Abstract
Neurotrophins (NTs) play important roles in brain growth and development. Cord blood (CB) brain-derived neurotrophic factor (BDNF) concentrations increase with gestational age but data regarding postnatal changes are limited. We measured BDNF concentrations after birth in 33 preterm infants <32 weeks gestation. Serum was collected at birth (CB), at day 2 (D2), between day 6–10 (D6), at day 30 (D30) and at day 60 (D60). BDNF concentrations fell on D2 (p=0.03), recovered by D6 (p=0.10) and continued to rise thereafter at D30 (p=0.06) and D60 (p=0.01) compared to CB. CB BDNF concentrations positively correlated with duration of rupture of membranes (r = 0.43, p=0.04). Antenatal steroids (ANS, p=0.02), postnatal steroids (PNS, p=0.04) and retinopathy of prematurity (ROP, p=0.02) were identified as significant factors in multivariate analyses. The median (25th–75th interquartile range) CB BDNF concentrations were higher in infants who received a complete course ANS compared to those who received a partial course (1461 (553–2064) pg/ml versus 281 (171–536) pg/ml, p= 0.04). BDNF concentrations negatively correlated with the use of PNS at D30 (r = −0.53, p=0.002) and at D60 (r = −0.55, p= 0.009). PNS use was associated with reduced concentrations of BDNF at D30 (733 (101–1983) vs. 2224 (1677–4400) pg/ml, p= 0.004) and at D60 (1149 (288–2270) pg/ml vs. 2560 (1337– 5166) pg/ml, p= 0.01). BDNF concentrations on D60 in infants who developed ROP (n=16) were lower than those who did not develop ROP (n=7) (1417 (553–2540) pg/ml vs. 3593 (2620–7433) pg/ml respectively, p=0.005). Our data suggests that BDNF concentrations rise beyond the first week of age. BDNF concentrations correlate with factors that influence neurodevelopment outcomes.
Keywords: Antenatal steroids, brain-derived neurotrophic factor, neurotrophins, postnatal steroids, retinopathy of prematurity
Neurotrophins (NTs)- nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophins 4 and 5 (NT- 4/5)- are a family of growth factors that play an important role in the growth and development of central and peripheral nervous systems (1, 2). Neurotrophins promote the growth, survival, proliferation and migration of neurons; regulate neurotransmitter synthesis and secretion, and the development of synaptic plasticity. In addition, they also modulate immune cells (3–6). NTs are also protective against apoptotic neuronal loss, and exogenous BDNF administration has been shown to attenuate various forms of brain injury in both animals and humans (7–9).
BDNF is synthesized in several neuronal and glial cell populations and also expressed in several non neural tissues such as immune cells and the vascular endothelium, but the contribution of the latter to the circulating pool is limited (10–14). NTs cross the blood brain barrier that is relatively immature in the preterm infants (15, 16). Karege et al have shown that changes in serum BDNF concentrations are similar to changes in BDNF concentrations in the brain (17). In addition to its local effects in the central and peripheral nervous systems, BDNF plays important roles in several non-neural tissues (18–21). For example, exogenous BDNF administration has been shown to modulate glucagon secretion and glucose homeostasis (22). Changes in BDNF concentrations also correlate with disease course and response to therapy under various clinical conditions (6, 23–26).
Prematurity is associated with increased morbidity and mortality, and outcomes are influenced by both antenatal and postnatal factors (27–30). Preterm brain cortical volume grows nearly four fold between 29–35 weeks of gestation and is a critical period for neuronal growth and migration. Infants born at this gestation period have been shown to have decreased cortical gray and white matter at term equivalent - secondary to apoptosis and neuronal atrophy- that impacts on long term neurodevelopmental outcomes (31–33).
There is little data regarding BDNF during this critical period of neuronal growth, especially in preterm infants. BDNF concentrations are lower in preterm infants compared to term, and both are decreased compared to adults (34–36). Cord blood (CB) BDNF concentrations increase with gestational age and with use of antenatal steroids (ANS), whereas low concentrations have been associated with intraventricular hemorrhage (IVH) (36). BDNF concentrations in cerebrospinal fluid are increased in infants with perinatal depression and meningitis (37, 38). In both animal models and humans, BDNF concentrations are decreased in infections (39, 40). There is little data in preterm infants regarding changes in BDNF concentrations following birth, or its relationship with factors that influence neurodevelopmental outcomes. Since ANS improve neurodevelopmental outcomes (28) and increase BDNF concentrations (36), and postnatal steroids (PNS) are detrimental to the developing brain (41), we hypothesized that BDNF concentrations would be decreased by PNS use. In this study, we measured serial BDNF concentrations from birth until discharge in preterm infants < 32 weeks gestation. Further, we sought to correlate BDNF concentrations with other factors such as birth weight and gestational age, and with clinical morbidities such as IVH, bronchopulmoary dysplasia (BPD) and necrotizing enterocolitis (NEC), which are known to influence outcomes following premature birth.
Material and Methods
The study was approved by the University of Kentucky (UK) Institutional Review Board and written informed consent was obtained from all parents. Parents were free to withdraw their infant from the study at any time during their infant’s stay in the neonatal intensive care unit (NICU).
Study Design and population
The study was conducted prospectively over a one-year period at the UK NICU. Fifty (50) infants < 32 weeks were enrolled. Infants with known congenital anomalies or in the absence of informed consent were excluded.
Data Collection and analyses
Cord blood was collected aseptically at delivery as reported previously (36). Serial samples were collected on day 2 (D2), between 6–10 days (D6), at 30 days (D30) and at 60 days (D60) of life. At the time of medically indicated blood draw, an additional 0.5 ml blood was collected, serum separated and stored at −80°C until analyses.
BDNF assay was determined using DuoSet ELISA kits (R&D Systems®, Minneapolis) according to manufacturer’s instructions. The minimum detection limit of this assay is 23.4 pg/ml (sensitivity) and there is no cross reactivity with other neurotrophins i.e. β-NGF, GDNF, NT-3 or NT-4 at 50 ng/ml. The inter-assay and intra-assay coefficient of variation were 8.0% and 6.2% respectively. Preliminary assays were done to determine ideal dilutions to achieve assay results within the standardized range. All samples were run in triplicate.
Clinical Data
Antenatal data including maternal age, gravida, race, smoking, chorioamnionitis (42), premature rupture of membranes (PROM) (43), duration of rupture of membranes (ROM), and antenatal steroid use for lung maturation (defined as complete if 2 doses of betamethasone 24 hours apart or 4 doses of dexamethasone every 12 hours in a 48 hour period were given prior to delivery, or partial or none) (44); and neonatal characteristics of birth weight, gender and gestational age (GA) (45, 46) were recorded. Clinical morbidities of grades of intraventricular hemorrhage (IVH) (47), BPD (oxygen dependency at 36 weeks postmenstrual age) (48), NEC (Bell’s stage 2 or higher) (49), retinopathy of prematurity (ROP) (50) and sepsis (defined by positive blood culture). Use of systemic PNS was also recorded.
Statistical Analyses
Descriptive analysis was used to describe changes in BDNF concentrations at different time points, and median, interquartile range reported. Wilcoxon test was used to test the differences in BDNF concentrations between clinical outcome groups. Pearson and Spearman correlation were used to describe the relationship between BDNF concentrations at different time points and clinical outcomes. Two way ANOVA with contrast analyses was used to determine the changes in BDNF concentrations along time, and the difference among the time points and clinical outcomes. Multivariate analyses were carried out to determine significant clinical factors for BDNF concentration changes with time. All data was analyzed using SAS 9.0 statistical software. A p value < 0.05 was considered significant. BDNF concentrations are reported as median (25th–75th interquartile range).
Results
Outcomes of enrollment
Of the 50 infants enrolled, 7 died before the end of first week of life, 3 gave consent only for CB collection, and 4 parents withdrew consent after initial samples were drawn. In 3 infants, serial samples could not be collected at appropriate times and therefore excluded from analyses.
Missing data
CB samples were not always available as some infants were transferred after birth. In some, clinical condition (6 infants on D2, 5 on D6), technical reasons (1 on D6, 1 on D30) and discharge home (2 on D30 and 10 on D60) precluded sample collection. We present data from patients where at least 3 serial samples beyond the first week of life were available (n=33). Samples were available for CB (n=22), D2 (n=27), D6 (n=27), D30 (n=30) and D60 (n=23).
Serial changes in BDNF
The clinical and demographic features are presented in Table 1. BDNF concentrations were 1099 (374 – 2035) pg/ml, 46 (23 – 347) pg/ml, 1857 (678 – 2721) pg/ml, 1957 (1271 –2669) pg/ml and 2339 (941 – 3425) pg/ml in CB, on D2, D6, D30 and D60 respectively.
Table 1.
Antenatal, clinical features and postnatal outcomes of the study population. For duration of ROM, those ruptured at delivery were assigned a value of 1.
| Antenatal Data | (n=33) (%) |
|---|---|
| Maternal race | |
|
| |
| White | 26 (78.8) |
| Black | 3 (9.1) |
| Other | 4 (12.1) |
|
| |
| Gender, female/male | 19 (57.6)/14 (42.4) |
|
| |
| Chorioamnionitis | 4 (12.1) |
|
| |
| PROM | 9 (27.3) |
|
| |
| Duration of ROM (in hours) * | 1 (1– 57.0) |
|
| |
| Antenatal steroids | |
| None | 3 (9.1) |
| Partial | 6 (18.1) |
| Complete | 24 (72.7) |
|
| |
| Smokers | 12 (36.4) |
|
| |
| Maternal Age, years | 24 ± 6 |
|
| |
| Gravida | 2.6 ± 1.8 |
|
| |
| Preeclampsia | 13 (39.4) |
|
| |
| Gestational Age, weeks Mean ± SD | 27.4 ± 2.1 |
|
| |
| Neonatal Outcomes | |
|
| |
| Birth Weight, grams Mean ± SD | 833 ± 271 |
|
| |
| IVH | |
| Gr I–II | 12 (36.4) |
| Gr III–IV | 0 (0) |
|
| |
| NEC | 3 (9.1) |
|
| |
| BPD | 22 (66.7) |
|
| |
| ROP | 16 (48.5) |
|
| |
| Surgical ROP** | 5 (15.1) |
|
| |
| PNS | 7 (21.2) |
|
| |
| Sepsis | 11 (33.3) |
|
| |
| Median age at diagnosis of 1st episode of sepsis (days) * | 14 (9–20) |
|
| |
| Median number of episodes of sepsis * | 1 (1–2) |
Data shown are Median (25th–75th interquartile range).
(stage 3 or higher and required surgical intervention)
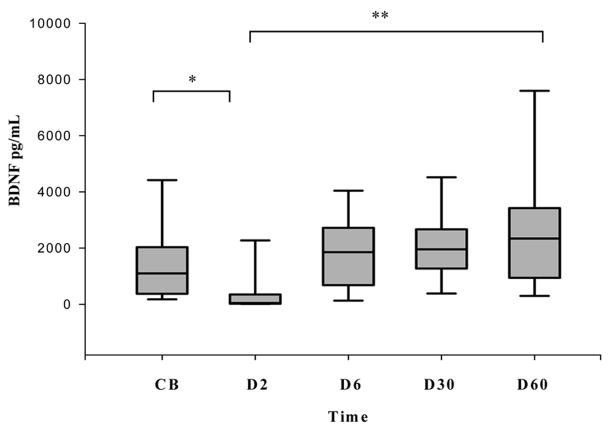
Following birth, there was a transient decline in BDNF concentrations followed by recovery between 6 to 10 days of life (Figure 1). BDNF concentrations on D2 were significantly lower than CB (p=0.03), and at D6, D30 and D60 (p<0.001 for all comparisons). BDNF concentrations recovered by D6 (p=0.10) and continued to rise at D30 (p=0.06) and D60 (p=0.01) compared to CB. There were no differences between D30 and D60 BDNF concentrations.
Figure 1.
Serial changes in BDNF levels in preterm infants < 32 weeks gestation. Box plots showing that BDNF levels decline at D2 compared to cord blood but continue to rise thereafter. BDNF levels at D2 were significantly lower than at all other times (*p=0.03, ** p < 0.001 for all comparisons). Data are shown as median (25–75th interquartile range).
BDNF and antenatal factors
CB BDNF concentrations correlated with duration of ROM (r = 0.43, p=0.04). CB BDNF concentrations (but not at other time points) were significantly higher in infants who received a complete course of antenatal steroids (n=16) compared to those (n=6) who had received a partial course of antenatal steroids (1461 (553–2064) pg/ml versus 281 (171–536) pg/ml, p=0.04, figure 2). CB was not available for the three infants who had received no ANS.
Figure 2.
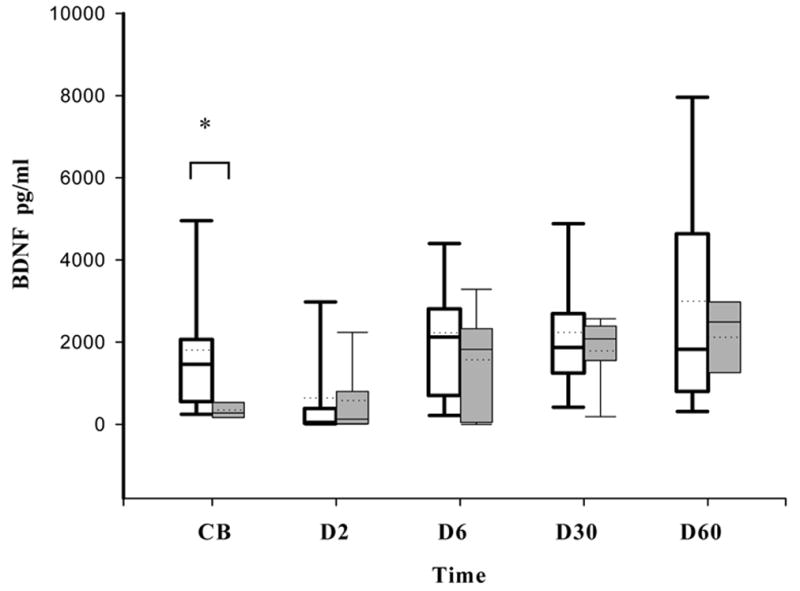
Effect of Antenatal Steroids on BDNF levels. Box plots showing BDNF levels at various time points in infants who received antenatal steroids (white) and those who did not receive antenatal steroids (grey). Data are shown as median (25–75th interquartile range). The dotted line in the box plots represents the mean value. (* p = 0.04).
CB BDNF concentrations did not correlate with birth weight, gestational age or any other antenatal or maternal factors.
BDNF and postnatal outcomes
CB BDNF concentrations did not correlate with IVH (r= −0.19, p=0.40) although they were lower in infants who developed IVH (n = 7) compared to those who did not develop IVH (n=12, 694 (344–1337) pg/ml vs. 1473 (375–2057) pg/ml; p= 0.36). IVH developed in 12 infants (grade I–II) with no infants with grades III or IV hemorrhage. 9/12 infants who developed IVH had received antenatal steroids, whereas the 3 infants who had not received any antenatal steroids all developed IVH.
BDNF concentrations were negatively correlated with the use of postnatal steroids (r = −&0.53, p=0.002) at D30 and at D60 (r = −0.55, p= 0.009).
In infants who received PNS (n=7) for either hypotension or chronic lung disease, BDNF concentrations were significantly decreased at D30 and at D60 (Figure 3). BDNF concentrations at D30 were 733 (101–1983) vs. 2224 (1677–4400) pg/ml, (p=0.004) and at D60 were 1149 (288–2270) pg/ml vs. 2560 (1337- 5166) pg/ml, (p=0.01) in infants who did and did not receive PNS respectively.
Figure 3.
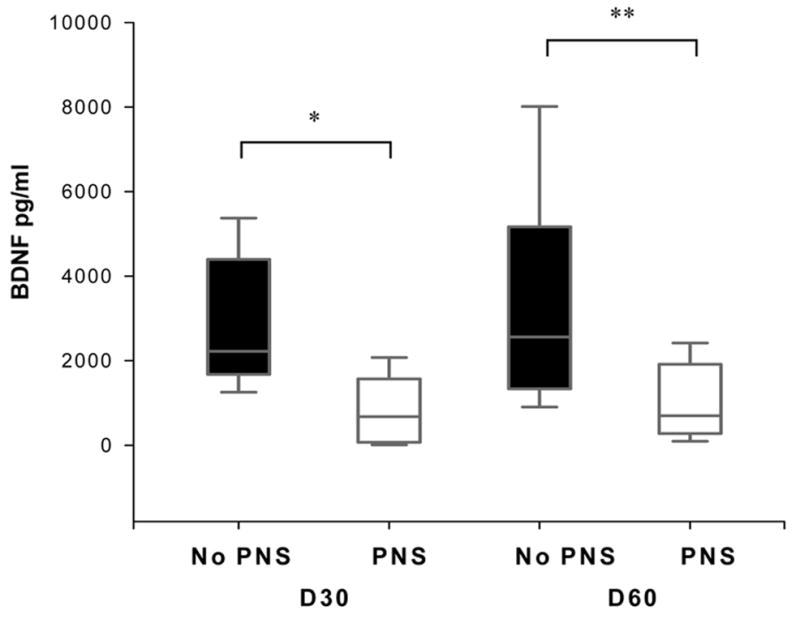
Effect of Postnatal Steroids on BDNF levels. Box plots showing that postnatal steroids use was associated with reduced BDNF levels at D30 (* p = 0.004) and D60 (** p = 0.014). Data are shown as median (25–75th interquartile range).
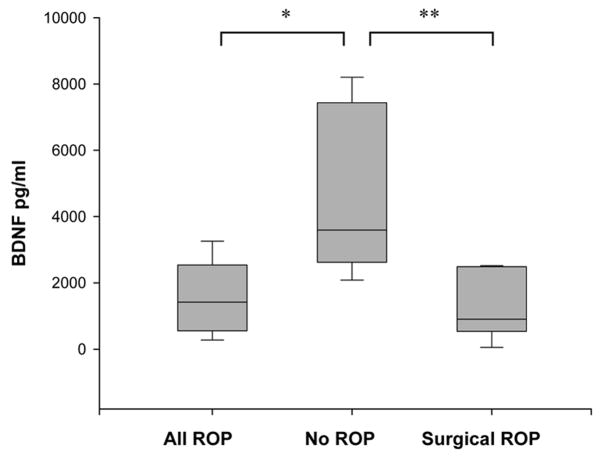
BDNF concentrations at D60 in infants who developed ROP (n=16) versus those who did not develop ROP (n=7) were 1417 (553–2540) pg/ml versus 3593 (2620–7433) pg/ml respectively (p=0.005, Figure 4). BDNF concentrations were significantly lower in those who required surgical intervention for Stage 3 or higher ROP (n=5) compared to those with no ROP (906 (538– 2489) pg/ml vs. 3593 (2620–7433) pg/ml respectively, p=0.02). In infants with ROP, BDNF concentrations in those who required surgical intervention trended lower than those who did not require surgical intervention (906 (538– 2489) pg/ml vs.1417 (553–2540) pg/ml, p= NS).
Figure 4.
BDNF concentrations in infants with ROP at D60. Box plots showing that BDNF concentrations are reduced in infants with ROP (p<0.005) and in infants with surgical ROP (p=0.02) compared to infants with no ROP. Data are shown as median (25–75th interquartile range).
BDNF concentrations did not correlate with clinical outcomes of BPD, sepsis and NEC.
Multivariate analyses
To determine significant factors that affected BDNF concentrations, multivariate analyses was carried out including each of the following covariates: GA, birth weight, smoking, ANS, IVH, BPD, ROP, NEC, sepsis and PNS. In this model, both ANS (p=0.02) and PNS (p=0.04) and ROP (p=0.02) were identified as significant factors.
Discussion
In this study, we have found that BDNF concentrations were correlated with factors known to be associated with deleterious developmental outcomes in preterm infants. To our knowledge, this is the first report of measured serial BDNF changes from birth until discharge in preterm infants less than 32 weeks gestation.
BDNF is synthesized in several neuronal populations in central and peripheral nervous systems, vascular endothelium and immune cells (12, 13, 51). Endogenous fetal synthesis, maternal passage and placenta serve as source of BDNF in the fetus, although maternal and amniotic fluid levels of BDNF fall with advancing gestational age (36, 39, 52–55). Abrupt removal of these sources could cause the sharp decline in BDNF levels after birth. The pattern of transient decline followed by rising levels is similar to that reported in term infants where BDNF concentrations fell on day 1 before rising on day 4 after birth (35), although in another study involving term and preterm infants, BDNF concentrations rose on day 1 in both groups but fell on day 4 only in preterm infants (34). In neither of these studies were BDNF concentrations followed beyond the first week of age.
Use of antenatal steroids for pulmonary maturation are associated with improved neurodevelopmental outcomes in preterm infants (28). Cord blood BDNF concentrations in this study were also significantly higher in infants whose mothers received a complete course of ANS (36). ANS mediated neuroprotection may be through increased BDNF synthesis or alternately, neuronal maturation following ANS may result in increased BDNF concentrations (36). Our data also suggests that the influence of ANS may last beyond birth, as BDNF levels were higher after the first week of age in infants who received a complete course. There was also no correlation between CB BDNF and IVH as reported previously (36). The higher antenatal steroid use, and lack of any grade 3 or 4 IVH in the current study may explain these differences.
In contrast to ANS, PNS use was associated with significant attenuation in BDNF concentrations. PNS used for treatment of chronic lung disease has declined due to their detrimental effect on cortical brain growth (41). Corticosteroids inhibit cellular proliferation in the subventricular zone, down regulate BDNF synthesis, and decrease BDNF expression in the developing brain and following ischemic injury (56–59). BDNF expression is also attenuated in conditions of neuronal atrophy or cell death and aging (60). Since BDNF crosses the blood brain barrier (14) and peripheral BDNF levels reflect central levels (17), lower BDNF levels associated with PNS could be a reflection of either a direct suppressive effect or neuronal apoptosis. BDNF also regulates neuronal migration (61) and Reelin expression- a metalloprotein that influences the “inside-out” layering during cortical migration (62). Likewise, BDNF is involved in synaptic development and plasticity associated with learning and memory (63). Alterations in BDNF concentrations following postnatal steroid use could therefore possibly affect migration of cortical neurons during this period of brain growth.
BDNF is expressed in the visual cortex and retina during development and plays an important role in development of visual plasticity (64, 65). We noted that BDNF concentrations were decreased in infants who developed ROP and trended lower in those with more severe ROP. ROP is characterized by abnormal neovascularization in which local concentrations of VEGF play a significant role in its pathogenesis (66). Conflicting data suggest that serum VEGF may be decreased or unchanged with severe ROP (67, 68). BDNF is expressed in the vascular endothelium, is involved in angiogenesis and increases VEGF expression (69, 70). The presence of BDNF in the retina and its possible role in angiogenesis suggests that BDNF may play an as yet unappreciated role in the development of ROP. Whether low BDNF concentrations are a marker for severe ROP remains to be determined.
BDNF concentrations were positively correlated with duration of ROM in our study, which, to the best of our knowledge, has not been reported previously. Premature ROM (PROM) increases risk of infections and can be considered a form of stress for the fetus (43). Alterations in expression of neuronal markers including decreased BDNF concentrations have been reported in animals exposed to prenatal stress and infections (39, 71, 72). However, only four infants were diagnosed with chorioamnionitis that precluded further analyses in this study. The correlation of BDNF concentrations and duration of ROM may perhaps be related to exposure to antenatal steroids the majority of infants received before delivery.
The strengths of our study include the serial measurement of BDNF concentrations in preterm infants. Our results support strong correlation between BDNF concentrations and antenatal steroid use; and postnatal factors of postnatal steroid use and severe ROP, all of which are known to influence neurodevelopmental outcomes.
In summary, BDNF concentrations rise following a transient decline after birth. BDNF concentrations were strongly correlated with several antenatal factors and postnatal outcomes that significantly impact neurodevelopmental outcomes in preterm infants. A larger study and developmental follow up of these infants would be required to further answer these questions.
Acknowledgments
Financial Disclosure: The study was supported by NIH/NINDS grant R01 NS040592 (G.M.S.).
We wish to thank Dr. Nitin Chouthai for his technical expertise and guidance in the successful completion of this work.
Abbreviations
- ANS
antenatal steroids
- BDNF
brain-derived neurotrophic factor
- IVH
intraventricular hemorrhage
- NTs
neurotrophins
- PNS
postnatal steroids
- ROP
retinopathy of prematurity
References
- 1.McAllister AK. Neurotrophins and neuronal differentiation in the central nervous system. Cell Mol Life Sci. 2001;58:1054–1060. doi: 10.1007/PL00000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernfors P. Local and target-derived actions of neurotrophins during peripheral nervous system development. Cell Mol Life Sci. 2001;58:1036–1044. doi: 10.1007/PL00000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 4.Twiss JL, Chang JH, Schanen NC. Pathophysiological mechanisms for actions of the neurotrophins. Brain Pathol. 2006;16:320–332. doi: 10.1111/j.1750-3639.2006.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans. 2007;35:424–427. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- 6.Schulte-Herbruggen O, Braun A, Rochlitzer S, Jockers-Scherubl MC, Hellweg R. Neurotrophic factors--a tool for therapeutic strategies in neurological, neuropsychiatric and neuroimmunological diseases? Curr Med Chem. 2007;14:2318–2329. doi: 10.2174/092986707781745578. [DOI] [PubMed] [Google Scholar]
- 7.Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husson I, Rangon CM, Lelievre V, Bemelmans AP, Sachs P, Mallet J, Kosofsky BE, Gressens P. BDNF-induced white matter neuroprotection and stage-dependent neuronal survival following a neonatal excitotoxic challenge. Cereb Cortex. 2005;15:250–261. doi: 10.1093/cercor/bhh127. [DOI] [PubMed] [Google Scholar]
- 9.Almli CR, Levy TJ, Han BH, Shah AR, Gidday JM, Holtzman DM. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000;166:99–114. doi: 10.1006/exnr.2000.7492. [DOI] [PubMed] [Google Scholar]
- 10.Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Radka SF, Holst PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709:122–301. doi: 10.1016/0006-8993(95)01321-0. [DOI] [PubMed] [Google Scholar]
- 12.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 13.Noga O, Englmann C, Hanf G, Grutzkau A, Seybold J, Kunkel G. The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin Exp Allergy. 2003;33:649–654. doi: 10.1046/j.1365-2222.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 14.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 15.Laterra J, Goldstein GW. Development of the Blood-Brain Barrier. In: Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. WB Saunders; Philadelphia: 2004. pp. 1699–1706. [Google Scholar]
- 16.Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain barrier to neurotrophins. Brain Res. 1998;788:87–94. doi: 10.1016/s0006-8993(97)01525-4. [DOI] [PubMed] [Google Scholar]
- 17.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279:33538–33546. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- 19.Calle M, Wang L, Kuijpers FJ, Cruijsen PM, Arckens L, Roubos EW. Brain-derived neurotrophic factor in the brain of Xenopus laevis may act as a pituitary neurohormone together with mesotocin. J Neuroendocrinol. 2006;18:454–465. doi: 10.1111/j.1365-2826.2006.01433.x. [DOI] [PubMed] [Google Scholar]
- 20.Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab. 2002;87:655–659. doi: 10.1210/jcem.87.2.8213. [DOI] [PubMed] [Google Scholar]
- 21.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanyu O, Yamatani K, Ikarashi T, Soda S, Maruyama S, Kamimura T, Kaneko S, Hirayama S, Suzuki K, Nakagawa O, Nawa H, Aizawa Y. Brain-derived neurotrophic factor modulates glucagon secretion from pancreatic alpha cells: its contribution to glucose metabolism. Diabetes Obes Metab. 2003;5:27–37. doi: 10.1046/j.1463-1326.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Wang YD, Guo T, Wei WN, Sun CY, Zhang L, Huang J. Identification of brain-derived neurotrophic factor as a novel angiogenic protein in multiple myeloma. Cancer Genet Cytogenet. 2007;178:1–10. doi: 10.1016/j.cancergencyto.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Pillai A. Brain-derived neurotropic factor/TrkB signaling in the pathogenesis and novel pharmacotherapy of schizophrenia. Neurosignals. 2008;16:183–193. doi: 10.1159/000111562. [DOI] [PubMed] [Google Scholar]
- 25.Noga O, Hanf G, Schaper C, O’Connor A, Kunkel G. The influence of inhalative corticosteroids on circulating Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Neurotrophin-3 in allergic asthmatics. Clin Exp Allergy. 2001;31:1906–1912. doi: 10.1046/j.1365-2222.2001.01249.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim TS, Kim DJ, Lee H, Kim YK. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci Lett. 2007;423:53–57. doi: 10.1016/j.neulet.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 27.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 28.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–1129. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 30.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 31.Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 33.Mewes AU, Huppi PS, Als H, Rybicki FJ, Inder TE, McAnulty GB, Mulkern RV, Robertson RL, Rivkin MJ, Warfield SK. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- 34.Malamitsi-Puchner A, Economou E, Rigopoulou O, Boutsikou T. Perinatal changes of brain-derived neurotrophic factor in pre- and fullterm neonates. Early Hum Dev. 2004;76:17–22. doi: 10.1016/j.earlhumdev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Nikolaou KE, Malamitsi-Puchner A, Boutsikou T, Economou E, Boutsikou M, Puchner KP, Baka S, Hassiakos D. The varying patterns of neurotrophin changes in the perinatal period. Ann N Y Acad Sci. 2006;1092:426–433. doi: 10.1196/annals.1365.041. [DOI] [PubMed] [Google Scholar]
- 36.Chouthai NS, Sampers J, Desai N, Smith GM. Changes in neurotrophin levels in umbilical cord blood from infants with different gestational ages and clinical conditions. Pediatr Res. 2003;53:965–969. doi: 10.1203/01.PDR.0000061588.39652.26. [DOI] [PubMed] [Google Scholar]
- 37.Korhonen L, Riikonen R, Nawa H, Lindholm D. Brain derived neurotrophic factor is increased in cerebrospinal fluid of children suffering from asphyxia. Neurosci Lett. 1998;240:151–154. doi: 10.1016/s0304-3940(97)00937-3. [DOI] [PubMed] [Google Scholar]
- 38.Chiaretti A, Antonelli A, Piastra M, Genovese O, Polidori G, Aloe L. Expression of neurotrophic factors in cerebrospinal fluid and plasma of children with viral and bacterial meningoencephalitis. Acta Paediatr. 2004;93:1178–1184. doi: 10.1080/08035250410031314. [DOI] [PubMed] [Google Scholar]
- 39.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 40.Marx CE, Vance BJ, Jarskog LF, Chescheir NC, Gilmore JH. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 levels in human amniotic fluid. Am J Obstet Gynecol. 1999;181:1225–1230. doi: 10.1016/s0002-9378(99)70113-4. [DOI] [PubMed] [Google Scholar]
- 41.Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107:217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 42.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 43.ACOG. Practice Bulletin No. 80: premature rupture of membranes. Obstet Gynecol. 2007;109:1007–1019. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- 44.ACOG. Antenatal Corticosteroid therapy for fetal maturation. Obstet Gynecol. 2008;111:805–807. doi: 10.1097/AOG.0b013e318169f722. [DOI] [PubMed] [Google Scholar]
- 45.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 46.Campbell S, Newman GB. Growth of the fetal biparietal diameter during normal pregnancy. J Obstet Gynaecol Br Commonw. 1971;78:513–519. doi: 10.1111/j.1471-0528.1971.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 47.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 48.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 49.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ICROP. The international classification of Retinopathy of Prematurity revisited. Arch Opthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 51.Rojas Vega S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortical response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 52.Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschlager M. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol. 2004;191:309–314. doi: 10.1016/j.ajog.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Lommatzsch M, Hornych K, Zingler C, Schuff-Werner P, Hoppner J, Virchow JC. Maternal serum concentrations of BDNF and depression in the perinatal period. Psychoneuroendocrinology. 2006;31:388–394. doi: 10.1016/j.psyneuen.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62:585–590. doi: 10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 55.Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han ZC. Human umbilical cord blood cells express neurotrophic factors. Neurosci Lett. 2005;380:322–325. doi: 10.1016/j.neulet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen JP, Mork A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006;1110:221–225. doi: 10.1016/j.brainres.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 57.Prickaerts J, van den Hove DL, Fierens FL, Kia HK, Lenaerts I, Steckler T. Chronic corticosterone manipulations in mice affect brain cell proliferation rates, but only partly affect BDNF protein levels. Neurosci Lett. 2006;396:12–16. doi: 10.1016/j.neulet.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Barbany G, Persson H. Regulation of Neurotrophin mRNA Expression in the Rat Brain by Glucocorticoids. Eur J Neurosci. 1992;4:396–403. doi: 10.1111/j.1460-9568.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 59.Yang JT, Chang CN, Lee TH, Hsu JC, Lin TN, Hsu YH, Hsieh Wu J. Effect of dexamethasone on the expression of brain-derived neurotrophic factor and neurotrophin-3 messenger ribonucleic acids after forebrain ischemia in the rat. Crit Care Med. 2002;30:913–918. doi: 10.1097/00003246-200204000-00034. [DOI] [PubMed] [Google Scholar]
- 60.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 61.Ohmiya M, Shudai T, Nitta A, Nomoto H, Furukawa Y, Furukawa S. Brain-derived neurotrophic factor alters cell migration of particular progenitors in the developing mouse cerebral cortex. Neurosci Lett. 2002;317:21–24. doi: 10.1016/s0304-3940(01)02412-0. [DOI] [PubMed] [Google Scholar]
- 62.Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, Ernfors P, Ibanez CF. BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron. 1998;21:305–315. doi: 10.1016/s0896-6273(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 63.Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- 64.Caleo M, Maffei L. Neurotrophins and plasticity in the visual cortex. Neuroscientist. 2002;8:52–61. doi: 10.1177/107385840200800110. [DOI] [PubMed] [Google Scholar]
- 65.Nag TC, Wadhwa S. Neurotrophin receptors (Trk A, Trk B, and Trk C) in the developing and adult human retina. Brain Res Dev Brain Res. 1999;117:179–189. doi: 10.1016/s0165-3806(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 67.Kwinta P, Bik-Multanowski M, Mitkowska Z, Tomasik T, Pietrzyk JJ. The clinical role of vascular endothelial growth factor (VEGF) system in the pathogenesis of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1467–1475. doi: 10.1007/s00417-008-0865-9. [DOI] [PubMed] [Google Scholar]
- 68.Pieh C, Agostini H, Buschbeck C, Kruger M, Schulte-Monting J, Zirrgiebel U, Drevs J, Lagreze WA. VEGF-A, VEGFR-1, VEGFR-2 and Tie2 levels in plasma of premature infants: relationship to retinopathy of prematurity. Br J Ophthalmol. 2008;92:689–693. doi: 10.1136/bjo.2007.128371. [DOI] [PubMed] [Google Scholar]
- 69.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 71.Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, Fleming AS. Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult female Sprague-Dawley rats. Brain Res. 2007;1158:28–38. doi: 10.1016/j.brainres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137:145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]