Abstract
Food restriction and experimentally-induced diabetes (streptozotocin) can modify serotonin (5-HT) neurotransmission and sensitivity to drugs acting on 5-HT systems. This study examined the effects of food restriction and streptozotocin on the hypothermic effects of the 5-HT1A receptor agonist (+)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT), the 5-HT2 receptor agonist (±)-2,5-dimethoxy-4-methylamphetamine hydrochloride (DOM), the 5-HT releaser fenfluramine, and the selective 5-HT reuptake inhibitor (SSRI) fluoxetine. All four drugs significantly decreased body temperature in free feeding rats. Limiting rats to 10 g/day of food for 7 days decreased body weight and sensitivity to 8-OH-DPAT induced hypothermia, without affecting sensitivity to DOM, fenfluramine, or fluoxetine induced hypothermia. Subsequently, 7 days of free feeding restored body weight and sensitivity to 8-OH-DPAT. Sensitivity to all drugs was significantly decreased 7 days after 50 mg/kg streptozotocin; subsequently, 10 days of insulin replacement restored sensitivity to all drugs. These results extend to body temperature the observation that food restriction and experimentally-induced diabetes differentially modify sensitivity to drugs acting on 5-HT systems and they further suggest that the clinical response to therapeutic drugs acting on 5-HT systems might be impacted by nutritional and insulin status.
Keywords: serotonin, body temperature, rat, streptozotocin, food restriction
1. Introduction
Serotonin (5-HT) systems are primary targets for many therapeutic drugs and a number of factors can impact the effectiveness of drugs acting on those systems. Those same factors might also be important in the etiology of diseases associated with dysfunction of 5-HT systems. For example, clinical depression is more common in diabetic than non-diabetic patients, including both type-1 (Barnard et al., 2006; Shaban et al., 2006) and type-2 diabetes (Ali et al., 2006; De Groot et al., 2001). Diabetic depressed patients often have a poorer response than non-diabetic depressed patients to treatment with antidepressant drugs (Lustman et al., 1997; Nagy et al., 2008), including drugs acting on 5-HT systems (e.g., selective 5-HT reuptake inhibitors [SSRIs]). The reason for this comorbidity is not known, although preclinical studies suggest that depression and diabetes might be associated with changes in common underlying mechanisms. For example, diabetic mice show greater immobility than non-diabetic mice in a tail suspension test (Miyata et al., 2004a) that is used to predict antidepressant drug effects in humans and the SSRI fluoxetine is less effective at decreasing immobility in diabetic as compared to non-diabetic mice (Miyata et al., 2004a). Experimentally-induced diabetes also decreases sensitivity to some behavioral effects of drugs acting at 5-HT receptors (Li and France, 2008).
A second factor that can impact sensitivity to drugs is diet. For example, dietary restriction is associated with decreased effectiveness of SSRIs in depressed patients (Slaiman, 1989) and antidepressant drugs acting on 5-HT systems are not indicated in depressed patients with eating disorders because they are often ineffective and they further decrease appetite (Barbarich et al., 2004; Kaye et al., 1998). In rats food restriction decreases sensitivity to drugs acting directly on 5-HT receptors (Li and France, 2008), decreases the clearance rate (i.e., uptake) of 5-HT from hippocampus and striatum, and decreases the effectiveness of the SSRI escitalopram in the forced swim test (France et al., submitted). Food restriction and experimentally-induced diabetes (streptozotocin) decreased the sensitivity of rats to some behavioral effects of the 5-HT1A receptor agonist (+)-8-hydroxy-2-(dipropylamino) tetralin hydrobromide (8-OH-DPAT; lower lip retraction and flat body posture) and the 5-HT2 receptor agonist (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI; head twitching). These changes were due to limiting food intake and to experimentally-induced diabetes because 7 days of free access to food or 10 days of insulin replacement, respectively, restored normal sensitivity to both drugs (Li and France, 2008). The current study examined the generality of those changes by testing whether the same food restriction or the administration of streptozotocin alters sensitivity of rats to the hypothermic effects of drugs acting on 5-HT systems. The current study also explored the mechanisms that might account for such changes by comparing the effects of drugs that have agonist actions directly at 5-HT receptors, including the 5-HT1A receptor agonist 8-OH-DPAT and the 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), to the effects of drugs that have indirect agonist actions at 5-HT receptors, including the 5-HT releaser fenfluramine and the SSRI fluoxetine.
2. Materials and Methods
2.1 Subjects
Thirty-two adult male Sprague-Dawley rats (Harlan, Indianapolis, Indiana, USA) were housed individually on a 12/12-h light/dark cycle (experiments were conducted during the light period) with free access to water in the home cage. Except for 7 days when access to food was limited to 10 g/day, all rats had free access to food in the home cage. Rats were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC, USA).
2.2 Experimental design
Body temperature was measured in a procedure room maintained at 23.5 ± 0.5° C; rats were habituated to the procedure room for 2 hours immediately prior to each test. The 32 rats were randomly assigned to four groups of 8 rats each. A cumulative dosing procedure was used with rats receiving vehicle (i.p.) prior to the first 30-min cycle followed by increasing doses of drug (i.p.) every 30 min (except for fenfluramine when the interinjection interval was 60 min) with the cumulative dose increasing by 0.5 log unit (except for DOM when the dose increased by 0.25 log unit) per injection. Body temperature was measured in the last minute of the 30- or 60-min cycle by inserting a rectal probe (5.0 cm) and recording temperature from the digital thermometer. Body temperature was measured at least 5 times prior to testing drugs in order to habituate rats to the procedure. The length of cycles for particular drugs was determined in preliminary experiments that examined the time course of drug effects on body temperature.
Each group of 8 rats was tested on five different occasions with the same drug and the drugs studied were the following: the 5-HT1A receptor agonist 8-OH-DPAT (0.01–0.32 mg/kg); 5-HT2A receptor agonist DOM (1.0–5.6 mg/kg); the 5-HT releaser fenfluramine (1.0–10 mg/kg); and the selective 5-HT reuptake inhibitor fluoxetine (1.0–10 mg/kg). Dose-response curves were determined for a given drug in the same 8 rats under the following conditions: 1) when rats had free access to food in the home cage; 2) after 7 days of limited access (10 g/day) to food in the home cage; 3) 7 days after resumption of free access to food in the home cage; 4) 7 days after a single i.p. injection of 50 mg/kg of streptozotocin; and, finally 5) 10 days after insulin replacement (i.e., two insulin pellets [i.e., Linplant] were surgically implanted [s.c.] under ketamine [36 mg/kg i.m.] and xylazine [4.8 mg/kg i.m.] anesthesia).
2.3 Drugs
8-OH-DPAT, fenfluramine hydrochloride, fluoxetine hydrochloride and streptozotocin were purchased from Sigma-Aldrich (St. Louis, MO). DOM was provided by the Research Technology Branch, National Institute of Drug Abuse (Rockville, MD). Linplant pellets (i.e., sustained-release insulin implants) were purchased from LinShin Canada, Inc (Scarborough, Ontario). According to the manufacturer, each Linplant releases 2 U of insulin over 24 h for at least 40 days (Wang, 1991). Except for ketamine hydrochloride and xylazine hydrochloride, both of which were purchased as commercially-available solutions (Vetus Animal Health, Burns Veterinary Supply Inc., Westbury, NY), drugs were dissolved in sterile 0.9% saline. Streptozotocin solutions were prepared immediately before administration. Injection volumes were 0.2–1.0 ml i.p.
2.4 Data analyses
Body temperature (°C, mean ± S.E.M.) is plotted as a function of dose. A two-way repeated measure ANOVA (including drug as a factor) failed to show a significant difference in body weight among the four groups of 8 rats; thus, data were pooled (n=32) for analyzing the effects of treatments on body weight. Body temperature and body weight data were analyzed with a two-way repeated measure ANOVA (one factor comprising treatment [control, food restriction, food replacement, streptozotocin treatment, and insulin replacement] and a second factor comprising dose) and one-way repeated measure ANOVA, respectively. A post-hoc Tukey-Kramer test was used to examine significant differences among treatments (P<0.05).
3. Results
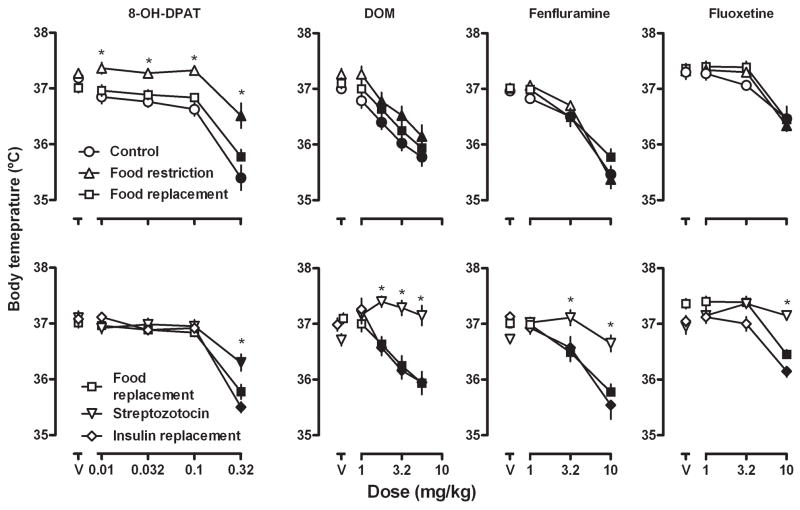
The average body temperature for all 32 rats after the administration of saline was 37.2 ± 0.1° C. Each of the four test compounds (8-OH-DPAT, DOM, fenfluramine, and fluoxetine) decreased body temperature in a dose-related manner (circles, upper panels, Fig 1). Over the dose ranges studied, 8-OH-DPAT decreased body temperature the most (−1.9° C at a dose of 0.32 mg/kg) and fluoxetine decreased body temperature the least (−0.5° C at a dose of 10 mg/kg).
Fig. 1.
Effects of 8-OH-DPAT, DOM, fenfluramine and fluoxetine on body temperature in rats with unlimited or limited (10 g/day) access to food (upper panels) and in the same rats after an injection of streptozotocin and, subsequently, after insulin replacement (lower panels). Ordinates: body temperature (°C). Abscissae: dose in mg/kg body weight; “V” indicates vehicle. Data were analyzed by two-way repeated measure ANOVA. Closed symbols indicate significantly different (P<0.05) from vehicle; * = P<0.05 in comparison with “Control” (upper) or with “Food replacement” (lower).
The average body weight of rats (n=32) before food restriction was 425.3 ± 3.4 g (mean ± S.E.M.); after 7 days of limiting rats to 10 g/day of food, the average body weight decreased significantly to an average of 395.5 ± 3.1 g (Table 1). Food restriction also significantly decreased sensitivity to the hypothermic effects of 8-OH-DPAT; however, sensitivity to the hypothermic effects of DOM, fenfluramine, or fluoxetine was not affected by food restriction (compare circles and triangles, upper panels, Fig 1). Subsequently, 7 days of unlimited access to food restored body weight to values that were not different from those obtained prior to food restriction (Table 1) and restored normal sensitivity to 8-OH-DPAT induced hypothermia (compare triangles and squares, upper left panel, Fig 1).
Table 1.
Average body weights of rats (mean ± S.E.M.; n=32) during different phases of the study
| Free feeding | 425.3 ± 3.4 |
| Food restriction | 395.5 ± 3.1a |
| Free feeding/food replacement | 428.8 ± 3.5 |
| Streptozotocin | 409.5 ± 4.2b |
| Insulin replacement | 433.4 ± 4.1 |
P<0.0001 compared with free feeding and with free feeding/food replacement
P<0.0001 compared with free feeding/food replacement and with insulin replacement
Seven days after a single injection of streptozotocin, body weight was significantly reduced (Table 1) and sensitivity was decreased to the hypothermic effect of 8-OH-DPAT, DOM, fenfluramine and fluoxetine (compare squares and inverted triangles, lower panels, Fig 1); however, the effect of streptozotocin varied among the four drugs. For example, prior to streptozotocin a dose of 0.32 mg/kg of 8-OH-DPAT decreased body temperature 1.9° C; after streptozotocin the same dose decreased body temperature 0.8° C. Moreover, doses of DOM (1.78, 3.2 and 5.6 mg/kg), fenfluramine (3.2 and 10 mg/kg), and fluoxetine (10 mg/kg) that significantly decreased body temperature prior to streptozotocin (i.e., filled symbols), failed to significantly change body temperature after streptozotocin. Ten days of insulin replacement restored body weights to values that were not different from those obtained prior to streptozotocin (Table 1) and restored sensitivity to the hypothermic effects of all four drugs (compare squares and diamonds, lower panels, Fig 1).
4. Discussion
It is well established that experimentally-induced diabetes or food restriction can significantly alter brain neurochemistry as well as sensitivity to drugs acting on monoamine systems, including dopamine (Owens et al., 2005; Sevak et al., 2007, 2008). Less is known regarding how diabetes or food restriction alters other neurotransmitter systems or drugs acting on those systems (e.g., 5-HT). The current study extends a prior study that investigated the observable behavioral effects of direct-acting 5-HT receptor agonists (Li and France 2008) and shows that diabetes and food restriction modify the hypothermic effects of some 5-HT receptor agonists. Many psychotherapeutic drugs target 5-HT systems and the clinical response to some of those drugs (e.g., SSRIs) can vary dramatically; for example, SSRIs are effective in less than half of depressed patients (Baghai et al., 2006; Tranter et al., 2002). Results of the current study suggest that nutritional status and insulin status impact 5-HT systems in a manner that might be relevant the varied clinical response to drugs acting on 5-HT systems and, possibly, to the comorbidity of diabetes and depression.
Body temperature is regulated by many different physiological and neurochemical systems, including 5-HT (Chase and Murphy, 1973), and a number of factors, including ambient temperature, can impact drug effects on body temperature. For example, in rats 8-OH-DPAT decreases body temperature at ambient temperatures ranging from 21 to 30° C and this effect is mediated by 5-HT1A receptors (Gudelsky et al., 1986; Eltayb et al., 2001), although 5-HT7 receptors might also play a role (Hedlund et al., 2004). Similarly, fluoxetine decreases body temperature at ambient temperatures of 8 and 22° C and is without effect at an ambient temperature of 30° C (Lin, 1978). The effects of DOM and fenfluramine on body temperature are more varied, with each drug increasing, decreasing or not affecting body temperature depending on the ambient temperature (Beaton et al., 1976; Malberg and Seiden, 1997). Antagonism studies conducted at an ambient temperature of 21° C indicate that the hypothermic effects of fenfluramine are mediated by multiple receptors including 5-HT1A, 5-HT2A, 5-HT2C, and dopamine D2 receptors (Cryan et al., 2000). In the current study, all four compounds decreased body temperature when studied at an ambient temperature of 23.5 ± 0.5° C.
One week of food restriction decreases sensitivity to some effects of 8-OH-DPAT, as reflected by a shift to the right in the dose response curves for 8-OH-DPAT induced lower lip retraction and flat body posture (Li and France, 2008). In the current study, the same food restriction protocol (10 g/day for 7 days) also decreased sensitivity to the hypothermic effects of 8-OH-DPAT. In both cases the decreased sensitivity appeared to be due to food restriction because 7 days of free feeding restored normal sensitivity to the behavioral and hypothermic effects of 8-OH-DPAT. In an earlier study (Li and France, 2008), food restriction also decreased sensitivity to a behavioral effect (head twitching) of the 5-HT2A receptor agonist DOI; however, in the current study food restriction had no effect on the hypothermic effects of the 5-HT2A receptor agonist DOM. It is not clear why food restriction decreases sensitivity to some and not other effects of 5-HT2A receptor agonists. It is possible that hypothermia and head twitching are mediated by different brain regions, by different 5-HT receptor subtypes, or that non 5-HT mechanisms play a role in these effects by DOM or DOI; however, these two phenethylamines have similar binding affinities and activities at 5-HT2A and 5-HT2C receptors (Pranzatelli, 1990). Food restriction also failed to modify sensitivity to the hypothermic effects of fenfluramine and fluoxetine, both of which are indirect acting 5-HT receptor agonists. Because the effects of fenfluramine and fluoxetine on body temperature are mediated by multiple mechanisms (e.g., receptor subtypes [Gudelsky et al., 1986; Cryan et al., 2000]), these results suggest that food restriction selectively alters sensitivity of some and not other 5-HT receptor subtypes.
Experimentally-induced diabetes can disrupt thermoregulation; for example, under some conditions diabetic animals do not maintain a stable body temperature when ambient temperature changes (Shalaby et al., 1989). Using telemetry studies in rats, it has been reported that streptozotocin decreases resting core temperature by 0.4° C (Howarth et al., 2005). Moreover, compared with untreated rats, diabetic rats are less sensitive to clonidine-induced hypothermia and more sensitive to apomorphine-induced hypothermia (O’Donnell et al., 1996; Bjorenson and Quock, 1988). In the current study, streptozotocin did not significantly alter body temperature, perhaps because of procedural differences between the two studies (current study versus Howarth et al., 2005, respectively): 7 versus 10 days after streptozotocin; 50 versus 60 mg/kg of streptozotocin; rectal probe versus telemetry; Sprague Dawley versus Wistar rats; testing in a separate procedure room versus in the home cage. Nevertheless, rats that received streptozotocin in the current study were significantly less sensitivity to the hypothermic effects of 8-OH-DPAT, DOM, fenfluramine and fluoxetine. The apparently greater effect of streptozotocin, as compared with food restriction, on hypothermic effects of direct- and indirect-acting 5-HT drugs might be due to the reduced 5-HT turnover rate and increased 5-HT clearance that occur in streptozotocin treated rats (Petrisic et al., 1997; Miyata et al., 2004b; Li and France, 2008). It is clear that decreased sensitivity to drugs was due to hypoinsulinemia because insulin replacement restored normal sensitivity to the hypothermic effects of all four compounds. Similarly, insulin replacement restores sensitivity to the behavioral effects of both 5-HT1A and 5-HT2A receptor agonists (Li and France, 2008). Together these results suggest that insulin status and diabetes can significantly change the sensitivity of 5-HT systems in a manner that could be relevant both for understanding the highly variable response of patients to psychotherapeutic drugs that target 5-HT systems as well as the comorbidity of diabetes and depression.
In conclusion, food restriction decreased sensitivity to the hypothermic effects of the 5-HT1A receptor agonist 8-OH-DPAT only, whereas streptozotocin decreased sensitivity to the hypothermic effects of the 8-OH-DPAT, DOM, fenfluramine, and fluoxetine. These data extend previous studies on behavioral effects of 5-HT agonists to show that nutritional status and insulin status significantly alter sensitivity to drugs acting on 5-HT systems, including a drug that is used extensively to treat depression (fluoxetine). Collectively these data suggest that dietary factors contribute to the clinical effectiveness of some psychotherapeutic drugs and they support that view that the comorbidity of diabetes and depression (De Groot et al., 2001; Ali et al., 2006; Barnard et al., 2006; Knol et al., 2006) might result, in part, from changes in common underlying neurochemical mechanisms.
Acknowledgments
The authors thank C. Cruz for expert technical assistance. CPF is supported by a Senior Scientist Award (DA17918).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of comorbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Baghai TC, Moller HJ, Rupprecht R. Recent progress in pharmacological and non-pharmacological treatment options of major depression. Current Pharmaceutical Design. 2006;12:503–515. doi: 10.2174/138161206775474422. [DOI] [PubMed] [Google Scholar]
- Barbarich NC, McConaha CW, Halmi KA, Gendall K, Sunday SR, Gaskill J, La Via M, Frank GK, Brooks S, Plotnicov KH, Kaye WH. Use of nutritional supplements to increase the efficacy of fluoxetine in the treatment of anorexia nervosa. Int J Eating Disord. 2004;35:10–15. doi: 10.1002/eat.10235. [DOI] [PubMed] [Google Scholar]
- Barnard KD, Skinner TC, Peveler R. The prevalence of comorbid depression in adults with type 1 diabetes: systematic literature review. Diabet Med. 2006;23:445–448. doi: 10.1111/j.1464-5491.2006.01814.x. [DOI] [PubMed] [Google Scholar]
- Beaton JM, Benington F, Bradley RJ, Kuhlemeier KV, Morin RD. Stereospecific actions of 2,5-dimethoxy-4-methylamphetamine (DOM) on colonic temperature in the rat at various ambient temperatures. Br J Pharmacol. 1976;57:547–550. doi: 10.1111/j.1476-5381.1976.tb10383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorenson JE, Quock RM. Enhanced apomorphine-induced hypothermia in alloxan-treated rats. Horm Metab Res. 1988;20:32–36. doi: 10.1055/s-2007-1010742. [DOI] [PubMed] [Google Scholar]
- Chase TN, Murphy DL. Serotonin and central nervous system function. Annu Rev Pharmacol. 1973;13:181–197. doi: 10.1146/annurev.pa.13.040173.001145. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Harkin A, Naughton M, Kelly JP, Leonard BE. Characterization of D-fenfluramine-induced hypothermia: evidence for multiple sites of action. Eur J Pharmacol. 2000;390:275–285. doi: 10.1016/s0014-2999(00)00012-1. [DOI] [PubMed] [Google Scholar]
- De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- Eltayb A, Lindblom S, Oerther S, Ahlenius S. Additive hypothermic effects of the 5-HT1A receptor agonist 8-OH-DPAT and the dopamine D2/3 receptor agonist 7-OH-DPAT in the rat. Acta Physiol Scand. 2001;172:205–209. doi: 10.1046/j.1365-201x.2001.00858.x. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;87:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Howarth FC, Jacobson M, Naseer O, Adeghate E. Short-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol. 2005;90:237–245. doi: 10.1113/expphysiol.2004.029439. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gendall K, Strober M. Serotonin neuronal function and selective serotonin reuptake inhibitor treatment in anorexia and bulimia nervosa. Biol Psychiatry. 1998;44:825–838. doi: 10.1016/s0006-3223(98)00195-4. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta analysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- Li JX, France CP. Food restriction and streptozotocin treatment decrease 5-HT1A and 5-HT2A receptor-mediated behavioral effects in rats. Behav Pharmacol. 2008;19:292–297. doi: 10.1097/FBP.0b013e328308f1d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT. Effects of specific inhibitors of 5-hydroxytryptamine uptake on thermoregulation in rats. J Physiol. 1978;284:147–154. doi: 10.1113/jphysiol.1978.sp012532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Freedland KE, Clouse RE. The course of major depression in diabetes. Gen Hosp Psychiatry. 1997;19:138–143. doi: 10.1016/s0163-8343(96)00170-3. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Administration of fenfluramine at different ambient temperatures produces different core temperature and 5-HT neurotoxicity profiles. Brain Res. 1997;765:101–107. doi: 10.1016/s0006-8993(97)00517-9. [DOI] [PubMed] [Google Scholar]
- Miyata S, Hirano S, Kamei J. Diabetes attenuates the antidepressant-like effect mediated by the activation of 5-HT1A receptor in the mouse tail suspension test. Neuropsychopharmacology. 2004a;29:461–469. doi: 10.1038/sj.npp.1300354. [DOI] [PubMed] [Google Scholar]
- Miyata S, Hirano S, Kamei J. Diabetes inhibits the DOI-induced head twitch response in mice. Psychopharmacology (Berl) 2004b;177:224–229. doi: 10.1007/s00213-004-1942-3. [DOI] [PubMed] [Google Scholar]
- Nagy G, Ronai Z, Somogyi A, Sasvari-Szekely M, Rahman OA, Mate A, Varga T, Nemoda Z. P2RX7 Gln460Arg polymorphism is associated with depression among diabetic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008 doi: 10.1016/j.pnpbp.2008.08.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Banyasz T, Kovacs T. Altered thermoregulatory responses to clonidine in streptozotocin-diabetic rats. Br J Pharmacol. 1996;117:938–942. doi: 10.1111/j.1476-5381.1996.tb15284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WA, Sevak RJ, Galici R, Chang X, Javors MA, Galli A, France CP, Daws LC. Deficits in dopamine clearance and locomotion in hypoinsulinemic rats unmask novel modulation of dopamine transporters by amphetamine. J Neurochem. 2005;94:1402–1410. doi: 10.1111/j.1471-4159.2005.03289.x. [DOI] [PubMed] [Google Scholar]
- Petrisi MS, Augood SJ, Bicknell RJ. Monoamine transporter gene expression in the central nervous system in diabetes mellitus. J Neurochem. 1997;68:2435–2441. doi: 10.1046/j.1471-4159.1997.68062435.x. [DOI] [PubMed] [Google Scholar]
- Pranzatelli MR. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropane)-2 (DOI) Neurosci Lett. 1990;115:74–80. doi: 10.1016/0304-3940(90)90520-j. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Galli A, France CP. Insulin replacement restores the behavioral effects of quinpirole and raclopride in streptozotocin-treated rats. J Pharmacol Exp Ther. 2007;320:1216–1223. doi: 10.1124/jpet.106.115600. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol. 2008;592:109–115. doi: 10.1016/j.ejphar.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban MC, Fosbury J, Kerr D, Cavan DA. The prevalence of depression and anxiety in adults with Type 1 diabetes. Diabet Med. 2006;23:1381–1384. doi: 10.1111/j.1464-5491.2006.02012.x. [DOI] [PubMed] [Google Scholar]
- Shalaby TH, Yousef MK, Dupré RK. Thermoregulatory responses of diabetic rats. Comp Biochem Physiol A. 1989;94:153–157. doi: 10.1016/0300-9629(89)90800-1. [DOI] [PubMed] [Google Scholar]
- Slaiman S. Restricted diets restrict antidepressant efficacy. Practitioner. 1989;233:972–975. [PubMed] [Google Scholar]
- Tranter R, O’Donovan CO, Chandarana P, Kennedy S. Prevalence and outcome of partial remission in depression. J Psychiatry Neurosci. 2002;27:241–247. [PMC free article] [PubMed] [Google Scholar]
- Wang PY. Sustained-release implants for insulin delivery. In: Pickup JC, editor. Biotechnology of insulin therapy. London, UK: Blackwell Scientific; 1991. pp. 42–74. [Google Scholar]