m/m males (top), and n=3 for both control and Prokr2m/m females (bottom). Note the greater hypothermic response to food deprivation in the Prokr2m/m mice, and their second bout of hypothermia on the day that they are returned to ad libitum diet.
Study 3: Feeding behavior and metabolic rate in Prokr2m/m mice
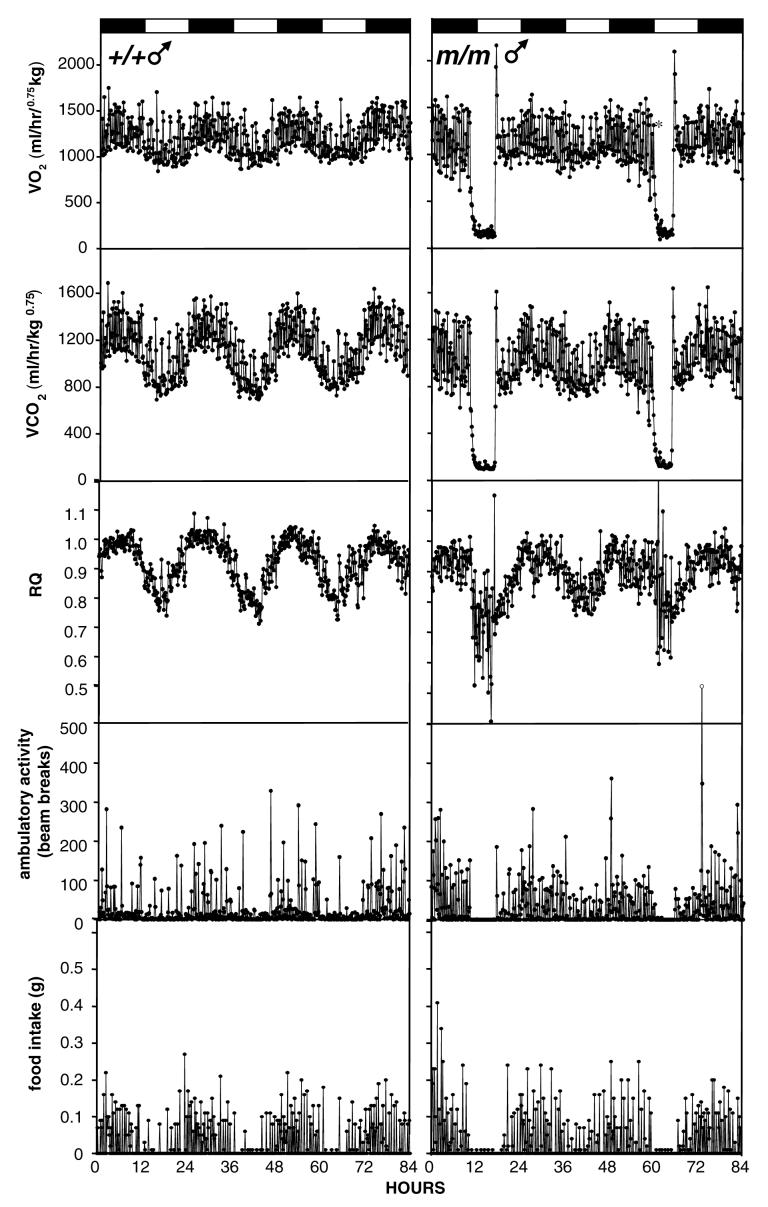
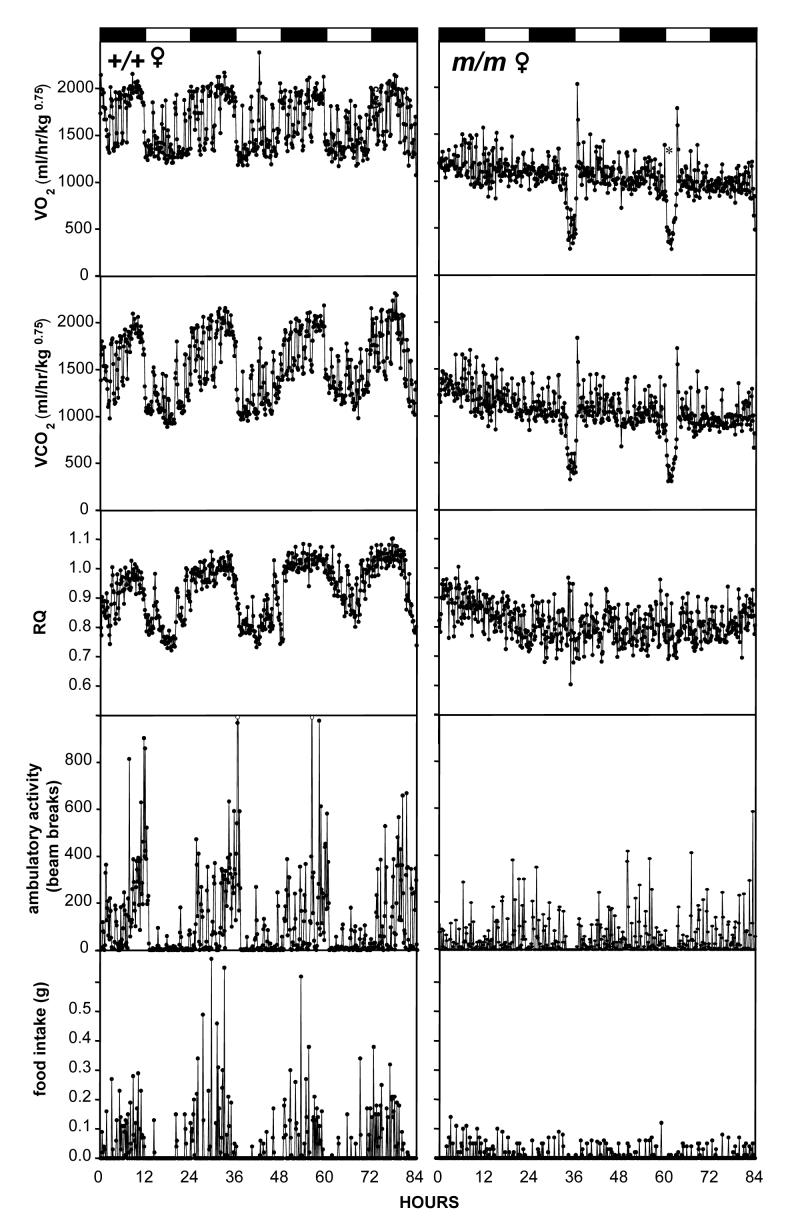
Control male (Fig. 6, left) and female (Fig. 7, left) mice displayed robust 24h variations in VO2, VCO2, RQ and feeding behavior while entrained to 12L:12D photocycle. The Prokr2m/m mice generally had less clearly defined circadian patterns of metabolic gas exchange (eg Fig. 7, right), and in 4 of 7 Prokr2m/m mice we observed one or more bouts of torpor during housing in the CLAMS cages (examples shown in Figs. 6 and 7, right panels). During these bouts of torpor, VO2 dropped to below 200ml/hr/kg0.75, and RQ decreased to approximately 0.7 (Figs. 6 and 7). RQ values below 0.7 occurred in some individual samples during torpor bouts (Fig. 6), but we consider these to be artefacts because when VO2 and VCO2 are extremely low, the ratio of these is much more sensitive to the variance (error) and limited resolution of the oxygen and carbon dioxide detectors. For subsequent statistical analyses of the impact of the Prokr2m/m mutation, data from 24h periods in which torpor bouts occurred were excluded from comparison. During these ‘non-torpor’ cycles, the Prokr2m/m mice had a significantly lower VO2 and VCO2 than their littermate controls during both the light and dark phases of the 12L:12D photocycle (Table 3), but RQ did not differ between the genotypes (Table 3). The lower VO2 and VCO2 occurred in both male and female Prokr2m/m mice (Table 3). There was a high degree of variability in the amount of locomotor activity within groups (Table 3), but during the dark phase there were significantly greater levels of activity in female mice (Table 3), and in both sexes significantly lower levels of activity in the Prokr2m/m mice (Table 3). During the light phase no significant effects of either genotype or gender on activity levels were detected (Table 3). Overall food intake was significantly lower in the Prokr2m/m mice in both the dark and light phases (Table 3). In the light phase there was a significant genotype × gender interaction suggesting that the decrease in food intake in mice only occurred in the females (Table 3). There were no significant differences in individual meal sizes between the genotypes, but a reduced frequency of meals during the light phase in the Prokr2m/m mice (Table 3).
Figure 6.
Representative examples of VO2 (top), VCO2 (second panel), RQ (middle panel), locomotor activity (fourth panel) and feeding bouts (bottom panel) in a male wild-type mouse (left) and a male Prokr2m/m mouse (right). Mice were entrained to a 12L:12D photocycle. Values for metabolic gases and activity were collected in 9 min epochs, bouts of food intake were recorded in real time. Note the two bouts of torpor and the reduced amplitude of the rhythm of O2 uptake and CO2 production in the Prokr2m/m mouse.
Figure 7.
Representative examples of VO2 (top), VCO2 (second panel), RQ (middle panel), locomotor activity (fourth panel) and feeding bouts (bottom panel) in a female Prokr2+/+ mouse (left) and a female Prokr2m/m mouse (right). Mice were entrained to a 12L:12D photocycle. Values for metabolic gases and activity were collected in 9 min epochs, bouts of food intake were recorded in real time. Note the two bouts of torpor and absence of clear rhythmicity of O2 uptake and CO2 production in the Prokr2m/m mouse.
Table 3.
Analysis of gas exchange and feeding behavior
| male | female | ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| +/+ or +/m |
m/m | +/+ or +/m |
m/m | genotype | gender | interaction | ||
| n | 5 | 4 | 3 | 3 | ||||
| Body weight1 (g) | 36.8±2.7 | 27.9±2.3 | 29.2±3.5 | 25.8±0.8 | F=2.9 P=0.12 |
F=4.7 P=0.05 |
F=0.9 P=0.36 |
|
| VO2 (ml/kg0.75/h) |
dark | 1315±71 | 1107±41 | 1556±251 | 994±18 | F=7.82 P<0.05 |
F=0.91 P=0.36 |
F=3.58 P=0.09 |
| light | 1319±58 | 1143±42 | 1310±147 | 1064±21 | F=6.86 P<0.05 |
F=0.30 P=0.60 |
F=0.19 P=0.66 |
|
| VCO2 (ml/kg0.75/h) |
dark | 1255±78 | 1030±32 | 1412±220 | 831±42 | F=12.3 P<0.005 |
F=0.03 P=0.86 |
F=2.40 P=0.15 |
| light | 1068±75 | 979±44 | 1176±26 | 841±20 | F=10.4 P<0.01 |
F=0.05 P=0.82 |
F=3.49 P=0.09 |
|
| RQ | dark | 0.95±0.02 | 0.93±0.03 | 0.91±0.02 | 0.84±0.03 | F=1.10 P=0.32 |
F=2.11 P=0.17 |
F=0.41 P=0.54 |
| light | 0.81±0.03 | 0.86±0.03 | 0.87±0.05 | 0.79±0.02 | F=0.17 P=0.69 |
F=0.01 P=0.94 |
F=3.28 P=0.10 |
|
| activity (arbitrary units) |
dark | 1213±267 | 709±235 | 2574±75 | 1155±491 | F=6.7 P<0.05 |
F=5.9 P<0.05 |
F=1,51 P=0.24 |
| light | 416±175 | 311±113 | 1314±568 | 368±27 | F=3.30 P=0.10 |
F=2.72 P=0.13 |
F=2.11 P=0.17 |
|
| total food intake (g per 12h) |
dark | 3.00±0.22 | 2.10±0.29 | 2.68±0.34 | 2.28±0.37 | F=4.9 P<0.05 |
F=0.08 P=0.78 |
F=0.58 P=0.46 |
| light | 1.55±0.25 | 1.53±0.16 | 3.34±0.55 | 0.91±0.37 | F=13.4 P<0.01 |
F=3.04 P=0.11 |
F=12.8 P<0.01 |
|
| meal freq (per h) |
dark | 2.32±0.40 | 1.73±0.29 | 2.00±0.52 | 2.17±0.27 | F=0.23 P=0.64 |
F=0.02 P=0.89 |
F=0.74 P=0.41 |
| light | 1.67±0.31 | 1.50±0.15 | 2.47±0.07 | 1.46±0.17 | F=5.01 P<0.05 |
F=2.10 P=0.18 |
F=2.58 P=0.14 |
|
| meal size (g) |
dark | 0.13±0.04 | 0.10±0.02 | 0.10±0.01 | 0.10±0.02 | F=0.94 P=0.35 |
F=0.14 P=0.71 |
F=0.07 P=0.80 |
| light | 0.09±0.02 | 0.09±0.02 | 0.11±0.02 | 0.05±0.02 | F=2.31 P=0.16 |
F=0.09 P=0.77 |
F=2.84 P=0.12 |
|
Weight at start of CLAMS studies. Values are group mean±SEM.
Study 4: Effects of food deprivation on metabolic rate in Prokr2m/m mice
Food deprivation beginning shortly before the onset of the dark phase caused a rapid decrease in VO2 and VCO2 in both groups of mice (Figure 8; effect of time VO2: F=11.9, P<0.001 VCO2: F=22.0, P<0.001). The decreases in these parameters were significantly greater in the Prokr2m/m mice compared to their littermates (time × genotype interaction VO2: F=8.0, P<0.05, VCO2: F=10.1, P<0.05). Post-hoc Bonferroni tests revealed that the significantly lower decreases in VO2 and VCO2 in the Prokr2m/m mice occurred after 11h food deprivation during the last 3h of the dark phase (Figure 8). There was also a significant decrease in RQ in both groups (Figure 8, effect of time F=45.2, P<0.0001), but this did not differ between the genotypes. There was no significant difference in the degree of locomotor activity between the two groups during food deprivation. Both groups showed a similar degree of compensatory hyperphagia when returned to the ad libitum food supply, the 24h food intake in the Prokr2m/m mice increased by 41±9% compared to the 24h prior to food deprivation, and in the control littermates food intake correspondingly increased by 54±7%.
Figure 8.
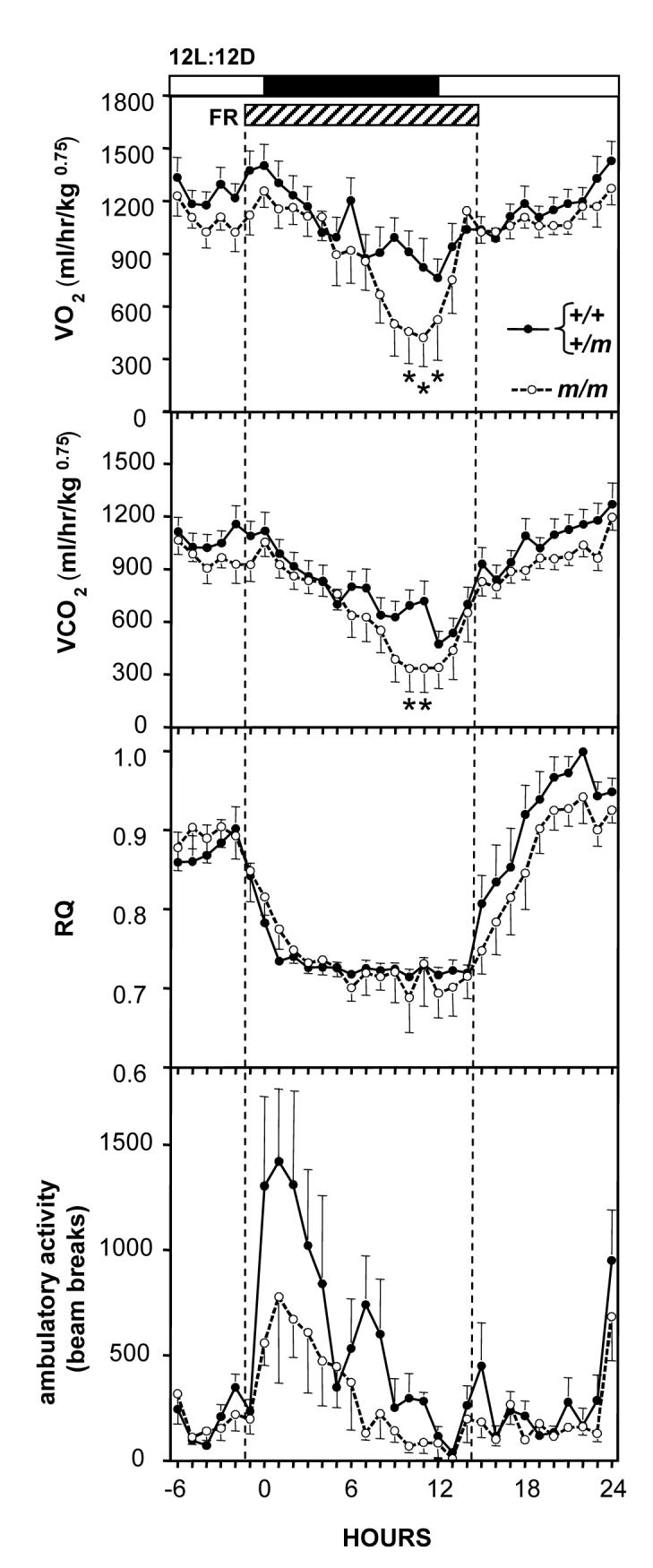
Metabolic parameters in control mice (Prokr2+/+ / Prokr2+/m, solid circles) and Prokr2m/m mice (open circles) entrained to a 12L:12D photocycle and subject a 16h period of food deprivation (FR: hatched bar) beginning at just before onset of the dark phase. Panels depict VO2 (top), VCO2 (second panel), RQ (middle panel), and locomotor activity (bottom panel). Values are group mean±SEM, n=8 control and n=7 Prokr2m/m mice (both sexes combined). *P<0.05 vs control mice. Note that both VO2 and VCO2 are lower in the Prokr2m/m mice compared to controls, and that all mice rapidly decrease RQ during food deprivation.
Body and organ weights
At the start of the study (mice aged ~5 months) there was no overall effect of genotype on body weight but there was a significant gender × genotype interaction, thus male Prokr2m/m mice weighed less than their litter-mate controls whereas female Prokr2m/m mice weighed more (Table 1). At the end of the study these significant differences in body weight had been lost (Table 1), and there were no significant differences in intra-abdominal fat depot weights (Table 1). There were clear differences (P<0.001) in the weight of the reproductive organs. Three of the four Prokr2m/m males had undeveloped testes (<10mg paired weight), the other had testes of intermediate size (paired weight 98mg vs range 166-249mg in wild-type males). Likewise, all the Prokr2m/m females had an undeveloped uterus (<20mg, range for wild-types 88-326mg).
Discussion
The major finding of the current study is that targeted genetic disruption of Prokr2 mediated signaling predisposes mice to torpor, a transient hypometabolic state. The majority of Prokr2m/m mice displayed spontaneous bouts of torpor despite being maintained at room temperature (21-22°C) on ad libitum food, and collectively the Prokr2m/m mice displayed significantly greater hypothermic and hypometabolic responses when challenged with acute feed deprivation. Although standard laboratory housing conditions of 21-22°C might be considered a mild thermal stress as this temperature is below the thermoneutral zone for mice (22), none of the heterozygote or wild-type litter mates (‘controls’) housed in these same conditions showed spontaneous torpor bouts. Similarly, whilst as expected all control mice showed a depression of body temperature and VO2 during acute food deprivation, these physiological responses were significantly less than those seen in the Prokr2m/m mice. Torpor is a physiological tactic that has evolved in small mammals as a means to reduce energy expenditure during metabolic stress, the main situations being exposure to cold ambient temperatures, or severe food shortage. Many small seasonal rodents have evolved the capacity to enter torpor spontaneously in anticipation of metabolically challenging conditions, for example short winter photoperiods will induce spontaneous bouts of torpor in Siberian hamsters, Phodopus sungorus (26, 28) and in several species of wild mouse (7, 17). Moreover, within the 24h light-dark cycle, circadian mechanisms play a key role in timing each individual torpor bout; ablation of the hypothalamic suprachiasmatic nucleus results in random timing of torpor bouts in food-restricted hamsters (24). However, in laboratory strains of mice, torpor has only been observed as a response to cold exposure (22), food deprivation (10) or to hormonal or pharmacological manipulations (8). The occurrence of spontaneous torpor bouts in Prokr2m/m mice therefore strongly implicates Prokr2-mediated signaling in the control of energy metabolism and thermoregulation, although the level at which this occurs is not yet known. It was noticeable that the timing of the onset of these torpor bouts generally occurred toward the end of the dark phase or in the early light phase, consistent with previous observations that the underlying circadian system functions normally in Prokr2m/m mice, despite some deficits in circadian output mechanisms (4, 23).
We can exclude the possibility that the occurrence of spontaneous torpor reflects an abnormality in sensing of ambient temperature in the Prokr2m/m mice because we saw no other signs of cold stress, for example shivering or piloerection. We also consider it unlikely that the impaired reproductive development and function clearly evident in the mice used in the current studies (and evident in other mutant mice with impaired Prokr2 signaling, 20) would predispose these mice to torpor. Whilst it is well established that high levels of circulating androgens can block the occurrence of torpor in hamsters exposed to short photoperiods (1), the opposite is not the case. Removal of testosterone from hamsters by means of surgical castration did not induce torpor bouts (28). Moreover, we have conducted studies in another strain of mutant mice which are hypogonadal (3) and never observed spontaneous torpor bouts in such mice (Ebling, unpublished data), so loss of bioactive GnRH per se is unlikely to contribute to the prevalence of torpor.
As torpor in mice occurs as a compensatory response to negative energy balance it is possible that Prokr2m/m mice have a deficiency in nutrient absorption from the gastrointestinal tract, particularly since prokineticins are expressed in the gut and act as potent stimulators of smooth muscle in the ileum (15). However, there are several lines of evidence that would argue against this. First, there is very limited expression of Prokr2 in the gastrointestinal tract, its expression being restricted just to the ilium-caecum junction, whereas Prokr1 is expressed widely in the stomach, small and large intestine (16). Second, one would predict that mice with impaired nutrient absorption would show a compensatory increase in food intake, however we observed that food intake was reduced in the Prokr2m/m mice rather than increased. Third, one might expect some degree of growth impairment and reduced body weight if nutrient absorption were impaired, but we observed at the end of this study that body weight and abdominal fat depots were similar in Prokr2m/m mice and their intact littermates. In other cohorts of transgenic and littermate control mice we also noted similar levels of adiposity and body weight (Ebling and Prosser, unpublished). Any early developmental effects of the mutation on growth could result from impaired olfaction and failure to locate and compete for maternal milk, or may reflect the abnormally low sex steroids concentrations in the mutant mice (20, 21). Finally, if gut absorption were impaired one might expect a lower respiratory quotient in Prokr2m/m mice reflecting greater reliance on catabolism of fat than carbohydrate. We did not observe this, despite observing an overall reduction in metabolic rate (ie lower VO2 and VCO2).
We consider it likely that the propensity of Prokr2m/m mice to enter torpor reflects an alteration in energy sensing and/or control of energy metabolism in hypothalamic and brain stem structures. Recent studies in the mouse indicate that Prokr2 gene expression is abundant in several structures known to be important in sensing of nutrients and peripheral endocrine signals (arcuate nucleus, area postrema, nucleus tractus solitarius) and in regulation of food intake including the paraventricular nucleus and dorsomedial nucleus of the hypothalamus (6). One interpretation of the current data is that the loss of Prokr2 signaling primarily causes a decrease in food intake, and compensatory strategies are therefore engaged to conserve energy expenditure. In support of this, we observed significantly lower food intake in Prokr2m/m mice, and infer that energy expenditure is decreased because even in cycles where torpor bouts did not occur, Prokr2m/m mice displayed a lower core body temperature and lower oxygen consumption across the circadian cycle when compared to their littermates. Moreover, Prokr2m/m mice exhibited a greater response to food deprivation, and in the study where such mice were food deprived for 24 h we even observed a second bout of hypothermia 24h after the initial response. Although we cannot rule out the possibility that this was simply a coincidental bout of ‘spontaneous’ torpor it seems more probable that the initial period of food deprivation produced a more extended period of negative energy balance such that the Prokr2m/m mice were more predisposed to hypothermia, and thus displayed a second bout of torpor when the circadian phase was appropriate. We also observed in the study in the metabolic cages that the RQ tended to take longer to increase after refeeding in the Prokr2m/m mice as compared to their littermates, despite the fact that they showed a comparable degree of hyperphagia after the food deprivation as did their control littermates, indicating that the period of food deprivation had induced a greater dependence catabolism fat reserves. Previous upon of mice studies in have demonstrated that the depth and duration of torpor bouts induced by food-deprivation can be enhanced by additional treatment with ghrelin (8), so it will be important to determine whether Prokr2m/m mice might have increased circulating concentrations of ghrelin or an increased responsiveness to ghrelin, as this may be a key factor in their propensity to show torpor.
One interesting though speculative possibility is that whatever the precise nature of the underlying metabolic disturbance in Prokr2m/m mice, it arises from the disruption of circadian output in these mutants. There is recent evidence that prokineticin 2 functions as a signal from the suprachiasmatic nucleus communicating circadian rhythmicity to Prokr2-expressing hypothalamic regions; loss of this signaling pathway results in less precise timing of patterns of locomotor activity, and in a reduced overall degree of activity, resulting in attenuated amplitude of rhythmicity (4, 23). A relationship between circadian dysfunction and metabolic abnormalities has been proposed recently (13 for review). One line of evidence for this link comprises observations of altered insulin production, glucose and lipid metabolism in humans (9) and laboratory animals (2) subjected to enforced changes in environmental light-dark cycles. Secondly, metabolic dysfunction has been observed in mice where the molecular basis of circadian rhythm generation has been perturbed (12, 27). Interestingly, disruption of rhythm generation in such clock mutants was associated with hyperphagia and increased energy storage leading to obesity (27), whereas disruption of circadian communication and the consequent dampened amplitude of locomotor and body temperature rhythms in the current study appears to be associated with mild hypophagia and decreased energy expenditure. This is reminiscent of a recent study by Zhang et al (30) where exposure of mice to constant darkness induced hypophagia and weight loss, decreases in blood glucose and increases in circulating fatty acids, phenomena indicative of increased catabolism of fat reserves (30). However, torpor was not observed during the studies in DD (30), whereas in the current study the propensity for torpor in the Prokr2m/m mice appears to have conserved energy expenditure such that catabolism of fat depots did not occur to an extent that would cause weight loss.
Perspectives and significance
To date the principal role of signalling via the prokineticin2 receptor in the hypothalamus has been considered to be the communication of circadian information. Our observations of hypothermia and spontaneous bouts of torpor in mice with disrupted Prokr2 signaling suggest an additional important role in sensing and control of energy balance. The loss of Prokr2 signaling predisposes the mice to a strategy to conserve energy; it is an intriguing hypothesis that this may be a consequence of an underlying attenuation of circadian rhythmicity. Our findings also raise the possibility that pharmacological enhancement of Prokr2 signaling would promote greater energy expenditure, and be a potential therapeutic strategy for promotion and maintenance of body weight loss.
Acknowledgements
We thank the staff at Biomedical Sciences Support Unit, University of Nottingham, for assistance with animal care. We thank Professor Allan Bradley for support with these studies.
Grants This research was supported by the Biotechnology and Biological Sciences Research Council, U.K (project grants BBS/B/10765, BB/D525064/1 and an ISIS Fellowship to HI), by the Wellcome Trust (HMP), and by the Medical Research Council, U.K (MHH, ESM).
References
- 1.Bartness TJ, Elliott JA, Goldman BD. Control of torpor and body weight patterns by a seasonal timer in Siberian hamsters. Am J Physiol. 1989;257:R142–R149. doi: 10.1152/ajpregu.1989.257.1.R142. [DOI] [PubMed] [Google Scholar]
- 2.Bartol-Munier I, Gourmelen S, Pevet P, Challet E. Combined effects of high-fat feeding and circadian desynchronization. International Journal of Obesity and Related Metabolic Disorders. 2006;30:60–67. doi: 10.1038/sj.ijo.0803048. [DOI] [PubMed] [Google Scholar]
- 3.Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotropin releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- 4.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 5.Cheng MY, Bittman EL, Hattar S, Zhou QY. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neuroscience. 2005;6:17. doi: 10.1186/1471-2202-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng MY, Leslie FM, Zhou QY. Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol. 2006;498:796–809. doi: 10.1002/cne.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiser F, McAllan BM, Kenagy GJ, Hiebert SM. Photoperiod affects daily torpor and tissue fatty acid composition in deer mice. Naturwissenschaften. 2007;94:319–324. doi: 10.1007/s00114-006-0193-z. [DOI] [PubMed] [Google Scholar]
- 8.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol. 2006;291:R1303–R1310. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- 9.Hampton SM, Morgan L, Lawrence N, Anastasiadou T, Norris F, Deacon S, Ribeiro D, Arendt J. Postprandial hormone and metabolic responses in simulated shift work. J Endocr. 1996;151:259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- 10.Hudson JW, Scott IS. Daily torpor in the laboratory mouse, Mus musculus var. albino. Physiological Zoology. 1979;52:205–218. [Google Scholar]
- 11.Jethwa PH, Warner A, Nilaweera KN, Brameld JM, Keyte JD, Carter WG, Bolton N, Bruggraber M, Morgan PJ, Barrett P, Ebling FJP. A VGF-derived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology. 2007;148:4044–4055. doi: 10.1210/en.2007-0038. [DOI] [PubMed] [Google Scholar]
- 12.Kennaway DJ, Owens JA, Voultsios A, Boden MJ, Varcoe TJ. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am J Physiol. 2007;293:1528–1537. doi: 10.1152/ajpregu.00018.2007. [DOI] [PubMed] [Google Scholar]
- 13.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS letters. 2008;582:142–151. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 14.Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Molecular Pharmacology. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 16.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 17.Lynch GR, White SE, Grundel R, Berger MS. Effects of photoperiod, melatonin administration and thyroid block on spontaneous daily torpor and temperature regulation in the white-footed mouse, Peromyscus leucopus. J Comp Physiol B. 1978;125:157–163. [Google Scholar]
- 18.Maldonado-Pe’rez D, Evans J, Denison F, Millar RP, Jabbour HN. Potential roles of the prokineticins in reproduction. Trends in Endocrinology and Metabolism. 2007;18:66–72. doi: 10.1016/j.tem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masumoto K, Nagano M, Takashima N, Hayasaka N, Hiyama H, Matsumoto S, Inouye S-I, Shigeyoshi Y. Distinct localization of prokineticin 2 and prokineticin receptor 2 mRNAs in the rat suprachiasmatic nucleus. Eur J Neurosci. 2006;23:2959–2970. doi: 10.1111/j.1460-9568.2006.04834.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto S, Yamazaki C, Masumoto K, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuickji K, Shigeyoshi Y. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 22.Overton JM, Williams TD. Behavioral and physiologic responses to caloric restriction in mice. Physiology and Behavior. 2004;81:749–754. doi: 10.1016/j.physbeh.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Prosser H, Bradley A, Chesham JE, Ebling FJP, Hastings MH, Maywood ES. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behaviour by the suprachiasmatic nuclei. Proc Natl Acad Sci USA. 2007;104:648–653. doi: 10.1073/pnas.0606884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruby NF, Zucker I. Daily torpor in the absence of the suprachiasmatic nucleus in Siberian hamsters. Am J Physiol. 1992;263:R353–R362. doi: 10.1152/ajpregu.1992.263.2.R353. [DOI] [PubMed] [Google Scholar]
- 25.Soga T, Matsumoto SI. Molecular cloning and characterization of prokineticin receptors. Biochemica et Biophysica Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 26.Steinlechner S, Heldmaier G. Role of photoperiod and melatonin in seasonal acclimatization of the Djungarian hamster, Phodopus sungorous. International Journal of Biometeorology. 1982;26:329–337. doi: 10.1007/BF02219503. [DOI] [PubMed] [Google Scholar]
- 27.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova E, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitale PM, Darrow JM, Duncan MJ, Shustak CA, Goldman BD. Effects of photoperiod, pinealectomy and castration on body weight and daily torpor in Djungarian hamsters (Phodopus sungorus) J Endocr. 1985;106:367–373. doi: 10.1677/joe.0.1060367. [DOI] [PubMed] [Google Scholar]
- 29.Willis CK. An energy-based body temperature threshold between torpor and normothermia for small mammals. Physiol Biochem Zool. 2007;80:643–651. doi: 10.1086/521085. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- 31.Zhou QY, Cheng MY. Prokineticin 2 and circadian clock output. FEBS Journal. 2005;272:5703–5709. doi: 10.1111/j.1742-4658.2005.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]