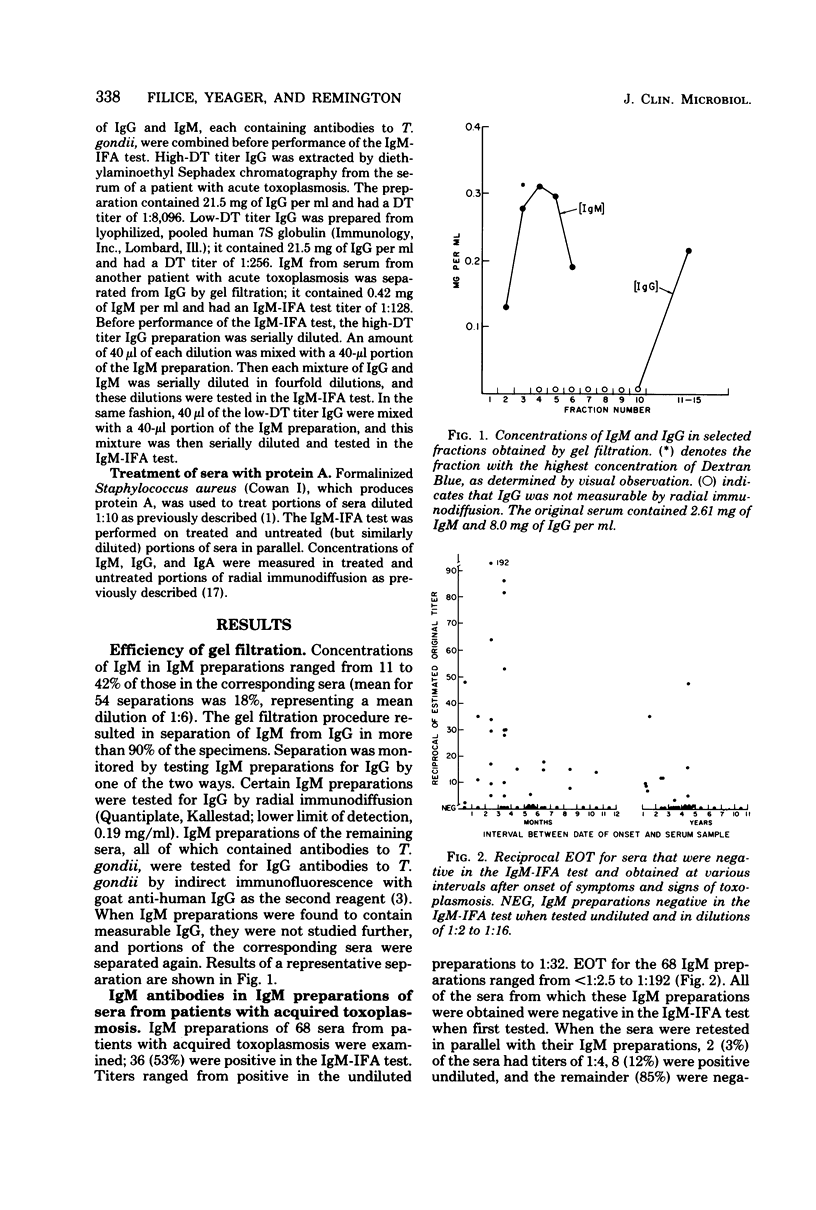
Abstract
Failure to demonstrate immunoglobulin M (IgM) antibodies by indirect immunofluorescence (IgM-IFA) in sera from some patients with acute acquired toxoplasmosis has recently been attributed to an inhibitory effect of high titers of IgG antibodies in these sera (Pyndiah et al. J. Clin. Microbiol. 9:170-174, 1979). To confirm these findings and define their importance for diagnosis, we used gel filtration to separate IgM from IgG antibodies in a series of sera that were negative in the IgM-IFA test. A total of 68 sera were from patients with acquired toxoplasmosis, 13 were from uninfected adults, 13 were from infants with congenital toxoplasmosis, and 7 were from uninfected neonates. Of the 68 sera from patients with acquired toxoplasmosis, IgM preparations (from the separated sera) were positive in the IgM-IFA test in 36 (53%). There was a significant (P = 0.00003) association between high titers of IgM-IFA antibodies in the IgM preparations (corrected for dilution of IgM antibodies by the gel filtration procedure) and recent acquisition of infection. IgM antibodies were also detected in 5 (38%) of the IgM preparations of 13 sera from congenitally infected infants but not in any of the IgM preparations of sera from uninfected neonates. IgG antibodies to Toxoplasma gondii were shown to interfere with demonstration of IgM antibodies in the IgM-IFA test. Treatment of sera with protein A resulted in greater dilution of IgM antibodies and less efficient separation of IgM from IgG antibodies than did separation of sera by gel filtration. Treatment of sera with protein A did not result in increased detection of IgM antibodies to T. gondii. Testing of IgM preparations (obtained by gel filtration) resulted in a significant increase in sensitivity of the IgM-IFA test for the diagnosis of recently acquired and congenital toxoplasmosis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankerst J., Christensen P., Kjellén L., Kronvall G. A rountine diagnostic test for IgA and IgM antibodies to rubella virus: absorption of IgG with Staphylococcus aureus. J Infect Dis. 1974 Sep;130(3):268–273. doi: 10.1093/infdis/130.3.268. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Barnett E. V., Gentry L. O., Remington J. S. False-positive anti-Toxoplasma fluorescent-antibody tests in patients with antinuclear antibodies. Appl Microbiol. 1971 Sep;22(3):270–275. doi: 10.1128/am.22.3.270-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Antigenemia in recently acquired acute toxoplasmosis. J Infect Dis. 1980 Feb;141(2):144–150. doi: 10.1093/infdis/141.2.144. [DOI] [PubMed] [Google Scholar]
- Camargo M. E., Leser P. G., Leser W. S. Diagnostic information from serological tests in human toxoplasmosis. I. A comparative study of hemagglutination, complement fixation, IgG and IgM-immunofluorescence tests in 3,752 serum samples. Rev Inst Med Trop Sao Paulo. 1976 Jul-Aug;18(4):215–226. [PubMed] [Google Scholar]
- Camargo M. E., Leser P. G., Rocca A. Rheumatoid factors as a cause for false positive IgM anti-toxoplasma fluorescent tests. A technique for specific results. Rev Inst Med Trop Sao Paulo. 1972 Sep-Oct;14(5):310–313. [PubMed] [Google Scholar]
- Desmonts G., Baufine-Ducrocq H., Couzineau P., Peloux Y. Anticorps toxoplasmiques naturels. Nouv Presse Med. 1974 Jun 15;3(24):1547–1549. [PubMed] [Google Scholar]
- Desmonts G., Couvreur J., Colin J., Peupion J. Vers un diagnostic précoce de la toxoplasmose aiguë. Etude critique du test de Remington. Nouv Presse Med. 1972 Jan 29;1(5):339–342. [PubMed] [Google Scholar]
- Dorfman R. F., Remington J. S. Value of lymph-node biopsy in the diagnosis of acute acquired toxoplasmosis. N Engl J Med. 1973 Oct 25;289(17):878–881. doi: 10.1056/NEJM197310252891702. [DOI] [PubMed] [Google Scholar]
- Field P. R., Shanker S., Murphy A. M. The use of protein A-sepharose affinity chromatography for separation and detection of specific IgM antibody in acquired rubella infection: a comparison with absorption by staphylococci containing protein A and density gradient ultracentrifugation. J Immunol Methods. 1980;32(1):59–70. doi: 10.1016/0022-1759(80)90117-9. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Krick J. A., Remington J. S. Toxoplasmosis in the adult--an overview. N Engl J Med. 1978 Mar 9;298(10):550–553. doi: 10.1056/NEJM197803092981006. [DOI] [PubMed] [Google Scholar]
- Pyndiah N., Krech U., Price P., Wilhelm J. Simplified chromatographic separation of immunoglobulin M from G and its application to toxoplasma indirect immunofluorescence. J Clin Microbiol. 1979 Feb;9(2):170–174. doi: 10.1128/jcm.9.2.170-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Miller M. J., Brownlee I. IgM antibodies in acute toxoplasmosis. II. Prevalence and significance in acquired cases. J Lab Clin Med. 1968 May;71(5):855–866. [PubMed] [Google Scholar]
- Teutsch S. M., Juranek D. D., Sulzer A., Dubey J. P., Sikes R. K. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. 1979 Mar 29;300(13):695–699. doi: 10.1056/NEJM197903293001302. [DOI] [PubMed] [Google Scholar]
- Yeager A. S. Variation of cord IgM level with birth weight. Pediatrics. 1973 Apr;51(4):616–619. [PubMed] [Google Scholar]


