Abstract
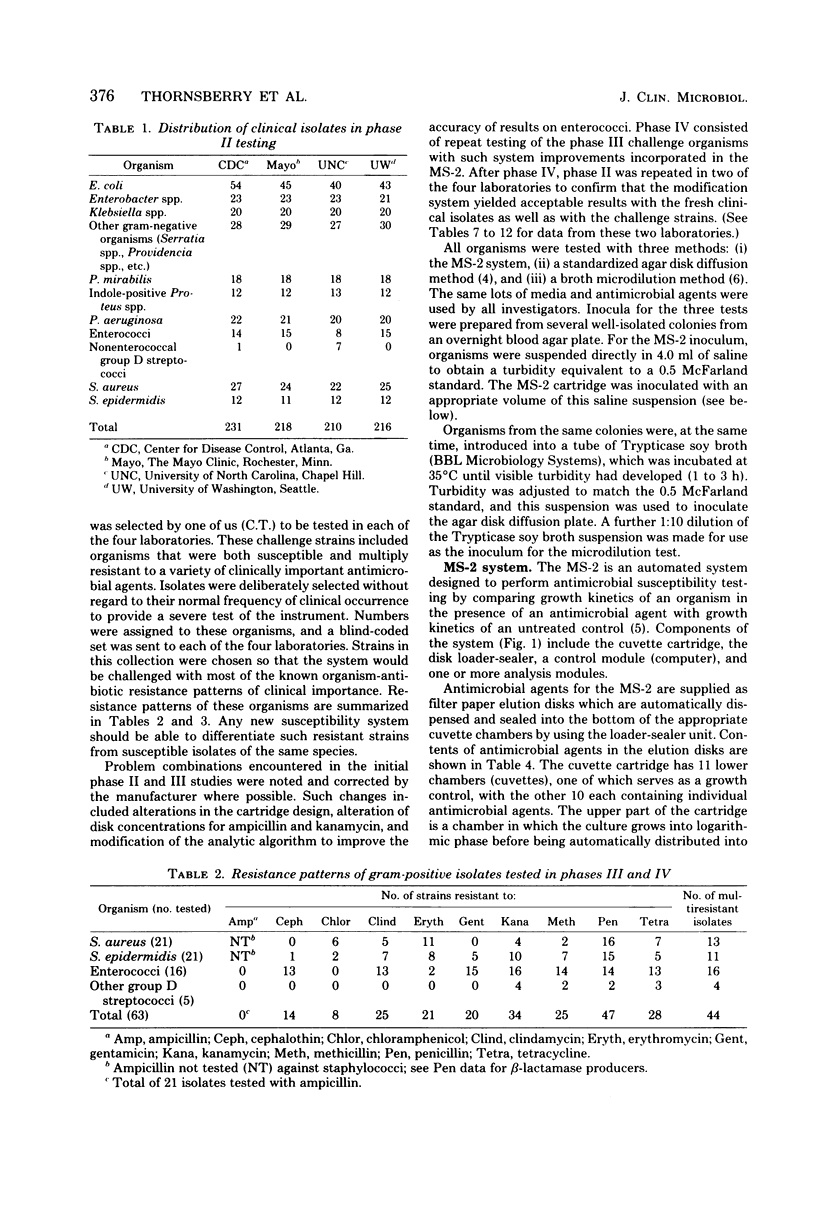
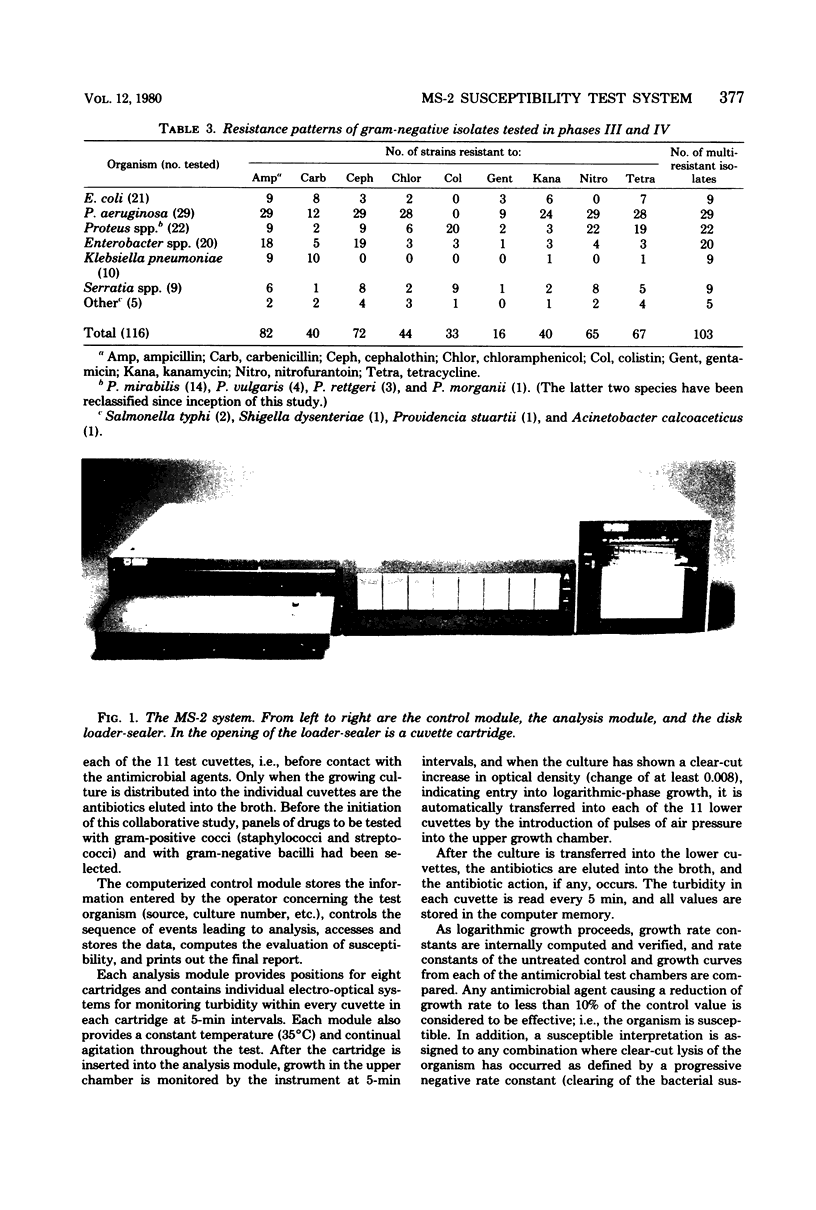
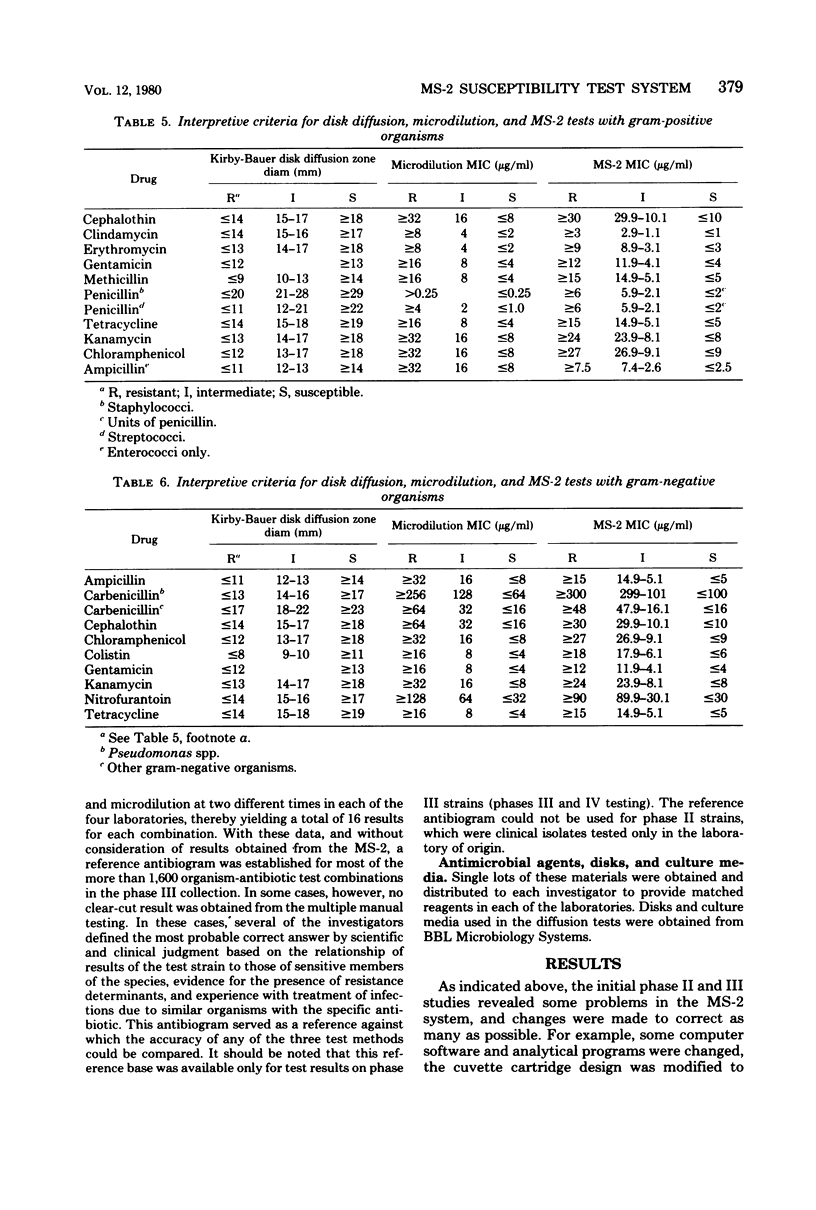
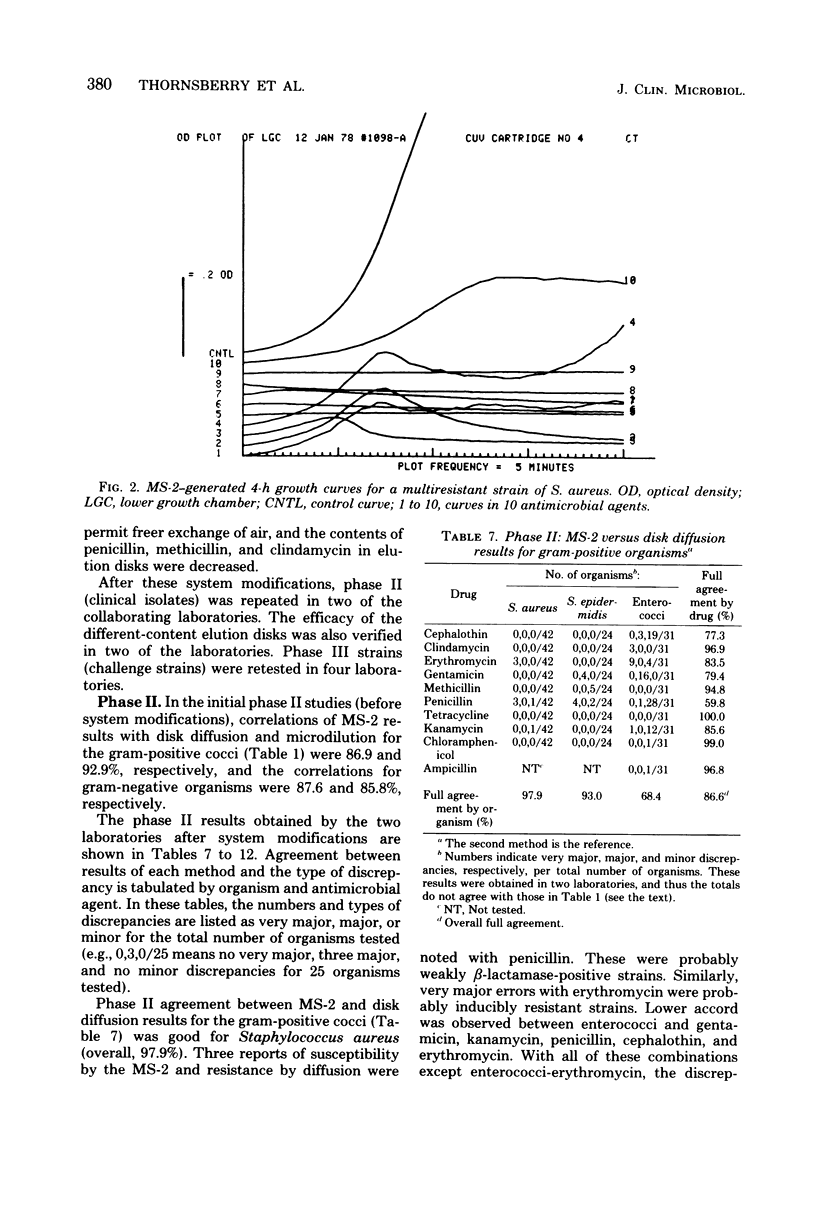
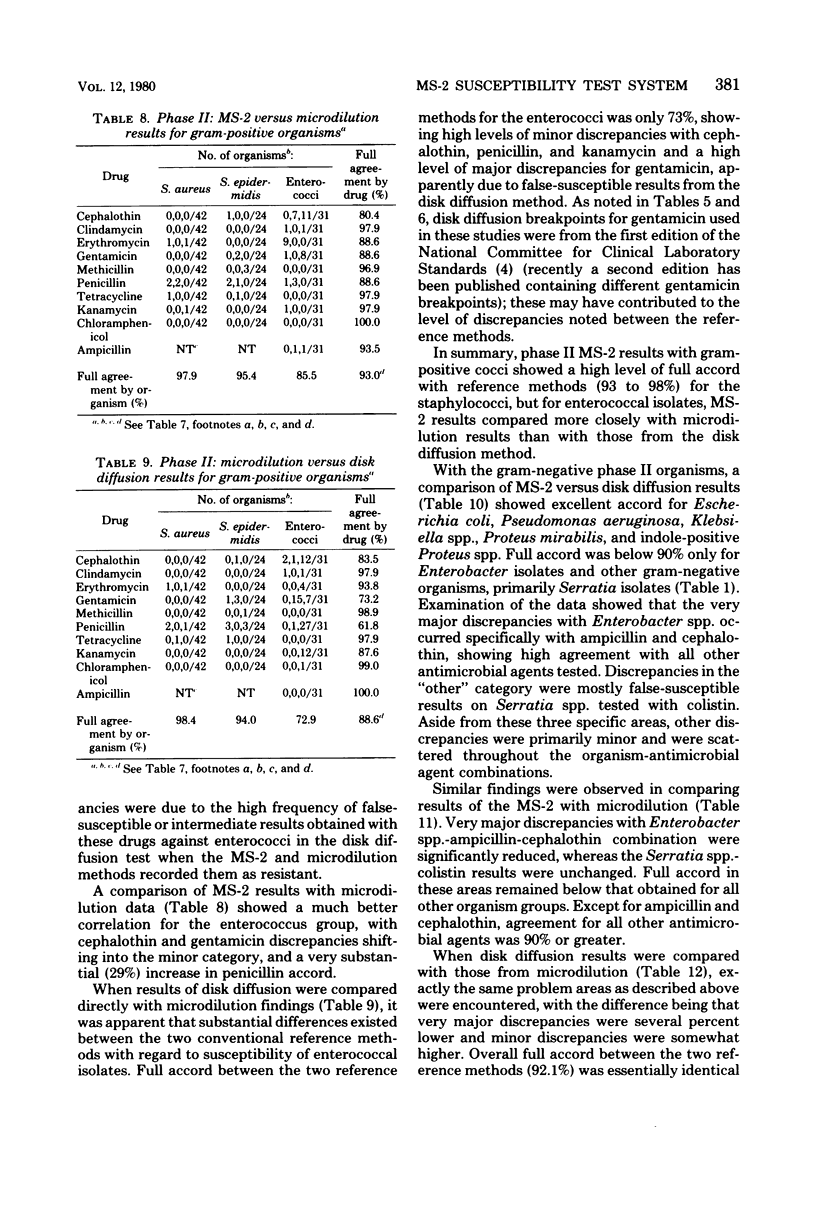
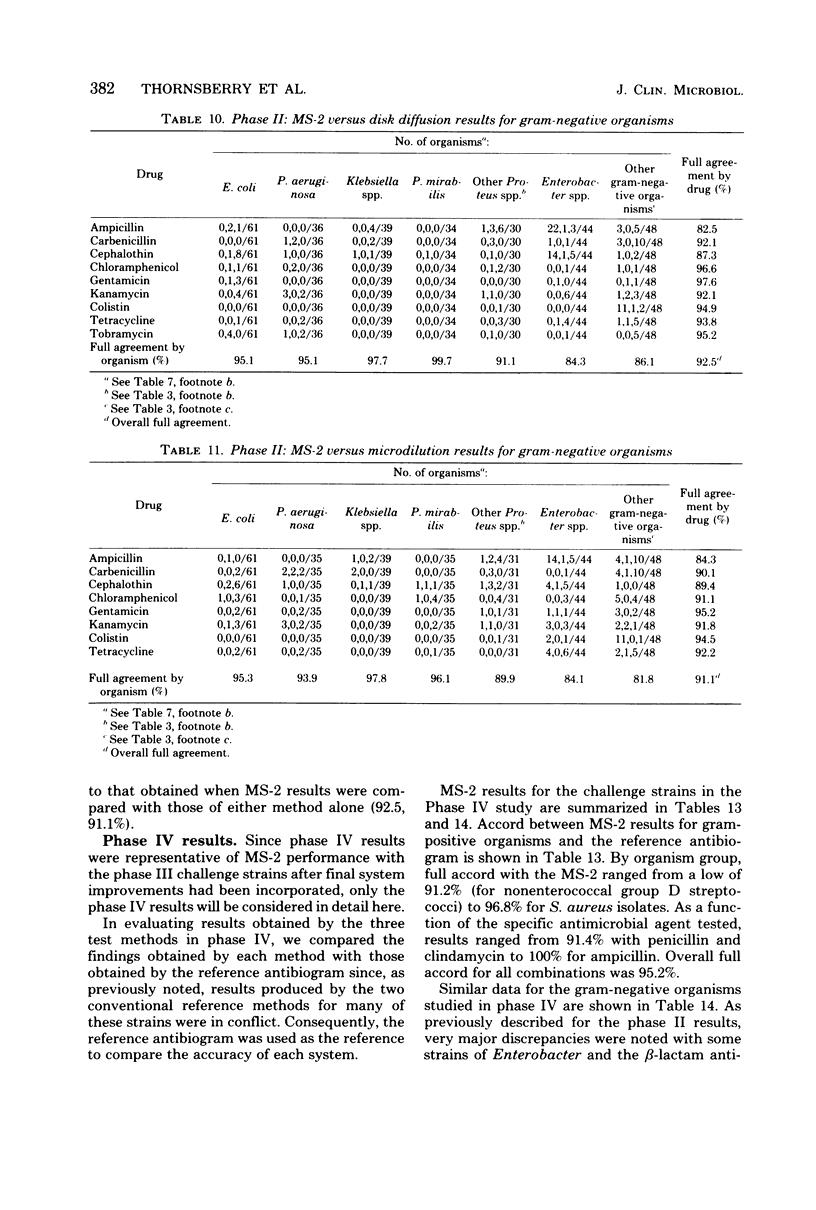
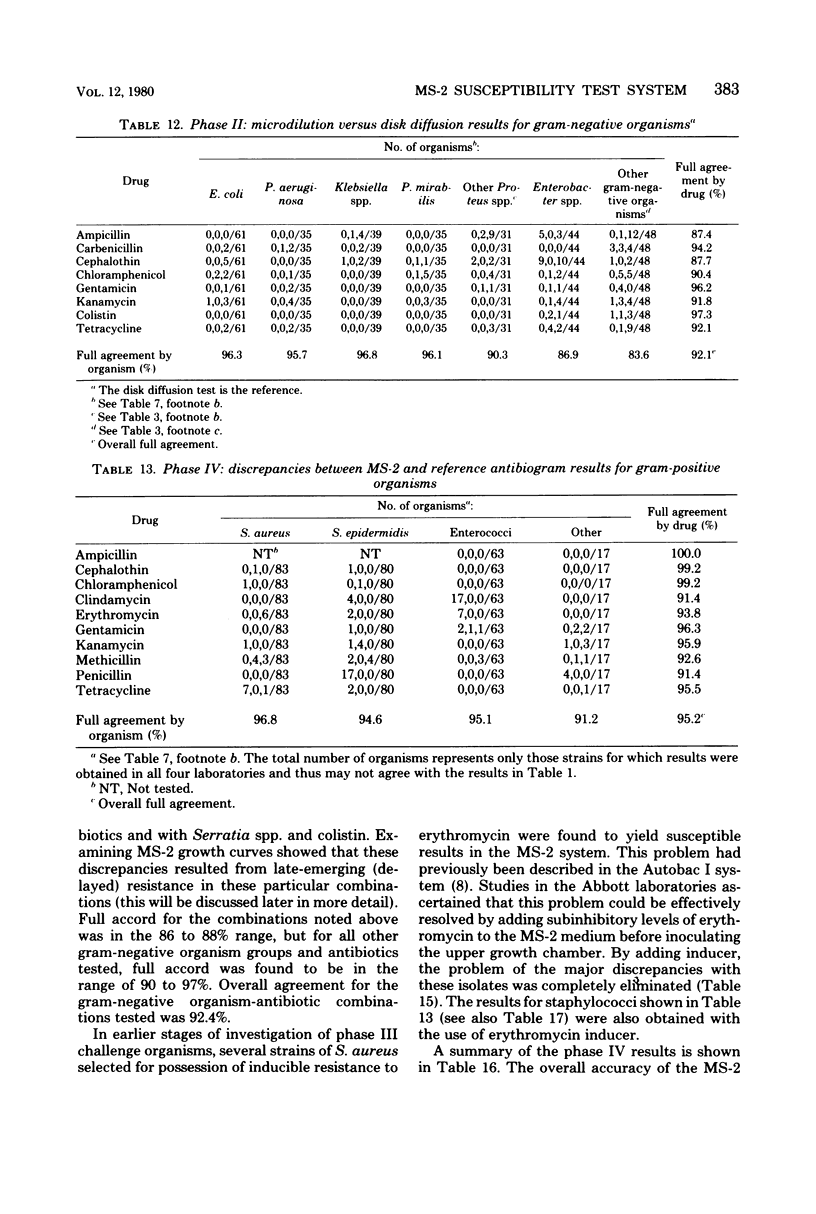
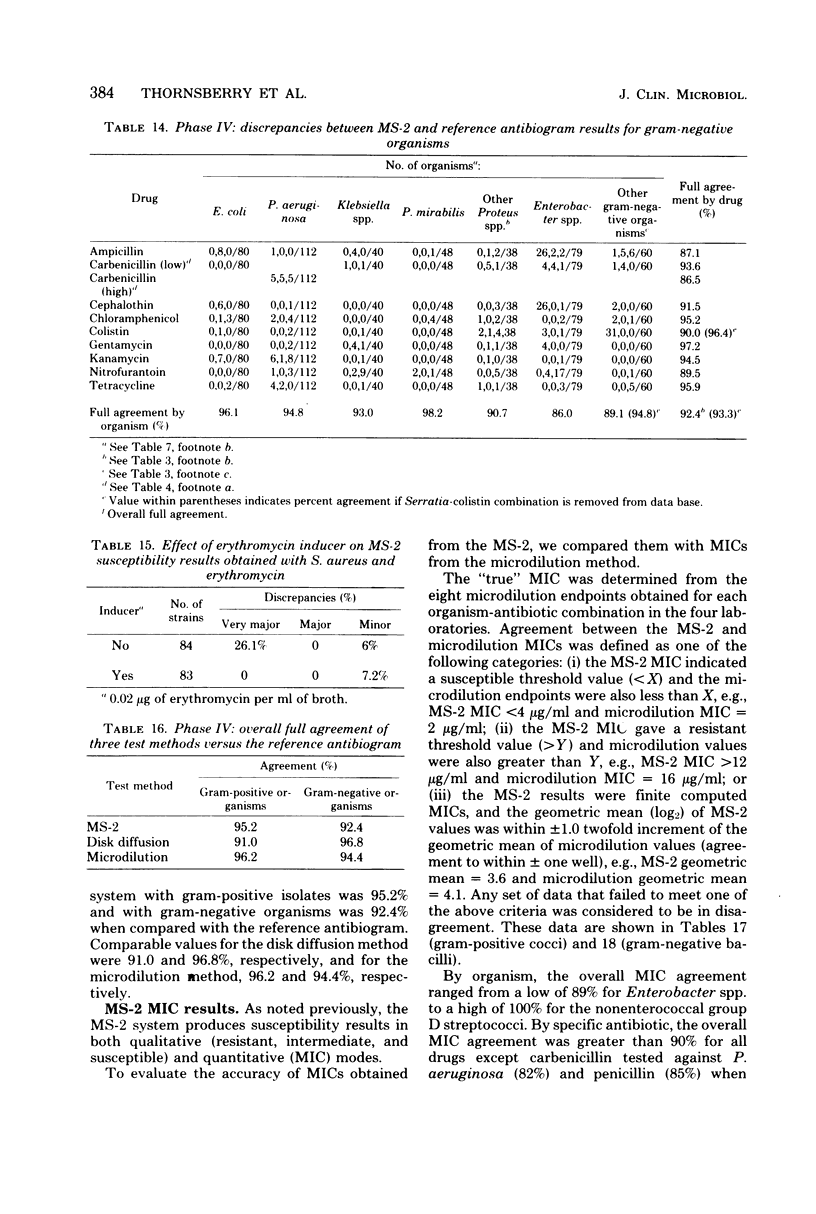
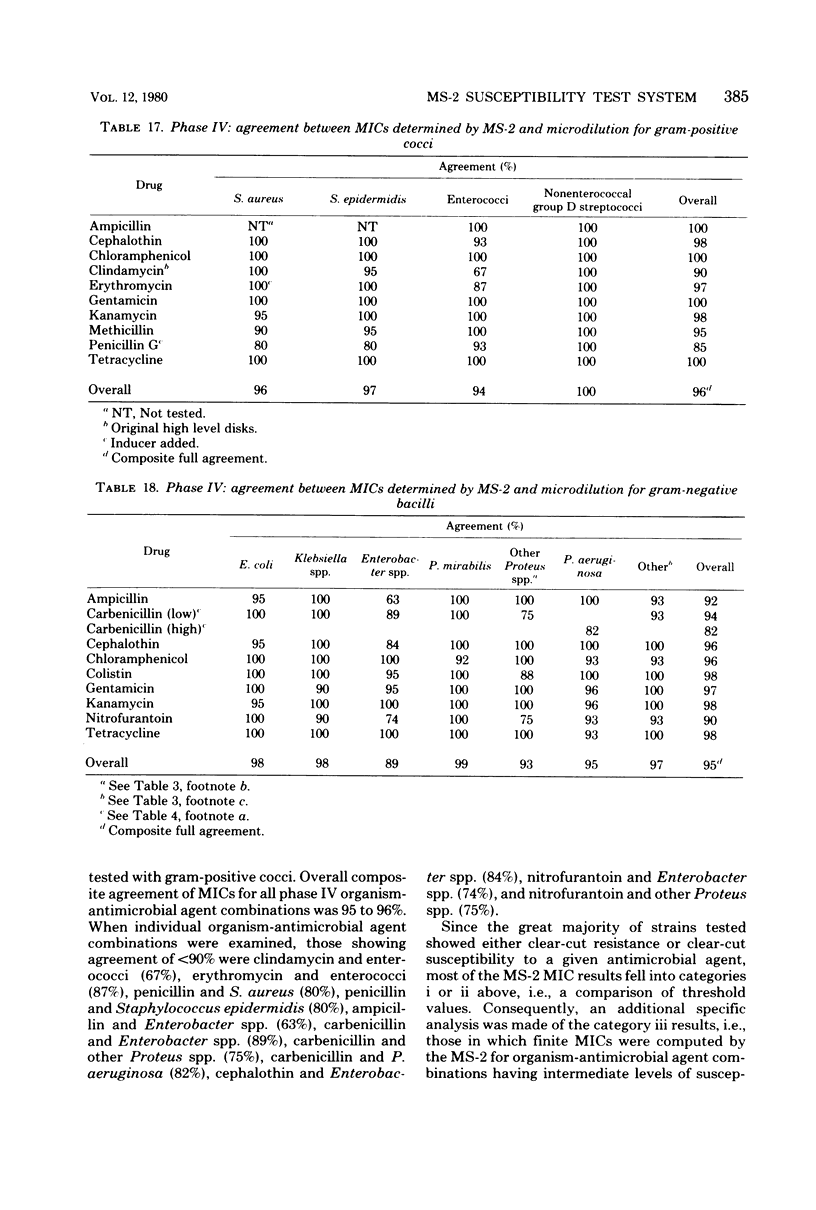
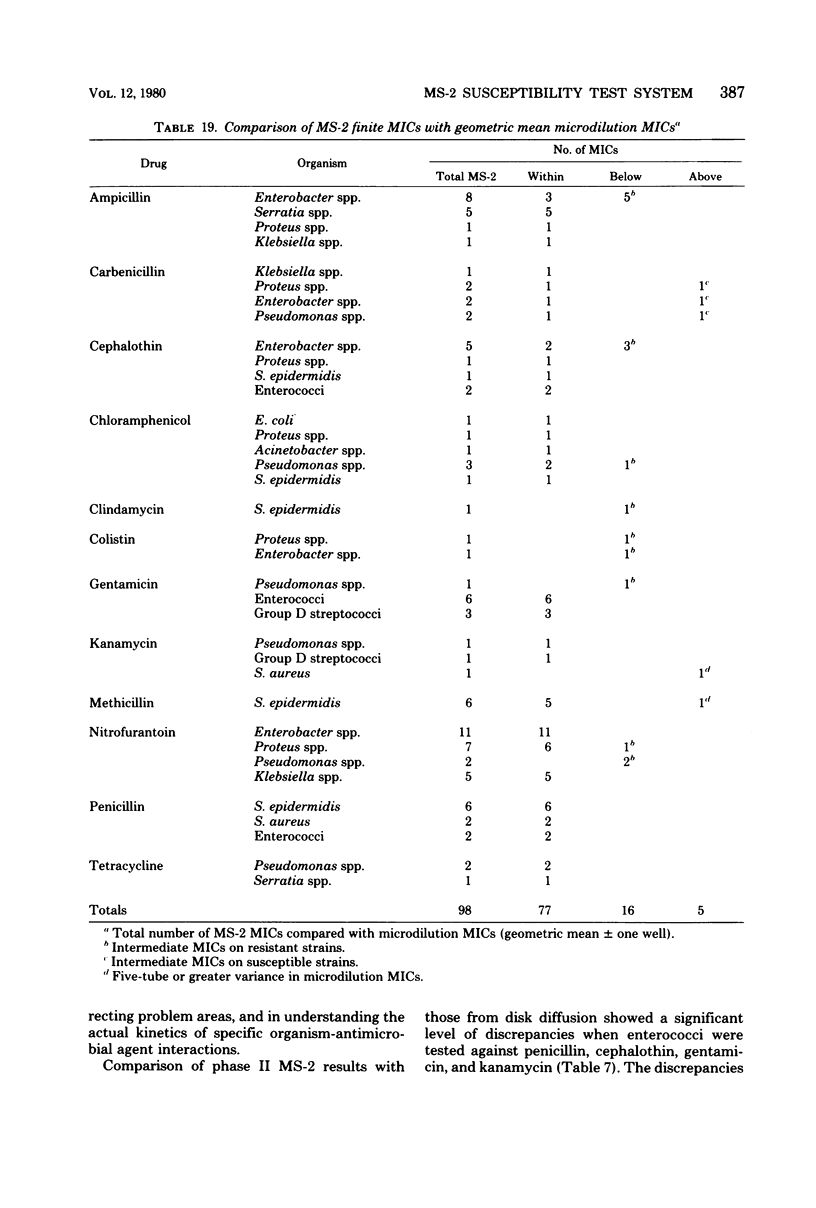
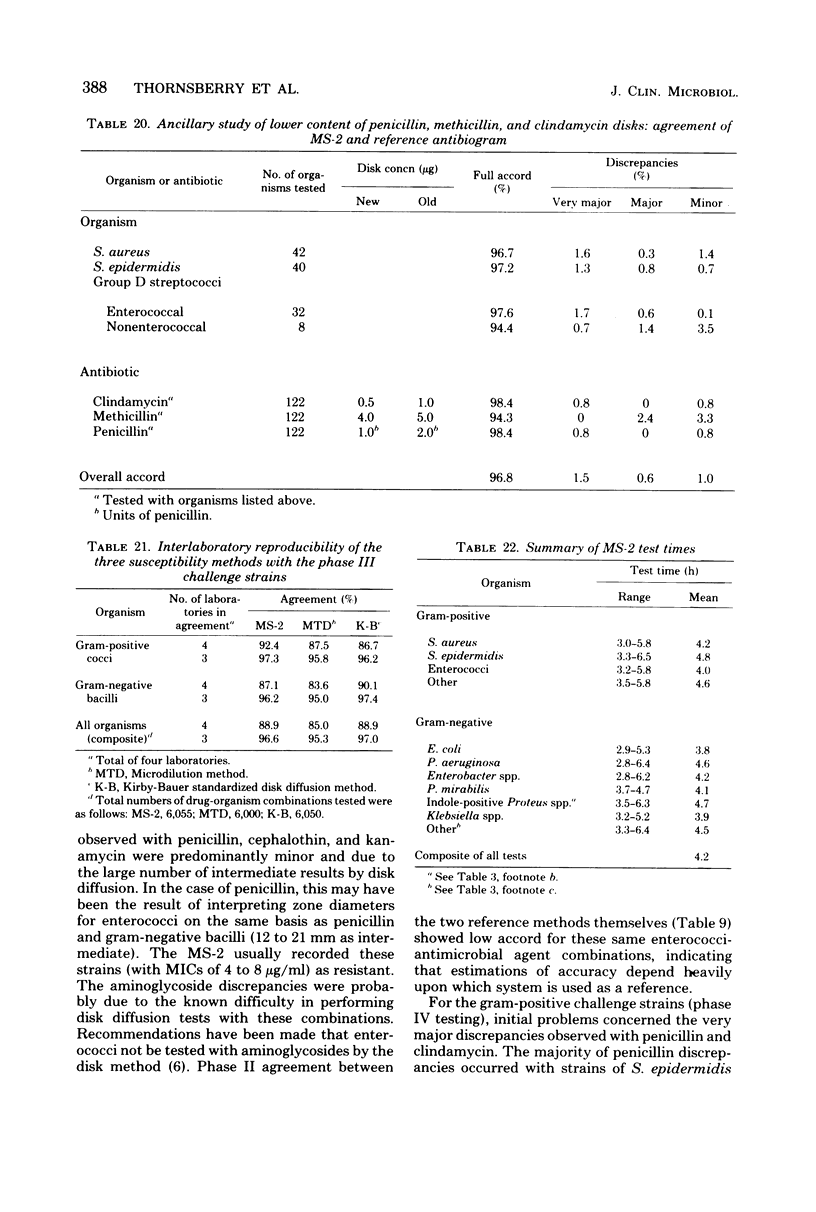
The MS-2 system (Abbott Diagnostics, Division of Abbott Laboratories, Dallas, Tex.) was evaluated for its efficacy in determining the susceptibilities of both clinical and selected challenge (nonfastidious, facultative, and aerobic) isolates. The MS-2 results were compared with standard Kirby-Bauer disk diffusion and microdilution results by using fresh clinical isolates. For gram-positive isolates other than enterococci, overall agreement between MS-2 and reference results was 93 to 98%. With enterococci, MS-2 agreement with disk diffusion was 68% but with microdilution was 86% (agreement between disk diffusion and microdilution was 73%). The main discrepancies with enterococci were with cephalothin, penicillin, gentamicin, and kanamycin. With clinical gram-negative isolates, the overall agreement was 91 to 93%, with most discrepancies occurring with Enterobacter spp. and beta-lactam antibiotics (MS-2 versus disk diffusion, 84%; MS-2 versus microdilution, 84%; disk diffusion versus microdilution, 87%) and with Serratia spp. and colistin (false-susceptible results). The agreement of MS-2 results with established reference antibiograms of a special collection of challenge strains was 91 to 97% for the gram-positive cocci and 86 to 98% for the gram-negative strains. (With Enterobacter spp., agreement was 86%, but was greater than 90% for all other organism groups.) Of the 98 finite MS-2 minimum inhibitory concentrations (MICs) that could be directly compared with microdilution MICs, 77 (79%) were within +/- 1 well of the geometric mean microdilution MIC. MS-2 analysis time ranged from 2.8 to 6.5 h (mean, 4.2 h). On the basis of these results, we conclude that the MS-2 can be expected to yield rapid and accurate results with most nonfastidious, facultative, and aerobic pathogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Isenberg H. D., Reichler A., Wiseman D. Prototype of a fully automated device for determination of bacterial antibiotic susceptibility in the clinical laboratory. Appl Microbiol. 1971 Dec;22(6):980–986. doi: 10.1128/am.22.6.980-986.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
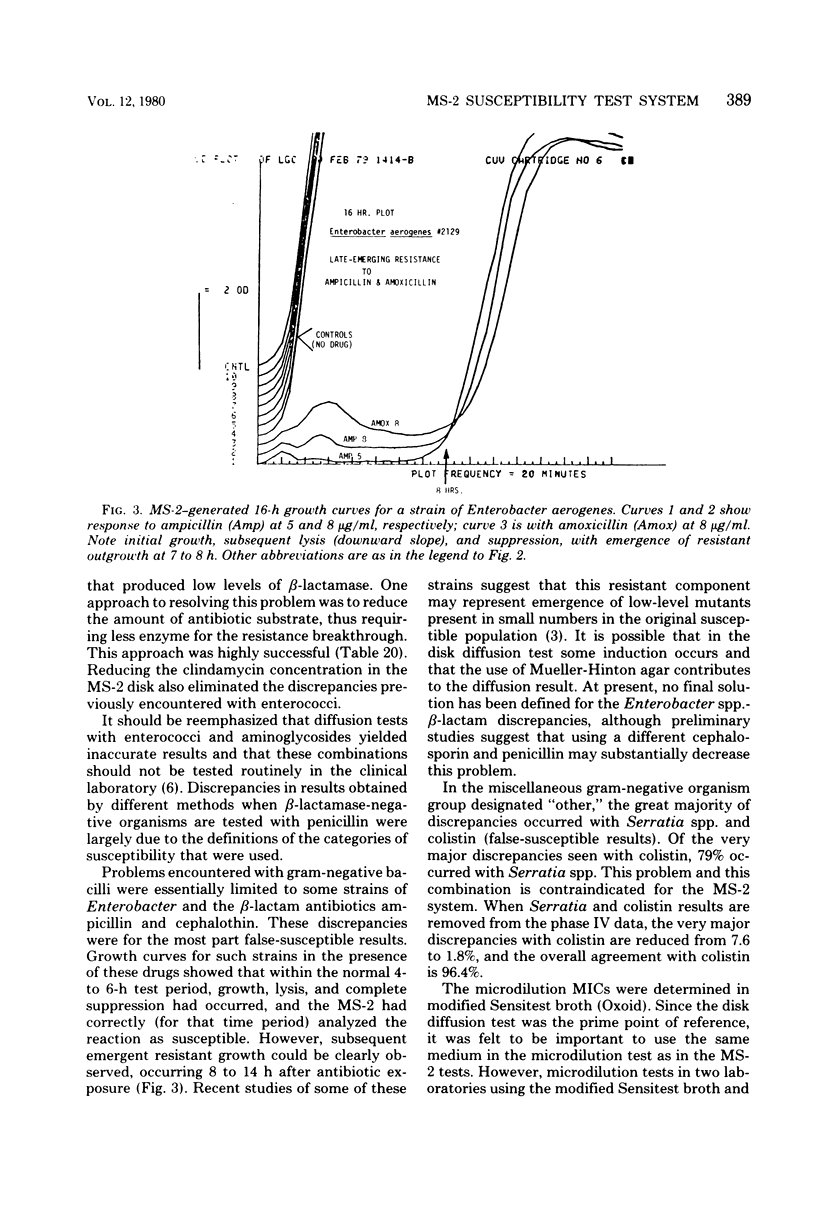
- Lampe M. F., Minshew B. H., Sherris J. C. In vitro response of Enterobacter to ampicillin. Antimicrob Agents Chemother. 1979 Oct;16(4):458–462. doi: 10.1128/aac.16.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth P. M. Letter: Automated sensitivity tests. J Antimicrob Chemother. 1976 Mar;2(1):104–106. doi: 10.1093/jac/2.1.104. [DOI] [PubMed] [Google Scholar]



