Abstract
Advances in computed tomography (CT) technology allow images to be obtained with high spatial and temporal resolution. These features now permit noninvasive coronary CT angiography (CCTA). Many studies addressing proof of concept, feasibility, and clinical robustness have been published since CCTA was first described. More recently, the scientific evaluation of CCTA has rightly focused less on technical aspects and more on multicenter trials of the diagnostic value of CCTA and on head-to-head comparisons with other noninvasive modalities for the detection of coronary artery disease (CAD), such as stress myocardial perfusion imaging (MPI) with radionuclides. Recent peer-reviewed publications that compare CCTA to invasive, selective coronary angiography (SCA) or MPI, or that address radiation protection issues related to CCTA, were reviewed and summarized. Overall, there is high agreement between CCTA and both SCA and MPI for the presence of CAD. However, CCTA can over- or underestimate the severity of CAD compared to SCA as a reference standard. Initial studies that compared CCTA to MPI found their accuracies for determining the presence of high-grade luminal obstructions comparable. Limitations of CCTA include inability to reliably assess the coronary artery lumen dimensions in patients with large amounts of coronary artery calcium, artifacts caused by coronary and respiratory motion, and the need for ionizing radiation and intravenous administration of iodinated contrast material. Various dose reduction methods for CCTA now exist that may substantially lower patient dose to levels less than those of SCA or MPI. Although current expert consensus does not call for CCTA to be a first-line test for CAD, particularly for screening in asymptomatic individuals, current data suggest a promising role in the evaluation of symptomatic patients for possible CAD.
Keywords: cardiology, computed tomography, coronary angiography, imaging, ionizing radiation
Coronary artery disease (CAD) and its consequences remain a leading cause of morbidity and mortality among most age groups in the USA and most Western countries.1 In 50–65% of all patients, myocardial infarction (MI) is the first clinical presentation of CAD in previously asymptomatic patients. Approximately 35% of these first MIs are lethal. In symptomatic patients with acute or chronic chest pain, establishing the presence of myocardial ischemia secondary to severe CAD as the cause can be challenging and expensive.2–4
Consequently, much clinical research has been devoted to establishing “new” techniques to predict MI and sudden cardiac death in intermediate- or high-risk populations (prognostication), and to diagnose high-grade CAD in symptomatic patients (diagnosis). Ultimately, the clinical objective of employing these techniques is to facilitate patient management decisions that will improve patients’ longevity or quality of life (therapy). Many tools exist to address prognostication and diagnosis of CAD, which all have different strengths and weaknesses.
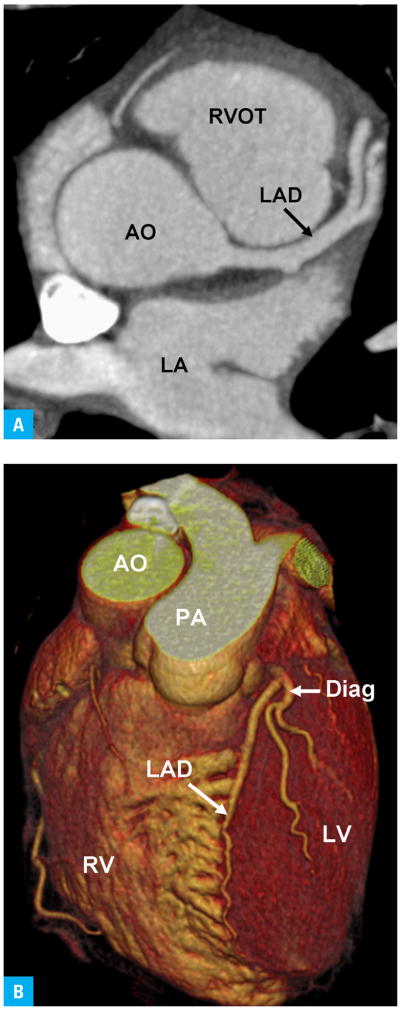
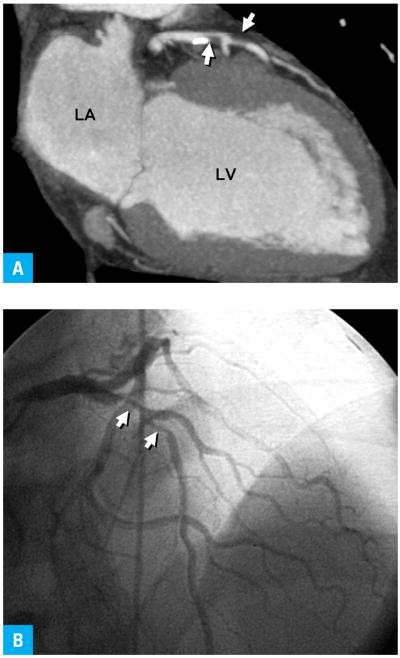
With recent progress in the technical development of computed tomography (CT) scanners, images can now be acquired very rapidly and with very high spatial resolution. In particular, the development of 64-slice CT scanners allows imagers to scan the heart with a temporal resolution that is a fraction of the length of the cardiac cycle (as little as 68 ms) and with near-isotropic spatial resolution of less than 0.5 mm (FIGURE 1).5 Coronary CT angiography (CCTA) holds the promise to noninvasively and, with little procedural risk, directly identify high-grade coronary artery stenoses and characterize coronary artery wall and plaque characteristics in hopes of identifying morphologic features that predict future plaque rupture (FIGURE 2).6,7
FIGURE 1.
Normal contrast-enhanced coronary computed tomography angiogram53
A Axial view (similar to horizontal long axis at the level of the aortic root)
B Volume rendering
Abbreviations: AO – aorta, Diag – diagonal branch, LA – left atrium, LAD – left anterior descending coronary artery, LV – left ventricle, PA – pulmonary artery, RV – right ventricle, RVOT – right ventricular outflow tract
FIGURE 2.
Abnormal coronary computed tomography angiogram with confirmation from diagnostic catheterization shows tandem high-grade stenoses (arrows) in the left anterior descending artery53
A Coronary computed tomography angiogram reformatted in vertical long axis
B Selective coronary angiogram in similar projection
Abbreviations: see FIGURE 1
What are our current approaches for the assessment of coronary artery disease?
The clinical stratification of cardiovascular risk in asymptomatic individuals currently relies on analyzing the presence and pattern of risk factors identified in population-based longitudinal studies such as the Framingham study. However, approximately ⅓ of cardiovascular events are not readily explained by these “traditional” cardiovascular (CV) risk factors.7 Therefore, a large body of literature has examined the predictive value of “novel” cardiac risk factors such as lipoprotein A, homocystein, highly sensitive C-reactive protein (CRP), or biomarkers of atherosclerosis and inflammation, such as CRP, interleukin 6, or matrix metalloproteinase.8 Another relatively new approach to cardiovascular risk stratification uses imaging of “subclinical” atherosclerosis. Examples include ultrasonic measurement of carotid intima-media thickness or scanning for coronary artery calcium by CT.8 The rationale for imaging to find non-obstructive, clinically silent plaque is to provide evidence for a genetic susceptibility for responding to the presence of CV risk factors with development of atherosclerosis. This approach could theoretically identify “vulnerable” patients at a time when aggressive risk factor modification can slow or halt the atherosclerotic process and reduce the risk of progression to the stage of symptomatic disease.
The noninvasive identification of ischemia in symptomatic patients relies on stress testing. Current guidelines by the American Heart Association (AHA) and American College of Cardiology9 suggest treadmill stress electrocardiography as the test of first choice. Stress testing combined with imaging in the form of echocardiography or myocardial perfusion imaging (MPI) with radionuclides is indicated only if the electrocardiography cannot be reliably interpreted for ischemic changes (i.e. ST-segment abnormalities at baseline, left bundle branch block). Treadmill exercise is recommended as stress modality of first choice over pharmacologic stress agents such as dobutamine or adenosine as long as the patient is able to exercise effectively.
A great strength of stress testing lies in the functional information it can provide. Common to all stress tests is the ability to detect impaired coronary flow reserve, which can serve as a “roadmap” to plan percutaneous or surgical revascularization if more than one anatomically “significant” stenosis is found eventually on selective coronary angiography (SCA), and has prognostic value if not only the presence but also the extent and degree of ischemia is considered. Unique to exercise tests is the prognostic information conveyed by a patient’s exercise capacity.
What are the shortcomings of our current approaches?
The traditional noninvasive tests for CAD rely on indirect evidence for high-grade coronary artery stenoses from the “ischemic cascade” in the form of myocardial perfusion defects (MPI), inducible regional myocardial dysfunction (stress echocardiography) or typical electrocardiographic abnormalities (treadmill exercise testing) for the diagnosis of significant CAD. Owing to this principle, imaging stress tests with echocardiography or MPI are somewhat limited in their sensitivity and specificity. For example, in prior meta-analysis the sensitivity of stress echocardiography was 79% (95% CI 78–81) and the specificity, 87% (95% CI 86–89). MPI was 88% sensitive (95% CI 87–90) and 73% specific (95% CI 69–77).3
For many decades, invasive, catheter-based SCA was the only means to directly visualize the coronary artery lumen. To date, SCA remains the reference standard for the evaluation of CAD, but there is ongoing debate among clinicians as to the appropriate indications and timing for coronary catheterization.
The shortcomings of SCA are well recognized. First, the risk of “major” procedural complications such as MI, stroke, and need for emergent bypass surgery are low but appreciable at approximately 1 in 1,000 procedures.10–12 Second, SCA is a “battered gold standard” with low accuracy compared to pathology and a worrisome degree of interobserver variability in the determination of the degree of luminal obstruction.13,14 Third, the degree of luminal obstruction does not reliably predict the functional significance of a stenosis, i.e. ischemia. The fractional flow reserve in diseased coronary arteries depends on many morphologic parameters15, and in studies and guidelines, “significant” coronary stenosis has variably been defined as 50% or 70% luminal narrowing compared to presumably normal reference segments16,17. Uncertainty about the functional significance of intermediate (50–70%) stenoses is a well known limitation of “anatomic” imaging modalities such as angiography. Fourth and final, most plaque ruptures that cause acute coronary thromboses occur in segments with no more than moderate stenoses18; hence, absence of high-grade stenoses does not guarantee freedom from cardiac events even in the near term.
How does coronary computed tomographic angiography compare to “traditional” diagnostic techniques?
A rapidly increasing body of literature is examining the place of CCTA in the contemporary clinical practice of cardiology. Although initially described in 1995 for a very specialized, rare type of CT scanner19, CCTA did not become possible on conventional CT scanners with mechanical rotation gantries until the late 1990s20. Early research focused on proof-of-concept, clinical feasibility and robust scanning protocols.21 Subsequently, experienced investigators from individual academic centers reported their experience with the diagnostic accuracy of CCTA compared to SCA in comparatively small numbers of patients. 22 Only more recently have multicenter trials involving 250 patients or more23,24 and studies comparing CCTA to other noninvasive diagnostic modalities25–30 become available.
Coronary computed tomography angiography vs. selective coronary angiography
Early studies of CCTA reported large proportions of nondiagnostic studies, mostly due to the comparatively low temporal and spatial resolution. Initial meta-analyses31 indicated higher diagnostic accuracy and lower number of nondiagnostic studies with newer compared to older CT scanners.
Most studies of CCTA have reported diagnostic accuracy by coronary artery segment, coronary artery, and per patient. In newly symptomatic patients without prior history of CAD, the per-patient accuracy is the most meaningful parameter among these three for classifying individual patients as having or not having CAD. A recent meta-analysis22 of 23 single-center studies that compared CCTA to SCA in a total of 2045 patients noted the following findings: for a significant coronary artery stenosis of ≥50% in patient-based analysis (the presence of coronary disease some-where in the coronary tree of a given patient), vessel-based analysis (the presence of disease somewhere in a particular coronary artery) and segment-based analysis (the presence of disease in a particular segment of a particular coronary artery), CCTA had sensitivities of ≥90%, specificities of 88 to ≥90%, variable positive predictive values (PPV) ranging from 69% to 93%, and negative predictive values (NPV) ranging from 96% to 100%. Given the dependence of PPV and NPV on the prevalence of disease, the comparatively high prevalence of significant CAD as determined by SCA in many of these selected study populations (61%) compared to the general population is a problem in appraising the value of CCTA in clinical practice. Therefore, this meta-analysis also reported positive (+LR) and negative (−LR) likelihood ratios as prevalence-independent indicators of diagnostic accuracy. The +LR values ranged from 8.0 to ≥9.7, and the −LR was <0.1 except for distal coronary segments. These findings indicate that negative CCTA examinations reliably exclude significant CAD, but abnormal CCTA examinations require further workup.22
Two recent prospective multicenter studies23,32 reported data on 64-slice multidetector CCTA in 360 and 291 subjects, respectively, who were referred for clinically indicated SCA. Using a coronary artery diameter reduction of ≥50% as significant, both studies found CCTA to be very sensitive for detecting overall significant CAD and at least moderately specific (TABLE 1). As stated above, per-patient analyses referred to the presence of at least one significant stenosis anywhere in the coronary system. This type of analysis did not necessarily imply that significant stenoses seen on CCTA were visible in the same coronary segment on SCA. When analysis of diagnostic accuracy was performed by vessel32 or segment23, the results were markedly different (TABLE 1), though comparable to the recent meta-analysis discussed above 22.
TABLE 1.
Diagnostic performance of 64-slice computed tomography in detecting significant (≥50% stenosis) coronary artery disease in patient-based and vessel-based analysis23,32
| Patient based analysis (95% CI) | Vessel based analysis (95% CI) | |||
|---|---|---|---|---|
| Meijboom et al. | Miller et al. | Meijboom et al.a | Miller et al. | |
| sensitivity (%) | 99(98–100) | 85(79–90) | 95(92–97) | 75(69–81) |
| specificity (%) | 64(55–73) | 90(83–94) | 77(74–80) | 93(90–94) |
| PPV (%) | 86(82–90) | 91(86–95) | 59(55–63) | 82(77–86) |
| NPV (%) | 97(94–100) | 83(75–89) | 98(96–99) | 89(86–92) |
segment based analysis
Abbreviations: PPV – positive predictive value, NPV – negative predictive value
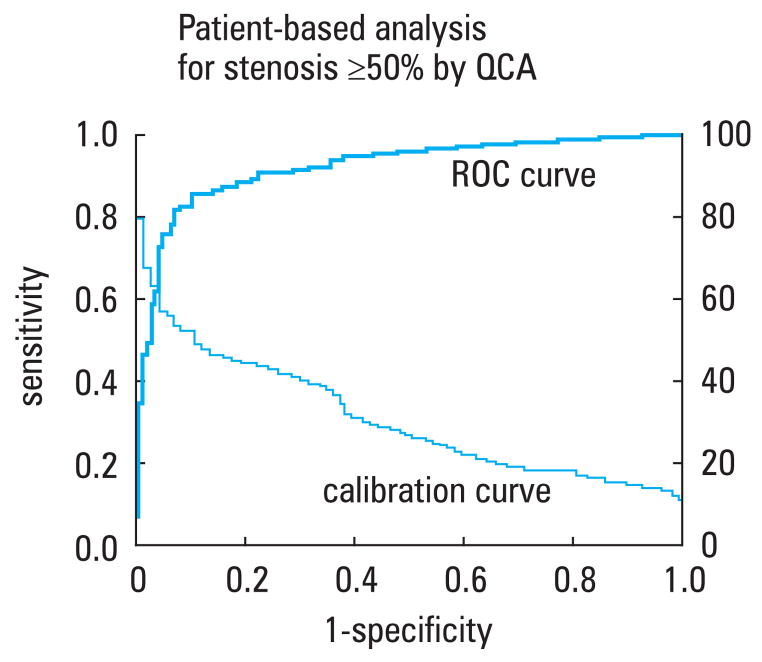
Receiver operator characteristics curves were generated in the study by Miller et al. demonstrating an area under the curve of 0.93 for the per-patient ability of CCTA to predict presence of at least one >50% stenosis diagnosed by SCA (FIGURE 3).32 The severity of disease expressed as a modified Duke score33,34 correlated well (r = 0.81) between CCTA and SCA.
FIGURE 3.
ROC curve (solid line) describing the diagnostic performance of CCTA to identify coronary stenosis of 50% or more in at least one vessel, as compared with invasive quantitative coronary angiography at the level of the patient. The area under the curve was 0.93 (95% CI, 0.90–0.96). The dotted line represents a calibration curve. A corresponding CCTA cutoff point can be determined by extending a vertical line from a point on the ROC curve to the calibration curve and then a horizontal line to the right ordinate, which describes the cutoff point.32
Abbreviations: CCTA – coronary computed tomographic angiography, QCA – quantitative coronary angiography, ROC – receiver-operating-characteristic
These two recent multicenter studies discussed several methodological limitations of CCTA. In the study by Miller et al.32, patients with high coronary calcium scores (Agatston calcium score of >600) were automatically excluded from the analysis, under the argument that such levels of calcium would obscure too much of the vessel to accurately evaluate. In the study by Meijboom et al.23, no segments were automatically excluded because of high calcium scores, but the authors noted that such calcifications limited the accuracy of vessel and segment analysis. Accordingly, the typically high levels of coronary calcium in older patients (>75 years) or patients with known CAD limit the use of CCTA in these patient populations.
Also of importance was the high prevalence of CAD with ≥50% diameter reduction of 68% and 31% in these studies, respectively, both substantially higher than in the general population, which also limits the ability to extrapolate their findings to the general population.
Of concern in the study by Meijboom et al. was the high number of false positive findings on CCTA.35 For example, of 98 patients diagnosed to have three-vessel disease by CCTA, only 19 were confirmed by SCA, and 9 patients had no disease at all. The overall weighted κ value for the agreement between CCTA and SCA in the determination of extent of disease was only moderate at 0.47.23 This finding, together with the low PPV of 47% in the per-segment analysis, exemplifies how limited CCTA was in precisely localizing significant coronary stenoses in a population with moderate prevalence of disease. Similarly, the study by Miller et al.32 also shows a high rate of misclassification of disease severity in CCTA compared to SCA. Indeed, Miller et al. themselves note that despite the overall excellent accuracy, sensitivity, and specificity of CCTA, “multidetector CT angiography cannot replace coronary angiography in this population of patients at present.”24 Conversely, both studies confirmed the previously noted very high NPV despite the high prevalence of disease. In both studies, if a patient’s CCTA was interpreted as being normal, significant CAD on SCA was virtually excluded.
Coronary artery computed tomographic angiography vs. stress nuclear imaging
The limited PPV of CCTA compared to SCA invites combined “hybrid” imaging with MPI, or at least comparative studies between CCTA and MPI. An initial feasibility study of hybrid imaging of CCTA and single photon emitted computed tomography (SPECT)36 showed excellent results, with substantially improved specificity (63–95%) and PPV (31–77%) in SPECT/CCTA examinations versus CCTA examination alone in the detection of significant (>50% narrowing) stenoses in per-segment analysis.36
Several studies have also compared CCTA to MPI25,28,29 or to both MPI and SCA26,27,30. The studies comparing CCTA to MPI examined how well significant stenoses on CCTA correlated with reversible myocardial perfusion defects on MPI. In keeping with the expected differences between anatomic and functional imaging, the results were mixed. In general, when high cut-off values for “significant” coronary artery stenoses (>70% or 75% stenosis, as opposed to the commonly used criterion of 50%) were used, these studies found that CCTA was useful in ruling out functionally significant CAD but was not a good predictor of ischemia (TABLE 2).25,29
TABLE 2.
Diagnostic performance of multidetector computed tomography in detecting significant perfusion abnormailites in myocardial perfusion imaging25,26,29
| Study | Gaemperli et al.(2007)a | Sato et al.b | Gaemperli et al.(2008)c |
|---|---|---|---|
| SCA data included | no | no | yes |
| sensitivity (%) | 75 | 79 | 95 |
| specificity (%) | 90 | 92 | 53 |
| PPV (%) | 68 | 66 | 58 |
| NPV (%) | 93 | 96 | 94 |
CI were not reported.
Data is for ≥75% stenosis correlating to any perfusion deficit.
Data is for ≥70% stenosis correlating to a reversible perfusion deficit.
Data is for ≥50% stenosis correlating to a reversible perfusion deficit.
Abbreviations: SCA – selective coronary angiography, others – see TABLE
Other recent studies compared CCTA to both MPI and SCA.26,30 Similar to the studies comparing CCTA to MPI only, CCTA demonstrated a high NPV for reversible perfusion defects on MPI. However, the sensitivities and specificities varied, in some cases substantially, between the studies. All the studies concluded, however, that normal CCTA examinations effectively ruled out significant functional abnormalities on MPI or high-grade stenoses on SCA, but that the PPV of abnormal CCTA examinations for ischemia was limited. The overall strength of evidence provided by these studies was limited based on the low numbers of study subjects (78–114 patients) and the selected nature of the patient populations based on the presence of at least intermediate pretest likelihood of CAD.27,30
More recently, a study comparing CCTA to MPI and SCA was performed in low-risk emergency room chest pain patients.27 In this nonrandomized study, subjects were recruited from a population of emergency room patients who presented with symptoms consistent with an acute coronary syndrome. These patients underwent both CCTA and MPI evaluations and, if certain clinical or imaging criteria were met, SCA. The results suggested that the accuracy in the prediction of significant clinical outcomes, acute coronary syndrome, or CAD was comparable between CCTA and MPI. CCTA was 86% sensitive and 92% specific, with PPV and NPV of 50% and 99%, respectively. MPI was 71% sensitive, 90% specific, and had PPV and NPV of 38% and 97%, respectively. The overall low prevalence of disease (8%) contributed to the low PPV for both imaging modalities. This study was limited in that SCA was not performed in all patients, raising the possibility of verification bias for those patients who underwent both CCTA and SCA. Importantly, 7 out of 96 (7%) study patients were excluded from the study due to non-diagnostic image quality on CCTA. However, this study overall suggests that CCTA may be useful and clinically relevant in low-risk chest pain patients presenting to the emergency room.
The discrepancies in the studies comparing CCTA, SCA, and MPI discussed above highlight the conceptual differences between anatomic and functional imaging modalities. In short, normal MPI does not exclude the presence of coronary atherosclerosis but does suggest a very low risk of short- to mid-term adverse cardiac events. Conversely, CCTA can detect coronary artery plaques that are not functionally significant.
But what about radiation dose?
An appraisal of the clinical value of CCTA cannot be complete without discussion of exposure to ionizing radiation. Biased reporting in the media frequently emphasizes the potential risks of ionizing radiation without addressing the potential benefits that medical imaging can provide by offering diagnostic information and guidance for management. A recent report from the National Council for Radiation Protection and Measurements37 showed that, compared to 1986, the number of CT imaging studies increased by >10% per year, and that the collective dose received from diagnostic medical radiation including radiography and nuclear medicine studies has increased by >700% and the annual per-capita dose, by almost 600%. However, the report also showed that 80% of the 67 million CT studies in the USA in 2006 were performed in presumably very ill or at-risk patient populations, namely in the hospital setting and in the elderly.
In order to understand the information on radiation exposure and dose that is often provided in passing in clinical studies of CCTA, it is important to have a basic working knowledge of radiation dosimetry and radiation biology.38 While the risk of malignancies at high radiation doses such as those received by the survivors of atomic bomb explosions or nuclear accidents is rarely disputed, the risk of cancer at the radiation dose levels in medical imaging is very controversial among medical physicists. Because no definite data on the dose-response relationship exist, and possibly never will, the risks of medical radiation are usually discussed with the conservative assumption that there is no dose threshold below which ionizing radiation cannot cause malignancies, and that the risk varies proportionally and linearly with dose (the so-called “linear no-threshold hypothesis”).39 Based on the linear no-threshold hypothesis, a recent study40 modeled the lifetime attributable risk of cancer of a typical 64-slice CCTA: the risk varied between 0.7% (1 in 143) for 20-year-old women to 0.044% (1 in 2273) for 80-year-old men. However, the linear no-threshold hypothesis is not universally supported41.
It is important to realize that there is a difference between dosimetry parameters that can be measured, such as the volume computed tomographic dose index or the dose length product, and parameters that are estimated based on modeling from complex assumptions, such as the effective dose (E) estimate. E, perhaps the dosimetry parameter most frequently quoted in CCTA studies, is an estimate of the biologic risk of a non-homogeneous irradiation of a part of the body (i.e. the chest) that is typical in medical imaging. E is a generic, not a patient-specific, estimate that is best used to compare the potential biologic risk between different CT imaging protocols, or between different types of radiological examinations, including comparisons between different types of radiation (i.e. X-ray-based CCTA vs. radionuclide-based MPI). It cannot be used to compare radiation doses between patients for the same imaging procedure. Given the various uncertainties related to the modeling process used to estimate E, E should be quoted as ranges, not numbers with several decimal places. TABLE 3 38,42 lists the representative values and ranges of E reported in the literature for selected radiological studies. For comparison, the average annual background exposure in the USA due to natural sources of radiation such as radon is approximately 3 mSv (range: 1–10 mSv).
TABLE 3.
Representative values and ranges of effective dose estimates reported in the literature for selected radiological studies38,42
| Examination | Representative effective dose value (mSv) | Range of reported effective dose values (mSv) | Administered activity (MBq) |
|---|---|---|---|
| chest X-ray PA and lateral | 0.1 | 0.05–0.24 | NA |
| coronary calcium CTa | 3 | 1–12 | NA |
| 64-slice CCTAb without tube current modulation | 15 | 12–18 | NA |
| 64-slice CCTAb with tube current modulation21 | 9 | 8–18 | NA |
| prospectively triggered CCTAb22 | 3 | 2–4 | NA |
| diagnostic invasive coronary angiogram | 7 | 2–16 | NA |
| percutaneous coronary intervention or radiofrequency ablation | 15 | 7–57 | NA |
| myocardial perfusion study | |||
| sestamibi (1-day) stress/rest SPECT | 9 | – | 1100 |
| thallium stress/rest SPECT | 41 | – | 185 |
| F-18 FDG PET | 14 | – | 740 |
| rubidium-82 PET | 5 | – | 1480 |
Data combine prospectively triggered and retrospectively gated protocols.
The representative effective dose is approximately 1 mSv for prospectively triggered coronary calcium CT scans and 3 mSv for retrospectively gated scans.
64-slice multidetector-row CT and dual-source CT studies published since 2005 only; data include a survey of the literature by Gerber et al.
Abbreviations: CT – computed tomography, CCTA – coronary CT angiography, FDG – fluorodeoxyglucose, NA – not applicable, PA – posteroanterior, PET – positron emission tomography, SPECT – single photon emitted computed tomography
The radiation output of CT scanners, and hence radiation dose estimates for CCTA, are related to several modifiable scanner settings. There is an inverse relationship between radiation dose and image noise. Radiation protection for the patient includes the challenge to keep patient dose as low as reasonably achievable while maintaining the image quality at a level that allows confident interpretation.
Traditionally, coronary multidetector CT angiography uses retrospective gating. In this mode, radiation is produced for the entire cardiac cycle over several cardiac cycles, until the patient table has moved through the gantry enough for the entire heart to be covered from its cranial to its caudal end. Planar, transaxial images are then reconstructed from the projection data at a retrospectively defined window during the cardiac cycle. This reconstruction window is chosen at a phase where cardiac motion is minimal, typically during mid-diastole just after passive ventricular filling is complete (diastasis, 60–70% of the R-to-R interval on the electrocardiogram). The remainder of the projection data, and the radiation invested to acquire it, is not used.
Several techniques exist to reduce patient dose from CCTA. Electrocardiographically controlled tube current modulation (ECTCM) reduces radiation output by approximately 80% during the portions of the cardiac cycle unlikely to be used for image reconstructions (i.e. typically during most of systole). In a recent international, multicenter survey of radiation dose43 in CCTA, ECTCM lowered E by 25% and was used in 73% of patients. Sequential scanning, sometimes also referred to as “prospective triggering,” is a new CT scanning technique that entirely shuts off the X-ray tube during the portions of cardiac cycle unlikely to be used for image reconstruction. Sequential scanning lowered E by 78% and was used in 6% of patients.43 Reduction of tube voltage from 120 to 100 kVp reduced E by 46% and was used in 5% of patients.43 The low utilization of the techniques that reduced radiation dose the most, namely sequential scanning and tube voltage reduction, are likely related to the facts that sequential scanning was not widely available in 2007, when the survey was conducted, and to concerns among the cardiac imaging community that use of these techniques might reduce image quality and diagnostic accuracy. Studies currently under way will hopefully alleviate these concerns and lead to wider acceptance of these highly effective means of radiation dose reduction.
So what are the recommended indications for coronary computed tomographic angiography?
Given the limited evidence base to date, no guidelines by any professional associations of healthcare providers in the USA exist for the use of CCTA in clinical practice. A scientific statement by the AHA5 summarized clinical studies predating the information discussed above. This statement considered the evaluation of the proximal course of known coronary anomalies (FIGURE 4) a meaningful indication for CCTA, based on expert consensus among the Writing Group. Coronary magnetic resonance (MR) angiography, where available, was recommended over CT angiography for this indication in younger patients because of the potentially harmful consequences of exposure to ionizing radiation. There was also consensus that CCTA was indicated as a second-line test in symptomatic patients who remained at intermediate probability of having CAD after initial evaluation by history taking, physical examination, and conventional stress testing. For this indication, CCTA was considered better suited than MR angiography given the higher diagnostic accuracy of the former.44
FIGURE 4.
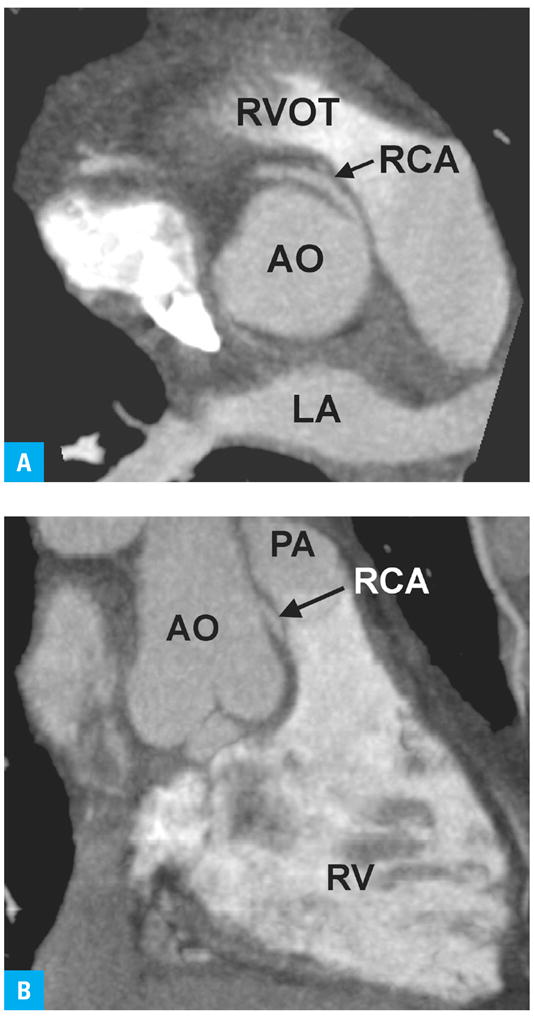
Coronary computed tomography angiogram demonstrating aberrant origin of the RCA from the left sinus of Valsalva, coursing between the PA and AO.
A Horizontal long axis at the level of the aortic root
B Vertical long axis. The potential for compression of the anomalous RCA between the PA from anteriorly and the AO from posteriorly becomes apparent.54
Abbreviations: RCA – right coronary artery, others – see FIGURE 1
The AHA scientific statement specifically discouraged use of CCTA for screening for subclinical CAD in asymptomatic patients, but encouraged research into the potential of CCTA to characterize and quantify coronary plaque burden as a means of risk stratification. Similarly, CCTA was not recommended for symptomatic patients with high probability of CAD because these patients were likely to need SCA given the fact that CCTA currently cannot be combined with percutaneous coronary revascularization.
The newer data discussed above22,23,32 do not warrant revision of these recommendations at the current time. However, given the consistently high NPV at many levels of disease prevalence, CCTA could perhaps in the future prove useful for “ruling out” significant coronary stenoses in patient groups where the predictive value of stress imaging is limited or where SCA is currently performed as a matter of course.45 Such scenarios include ruling out CAD in patients with unexplained left ventricular dysfunction46, left bundle branch block47, before non-coronary cardiac surgery48, or after heart transplantation49.
Conclusions
Finding the place for CCTA in current clinical practice means weighing its known strengths against its potential risks. There are currently no generally accepted first-line indications for CCTA except for the evaluation of congenitally abnormal coronary arteries.
The value of atherosclerosis imaging in general (not limited to CCTA but also including coronary artery calcium scanning or carotid intima-media thickness by ultrasound) for prognostication and for improving patient outcomes as discussed above is controversial because no data from controlled randomized trials exist.50 In particular, the rapidly increasing use of CCTA in patients with risk factors for CAD but no symptoms has drawn criticism in the USA for its high cost in the face of unproven value.51,52 The optimal management of non-obstructive, subclinical CAD is not established. On this background, we believe that the small hypothetical risk outweighs the unproven, potential benefit, and we advise against the use of CCTA for risk stratification in asymptomatic patients.
CCTA is clearly not useful in patients with enzymatic or electrocardiographic evidence for myocardial compromise where SCA should be used because it can readily be combined with percutaneous coronary revascularization if indicated. The limitations imposed by high levels of coronary calcium on confident image interpretation makes CCTA unsuitable for the assessment of patients with established CAD. Assessment of coronary artery bypass grafts is an interesting but currently unproven use of CCTA.
In symptomatic patients in whom the diagnosis of CAD remains unclear after conventional evaluation, the high sensitivity of CCTA in our opinion more than balances the potential risk of future malignancies, considering the possibly catastrophic consequences of missing high-grade coronary stenoses. This is particularly true for patients who present to the emergency department acutely. In addition, the typical chest pain patient with intermediate probability of CAD is at an age where they are likely to die of other causes before the 10–30 year latency period of radiation-induced malignancies has passed. Other potential indications that exploit the high NPV of CCTA await further study.
The substantial reduction of radiation dose to be expected from widespread implementation of current and future dose-sparing scanning protocols may well shift the risk-benefit balance for many patient groups, but concerns about cost-efficiency remain. Studies of the value of detecting and treating subclinical atherosclerosis in the form of noncalcified plaque for improving longevity are pivotal if the use of CCTA in asymptomatic patients with risk factors is to be justified.
Acknowledgments
Conflict of interest: this work was supported in part by National Institutes of Health (NIH) grant 1R01EB007986-02 (“Non-Invasive Localization of Vulnerable Plaques”) awarded to Dr Birgit Kantor.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. J Nucl Cardiol. 2002;9:133–134. doi: 10.1067/mnc.2002.120681. [DOI] [PubMed] [Google Scholar]
- 3.Heijenbrok-Kal MH, Fleischmann KE, Hunink MG. Stress echocardiography, stress single-photon-emission computed tomography and electron beam computed tomography for the assessment of coronary artery disease: a meta-analysis of diagnostic performance. Am Heart J. 2007;154:415–423. doi: 10.1016/j.ahj.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Kuntz KM, Fleischmann KE, Hunink MG, Douglas PS. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med. 1999;130:709–718. doi: 10.7326/0003-4819-130-9-199905040-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008;118:586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder S, Kopp AF, Baumbach A, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001;37:1430–1435. doi: 10.1016/s0735-1097(01)01115-9. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder S, Kopp AF, Ohnesorge B, et al. Virtual coronary angioscopy using multislice computed tomography. Heart. 2002;87:205–209. doi: 10.1136/heart.87.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenland P, Abrams J, Aurigemma GP, et al. Prevention Conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden: Writing Group III. Circulation. 2000;101:E16–22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 10.Ammann P, Brunner-La Rocca HP, Angehrn W, et al. Procedural complications following diagnostic coronary angiography are related to the operator‘ s experience and the catheter size. Catheter Cardiovasc Interv. 2003;59:13–18. doi: 10.1002/ccd.10489. [DOI] [PubMed] [Google Scholar]
- 11.Batyraliev T, Ayalp MR, Sercelik A, et al. Complications of cardiac catheterization: a single-center study. Angiology. 2005;56:75–80. doi: 10.1177/000331970505600110. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekar B, Doucet S, Bilodeau L, et al. Complications of cardiac catheterization in the current era: a single-center experience. Catheter Cardiovasc Interv. 2001;52:289–295. doi: 10.1002/ccd.1067. [DOI] [PubMed] [Google Scholar]
- 13.Arnett EN, Isner JM, Redwood DR, et al. Coronary artery narrowing in coronary heart disease: comparison of cineangiographic and necropsy findings. Ann Intern Med. 1979;91:350–356. doi: 10.7326/0003-4819-91-3-350. [DOI] [PubMed] [Google Scholar]
- 14.Marcus ML, Skorton DJ, Johnson MR, et al. Visual estimates of percent diameter coronary stenosis: “a battered gold standard”. J Am Coll Cardiol. 1988;11:882–885. doi: 10.1016/0735-1097(88)90226-4. [DOI] [PubMed] [Google Scholar]
- 15.Kern MJ. Coronary physiology revisited: practical insights from the cardiac catheterization laboratory. Circulation. 2000;101:1344–1351. doi: 10.1161/01.cir.101.11.1344. [DOI] [PubMed] [Google Scholar]
- 16.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110:1168–1176. doi: 10.1161/01.CIR.0000138790.14877.7D. [DOI] [PubMed] [Google Scholar]
- 17.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention–summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:156–175. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 18.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 19.Moshage WE, Achenbach S, Seese B, et al. Coronary artery stenoses: three-dimensional imaging with electrocardiographically triggered, contrast agent-enhanced, electron-beam CT. Radiology. 1995;196:707–714. doi: 10.1148/radiology.196.3.7644633. [DOI] [PubMed] [Google Scholar]
- 20.Ohnesorge B, Flohr T, Becker C, et al. Cardiac imaging by means of electrocardiographically gated multisection spiral CT: initial experience. Radiology. 2000;217:564–571. doi: 10.1148/radiology.217.2.r00nv30564. [DOI] [PubMed] [Google Scholar]
- 21.Gerber TC, Kuzo RS, Karstaedt N, et al. Current results and new developments of coronary angiography with use of contrast-enhanced computed tomography of the heart. Mayo Clin Proc. 2002;77:55–71. doi: 10.4065/77.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Stein PD, Yaekoub AY, Matta F, Sostman HD. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med. 2008;121:715–725. doi: 10.1016/j.amjmed.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 24.Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. Eur Radiol. 2009;19:816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaemperli O, Schepis T, Koepfli P, et al. Accuracy of 64-slice CT angiography for the detection of functionally relevant coronary stenoses as assessed with myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2007;34:1162–1171. doi: 10.1007/s00259-006-0307-z. [DOI] [PubMed] [Google Scholar]
- 26.Gaemperli O, Schepis T, Valenta I, et al. Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology. 2008;248:414–423. doi: 10.1148/radiol.2482071307. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher MJ, Ross MA, Raff GL, et al. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49:125–136. doi: 10.1016/j.annemergmed.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Lin F, Shaw LJ, Berman DS, et al. Multidetector computed tomography coronary artery plaque predictors of stress-induced myocardial ischemia by SPECT. Atherosclerosis. 2008;197:700–709. doi: 10.1016/j.atherosclerosis.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Sato A, Hiroe M, Tamura M, et al. Quantitative measures of coronary stenosis severity by 64-Slice CT angiography and relation to physiologic significance of perfusion in nonobese patients: comparison with stress myocardial perfusion imaging. J Nucl Med. 2008;49:564–572. doi: 10.2967/jnumed.107.042481. [DOI] [PubMed] [Google Scholar]
- 30.Schuijf JD, Wijns W, Jukema JW, et al. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48:2508–2514. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 31.Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006;48:1896–1910. doi: 10.1016/j.jacc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 33.Mark DB, Hlatky MA, Harrell FE, Jr, et al. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106:793–800. doi: 10.7326/0003-4819-106-6-793. [DOI] [PubMed] [Google Scholar]
- 34.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 35.Nissen SE. Limitations of computed tomography coronary angiography. J Am Coll Cardiol. 2008;52:2145–2147. doi: 10.1016/j.jacc.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Rispler S, Keidar Z, Ghersin E, et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49:1059–1067. doi: 10.1016/j.jacc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 37.National Council on Radiation Protection and Measurements (NCRP) Ionizing radiation exposure of the population of the United States. Publication No. 160. Bethesda, MD: Nartional Council on Radiation Protection and Measurements; 2009. [Google Scholar]
- 38.Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056–1065. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 39.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation Board on Radiation Effects Research Division on Earth and Life Studies National Research Council of the National Academies. Health risks from exposure to low levels of ionizing radiation: BEIR VII-Phase 2. Washington, D.C: National Academies Press; 2006. [PubMed] [Google Scholar]
- 40.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 41.Burk RJ. Radiation risks in perspective. Position Statement of the Health Physics Society. 2004 [Google Scholar]
- 42.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 43.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 44.Kefer J, Coche E, Legros G, et al. Head-to-head comparison of three-dimensional navigator-gated magnetic resonance imaging and 16-slice computed tomography to detect coronary artery stenosis in patients. J Am Coll Cardiol. 2005;46:92–100. doi: 10.1016/j.jacc.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 45.Kantor B, Anavekar NS, Gerber TC. Good news on coronary computed tomographic angiography: answers that have questions! Eur Heart J. 2008 doi: 10.1093/eurheartj/ehn338. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghostine S, Caussin C, Habis M, et al. Non-invasive diagnosis of ischaemic heart failure using 64-slice computed tomography. Eur Heart J. 2008 Apr 1; doi: 10.1093/eurheartj/ehn072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Ghostine S, Caussin C, Daoud B, et al. Non-invasive detection of coronary artery disease in patients with left bundle branch block using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1929–1934. doi: 10.1016/j.jacc.2006.04.103. [DOI] [PubMed] [Google Scholar]
- 48.Meijboom WB, Mollet NR, Van Mieghem CA, et al. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol. 2006;48:1658–1665. doi: 10.1016/j.jacc.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 49.Sigurdsson G, Carrascosa P, Yamani MH, et al. Detection of transplant coronary artery disease using multidetector computed tomography with adaptative multisegment reconstruction. J Am Coll Cardiol. 2006;48:772–778. doi: 10.1016/j.jacc.2006.04.082. [DOI] [PubMed] [Google Scholar]
- 50.Gerber TC, Taylor AJ. Carotid intima-media thickness: can it close the “detection gap” for cardiovascular risk? Mayo Clin Proc. 2009;84:218–220. doi: 10.4065/84.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caplan S, Rollins JA, Jacques LB, Phurrough SE. Commentary: Medicare Coverage Advisory Committee meeting on noninvasive imaging for coronary artery disease. Am Heart J. 2007;153:159–160. doi: 10.1016/j.ahj.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Redberg RF. Evidence, appropriateness, and technology assessment in cardiology: a case study of computed tomography. Health Aff (Millwood) 2007;26:86–95. doi: 10.1377/hlthaff.26.1.86. [DOI] [PubMed] [Google Scholar]
- 53.Gerber TC, Walser EM. Cardiovascular computed tomography and magnetic resonance imaging. In: Murphy JG, Lloyd MA, editors. Mayo Clinic Cardiology: Concise Textbook. 3. Rochester (MN): Mayo Clinic Scientific Press; 2007. pp. 185–204. [Google Scholar]
- 54.Deibler AR, Kuzo RS, Vohringer M, et al. Imaging of congenital coronary anomalies with multislice computed tomography. Mayo Clin Proc. 2004;79:1017–1023. doi: 10.4065/79.8.1017. [DOI] [PubMed] [Google Scholar]