Abstract
Background
Because the course of Legg-Calvé-Perthes disease (LCPD) is highly variable, its appropriate diagnostic evaluation and treatment are still debated.
Methods
The authors selectively review the literature, present their own study findings, and discuss the guidelines of the German Society for Orthopedics and Orthopedic Surgery.
Results
The main prognostic factors are the patient’s age at the onset of the disease, the degree of limitation of range of motion, the extent of involvement of the femoral epiphysis, and any additional radiographic "head-at-risk" signs. Depending on the severity of the disease, the treatment options range from observation and frequent follow-up to reconstructive hip surgery. The goal of all treatments is to prevent a prearthrotic deformity and the ensuing premature coxarthrosis. This goal is best met by adherence to the principle of containment, i.e., the maintenance or restoration of joint congruence while biological plasticity is still present.
Conclusions
In view of the variable course of LCPD, the proper course of treatment must be determined individually in each case. Every child with LCPD must receive individually adapted treatment and continued follow-up into adulthood.
Keywords: Legg-Calvé-Perthes disease, pediatric disease, prognosis, osteochondrosis, joint diagnosis
Perthes disease (Legg-Calvé-Perthes disease) is a relatively common disease of the hip in childhood, affecting 10.8 of every 100 000 children. Its cause and treatment are still topics of controversy. The disease was described nearly simultaneously in the year 1910 by G. C. Perthes in Germany, A. T. Legg in the United States, and J. Calvé in France. Despite nearly 100 years of research and detailed characterization of the pathophysiology, clinical and radiological features, and spontaneous course of this disease, its etiology remains essentially unknown.
Because the disease takes a variable course, the appropriate manner of treatment and the benefit to be expected from treatment are still matters of debate. In particular, the use of so-called weight-relieving orthoses is now viewed with increasing disfavor.
While conservative and operative treatments to preserve containment are already well established forms of therapy for the early stage of the disease, increasing attention is now being paid to treatment of the residual deformity in adolescent patients, with restoration of the normal anatomy.
In this article, we provide an overview of current diagnostic and therapeutic strategies for Perthes disease, in the light of a selective literature review and the findings of our own clinical studies.
Etiology and pathogenesis
Perthes disease belongs to the class of aseptic osteochondroses of childhood. It is characterized by avascular necrosis of the epiphysis, which, in turn, impairs the enchondral ossification of the femoral head.
The etiology of Perthes disease remains unknown. A number of possible causes have been proposed, including repetitive microtrauma, skeletal retardation, and vascular insufficiency. It is suspected that repetitive microtrauma of the femoral head leads to small fractures in the fragile spongiosa framework of the immature femoral head; this hypothesis is supported by the observation that the disease is more common in hyperactive children (1).
The vascular hypothesis is supported by the fact that the blood supply of the femoral head, derived from vessels that take an intra-articular course at the femoral neck, is especially vulnerable in children of this age group (e1).
Abnormalities of blood coagulation or blood viscosity, as well as blood vessel changes, can lead to epiphyseal bone necrosis (e2).
In addition, most children with Perthes disease also manifest skeletal retardation (e3). It has been shown that the urinary desoxypyridinoline/creatinine quotient is abnormally low in the condensation phase of the disease; this is also an indication of abnormally hypoactive skeletal metabolism and thus points to a systemic cause of the disease (e4).
Clinical features
The disease typically arises in children aged 3 to 7 years. It affects boys more commonly than girls, in a 4:1 ratio.
The affected children come to medical attention because of limping. Pain is typically located in the groin area and is often present only during physical activity; in 25% of patients, the pain radiates into the thigh and knee. There may also be knee pain without groin or thigh pain, and, in such cases, the diagnosis is often delayed. It follows, therefore, that any child presenting with knee pain should have the hip carefully examined as well (2, 4). In 10% to 15% of children with Perthes disease, both hips are affected, usually in temporally staggered fashion, rather than simultaneously.
Physical examination usually reveals a limping gait. The mobility of the hip is limited mainly in internal rotation and abduction. In addition, the legs may be of different length because of an adduction contracture or a collapsed epiphysis.
Clinical risk factors for a poor outcome include a later age of onset, overweight, severe limitation of the range of motion, and female sex.
The differential diagnosis of Perthes disease is summarized in Box 1. In particular, when both hips are affected, multiple epiphyseal or spondyloepiphyseal dysplasia must be ruled out. Meyer’s dysplasia is also very difficult to differentiate from Perthes disease on radiological grounds alone. The diagnosis of Meyer’s dysplasia can be made in the light of the absence of clinical symptoms and of the staged radiological progression that typifies Perthes disease (2).
Box 1.
Major differential diagnoses of Perthes disease
Coxitis fugax
Juvenile idiopathic arthritis
Osteomyelitis
Meyer’s dysplasia
Epiphyseal dysplasia
Spondyloepiphyseal dysplasia
Chondroblastoma
So-called "Perthes" due to hip dysplasia
Cortisone-induced necrosis of the femoral head
Other diseases associated with osteonecroses similar to those of Perthes disease
Sickle-cell anemia
Thalassemia
Trisomy 21
Trichorhinophalangeal syndrome
Achondroplasia
Gaucher’s disease
Hemophilia
Hypothyroidism
Klinefelter syndrome
Diagnostic evaluation
The diagnostic evaluation is performed in standard fashion with x-ray views in two planes. The pelvic survey film and lateral hip x-ray serve not only to establish the diagnosis, but also to enable classification, prognostic assessment, and follow-up of the course of the disease.
Ultrasonography can be used as a supplementary technique to diagnose changes of the femoral head and, in particular, any accompanying synovitis or effusion (3). Whenever a patient of the typical age for Perthes disease suffers from coxitis fugax that persists for several weeks, plain x-rays or a magnetic resonance scan should be performed to rule out early Perthes disease (2).
Magnetic resonance imaging (MRI) is of particular value in identifying the early stage of Perthes disease in the absence of changes on plain films, as well as in cases where the differential diagnosis would otherwise be difficult. Dynamic MRI, like arthrography, can also be helpful for diagnosing any accompanying "hinge abduction" (in which the lateral side of the femoral head contacts the acetabular margin) during preoperative planning. Nonetheless, MRI has not been shown to be of prognostic value, and it is therefore not used as a standard diagnostic technique (4, 5).
The radiologically demonstrable stages that all children with Perthes disease pass through are based on the scheme devised by Waldenström (6). The course of the disease is divided into the initial, condensation, fragmentation, repair, and healing stages.
In 1971, Catterall propsed a fourfold classification of the disease, based on the extent of involvement of the femoral head (7). He supplemented this classification with what he called "head-at-risk signs." The Catterall classification has only limited reliability and prognostic value (e5– e7).
In 1984, Salter and Thompson described a classification involving only two groups, which were defined by the extent of subchondral fracture seen in axial x-rays in the early stage of the disease (e8). The disadvantage of this classification is that not all patients are diagnosed in the early stage.
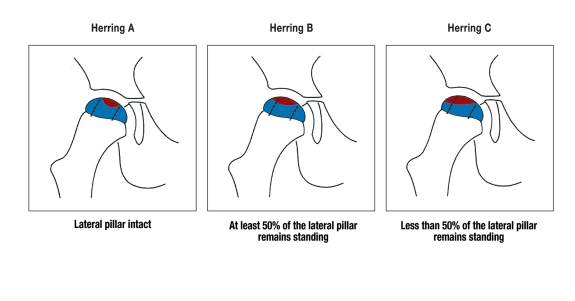
The most recent classification was proposed by Herring in 1992 (8, 9). It is based on the height of the lateral pillar of the epiphysis of the femoral head, as seen on an AP x-ray view in the early fragmentation phase (figure 1). This "lateral pillar" classification, when determined in the fragmentation phase, is of greater prognostic value and has a greater inter-observer reliability than the Catterall classification (8– 10).
Figure 1.
Herring’s "lateral pillar" classification, depending on the height of the lateral pillar (necrotic area marked in red)
The predictive value of the Herring classification is higher when the patient’s age at the onset of the disease is also taken into account (11).
Yet more recently, Herring has added a further group to his classification, the B/C "border group," because his multicenter study revealed that many affected hips have a radiological appearance that is intermediate between types B and C (12).
Prognosis
Retrospective studies on late outcomes have shown that more than 80% of affected hips have good or very good outcomes that persist into the fourth decade of life (7, 13). Nonetheless, a retrospective study by McAndrew et al. revealed that half of all patients eventually required an artificial hip after a median follow-up interval of 50 years (13).
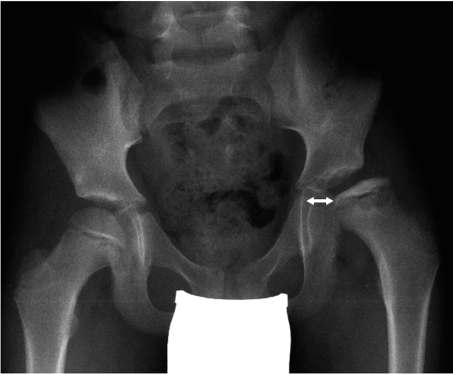
Important prognostic factors include age, limitation of movement, radiologically visible involvement of the femoral head, and any accompanying "head-at-risk signs." Typical "head-at-risk signs" include lateralization of the femoral head in the acetabulum (subluxation), lateral epiphyseal calcification, and metaphyseal cyst formation (figure 2).
Figure 2.
Involvement of the entire epiphysis, with the height of the lateral pillar less than half normal (Herring type C, Catterall type IV). In addition, note lateralization (arrow) and metaphyseal cysts, both of which are "head-at-risk" signs.
Young age at onset of the disease (i.e., below age 6) is prognostically favorable because of the higher remodeling potential. Hip joints classified as Catterall types III and IV and Herring type C, as well as those in which "head-at-risk signs" are present, are prognostically unfavorable (4, 5). If a subluxed femoral head has also developed a lateral outgrowth that impinges on the acetabular margin (the "hinge abduction" phenomenon), this, too, is a prognostically unfavorable sign.
The deformity and incongruence of the hip joint that are present when the patient reaches adulthood are important long-term prognostic factors (14, 15). The Stulberg classification of the final stage of the disease divides patients into subtypes and thereby enables better assessment of the prognosis (15). Even if an affected child or adolescent is still asymptomatic, an assessment of the prognosis is important for counseling (e.g., with respect to participation in sports or choice of occupation).
Treatment
The goal of all forms of treatment is to prevent deformity of the femoral head and incongruence of the affected hip. The extent of incongruence in adolescence determines the severity of the prearthrotic deformity, and thus also the probability of early secondary coxarthrosis.
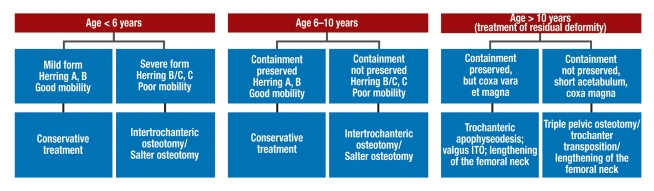
In order to achieve these goals, treatment is based on the principle of containment, i.e., the maintenance or restoration of the central position of the femoral head. The type of treatment that is to be provided is determined on the basis of the radiological severity of the disease, the presence or absence of "head-at-risk signs," the xtent of limitation of mobility of the hip joint, and the age of the patient (figure 3).
Figure 3.
Treatment algorithm for Perthes disease, depending on the patient’s age and risk factors
In the past, attempts were made to prevent deformity of the femoral head by prolonged mechanical unloading and immobilization of the hip joint. Thus, the hip was immobilized in a cast, with prolonged bed rest, with crutches for walking, or with cumbersome orthoses and ambulating devices, for the entire duration of the illness.
Intra-articular pressure measurements, however, have revealed that immobilization in an orthosis can actually elevate the intra-articular pressure. Furthermore, long-term immobilization has considerable negative consequences, including muscle atrophy, contractures, weight gain, and social exclusion. It has thus been largely abandoned in favor of functional physiotherapeutic measures and, in cases with severe progression, operative containment therapy (2, 16).
A poll taken by our department in Ulm to assess the current state of care for Perthes disease in the German-speaking countries has revealed that the use of weight-relieving orthoses has declined markedly in comparison to earlier treatment recommendations. 70% of the hospital physicians questioned stated that they seldom, if ever, used such orthoses (17).
Strict mechanical unloading of the affected hip joint is not necessary, nor should the patient be strictly forbidden to participate in sports. There is no objection to lighter sporting activities such as swimming and cycling, but extreme stresses, such as can arise in types of sport involving jumping and/or physical contact, should be avoided (4).
Depending on the patient’s age and the severity of disease, treatment may at first consist of no more than mechanical stress reduction and further observation. This is usually possible only for children under age 6 who have a good range of motion of the hip (18).
Initial clinical trials, involving only a small number of patients, have shown good results from the additional (off-label) use of vasoactive prostacycline analogues in the early phase of the disease, when the x-rays are still normal, with improved range of motion, diminished pain, and revascularization of the epiphysis. Long-term results are still pending (19).
The range of motion of the hip is usually already restricted at the time of diagnosis, however, and the reduction of mechanical stress must therefore be combined with physiotherapeutic mobilization so that the range of motion can be maintained or, if possible, improved. A regular program of physiotherapy is needed to optimize mobility (Box 2).
Box 2.
The goals of treatment for Perthes disease
Improved mobility
Reduction of mechanical stress
Preservation of joint congruence (containment)
The treatment of pain is important mainly in the initial phase, in which acute inflammation is present. Until the acute pain subsides, the joint should be moved as little as possible, and a non-steroidal anti-inflammatory drug such as ibuprofen should be given. Analgesic medication is of no use in the long-term treatment of the disease.
If contracture of the adductor musculature is also present, treatment with botulinum toxin, combined with intensive physiotherapy, may increase the range of motion in abduction and thereby improve containment.
During the entire course of treatment, the foremost treatment goal is the attention of free mobility of the hip in all directions, and especially free rotation and abduction, with maintenance of the central position of the femoral head in the acetabulum ("motion and containment") (2).
If the disease takes an unfavorable course, or if conservative therapy fails, a number of operative methods of improving containment may be indicated.
Good range of motion of the hip, with at least 30° of abduction, is a prerequisite for the success of operative containment therapy. Any greater restriction of hip mobility is considered to contraindicate surgery (5).
Ideally, the child should be in the early phase of the disease (the fragmentation or early repair phase) at the time of surgery, so that the femoral head will still possess the remodeling potential that is present in this phase.
Herring et al. showed in their prospective, multicenter trial (level II-1) that patients who first manifest the disease after age 8, with a Herring type B or B/C involvement of the femoral head, can benefit from operative containment therapy (20).
Wiig et al., in their report of a current collective study from Norway (level II-1), recommend intertrochanteric varus osteotomy in children above age 6 with more than 50% involvement of the femoral head (21).
An osteotomy in the late stage of the disease (age 10 or older), however, yields markedly worse results (22).
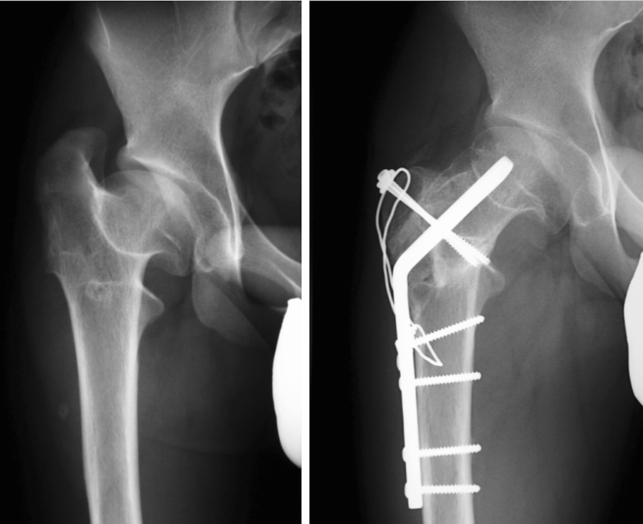
Typically, surgery to improve containment involves an intertrochanteric varus osteotomy. The CCD angle should be reduced to the extent that is necessary to reposition the femoral head in the center of the acetabulum (figure 4), yet not so much as to produce an overcorrection, with a high-standing trochanter and coxa vara. Thus, the CCD angle should not be reduced below 105° (5).
Figure 4.
Restoration of containment by means of varus osteotomy (left) or Salter’s pelvic osteotomy (right), after "hinge abduction" has been ruled out intraoperatively
If intertrochanteric varus osteotomy alone does not produce adequate containment, a pelvic osteotomy is performed in the same sitting.
Alternatively, centralization can also be restored with a Salter pelvic osteotomy alone (figure 4) or with a triple pelvic osteotomy. With these techniques, the leg shortening and high-standing trochanter that often follow varus osteotomy can be avoided.
Before surgery is performed, the so-called hinge abduction phenomenon must be ruled out. Hinge abduction is assumed to be present when the femoral head is lateralized by more than 2 mm on the abduction x-ray (5).
If the range of motion of the hip is restricted, an adequate range of abduction must be achieved before surgery. It may be necessary for the patient to be hospitalized for intensive physiotherapy, intermittent traction treatment, and supportive therapy with anti-inflammatory drugs; sometimes, adductor tenotomy will be necessary. In case of doubt, hinge abduction can also be demonstrated by intraoperative arthrography (e9).
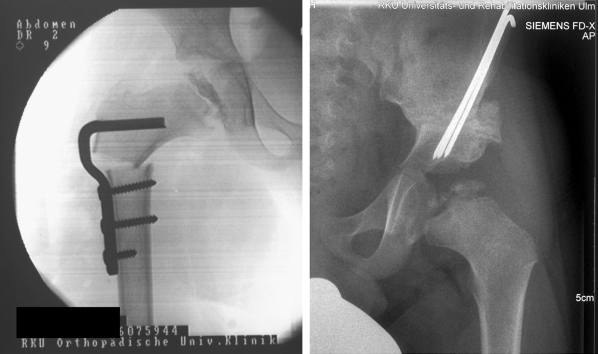
The disturbed growth of the epiphysis of the femoral head can lead to coxa vara et magna in the late stage of the disease, with relative shortening of the femoral neck and a high-standing trochanter. In such cases, the indication for surgical lengthening of the femoral neck (figure 5) or a valgus osteotomy should be evaluated, so that leg shortening and muscular insufficiency, if present, can be treated, and the optimal anatomy and function of the proximal femur can be restored (23, 24, e10).
Figure 5.
Treatment of a short femoral neck and high trochanter with osteotomy and lengthening of the femoral neck
If the growth plate is still open, the physician should assess whether the growth of the proximal femur might be benefited by a trochanteric apophyseodesis (25).
Overview
As the course of Perthes disease is variable, the appropriate treatment must be determined individually for each patient. The therapeutic spectrum ranges from observation and follow-up all the way to extensive surgical reconstructions of the hip. The goal of all treatments is to prevent deformation of the femoral head, in order to avert a prearthrotic deformity and early coxarthrosis.
The main goals of treatment are, therefore, the restoration of the central position of the femoral head in the acetabulum together with a reduction of mechanical stress and improvement of the restricted range of motion. For adolescent patients, the indication for surgical treatment of the residual deformity of the proximal femur should be assessed. The disease should be treated by a board-certified orthopedist with experience in pediatric orthopedic surgery.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors state that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Loder RT, Schwartz EM, Hensinger RN. Behavioral characteristics of children with Legg-Calvé-Perthes disease. J Pediatr Orthop. 1993;13:598–601. [PubMed] [Google Scholar]
- 2.Krauspe R, Raab P. Morbus Perthes. Orthopäde. 1997;26:289–302. [PubMed] [Google Scholar]
- 3.Eggl H, Drekonja T, Kaiser B, Dorn U. Ultrasonography in the diagnosis of transient synovitis of the hip and Legg-Calvé-Perthes disease. J Pediatr Orthop B. 1999;8(3):177–180. doi: 10.1097/01202412-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Hefti F. Kinderorthopädie in der Praxis. 2. Aufl. Berlin, Heidelberg, New York: Springer; 2006. [Google Scholar]
- 5.Thompson GH, Price CT, Roy D, Meehan PL, Richards BS. Legg-Calvé-Perthes disease AAOS. In: Birch JG, editor. Instructional Course Lectures. Rosemont: American Academy of Orthopedic Surgeons; 2006. pp. 27–44. [Google Scholar]
- 6.Waldenström H. The defintive forms of coxa plana. Act Radiol. 1922;1 [Google Scholar]
- 7.Catterall A. The natural history of Perthes disease. J Bone Joint Surg Br. 1971;53:37–53. [PubMed] [Google Scholar]
- 8.Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop. 1992;12:143–150. doi: 10.1097/01241398-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Herring JA. Current Concepts Review: The treatment of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1994;76:448–458. doi: 10.2106/00004623-199403000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Ismail AM, Macnicol MF. Prognosis in Perthes’ disease: a comparison of radiological predictors. J Bone Joint Surg Br. 1998;80:310–314. doi: 10.1302/0301-620x.80b2.8054. [DOI] [PubMed] [Google Scholar]
- 11.Gigante C, Frizziero P, Turra S. Prognostic value of Catterall and Herring classification in Legg-Calvé-Perthes disease: follow-up to skeletal maturity of 32 patients. J Pediatr Orthop. 2002;22:345–349. [PubMed] [Google Scholar]
- 12.Herring JA, Kim HT, Browne R. Part I: Classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am. 2004;86:2103–2120. [PubMed] [Google Scholar]
- 13.McAndrew MP, Weinstein SL. A long-term follow-up of Legg-Perthes-Calvé disease. J Bone Joint Surg Am. 1984;66:860–869. doi: 10.2106/00004623-198466060-00006. [DOI] [PubMed] [Google Scholar]
- 14.Niethard FU. Kinderorthopädie. Stuttgart: Thieme Verlag; 1997. [Google Scholar]
- 15.Stulberg SD, Cooperman DR, Wallenstein R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1981;63:1095–1108. [PubMed] [Google Scholar]
- 16.Wild A, Westhoff B, Raab P, Krauspe R. Die nichtoperative Therapie des Morbus Perthes. Orthopäde. 2003;32:139–145. doi: 10.1007/s00132-002-0429-3. [DOI] [PubMed] [Google Scholar]
- 17.Lagos R, Nelitz M, Günther KP. Diagnostik und Therapie des Morbus Perthes: Ergebnisse einer Befragung zur Versorungssituation im deutschsprachigen Raum. Z Orthop Ihre Grenzgeb. 2003;141 [Google Scholar]
- 18.Canavese F, Dimeglio A. Perthes disease. Prognosis in children under six years of age. J Bone Joint Surg Br. 2008;90:940–945. doi: 10.1302/0301-620X.90B7.20691. [DOI] [PubMed] [Google Scholar]
- 19.Suda R, Petje G, Radler C, Ganger R, Grill F. Osteonekrotische Erkrankungen in der Pädiatrie. J Miner Stoffwechs. 2007;14:27–31. [Google Scholar]
- 20.Herring JA, Kim HT, Browne R. Part I: Legg-Calvé-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86:2121–2134. [PubMed] [Google Scholar]
- 21.Wiig O, Terjesen T, Svenningsen S. Prognostic factors and outcome of treatment in Perthes’ disease: a prospective study of 368 patients with five year follow-up. J Bone Joint Surg Br. 2008;90:1364–1371. doi: 10.1302/0301-620X.90B10.20649. [DOI] [PubMed] [Google Scholar]
- 22.Noonan KJ, Price CT, Kupiszewski SJ, Pyevich M. Results of femoral varus osteotomy in children older than 9 years of age with Perthes disease. J Pediatr Orthop. 2001;21:198–204. [PubMed] [Google Scholar]
- 23.Bankes MJK, Catterall A, Hashemi-Nejad A. Valgus extension osteotomy for hinge abduction’ in Perthes’ disease. J Bone Joint Surg Br. 2000;82:548–554. doi: 10.1302/0301-620x.82b4.10339. [DOI] [PubMed] [Google Scholar]
- 24.Wenger DR, Kishan S, Pring ME. Impingement and childhood hip disease impingement. J Pediatr Orthop B. 2006;15:233–243. doi: 10.1097/01202412-200607000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Schneidmüller D, Carstens C, Thomsen M. Surgical treatment of overgrowth of the greater trochanter in children and adolescents. J Pediatr Orthop. 2006;26:486–490. doi: 10.1097/01.bpo.0000226281.01202.94. [DOI] [PubMed] [Google Scholar]
- e1.Atsumi T, Yamano K, Muraki M, Yoshihara S, Kajihara T. The blood supply of the lateral epiphyseal arteries in Perthes’ disease. J Bone Joint Surg Br. 2000;82:392–398. doi: 10.1302/0301-620x.82b3.10193. [DOI] [PubMed] [Google Scholar]
- e2.Gallistl S, Reitinger T, Linhart W, Muntean W. The role of inherited thrombotic disorders in the etiology of Legg-Calvé-Perthes Disease. J Pediatr Orthop. 1999;19:82–83. [PubMed] [Google Scholar]
- e3.Kristmundsdottir F, Burwell RG, Harrison MH. Delayed skeletal maturation in Perthes’ disease. Acta Orthop Scand. 1987;58:277–279. doi: 10.3109/17453678709146484. [DOI] [PubMed] [Google Scholar]
- e4.Westhoff B, Krauspe R, Kalke AE, Hermsen D, Kowall B, Willers R, Schneider U. Urinary excretion of deoxypyridinoline in Perthes’ disease: a prospective, controlled comparative study in 83 children. J Bone Joint Surg Br. 2006;88:967–971. doi: 10.1302/0301-620X.88B7.16564. [DOI] [PubMed] [Google Scholar]
- e5.Christensen F, Séballe K, Ejsted R. The Catterall classification of Perthes disease: an assessment of reliability. J Bone Joint Surg Br. 1978;68:614–615. doi: 10.1302/0301-620X.68B4.3733840. [DOI] [PubMed] [Google Scholar]
- e6.Hardcastle PH, Ross R, Hamalainen M, Mata A. Catterall grouping of Perthes’ disease. An assessment of observer error and prognosis using the Catterall classification. J Bone Joint Surg Br 1. 980;62:428–431. doi: 10.1302/0301-620X.62B4.7430217. [DOI] [PubMed] [Google Scholar]
- e7.Sambandam Nathan S, Gul A, Shankar R, Goni V. Reliability of radiological classifications used in Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2006;15:267–270. doi: 10.1097/01202412-200607000-00006. [DOI] [PubMed] [Google Scholar]
- e8.Salter RB, Thompson GH. Legg-Calvé-Perthes disease. The prognostic significance of the subchondral fracture and a two-group classification of the femoral head involvement. J Bone Joint Surg Am. 1984;66:479–489. [PubMed] [Google Scholar]
- e9.Bennett JT, Stuecker R, Smith E, Winder C, Rice J. Arthrographic findings in Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2002;11:110–116. doi: 10.1097/00009957-200204000-00005. [DOI] [PubMed] [Google Scholar]
- e10.Patil S, Sherlock D. Valgus osteotomy for hinge abduction in avascular necrosis. J Pediatr Orthop B. 2006;15:262–266. doi: 10.1097/01202412-200607000-00005. [DOI] [PubMed] [Google Scholar]