Abstract
Co-stimulatory signals are important for development of effector and regulatory T cells. In this case, CD28 signaling is usually considered inert in the absence of signaling through the TCR. By contrast, mitogenic rat CD28 mAbs reportedly expand regulatory T cells without TCR stimulation. We found that a commercially available human CD28 mAb (ANC28) stimulated PBMCs without TCR co-ligation or cross-linking; ANC28 selectively expanded CD4+CD25+FoxP3−(T effector) and CD4+CD25+FoxP3+ (Treg) cells. ANC28 stimulated the CD45RO+ CD4+ (memory) population whereas CD45RA+CD4+ (naïve) cells did not respond. ANC28 also induced inflammatory cytokines. Treg induced by ANC28 retain the Treg phenotype longer than did co-stimulated Treg. Treg induced by ANC28 suppressed CD25− T cells through a contact-dependent mechanism. Purity influenced the response of CD4+CD25+ cells because bead-purified CD4+CD25+ cells (85–90% pure) responded strongly to ANC28, whereas 98% pure FACS-sorted CD4+CD25 bright (T-reg) did not respond. Purified CD4+CD25int cells responded similarly to the bead-purified CD4+CD25+ cells. Thus, pre-activated CD4+ T cells (CD25int) respond to ANC28 rather than Treg (CD25bright). The ability of ANC28 to expand both effectors producing inflammatory cytokines as well as suppressive regulatory T cells might be useful for ex vivo expansion of therapeutic T cells.
Keywords: Human T Cells, Regulatory T cells, T Cell Activation, Co-stimulation, Inflammation
Introduction
A central dogma of cellular immunology is that activation of naive T cells requires two signals, one transmitted through the T cell receptor and the second signal received through CD28 or other co-stimulatory receptors[1]. In this model, TCR activation without a co-stimulatory signal leads to T cell inactivation (paralysis, anergy); co-stimulatory signals block the inactivation step, leading to T cell activation. In the original descriptions of the Two Signal Model, receipt of Signal Two alone has little effect on the T cell because, otherwise, bystander activation or inactivation would result.
CD28 has emerged as the most important co-stimulatory receptor of T-lymphocytes, at least for activation of naive T cells. In experimental systems, the natural ligands of the TCR, the cognate MHC–peptide complexes, and of CD28, the B7 family members CD80 and CD86, can be replaced by CD3 and CD28 mAbs, which are used to study T cell activation in vitro. There is growing evidence that certain human [2–4], rat [4–8] and mouse [4] CD28 mAb trigger T cell activation and mitogenesis in the absence of overt co-ligation of the TCR. These antibodies have been called superagonistic or mitogenic CD28. However, the molecular mechanism of their action is still unclear. Mitogenic CD28 bind to CD28 differently than conventional CD28 antibodies. Conventional CD28 antibodies bind at the “top” of the molecule, i.e. in the area of natural ligand’s binding site, whereas a mitogenic CD28 mAb binds laterally to the IgV domain, involving the C-D loop at its bottom [4] A kinetic segregation model was proposed in which cross–linking of CD28 by mitogenic mAb to a plastic surface or an Fc receptor on an APC leads to a much closer approximation of the T cell membrane to the opposing surface than in the case with conventional CD28-specific mAb. This allows kinases to dominate over phosphatases which are excluded because of large extracellular domains, and signaling is thus initiated [9]. The Hünig/Hanke groups extensively characterized the mitogenic anti-rat CD28 mAb JJ316, which preferentially expanded regulatory T cells (Treg) and induced anti-inflammatory cytokines in vitro and in vivo with therapeutic abilities in vivo [6–13]. In particular, JJ316 prevented EAE and adjuvant arthritis in rats [6, 10]. This model rationalized a phase I in vivo trial of a humanized mitogenic anti-human CD28 mAb, TGN1412, the properties of which are known from the report prepared by the British Expert Scientific Group (ESG) on phase I clinical trials[14].The trial was stopped due to rapid induction of multi-organ failure in six volunteers receiving the mAb. Contamination with known agents causing toxic shock (such as LPS) was ruled out and the symptoms were likely caused by a cytokine storm [15, 16]. The failure of experiments using TGN1412 in cynomolgus macaques to reveal toxicity [17] underscores the need for better understanding the mechanism of mitogenic CD28 mAbs.
The reported preferential activation of only regulatory T cells by JJ316 was a key finding because Treg are a useful subset of T cells with therapeutic implications for modulating the immune system. Treg are normally unresponsive using conventional CD3 and CD28 antibody stimulation and do not proliferate. However, in those studies reporting expansion of regulatory T cells by JJ316, the purity of the starting cells was not well defined [7, 18]. Thus, in those studies, CD4+CD25+ cells were isolated by magnetic columns with about 85–90% purity, so that a wide range of CD25 expressing cells were obtained including the CD25 bright (Treg). In addition, data about the Teff /Treg ratio before and after JJ316 stimulation were not reported. Because of the reduced purity, it is therefore possible that mitogenic CD28 might stimulate other subsets of human T cells in addition to Treg.
We show here that a commercially available CD28 antibody, ANC28, expands and activates human PBMCs, purified CD4 T cells and the CD45RO+CD4+ subset (memory CD4 T cells) with secretion of inflammatory cytokines. It does not require cross-linking. ANC28 preferentially expands most CD4+ CD25+ cells, which include effector and regulatory T cells, rather than only Treg. ANC28-activated Treg have effector mechanisms resembling Treg described by others [19]. Magnetically purified CD4+ CD25+ or CD25int responded strongly to ANC28. By contrast, 98% pure CD4CD25bright (FACS-sorted) cells did not respond to ANC28, suggesting that, in contrast to previous reports, mitogenic CD28 antibodies are not specific for Treg. The likely explanation of the failure of phase I in vivo trial of a humanized mitogenic anti-human CD28 mAb, TGN1412 may be related to these observations.
Results
ANC28 induces T cell proliferation, activation and cytokine production independently of TCR co-ligation
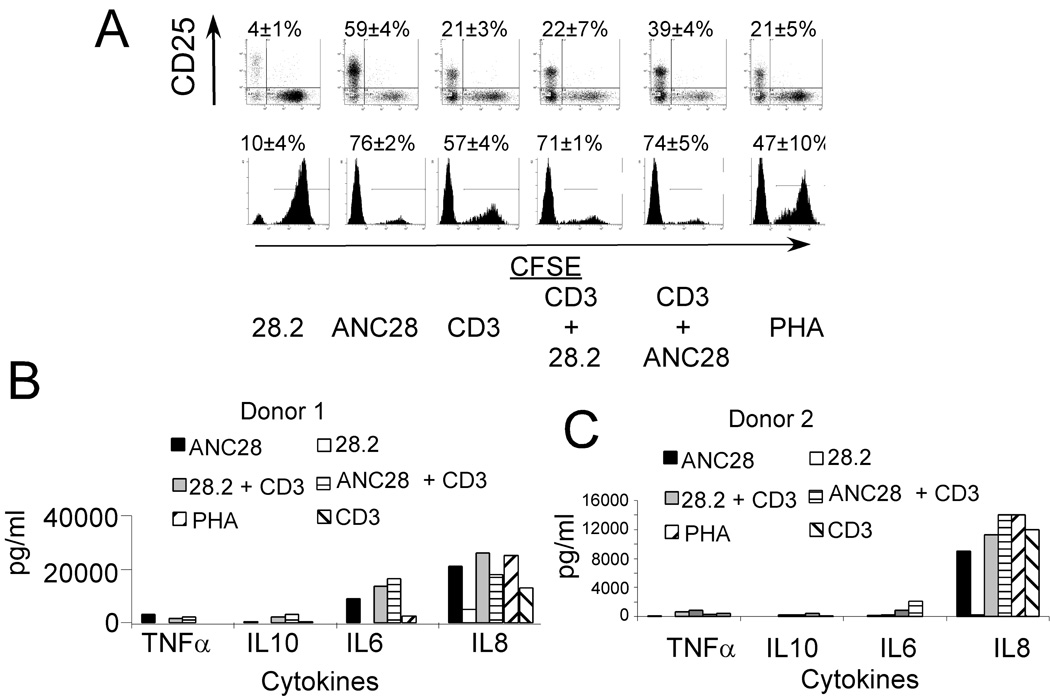
Based on preliminary observations suggesting that ANC28 signaled in the absence of TCR co-ligation, we asked whether ANC28 had other properties characteristic of ‘mitogenic’ CD28 antibodies for primary resting T cells. We analyzed the expansion and activation of CFSE-labeled unfractionated PBMC stimulated with CD3, PHA, ANC28 or 28.2 alone, or CD28 mAb in combination with CD3. Significant expansion (76.5±2.1%; p<0.05, ANC28 vs 28.2) and activation (59.85±4.1%; p<0.05, ANC28 vs 28.2) were induced by ANC28, and were superior to other stimuli including co-stimulation (Fig. 1A).
Figure 1. ANC28 has properties of ‘mitogenic’ CD28 antibody.
(A) CFSE-labeled PBMCs stimulated with CD3, PHA, ANC28 or 28.2 alone, or CD28 mAb in combination with CD3 were analyzed for activation (CD25 up-regulation) and expansion (CFSE dye dilution) by flow cytometry on day 6. Mean percent of dividing cells ± SD, p<0.05 ANC28 vs. 28.2, N = 2 different donors. (B) Culture supernatants from PBMC stimulated as indicated for 2 days tested by cytokine bead array.
Without TCR/CD3 co-ligation, cross-linked but not soluble TGN1412 CD28 mAb induced TNF-α, IL-6, and IL-8 from human PBMCs [20]. By contrast, soluble ANC28 induced high levels of similar cytokines including TNF-α, IL-6 and IL-8 from one donor and IL-8 from another donor (Fig. 1B). These results indicate that ANC28 does not require TCR- co-ligation or cross-linking to activate human PBMCs and, with respect to cytokine production, it resembles TGN1412 CD28 mAb.
Selectively expansion of CD4CD45RO by ANC28
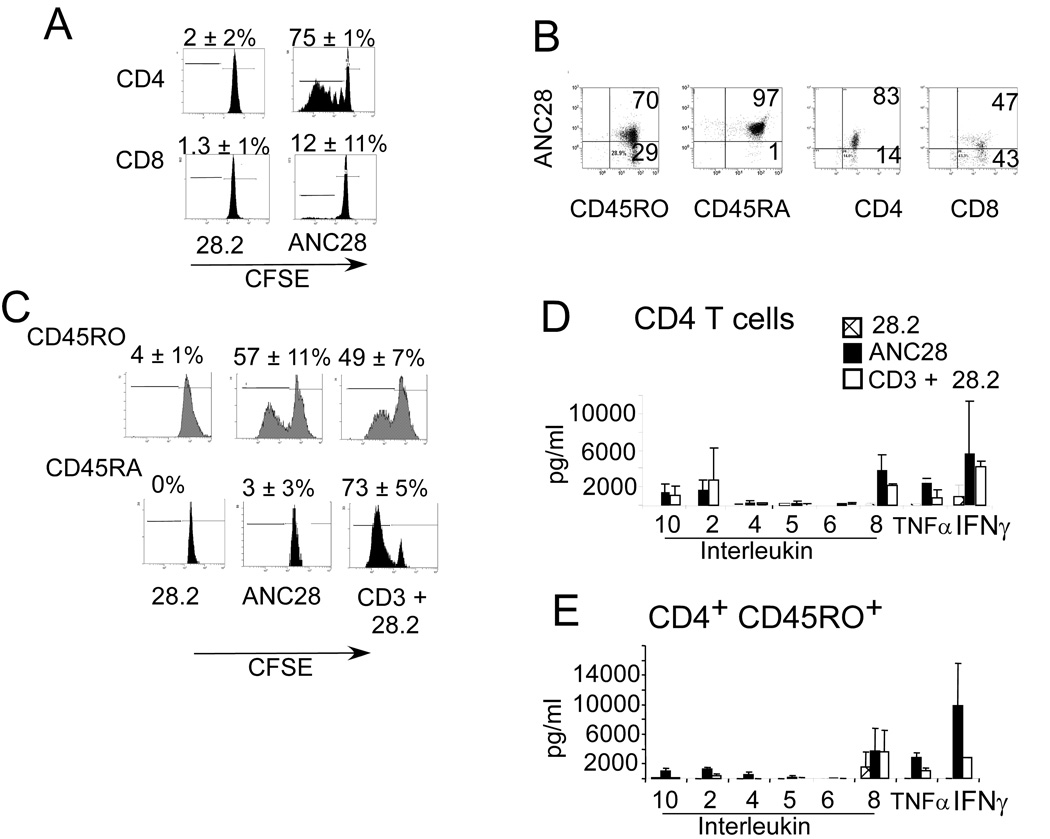
The stimulatory capacity of ANC28 prompted us to further characterize the cell populations affected by ANC28.ANC28, but not 28.2, induced proliferation in about three quarters of CFSE-labeled CD4+ T cells and a minor fraction of CD8+ T cells (Fig. 2A).
Figure 2. Selective expansion of CD4CD45RO cells by ANC28.
CFSE-labeled purified (A) CD4, CD8 cells stimulated with 28.2 or ANC28 and (C) CD4CD45RO, CD4CD45RA stimulated with ANC28, 28.2 or CD3+28.2 were analyzed by flow cytometry on day 6. Mean percent of dividing cells ± SD, p<0.05 ANC28 vs. 28.2 and p<0.05 CD4ANC28 vs CD8ANC28, N = 3 different donors. Expansion of CD45RO was comparable with ANC28 or CD3+28.2 stimulation and the CD4CD45RA subset of T cells was unresponsive to ANC28. (B) Purified CD4, CD8, CD45RA or CD45RO were stained with PE–conjugated ANC28 and analyzed by flow cytometry for CD28 expression. Low percentages of CD28+cells were present in CD8+ sub-population (N=2).
Mean ± SD indicate the production levels of indicated cytokines from (D) CD4 or (E) CD4CD45RO cells. Culture supernatants two days after of stimulation by ANC28, 28.2 and 28.2+CD3 were assayed by cytokine bead arrays. ANC28 but not 28.2 induced high levels of inflammatory cytokines including IL-2, IL-8, and TNF-α and IFN-γ. N = 3 different donors. (E) Purified CD4, CD8, CD45RA or CD45RO were stained with PE–conjugated ANC28 and analyzed by flow cytometry for CD28 expression. Low percentages of CD28+cells were present in CD8+ sub-population (N=2).
The difference in expansion of CD4+ (74.7% ± 0.6) and CD8+ (12%±11.6) T cells was reproducible and significant (p ≤ 0.01). The poor responses of CD8+ T cells might be due to the low percentage of CD28+CD8+ T cells (CD4 vs. CD8, 85±6.2% vs. 48.6±4.7% of cells; Fig 2B). A previously described human mitogenic CD28 antibody, BW828 [2] expanded CD4 memory cells whereas another human antibody 5.11A1[21] expanded both naïve CD45RA+ and CD45RO+ CD4 T cells suggesting differential reactivity of T cells when stimulated with different mAbs. To test whether ANC28 expanded only memory CD4 cells, we compared responses of negatively selected CD45RO+ or CD45RA+ CD4 T cells. ANC28 induced proliferation in the CD4CD45RO+ subset but not in CD4CD45RA+ cells. However, both subsets expanded in response to conventional co-stimulation with CD3 and 28.2. Expansion of CD4+CD45RO+ T cells by ANC28 was comparable to CD3 and 28.2 stimulation (Fig 2C). Contrary to CD8, most CD4CD45RA (92±6.4%) express higher levels of CD28 whereas about 75% (75±7) of the CD4CD45RO T cells express CD28 (Fig.2B). Naive cells need stronger activation signals than memory T cells, which may be why they do not respond to ANC28. However, when IL-2 was added, naïve cells could be expanded by ANC28 (data not shown), indicating that the limiting factor is the ability to make IL-2.
We also examined Th1/Th2 and inflammatory cytokines after ANC28, 28.2 or combination (28.2 and CD3) treatment in bulk CD4+ T cells or in CD4+CD45RO+memory cells. ANC28, but not 28.2, induced the inflammatory cytokines, IL-8, IFN-γ, TNF-α and IL-10 from both subsets (Fig.2D&E). Levels of IL-2 were significantly higher in ANC28-treated CD4+CD45RO+ cultures than in co-stimulated cultures (1248±288 vs 430±271pg/ml; ANC28 vs 28.2 and CD3; p= 0.02). By contrast, levels of IL-2 were lower in ANC28-treated bulk CD4 cultures. We believe that lower levels of IL-2 in bulk CD4 culture might be due to the consumption of IL-2 by CD45RA cell, as purified CD45RA did not respond to ANC28 but did respond when IL-2 was added (data not shown). By contrast, co-stimulation has a direct effect on both CD45RA and CD45RO populations so they both expand and produce IL-2. Except for IL-2, the levels of cytokines produced by ANC28 stimulation were greater in both CD4 and CD45RO cultures than those produced after CD3 and 28.2 stimulation and TNF- α levels were significantly greater in ANC28-treated CD4 cultures (2244 pg/ml ±705 vs 78±73; ANC28vs28.2 and CD3; p≤ 0.01). In contrast to the previously reported work with mitogenic rat CD28 mAb JJ316 [8, 10], these data suggest that ANC28 induces inflammatory, rather than anti-inflammatory cytokines.
ANC28 stimulates T cells without cross-linking
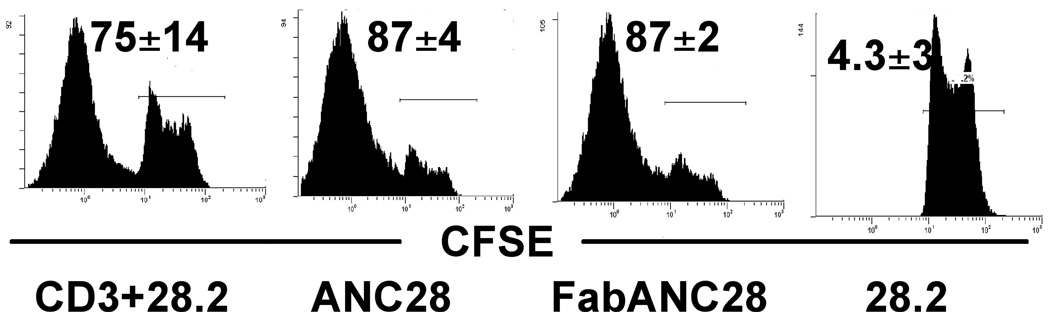
Previously described mitogenic CD28 antibodies BW828[2] and JJ316[5] required cross-linking for their stimulatory capacity. In addition, immobilized but not soluble TGN1412 induced secretion of inflammatory cytokines from human PBMCs [20]. By contrast, soluble ANC28 was active in all our experiments. Moreover, negatively selected CD4 T cells free of contaminating monocytes to mediate cross-linking also were expanded by soluble ANC28 (data not shown). To further test the requirement of cross-linking, we used F (ab’)2 ANC28 and found that CD4 T cells expand similarly to both intact and F(ab’)2 ANC28 (Fig 3). These results suggest that cross-linking via FcR is not required for ANC28 to activate T cells.
Figure 3. ANC28 does not require cross-linking to cause cell division.
CFSE-labeled purified CD4 cells were stimulated with ANC28 or its F (ab)2 fragment and analysed after 6 days. Similar level of proliferation were induced (Mean ±SD indicates percentage of dividing cells;N = 2 different donors).
ANC28 expands CD4+CD25+Foxp3− and CD4+CD25+Foxp3+ cells
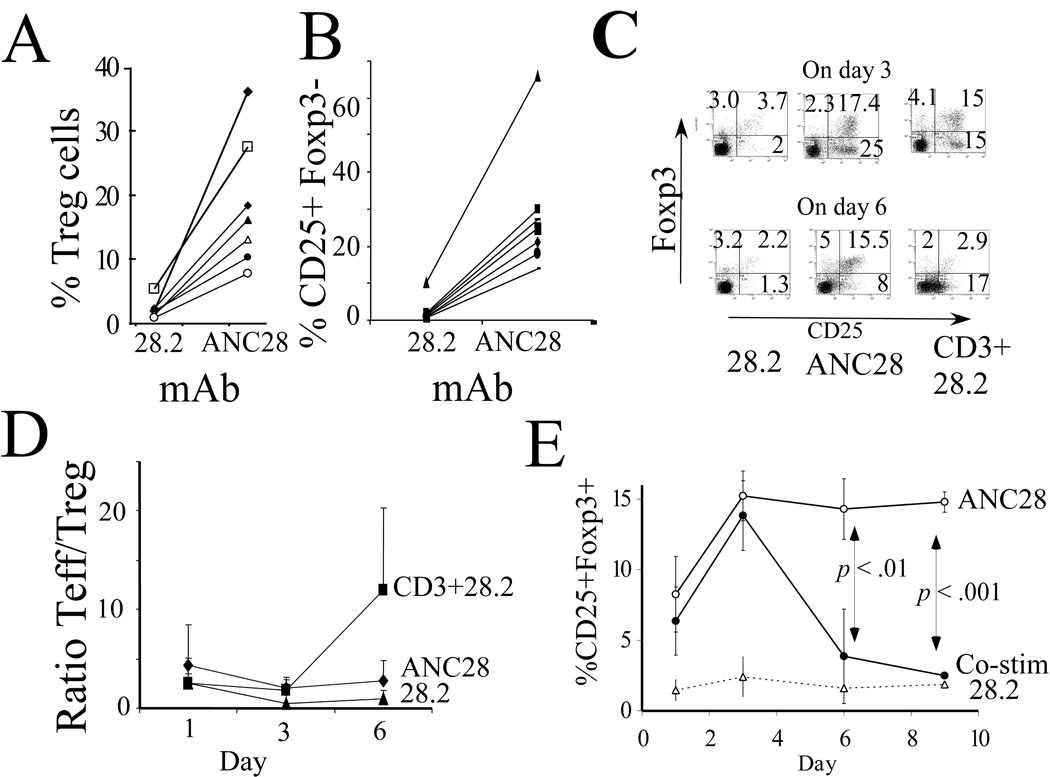
Recent studies indicated that mitogenic CD28 mAb selectively expand Treg cells in vitro [7, 18] and in rats, mice and humanized mice [8, 22]. To test if ANC28 also selectively expands Treg, we examined Treg markers after expansion of PBMCs from seven healthy donors (age 25–35 years). The fraction of CD4+CD25+Foxp3+ T cells increased five- to 18-fold after ANC28 (18.4%±10) compared to 28.2 treatment (2.23%±1.5; p<0.004, Fig.4A). However, CD4+CD25+Foxp3− T cells were also expanded 6 to 23-fold more in ANC28-treated cultures than in those treated with 28.2 (29%±17 vs 2.5%±3; p <0.002; Fig 4B), indicating that ANC28 preferentially expands bulk CD4+CD25+ rather than just CD4+CD25+Foxp3+ (Treg). ANC28 also expanded both CD25+ Foxp3+ and CD25+ Foxp3− cells when starting with purified CD4 T cells (p <0.005 compared to 28.2; Fig4C). The ratio of Teff / Treg (mean±SD of three experiments) was greater than one at every time point (Fig 4D).This is in contrast to a previous report in which selective expansion of T regs by mitogenic CD28 apparently contracted T effectors cells [18]. The fraction of CD25+ Foxp3+ cells were more stable in ANC28- induced culture than after co-stimulation (CD3 and CD28.2; Fig. 4E; p < 0.05). Treg induced by ANC28 increased in number until about day 3 of culture and then remained at a stable level for more than 6 days without exogenous IL-2 (Fig 4E). By contrast, co-stimulated CD4 cells that expressed FoxP3 returned to baseline expression after six days, indicating that TCR signals might inhibit FoxP3 expression in vitro. The numbers of CD4 T cells increased about 6–8 fold in ANC28 –treated and co-stimulated cultures starting with 5×105 cells. The number of CD25+FoxP3+ CD4 T cells in the starting population constituted about 2.5×104 about (5–7%).These Tregs increased 20–22 fold after 3 days and were similar in co-stimulated culture and ANC28 stimulated cultures (4.5×105±1.2×105 vs 4.7×105±1.1×105 ANC28 vs co-stimulation). After 6 days of culture, more T regs were present in ANC28 treated cultures than in co-stimulated cultures (4.2×105 ± 6×104 vs 1.3×105 ±1 ×104; ANC28 vs co-stimulation; p < 0.05). These data suggest that after 6 days the absolute numbers of Tregs were also more after ANC28 treatment compared to co-stimulation.
Figure 4. ANC28 induces CD4+CD25+ phenotype ( Treg and T effectors).
PBMC from seven different donors were assayed for (A) CD4+CD25+FoxP3+ or (B) CD4+CD25+ expression 6 days after culture with ANC28 or conventional mAb 28.2. Lines connect cultures from the same donor. (C) Negatively-selected CD4 cells were stimulated with ANC28, 28.2 or co-stimulated with anti-CD3 and 28.2 and assayed on days 3 and 6. Data are representative of three independent experiments with different donors. (D) Ratio of T effectors/Treg >1; mean percentage ± SD of CD4+CD25+ cells were greater than mean percentage ± SD of CD4+CD25+FoxP3+ in both ANC28 and CD3+28.2 treated cultures at every time point (N=3 different donors). (E) Percentages of CD4+CD25+FoxP3+ cells in two series of experiments using three donors each. Results are means ± SD, ANC28 values exceed those of 28.2-treated cultures at every time point (p <0.05). ANC28 values exceed those of co-stimulated cultures on days 6 (p <.01, N = 6) and 9 (p <.001; N = 3)
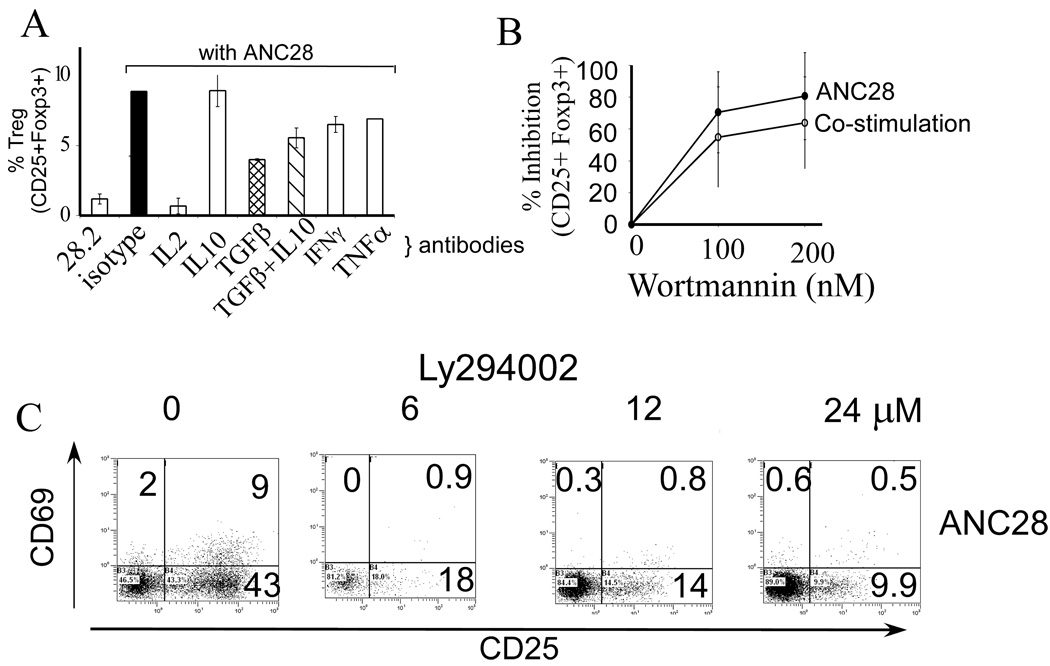
IL-2 is important for natural Treg development in mice and IL-2R signaling is required for Treg function[23]. Therefore, we tested whether IL-2 was required for ANC28-induced Treg development. Anti-IL-2 inhibited Treg formation by 92% (p <0.001). TGF-β is required for inducing Treg cells in some systems [24]. Anti-TGF-β antibodies blocked about 50% of CD25+Foxp3+ cell generation from bulk CD4+ cells, whereas anti-IFN-γ and anti-TNF-α antibodies inhibited Treg formation only 20–25% (Fig. 5A). The latter inhibitory effects were not significant after Bonferroni correction for multiple comparisons. These findings suggest that at least IL-2 is critical for the induction and/or expansion of Treg by ANC28, consistent with the importance of IL-2 in Tregs development, whereas IFN-γ, TNF-α and TGF-β might have more limited roles.
Figure 5. (A) Regulation of Treg formation.
IL-2 is required for generation of Treg by ANC28. 10 µg neutralizing antibody for each indicated cytokine or isotype matched control antibodies were added to CD4 cultures 30 min prior to ANC28 treatment and analyzed on day 3. Inhibition by anti-IL-2 was significant (p <0.001,* whereas anti-TGF-β, anti-IFN-γ and anti-TNF-α had moderate effects (N = 2 different donors).
ANC28 stimulates T cells through PI3K-like pathway (B) Wortmannin inhibits Treg formation. CD4 cells were cultured with up to 200 nM wortmannin for 30 min, before adding ANC28 or co-stimulatory treatments and analyzed on day 3, (N = 3 different donors) (C) LY294002 inhibits activation (CD69 & CD25 up-regulation) in a dose dependent fashion. CD4 cells were cultured with indicated amounts of LY294002 30 min. before adding ANC28 and analyzed on day 3. Data representive of three independent experiments (N=3 different donors)
ANC28 stimulates T cells through PI3K-like pathway
The cytoplasmic tail of CD28 binds PI3K after T cell activation and a role for PI3K activation has been implicated in some, but not all, CD28 signaling pathways [25–29]. T cell activation during co-stimulation does not require PI3K signaling. However, TCR-independent CD28 signaling involved in glucose metabolism [25], trafficking of cells [29] and mitogenic responses does require PI3K. A recent report showed superagonistic anti-CD28 (5.11A1) stimulated T cells independently of PI3K [21]
To test whether ANC28-mediated induction of T cells requires PI3K kinase, we assayed CD25+ and CD25+ Foxp3+ formation in the presence of wortmannin. This compound is highly selective for the serine-threonine kinases PI3K and polo-like kinases, with an IC50 in vitro of about 5 nM for each enzyme [30, 31]. However, the half-life of aqueous wortmannin at 37°C is about 10 min. Therefore, to ensure an inhibitory dose throughout the culture period, we, like others, used wortmannin in the range of 100–200 nM. In three experiments (Fig. 5B), 100 nM wortmannin inhibited ANC28-induced Treg formation by 70–98%, consistent with the involvement of PI3K and/or PLK1. In each experiment, the reduction of CD25+Foxp3+ cells paralleled the reduction in CD25+Foxp3− cells (not shown). Moreover, LY294002, a stable inhibitor of PI3 and polo-like kinases, dose dependently inhibited CD69 and CD25 up-regulation after 6 hrs (without LY294002 vs with 24uM LY294002; 39±4 vs 11±1; p=0.003) as well as after 3 days (Fig 5C). Treg formation induced by ANC28 was also inhibited (data not shown) by LY294002. These results suggest that PI3K is involved in the T cell stimulation caused by ANC28.
ANC28 induces functional Treg
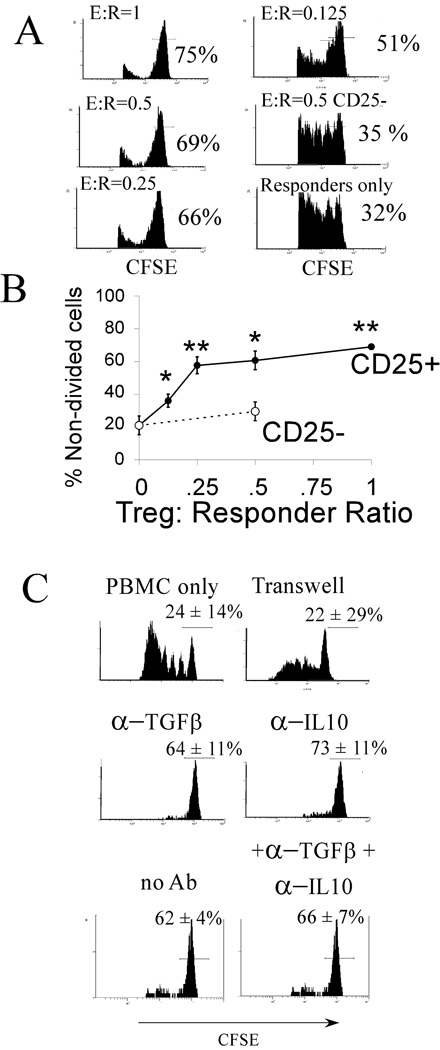
To assess the functional state of ANC28-induced Treg, we tested ANC28-stimulated T cell subsets for their ability to suppress co-stimulated proliferation of autologous PBMC (Figs. 6A & 6B). 80% of CFSE-labeled PBMC proliferated in the absence of added Treg. ANC-induced CD25+ cells dose-dependently suppressed up to 60% of the proliferation at effector: responder ratios of 1:1 and ANC28-induced CD25+cells suppressed better than added CD25− cells at all ratios ( p ≤ 0.005; Fig. 6B).
Figure 6. ANC28 induces functional Treg cells.
(A) CD4+CD25+ cells were isolated using magnetic beads after 3 days from ANC28 treated CD4 cultures and different amounts of these cells were co-cultured with CFSE-labeled responder PBMCs in the presence of CD3+28.2 and analyzed on day 6 for measuring the proliferation of responder PBMCs. Results are gated on CFSE+ cells to exclude unlabeled CD4+CD25+cells. (B) CD25+ (●) from ANC28-activated cultures but not mock treated CD25− (○) cells suppressed PBMCs dose-dependently (N = 3 different donors; p <0.05*, ** compared to without Treg in the culture). (C) Suppression was blocked by a permeable membrane but not by anti-IL-10, anti-TGF-β or anti-IL-10 and anti-TGF-β together. Data are representative of two independent experiments with means ± SD.
Natural CD4+ Treg cells suppress through a contact-dependent mechanism, whereas suppression by other “Tr1” or “Th3” regulatory cells requires cytokines [19]. To test whether suppression by ANC-induced Treg cells required cell-contact or was due to soluble factors, co-cultures of Treg and responder cells were separated by a Transwell™ membrane. No suppression was detected in these cultures (Fig. 6C). By contrast, cultures containing anti-IL-10 or anti-TGF-β or anti-IL-10 and anti-TGF-β together had profound suppression (p <0.025 in each case). These results showed that contact was responsible for suppression caused by ANC induced T regs.
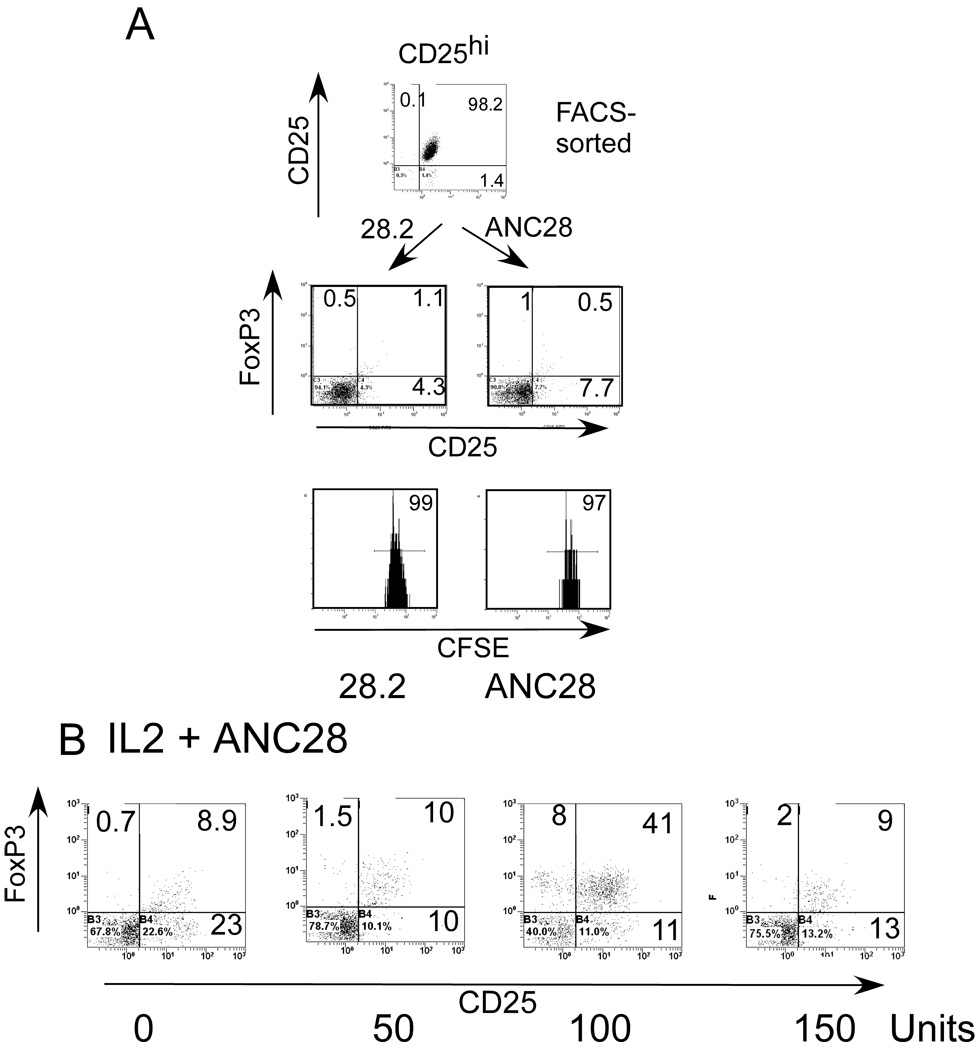
ANC28 does not expand CD25bright (naturally occurring Treg)
The Hünig/Hanke labs reported that mitogenic CD28 mAb selectively caused proliferation of rodent Treg cells in vitro and in vivo [6–8, 18, 22]. These results are consistent with our data using bead - purified CD25+ cells. By contrast, we found that the FACS-sorted 98% pure CD25 bright fraction of CD4 T cells did not respond to ANC28 (Fig. 7A), which suggests that ANC28 must target other CD4 memory T cells. 80–90% of CD25bright starting population expressed FoxP3 but after 3 days in ANC28- treated cultures this percentage decreased dramatically to from 1 to 12% (7±4.3%). Loss of FoxP3 and CD25 expression by 3 days might be due to an unresponsiveness of this population to ANC28 and /or lack of IL-2. When we repeated this same experiment in the presence of IL-2, we found that 100U/ml of exogenous IL-2 maintained the survival of some cells as well as CD25 and FoxP3 expression in CD25bright cells (44±7% vs 7±4.3%; with vs without IL-2, p = 0.004, Fig 7B), with only a small expansion in ANC28 treated cultures. However, 50U/ml IL-2 was not enough to maintain FoxP3 and CD25 expression and the cells were killed with 150 –200 U/ml IL 2(Fig 7B). Indeed, when we purified CD25+ cells by magnetic columns, the cells were 85–90% pure and thus contained CD25 bright, CD25int as well as a few CD25− CD4 T cells. The representation of FoxP3 positive cells in this starting population was between 25–30% (data not shown). This semi-pure cell population responded strongly to ANC28 and almost all (90 ± 7%, Fig 8A) CFSE labeled cells proliferated in response to ANC28. In addition, after three days of ANC28 treatment; about 40% of the cells expressed FoxP3 (Fig8A).
Figure 7. CD4CD25bright (Treg) do not respond to ANC28.
CFSE-labeled or unlabeled, FACS sorted (A) CD25bright T cells were stimulated with ANC28 or 28.2 and analyzed after 3 days for CD25+FoxP3+ expression (Treg phenotype) and after 6 days for expansion by flow-cytometry. Data representive of 4 independent experiments (N= 4 different donors) (B) Exogenous IL-2 (100U/ml) maintains survival and CD25 and FoxP3 expression of CD25bright cells; After 30 min. of ANC28 treatment 50, 100 or 150U/ml IL-2 were added in CD25bright culture, and analyzed after 3 days for CD25 and FoxP3 expression by flow cytometry (N=2).
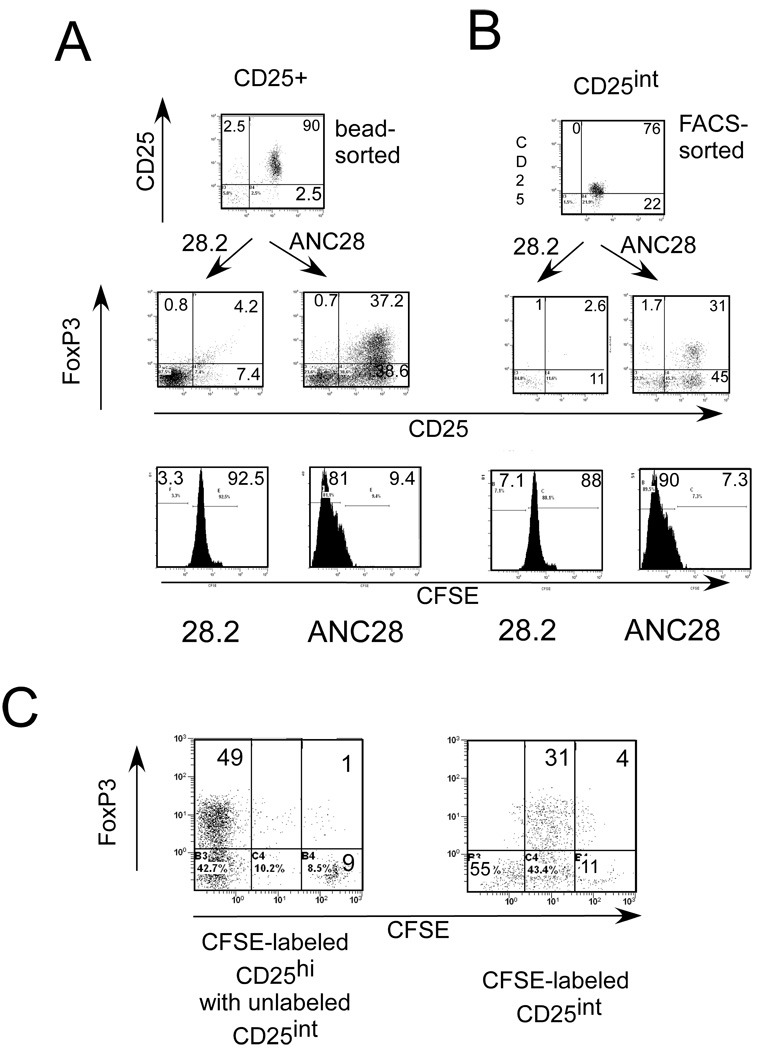
Figure 8. CFSE-labeled or unlabeled, bead- purified.
(A) CD25+ or (B) FACS sorted CD25int were stimulated with ANC28 or 28.2 and analyzed after 3 days for CD25+FoxP3+ expression (Treg phenotype) and after 6 days for expansion by flow-cytometry. Data representive of 4 independent experiments (N= 4 different donors) (C) CD25bri cells were not expanded in the presence of CD25int cells; CFSE labeled CD25bri cells were co-cultured with CD25int cells and analyzed after 5 days for expansion and CD25 and FoxP3 expression. Data are representative of two independent experiments.
To further test whether only CD25int cells responded to ANC28, we sorted CD4+ cells into CD25+, CD25int or CD25− cells by FACS. As expected, FACS-sorted CD25int (2–3% FoxP3+) responded similarly to the bead-purified CD25+cells. In addition, the Treg phenotype (34±9%) was detected after 3 days (Fig 8B), whereas only a small percentage (6±5%) of CD25− cells expanded in response to ANC28 (data not shown).These findings indicate that CD25int CD4+ memory cells (recently activated T cells) rather than CD25brightcells respond to ANC28.
To explore whether CD25int T cells were responsible for the expansion of CD25bright T cells in magnetically purified semi-pure populations, we mixed FACS sorted CD25int and CFSE labeled CD25 bright T cells at a 2:1 ratio and treated the cells with ANC28. CD25bright did not expand even in the presence of CD25int cells (Fig.8C), which suggests that ANC28 specifically expands only CD25int cells.CD25bright cells neither expand nor maintain FoxP3 expression after ANC28 treatment even when IL-2 is added or when CD25int CD4 T cells are mixed with CD25bright cells, therefore we conclude that ANC induced functional Tregs in bulk CD4 T cells culture (Fig.4C & D) specifically generate from CD25int cells not from CD25bright cells.
Discussion
In this work, we found that a commercially available human CD28 mAb “ANC28.1” (ANC28; clone 5D10; mouse IgG1κ; Ancell Corp., Bayport, MN) is mitogenic for human T cells, especially for CD4 memory T cells. Previously, Schafer et al. (1999) showed that, similar to a conventional anti-CD28 antibody (28.2), cross-linked ANC28 caused activation of p38α mitogen-activated protein kinase in memory CD45RO T cells [32].In that study, the ability to stimulate p38α through CD28 in memory cells was not limited to mitogenic CD28 mAb[32]. Previously,we reported that ANC28 not CD28.2 mediates down-regulation of CD28 mRNA, similar to that caused by CD80 [33]. In the present study, neither plate-bound nor soluble 28.2 stimulated T cells, but both forms of ANC28 were potent mitogens for T cells, especially for CD4+CD45RO+ T cells. ANC28 resembles previously described human mitogenic CD28 mAbs (Table 1). Similar to BW828 [2, 3], ANC28 selectively expanded only the CD4CD45RO subset of T cells, and similar to TGN1412 [20], it induces inflammatory cytokines, TNF-a, IL-6, and IL-8 from human PBMCs. However, in contrast to ANC28, all other previous described mitogenic or superagonistic CD28 required cross- linking for their action. Use of the F(ab)’2 fragment of ANC28 shows that FcR - mediated cross-linking is not required.
Table: 1.
Comparison of ANC28 with other mitogenic CD28 abs
| Mitogenic CD28 antibodies | |||||
|---|---|---|---|---|---|
| ANC28current study | BW828[1] | 5.11A1[2] | TGN1412[3, 4] | JJ316[5] | |
| Specificity | Human | Human | Human | Human | Rat |
| Isotype | IgG1k | IgG2a | IgG1 | IgG4 k | IgG1k |
| Study | in vitro | in vitro | in vitro | in vivo/invitro | invivo/invitro |
| Cross-linking needed | no | yes | yes | yes | yes |
| Activation | CD4 CD45RO | CD4 CD45RO | CD4 CD45RO, RA | − | CD4CD45RC+, RC− |
| Cytokines | Inflammatory | IL-2 | IL-2, IFN-γ | Inflammatory | mainly IL-4 |
| Selective expansion | CD25+ (Teff/Treg) | − | Treg | − | Treg |
| PI3K involvement | yes | − | no | − | − |
(−) not known
inflammatory cytokines- IFN-γ,IL-8,TNF-α
The evidence that T cell activation by mitogenic CD28 mAb does not require simultaneous TCR co-ligation by MHC:peptide complexes is compelling. First, the fraction of CD4+ T cells that respond to these antibodies is large. Thus, the majority of rat CD4+ T cells [7, 8] and nearly all T cells from RAG-deficient mice expressing the ovalbumin-specific OT2 transgenic TCR [22] responded to mitogenic CD28 mAb. The latter result is especially persuasive because it is unlikely that the OT2 T cells would be cryptically activated. These findings in rats and mice are supported by the observations here and by other [2] that mitogenic human CD28 mAbs activate a large fraction of CD4+ T cells.
Second, in a study using cell lines, Dennehy et al. found that mitogenic CD28 signaling required components typically associated with the TCR: CD3-ζ, the CD3-ζ-associated kinase Zap70, and the adaptor molecule SLP-76 [4]. This group argued that mitogenic CD28 mAb enhance “tonic” signaling by the TCR complex, but studies using mice deficient in both class I and class II MHC molecules will be required to rule out low affinity MHC:TCR interactions. The studies of Deenehy et al. were consistent with those of Raab et al. who found (using a non-mitogenic CD28 mAb or CHO cells expressing B7) that CD28 can signal independently of TCR if VAV and SLP-76 are over-expressed [34, 35]. In their studies, CD28 ligation led to PI3-kinase activation and recruitment of VAV and SLP-76 through the plectin-homology domains. This pathway was invoked to explain “in trans” activation in which B7 expressed by “third party” cells can co-stimulate T cells that had previously received Signal One. The TCR-independent CD28 signaling pathway described by Raab et al. [34, 35] might be different from that used by mitogenic CD28 mAb, but a major role for PI3-kinase in signaling through ANC28 was suggested in our work by the inhibition of T cell activation by wortmannin and Ly294002. This result is different than that reported for another mitogenic CD28 antibody, 5.11A1 [21]. Our results, however, do not exclude the possibility that mitosis-associated Polo-like kinases are additional targets [30, 36].
Memory T cells are likely candidates for CD28 signaling since they exhibit enhanced activation properties relative to naïve T cells and have a reduced activation threshold [37, 38]. Normal function by memory CD4 and CD8 T cells were seen in CD28−/− mice [39, 40]. In vitro activation of memory T cells in the absence of CD28 engagement by B7 ligands was also normal [37, 41] The CD28/B7 co-stimulatory pathway was thus generally considered dispensable for memory T cell responses. However, Ndejembi et al reported that, although recall functions like up-regulation of activation marker expression on memory CD4 cells are independent of CD28 co-stimulation, optimal IL-2 production and, most importantly, expansion of memory CD4 T cells, required CD28 costimulation [42]. Interestingly, our findings suggest that signaling through CD28 alone can be sufficient for activation and expansion of memory CD4 T cells.
Like other studies, we observed that ANC28 preferentially expands CD4 T cells over CD8 T cells in unfractionated PBMCs (data not shown), however, we did not find expansion of just Treg by ANC28. Indeed, T-effector cells (CD4+CD25+) expanded more than Treg (CD4+CD25+FoxP3+) in unfractionated PBMCs and in purified CD4 T cells (Fig 4). This is in contrast to an in vitro study using mitogenic CD28 JJ316 [8, 18], which reported a 4-fold greater expansion of Treg than effector CD4 T cells. A major criticism of that study is that CTLA-4 rather than FoxP3 was used to identify Treg.
Lin et al. [18] and Beyersdorf et al [7] stated that mitogenic CD28 expanded purified regulatory T cells. However, in those studies, bead- purified CD4+CD25+ were considered Treg. When we used a similar approach for CD4+CD25+ purification, we also found massive expansion of CD4+CD25+ cells by ANC28 (Fig 7C). However, bead- purified CD4+CD25+ preparations contained not only CD25bright (Treg) but also included CD25int (mixture of T-effectors and Treg) CD4 T cells. When CD4+CD25 bright (highly purified Treg), were purified by flow sorting, they did not respond to ANC28 (Fig 7B), suggesting, that bead – purified CD25+ CD4 T cells (85–90%, pure) contain both CD25int and CD25bright cells, which ultimately confuse interpretation of results. Thus it is entirely possible that the previously reported JJ316 did not selectively expand rat Treg.
Our study offers an explanation for previous in vitro studies in which JJ316 was reported to expand only Treg. Our findings, however, can not explain the reported therapeutic effects and the lack of production of inflammatory cytokines in autoimmune rodent models by JJ316. However, the therapeutic effect might have been due to blockade of CD28 by JJ316, rather than activation of Treg.
The differences observed between anti-human ANC28 and anti-rat JJ316, however, underscore the difficulty of extrapolating from one mAb to another or between species. Indeed, ANC28 is different from other mitogenic CD28 antibodies because it does not require cross-linking and it signals via PI3 kinase.
Stebbings et al did show that the TGN1412 can induces inflammatory cytokines from human PBMCs [20], but it was unclear how this mitogenic CD28 antibody induced inflammatory cytokines if it selectively expanded regulatory T cells. Typically the production of Tregs correlates with anti-inflammatory cytokines, not inflammatory cytokines. Those authors did not explore the mechanism of inflammatory cytokine production nor did they characterize subpopulations of cells expanded by mitogenic CD28. Our results indicate that, with respect to cytokine production, ANC28 resembles the mitogenic human CD28 mAb TGN1412 [20]. If TGN1412 and ANC28 share similar molecular mechanisms and specificity for T cells, our study provides an explanation of the recent failure to translate CD28-driven Treg activation to humans using TGN1412[15].The clinical use of TGN1412 was predicated on the assumption that mitogenic CD28 antibodies selectively expand Treg. However, ANC28 activates and expands all CD4+CD25+ cells (CD25int) including T effectors and Treg rather than just Treg. Our results are consistent with the hypothesis that TGN1412 initiated a “cytokine storm” in the volunteers through polyclonal activation of CD4 CD25int memory T cells, releasing toxic levels of IFN-γ, TNF-α, IL-8 and other inflammatory cytokines.
It now appears that mitogenic CD28 mAbs induce high levels of inflammatory cytokines both in vivo and in vitro challenging the clinical efficacy of these antibodies. The Hünig/Hanke groups reported selective expansion of regulatory T cells by mitogenic rat or human CD28 [6–13], yet the mitogenic anti-human CD28 caused a cytokine storm in the phase I in vivo trial [15, 16]. Our study provides a plausible explanation for this contradiction; (1) stimulation through mitogenic CD28 activates pathogenic effector T cells as well as protective regulatory T cells. (2)ANC28 selectively expands only memory cells whereas naïve cells do not respond with ANC28. Since the activation requirements of memory cells are generally much less stringent than those of naive cells, it is likely that other reported mitogenic CD28 also preferentially activate memory T cells. Therefore, mitogenic antibodies that do not induce widespread immune activation in mice and rats (with predominantly naive T cells) are capable of doing so in humans (who have more memory cells).
This hypothesis predicts that mitogenic CD28 should be pathogenic in mice expressing large numbers of memory cells, but does not explain the failure of TGN1412 to induce inflammation in cynomolgus or rhesus monkeys. In that case, we speculate that subtle difference in the cynomolgus and rhesus CD28 molecules limit the ability of TGN1412 to activate the TCR-independent signaling pathway. If so, studies using mutagenized CD28 molecules should reveal the structural features of CD28 critical for permitting TCR-independent signaling. Although our studies have excluded a requirement for FcR molecules for T cell activation in vitro, it remains possible that species differences between the binding characteristics of monkey and human FcR could contribute to in vivo toxicity.
Stimulation through mitogenic CD28 mAb can be quite distinct from stimulation through other classes of ligands (conventional CD28 mAb; soluble B7; plate-bound ligands). Just as pharmacological agents can imperfectly mimic the natural “physiological” ligands of receptors, yet provide important insights into the function of those receptors, we believe that ANC28 and similar mAb can give us insight into the functions of CD28.
An important observation of our study is that mitogenic CD28 (ANC28) induces stable FoxP3 expression and the cells have suppressive activity. This is in contrast to CD3 stimulation which induces transient FoxP3 expression and cells with no suppressive activity [43]. However, direct comparison between these two studies is not possible because ANC28 generates Tregs from CD25int whereas in other study FoxP3+ expressing T cells are generated from CD25− T cells [43].
ANC28 might be useful tool for dissecting the poorly understood CD28/B7 pathway involved in memory T cell activation and may be useful for ex vivo expansion of antigen specific effecter and regulatory T cells for therapeutic purpose.
Materials and Methods
Isolation of human PBMCs, and purification of different subsets of T cells
All studies using samples from human volunteers follow protocols approved by the BCM institutional review board. PBMC from healthy donors were obtained after informed consent and purified over Ficoll-Hypaque gradients (Sigma Aldrich, St. Louis, MO). CD4+ or CD8+ T cells were purified via negative selection by magnetic cell sorting (Miltenyi Biotech, Auburn, CA) as per manufacturer’s protocols. The purity of subsets averaged 95% as determined by flow cytometry. CD45RA+ or CD45RO+ subsets of CD4 T cells were also purified by negative selection by bead cell sorting (Stem Cell, Vancouver, Canada) with purity greater than 97%. Negatively selected CD4 cells, immunostained with CD25 PE and CD4 FITC were then sorted into three groups; bright CD25+, CD25int or CD25− CD4+ T cells by a FACS-ARIA cell sorter (Becton Dickinson,) with post-sort purity of 97–99%. CD4+CD25+ cells were also purified by magnetic columns (Miltenyi Biotech, Auburn, CA) with 85–90% purity.
CFSE staining
One million cells were suspended in 0.5 µm carboxyfluorescein succinimidyl ester diacetate (CFSE; Sigma-Aldrich) in PBS + 2%FBS and incubated at 37°C for 10 min. Cells were diluted in 10% FBS containing RPMI and washed 3 times in fresh media.
mAb and inhibitors
The following antibodies were obtained from BD Biosciences, San Jose, CA, USA: FITC–conjugated anti-CD4, CD25, CD45RO; PE-conjugated antibodies for CD25, CD45RA and CD69; PE-Cy5 and PE-Cy7-conjugated anti-CD4, CD8 respectively; and unlabeled anti-CD3 and anti-CD28. A conventional, non-mitogenic, CD28 mAb served as negative control (28.2, Mouse IgG1κ, BD Biosciences, Franklin Lakes, NJ). The mitogenic CD28 mAb was “ANC28.1” (unlabeled and PE-conjugated) and its F(ab')2 fragment (clone 5D10; mouse IgG1κ; Ancell Corp., Bayport, MN). To avoid confusion with a conventional mAb named “ANC28.1 clone 15E8” [44], we refer to the 5D10 mAb as “ANC28”. Neutralizing antibodies were IL-10 (JES3-19F1), IL-2, an isotype-matched control from BD Biosciences, TGF-β123 (1D11), TNF-α, and IFN-γ (R&D Systems, Minneapolis, MN). PI3-kinase inhibitors, wortmannin and LY294002, were obtained from Upstate (Temecula, CA) and Calbiochem (San Diego, CA), respectively.
Cell culture
PBMC’s were cultured at 1×106 cells/well or 0.1−0.5×106 cells/well of purified subsets (CD4, CD8, CD25int,CD25+ or CD25−) at 37°C in 5% CO2 in 12 or 24 or 48 well plates in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, HEPES buffer,1 mM sodium pyruvate, non-essential amino acids, 2 mM L-glutamine and 100 U/ml penicillin. Antibodies were used at 3 µg/ml mitogenic (ANC28), 3 µg/ml (conventional 28.2), 10 µg/ml plate-bound anti-CD3, or 1.5 µg/ml soluble anti-CD28 (BD).
Cytokine measurements
Supernatants from cells cultured with CD3, ANC28, 28.2, or co-stimulated with CD3 and either 28.2 or ANC28 were collected after 48 or 72 hrs and cytokines measured by Th1/Th2 and inflammatory cytokine bead array (CBA kit, Becton-Dickinson, San Jose, CA).
Flow cytometry
To measure intracellular Foxp3, cells were stained with PE-Cy5-antiCD4 and FITC or PE-conjugated anti-CD25 before incubating in Fixation Buffer (E-Bioscience, San Diego, CA, USA) for 30 min at 4°C and permeabilisation Buffer (E-bioscience). Cells were blocked with rat serum before staining with PE or FITC–conjugated anti-Foxp3 antibody (PCH101 and 236A/E7; E-Bioscience). Because of a controversy about anti-FoxP3 clone PCH101 specificity[24], we used two different clones for FoxP3 staining. About 1–2% more FoxP3+ CD4 T cells were found using PCH101 compared to 236A/E7. Samples were acquired by flow cytometry (XL2, Beckman Coulter, Inc, Miami, FL) and analyzed by Expo32 software.
Suppression assay
CFSE-labeled, fresh PBMC were cultured with or without the indicated numbers of CD4+CD25+ and co-stimulated with 2 µg/ml soluble anti-CD3 and 2 µg/ml mAb CD28.2 for 5 days. Cell mixtures were cultured in 6-well plates with or without 20 µg/ml anti-TGF-β, anti-IL-10 or isotype-matched control abs or in 4 µM Transwell plates (CoStar, Corning, NY) to separate Treg and responder cells.
Statistical analyses
Results are expressed as the mean ± standard deviation. Differences were tested using paired or unpaired Student’s t tests, as appropriate.
Acknowledgements
We thank Cassandra Horne for cell sorting in preliminary experiments and Richard Cook for critiquing an early version of this manuscript.
This study was supported by NIH grants R21- AI054251, R37-AI36682 to Dorothy E Lewis.
Abbreviations
- PLK
polo-like kinases
- PBMCs
peripheral blood mononuclear cells
Footnotes
Conflict of Interest
We do not have any financial conflicts of interest.
References
- 1.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96:185–190. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siefken R, Kurrle R, Schwinzer R. CD28-mediated activation of resting human T cells without costimulation of the CD3/TCR complex. Cell. Immunol. 1997;176:59–65. doi: 10.1006/cimm.1996.1066. [DOI] [PubMed] [Google Scholar]
- 3.Siefken R, Klein-Hessling S, Serfling E, Kurrle R, Schwinzer R. A CD28-associated signaling pathway leading to cytokine gene transcription and T cell proliferation without TCR engagement. Journal of Immunology. 1998;161:1645–1651. [PubMed] [Google Scholar]
- 4.Dennehy KM, Elias F, Na SY, Fischer KD, Hunig T, Luhder F. Mitogenic CD28 signals require the exchange factor Vav1 to enhance TCR signaling at the SLP-76-Vav-Itk signalosome. J Immunol. 2007;178:1363–1371. doi: 10.4049/jimmunol.178.3.1363. [DOI] [PubMed] [Google Scholar]
- 5.Tacke M, Hanke G, Hanke T, Hunig T. CD28-mediated induction of proliferation in resting T cells in vitro and in vivo without engagement of the T cell receptor: evidence for functionally distinct forms of CD28. Eur J Immunol. 1997;27:239–247. doi: 10.1002/eji.1830270136. [DOI] [PubMed] [Google Scholar]
- 6.Beyersdorf N, Gaupp S, Balbach K, Schmidt J, Toyka KV, Lin CH, Hanke T, Hunig T, Kerkau T, Gold R. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:445–455. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyersdorf N, Balbach K, Hünig T, Kerkau T. Large-scale expansion of rat CD4+ CD25+ Treg cells in the absence of T-cell receptor stimulation. Immunology. 2006 doi: 10.1111/j.1365-2567.2006.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyersdorf N, Hanke T, Kerkau T, Hünig T. CD28 superagonists put a break on autoimmunity by preferentially activating CD4+CD25+ regulatory T cells. Autoimmun Rev. 2006;5:40–45. doi: 10.1016/j.autrev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse B, Hunig T, Sorensen P, Stuart DI, Davis SJ. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Palmero M, Franch A, Castell M, Pelegri C, Perez-Cano FJ, Kleinschnitz C, Stoll G, Hunig T, Castellote C. Effective treatment of adjuvant arthritis with a stimulatory CD28-specific monoclonal antibody. J Rheumatol. 2006;33:110–118. [PubMed] [Google Scholar]
- 11.Schmidt J, Elflein K, Stienekemeier M, Rodriguez-Palmero M, Schneider C, Toyka KV, Gold R, Hunig T. Treatment and prevention of experimental autoimmune neuritis with superagonistic CD28-specific monoclonal antibodies. J Neuroimmunol. 2003;140:143–152. doi: 10.1016/s0165-5728(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 12.Tischner D, Weishaupt A, van den Brandt J, Muller N, Beyersdorf N, Ip CW, Toyka KV, Hunig T, Gold R, Kerkau T, Reichardt HM. Polyclonal expansion of regulatory T cells interferes with effector cell migration in a model of multiple sclerosis. Brain. 2006;129:2635–2647. doi: 10.1093/brain/awl213. [DOI] [PubMed] [Google Scholar]
- 13.Urakami H, Ostanin DV, Hunig T, Grisham MB. Combination of donor-specific blood transfusion with anti-CD28 antibody synergizes to prolong graft survival in rat liver transplantation. Transplant Proc. 2006;38:3244–3246. doi: 10.1016/j.transproceed.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Duff G. Expert Scientific Group [ESG] on Phase One Clinical Trials, Final report. Stationery Office, Norwich UK: 2006. Nov 30, (Chairman) 2006 http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_073165.pdf. [Google Scholar]
- 15.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 16.Corry DB, Lewis DE. Cytokine storm and an anti-CD28 monoclonal antibody. N Engl J Med. 2006;355:2592. [PubMed] [Google Scholar]
- 17.Ohresser M, Olive D, Vanhove B, Watier H. Risk in drug trials. Lancet. 2006;368:2205–2206. doi: 10.1016/S0140-6736(06)69883-8. [DOI] [PubMed] [Google Scholar]
- 18.Lin CH, H T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003 Mar;33:626–638. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- 19.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stebbings R, Findlay L, Edwards C, Eastwood D, Bird C, North D, Mistry Y, Dilger P, Liefooghe E, Cludts I, Fox B, Tarrant G, Robinson J, Meager T, Dolman C, Thorpe SJ, Bristow A, Wadhwa M, Thorpe R, Poole S. "Cytokine Storm" in the Phase I Trial of Monoclonal Antibody TGN1412: Better Understanding the Causes to Improve PreClinical Testing of Immunotherapeutics. J Immunol. 2007;179:3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- 21.Sester U, Wabnitz GH, Kirchgessner H, Samstag Y. Ras/PI3Kinase/cofilin-independent activation of human CD45RA(+) and CD45RO(+) T cells by superagonistic CD28 stimulation. Eur J Immunol. 2007;37:2881–2891. doi: 10.1002/eji.200737206. [DOI] [PubMed] [Google Scholar]
- 22.Legrand N, Cupedo T, van Lent AU, Ebeli MJ, Weijer K, Hanke T, Spits H. Transient accumulation of human mature thymocytes and regulatory T cells with CD28 superagonist in "human immune system" Rag2−/−γc−/− mice. Blood. 2006;108:238–245. doi: 10.1182/blood-2006-01-0190. [DOI] [PubMed] [Google Scholar]
- 23.Furtado GC, Curotto de LaFaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J. Exp. Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3- T cells by T cell receptor stimulation is TGF{beta}-dependent but does not confer a regulatory phenotype. Blood. 2007 doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 26.Hanawa H, Ma Y, Mikolajczak SA, Charles ML, Yoshida T, Yoshida R, Strathdee CA, Litchfield DW, Ochi A. A novel costimulatory signaling in human T lymphocytes by a splice variant of CD28. Blood. 2002;99:2138–2145. doi: 10.1182/blood.v99.6.2138. [DOI] [PubMed] [Google Scholar]
- 27.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110 isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 28.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B, Okkenhaug K. Cutting edge: the phosphoinositide 3-kinase p110δ is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 29.Mirenda V, Jarmin SJ, David R, Dyson J, Scott D, Gu Y, Lechler RI, Okkenhaug K, Marelli-Berg FM. Physiologic and aberrant regulation of memory T-cell trafficking by the costimulatory molecule CD28. Blood. 2007;109:2968–2977. doi: 10.1182/blood-2006-10-050724. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Jiang N, Wu J, Dai W, Rosenblum JS. Polo-like kinases inhibited by wortmannin. Labeling site and downstream effects. J Biol Chem. 2007;282:2505–2511. doi: 10.1074/jbc.M609603200. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol. 2005;12:99–107. doi: 10.1016/j.chembiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Schafer PH, Wadsworth SA, Wang L, Siekierka JJ. p38 alpha mitogen-activated protein kinase is activated by CD28-mediated signaling and is required for IL-4 production by human CD4+CD45RO+ T cells and Th2 effector cells. J Immunol. 1999;162:7110–7119. [PubMed] [Google Scholar]
- 33.Lewis DE, Merched-Sauvage M, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-alpha and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J Biol Chem. 2004;279:29130–29138. doi: 10.1074/jbc.M402194200. [DOI] [PubMed] [Google Scholar]
- 34.Raab M, Pfister S, Rudd CE. CD28 signaling via VAV/SLP-76 adaptors. Regulation of cytokine transcription independent of TCR ligation. Immunity. 2001;15:921–933. doi: 10.1016/s1074-7613(01)00248-5. [DOI] [PubMed] [Google Scholar]
- 35.Rudd CE, Raab M. Independent CD28 signaling via VAV and SLP-76: a model for in trans costimulation. Immunol Rev. 2003;192:32–41. doi: 10.1034/j.1600-065x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 36.Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem Biol. 2005;12:621–637. doi: 10.1016/j.chembiol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 37.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J. Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 38.Dutton RW, B L, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 39.Ekkens MJ, Liu Z, Liu Q, Foster A, Whitmire J, Pesce J, Sharpe AH, Urban JF, Gause WC. Memory Th2 effector cells can develop in the absence of B7-1/B7-2, CD28 interactions, and effector Th cells after priming with an intestinal nematode parasite. J Immunol. 2002;168:6344–6351. doi: 10.4049/jimmunol.168.12.6344. [DOI] [PubMed] [Google Scholar]
- 40.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 41.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 42.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 44.Romano MF, Bisogni R, Lamberti A, Turco MC, Petrella A, Tassone P, Van LR, Formisano S, Venuta S. Regulation of NFκB nuclear activity in peripheral blood mononuclear cells: role of CD28 antigen. Cellular Immunology. 156:371–377. doi: 10.1006/cimm.1994.1182. [DOI] [PubMed] [Google Scholar]