Abstract
Purpose
To assess the incidence of vitreomacular adhesion and traction in age-related macular degeneration (AMD), and to evaluate surgical treatment in a subset of patients with choroidal neovascularization (CNV) nonresponsive to anti-neovascular growth factor (anti-VEGF) treatment.
Design
Retrospective observational case-control and interventional case series.
Methods
Spectral optical coherence tomography, combined with simultaneous scanning laser ophthalmoscope (Spectral OCT/SLO), was performed in 170 eyes of 94 elderly patients, 61 with exudative AMD, 59 with nonexudative AMD, and 50 control eyes. The presence of hyaloid adhesion to the posterior pole, and vitreomacular traction (VMT) were determined. Five patients with VMT underwent surgical hyaloid removal. Best-corrected visual acuity (BCVA) and retinal thickness were evaluated as outcomes.
Results
Hyaloid adhesion was present in 17 eyes with exudative AMD (27.8%), 15 eyes with nonexudative AMD (25.4%), and eight control eyes (16%). Significant difference was found among the groups (P = .002). Among the eyes with hyaloid adhesion, VMT was shown in 10 eyes (59%) with exudative AMD, two eyes (13%) with nonexudative AMD, and one control eye (12%). VMT was associated with the severity of AMD (P = .0082). The area of hyaloid adhesion was significantly smaller than and concentric to the area of CNV complex in eyes with exudative AMD. Eyes with VMT that underwent surgery experienced a modest improvement of BCVA and decrease of retinal thickness.
Conclusions
Hyaloid adhesion to the macula is associated with AMD, and frequently causes VMT in eyes with CNV. Tractional forces may antagonize the effect of anti-VEGF treatment, and cause pharmacological resistance in a subpopulation of patients. Future studies are needed to define the role of vitreoretinal surgery in such cases. Spectral OCT/SLO allows careful diagnosis and follow-up.
Age-related macular degeneration (AMD) IS the leading cause of severe visual acuity (VA) loss in industrialized countries.1–3 Although the pathogenesis of the disease is still under investigation, it is clear that the defect lies in the outer retina and retinal pigment epithelium (RPE), and that genetic predisposition has been shown to play a major role in its development.4–6 The identified genes mainly control the immune responses in the posterior segment, directly or secondarily affecting the RPE environment and function.7 Although AMD primarily involves the outer retinal layers, several authors have suggested that the vitreous may possibly play a role in the pathogenesis and/or progression of AMD. A high incidence of posterior vitreous attachment was intraoperatively observed by different authors.8 Subsequent studies of the vitreous in AMD have been performed using ultrasound, which suggest that complete posterior vitreous detachment (PVD) occurs less frequently in AMD than in the age-matched elder normal population, and that a higher incidence of vitreomacular adhesion is detected in both exudative and nonexudative AMD.9,10 Conventional time-domain optical coherence tomography (OCT) has also been used to evaluate the vitreous in AMD, and such studies have suggested that there is a higher rate of vitreomacular adhesion in exudative AMD, while no difference was found between nonexudative AMD and normal population.11 Different hypothesis have been formulated to explain the role of vitreomacular adhesion in AMD, focusing on both mechanical and biochemical factors underlying the observed phenomenon.10
We wished to determine the relationship between exudative AMD, nonexudative AMD, and elder normal eyes in terms of the presence and extent of the hyaloid attachment to the retina. We elected to evaluate this using a new technology, Spectral domain OCT (SD-OCT), which provides dramatic improvements in the speed and resolution of imaging of the vitreoretinal interface and other macular diseases.12–15 Here, we evaluated a novel such instrument, which couples SD-OCT with the scanning laser ophthalmoscope (SLO), allowing a highly precise co-localization of optically detected lesion in the 2-dimensional image of the posterior pole (OTI-Spectral OCT/SLO; OTI Inc, Toronto, Canada). With this dual simultaneous imaging, it is possible to correlate fundus details with ultrahigh resolution OCT, while the high sensitivity of the instrument allows observation of subtle details of the vitreous that time-domain OCT cannot visualize.
The purpose of our study was to determine the frequency of persistent hyaloid adherence to the posterior pole in patients with AMD, and to determine whether this phenomenon diverges from the physiological PVD process. The hypothesis is that a local inflammation induced by AMD is causing focal corresponding adhesion, potentially inducing tractional forces on the retina. This is important because the unresponsive chronic macular edema overlying the choroidal neovascularization (CNV) process could in theory be related to vitreomacular traction (VMT) and might explain the resistance of some eyes to new anti-vascular endothelial growth factor (anti-VEGF) treatments.
We chose to use OTI-Spectral OCT/SLO because we have previously demonstrated that this device allows excellent detection of subtle inner retina pathology, and in some eyes it even allows visualization of the intact vitreous cortex (Nigam N, et al. IOVS 2006;116:ARVO E-Abstract B225).
Methods
A consecutive case series of nonexudative and exudative AMD patients, who were referred to the Jacobs Retina Center at the University of California San Diego, in La Jolla, California and who agreed to Spectral OCT scanning, was reviewed. All patients had AMD and underwent a complete ophthalmologic examination, VA testing with standardized refraction using Early Treatment Diabetic Retinopathy Study (ETDRS) charts, and slit-lamp biomicroscopy was performed in mydriasis with either a 78 or a 90 diopters (D) indirect lens. The finding of the Weiss ring, or a clearly visible posterior vitreous cortex that moved freely in the vitreous cavity, indicated the presence of a complete PVD.16 The diagnosis of AMD was based on the clinical presence of drusen with RPE changes, geographic atrophy or CNV. All eyes underwent fluorescein angiography (FA) exam and fundus color photographs. We excluded eyes with coexisting diseases involving the posterior pole or that would affect the presence of vitreomacular adhesion, such as diabetic retinopathy, inflammatory diseases, vascular occlusions, and high myopia. Eyes that had previous vitreoretinal surgery were also excluded, as well as eyes with the presence of media opacities, which would prevent good visualization of the fundus. A control group of consecutive patients seen in clinic, who did not have AMD or any visible retinopathy, was also analyzed with the same technique to determine the presence of PVD.
This was a retrospective analysis of a consecutive series of patients. All patients were examined using a Spectral OCT (OTI-Spectral OCT/SLO, OTI Inc).
Imaging Technique
The light source of the OTI-Spectral OCT/SLO is a modified superluminescent diode, with a bandwidth of 40 nm centered at an 830 nm wavelength. The axial resolution is equal to up to 5 microns, and the transverse one is 16 microns. The speed is 28,000 A-scans/sec. Every B-scan is composed of 512 A-scans and is acquired in 0.18 seconds. The OCT is coupled to a SLO. In the sensing arm of the OCT, a beam splitter redirects part of the light returned from the eye toward a confocal optical receiver. Both OCT and confocal signals carrying the information about the reflectivity of the target are collected by a dual-input, which displays two images on a screen under computer control. A strict topographic correspondence of the OCT cross-sectional image and the SLO image allows the operator to sweep the cursor all over the posterior pole, focusing on specific notable areas. Each posterior pole series of B-scans contains 128 images, covering an area of 8 mm × 8 mm throughout the posterior pole. In the current study, high quality series of scans (horizontally and vertically oriented, respectively) were performed for each eye. The SLO image was centered on the fovea or on the exudative lesion, when present, on the basis of the clinical and angiographic findings. The optic nerve was scanned as well, to evaluate the attachment of the vitreous to its edge. The evidence of PVD was determined by focusing the instrument on superficial retinal layers and into the vitreous cavity. According to the absence or presence of any adhesion of the hyaloid to the retina surface in the scanned area, the eye was classified into one of the following three categories (modified from Uchino and Johnson17,18): 1) no evidence of posterior hyaloid adhesion; 2) persistent adhesion of hyaloid only to the optic disk; or 3) persistent adhesion of hyaloid to the macula (in any of the four quadrants). Statistically, we ordered the categories from least to most pathology as follows: no adhesion < adhesion to optic disk < persistent adhesion to macula.
Determination of Vitreomacular Traction in Eyes with Hyaloid Adhesion
In those eyes that showed an attachment of the hyaloid to the macula, the adhesion area was carefully analyzed using Spectral OCT; a VMT was diagnosed when a steep slope of the inner macular surface, or a sharp angulation and localized deformation of the retinal profile, was detected at the adhesion site of the hyaloid. Otherwise, VMT was considered to be not present.
Analysis of Hyaloid Adhesion Area, Choroidal Neovascularization Area, and Respective Location in Eyes with Exudative Age-Related Macular Degeneration
Those eyes with exudative AMD and macular attachment of the hyaloid, with well detected hyaloid in the vertical and horizontal scans, were evaluated for further analysis, as reported. The location and extent of the AMD lesion was identified with the Spectral OCT. All the areas involved in the CNV complex were considered: CNV, fibrosis, pigment epithelial detachment (PED), subretinal or intraretinal fluid, diffusely thickened retina, and hard exudates. The simultaneous SLO corresponding image allowed the operator to identify their precise location, as related to posterior pole anatomic landmarks. The vertical and horizontal diameters of the affected region were measured in mm with calipers provided on the OTI-Spectral OCT/SLO software and used to determine the lesion area. The same procedure was performed for the hyaloid area that showed adhesion to the inner retina. The formula for an ellipse was used to estimate both area measurements. The anatomic location of the exudative lesion and the hyaloid adhesion were then analyzed; for this purpose the horizontal scan through the fovea was reviewed. The distance in between the center of the hyaloid attachment and the center of the fovea was measured, and the same procedure was repeated using the center of the CNV complex. The two distances were then compared.
Surgical Intervention
A small number of patients, who showed persistent attachment of hyaloid to the macula, with evidence of VMT, were surgically treated. These patients had a history of poorly responsive CNV despite aggressive anti-VEGF therapy (≥6 intravitreal injections of Bevacizumab or Ranibizumab), demonstrable vitreomacular adhesion, and were willing to undergo surgery. One patient had progressive drusenoid RPE detachment with a decrease in VA. All surgeries were performed by the same surgeon (W.R.F.). A tran pars plana vitrectomy (TPPV) was performed with 25-gauge three port instruments (Accurus Surgical System; Alcon Inc, Fort Worth, Texas, USA). After core vitrectomy, the posterior hyaloid was engaged with a soft-tipped cannula, or a vitreous cutter, to create posterior hyaloid detachment. Particular care was taken during this surgical phase of removing the hyaloid adhesion. The surgery was monitored with video recording and a subsequent review of the findings was performed by another author (F.M.) to confirm that the hyaloid traction was removed. In some cases SF6 or C3F8 were used for tamponade. Intravitreal Bevacizumab was administered at the end of the surgery and on a monthly basis in all of the exudative AMD cases. Neither triamcinolone acetonide nor other treatments for CNV were used. A complete eye exam was performed at day one, seven, and 14 of the follow-up, and at each subsequent month. Best-corrected visual acuity (BCVA) was measured with ETDRS charts at baseline and at each follow-up. Spectral OCT scanning was repeated at month one and subsequently every three months.
Statistical Analysis
To delineate any association between macular abnormality and vitreomacular adhesion, an ordinal logistic regression was simulated using the graded vitreomacular adhesion as the response variable, and using the macular disease status as a predictor. The eyes were classified into three categories, normal (0), nonexudative AMD (1), and exudative AMD (2) (severity order). The adhesion of hyaloid to the posterior pole was also graded in order of severity: no adhesion presence < adhesion to the disk < adhesion to the macula. In addition, the vitreomacular adhesion was also classified as binary response (presence of macular hyaloid adhesion or no macular adhesion; eyes with hyaloid adhesion to the disk and no adhesion were considered as part of the same group). Both an ordinary logistic regression and a binary logistic regression were performed. Attributable to the fact that both eyes of the same subject were used in this study, Generalized Estimating Equations (GEE) was applied to analyze the data to control for intereye dependence; gender, age and lens status were analyzed as the covariates.19,20P values are reported as two-tailed test results.
For the subanalysis of the eyes with hyaloid adhesion to the macula, the adhesion was graded as tractional or nontractional according the configuration of the retinal profile. The Chi-square along with the Cochran-Armitage trend test were utilized.
In addition, within the exudative AMD group, we analyzed the possible correlation between vitreomacular adhesion and types of CNV using GEE, and the possible relationship between tractional vitreoretinal adhesion and the type of CNV using the Fisher exact test. We also analyzed the topographic relationship between the CNV complex and the vitreomacular adhesion by correlating the distance between the fovea and the center of the CNV complex and the hyaloid adhesion, respectively. LogMAR VA was used for the statistic, as the eyes were all tested on the ETDRS chart. All statistical analysis was performed using SAS software version 9.1 (SAS Inc, Cary, North Carolina, USA).
Results
OTI-Spectraloct/SLO was performed in 170 eyes of 94 patients. Fifty-eight patients were females, 36 were males. The average age for the entire group was 76 years (SD ± 8.4). Eighty-three eyes were phakic and 87 were pseudophakic. AMD was diagnosed in 120 eyes (61 eyes had exudative AMD and 59 nonexudative AMD). Fifty eyes showed no evidence of AMD and constituted the control group of the study.
The average age of patients with exudative AMD was 79.02 years (SD ± 7.21), while it was 75.29 years (SD ± 9.08) in the group of nonexudative AMD and in the control group it was 72.12 years (SD ± 7.48). At a careful biomicroscopical exam, 16 eyes showed a complete PVD in the exudative AMD group (26%), 13 in the nonexudative group (22%), and 13 in the control group (26%).
The ordinal logistic regression revealed that age and gender were the significant covariates (P < .0001 and P = .0065). After adjustment for these effects, vitreomacular adhesion was significantly associated with macular abnormality (P = .002; Table 1). Further analysis showed that, when compared with the controls, both exudative and nonexudative AMD eyes were significantly more likely to have vitreomacular adhesion (odds ratio (OR), 6.3 for exudative vs control and 3.2 for nonexudative vs control; P = .0012 and P = .0141, respectively). In another analysis, eyes with the hyaloid attached only to the optic disk and eyes with no adhesion were considered to be part of the same group: the binary logistic regression showed similar results. The significant covariates were age and gender (P = .0018 and P = .0065). After adjustment of these effects, vitreomacular adhesion was again associated with macular abnormality (P = .01). The analysis revealed that, when compared with the control eyes, exudative AMD were significantly more likely to have vitreomacular adhesion (OR, 5.3; P = .0052). Compared with control eyes, nonexudative AMD showed a statistically marginal association with vitreomacular adhesion (OR, 2.7; P = .06). No statistical significance was found when comparing exudative and nonexudative AMD eyes. (OR, 1.9; P = .15) (Table 2).
TABLE 1.
Ordinal Logistic Regression of Hyaloid Appearance in Exudative Age-Related Macular Degeneration, Nonexudative Age-Related Macular Degeneration and Control Group with Spectral Optical Coherence Tomography
| No Evidence of Hyaloid Adhesion | Hyaloid Adhesion to Disk Only | Hyaloid Adhesion to Macula | Total | |
|---|---|---|---|---|
| Exudative AMD (%) | 40 (65.6) | 4 (6.6) | 17 (27.8) | 61 |
| Nonexudative AMD (%) | 38 (64.4) | 6 (10.2) | 15 (25.4) | 59 |
| Control (%) | 39 (78) | 3 (6) | 8 (16) | 50 |
| Total | 117 | 13 | 40 | 170 |
| *P =.002 |
AMD = age-related macular degeneration.
In parenthesis are reported the percent of eyes.
Ordinal Logistic Regression (GEE) for decrease severity association with hyaloid adhesion corrected for covariates: gender and age.
TABLE 2.
Binary Logistic Regression of Vitreomacular Adhesion in Exudative AMD, Nonexudative Age-Related Macular Degeneration, and Control Group
| Group | Hyaloid Adhesion to Macula (%) | *P | Odds Ratio (Confidence Intervals) |
|---|---|---|---|
| Exudative AMD | 17 (27.8) | .005 | 5.3 (1.64 to 17.45) |
| Control | 8 (16) | ||
| Exudative AMD | 17 (27.8) | .15 | 1.975 (0.78 to 4.99) |
| Nonexudative AMD | 15 (25.4) | ||
| Nonexudative AMD | 15 (25.4) | .06 | 2.7 (0.96 to 7.65) |
| Control | 8 (16) |
AMD = age-related macular degeneration; vs = versus.
In parenthesis are reported the percent of eyes.
Binary Logistic Regression (GEE).
The 40 eyes that had hyaloid adhesion to the macula were further analyzed for the presence of a tractional component. The analysis showed that VMT was significantly associated with the presence of AMD (P = .0082 likelihood ratio, Chi-square test) and that there was a significant increase in VMT when the disease category changed from control to nonexudative AMD to exudative AMD (P = .007 Cochran-Armitage trend test; Table 3; Figure 1).
TABLE 3.
Role of Traction in Eyes with Vitreomacular Adhesion. SubAnalysis of Eyes with Vitreomacular Adhesion (n = 40); Spectral Optical Coherence Tomography Proportion of Vitreomacular Traction vs Absence of Traction in Exudative Age-Related Macular Degeneration, Nonexudative Age-Related Macular Degeneration, and Control Eyes
| Hyaloid Adhesion to Macula without VMT | Hyaloid Adhesion to Macula with VMT | |
|---|---|---|
| Exudative AMD (%) | 7 (41) | 10 (59) |
| Nonexudative AMD (%) | 13 (87) | 2 (13) |
| Control (%) | 7 (88) | 1 (12) |
| Total (%) | 27 (68) | 13 (32) |
| P = .0082 (likelihood ratio, Chi-square) | ||
| P = .007 (Cochran-Armitage trend test) | ||
AMD = age-related macular degeneration; OCT = optical coherence tomography; VMT = vitreomacular traction.
In parenthesis are reported the percent of eyes.
FIGURE 1.
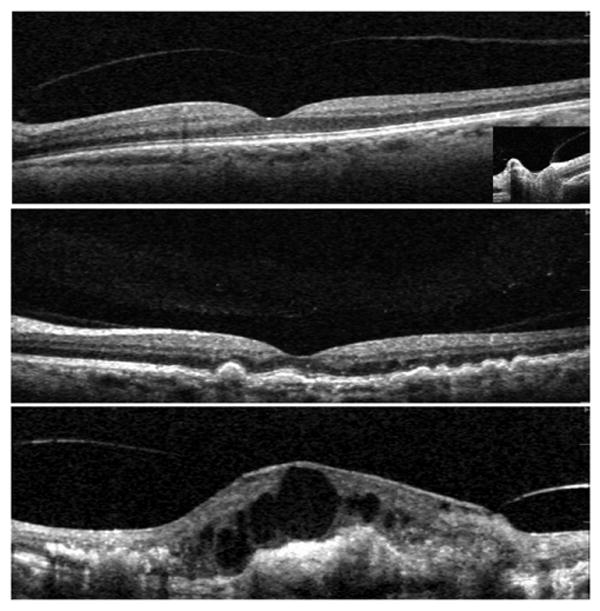
Classification scheme of hyaloid adhesion and vitreomacular traction (VMT) seen with Spectral optical coherence tomography (OCT). (Top) A normal eye of an elder patient: the hyaloid membrane is visible and completely detached from the fovea, though there is a persistent adhesion to the optic nerve (right frame). (Middle) Eye with nonexudative age-related macular degeneration (AMD) and drusen: the hyaloid is attached over the entire macula, including the fovea. We considered this as a “no traction” configuration, since no distortion is visible on the retinal surface and the angle of insertion of the hyaloid onto the retina is not steep. The lucency between the center of the macula and the vitreous collagen is likely the posterior precortical vitreous pocket. (Bottom) Eye with choroidal neovascularization (CNV). The persistence of hyaloid adhesion causes VMT over the CNV complex: a focal distortion of the retinal profile is visible at the site of hyaloid attachment.
Among the eyes with exudative AMD, it was possible to clearly detect the attachment site of the hyaloid to the inner retina in both the vertical and horizontal scans in 13 eyes, which were considered for the anatomic analysis. The average area of the exudative lesion in these eyes was 26.62 mm2 (SD ± 17.08), while the average area of attached hyaloid was 9.94 mm2 (SD ± 9.76). The smaller size of the vitreous adhesion area as compared with the CNV complex was statistically significant (P = .01). There was no significant correlation among the size of the two entities. In these 13 eyes, the 2-dimensional relationship between the CNV complex and the vitreomacular adhesion was analyzed by correlating the distance between the fovea and the center of the CNV complex and adhesion. This was done to determine if the hyaloid adhesion and the CNV complex were concentric. The mean distance between the center of the hyaloid adhesion and the fovea was 0.5 mm (SD ± 0.46), while the mean distance between the center of the CNV complex and the fovea was 0.49 mm (SD ± 0.40). The anatomic location of the hyaloid adhesion was significantly correlated to the anatomic location of the CNV complex (P = .048, pairwise correlation; Figure 2).
FIGURE 2.
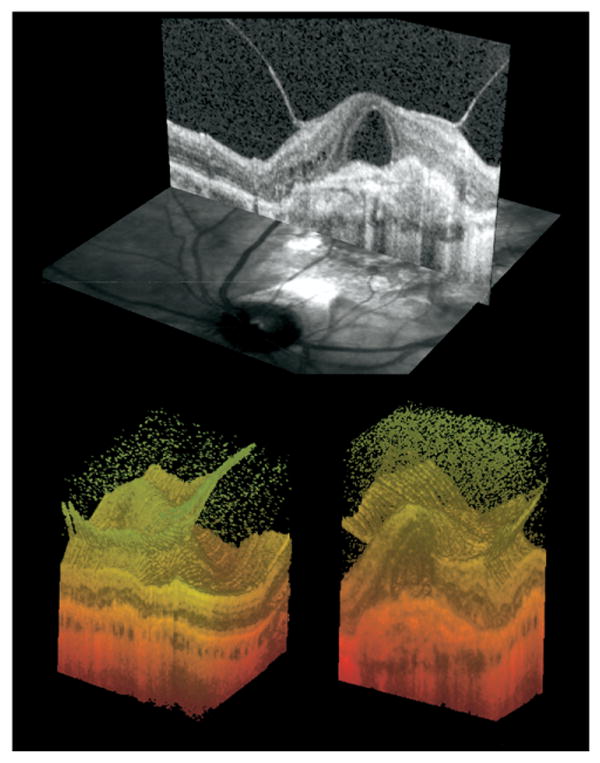
Anatomic configuration of hyaloid adhesion and CNV complex. (Top) Cross-sectional view of Spectral OCT B-scan and corresponding scanning laser ophthalmoscope (SLO) image. The simultaneous images allow for precise topographic correlation of the OCT findings. (Bottom) Three-dimensional reconstruction of the same eye in two different perspectives. On the left, the surface of the retina and the continuous adhesion of the hyaloid are clearly seen. On the right, a slice through the fovea is showing the exudative complex and its relationship to the hyaloidal adhesion.
In the subgroup analysis (exudative AMD), we found that minimally classic lesions showed significantly higher incidence of local vitreomacular adhesion than classic (OR, 7.7, 1.72 to 34.8; P = .0077) and occult (OR, 7.0, 1.17 to 41.6; P = .0327). However, a Chi-square analysis did not show association between tractional vitreomacular adhesion and types of CNV (classic 3/10 vs minimally classic 6/10 vs occult 1/10; P = .4).
Five patients with evidence of VMT had 25-gauge TPPV with hyaloid removal. Four patients experienced an improvement of VA after surgery, as assessed at the end of the follow-up. In one eye the VA remained stable. The mean VA improvement was 1.4 ETDRS lines (SD ± 1.14; P = .052, two-tailed paired Student t test). Four patients had showed a decrease of OCT foveal thickness, when compared with the preoperative exam. The patient with the shortest follow-up showed a stable retinal thickness on OCT. The mean retinal thickness decrease on Spectral OCT was −365.4 micron (SD ± 385 micron; P = .092, two-tailed paired Student t test). No complications occurred during the surgeries and the follow-up period. All the patients affected by exudative AMD, after surgery, were treated with intravitreal Bevacizumab injection (2.5 mg) every four weeks. The treatment was ongoing at the last follow-up. Details are reported in Table 4; Figures 3 and 4 show examples of OCT changes in two patients.
TABLE 4.
Surgical Results of Hyaloid Removal in Five Consecutive Cases with Vitreomacular Traction
| Patient Number |
Group | Lens Status | Follow-up (weeks) |
Preoperative Treatments |
Surgical Adjuvant |
Postoperative Treatments |
Baseline VA (ETDRS Chart) |
Final VA (ETDRS Chart) |
Change in VA (ETDRS Lines) |
Baseline OCT Foveal Thickness (micron) |
Final OCT Foveal Thickness (micron) |
Change in OCT Foveal Thickness (micron) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nonexudative AMD (Drusenoid PED) |
Phakic | 24 | None | None | None | 20/80 | 20/40 | +3 | 409 | 160 | −249 |
| 2 | Exudative AMD | Pseudophakic | 32 | PDT#2 Ranibizumab#3 Bevacizumab#3 |
C3F8 Bevacizumab | Bevacizumab#4 (2.5 mg) |
20/40 | 20/40 | 0 | 343 | 296 | −47 |
| 3 | Exudative AMD | Pseudophakic | 36 | Bevacizumab#6 | SF6 Bevacizumab | Bevacizumab#3 (2.5 mg) |
2/200 | 20/400 | +2 | 1103 | 224 | −879 |
| 4 | Exudative AMD | Pseudophakic | 24 | Bevacizumab#8 | Bevacizumab | Bevacizumab#6 (2.5 mg) |
20/400 | 20/320 | +1 | 1156 | 504 | −652 |
| 5 | Exudative AMD | Pseudophakic | 8 | FVT#2 PDT#1 Ranibizumab#9 |
SF6 Bevacizumab | Bevacizumab#2 (2.5 mg) |
20/400 | 20/320 | +1 | 858 | 858 | 0 |
| Mean ± SD | 20/250 | 20/150 | +1.4 ± 1.14 | 773.8 | 408.4 | −365.4 ± 385 | ||||||
| *P | .052 | .092s | ||||||||||
AMD = age-related macular degeneration; ETDRS = Early Treatment Diabetic Retinopathy Study; FVT = feeder vessel treatment; PED = pigment epithelial detachment; PDT = photodynamic therapy; SD = standard deviation.
Two-tailed paired Student t test.
FIGURE 3.
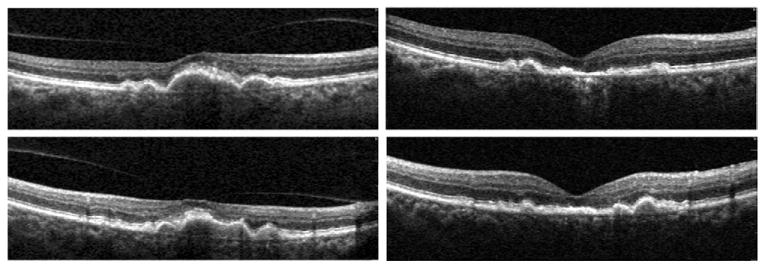
Drusenoid retinal pigment epithelial (RPE) detachment with VMT, surgical outcome (Patient 1). This patient experienced a recent decrease in vision to 20/80. (Top left) Horizontal Spectral OCT scan through the fovea shows hyaloid attachment to the apex of the pigment epithelial detachment (PED) causing macular traction. Postoperatively (Top right), a scan in the same anatomic location shows collapse of the large PED, some residual RPE changes, and a focal photoreceptor defect. Vitreous is absent. Vision improved to 20/40. The corresponding vertical scans done preoperatively (Bottom left) and postoperatively (Bottom right) confirm the release of VMT and the PED collapse.
FIGURE 4.
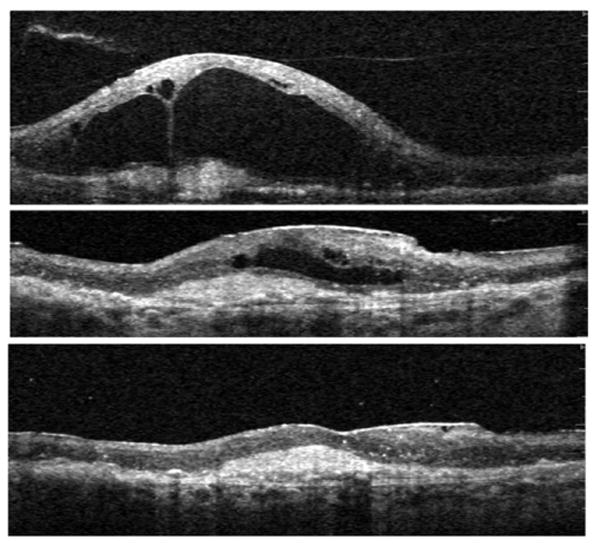
Exudative AMD with VMT, surgical outcome (Patient 4). Vertical Spectral OCT scans through the center of the macula are shown. (Top) Baseline scan shows vitreous adherent to the apex of large cavities within edematous retina, and an underlying CNV complex; this fluid persisted despite eight previous monthly injections of intravitreal Bevacizumab. (Middle) Image shows center of macula one month after vitrectomy with removal of the attached hyaloid membrane. Fluid is reabsorbing (note: the scale is adjusted to facilitate direct comparison with the other scans). (Bottom) (same magnification as top) six months follow-up: complete reabsorption of intra- and sub-retinal fluid. Monthly Bevacizumab treatment was continued after surgery. Vision improved by one line, from 20/400 to 20/320.
One patient with exudative AMD and VMT, caused by a small area of focal hyaloid adhesion, experienced a spontaneous resolution of the attachment, with decrease of macular thickness. Vision did not improve despite ongoing monthly treatments with intravitreal injections of Bevacizumab (Figure 5).
FIGURE 5.
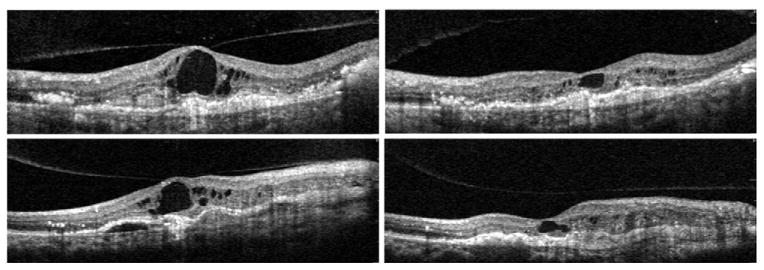
Spontaneous resolution of VMT in exudative AMD. Four scans through the fovea of the same eye. (Top left) Large amount of intraretinal fluid with cystic formation that persisted after three monthly intravitreal injections of Bevacizumab. (Top right) Same view after five months. A spontaneous release of the central VMT is detected by Spectral OCT. (Bottom left) Vertical scan through the fovea at the same time as the top left panel. (Bottom right) Vertical view after the spontaneous release of traction. There was no improvement in vision despite ongoing treatment.
Discussion
Our study clearly shows that eyes with exudative AMD have a significantly higher incidence of incomplete PVD than elder normal eyes and a similar trend exists also when comparing nonexudative AMD with the control group. We have also shown that there is a higher incidence of tractional configuration of the attached posterior hyaloid in eyes with exudative AMD. Other investigators have also suggested a possible role of persistent vitreomacular adhesion in eyes with exudative macular degeneration.9–11,21 Those studies were limited in several ways, and particularly in the use of ultrasound and/or time domain OCT to evaluate the phenomenon. Our evaluative technique, confocal simultaneous SLO fundus imaging performed simultaneously with ultra-high speed and ultra-high resolution Spectral OCT, allows the precise anatomic localization of the vitreoretinal adhesion in relationship to the CNV complex. Our study confirms some of the findings of other authors and gives new and statistically significant information, including the 3-dimensional configuration of the VMT in exudative AMD cases, the size of the hyaloid adhesion, and its concentricity to the CNV complex. We furthermore studied the response of a subpopulation of eyes to surgical intervention. Discrepancies exist in the literature when nonexudative AMD eyes are analyzed. Ondes found no statistically significant difference between the exudative and nonexudative groups with respect to PVD.10 In contrast, Krebs reported no statistically significant difference when comparing nonexudative eyes with a control group.11 Our analysis was conducted with the more advanced instrumentation and showed that the hyaloid adhesion is more frequent in nonexudative AMD than in control eyes.
We did not find a correlation between the size of the hyaloid attachment and the area of the CNV complex. This is not surprising, as the duration of the disease and other factors may affect this finding. It is interesting, and probably physiologically important, that in our study we did find a concentric configuration of the two processes, suggesting a causal relationship. Nonexudative AMD is mainly represented by multiple lesions variably spread throughout the macula. Being impossible to define the exact anatomical border in these cases, we could just evaluate the topographical relationship existing among the CNV complex in exudative AMD and the incomplete PVD. A trend was nevertheless seen when the group of nonexudative AMD patients was compared with controls, suggesting that even initial AMD stages might induce a reaction at the inner interface of the retina.
It is interesting to speculate on the cause of vitreomacular adhesion, which is seen most frequently in exudative AMD eyes, but also more often in nonexudative eyes than elder normal eyes. Furthermore, in exudative AMD patients, minimally classic lesions had a higher incidence of focal vitreomacular adhesion. The results are indicative that a slower, more chronic, and indolent process, and the consequent persistent low grade of local inflammation, are more likely to induce structural changes at the level of the vitreoretinal interface. The majority of eyes in our study had minimally classic CNV and we therefore do not have an adequate number of eyes to test whether VMT is statistically associated with minimally classic CNV as opposed to other types. The persistent adhesion of the hyaloid to the posterior pole, with or without VMT, is not the underlying cause of AMD. It has become clear, recently, that the cause of AMD is one of several genetic defects, which affect the outer retina and RPE. Other authors suggested that vitreomacular adhesion might be considered a risk factor for the natural history of this disease.11,22–24 This would facilitate in fact the progression from nonexudative to exudative forms of AMD. The hypotheses are multiple: persistent vitreomacular adhesion might induce chronic low-grade inflammation, prevent normal oxygen and nutrients diffusion to the macula, and/or confine proangiogenic cytokines in the macula. Thus, there is no suggestion that vitrectomy may be useful in preventing the progression of AMD. We know that both inflammation and disruption of the photoreceptor-RPE interface, resulting in cellular apoptosis in the outer retina, can induce the recruitment of Müller cells and possibly astrocytes to become reactive. Reactive gliosis is associated with physical changes such as hypertrophy as well as biochemical changes, including the secretion of various inflammatory cytokines and reversal of ion pumps and homeostatic buffering.25–28 These changes could directly affect retinal-vitreal adhesion points that induce firmer fibrous attachments between the retina and vitreous. Nonexudative AMD precedes exudative AMD by years.29,30 We have shown that nonexudative AMD eyes also have a higher incidence of vitreomacular adhesions than control eyes, but not as much as in exudative AMD eyes. The additional insult of CNV in eyes with exudative AMD likely increases the VMT through interactions with the Müller cell footplates and posterior hyaloid membrane. Our study clearly shows that anterior-posterior forces, present at the surface of the retina, may exacerbate the macular edema, which originally occurs secondary to the presence of leaking vessels in the CNV complex. Some authors have hypothesized that increases in the mechanical forces associated with abnormal vitreal attachments may result in the secretion of signaling factors by the Müller cells with both paracrine and autocrine effects. This process leads to a cascade of inflammatory factors and local vascular changes, which may include vascular leakiness, and subsequent cystoid macular edema (CME).31 In turn, in eyes with CNV, this could set up a vicious cycle, in which the inflammation, the reactive gliosis, and the tractional forces may result in worsening the chronic exudation of the underlying disease.
In a subset of eyes, we studied the role of surgical intervention. These eyes showed clinical resistance to fluid reabsortion despite frequent and aggressive anti-VEGF therapy, given in the form of monthly intravitreal injections of Bevacizumab or Ranibizumab. None of the five patients, at the time of the surgery, had signs of epiretinal membrane (ERM). Pseudophakic CME was excluded attributable to the temporal sequence of cataract surgery and CME: all the cases had an interval of time of at least one year without a history of CME, before the onset of the CNV. This was a consecutive series of patients without a prospective, standardized surgical methodology. No other patients in the study population had surgery during the study period. Gas tamponade was used in three out of five cases in the hope that it would assist in flattening the macular fluid. We cannot comment further on whether gas should be used and which gas should be used. Our surgical outcome showed an improvement in vision, which was of borderline significance using the two-tailed Student t test (P = .052). This marginal result may be attributable to the fact that most eyes in our series were operated on after disease was long standing despite aggressive intravitreal treatment. It is interesting that the eye with the most visual improvement had a progressive drusenoid RPE detachment. Less improvement was seen in the other eyes, but was present in three of the four remaining eyes with active CNV. Our results suggest that there may be a subpopulation of CNV eyes that do not respond to anti-VEGF therapy and in which VMT may play a component in the resistance to such therapy. We do not know if, in this group of patients, the visual improvement is secondary just to the release of VMT (combined with ongoing anti-VEGF treatment). Other factors may play an ancillary role: vitrectomy is in fact known to improve retinal oxygenation and the compressive effect of gas is also likely to facilitate a reabsortion of the intraretinal fluid. This series has a small number of patients and we are not advocating surgery in all eyes with CNV and VMT. Further studies are necessary. Surgery in such eyes can result in complications and vitrectomy may also complicate anti-VEGF treatment, as it may shortens the half-life of intravitreal anti-VEGF drugs, so that higher doses of anti-VEGF drugs may be needed postoperatively.32
Mild ERM was present in a minority of patients; none of our patients had a history of CME or ERM causing vision loss prior to the development of CNV. All the patients who showed some edema and were considered affected by exudative AMD had clear FA signs of CNV. All the patients that had surgery did not show any ERM at the baseline exam. The presence of ERM can be another cause of chronic CME in AMD patients, and should also be considered in the clinical practice. As for VMT, new OCT technology nowadays represents the most sensitive test to identify this subpopulation of patients.
In our study, we utilized the OTI-Spectral OCT/SLO with gold plated mirrors, which improved the visualization of the vitreous. Gold plated mirrors have, in fact, a higher reflectivity than aluminum and silver coating at the operating wavelength of the instrument (infrared). We do not know which coating material is used by the other manufacturers, but in our experience the visualization of the vitreoretinal interface, including hyaloid adhesions, ERM, VMT, and vitreous cortex detection, is highly enhanced with this device (results unpublished). We did combine that study with careful slit-lamp biomicroscopy to evaluate the vitreous planes. We recognize that ultrasound is the standard technique to detect PVD; however, subtle adhesion to the retina or vitreal schisis (as other authors have previously described) are difficult to detect and quantify with ultrasound.33 We recognize that a complete PVD could be missed in the clinical exam and that OCT is not the standard technique to tell a complete PVD from a completely attached vitreous. It is important to note that this would not affect our results. Our study was not designed to determine a correlation of complete PVD or completely attached vitreous with AMD, but the specific correlation of AMD with focal vitreomacular adhesion and eventual traction. In fact, our statistical analysis is not based on the presence or absence of a complete vitreous detachment, but only on the presence of partial adhesion. The hypothesis was that a local inflammation induced by AMD is causing a focal corresponding adhesion, the rest of the retina acting as a normal tissue. Future studies may wish to also incorporate dynamic ultrasound and a larger number of patients to confirm and expand our findings. We do know that the instrument we utilized does allow a focal offset of up to 10 mm, which we used to our advantage in searching for the posterior hyaloid membrane.
In conclusion, persistence of hyaloid adhesion to the posterior pole is highly associated with AMD. This adhesion is often responsible for VMT in eyes affected by exudative AMD. Mechanical factors related to the traction forces may antagonize the effect of anti-VEGF treatment, thus being responsible for pharmacological resistance in a subpopulation of AMD patients. Further studies need to be conducted to assess if surgery may be considered as a treatment option in these cases. New Spectral OCT technology, combined with simultaneous SLO, allows a highly sensitive diagnosis and a subsequent selection of cases, and a careful follow-up.
Acknowledgments
THIS STUDY WAS SUPPORTED BY THE RESEARCH TO PREVENT BLINDNESS (RPB), NEW YORK, NEW YORK, THE DEPARTMENT National Eye Institute (NIH-NEI) Grant No. EY16323 (Dr Bartsch), and the National Institutes of Health (NIH) Grant No. EY07366 (Dr Freeman). Dr Freeman is the recipient of an RPB Physician Scientist award. Dr Bartsch has received discounted products and specialized software from OTI, Inc. Involved in design and conduct of study (F.M., L.C., W.R.F.); collection (F.M.); management (F.M., L.C., W.R.F.); analysis and interpretation of the data (F.M., L.C., G.A.S., W.R.F.); and preparation, review, or approval of the manuscript (F.M., L.C., D.-U.G.B., G.A.S., W.R.F.). The University of California San Diego Institutional Review Board approval was obtained to conduct this study.
Biography

Francesca Mojana, MD, graduated at the Universita' degli Studi di Milano, Milano, Italy, in 2002. In 2006, she completed her residency at the Department of Ophthalmology of the same university. Since then, she started a fellowship at Jacobs Retina Center, Shiley Eye Center, University of California San Diego, California. Dr Mojana's concentration is in the areas of research and clinical work in macular pathologies, especially focusing on new optical coherence tomography technology and angiography.
References
- 1.La Cour M, Kiilgaard JF, Nissen MH. Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging. 2002;19:101–133. doi: 10.2165/00002512-200219020-00003. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 6.Mori K, Horie-Inoue K, Kohda M, et al. Association of the HTRA1 gene variant with age-related macular degeneration in the Japanese population. J Hum Genet. 2007;52:636–641. doi: 10.1007/s10038-007-0162-1. [DOI] [PubMed] [Google Scholar]
- 7.Nussenblatt RB, Ferris F., III Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert HM, Capone A, Aaberg TM, et al. Surgical excision of subfoveal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1992;113:257–262. doi: 10.1016/s0002-9394(14)71576-4. [DOI] [PubMed] [Google Scholar]
- 9.Weber-Krause B, Eckardt U. Incidence of posterior vitreous detachment in eyes with and without age-related macular degeneration. An ultrasonic study. Ophthalmologe. 1996;93:660–665. doi: 10.1007/s003470050054. [DOI] [PubMed] [Google Scholar]
- 10.Ondes F, Yilmaz G, Acar MA, Unlu N, Kocaoglan H, Arsan AK. Role of the vitreous in age-related macular degeneration. Jpn J Ophthalmol. 2000;44:91–93. doi: 10.1016/s0021-5155(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 11.Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S. Posterior Vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144:741–746. doi: 10.1016/j.ajo.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier-domain optical coherence tomography. J Biomed Opt. 2002;7:457–463. doi: 10.1117/1.1482379. [DOI] [PubMed] [Google Scholar]
- 13.De Boer JF, Cense B, Park BH, Pierce MC, Tearney GJ, Bouma BE. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Optics Letters. 2003;28:2067–2069. doi: 10.1364/ol.28.002067. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Erfurth U, Leitgeb RA, Michels S, et al. Three-dimensional ultra-high-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci. 2005;46:3393–3402. doi: 10.1167/iovs.05-0370. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan VJ, Wojtkowski M, Witkin AJ, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultra-high-resolution optical coherence tomography. Ophthalmology. 2006;113:2054 e1–14. doi: 10.1016/j.ophtha.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakehashi A, Kado M, Akiba J, Hirokawa H. Variations of posterior vitreous detachment. Br J Ophthalmol. 1997;81:527–532. doi: 10.1136/bjo.81.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475–1479. doi: 10.1001/archopht.119.10.1475. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–567. [PMC free article] [PubMed] [Google Scholar]
- 19.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 20.Lipsitz SH, Kim K, Zhao L. Analysis of repeated categorical data using generalized estimating equations. Statistics in Medicine. 1994;13:1149–1163. doi: 10.1002/sim.4780131106. [DOI] [PubMed] [Google Scholar]
- 21.Quaranta-El Maftouhi M, Mauget-Faysse M. Anomalous vitreoretinal adhesions in patients with exudative age-related macular degeneration: an OCT study. Eur J Ophthalmol. 2006;16:134–137. [PubMed] [Google Scholar]
- 22.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 23.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PT, Lewis GP, Talaga KC, et al. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003;44:4481–4488. doi: 10.1167/iovs.03-0436. [DOI] [PubMed] [Google Scholar]
- 26.Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early vs late AMD: a quantitative analysis. Br J Ophthalmol. 2003;87:1159–1166. doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez JM, Ramirez AI, Salazar JJ, de Hoz R, Trivino A. Changes of astrocytes in retinal aging and age-related macular degeneration. Exp Eye Res. 2001;73:601–615. doi: 10.1006/exer.2001.1061. [DOI] [PubMed] [Google Scholar]
- 28.Petrukhin K. New therapeutic targets in atrophic age-related macular degeneration. Expert Opin Ther Targets. 2007;11:625–639. doi: 10.1517/14728222.11.5.625. [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 30.Van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam Study. Arch Ophthalmol. 2003;121:519–526. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 31.Schubert HD. Cystoid macular edema: the apparent role of mechanical factors. Prog Clin Biol Res. 1989;312:277–291. [PubMed] [Google Scholar]
- 32.Freeman WR, Falkenstein I. Avastin and new treatments for AMD: where are we? Retina. 2006;26:853–858. doi: 10.1097/01.iae.0000244722.35073.7c. [DOI] [PubMed] [Google Scholar]
- 33.Chu TG, Lopez PF, Cano MR, et al. Posterior vitreoschisis. An echographic finding in proliferative diabetic retinopathy. Ophthalmology. 1996;103:315–322. doi: 10.1016/s0161-6420(96)30698-2. [DOI] [PubMed] [Google Scholar]


