Abstract
Rationale
The recent emergence of hydrogen sulfide (H2S) as a potent cardioprotective signaling molecule necessitates the elucidation of its cytoprotective mechanisms.
Objective
The current study evaluated potential mechanisms of H2S-mediated cardioprotection using an in vivo model of pharmacological preconditioning (PC).
Methods and Results
H2S (100 μg/kg), or vehicle was administered to mice via an intravenous injection 24 hr prior to myocardial ischemia. Treated and untreated mice were then subjected to 45 min of myocardial ischemia followed by reperfusion for up to 24 hr, during which time the extent of myocardial infarction was evaluated, circulating troponin-I levels were measured, and the degree of oxidative stress was evaluated. In separate studies myocardial tissue was collected from treated and untreated mice during the early (30 min and 2hr) and late (24 hr) PC periods to evaluate potential cellular targets of H2S. Initial studies revealed that H2S provided profound protection against ischemic injury as evidenced by significant decreases in infarct size, circulating troponin-I levels, and oxidative stress. During the early PC period H2S increased the nuclear localization of Nrf2, a transcription factor that regulates the gene expression of a number of antioxidants, and increased the phosphorylation of PKCε and STAT-3. During the late PC period, H2S increased the expression of antioxidants (HO-1 and Trx1), increased the expression of HSP90, HSP70, Bcl-2, Bcl-xL, and COX-2 and also inactivated the pro-apoptogen Bad.
Conclusions
These results reveal that the cardioprotective effects of H2S are mediated in large part by a combination of antioxidant and anti-apoptotic signaling.
Keywords: hydrogen sulfide, cardioprotection, antioxidant signaling, myocardial infarction, Nrf2
Introduction
Hydrogen sulfide (H2S) is an endogenously produced gaseous signaling molecule with a diverse physiological profile. Its production in mammalian systems has been attributed to two key enzymes in the cysteine biosynthesis pathway, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL). The rate of H2S production in tissue homogenates is in the range of 1–10 pmoles per second per mg protein, resulting in low micromolar extracellular concentrations.1,2 It is at these physiological concentrations that H2S is cytoprotective in various models of cellular injury.3,4 The reported cytoprotective effects of H2S are partially related to its ability to neutralize reactive oxygen species (ROS), to inhibit leukocyte-endothelial cell interactions, to promote vascular smooth muscle relaxation, to reduce apoptotic signaling, and to reversibly modulate mitochondrial respiration.5 Pretreatment with NaHS has been reported to reduce the number and duration of arrhythmias in isolated hearts subjected to global ischemia-reperfusion (I/R)6 and to enhanced the viability of isolated rat ventricular myocytes exposed to glucose deprivation and 2-deoxy-glucose.4 Recently, Elrod et al7 reported that the administration of H2S at the time of reperfusion decreased infarct size and preserved left ventricular function in an in vivo model of myocardial I/R. Additional findings from this study also demonstrated that cardiac-specific overexpression of CGL likewise limited the extent of myocardial I/R injury. The findings of these studies and others suggest that H2S is cytoprotective during myocardial I/R injury and that either direct H2S administration or the modulation of endogenous H2S production may be of clinical importance.
Although the physiological and cardioprotective effects of H2S have previously been documented, the signaling mechanisms that mediate these effects have not been fully evaluated. Moreover, the signaling mechanisms that have been attributed to the cardioprotective effects of H2S have predominantly been studied in in vitro model systems with very few studies actually exploring the protective effects in in vivo systems. Therefore, the purpose of the current study was to evaluate signaling mechanisms triggered by H2S treatment using an in vivo model of pharmacological preconditioning (PC). This model was chosen as a model for which early and delayed cellular targets responsible for H2S-mediated cardioprotection could be identified. Additionally, a cardiac-specific transgenic mouse that overexpresses CGL was utilized to evaluate signaling mechanisms mediated by endogenous H2S.
Material and Methods
An expanded Materials and Methods section is available in the online data supplement
Animals
Male C57BL6/J mice, 8–10 weeks of age, were utilized (Jackson Labs, Bar Harbor, ME). The generation of cardiac-specific transgenic mice overexpressing CGL (αMHC-CGL-Tg; FVB background)7, as well as the generation of Nrf2 deficient mice (Nrf2 KO; ICR background)8 has been described previously. All experimental mouse procedures were approved by the Institute for Animal Care and Use Committee at Albert Einstein College of Medicine and Emory University and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86-23, Revised 1996) and with federal and state regulations.
Materials
H2S was administered in the form of sodium sulfide (Na2S). Na2S was produced by Ikaria Holdings Inc. (Seattle, WA) using H2S gas (Matheson, Newark, CA) as a starting material and was formulated to pH neutrality, and iso-osmolarity. Na2S (stock solution at 0.55 mg/ml and 7.1 mM) was diluted in normal (0.9%) saline to the desired concentration in a rapid fashion, immediately before administration For all experiments, normal saline (100 μL) or Na2S (100 μg/kg) in a final volume of 100 μL was injected intravenously into the femoral vein using a 32-gauge needle. For this study, H2S denotes Na2S.
Myocardial Ischemia-Reperfusion (I/R) Protocol and Myocardial Injury Assessment
Surgical ligation of the left coronary artery (LCA), myocardial infarct size determination, Troponin-I measurements and LV echocardiography was performed similar to methods described previously.9
Gluthathione and Lipid hydroperoxide assays
Cardiac glutathione and lipid hydroperoxides were measured in heart tissue collected from mice subjected to 45 min of myocardial ischemia and 1, 4, or 24 hr of reperfusion using commercially available kits (Cayman Chemicals) as previously described.3 GSH and GSSG values were used to calculate the steady-state redox potential using the Nernst equation, as described previously.10
Tissue Collection for Western Blot Analysis
For the evaluation of cellular targets during early and late phase preconditioning, mice were administered H2S as described above and then sacrificed 30 min, 2 hr, and 24 hr after the injection. The hearts were rapidly excised and the LV was isolated and snap frozen in liquid nitrogen. The samples were then stored at −80°C. In a separate group of mice, heart samples were obtained following 45 min of myocardial ischemia and 4 hr of reperfusion.
Western Blot Analysis
Whole cell, cytosolic, membranous, nuclear, and mitochondrial fractions were prepared as described previously.11 Equal amounts of protein were loaded into lanes of polyacrylamide-SDS gels and Western blot analysis was performed as previously described.9
TUNEL Staining
Following 45 min of ischemia and 4 hr of reperfusion hearts from sham, vehicle, and H2S PC treated mice were rapidly excised, cross-sectioned into 3 sections and fixed in 10% buffered formalin. Fixed tissue was then paraffin embedded and sectioned in a standard fashion. TUNEL staining was conducted using a kit according to the manufacturer's instructions (ApopTag HRP kit, DBA). The number of TUNEL-positive nuclei and the total number of nuclei per high-powered field were counted in a minimum of 10 fields from the area-at-risk portion of the myocardium for each section (a total of 30 fields per heart).
Statistical Analysis
All data in this study are expressed as mean ± standard error (SEM). Differences in data between the groups were compared using Prism 4 (GraphPad Software, Inc) with Student's paired 2-tailed t test, one-way analysis of variance (ANOVA) with post hoc Tukey test, or two-way ANOVA with post hoc Bonferroni analysis where appropriate. A p value less than 0.05 was considered significant.
Results
H2S PC limited the extent of myocardial injury following ischemia-reperfusion
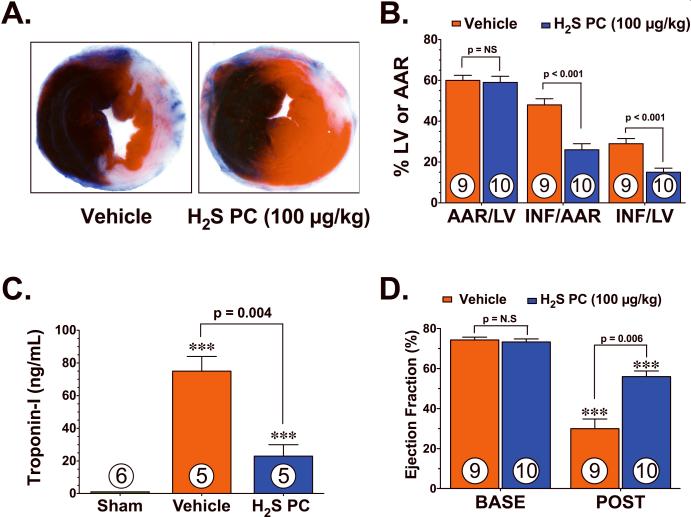
Initial experiments were conducted to investigate if H2S PC could limit myocardial I/R injury, For these experiments, mice were subjected to 45 min of LCA ischemia followed by 24 hr of reperfusion. H2S (Na2S; 100 μg/kg) or vehicle was administered 24 hr prior to ischemia via an intravenous injection. The extent of myocardial infarction was then evaluated at 24 hr of reperfusion. Representative photomicrographs of mid-ventricular cross sections of TTC-stained hearts taken from vehicle and H2S PC treated mice are shown in Figure 1A. The area-at-risk (AAR) per LV was similar (p=not significant, NS) in all of the groups (Figure 1B). H2S PC decreased the infarct (Inf) relative to the AAR (Inf/AAR) by 46% (48±3 vs. 26±3%, p<0.001) and the Inf relative to the entire LV (Inf/LV) by 48% (29±2.5 vs. 15±2%, p<0.001) when compared to vehicle treated mice. Circulating levels of troponin-I were evaluated as an additional marker of myocardial injury at 24 hr of reperfusion (Figure 1C). Following myocardial I/R, circulating levels of troponin-I rose from undetectable levels in sham operated mice to 75±9 ng/mL in the vehicle treated group (p<0.001 vs. Sham). H2S PC significantly (p=0.026) attenuated the rise in circulating troponin-I by 69% (75±9 vs. 23±7 ng/mL), thus confirming the cardioprotective effects of H2S PC.
Figure 1.
Hydrogen sulfide preconditioning (H2S PC) reduced the extent of injury and improved left ventricular function in mice following myocardial ischemia and reperfusion. (A) Representative mid-ventricular photomicrographs of hearts treated with vehicle and H2S. (B) Area-at-risk (AAR) with respect to the left ventricle (LV) was similar between all groups. H2S PC significantly attenuated myocardial infarct size with respect to the area-at-risk (Inf/AAR) and with respective to the LV (Inf/LV). (C) Circulating levels of troponin-I (ng/mL) were measured 24 hr after reperfusion. (D) Ejection fraction (%) was calculated in separate groups of mice using high-resolution, two-dimensional B-mode echocardiography images at baseline (BASE) and 7 days following myocardial ischemia. Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. ***p<0.001 vs. Sham or BASE. H2S denotes Na2S.
The effects of H2S PC on LV structure and function following myocardial I/R were evaluated in separate groups of mice using in vivo transthoracic echocardiography. For these experiments, mice were subjected to 45 min of myocardial ischemia and 7 days of reperfusion. Myocardial I/R increased left ventricular end-diastolic diameter (LVEDD, p=NS) and LV end-systolic diameter (LVESD, p<0.05) in both groups compared to their respective baseline (BASE) readings (Online Figure). However, the increase in LVEDD (p=0.12 vs. I/R+Veh) and LVESD (p=0.03 vs. I/R+Veh) was attenuated in mice pretreated with H2S. Following myocardial I/R, LV ejection fraction (Figure 1D) decreased in both groups (p<0.001 vs. BASE). H2S PC did, however, significantly improve ejection fraction by 87% (p=0.006 vs. I/R+Veh). Together, these results suggest that H2S PC limits the extent of damage to the myocardium following I/R injury.
H2S PC reduced oxidative stress and apoptosis following ischemia-reperfusion
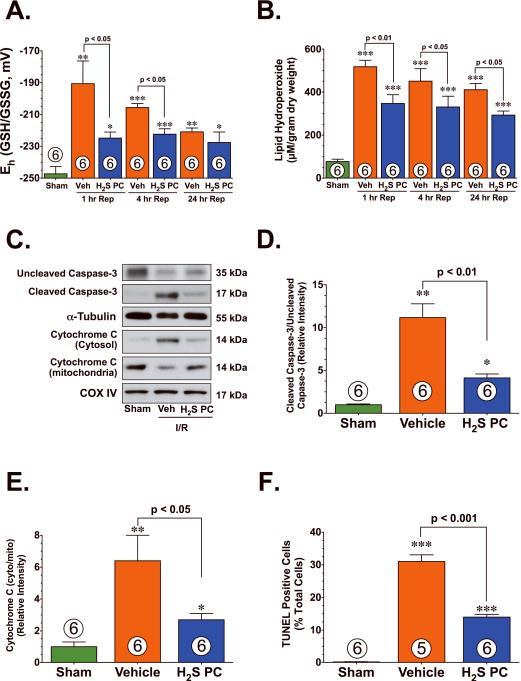
There is considerable evidence that implicates the production of ROS as an initial cause of injury to the myocardium following I/R.12 To evaluate the effects of H2S PC on cellular oxidative stress, lipid hydroperoxide levels were measured in tissue isolated from the hearts of vehicle and H2S PC treated mice. In response to myocardial I/R injury, the redox potential (Figure 2A, Eh) significantly increased in the hearts of vehicle treated mice. This change in redox potential was accompanied by a significant rise in lipid hydroperoxide levels (Figure 2B, p<0.001 vs. Sham). In contrast, the redox potential was preserved in the H2S PC treated hearts (p<0.05 vs. I/R+Veh) and a significant less rise in lipid hydroperoxide levels was also evident (p<0.05 vs. I/R+Veh). To investigate the effects of H2S PC on apoptosis, the expression of uncleaved caspase-3, cleaved caspase-3 and cytochrome C, as well as the extent of TUNEL-positive staining was evaluated after 45 min of LCA and 4 hr of reperfusion in heart samples from sham, vehicle, and H2S PC-treated mice (Figure 2C–F). Myocardial I/R induced a significant decrease in the expression of uncleaved caspase-3 and induced a significant increase in the expression of cleaved caspase-3 as well as induced the translocation of cytochrome C from the mitochondria to the cytosol in the hearts of vehicle-treated mice (p<0.01 vs. Sham). This was accompanied by a significant increase in the number of TUNEL-positive nuclei (p<0.001 vs. Sham). In contrast, the hearts of H2S PC-treated mice when compared to vehicle treated hearts exhibited a significant preservation of uncleaved caspase-3, a significant reduction in cleaved caspase-3 (p<0.01 vs. I/R+Veh), a significant reduction in the translocation of cytochrome C (p<0.05 vs. I/R+Veh), and fewer TUNEL-positive nuclei (p<0.001 vs. I/R+Veh).
Figure 2.
H2S PC reduced oxidative stress and apoptotic cell death following myocardial ischemia and reperfusion. Cardiac (A) redox state (Eh) for GSH and GSSG and (B) lipid hydroperoxide levels (μM) from Sham controls, Vehicle (Veh), and H2S PC treated mice at 1–24 hr of reperfusion following myocardial ischemia. (C) Representative immunoblots of uncleaved caspase-3, cleaved caspase-3, cytosolic cytochrome C, and mitochondrial cytochrome C from the hearts of Sham controls, Veh, and H2S PC treated mice at 4 hr of reperfusion following myocardial ischemia. (D) Ratio of cleaved caspase-3 to uncleaved caspase-3, (E) ratio of cytosolic cytochrome C to mitochondrial cytochrome C, and (F) the number of TUNEL positive cells (% of total nuclei) from the hearts of Sham controls, Veh, and H2S PC treated mice at 4 hr of reperfusion following myocardial ischemia. Values are means ± S.E.M. Numbers inside bars indicate the number of animals that were investigated in each group. *p<0.05, **p<0.01, ***p<0.001 vs. Sham.
H2S increased the nuclear accumulation of Nrf2 and increased the protein expression of Trx1 and HO-1
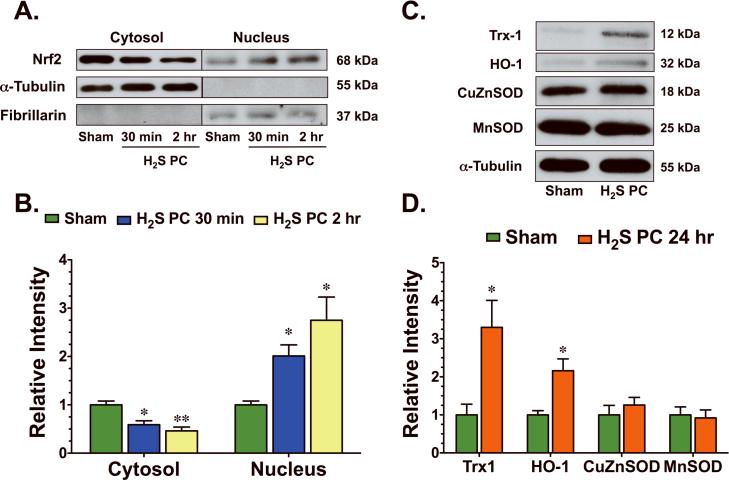
Nuclear-factor-E2-related factor (Nrf2) is a key transcription factor that regulates antioxidant genes as an adaptive response to oxidative stress or pharmacological stimuli. To investigate if H2S induced Nrf2 signaling, Na2S was administered to mice via an intravenous injection and heart tissue was excised at different times following this administration. As early as 30 min following the administration of H2S, Nrf2 accumulated in the nucleus of cardiac tissue and remained at an elevated level for at least 2 hr (Figure 3A–B). Subsequently, the protein expression (Figure 3C–D) of two downstream targets of Nrf2, Thioredoxin-1 (Trx1) and heme oxygenase-1 (HO-1), were elevated 24 hr following the administration H2S. H2S did not increase the protein expression of either CuZnSOD or MnSOD (Figure 3C–D) at this latter time point.
Figure 3.
H2S upregulated cellular antioxidant defenses. (A) Representative immunoblots and (B) densitometric analysis of cardiac Nrf2 in the cytosolic and nuclear fractions 30 min and 2 hr following the administration of H2S. (C) Representative immunoblots and (D) densitometric analysis of cardiac thioredoxin-1 (Trx1), heme oxygenase-1 (HO-1), copper zinc superoxide dismutase (CuZnSOD), and manganese SOD (MnSOD) 24 hr following the administration of H2S. Values are means ± S.E.M. for an n of 4–5 animals for each group. *p<0.05, **p<0.01 vs. Sham.
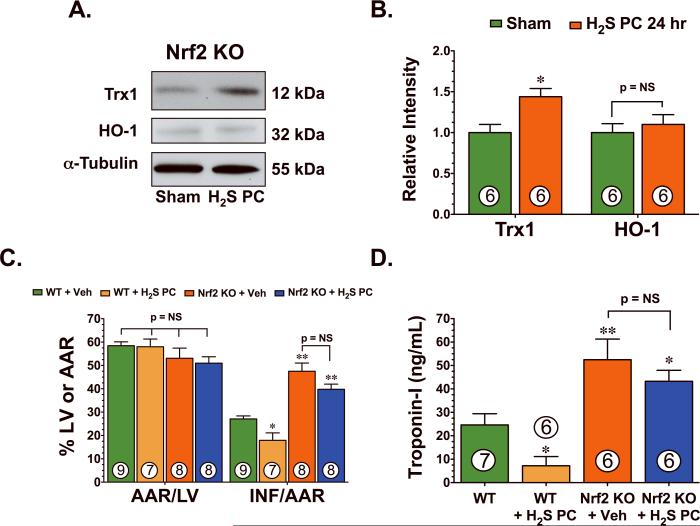
To determine if H2S upregulated HO-1 and Trx1 in an Nrf2-dependent manner, subsequent experiments were performed with mice deficient in Nrf2 (Nrf2 KO). For these experiments, H2S was administered as noted above and heart tissue was excised 24 hr later (Figure 4A–B). H2S slightly increased the expression of Trx1 (p<0.05 vs Sham), but failed to increase the expression of HO-1 in the hearts of Nrf2 KO mice. Subsequent experiments were then conducted to determine if Nrf2 was critical for the cardioprotective actions of H2S PC. For these experiments, H2S (Na2S; 100 μg/kg) or vehicle was administered 24 hr prior to ischemia via an intravenous injection to Nrf2 KO mice and Wild-Type littermates. H2S PC reduced Inf/AAR and reduced circulating troponin-I levels when it was administered to Wild-Type mice. Myocardial injury following I/R was found to be exacerbated in Nrf2 KO mice compared with Wild-Type mice (Figure 4C–D), as evidenced by an increase in Inf/AAR (44±5 vs. 25±2, p<0.01) and circulating troponin-I levels (52.4±9.0 vs. 26±5.2, p<0.01). H2S failed to provide protection in the Nrf2 KO mice, suggesting that Nrf2 plays a role in the cardioprotection actions mediated by H2S. The smaller infarct size reported here for the Wild-Type mice in these experiments as compared to the size reported in Figure 1 is indicative of the background strain of these mice (ICR), as past studies have reported the different susceptibility of various strains of mice to myocardial injury.9,13
Figure 4.
Nrf2 mediates the cardioprotective effects of H2S. (A) Representative immunoblots and (B) densitometric analysis of cardiac Trx1 and HO-1 from the hearts of Nrf2 deficient (Nrf2 KO) mice ± H2S. (C) Myocardial infarct size and (D) circulating levels of troponin-I were measured 24 hr after LCA ischemia in Wild-Type (WT) and Nrf2 KO mice receiving either vehicle or H2S PC (100 μg/kg) treatment. Nrf2 KO mice experienced exacerbated myocardial injury when compared to wild-type mice. However, no differences in myocardial infarct size or circulating troponin-I levels were observed in Nrf2 KO following H2S PC treatment. Numbers inside bars indicate the number of animals that were investigated in each group. *p<0.05, **p<0.01 vs. Sham or WT.
H2S activated a PKCε-p44/42-STAT-3 pro-survival signaling pathway
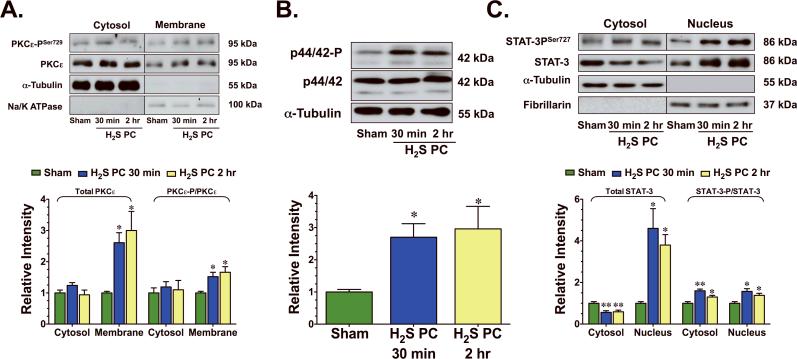
H2S has been reported to induce PKC activation in isolated rat cardiomyocytes.14 To investigate if H2S induced PKCε activation in vivo, the translocation of PKCε from the cytosolic fraction to the membranous fraction was evaluated after a single administration of H2S. A marked increase in the phosphorylated form of PKCε at serine residue 729 (PKCε–PSer729) was evident in the membranous fraction (p<0.05 vs. Sham, Figure 5A) 30 min and 2 hr following the administration of H2S. The total membrane levels of PKCε were also increased during this time period (p<0.05 vs. Sham,), indicating translocation of PKCε to the membrane. In addition, H2S increased the phosphorylation of p44/42, a downstream effector of PKCε (p<0.05 vs. Sham, Figure 5B). H2S did not change the total levels of PKCε or alter the phosphorylation state PKCε in the cytosolic fraction and did not change the total levels of p44/42.
Figure 5.
H2S activated PKCε-p/44/42-STAT-3 signaling. Representative immunoblots and densitometric analysis of (A) phosphorylated PKCε at serine residue 729 (PKCεSer729) and total PKCε (cytosolic and membranous fractions), (B) phosphorylated p44/42 and total p44/42 (cytosolic fraction), and (C) phosphorylated STAT-3 at serine residue 727 (STAT-3Ser727) and total STAT-3 (cytosolic and nuclear fractions) 30 min and 2 hr following the administration of H2S. Values are means ± S.E.M. for an n of 4–5 animals for each group. *p<0.05, **p<0.01 vs. Sham.
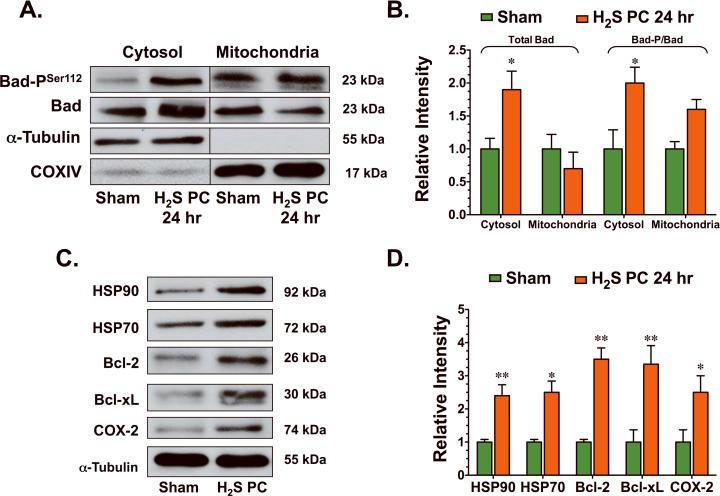
The activation of pro-survival kinases, such as PKCε and p44/42, has been demonstrated to confer cardioprotection through an upregulation of anti-apoptotic signaling mediated in part by signal transducers and activators of transcription-3 (STAT-3).15 In the present study, H2S increased the translocation of STAT-3 to the nucleus (Figure 5C), as evidenced by a decrease in the total cytosolic levels of STAT-3 (p<0.01 vs. Sham) and an increase in the total nuclear levels of STAT-3 (p<0.05 vs. Sham) from 30 min to 2 hr after its administration. A marked increase in the phosphorylated form of STAT-3 (p<0.01 and p<0.05 vs. Sham) at serine residue 727 (STAT-3PSer727) was also evident in both the cytosolic fraction and nuclear fraction of H2S treated hearts during this time period. H2S also increased the phosphorylation of Bad (Figure 6A–B) at serine residue 112 (Bad-PSer112) in both the mitochondrial (p=NS) and the cytosolic fraction (p<0.05 vs. Sham) 24 hr after its administration. As a result of this phosphorylation, H2S induced the translocation of Bad from the mitochondria to the cytosol, as evidenced by a significant increase in the total cytosolic levels of Bad (p<0.05 vs. Sham). Further analysis revealed that H2S increased the protein expression of HSP90, HSP70, Bcl-2, Bcl-xL, and cyclooxygenase-2 (COX-2) (Figure 6C–D, p<0.05 and p<0.01 vs. Sham) 24 hr after its administration. These data suggest that in addition to promoting antioxidants, H2S also increased anti-apoptogens.
Figure 6.
H2S increased the expression of anti-apoptogens. (A–B) Representative immunoblots and densitometric analysis of phosphorylated Bad at serine residue 112 (Bad-PSer112) and total Bad (cytosolic and mitochondrial fractions) and (C–D) HSP90, HSP70, Bcl-2, Bcl-xL, and COX-2 24 hr following the administration of H2S. Values are means ± S.E.M. for an n of 4–5 animals for each group. *p<0.05, **p<0.01 vs. Sham.
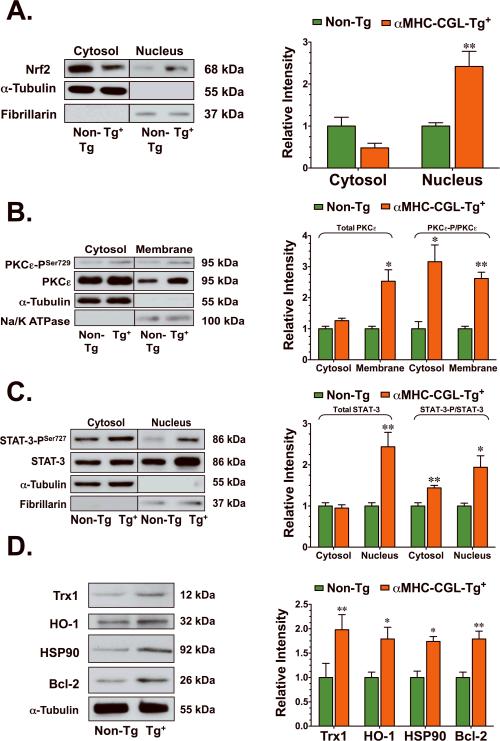
The overexpression of CGL increased Nrf-2 nuclear accumulation and activated PKCε, p44/42, and STAT-3
Previously, the cardiac-specific overexpression of CGL was shown to increase H2S production in the heart and reduce the degree of injury following myocardial I/R.7 Therefore, the next series of experiments were conducted to evaluate if endogenous H2S activated the same cardioprotective signaling pathways as exogenous H2S. The overexpression of CGL resulted in the accumulation of Nrf2 in the nucleus of hearts isolated from transgenic mice (p<0.01 vs. Non-Tg, Figure 7A). Although there was a trend for a decline, cytosolic Nrf2 levels were not significantly different between the groups of mice. A marked increase in PKCε–PSer729 was evident in both the cytosolic (p<0.05 vs. Non-Tg, Figure 7B) and membranous fractions (p<0.01 vs. Non-Tg) of hearts isolated from CGL-Tg+ mice. The total membrane levels of PKCε were also increased in the hearts of CGL-Tg+ mice (p<0.05 vs. Non-Tg), indicating translocation of PKCε to the membrane. CGL overexpression also induced the translocation of STAT-3 to the nucleus, as evidenced by an increase in the total nuclear levels of STAT-3 (p<0.01 vs. Non-Tg, Figure 7C). A marked increase in STAT-3PSer727 was also evident in the cytosolic (p<0.01 vs. Non-Tg) and nuclear fractions (p<0.05 vs. Non-Tg) of CGL-Tg+ hearts. Further analysis revealed that hearts from CGL-Tg+ mice have increased protein expressions of HSP90, HO-1, Trx-1, and Bcl-2 (p<0.05 and p<0.01 vs. Non-Tg, Figure 7D).
Figure 7.
Cardiac-specific overexpression of CGL increased Nrf2 nuclear accumulation and PKCε-STAT-3 signaling. Representative immunoblots and densitometric analysis of (A) Nrf-2 (cytosolic and nuclear fractions), (B) phosphorylated PKCεSer729 and total PKCε (cytosolic and membranous fractions), (C) phosphorylated STAT-3Ser727 and total STAT-3 (cytosolic and nuclear fractions), (D) Trx1, HO-1, HSP90, and Bcl-2 from the hearts of CGL-Tg+ (n=4) and Non-Tg (n=4) mice. Values are means ± S.E.M. *p<0.05, **p<0.01 vs. Non-Tg.
Discussion
As a gaseous signaling molecule, H2S is able to freely diffuse across cell membranes in a receptor-independent manner and activate various cellular targets. This distinct ability makes H2S an attractive pharmacological agent for the treatment of cardiovascular disease. The current study highlights two signaling cascades which lead to the upregulation of antioxidants and anti-apoptogens and provides evidence that pharmacological preconditioning with H2S results in profound protection against myocardial I/R injury, as evidenced by a significant decrease in infarct size and a preservation of LV geometry and cardiac function.
Under physiological conditions, small amounts of ROS produced in cells are quenched by cellular antioxidant defense systems. Antioxidants act by scavenging oxidative species and their precursors, inhibiting their formation and enhancing endogenous antioxidant defenses.16 There is considerable evidence that implicates the production of ROS and subsequent related cellular damage as an initial cause of injury to the myocardium following I/R injury.12 Therefore, the capacity of cardiac myocytes to maintain homeostasis during periods of oxidative stress resides in the ability to activate or induce protective enzymes.17 However, during I/R the activity of many of the endogenous antioxidant enzyme systems are compromised or even abolished18, suggesting that increasing the activity of cellular antioxidant enzymes may protect tissues against reperfusion damage.19 Previous studies have reported that H2S protects various cell types, including myocytes20 from oxidative stress. There is some debate, however, regarding the nature in which H2S reduces oxidative stress. H2S acts as a direct scavenger of ROS and up-regulates endogenous antioxidant defenses. Kimura and colleagues21 demonstrated that H2S protects neurons from cell death by increasing GSH levels through an enhancement of γ-glutamylcysteine synthetase activity and an up-regulation of cystine transport. A major finding of the current study supports the latter notion of H2S inducing a signaling mechanism to combat oxidative stress, as evidenced by the ability of H2S to up-regulate cellular antioxidants in the heart in a Nrf2-dependent manner. Nrf2, a member of the NF-E2 family of nuclear basic leucine zipper transcription factors, regulates the gene expression of a number of enzymes that serve to detoxify pro-oxidative stressors.22 This regulation is mediated by Nrf2 binding to the antioxidant responsive element (ARE), a cis-acting regulatory element or enhancer sequence, found in the promoter region of certain genes, including HO-1 and Trx1. In the current study, H2S was shown for the first time to induce the nuclear accumulation of Nrf2 very rapidly after its administration and to subsequently increase the protein expression of HO-1 and Trx1. Additionally, this is the first study to report that Nrf2 deficient mice experienced an exacerbated injury in response to myocardial I/R, suggesting that Nrf2 is an important endogenous cardioprotective signal that protects against oxidant-mediated injury. This is further supported by previous reports indicating that Nrf2 deficiency is associated with enhanced oxidative stress and cell death.23,24
In the current study, H2S modestly increased the expression of Trx1, but failed to increase the expression of HO-1 in the hearts of Nrf2 KO mice. This is an important finding because the increase in Trx1 was found to be only 58% of the increase observed in wild-type mice (1.4±0.1 vs. 3.3±0.7), suggesting that H2S upregulates HO-1 in an Nrf2-dependent manner, but only partially upregulates Trx1 in an Nrf2-dependent manner, suggesting that H2S regulates Trx1 expression through an additional unidentified mechanism. Interestingly, the modest increase in Trx1 levels in the Nrf2 KO hearts was not sufficient to induce protection, as H2S failed to reduce myocardial injury following I/R in these mice. Our data indicate that Nrf2 plays an important role in mediating the cardioprotective effects of H2S and provides important evidence linking Nrf2 and its downstream effectors to the antioxidant effects of H2S. Moreover, these results suggest that H2S therapy enhances the endogenous antioxidant defenses of myocytes and create an environment resistant to the oxidative stress associated with myocardial I/R injury as evidenced by the preservation of redox state and a reduction in lipid peroxidation.
Another major finding of the current study relates to H2S-medaited activation of a PKCε-STAT-3 signaling cascade. Although, there is in vitro evidence in the literature suggesting that H2S activates PKC14, the current study is the first to report that H2S activates PKCε in vivo. The activation of this pro-survival signaling cascade has previously been shown to confer cardioprotection against myocardial I/R through an inhibition of apoptotic cell death25, an activation of COX-2, and has been shown to play a prominent role in the cardioprotective signaling of ischemic PC.26 The anti-apoptotic actions of this pathway are mediated in part by the phosphorylation and inhibition of the pro-apoptotic factor Bad27, an upregulation of the pro-survival factors Bcl-2 and Bcl-xL28, and an upregulation of HSPs.29 p44/42 promotes the phosphorylation of Bad through the actions of p90RSK, whereas it upregulates Bcl-2, Bcl-xL, and HSPs through STAT-3. The STAT pathway has recently been shown to be an integral part of the response of the myocardium to various cardiac insults, including myocardial infarction.30 In particular, the overexpression of STAT-3 results in cardioprotection31, whereas cardiac-specific deficiency of STAT-3 exacerbates cardiac injury.32 HSPs have also been demonstrated to provide cardioprotection in the setting of I/R.33 In particular, HSP70 suppresses apoptosis in a caspase-dependent34 and caspase-independent manner.35 The findings of the current study suggest that H2S therapy does not simply reduce apoptotic cell death following myocardial I/R through a reduction in oxidative stress, but actually promotes direct anti-apoptotic signaling.
The activation of multiple pathways by H2S in the current study highlights the diversity of this gasotransmitter. Unlike other pharmacological agents that rely on receptor-mediated signaling, H2S activates multiple pathways simultaneously. In addition to the pathways studied here, H2S could promote cardioprotection via the activation of KATP channels and by inhibiting leukocyte-endothelial interactions and subsequent inflammation.2 An important question that remains unanswered relates to the mechanism by which H2S induces the nuclear accumulation of Nrf2. Under basal conditions, Kelch ECH associating protein 1 (Keap1) represses the ability of Nrf2 to induce endogenous antioxidants by binding very tightly to Nrf2, anchoring it in the cytoplasm, and targeting it for ubiquitination and proteasome degradation.36 Only when this association is disrupted can Nrf2 translocate to the nucleus and bind AREs. It is thought that several critical cysteine residues in Keap1 serve as the primary sensor for stress signals (i.e. ROS) and that their modification leads to conformational changes in Keap1, which results in the release of Nrf2.36 In addition to Keap1 being a target, Nrf2 can also be directly modified by kinases, such as PKC, to induce its release.37 Whether H2S alters Keap1 and/or Nrf2 directly or through upstream signaling (i.e. PKC) remains an important unanswered question that requires further study.
Together, the findings of the current study indicate that the cardioprotective effects of H2S are mediated in large part by a combination of antioxidant and anti-apoptotic signaling and highlight a novel signaling cascade involving Nrf2. Furthermore, this study suggests that either the administration of H2S donors or the modulation of the endogenous production of H2S may be of therapeutic benefit in the setting of myocardial I/R.
Supplementary Material
Acknowledgments
The authors would like to thank D.B. Grinsfelder and M. Elston for invaluable technical expertise in conducting these experiments.
Sources of funding Supported by grants from the NIH (2 RO1 HL-060849-09 and 5R01 HL-092141-01) and the American Diabetes Association (7-04-RA-59) to D.J.L and by a grant from the NIH (F32 DK 077380-01) to J.W.C. as well as funds from the Carlyle Fraser Heart Center of Emory University Hospital Midtown.
Non-standard Abbreviations and Acronymst
- AAR
area-at-risk
- ARE
antioxidant response element
- BASE
baseline
- CBS
cystathionine β-synthase
- CGL
cystathionine γ-lyase
- COX-2
cyclooxygenase-2
- Eh
redox potential
- H2S
hydrogen sulfide
- HO-1
heme oxygenase 1
- HSP
heat shock protein
- Inf
Infarct
- Keap1
Kelch ECH associating protein
- KO
knockout
- LCA
left coronary artery
- LVEDD
left ventricular end-diastolic diameter
- LVESD
left ventricular end-systolic diameter
- Na2S
sodium sulfide
- Non-Tg
non-transgenic
- Nrf2
Nuclear-factor-E2-related factor
- PC
preconditioning
- PKC
protein kinase c
- ROS
reactive oxygen species
- SEM
standard error
- STAT-3
signal transducers and activator of transcription 3
- Tg
transgenic
- TTC
2,3,5-triphenyltetrazolium chloride
- Trx1
thioredoxin 1
- Veh
vehicle
Footnotes
Disclosures Ikaria Holdings, Inc provided the Na2S.
References
- 1.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Jr., Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 3.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci U S A. 2007;104:17907–17908. doi: 10.1073/pnas.0709010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 7.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince M, Li Y, Childers A, Itoh K, Yamamoto M, Kleiner HE. Comparison of citrus coumarins on carcinogen-detoxifying enzymes in Nrf2 knockout mice. Toxicol Lett. 2009;185:180–186. doi: 10.1016/j.toxlet.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 10.Go YM, Ziegler TR, Johnson JM, Gu L, Hansen JM, Jones DP. Selective protection of nuclear thioredoxin-1 and glutathione redox systems against oxidation during glucose and glutamine deficiency in human colonic epithelial cells. Free Radic Biol Med. 2007;42:363–370. doi: 10.1016/j.freeradbiomed.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: Roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol. 2007;292:R800–809. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- 12.Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem. 2007;14:1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol. 2008;294:C169–177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- 15.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription1/3 pathway. Circulation. 2007;116:535–544. doi: 10.1161/CIRCULATIONAHA.107.689471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation--the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 17.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 18.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 20.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 21.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. Faseb J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 22.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 23.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagishi S, Morito N, Nakano T, Ojima M, Shimohata H, Itoh K, Takahashi S, Yamamoto M. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13:1159–1170. doi: 10.1111/j.1365-2443.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 25.Yellon DM, Baxter GF. Reperfusion injury revisited: is there a role for growth factor signaling in limiting lethal reperfusion injury? Trends Cardiovasc Med. 1999;9:245–249. doi: 10.1016/s1050-1738(00)00029-3. [DOI] [PubMed] [Google Scholar]
- 26.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertolotto C, Maulon L, Filippa N, Baier G, Auberger P. Protein kinase C theta and epsilon promote T-cell survival by a rsk-dependent phosphorylation and inactivation of BAD. J Biol Chem. 2000;275:37246–37250. doi: 10.1074/jbc.M007732200. [DOI] [PubMed] [Google Scholar]
- 28.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 29.Madamanchi NR, Li S, Patterson C, Runge MS. Thrombin regulates vascular smooth muscle cell growth and heat shock proteins via the JAK-STAT pathway. J Biol Chem. 2001;276:18915–18924. doi: 10.1074/jbc.M008802200. [DOI] [PubMed] [Google Scholar]
- 30.Barry SP, Townsend PA, Latchman DS, Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med. 2007;13:82–89. doi: 10.1016/j.molmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, Okabe M, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci U S A. 2000;97:315–319. doi: 10.1073/pnas.97.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Wu XJ, Lu XY, Zhu L, Wang LP, Yang HT, Chen HZ, Yuan WJ. Phosphorylated heat shock protein 27 is involved in enhanced heart tolerance to ischemia in short-term type 1 diabetic rats. Acta Pharmacol Sin. 2005;26:806–812. doi: 10.1111/j.1745-7254.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 34.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 35.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.