Summary
The entorhinal cortex (EC) is regarded as the gateway to the hippocampus and thus is essential for learning and memory. Whereas the EC expresses a high density of GABAB receptors, the functions of these receptors in this region remain unexplored. Here we examined the effects of GABAB receptor activation on neuronal excitability in the EC and spatial learning. Application of baclofen, a specific GABAB receptor agonist, inhibited significantly neuronal excitability in the EC. GABAB receptor-mediated inhibition in the EC was mediated via activating TREK-2, a type of two-pore domain K+ channels and required the functions of inhibitory G proteins and protein kinase A pathway. Depression of neuronal excitability in the EC underlies GABAB receptor-mediated inhibition of spatial learning as assessed by Morris water maze. Our study indicates that GABAB receptors exert a tight control over spatial learning by modulating neuronal excitability in the EC.
Introduction
γ-Aminobutyric acid (GABA) is a major inhibitory neurotransmitter in the central nervous system where it acts at ionotropic (GABAA and GABAC) and metabotropic (GABAB) receptors. Whereas the functions of GABAA and GABAC receptors are to mediate fast inhibitory synaptic transmission, stimulation of GABAB receptors (GABABRs) modulates synaptic function via G proteins and intracellular signals (Couve et al., 2000). GABABRs consist of heterodimers of GABAB1 and GABAB2 subunits that are coupled to inhibitory G proteins (Gαi and Gαo) which inhibit adenylyl cyclase (AC) resulting in a reduction in cyclic AMP (cAMP) generation and an inhibition of protein kinase A (PKA) (Couve et al., 2000). GABABRs play important modulatory roles in cognition, nociception, neuroprotection, depression, addiction and epilepsy (Couve et al., 2000). Anatomically, the entorhinal cortex (EC) mediates the majority of the connections between the hippocampus and other cortical areas (Witter et al., 1989; Witter et al., 2000a). Sensory inputs converge onto the superficial layers (layers II–III) of the EC (Burwell, 2000) which give rise to dense projections to the hippocampus; the axons of the stellate neurons in layer II of the EC form the perforant path that innervates the dentate gyrus and CA3 (Steward and Scoville, 1976) whereas those of the pyramidal neurons in layer III form the temporoammonic pathway that synapses onto the distal dendrites of pyramidal neurons in CA1 and the subiculum (Steward and Scoville, 1976; Witter et al., 2000a; Witter et al., 2000b). Moreover, neurons in the deep layers of the EC (layers V–VI) relay a large portion of hippocampal output projections back to the superficial layers of the EC (Dolorfo and Amaral, 1998a, b; Kohler, 1986; van Haeften et al., 2003) and to other cortical areas (Witter et al., 1989). The EC is part of a network that aids in the consolidation and recall of memories (Haist et al., 2001; Squire et al., 2004; Steffenach et al., 2005). Neuronal pathology and atrophy of the EC are potential contributors to Alzheimer's disease (Hyman et al., 1984; Kotzbauer et al., 2001) and schizophrenia (Falkai et al., 1988; Prasad et al., 2004). Furthermore, the EC participates in the induction and maintenance of temporal lobe epilepsy (Avoli et al., 2002; Spencer and Spencer, 1994).
GABABRs are densely expressed in the principal cells, especially the stellate neurons, of the EC (Mizukami et al., 2002). However, the functions of GABABRs in this brain region remain unexplored. Because GABABRs and the EC are closely associated with cognitive function in the brain, we tested the hypothesis that GABABRs expressed in the EC modulate neuronal excitability and participate in learning and memory. Our results demonstrate that GABABR activation drastically inhibited neuronal excitability in the superficial layers of the EC via activation of TREK-2, a type of two-pore domain K+ (K2P) channels. We also found that the functions of pertussis toxin (PTX)-sensitive G proteins, PKA and A-kinase anchoring proteins (AKAPs) are necessary for GABABR-mediated inhibition in the EC. Microinjection of the GABABR agonist, baclofen, and Rp-cAMPS, a specific PKA inhibitor, into the EC of rats prevented spatial learning whereas down-regulation of TREK-2 channels using siRNA significantly reduced the effect of baclofen on spatial learning suggesting that GABABR-mediated inhibition of neuronal excitability contributes significantly to spatial learning.
Results
GABABR activation inhibits action potential firing in the EC
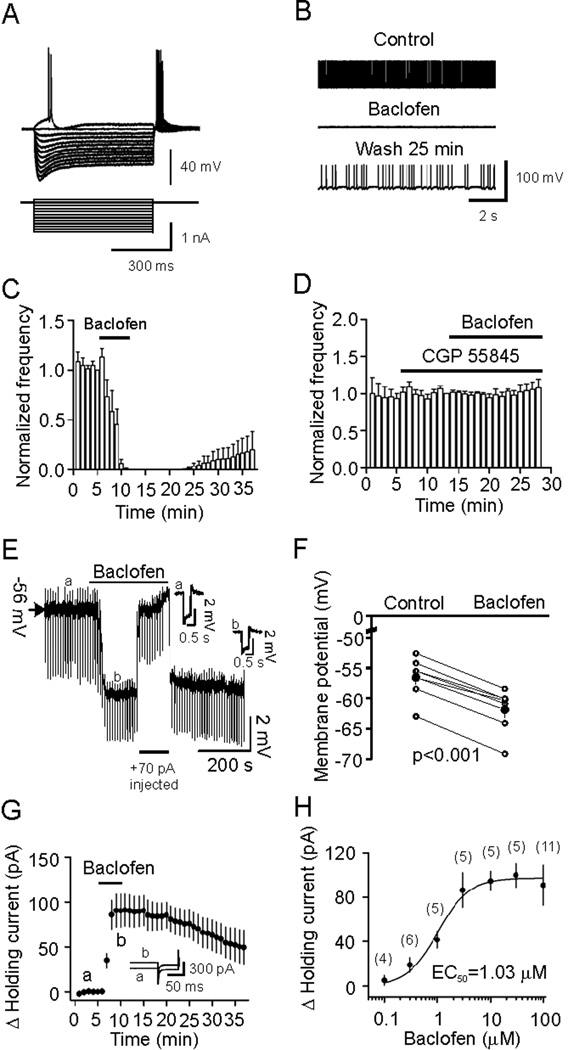
GABABRs are densely expressed in the EC, especially in the stellate neurons of the superficial layers (Mizukami et al., 2002). Accordingly, we examined first the effects of GABABR activation on the excitability of stellate neurons. The extracellular solution contained DNQX (10 µM), dl-APV (50 µM) and bicuculline (10 µM) to block synaptic transmission. Stellate neurons were differentiated by their location, morphology and electrophysiology properties (Deng et al., 2007). Figure 1A shows the current-voltage responses from a stellate neuron in layer II of the EC. This neuron showed profound depolarizing voltage sags in response to hyperpolarizing current pulses (Deng et al., 2007). Application of baclofen (100 µM), a specific GABABR agonist, completely blocked action potential (AP) firing within 5–6 min of application (n=6, p<0.001, Figure 1B and 1C). AP firing frequency recovered partially after wash for ~25 min (Figure 1C). The effect of baclofen was mediated by GABABRs because application of CGP 55845 (2 µM), a specific GABABR inhibitor, completely blocked baclofen-induced inhibition of AP firing (101±2% of control, n=7, p=0.76, Figure 1D). These results indicate that GABABR activation drastically inhibits neuronal excitability in the EC.
Figure 1.
Baclofen reduces the excitability of stellate neurons by generating membrane hyperpolarization. A, Voltage responses (upper panel) generated by current injection from +0.1 nA to −1 nA at an interval of −0.1 nA (lower panel) recorded from a stellate neuron in layer II. Note the depolarizing voltage sags in response to hyperpolarizing current pulses. B, AP firing recorded prior to, during and after application of baclofen from the stellate neuron in A. C, Pooled time course of AP firing frequency before, during and after application of baclofen. D, Baclofen-mediated inhibition of AP firing was blocked in the presence of the GABABR blocker, CGP 55845. E, Baclofen generated membrane hyperpolarization and reduced input resistance. A negative current (−50 pA for 500 ms) was injected every 5 s to assess the changes of input resistance. Insets are the voltage traces taken before (a) and during (b) the application of baclofen. Note that baclofen induced membrane hyperpolarization and reduced the voltage responses induced by the negative current injections suggesting a reduction in input resistance. To exclude the influence of baclofen-induced membrane hyperpolarization on input resistance, a constant positive current (+70 pA indicated by the horizontal bar) was injected briefly to elevate the membrane potential to the initial level. Under these conditions, the voltage responses induced by the negative current injections (−50 pA) were still smaller compared with control suggesting that baclofen-induced reduction in input resistance is not secondary to its effect on membrane hyperpolarization. F, Summarized data for baclofen-induced changes in RMPs. Filled circles denote the averaged values. G, Baclofen induced an outward HC. HCs were averaged per min and zeroed to the level just prior to the application of baclofen. Inset shows the averaged HCs recorded at the time points denoted in the figure. A −5 mV hyperpolarizing voltage step was used at the end of each trace to monitor potential changes of series resistance during recordings. H, Concentration-response curve for baclofen-induced changes in HCs. Numbers in the parenthesis were number of cells recorded.
GABABR activation generates hyperpolarization
Inhibition of AP firing could be attributable to GABABR-induced membrane hyperpolarization. We therefore recorded the resting membrane potentials (RMPs) in current-clamp in the presence of TTX (0.5 µM) to block potential indirect effects from synaptic transmission. A negative current (−50 pA for 500 ms) was injected every 5 s to assess the changes of input resistance. Application of baclofen (100 µM) generated membrane hyperpolarization (control: −56.5±1.3 mV, baclofen: −61.8±1.4 mV, n=7, p<0.001, Figure 1E, 1F) and reduced the input resistance (control: 98.3±13.1 MΩ, baclfen: 75.0±7.7 MΩ, n=7, p<0.006, Figure 1E) suggesting that GABABR activation increases membrane conductance. We then recorded the holding currents (HCs) in voltage-clamp at −60 mV, a potential close to the RMPs. Under these conditions, application of baclofen (100 µM) generated an outward HC. The maximal effect usually occurred 4–6 min after the beginning of baclofen application (90.9±17.9 pA, n=11, p<0.001, Figure 1G) and was used for statistical analysis thereafter. The EC50 value for baclofen was calculated to be 1.03 µM (Figure 1H).
Baclofen-induced hyperpolarization is G protein-dependent and requires the functions of PKA and AKAPs
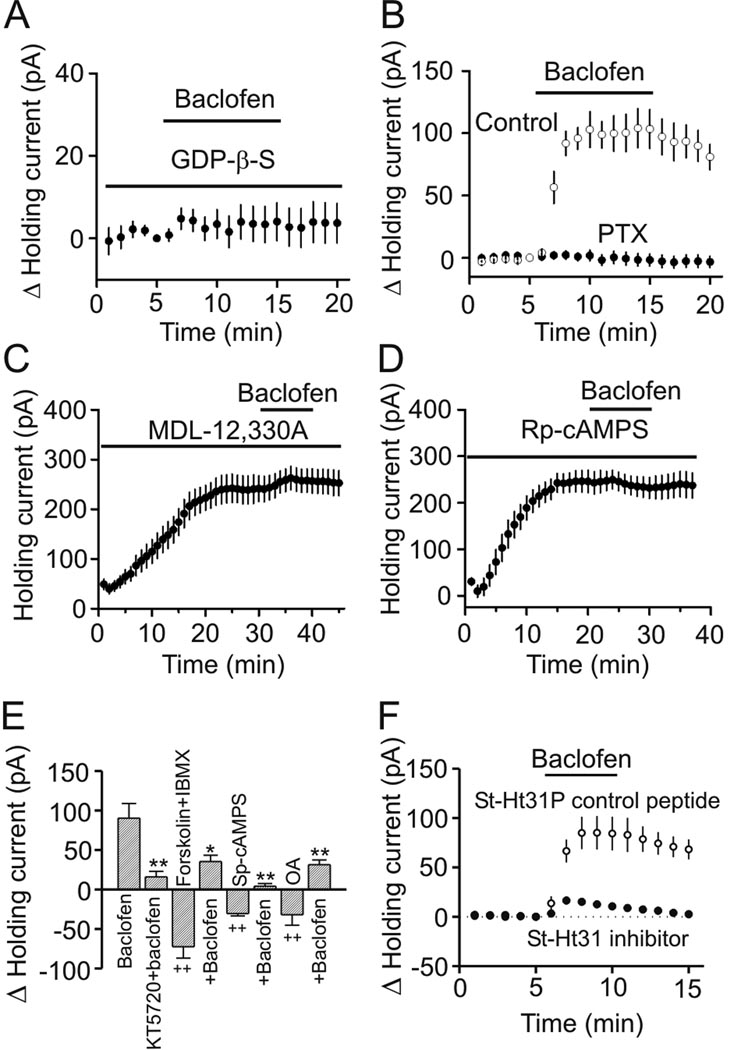
GABABRs are G protein-coupled (Couve et al., 2000). We next examined the requirement of G proteins in baclofen-induced hyperpolarization. We replaced the intracellular GTP with GDP-β-S (4 mM), a G protein inactivator and recorded baclofen-induced changes in HCs at −60 mV. In the presence of GDP-β-S, baclofen did not significantly change the HCs (4.3±2.7 pA, n=12, p=0.13, Figure 2A) suggesting that baclofen-induced hyperpolarization is mediated by G-proteins. We then identified the type of G proteins involved in baclofen-induced hyperpolarization. GABABRs activate the inhibitory G proteins (Gαi and Gαo, Couve et al., 2000) and these G proteins are sensitive to PTX. We therefore pretreated slices with PTX (500 ng/ml) in the extracellular solution continuously oxygenated with 95% O2 and 5% CO2 for ~8 h. Bath application of baclofen (100 µM) failed to significantly increase the outward HCs in slices pretreated with PTX (2.5±3.2 pA, n=8, p=0.47, Figure 2B) whereas baclofen still increased the outward HCs in slices treated in the same fashion without PTX (98.8±10.6 pA, n=6, p<0.001, Figure 2B) suggesting that PTX-sensitive G proteins (Gαi or Gαo) are involved in baclofen-induced hyperpolarization.
Figure 2.
GABABR-mediated inhibition requires the functions of inhibitory G proteins, AC, PKA and AKAPs. A, Intracellular application of GDP-β-S via the recording pipettes blocked baclofen-induced increases in outward HCs. B, Treatment of slices with PTX abolished the effects of baclofen on HCs, whereas treatment of the slices in the same fashion without PTX failed to alter the effect of baclofen. C, Intracellular dialysis of MDL-12,330A induced an outward HC and significantly inhibited baclofen-induced increases in outward HCs. HCs at −60 mV were recorded immediately after the formation of whole-cell configuration and the actual HCs were used to plot the figure. D, Application of Rp-cAMPS in the recording pipettes induced an outward HC and blocked baclofen-induced increases in outward HCs. The actual HCs were used to plot the figure. E, Baclofen-induced increases in outward HCs were significantly reduced by co-application of KT5720, forskolin plus IBMX, Sp-cAMPS or okadaic acid (OA) (*p<0.05, **p<0.01 vs. baclofen alone; ++p<0.01 vs. baseline=0). F, Inclusion of St-Ht31, the AKAP inhibitory peptide, in the recording pipettes significantly inhibited baclofen-induced increases in outward HCs whereas application of St-Ht31P, the control peptide, had no effects.
GABABR activation inhibits AC and reduces the levels of cAMP thereby inhibiting PKA (Couve et al., 2000). We next determined the roles of this pathway in GABABR-induced hyperpolarization. Intracellular dialysis of MDL-12,330A (2 mM), an AC inhibitor, via the recording pipettes for ~30 min immediately after the formation of whole-cell configuration induced an outward HC (192.7±19.6 pA, n=7, p<0.001, Figure 2C) and extensively reduced baclofen-induced increases in outward HCs (15.7±6.4 pA, n=7, p=0.005 vs. baclofen alone, Figure 2C) suggesting that activity of AC is required. Likewise, intracellular application of Rp-cAMPS (1 mM), a specific PKA inhibitor, in the recording pipettes for ~20 min induced an outward HC (215.1±22.9 pA, n=7, p<0.001, Figure 2D) and completely blocked baclofen-induced increases in outward HCs (3.8±7.2 pA, n=7, p=0.62, Figure 2D). Furthermore, intracellular application of KT5720 (1 µM), another PKA inhibitor, also induced an outward HC (152.3±20.5 pA, n=6, p<0.001, not shown) and significantly reduced baclfen-induced outward HCs (13.5±6.1 pA, n=6, p=0.006 vs. baclofen alone, Figure 2E). These results together demonstrate that PKA pathway is necessary for baclofen-induced increases in outward HCs.
We further corroborated the roles of PKA pathway by applying forskolin (an AC activator) and 3-isobutyl-1-methylxanthine (IBMX, a phosphodiesterase inhibitor) to elevate intracellular cAMP level. Co-application of forskolin (10 µM) and IBMX (1 mM) generated an inward HC per se (−72.4±14.4 pA, n=6, p<0.001, Figure 2E) and significantly reduced baclofen-induced outward HCs (35.2±8.0 pA, n=6, p=0.011 vs. baclofen alone, Figure 2E). Moreover, application of the specific PKA activator, Sp-cAMPS (100 µM), induced an inward HC by itself (−30.5±7.2 pA, n=6, p<0.001, Figure 2E) and significantly diminished the effect of baclofen (4.1±3.4 pA, n=6, p<0.001 vs. baclofen alone, Figure 2E). Finally, bath application of okadaic acid (OA, 0.1 µM), a protein phosphatase inhibitor that inhibits the dephosphorylation process, induced an inward HC (−31.9±13.2 pA, n=6, p<0.001) and significantly reduced baclofen-induced outward HC (31.3±6.0 pA, n=6, p=0.006 vs. baclofen alone, Figure 2E). These results together demonstrate that PKA-mediated phosphorylation exerts a tonic control over neuronal excitability in the stellate neurons of the EC.
AKAPs play a crucial role in the functional expression of PKA by tethering PKA to other signaling substrates (Beene and Scott, 2007). Whereas AKAPs are a diverse family of more than 50 scaffolding proteins, they share a structurally conserved PKA-binding domain (Hundsrucker and Klussmann, 2008). Disruption of AKAPs by application of the PKA-anchoring inhibitory peptide has been shown to prevent a myriad of functions ascribed to PKA (Hundsrucker and Klussmann, 2008). Intracellular perfusion of the AKAP inhibitory peptide (St-Ht31, 20 µM) dramatically inhibited baclofen-induced increases in outward HCs (16.5±2.9 pA, n=6, p=0.004, Figure 2F) compared with those when the control peptide (St-Ht31P, 20 µM) was applied via the recording pipettes (85.1±16.9 pA, n=7, Figure 2F) demonstrating that the function of AKAPs is required for baclofen-induced hyperpolarization. Together, these results indicate that GABABRs exert powerful inhibition on neuronal excitability via PTX-sensitive G protein-mediated inhibition of AC-PKA pathway.
GABABR-induced hyperpolarization is mediated by activation of K2P channels
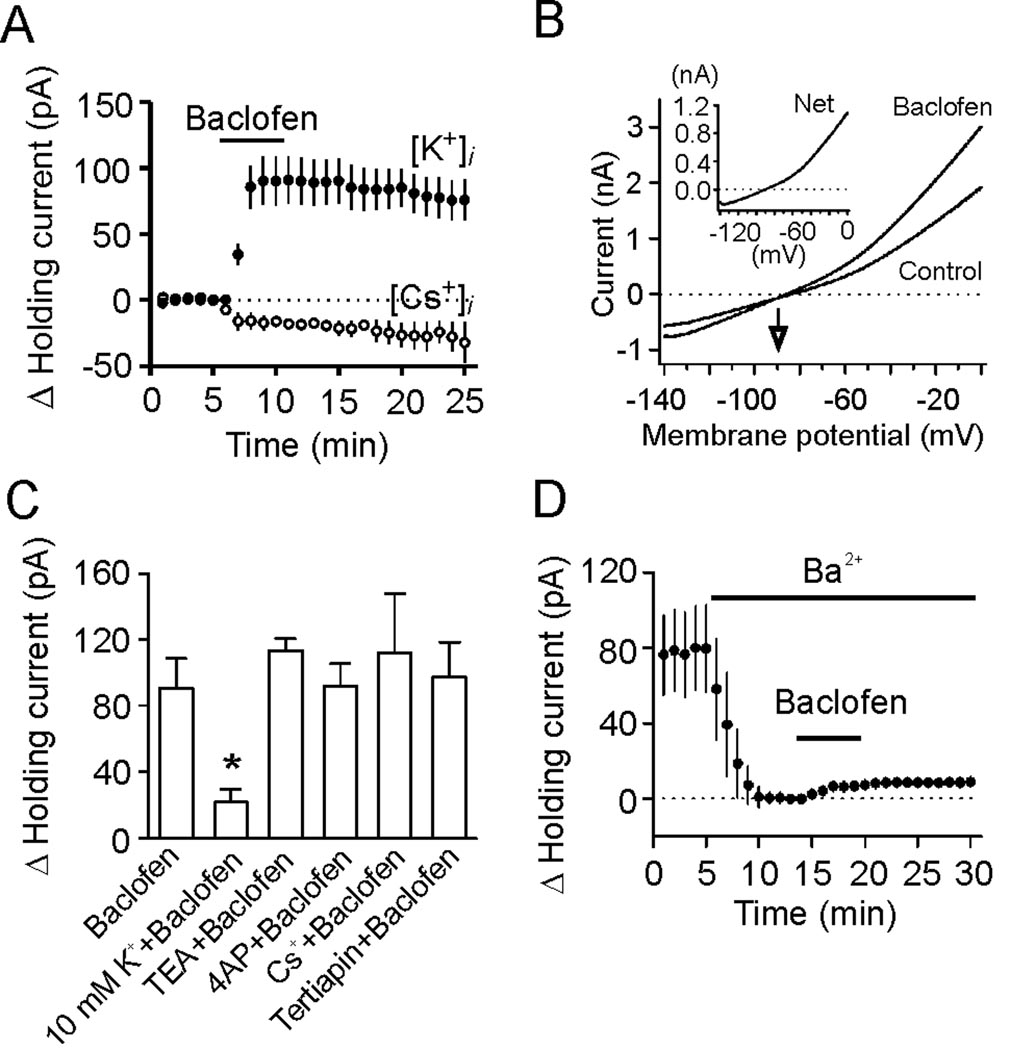
We next tested the hypothesis that GABABR activation opens background K+ channels to generate membrane hyperpolarization in the EC. We first replaced the intracellular K+ with the same concentration of Cs+ on the basis that if K+ channels are involved, replacement of intracellular K+ with Cs+ should block baclofen-induced increases in outward HCs because K+ channels are not permeable to intracellular Cs+. When the intracellular K+ was replaced by Cs+, instead of inducing an outward HC, application of baclofen (100 µM) induced a small inward HC (−16.3±3.5 pA, n=5, p=0.01, Figure 3A) suggesting that K+ channels are required for baclofen-induced hyperpolarization. One plausible explanation for baclofen-induced inward currents under this condition was the reverse K+ flowing via K+ channels from extracellular side when intracellular K+ was replaced by Cs+. We then measured the reversal potential of baclofen-induced currents using a ramp protocol prior to and during the application of baclofen because if K+ channels are involved, the currents generated by GABABR activation should have a reversal potential close to the K+ reversal potential. Application of baclofen (100 µM) in the presence of 3.5 mM K+ induced a net current that had a reversal potential of −90.1±4.7 mV (n=9, Figure 3B), close to the theoretical K+ reversal potential calculated by the Nernst equation (−92.2 mV) suggesting that baclofen produces membrane hyperpolarization by activating a background K+ conductance. The net current generated by baclofen had an outward rectification property (Figure 3B, inset). Further evidence to support the involvement of K+ channels was that elevating extracellular K+ concentration to 10 mM significantly reduced baclofen-induced outward HCs (21.9±7.9 pA, n=6, p=0.02 vs. baclofen alone, Figure 3C). Together, these data indicate that GABABR activation induces hyperpolarization by activating a background K+ conductance.
Figure 3.
GABABR-mediated increases in outward HCs are mediated by activation of K2P channels. A, Bath application of baclofen induced a small inward current when Cs+-gluconate-containing intracellular solution was used in contrast to baclofen-induced outward currents when the intracellular solution contained K+-gluconate. B, Voltage-current relationship induced by a ramp protocol from −140 mV to 0 mV at a speed of 0.1 mV/ms prior to and during the application of baclofen. Subtraction of the current prior to the application of baclofen generated a net current of outward rectification (inset). The traces were averages from 9 cells. Note that the reversal potential was ~ −90.1 mV, close to the calculated K+ reversal potential (−92.2 mV). C, Baclofen-induced increases in outward HCs were insensitive to extracellular application of TEA, 4-AP, Cs+ and tertiapin, but reduced when the extracellular K+ concentration was increased to 10 mM (* p=0.02). D, Inclusion of Ba2+ in the extracellular solution induced an inward HC, but significantly reduced baclofen-induced increases in outward HCs.
We then identified the properties of the involved K+ channels. Baclofen-induced increases in outward HCs were not significantly altered (vs. baclofen alone) in the extracellular solution containing tetraethylammonium (TEA, 10 mM, n=8, p=0.32, Figure 3C), 4-aminopyridine (4-AP, 2 mM, n=6, p=0.96, Figure 3C), Cs+ (3 mM, n=5, p=0.57, Figure 3C) or tertiapin (50 nM, n=7, p=0.82, Figure 3C) suggesting that baclofen-activated K+ channels are insensitive to the classic K+ channel blockers.
K2P channels are involved in controlling RMPs and they are insensitive to the classic K+ channel blockers. We next examined the roles of K2P channels in baclofen-induced membrane hyperpolarization. K2P channels can be divided into 6 subfamilies: TWIK, THIK, TREK, TASK, TALK and TRESK (Bayliss et al., 2003; Lesage, 2003), some of which are sensitive to Ba2+. We therefore tested the role of Ba2+ in baclofen-induced membrane hyperpolarization. Inclusion of Ba2+ (2 mM) in the extracellular solution per se generated an inward HC (−79.6±22.9 pA, n=7, p=0.01, Figure 3D) and subsequent application of baclofen (100 µM) induced a significantly smaller outward HC (8.6±1.9 pA, n=7, p=0.002 vs. baclofen alone, Figure 3D). Together, our results suggest that GABABR activation generates membrane hyperpolarization by activating Ba2+-sensitive K2P channels.
TREK-2 channels are involved in baclofen-induced hyperpolarization
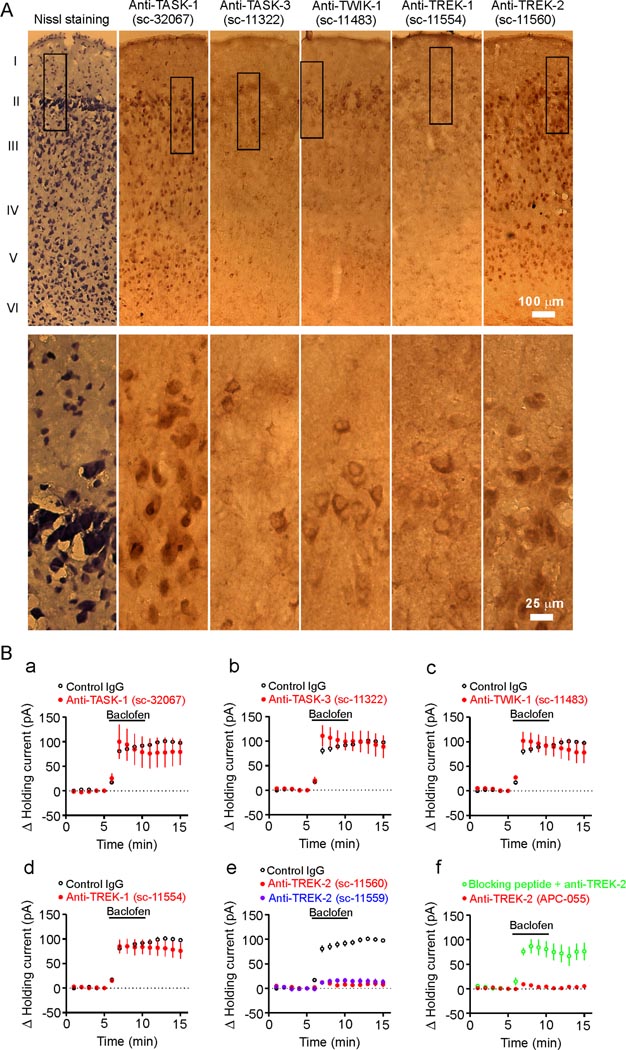
Among the K2P channels, TASK-1 (Han et al., 2002), TASK-3 (Han et al., 2002; Kim et al., 2000), TREK-1 (Fink et al., 1996), TREK-2 (Han et al., 2002), TWIK-1 (Lesage et al., 1996) and TRESK (Kang et al., 2004; Sano et al., 2003) are sensitive to Ba2+. TRESK channels are unlikely to be involved because they are expressed in the spinal cord and other organs but not in the brain (Kang et al., 2004; Sano et al., 2003). We examined the immunoreactivity of TASK-1, TASK-3, TWIK-1, TREK-1 and TREK-2 in the EC using two sets of antibodies (ABs, Santa Cruz Biotechnology Inc.). Immunoreactivities for all these K2P channels especially for TASK-1 and TREK-2 were detected in the EC (Figure 4A). Similar patterns of immunoreactivities were detected in the EC when the second set of K2P ABs against distinct domains of the channels were used (Figure S1A) whereas preabsorption of the ABs with their corresponding blocking peptides blocked the detection of the immunoreactivities (Figure S1B).
Figure 4.
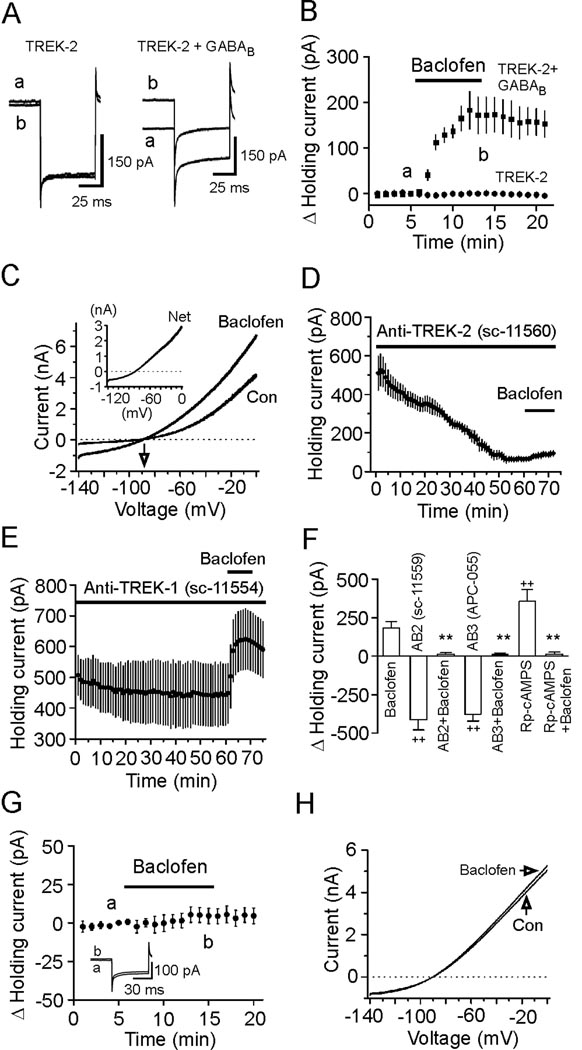
TREK-2 channels are involved in GABABR-mediated hyperpolarization. A, Immunocytochemical staining of K2P channels in the EC (layer I–VI). Upper panels: low magnification, Low panels: high magnification of the regions marked in the upper panels. The catalog numbers of the ABs were labeled on the top. B, a–d, Intracellular infusion of ABs to TASK-1 (a), TASK-3 (b), TWIK-1 (c) and TREK-1 (d,) at 40 µg/ml failed to significantly change baclofen-induced increases in outward HCs. e, Intracellular dialysis of two TREK-2 ABs (40 µg/ml) drastically reduced baclofen-induced increases in outward HCs. f, Intracellular application of the third TREK-2 AB (APC-055, Alomone Labs, 40 µg/ml) significantly reduced the effect of baclofen whereas application of the TREK-2 AB preabsorbed with the corresponding blocking peptide via the recording pipettes significantly reduced the inhibitory effect of TREK-2 AB.
We then identified the roles of these K2P channels in baclofen-induced hyperpolarization by applying, via the recording pipettes, specific ABs against each channel type. The rationale for this experiment was that the epitopes for all these ABs are against intracellular C- or N-terminal domains of the channels (Goldstein et al., 2001) and intracellular application of these ABs would interfere with the function of the corresponding K2P channels and block or at least reduce the effects of baclofen. To ensure a complete intracellular diffusion of the ABs, we waited for ~60 min after formation of whole-cell recordings. Under these conditions, application of control Ig-G (sc-2028, n=5, p=0.42 vs. baclofen alone, Figure 4B) and ABs to TASK-1 (sc-32067, n=5, p=0.53, Figure 4Ba), TASK-3 (sc-11322, n=6, p=0.97, Figure 4Bb), TWIK-1 (sc-11483, n=7, p=0.71, Figure 4Bc) and TREK-1 (sc-11554, n=6, p=0.31, Figure 4Bd) failed to change baclofen-induced increment of outward HCs significantly (vs. control Ig-G) whereas inclusion of the AB to TREK-2 (sc-11560) dramatically reduced baclofen-induced augmentation of outward HCs (10.9±4.7 pA, n=6, p<0.001 vs. control IgG, Figure 4Be). We used the following approaches to confirm the specificities of the ABs further. First, intracellular application of the second TREK-2 AB (sc-11559) against distinct epitopes of TREK-2 channels also significantly reduced baclofen-induced increases in outward HCs (14.5±4.9 pA, n=7, p=0.002 vs. control Ig-G, Figure 4Be). Similar results were obtained when the third TREK-2 AB (APC-055, Alomone Labs) was included in the recording pipettes (11.8±2.9 pA, n=6, p=0.001 vs. control Ig-G, Figure 4Bf) whereas intracellular application of the TREK-2 AB preabsorbed with the corresponding blocking peptide significantly reduced the inhibitory effect of the TREK-2 AB (86.6±13.9 pA, n=6, p<0.001 vs. anti-TREK-2 alone, Figure 4Bf) indicating that TREK-2 channels are indeed required. Second, we repeated the above electrophysiological experiments by intracellular application of the second set of the ABs to TASK-1, TASK-3, TWIK-1 and TREK-1. Similar results were obtained (Figure S2) suggesting that these K2P channels are not involved. Third, we transfected HEK293 cells with GFP and individual K2P channels and immunostained the transfected cells with the K2P ABs. Immunoreactivity of individual K2P channels was detected only in HEK293 cells transfected with the corresponding channels and there were no cross reactions among those ABs (Figure S3A). Finally, we tested the effectiveness of intracellular application of the ABs to TASK-1, TASK-3, TREK-1 and TWIK-1 at inhibiting the functions of these channels in transfected cells. We applied via the recording pipettes the individual K2P channel ABs to the HEK293 cells expressing the corresponding K2P channels and recorded the changes of the HCs at −60 mV. Intracellular dialysis of these ABs inhibited the channel function by producing an inward HC whereas intracellular application of an irrelevant AB (anti-TREK-2) failed to change the HCs significantly (Figure S3B–S3E). These data indicate that the ineffectiveness of intracellular application of ABs to TASK-1, TASK-3, TREK-1 and TWIK-1 to block the effect of baclofen in the stellate neurons of the EC was unlikely due to the inability of the ABs to inhibit these channels.
The function of TREK-2 channels is up-regulated by intracellular acidification, heat and arachidonic acid (AA) (Huang and Yu, 2008; Kim, 2005). We next tested the effects of these factors on GABABR-mediated hyperpolarization in the EC. Intracellular acidification achieved by substituting 90 mM NaCl with 90 mM NaHCO3 in the extracellular solution (Fakler et al., 1996; Maingret et al., 1999) induced an outward HC and significantly reduced baclofen-induced increases in outward HCs (Figure S4). Elevation of the temperature in the recording chamber to 33°C also induced an outward HC by itself and significantly diminished baclofen-induced enhancement of outward HCs (Figure S4). However, bath application of AA (10 µM) alone induced an inward HC instead (Figure S4). Because AA interacts with many ion channels (Meves, 1994) including the cationic channels such as H- (Fogle et al., 2007) and TRP (Oike et al., 2006) channels and these channels are expressed in the EC (Dickson et al., 2000; von Bohlen Und Halbach et al., 2005), one explanation for this unexpected result is that AA-induced inward HCs might be the neutralized effect of AA on TREK-2 and other channels such as the cationic channels. To test this possibility, we replaced extracellular NaCl with the same concentration of N-methyl-D-glucamine (NMDG)-Cl and omitted extracellular Ca2+ to minimize the contribution of cationic channels to RMPs. Under these circumstances, application of AA indeed induced an outward HC per se (Fig. S4) and also significantly reduced baclofen-induced enhancement of outward HCs compared with the effect of baclofen in this condition without AA (Fig. S4). Collectively, these results further support the involvement of TREK-2 channels in GABABR-mediated hyperpolarization.
We then co-transfected GABABRs with TREK-2 channels in HEK293 cells and recorded the HCs at −60 mV from the transfected cells. Application of baclofen (100 µM) generated an outward HC in cells co-transfected with GABABRs and TREK-2 channels (183±41 pA, n=7, p=0.004, Figure 5A and 5B) whereas application of the same concentration of baclofen failed to significantly change the HCs in HEK293 cells transfected with either TREK-2 channels (0.6±1.9 pA, n=6, p=0.78, Figure 5A and 5B) or GABABRs (−1.5±1.5 pA, n=6, p=0.35, not shown). The reversal potential for baclofen-induced current was −89.2±2.2 mV in cells cotransfected with GABABRs and TREK-2 (n=6, Figure 5C), which was close to the calculated K+ reversal potential (−96.1 mV) in our recording conditions. Application of TREK-2 AB (sc-11560, 40 µg/ml) via the recording pipettes to the HEK293 cells co-transfected with GABABRs and TREK-2 channels also generated an inward HC (−446.3±84.8 pA, n=6, p=0.003, Figure 5D) and significantly reduced baclofen-induced increases in outward HCs (27.9±5.3 pA, n=6, p=0.005 vs. baclofen alone in transfected cells, Figure 5D) whereas intracellular application of TREK-1 AB (sc-11554, 40 µg/ml) failed to change baclofen-induced outward HCs (181.1±18.4 pA, n=6, p=0.97 vs. baclofen alone, Figure 5E). Similar results were obtained by using other two TREK-2 ABs (Figure 5F) further demonstrating the requirement of TREK-2 channels. Finally, dialysis of Rp-cAMPS (1 mM) into the HEK293 cells expressing GABABRs and TREK-2 channels induced an outward HC (357.1±74.7 pA, n=7, p=0.003, Figure 5F) and significantly reduced the effect of baclofen (12.6±14.3 pA, n=7, p=0.002 vs. baclofen alone, Figure 5F) demonstrating the involvement of PKA. Collectively, these results indicate that GABABR activation inhibits PKA resulting in increases in the function of TREK-2 channels to generate hyperpolarization in the EC. In agreement with our results, both TREK-1 (Fink et al., 1996; Murbartián et al., 2005) and TREK-2 (Bang et al., 2000; Lesage et al., 2000) channels are inhibited by PKA-mediated phosphorylation.
Figure 5.
GABABR-induced hyperpolarization is dependent on PKA-mediated phosphorylation site on TREK-2 channels. A, Left panel: HCs recorded at −60 mV from a HEK293 cell transfected with TREK-2 alone before (a) and during (b) the application of baclofen (100 µM). Right panel: HCs recorded at −60 mV from a HEK293 cell cotransfected with GABABRs and TREK-2 channels before (a) and during (b) the application of baclofen. B, Summarized data from 6 HEK293 cells transfected with TREK-2 channels alone and 7 HEK293 cells cotransfected with GABABRs and TREK-2 channels. C, Baclofen-induced currents had a reversal potential (−89.2±2.2 mV, n=6) close to the reversal potential of K+ (−96.1 mV). Inset, net current induced by baclofen. D, Intracellular application of the first TREK-2 AB (40 µg/ml) to the HEK293 cells co-expressing GABABRs and TREK-2 channels generated an inward HC per se and significantly reduced baclofen-induced increases in outward HCs. HCs at −60 mV were recorded immediately after the formation of whole-cell configuration and the actual HCs were used to plot the figure. E, Intracellular application of TREK-1 AB (40 µg/ml) had little effects on HCs and did not significantly change baclofen-induced increases in outward HCs. HCs at −60 mV were recorded immediately after the formation of whole-cell configuration and the actual HCs were used to plot the figure. F, Intracellular dialysis of the second TREK-2 AB (AB2, sc-11559, 40 µg/ml) into the HEK293 cells co-transfected with GABABRs and TREK-2 channels generated an inward holding current (n=6, ++p<0.01 vs. baseline=0) and significantly inhibited baclofen-induced increases in outward holding currents (n=6, **p<0.01 vs. baclofen alone). Intracellular perfusion of the third TREK-2 AB (AB3, APC-055, Alomone labs, 40 µg/ml) induced an inward holding current (n=6, ++p<0.001 vs. baseline=0) and significantly inhibited the effect of baclofen (n=6, **p<0.01 vs. baclofen alone). Intracellular application of Rp-cAMPS (1 mM) into the HEK293 cells co-transfected with GABABRs and TREK-2 channels induced an outward holding current (n=7, ++p<0.01 vs. baseline=0) and significantly reduced the effect of baclofen (n=7, **p<0.01 vs. baclofen alone). G, Application of baclofen did not change the HCs significantly in HEK293 cells co-transfected with S359A mutant TREK-2 channels and GABABRs. Inset shows the holding currents before and during the application of baclofen. H, Application of baclofen did not induce significant changes in the voltage-current relationship recorded from HEK293 cells co-expressing GABABRs and S359A mutant TREK-2 channels.
TREK-2 channels are phosphorylated by PKA on serine 359 of the C-terminus (Bang et al., 2000). Because our preceding results demonstrate that PKA is required for GABABR-induced hyperpolarization, we tested the role of the PKA phosphorylation site on TREK-2 channels in GABABR-mediated hyperpolarization in HEK293 cells co-expressing GABABRs and the mutant TREK-2 channels in which serine 359 was mutated to alanine (S359A) to nullify PKA-mediated phosphorylation of TREK-2 channels (Kang et al., 2007a). Alanine substitution of this site did not prevent the expression and functions of TREK-2 channels in HEK293 cells (Kang et al., 2007a). However, application of baclofen (100 µM) failed to change the HCs significantly in HEK293 cells co-expressing S359A mutant and GABABRs (5.4±5.0 pA, n=9, p=0.32, Figure 5G) and there was little change in the voltage-current relationship when baclofen was applied (Figure 5H). Together, these results indicate that serine 359, the PKA phosphorylation site on TREK-2 channels is necessary for GABABR-mediated hyperpolarization.
GABABR activation in the EC impairs spatial learning in Morris water maze via PKA-dependent activation of TREK-2 channels
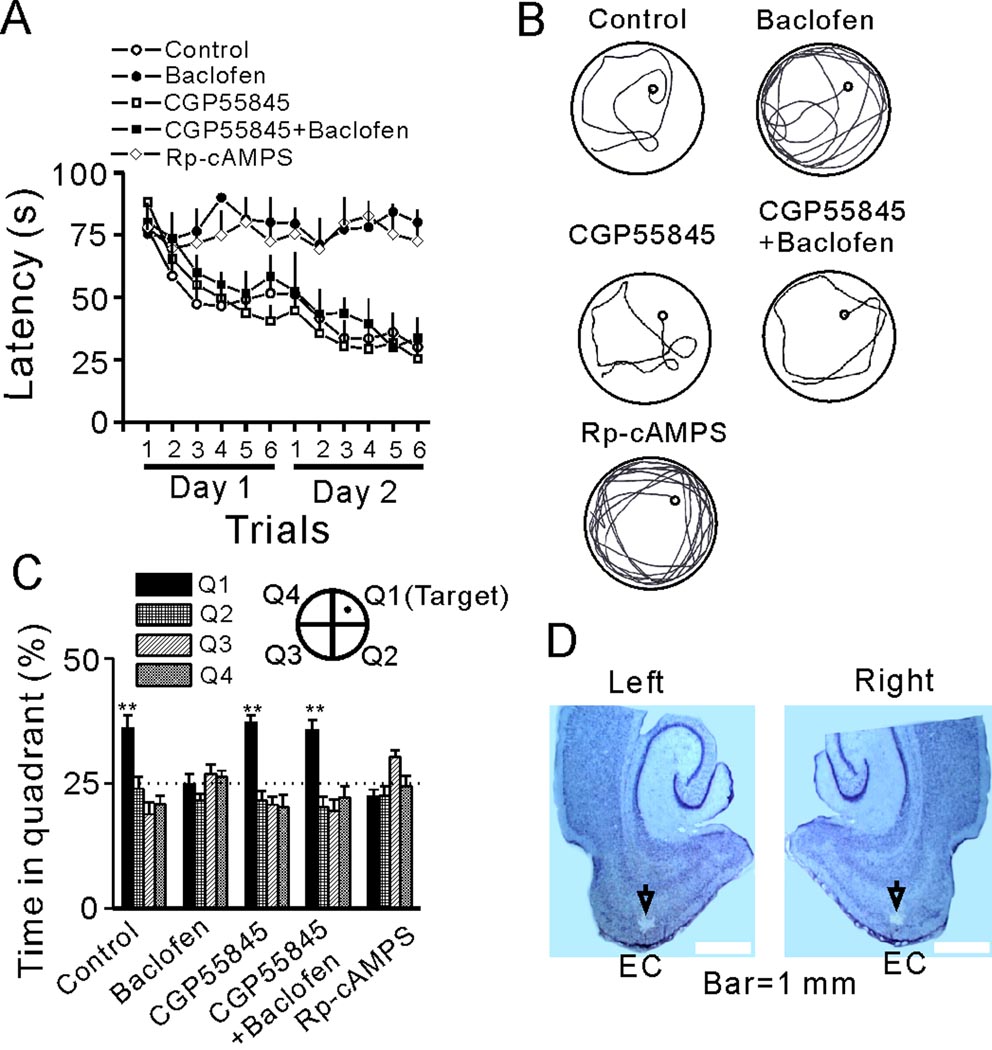
We then tested the roles of GABABR activation in spatial learning using Morris Water Maze. Rats receiving intra-EC infusion of normal saline (0.9% NaCl) (control, 1 µl/rat, n=7 rats) acquired spatial learning rapidly as shown by a significant decrease in latency to find the hidden platform throughout the trials (F(11,66)=5145.33, p<0.001, Figure 6A). Intra-EC infusion of baclofen (5 µg/rat in 1 µl, n=8 rats) completely prevented the spatial learning of rats because the escape latencies were not reduced throughout the trials (F(11,77)=1.682, p=0.093, Figure 6A and 6B). The effect of baclofen was mediated via activation of GABABRs because microinjection of the GABABR antagonist, CGP55845 (1 µg/rat in 1 µl, n=8 rats) completely blocked baclofen-induced impairment of spatial learning (F(1,14)=47.89, p<0.001 between CGP55845+baclofen group and baclofen group, Figure 6A and 6B). Because the function of PKA is involved in GABABR-mediated depression of neuronal excitability in the EC, we then tested the roles of PKA in spatial learning. Intra-EC application of Rp-cAMPS (18 µg/rat in 1 µl, n=8 rats) prevented spatial learning as the latencies were not significantly reduced throughout the trials (F(11,77)=0.55, p=0.862, Figure 6A, 6B) suggesting that endogenous PKA controls spatial learning (Arnsten et al., 2005). In the probe trials, control rats and rats treated with CGP55845 or CGP55845 followed by baclofen showed a significant bias for the target quadrant where the platform had been originally located (p<0.01 for each group compared with the chance level 25%). However, rats in other groups failed to show preference for the target quadrant (Figure 6C). The microinjection sites were confirmed at the end of each experiment (Figure 6D). Together with the electrophysiological data, these results suggest that GABABR activation depresses spatial learning via inhibition of PKA.
Figure 6.
Activation of GABABRs impairs spatial learning in Morris water maze. A, Mean latencies to the platform from the acquisition trials were presented by groups. Rats were microinjected with normal saline (control), baclofen, Rp-cAMPS, CGP55845 or CGP55845 followed by baclofen. B, Representative swimming traces from the last trial of day 2. Note that baclofen markedly lengthened the swimming path, which was mimicked by Rp-cAMPS. C, Probe trial performance of each group as presented by the proportion of total time spent in each quadrant of the Morris water maze (**p<0.01 vs. 25% chance in each quadrant). D, Horizontal brain sections showing the microinjection sites indicated by the arrows.
The water maze performance is subject to the influences of other non-spatial learning factors such as the sensory, motivational, emotional, or motor functions of the tested subjects. We took the following measures to ensure that the effects of baclofen and Rp-cAMPS were not produced by non-spatial learning factors. First, we examined the recorded swimming speed of the rats. Intra-EC applications of the drugs failed to alter the swimming speed of the rats (Figure S5A). Second, we tested the performance of the rats microinjected with 0.9% NaCl (control, 1 µl/rat, n=7 rats), baclofen (5 µg/rat in 1 µl, n=7 rats) and Rp-cAMPS (18 µg/rat in 1 µl, n=7 rats) in a visible platform water maze in which no learning is involved (Rudy, 2008). There were no significant differences for the latencies to find the visible platform among control, baclofen- and Rp-cAMPS-treated rats (Figure S5B). Third, we tested whether baclofen and Rp-cAMPS specifically impact the learning phase. Rats were randomly divided into 3 groups and received 2 days of the same acquisition trials in the hidden-platform water maze without microinjection. On day 3, the three groups of the rats were microinjected with normal saline (Group 1, n=7 rats), baclofen (Group 2, n=7 rats) and Rp-cAMPS (Group 3, n=7 rats), respectively. A probe trial with the platform unavailable was conducted for these three groups of rats 15 min after microinjection. Whereas each group of rats acquired spatial learning rapidly as demonstrated by significant decreases in latency to find the platform throughout the trials (Figure S5C1), there were no significant differences of escape latency among these three groups (Figure S5C1). In the probe trials, all the 3 groups of rats showed a bias for the target quadrant and there were no significant differences in the time spent in the target quadrant among the 3 groups (Figure S5C2) demonstrating that baclofen and Rp-cAMPS specifically depress the learning phase of the performance.
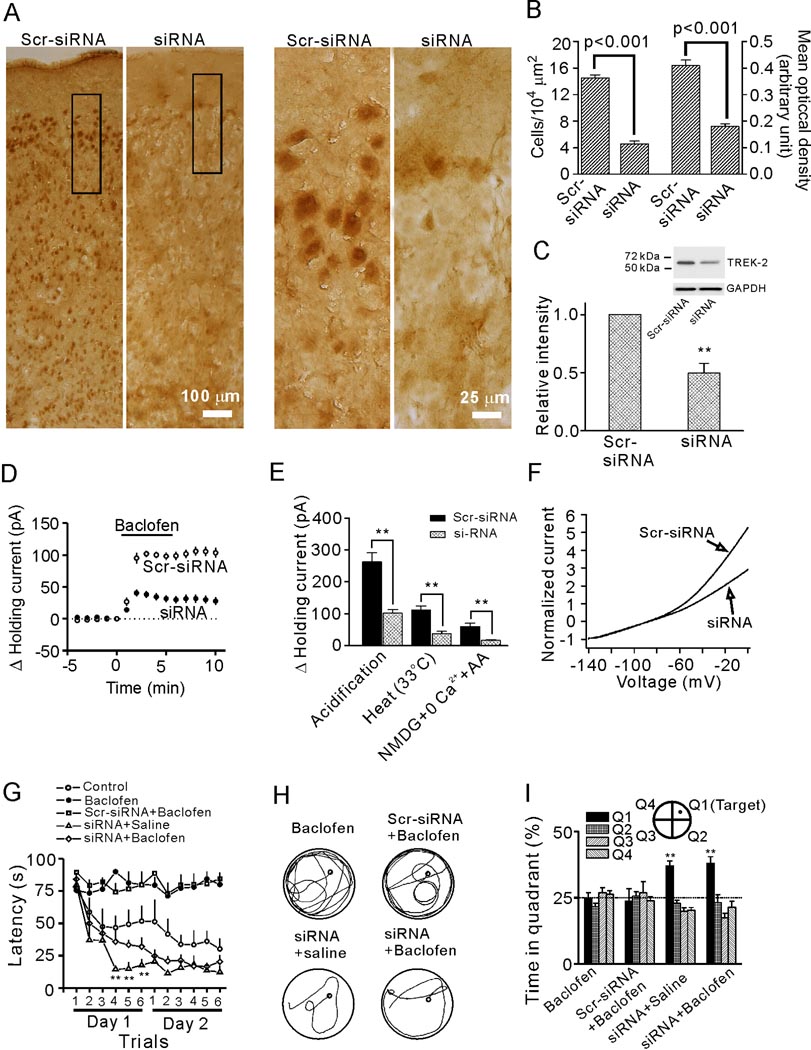
We then tested the role of TREK-2 channels in GABABR-mediated inhibition of spatial learning by applying siRNA of TREK-2 channels into the EC through osmotic pumps to knockdown TREK-2 channel expression. We used several approaches to confirm the effectiveness of siRNA. First, we immunostained the EC for TREK-2 channels in rats injected with TREK-2 siRNA or the scrambled siRNA (Scr-siRNA). Because the immunoreactivity of TREK-2 channels was lower usually in the region of ~400–500 µm around the injection site in slices from siRNA-treated rats (Figure S6), the immunocytochemical quantification and electrophysiological recordings thereafter were limited to this range in slices from both siRNA- and Scr-siRNA-treated rats. We employed two indexes (the number and the mean optical density of TREK-2-positive cells) to quantify the results. The number of the TREK-2-positive cells in layer II of the EC from siRNA-treated rats (4.6±0.4 cells/104 µm2, n=6 rats) was markedly reduced compared with that from Scr-siRNA-treated rats (14.5±0.5 cells/104 µm2, n=6 rats, p<0.001, Figure 7A, 7B). The mean optical density of positive cells from siRNA-treated rats (0.18±0.01 arbitrary units) was significantly lower than that of Scr-siRNA-treated rats (0.41±0.02 arbitrary units, p<0.001, Figure 7B). Second, western blot showed that the level of TREK-2 proteins in the EC from rats treated with siRNA was significantly lower than that of rats treated with Scr-siRNA (Figure 7C). Third, siRNA treatment significantly reduced baclofen-induced outward HCs (101.9±4.7 pA, n=16 slices from 4 Scr-siRNA-treated rats vs. 38.2±5.3 pA, n=13 slices from 4 siRNA-treated rats, p<0.001, Figure 7D). Fourth, the characteristic increases of TREK-2 channels in response to intracellular acidification, heat and AA (in the presence of NMDG and 0 Ca2+) were significantly reduced in rats treated with siRNA compared with those of the rats treated with the Scr-siRNA (Figure 7E). Finally, the outward rectification of the voltage-current relationship of the cells in rats treated with siRNA was obviously reduced (Figure 7F) suggesting a proportional loss of TREK-2 channels. However, the RMPs of the stellate neurons from siRNA-treated rats (−55.8±1.2 mV, n=16) were not significantly different from those of the Scr-siRNA-treated rats (−56.3±1.5 mV, n=13, p=0.86, not shown). One explanation is that the proportional contribution of TREK-2 channels to RMPs in siRNA-treated rats could be compensated by other K2P channels as has been observed in TREK-1 (Heurteaux et al., 2004) and TASK-1 (Aller et al., 2005) knockout mice in which the RMPs of neurons were not changed.
Figure 7.
Knockdown of TREK-2 channels by siRNA annuls baclofen-induced impairment of spatial learning. A, TREK-2 channels were significantly knocked down after delivery of TREK-2 siRNAs to the EC. Left two panels: immunoreactivity of TREK-2 channels in a region of the EC adjacent to the injection site from rats treated with Scr-siRNA or siRNA in low magnification. Right two panels: high magnification of the regions denoted in the left two panels. B, siRNA-treatment significantly deceased the number of TREK-2-positive cells (left) and the mean optical density of TREK-2-positive cells (right) in layer II of the EC. C, Western blot showed that siRNA-treatment significantly decreased the level of TREK-2 channel proteins in the EC (**p<0.001). A band that had a molecular mass of ~60 kDa corresponding to the reported molecular mass of TREK-2 channels (Kang et al., 2007b; Simkin et al., 2008) was detected in the lysates of the EC. D, Baclofen-induced increases in outward HCs were significantly reduced in slices cut from rats treated with siRNA. E, siRNA treatment significantly reduced the increases in outward HCs induced by intracellular acidification (262.1±28.4 pA, n=10 slices from 3 Scr-siRNA treated rats vs. 101.8±11.5 pA, n=12 slices from 4 siRNA treated rats), heat (111.1±13.2 pA, n=10 slices from 3 Scr-siRNA treated rats vs. 37.5±7.4 pA, n=11 slices from 3 siRNA treated rats) and AA (in the presence of NMDG and 0 Ca2+; 59.9±10.6 pA, n=10 slices from 3 Scr-siRNA treated rats vs. 16.5±3.0 pA, n=12 slices from 4 siRNA treated rats) (**p<0.001). F, siRNA treatment conspicuously reduced the extent of outward rectification of the voltage-current relationship of the stellate neurons. Currents at different voltages from each cell were normalized to the absolute value of the current at −140 mV to minimize the influence of current sizes on voltage-current relationship. Each trace shows the averaged voltage-current relationship from 10 cells from 3 rats treated with siRNA or Scr-siRNA. G, Summarized mean latencies. Note that siRNA knockdown of TREK-2 channels blocked baclofen-induced impairment of spatial learning (**p<0.01 vs. the corresponding values in control group). H, Representative swimming traces from the last trial of day 2 for each group. I, Probe trial performance of each group as presented by the proportion of total time spent in each quadrant. The siRNA-treated rats showed a preference for the target quadrant and intra-EC injection of baclofen into these rats did not prevent the preference of the rats (** p<0.01 vs. 25% chance level).
We then tested the water maze performance of rats treated with TREK-2 siRNA and Scr-siRNA. Treatment of rats with siRNA (n=8 rats) abolished baclofen-induced inhibition of spatial learning, as demonstrated by decreasing latencies of target acquisition (siRNA+baclofen group vs. baclofen group, F(1,14)=111.26, p<0.001, Figure 7G and 7H). However, Scr-siRNA treatment (n=7 rats) failed to alter the effect of baclofen (Scr-siRNA+baclofen group vs. baclofen group, F(1,13)=0.208, p=0.656, Figure 7G and 7H). siRNA treatment tended to improve the learning ability of rats when compared with controls, as shown by the decreased latencies of the last 3 trials on the first day (Figure 7G, p<0.01, multivariate ANOVA), which is consistent with the notion that TREK-2 channels are involved in learning and memory (Huang and Yu, 2008; Pan et al., 2003). In the probe trials, preferences for target quadrant were observed only in siRNA-treated rats injected with saline (36.9±2.0%, p<0.01, Figure 7I) or baclofen (37.9±2.6%, p<0.01, Figure 7I) whereas Scr-siRNA-treated rats injected with baclofen failed to show bias for the target quadrant (23.6±4.8%, p=0.75 vs. 25% chance level, Figure 7I). Again, siRNA treatment failed to change the swimming speed of rats (Figure S5D). Together, these results demonstrate that GABABR activation impairs spatial learning via activation of TREK-2 channels in the EC.
Discussion
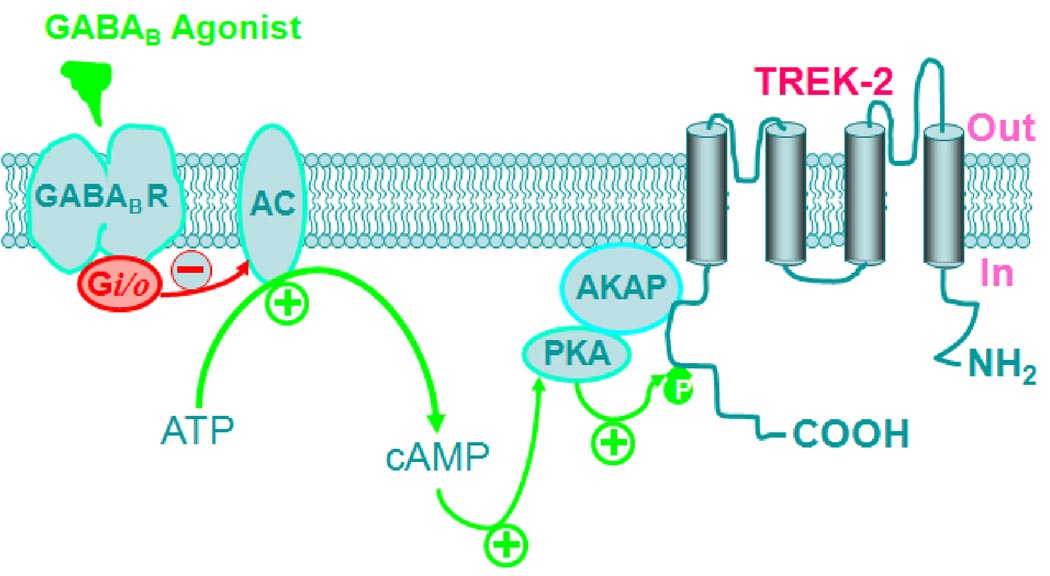
Our results demonstrate that GABABR activation drastically inhibits neuronal excitability in the EC. GABABR-induced inhibition is mediated by TREK-2, a type of K2P channels and requires the functions of Gαi proteins and AC-cAMP-PKA pathway. We further demonstrate that PKA and TREK-2 channels are essential for GABABR-mediated inhibition of spatial learning assessed by Morris Water Maze. As schematically illustrated in Figure 8, our results support an action mode in which GABABR activation depresses AC-cAMP-PKA pathway and disinhibits the tonic inhibition of PKA on TREK-2 channels resulting in depression of neuronal excitability in the EC and spatial learning.
Figure 8.
Schematic illustration of signaling cascade leading to the activation of TREK-2 channels by GABABRs. Red arrows and minus mark indicate inhibition whereas green arrows and plus marks denote facilitation. GABABR agonists activate GABABRs resulting in activation of the inhibitory G proteins (Gi/o). Activation of Gi/o inhibits the activity of AC leading to a reduction in the production of cAMP from ATP and an inhibition of PKA activity. Normally, PKA exerts a tonic inhibition on TREK-2 channels by phosphorylating serine 359 on TREK-2 channels. The effects of PKA require the function of AKAPs which tether PKA to TREK-2 channels. GABABR-mediated inhibition of PKA annuls PKA-mediated tonic inhibition of TREK-2 channels resulting in an increase in the function of TREK-2 channels. The ultimate result is the inhibition of neuronal excitability in the EC and depression of spatial learning.
Ionic mechanisms underlying GABABR-mediated inhibition of neuronal excitability and spatial learning
Resting K+ channels are the major determinants of neuronal membrane potential and their modulation is one of the principal mechanisms by which neurotransmitters regulate neuronal excitability. The following lines of evidence clearly demonstrate that GABABR activation generates membrane hyperpolarization by activating a resting K+ conductance. First, replacement of intracellular K+ with Cs+ failed to induce hyperpolarization but instead a slight depolarization. Second, the net current generated by GABABR activation had a reversal potential close to the K+ reversal potential. Third, elevation of extracellular K+ concentration significantly reduced baclofen-induced hyperpolarizaton. We have also shown that the K+ channels activated by GABABRs are insensitive to the classic K+ channel blockers, but sensitive to Ba2+. Whereas Ba2+ blocks the inward rectifier K+ channels which are involved in controlling RMPs and GABABR activation has been shown to activate the inward rectifier K+ channels in other neurons (Misgeld et al., 1995), the inward rectifier K+ channels are unlikely to be the targets for GABABRs in the EC based on the following two lines of evidence. First, the inward rectifier K+ channels are sensitive to TEA and Cs+ whereas application of these classic K+ channel blockers failed to significantly change baclofen-induced increases in outward currents. Second, application of tertiapin, a selective inward rectifier K+ channel inhibitor, did not alter the effects of baclofen. Together, all our results indicate the involvement of K2P channels in GABABR-mediated hyperpolarization in the EC. To identify the involved types of K2P channels, we took the advantage of the findings that the Ba2+-sensitivity to different K2P channels has been well-characterized. Among all the K2P channels, TASK-1 (Han et al., 2002), TASK-3 (Han et al., 2002; Kim et al., 2000), TREK-1 (Fink et al., 1996), TREK-2 (Han et al., 2002), TWIK-1 (Lesage et al., 1996) and TRESK (Kang et al., 2004; Sano et al., 2003) are sensitive to Ba2+. We therefore examined the roles of these K2P channels in baclofen-induced hyperpolarization. Our immunocytochemical results showed that TREK-2 channels are expressed in the EC and intracellular application of TREK-2 ABs significantly reduced baclofen-induced increases in outward HCs indicating that GABABR activation inhibits neuronal excitability by activating TREK-2 channels. Further evidence to support the requirement of TREK-2 channels is that application of baclofen to HEK293 cells co-expressing GABABRs and TREK-2 channels induces hyperpolarization whereas intracellular application of TREK-2 ABs to these cells significantly reduced the effects of baclofen.
Signaling mechanisms whereby GABABR activation inhibits neuronal excitability in the EC
K2P channels are modulated by a variety of G protein-coupled receptors (Chemin et al., 2003; Chen et al., 2006; Deng et al., 2006; Honoré, 2007; Kim, 2005; Lesage et al., 2000; Mathie and Veale, 2007; Talley et al., 2000). The α subunits of G proteins are responsible for the modulation of K2P channels (Chen et al., 2006; Deng et al., 2006; Lesage et al., 2000). However, Gα subunits modulate K2P channels in distinct ways; stimulation of the Gs- or Gq-coupled receptors inhibits (Chemin et al., 2003; Chen et al., 2006; Deng et al., 2006; Lesage et al., 2000) whereas activation of Gi-coupled receptors (Lesage et al., 2000) activates K2P channels. Gq-mediated inhibition of K2P channels is mediated via either intracellular signaling molecules (Chemin et al., 2003; Kang et al., 2006; Mathie, 2007; Veale et al., 2007) or direct G protein-coupling (Chen et al., 2006; Deng et al., 2006) depending on the channel type whereas Gi-induced activation requires intracellular signals because it has been reported that Gi does not directly interact with K2P channels (Chen et al., 2006). Our results are consistent with this mechanistic framework because we have shown that GABABR-mediated increases in outward HCs were blocked by intracellular application of GDP-β-S or in slices pretreated with PTX suggesting the involvement of Gi. PKA is an important intracellular messenger downstream of Gi proteins (Couve et al., 2000). Our results indicate that PKA is necessary for GABABR-induced increases in outward HCs. Consistent with our results, both TREK-1 (Fink et al., 1996; Murbartián et al., 2005) and TREK-2 (Lesage et al., 2000) channels contain the phosphorylation site for PKA. Serine 333 in TREK-1 (Murbartián et al., 2005) and Serine 359 in TREK-2 (Bang et al., 2000) are the putative PKA phosphorylation sites and activation of PKA down-regulates the channel activities. We further showed that mutation of serine 359 to alanine in TREK-2 channels annulled baclofen-mediated hyperpolarization suggesting that the effects of GABABRs are dependent on PKA-mediated phosphorylation of TREK-2 channels. These results could also explain the data that TASK-1 channels for which high immunoreactivity was detected in the EC (Figure 4A, Figure S1A) are not involved in GABABR-mediated hyperpolarization because TASK channels do not contain PKA phosphorylation sites and are not modulated by PKA (Mathie, 2007).
AKAPs are the proteins that tether PKA to other intracellular signaling substrates (Beene and Scott, 2007; Hundsrucker and Klussmann, 2008) and both TREK-1 and TREK-2 channels interact with AKAP150 (Sandoz et al., 2008; Sandoz et al., 2006). We therefore tested the roles of AKAPs by application of the AKAP inhibitory peptide that selectively blocks the conserved binding domain of different forms of AKAPs to PKA (Beene and Scott, 2007; Hundsrucker and Klussmann, 2008). Our results demonstrate that infusion of the AKAP inhibitory peptide via the recording pipettes remarkably reduced baclofen-induced hyperpolarization whereas application of the control peptide was without effect. Together, our results support a scenario in which GABABR-mediated activation of Gαi leads to a down-regulation of PKA activity resulting in a reduction in the tonic inhibition of TREK-2 channels mediated by PKA. The ultimate result is an up-regulation of TREK-2 channel activity leading to hyperpolarization of entorhinal cortical neurons and depression of spatial learning (Figure 8).
Roles of GABABRs in spatial learning
GABABRs have been implicated in spatial learning (Helm et al., 2005; McNamara and Skelton, 1996). However, the mechanisms by which GABABRs affect spatial learning have not been determined. The EC is one of the structures essential for the consolidation and recall of memories (Haist et al., 2001; Squire et al., 2004; Steffenach et al., 2005). The findings that a high density of GABABRs is expressed in the EC (Mizukami et al., 2002) and that activation of GABABRs remarkably depresses neuronal excitability in the EC suggest that activation of GABABRs controls learning and memory. Our results using Rp-cAMPS to mimic the effects of baclofen on spatial learning suggest that GABABR-induced impairment of spatial learning is mediated by down-regulation of PKA activity. The target of PKA is likely to be TREK-2 channels because down-regulation of TREK-2 channels by siRNA prevents GABABR-mediated impairment of spatial learning. Consistent with our results, TREK-2 channels are involved in the modulation of memory (Huang and Yu, 2008). In conclusion, our study provides a novel cellular and molecular mechanism to explain the roles of GABABRs in learning and memory.
Experimental Procedures
Electrophysiology
Whole-cell recordings were performed from layer II stellate neurons in entorhinal slices and transfected HEK293 cells as described previously (Deng et al., 2007). Detailed methods for slice preparation, expression of GABABRs and K2P channels in HEK293 cells and electrophysiological recordings are provided in Supplemental data.
Immunocytochemistry and Western blot
The detailed procedures for immunocytochemistry and Western blot analysis of K2P channels are described in Supplemental Data.
Behavioral test
Cannulation, siRNA injection and Morris Water Maze test were performed on Sprague-Dawley male rats weighing 150–200 g. For details, please see Supplemental Data.
Data analysis
Data are presented as the means ± S.E.M. Baclofen concentration-response curve was fit by the Hill equation: I = Imax × {1/[1 + (EC50/[ligand])n]}, where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and n is the Hill coefficient. Paired or unpaired t-test was used to compare electrophysiological, quantified immunohistochemical and western blot data as appropriate. For the data obtained from the water maze experiments, we used the repeated measures and multivariate ANOVA process of the general linear model in SPSS 11.0 statistical software and gave comparison among different groups and different measuring time points pairwise. P values are reported throughout the text and significance was set as P<0.05. N number in the text represents the cells examined, unless stated otherwise.
Supplementary Material
Acknowledgements
This work was supported by National Institute of Mental Health Grant 1R01MH08288. We sincerely appreciate the valuable advices for the water maze experiments from Dr. Fred J. Helmstetter in the University of Wisconsin-Milwaukee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol. 2007;19:192–198. doi: 10.1016/j.ceb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003;22:5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci U S A. 2006;103:3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Deng PY, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol. 2006;577:497–511. doi: 10.1113/jphysiol.2006.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol Pharmacol. 2007;72:208–218. doi: 10.1124/mol.107.034389. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Magistretti J, Shalinsky MH, Fransen E, Hasselmo ME, Alonso A. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol. 2000;83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998a;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998b;398:25–48. [PubMed] [Google Scholar]
- Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: the entorhinal region--a morphometric study. Biol Psychiatry. 1988;24:515–521. doi: 10.1016/0006-3223(88)90162-x. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fogle KJ, Lyashchenko AK, Turbendian HK, Tibbs GR. HCN pacemaker channel activation is controlled by acidic lipids downstream of diacylglycerol kinase and phospholipase A2. J Neurosci. 2007;27:2802–2814. doi: 10.1523/JNEUROSCI.4376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KA, Haberman RP, Dean SL, Hoyt EC, Melcher T, Lund PK, Gallagher M. GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology. 2005;48:956–964. doi: 10.1016/j.neuropharm.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Huang D, Yu B. Recent advance and possible future in TREK-2: a two-pore potassium channel may involved in the process of NPP, brain ischemia and memory impairment. Med Hypotheses. 2008;70:618–624. doi: 10.1016/j.mehy.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Hundsrucker C, Klussmann E. Direct AKAP-mediated protein-protein interactions as potential drug targets. Handb Exp Pharmacol. 2008:483–503. doi: 10.1007/978-3-540-72843-6_20. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Kang D, Choe C, Cavanaugh E, Kim D. Properties of single two-pore domain TREK-2 channels expressed in mammalian cells. J Physiol. 2007a;583:57–69. doi: 10.1113/jphysiol.2007.136150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol. 2006;291:C649–C656. doi: 10.1152/ajpcell.00047.2006. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim SH, Hwang EM, Kwon OS, Yang HY, Kim ES, Choi TH, Park JY, Hong SG, Han J. Expression of thermosensitive two-pore domain K+ channels in human keratinocytes cell line HaCaT cells. Exp Dermatol. 2007b;16:1016–1022. doi: 10.1111/j.1600-0625.2007.00626.x. [DOI] [PubMed] [Google Scholar]
- Kang D, Mariash E, Kim D. Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J Biol Chem. 2004;279:28063–28070. doi: 10.1074/jbc.M402940200. [DOI] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K(+) channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986;246:149–169. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer's disease. J Mol Neurosci. 2001;17:225–232. doi: 10.1385/jmn:17:2:225. [DOI] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Veale EL. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr Opin Investig Drugs. 2007;8:555–562. [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav. 1996;53:303–308. doi: 10.1016/0091-3057(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Meves H. Modulation of ion channels by arachidonic acid. Prog Neurobiol. 1994;43:175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Mizukami K, Ishikawa M, Hidaka S, Iwakiri M, Sasaki M, Iritani S. Immunohistochemical localization of GABAB receptor in the entorhinal cortex and inferior temporal cortex of schizophrenic brain. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:393–396. doi: 10.1016/s0278-5846(01)00247-0. [DOI] [PubMed] [Google Scholar]
- Murbartián J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- Oike H, Wakamori M, Mori Y, Nakanishi H, Taguchi R, Misaka T, Matsumoto I, Abe K. Arachidonic acid can function as a signaling modulator by activating the TRPM5 cation channel in taste receptor cells. Biochim Biophys Acta. 2006;1761:1078–1084. doi: 10.1016/j.bbalip.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Pan YP, Xu XH, Wang XL. [mRNA expression alteration of two-pore potassium channels in the brain of beta-amyloid peptide25–35-induced memory impaired rats] Yao Xue Xue Bao. 2003;38:721–724. [PubMed] [Google Scholar]
- Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- Rudy JW. The Neurobiology of Learning and memory. Sinauer Associates, Inc.; 2008. Making memories:conceptual issues and methodologies; p. 142. [Google Scholar]
- Sandoz G, Tardy MP, Thummler S, Feliciangeli S, Lazdunski M, Lesage F. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J Neurosci. 2008;28:8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Thummler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, Guy N, Lazdunski M, Lesage F. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K(+) channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003;278:27406–27412. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- Simkin D, Cavanaugh EJ, Kim D. Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: role of alternative translation initiation. J Physiol. 2008;586:5651–5663. doi: 10.1113/jphysiol.2008.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus. 2003;13:943–952. doi: 10.1002/hipo.10144. [DOI] [PubMed] [Google Scholar]
- Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. G(alpha)q-mediated regulation of TASK3 two-pore domain potassium channels: the role of protein kinase C. Mol Pharmacol. 2007;71:1666–1675. doi: 10.1124/mol.106.033241. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O, Hinz U, Unsicker K, Egorov AV. Distribution of TRPC1 and TRPC5 in medial temporal lobe structures of mice. Cell Tissue Res. 2005;322:201–206. doi: 10.1007/s00441-005-0004-4. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000a;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000b;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.