Abstract
Thiamin and thiamin pyrophosphate (TPP) are well known for their important roles in human nutrition and enzyme catalysis. In this work, we present new evidence for an additional role of these compounds in the protection of cells against oxidative damage. Arabidopsis (Arabidopsis thaliana) plants subjected to abiotic stress conditions, such as high light, cold, osmotic, salinity, and oxidative treatments, accumulated thiamin and TPP. Moreover, the accumulation of these compounds in plants subjected to oxidative stress was accompanied by enhanced expression of transcripts encoding thiamin biosynthetic enzymes. When supplemented with exogenous thiamin, wild-type plants displayed enhanced tolerance to oxidative stress induced by paraquat. Thiamin application was also found to protect the reactive oxygen species-sensitive ascorbate peroxidase1 mutant from oxidative stress. Thiamin-induced tolerance to oxidative stress was accompanied by decreased production of reactive oxygen species in plants, as evidenced from decreased protein carbonylation and hydrogen peroxide accumulation. Because thiamin could protect the salicylic acid induction-deficient1 mutant against oxidative stress, thiamin-induced oxidative protection is likely independent of salicylic acid signaling or accumulation. Taken together, our studies suggest that thiamin and TPP function as important stress-response molecules that alleviate oxidative stress during different abiotic stress conditions.
Thiamin pyrophosphate (TPP) is an essential cofactor required by enzymes involved in a number of important metabolic processes, including the production of acetyl-CoA, the tricarboxylic acid cycle, the pentose phosphate pathway/Calvin cycle, branched chain amino acid biosynthesis, and isoprenoid biosynthesis (Hohmann and Meacock, 1998). Due to its central role in intermediary metabolism, TPP is required by all biological systems. While TPP is synthesized de novo in plants and microbes, animals and humans must derive this cofactor through the intake of free (unphosphorylated) thiamin (vitamin B1). Due to the abundance of thiamin in whole grains and green vegetables, plants represent the primary dietary source of vitamin B1 in human and animal diets.
In addition to thiamin's well-known role in human nutrition and as an enzyme cofactor; recent studies suggested that thiamin could also function to alleviate stress in different organisms. Observations supporting this role for thiamin have been reported in bacteria, fungi, animals, and plants. In Escherichia coli, thiamin has been shown to protect against paraquat-induced damage, possibly as an antioxidant (Jung and Kim, 2003). In rat liver microsomes, thiamin functioned to inhibit lipid peroxidation and free radical oxidation of oleic acid in vitro (Lukienko et al., 2000). Additionally, lead-induced oxidative stress in liver cells was decreased when supplemented with a combination of thiamin and ascorbate (Wang et al., 2007). It was also found that thiamin prevents cell death stimulated by carbonyl stress and mitochondrial toxins in rats (Mehta et al., 2008). Sheline and Choi (2004) reported that thiamin is a potential therapeutic agent for Wilson's disease, because it restored thiamin-dependent enzyme activity, which is inhibited by Cu2+-induced reactive oxygen species (ROS). While in vitro studies suggest that thiamin can directly act as an antioxidant, the association between thiamin-dependent enzymes and oxidative stress may also indicate a vital cofactor role for thiamin under stress conditions.
Thiamin has been reported to alleviate the effects of several environmental stresses in plants. The exogenous application of thiamin was shown to counteract the harmful effects of salinity on growth (Sayed and Gadallah 2002) and to confer resistance to fungal, bacterial, and viral infections in rice (Oryza sativa), Arabidopsis (Arabidopsis thaliana), and specific vegetable crop species (Ahn et al., 2005). Thiamin was also implicated in responses to stress conditions such as sugar deprivation and hypoxia in Arabidopsis (Ribeiro et al., 2005). Protein levels of the important thiamin biosynthetic enzyme hydroxyethyl thiazole phosphate (HET-P) synthase have been shown to be modulated upon heat stress in Populus euphratica (Ferreira et al., 2006), and the rice homolog of this enzyme is connected to disease resistance (Wang et al., 2006). Interestingly, the yeast and Arabidopsis HET-P synthase gene homologs were also found to complement bacterial defects in DNA repair (Machado et al., 1996). Nevertheless, genetic and metabolic evidence for a direct role of thiamin in alleviating abiotic stress in plants was not, to the best of our knowledge, presented so far.
Although the mechanisms behind thiamin's stress protective activity have not been elucidated in plants, the thiamin biosynthetic pathway is fairly well known. Thiamin is synthesized from two major intermediates, HET-P and hydroxymethylpyrimidine monophosphate (HMP-P), through the action of HET-P synthase (Chatterjee et al., 2007) and HMP-P synthase (Lawhorn et al., 2004). Subsequently, a bifunctional enzyme, thiamin monophosphate pyrophosphorylase (TMP-PPase)/HMP-P kinase, condenses HET-P and HMP-P to form thiamin monophosphate (TMP; Kim et al., 1998; Ajjawi et al., 2007b). TMP is then dephosphorylated to free thiamin (Hohmann and Meacock, 1998), and thiamin pyrophosphokinase (TPK) catalyzes the conversion of free thiamin to its cofactor form, TPP (Ajjawi et al., 2007a).
Plants accumulate a number of defense compounds such as ascorbate (vitamin C), glutathione, N-acetyl Cys, tocopherols (vitamin E), carotenoids, phenylpropanoids, polyamines, and indoles in response to abiotic stresses (Iriti and Faoro, 2007). Protective effects of some of these metabolites against oxidative stress in plants have been shown by their exogenous application or by measuring altered levels of them in plants with enhanced resistance. For instance, exogenous ascorbate increases resistance to salt stress and reduces lipid peroxidation (Shalata and Neumann, 2001), and altered levels of glutathione can increase plant biomass under paraquat-induced oxidative stress (Gossett et al., 1996).
Here, we report that thiamin biosynthesis is induced in Arabidopsis in response to different abiotic stresses. Furthermore, we show that thiamin application increased paraquat tolerance in both wild-type Arabidopsis and the ROS-sensitive ascorbate peroxidase1 (apx1) mutant. Interestingly, thiamin biosynthetic mutants were more tolerant to oxidative stress and constitutively expressed transcripts encoding different ROS-scavenging enzymes, suggesting that thiamin deficiency is associated with activation of ROS defenses. Our studies suggest that thiamin and TPP could play important roles as stress-response molecules that alleviate oxidative stress under various abiotic stress conditions.
RESULTS
Oxidative Stress Increases Thiamin Levels
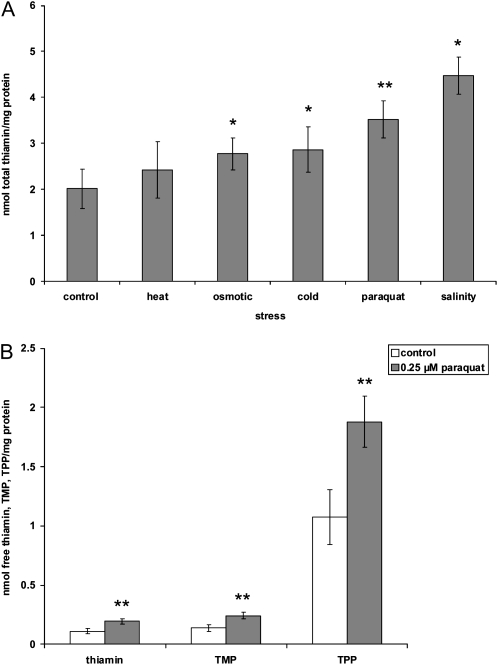
To test the hypothesis that plants accumulate thiamin in response to environmental stresses, thiamin levels were measured in Arabidopsis seedlings subjected to various abiotic stress conditions. The results of these analyses showed that relative to control plants, the total thiamin content was significantly increased in Arabidopsis seedlings subjected to cold, osmotic, and salinity conditions as well as the application of the superoxide radical-generating compound paraquat (Fig. 1A). Of these four stresses, the largest increases in thiamin content were found in seedlings subjected to paraquat and salt treatments, where 1.75- and 2.2-fold increases were observed, respectively. Lower increases in thiamin content were observed in osmotic stress (1.4-fold) and cold stress (1.4-fold) conditions (Fig. 1A). While a slight increase in total thiamin content was observed in heat-treated plants, it was not statistically significant. Closer examination revealed that all three forms of thiamin, specifically free thiamin, TMP, and TPP, were elevated by approximately the same degree relative to each other in all stresses (Supplemental Fig. S1). An example of this is shown for paraquat-treated plants in Figure 1B, where free thiamin, TMP, and TPP increased 1.75-fold. Because oxidative damage is one of the major outcomes associated with cold, salt, and paraquat treatments (Hernandez et al., 1993; Prassad et al., 1994; Durmuş and Kadioğlu, 2005; Bogdanović et al., 2008), we narrowed the focus of our investigation to study the relationship between thiamin biosynthesis and oxidative stress induced via paraquat. Paraquat is particularly appropriate for these studies because it induces the production of superoxide through an electron transfer mechanism involving PSI in the chloroplast and the respiratory electron transport chain in the mitochondria (Bowler et al., 1983) and therefore is highly effective at inducing ROS-mediated oxidative stress globally throughout the plant.
Figure 1.
Effects of various environmental stresses on thiamin content. A, Thiamin levels of 9-d-old whole Arabidopsis plants grown under heat (24 h at 38°C), osmotic (50 mm sorbitol), cold (24 h at 4°C), oxidative (0.25 μm paraquat), and salt (50 mm NaCl) stresses analyzed using HPLC. B, Free thiamin, TMP, and TPP levels of 9-d-old whole plants exposed to paraquat-induced oxidative stress. All plants were grown in 100 μmol m−2 s−1 constant light. Values represent means ± sd of three to five independent experiments. Student's t tests were conducted to compare the thiamin content/composition of stressed plants with control plants. **, Student's t test significant at P < 0.01; *, Student's t test significant at P < 0.05.
Oxidative Stress Enhances the Accumulation of Transcripts Encoding Thiamin Biosynthetic Enzymes
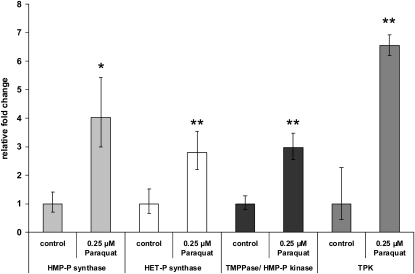
Quantitative reverse transcription (RT)-PCR analyses were performed to determine if the accumulation of thiamin (and its phosphate esters), observed in response to the paraquat treatment, correlated with the accumulation of transcripts encoding thiamin biosynthetic enzymes. In these studies, transcript levels corresponding to the four major thiamin biosynthetic enzymes, HMP-P synthase, HET-P synthase, TMPPase/HMP-P kinase, and TPK, were compared between paraquat-treated and untreated control plants. Results from these studies show that all four transcripts increased significantly in plants grown under oxidative stress conditions. While the largest induction (>6-fold) was observed for the TPK gene, an approximate 3- to 4-fold increase in transcript abundance was observed for HMP-P synthase, HET-P synthase, and TMPPase/HMP-P kinase genes (Fig. 2). These results correlated with the accumulation of thiamin in stressed plants (Fig. 1) and suggest that plants increase de novo thiamin biosynthesis in response to paraquat-induced oxidative stress.
Figure 2.
Effects of paraquat on the transcript abundance of thiamin biosynthetic genes in Arabidopsis. Four thiamin biosynthetic genes (HMP-P synthase [At2g29630], HET-P synthase [At5g54770], TMPPase/HMP-P kinase [At1g22940], and TPK [At1g02880]) were tested for their steady-state transcript levels in 9-d-old whole Arabidopsis plants grown on 0.25 μm paraquat in 100 μmol m−2 s−1 constant light. Values represent means ± sd of three to five independent experiments. Student's t tests were conducted to compare the relative fold change in mRNA abundance of stressed plants with control plants. **, Student's t test significant at P < 0.01; *, Student's t test significant at P < 0.05.
Stress-Induced Accumulation of Thiamin Is Reversible
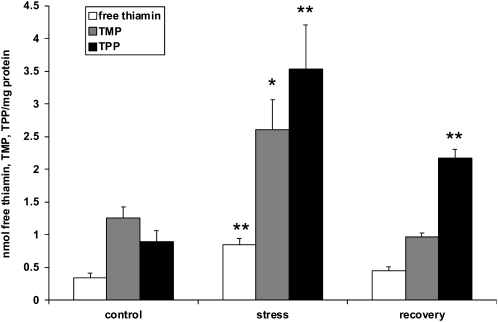
While thiamin accumulated in response to oxidative stress (i.e. paraquat treatment), it was not known if it would revert back to prestress levels once the stress is removed. To study the accumulation of thiamin in plants during stress and following recovery, plants were subjected to high-light stress. Thiamin content and composition were determined for wild-type plants prior to high-light treatment, during high-light treatment, and following recovery. Thiamin, TMP, and TPP levels all increased in response to the stress treatment (Fig. 3). However, when the stressed plants were transferred back to control light conditions, all three forms of thiamin decreased, indicating that the high-light-induced increases in thiamin, TPP, and TMP levels were transient and reversible once the stress was removed. Interestingly, while thiamin and TMP returned to prestress levels, TPP levels, although lower than those observed under stress conditions, remained relatively high, approximately 2-fold higher than that in prestressed plants (Fig. 3). In addition, compared with salinity and paraquat stress, the level of thiamin induction during light stress was higher. Total thiamin content increased 2.2- and 1.75-fold in seedlings exposed to salinity and paraquat stresses, respectively, while it increased 2.9-fold under high-light stress (Figs. 1A and 3).
Figure 3.
Thiamin composition of wild-type Arabidopsis leaves during high-light stress and subsequent recovery. Thiamin content of leaves harvested from 3-week-old wild-type plants exposed to high-light stress (850–900 μmol m−2 s−1) for 8 d was measured with HPLC. Values represent means ± sd of three to five independent experiments. Student's t tests were conducted to compare the thiamin content/composition of stressed and recovered plants with control plants. **, Student's t test significant at P < 0.01; *, Student's t test significant at P < 0.05.
Application of Thiamin Alleviates Growth Retardation Caused by Oxidative Stress
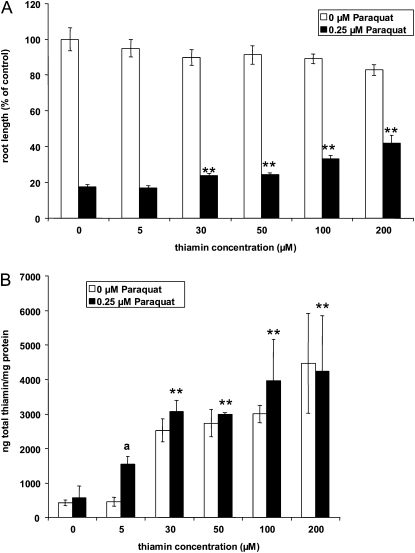
To determine if stressed plants provided with an exogenous source of thiamin would fare better than untreated stressed plants, Arabidopsis seedlings were germinated on thiamin at 0 to 200 μm on agar medium prepared with or without 0.25 μm paraquat. The germinated seedlings were grown vertically on agar medium for 7 d, after which relative seedling growth was determined for each treatment group as a function of final root length. Significant decreases in growth were observed in paraquat-treated plants relative to controls (Fig. 4A). However, when supplemented with thiamin, partial relief of the paraquat-induced growth inhibition was observed. Seedlings supplemented with thiamin at 30 μm showed statistically significant increases in paraquat tolerance. The protective role of thiamin appeared to be cumulative, because incremental increases in root growth were observed in plants supplemented with increasing amounts of exogenous thiamin. When paraquat-treated plants were grown on 200 μm thiamin, root lengths were approximately twice as long as those of paraquat-treated plants grown in the absence of thiamin.
Figure 4.
Thiamin improves root growth of wild-type seedlings subjected to oxidative stress. A, Root growth of wild-type seedlings grown on 0.25 μm paraquat in the presence or absence of different concentrations of thiamin for 7 d in 100 μmol m−2 s−1 constant light. Ordinate values were calculated as a percentage of the average root length of the 0 μm paraquat/0 μm thiamin control treatment such that 100% of control equals 33 mm. Values represent means ± sd of three to five independent experiments. Student's t tests were conducted to compare the root length of plants grown on paraquat in the presence of thiamin with plants grown on paraquat in the absence of thiamin. **, Student's t test significant at P < 0.01. B, Thiamin content of whole wild-type plants supplemented with different concentrations of thiamin in the presence or absence of paraquat for 7 d. Values represent means ± sd of three to five independent experiments. Student's t tests were conducted to compare the total thiamin content. Statistically significant differences between plants grown on thiamin in the presence of paraquat and plants grown on thiamin in the absence of paraquat are shown by a letter: a, Student's t test significant at P < 0.05. Statistically significant differences between plants grown on paraquat in the presence of thiamin and plants grown on paraquat in the absence of thiamin are shown by asterisks: **, Student's t test significant at P < 0.01.
HPLC analysis was performed to determine if the degree of paraquat tolerance observed in thiamin-supplemented plants correlated with the amount of thiamin found in plants. The results of these analyses showed that plants accumulated more thiamin as the amount of exogenous thiamin increased in the growth medium (Fig. 4B). When relating these results to those presented in Figure 4A, a positive correlation can be observed between the amount of thiamin found in plants and the degree of paraquat tolerance. Additionally, the pattern of thiamin accumulation in stressed plants did not differ significantly from that observed in unstressed plants, suggesting that the paraquat tolerance observed in the thiamin-supplemented plants was not due to stress-induced changes in thiamin import. In addition, these results suggest that plants subjected to oxidative stress are able to maintain thiamin levels possibly due to an increased biosynthetic rate (Fig. 1).
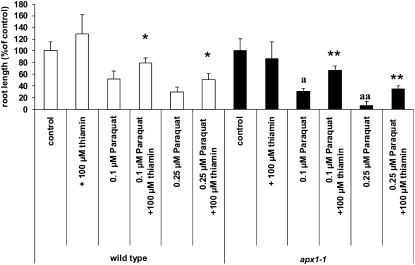
Thiamin Application Enhances the Stress Tolerance of the ROS-Sensitive apx1-1 Mutant
To test whether thiamin would also protect a plant that was significantly more sensitive to oxidative damage, we grew the Arabidopsis apx1-1 mutant, which is deficient in cytosolic APX1 activity and highly susceptible to ROS (Pnueli et al., 2003; Davletova et al. 2005), on agar medium containing paraquat with and without the addition of thiamin. In agreement with previous studies (Davletova et al. 2005), paraquat treatment inhibited root growth in the apx1-1 mutant to a higher degree than that observed for wild-type plants (Fig. 5). This observation could be attributed to the fact that the apx1-1 plants experience chronic oxidative stress due to the lack of APX1 activity (Davletova et al., 2005). However, when supplemented with 100 μm thiamin, the paraquat-induced root growth inhibition was alleviated in both the wild type and apx1-1. Similar results were also obtained with an Arabidopsis mutant carrying a different mutant allele of the APX gene (apx1-2; Supplemental Fig. S2). Interestingly, proteomics analysis of the apx1-1 mutant revealed that during high-light stress this plant accumulated the thiamin biosynthetic enzyme HET-P synthase, suggesting that at least part of the thiamin biosynthetic pathway was activated in these plants (Supplemental Fig. S2).
Figure 5.
Thiamin improves root growth of apx1 seedlings subjected to oxidative stress. All lines germinated simultaneously with a germination rate of 97% to 100%. All plants were grown in 100 μmol m−2 s−1 constant light for 7 d. Ordinate values for wild-type plants (white bars) and apx1-1 plants (black bars) were calculated as a percentage of the average root length of their corresponding controls, such that 100% of the wild-type and apx1-1 controls equaled 5.9 and 5.2 mm, respectively. Values represent means ± sd of three to five independent experiments. Statistically significant differences between wild-type (ecotype Wassilewskija) and apx1-1 plants treated with the same amount of paraquat are shown by letters: aa, Student's t test significant at P < 0.01; a, Student's t test significant at P < 0.05. Statistically significant differences between plants grown on paraquat in the presence of thiamin and plants grown on paraquat in the absence of thiamin are shown by asterisks: **, Student's t test significant at P < 0.01; *, Student's t test significant at P < 0.05.
Thiamin Suppresses Protein Oxidation and ROS Accumulation
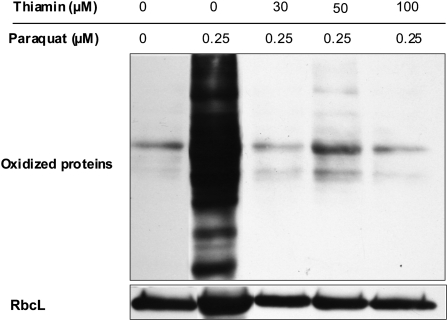
To test whether thiamin protected plants by decreasing oxidative damage, paraquat-treated Arabidopsis seedlings were germinated on thiamin at varying concentrations and subsequently examined for protein oxidation. As shown in Figure 6, providing exogenous thiamin greatly decreased the amount of protein carbonylation in paraquat-treated plants, suggesting that thiamin application reduced the effect of ROS on protein oxidation.
Figure 6.
Inhibition of paraquat-induced protein oxidation by thiamin. Plants were grown on plates in the presence 0.25 μm paraquat and supplemented with thiamin at the indicated concentrations for 10 d in 100 μmol m−2 s−1 constant light. Protein oxidation was determined on whole plant tissue as described in “Materials and Methods.” The level of RbcL was used as an internal control.
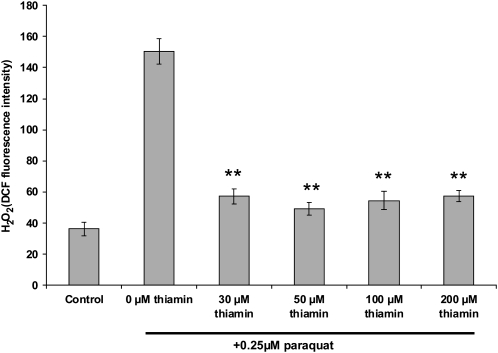
To test whether thiamin caused a reduction in ROS accumulation in plants, paraquat-treated Arabidopsis seedlings in the presence or absence of thiamin were stained with dichlorofluorescein diacetate (DCF) to determine the relative levels of hydrogen peroxide. In correlation with the results of the protein oxidation study (Fig. 6), the DCF staining revealed that when supplemented with thiamin, paraquat-treated plants accumulated significantly less hydrogen peroxide relative to unsupplemented paraquat-treated controls (Fig. 7). The combined results from the protein carbonylation and hydrogen peroxide staining experiments strongly suggest that thiamin treatment increases plant tolerance to paraquat through the reduction of ROS accumulation and subsequent oxidative damage.
Figure 7.
Thiamin effect on hydrogen peroxide (H2O2) accumulation in 7-d-old plants treated with paraquat. Hydrogen peroxide measurements were performed on plants grown under the conditions used in Figure 5. Whole plant tissue was subjected to these measurements. Control is 0 μm paraquat and 0 μm thiamin. Values represent means ± sd of three to five independent experiments. Student's t tests were conducted to compare the fluorescence intensity of plants grown on 0.25 μm paraquat in the presence of thiamin at the indicated concentrations with plants grown on 0.25 μm paraquat in the absence of thiamin. **, Student's t test significant at P < 0.01.
Oxidative Stress Resistance Conferred by Thiamin Is Independent of Salicylic Acid
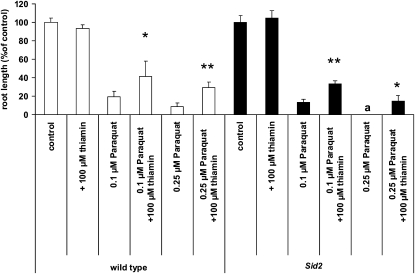
It has recently been reported that application of thiamin causes the induction of systemic acquired pathogen resistance through the salicylic acid (SA) signaling pathway (Ahn et al., 2005, 2007). Because SA has also been shown to induce paraquat tolerance in plants (Ananieva et al., 2004; Silverman et al., 2005), experiments were performed to determine if this phytohormone signaling pathway was required for thiamin-induced paraquat tolerance. To define the role of SA in this process, the Arabidopsis salicylic acid induction-deficient2 (sid2) mutant was examined for its ability to grow on paraquat in the presence or absence of thiamin. sid2 is deficient in SA biosynthesis and therefore blocks SA signal transduction (Wildermuth et al., 2001). If SA signaling is required for thiamin-induced paraquat tolerance, thiamin supplementation should fail to confer paraquat tolerance to sid2 mutant plants. However, because the results of these experiments showed that thiamin was able to confer paraquat tolerance to the sid2 mutant (Fig. 8), it is likely that SA signaling is not required for oxidative protection by thiamin.
Figure 8.
Enhancement of sid2 root growth under oxidative stress by thiamin. sid2 plants along with wild-type plants were grown on 0.1 or 0.25 μm paraquat in the presence or absence of 100 μm thiamin for 7 d in 100 μmol m−2 s−1 constant light. Values are expressed as percentages of the root length of untreated plants. Ordinate values for wild-type plants (white bars) and sid2 plants (black bars) were calculated as a percentage of the average root length of their corresponding controls, such that 100% of the wild-type and sid2 controls equaled 5.2 and 7.8 mm, respectively. All lines germinated simultaneously with a germination rate of 97% to 100%. Values represent means ± sd of three to four independent experiments. Statistically significant differences between wild-type and sid2 plants treated with same amount of paraquat are shown by a letter: a, Student's t test significant at P < 0.05. Statistically significant differences between plants grown on paraquat in the presence of thiamin and plants grown on paraquat in the absence of thiamin are shown by asterisks: **, Student's t test significant at P < 0.01; *, Student's t test significant at P < 0.05.
Responses of Arabidopsis Thiamin Biosynthetic Mutants, py and tz, to Oxidative Stress
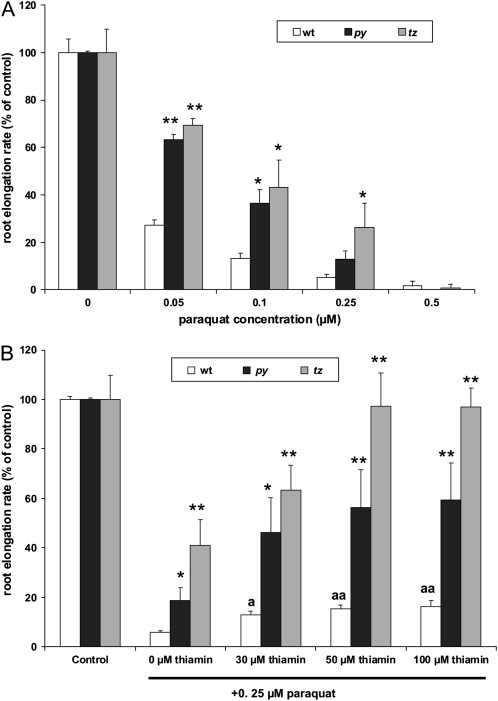
The findings described above indicate that in plants, elevated thiamin levels could increase tolerance to oxidative stress. Therefore, we hypothesized that the suppression of thiamin biosynthesis should result in greater susceptibility to oxidative stress. To test our hypothesis, we examined the oxidative sensitivity of two Arabidopsis thiamin auxotrophs, specifically the HMP-P synthase mutant py and the HET-P synthase mutant tz (Li and Rédei, 1969). While these auxotrophs cannot survive on thiamin-free medium, if supplemented with exogenous thiamin viable seeds can be obtained. Furthermore, thiamin stores present in seeds harvested from thiamin-supplemented mutants are sufficient to maintain growth for several days on thiamin-free medium. In these studies, seeds harvested from thiamin-supplemented wild-type and mutant plants were grown on paraquat at 0 to 0.5 μm on agar medium prepared without thiamin (Fig. 9A). Because the thiamin auxotrophs are stunted relative to wild-type seedlings when grown on thiamin-free medium (Papini-Terzi et al., 2003; Kong et al., 2008), relative root growth rate, instead of changes in absolute root length, was used to compare the effect of paraquat treatments between mutant and wild-type seedlings. Interestingly, our results showed that both the py and tz mutants were more resistant to the paraquat treatment (except for 0.5 μm paraquat). Although higher concentrations of paraquat reduced the root elongation rate of all the plants analyzed, both thiamin auxotrophs had at least 2-fold higher root growth rates than their wild-type counterparts in paraquat at 0.05 to 0.25 μm. The tz mutant was more resistant to the paraquat treatment relative to the py mutant, particularly on 0.25 μm paraquat plates, where the root elongation rate of tz was 2-fold higher than that of py and 5-fold higher than that of the wild type.
Figure 9.
Responses of Arabidopsis thiamin mutants, py and tz, to oxidative stress. A, Root elongation rate of Arabidopsis wild-type, py, and tz seedlings grown on 0, 0.05, 0.1, or 0.25 μm paraquat in 100 μmol m−2 s−1 constant light. Ordinate values for wild-type (white bars), py (black bars), and tz (gray bars) plants were calculated as a percentage of the average root elongation rate of their corresponding controls, such that 100% of the wild-type, py, and tz controls (0 μm paraquat and 0 μm thiamin) equaled 8.75, 1.95, and 2.65 mm d−1, respectively. B, Root elongation rate of Arabidopsis wild-type, py, and tz seedlings grown on thiamin at the indicated concentrations in the presence or absence of 0.25 μm paraquat in 100 μmol m−2 s−1 constant light. Ordinate values for wild-type (white bars), py (black bars), and tz (gray bars) plants were calculated as a percentage of the average root elongation rate of their corresponding controls (0 μm paraquat and 0 μm thiamin), such that 100% of wild-type, py, and tz controls equaled 8.90, 1.35, and 1.40 mm d−1, respectively. All lines germinated simultaneously with a germination rate of 97% to 100%. Values represent means ± sd of three to five independent experiments. Statistically significant differences between wild-type plants grown on paraquat in the presence of thiamin and plants grown on paraquat in the absence of thiamin are shown by letters: aa, Student's t test significant at P < 0.01; a, Student's t test significant at P < 0.05. Student's t tests were also conducted to compare the root elongation rates of thiamin auxotrophs represented with dark and light gray bars with wild-type plants represented with white bars in each treatment and are shown by asterisks: **, Student's t test significant at P < 0.01; *, Student's t test significant at P < 0.05.
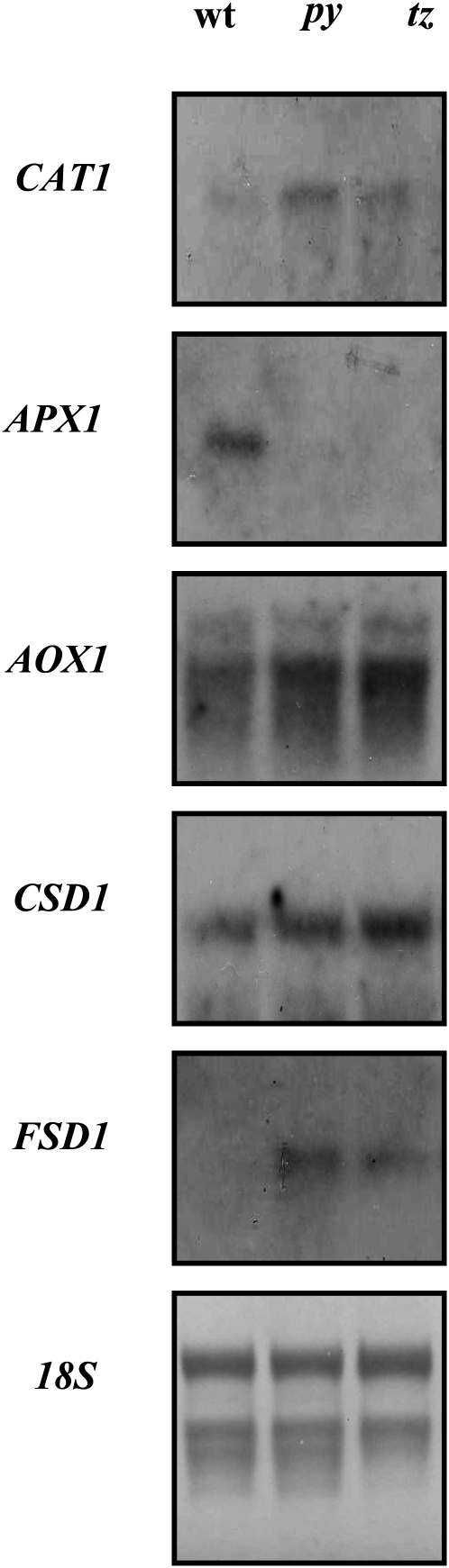
The increased paraquat tolerance observed in the py and tz plants could be attributed to their predisposition to oxidative stress resulting from thiamin deficiency, which results in enhanced expression of various ROS protection pathways. To test this possibility, mRNA levels corresponding to the major ROS protective enzymes, ascorbate peroxidase (APX1), catalase (CAT1), Fe-superoxide dismutase (FSD1), copper/zinc-superoxide dismutase (CSD1), and alternative oxidase (AOX1), were determined in py, tz, and wild-type seedlings grown under nonstressed control conditions. These studies showed that the CAT1, FSD1, CSD1, and AOX1 transcript levels were higher in the unstressed py and tz seedlings relative to unstressed wild-type controls (Fig. 10). Surprisingly, while the APX1 transcript was present in the control plants, it was not detectable in either of the thiamin auxotrophs.
Figure 10.
RNA gel-blot analysis showing the expression of transcripts encoding the reactive oxygen-scavenging enzymes CAT1 (At1g20630), APX1 (At1g07890), AOX1 (At3g22370), FSD1 (At4g25100), and CSD1 (At1g08830) in the wild type (wt) and thiamin auxotroph mutants py and tz. Total RNA was extracted from whole plants grown in 100 μmol m−2 s−1 constant light. Ribosomal RNA was used to control for RNA loading.
We also examined the ability of exogenous thiamin to rescue the thiamin auxotrophs from paraquat-induced oxidative damage. For these experiments, wild-type, tz, and py plants were grown on 0.25 μm paraquat and supplemented with 30, 50, and 100 μm thiamin. In all cases, thiamin supplementation resulted in significantly higher tolerance to paraquat (Fig. 9B), with the tz and py mutants responding better than the wild type to the thiamin treatment.
DISCUSSION
Arabidopsis seedlings responded to a variety of stress treatments by accumulating thiamin and its phosphate esters (Fig. 1A). In high-light stress experiments, we were able to show that the stress-induced thiamin response was transient and that thiamin levels returned to prestressed levels once the stress treatment was removed. Interestingly, the magnitude of the response appeared to be dependent on the particular stress applied. For example, with salinity, high light, and paraquat, 2.2-, 2.9-, and 1.75-fold increases were observed, respectively (Figs. 1A and 3).
A closer examination of cellular thiamin pools revealed that upon paraquat treatment, free thiamin, TMP, and TPP all accumulated in parallel. Furthermore, the relative proportions of each of these different forms of thiamin did not change significantly from those observed in untreated controls. Because similar results were observed in plants subjected to osmotic, cold, and salt stresses (Supplemental Fig. S1), it is likely that plants up-regulate thiamin metabolism in a similar manner when responding to these diverse stress conditions. The fact that stressed plants maintained wild-type thiamin compositions suggests that the three different forms of thiamin are maintaining their normal metabolic balance instead of functioning in a specialized individual manner.
Because the cofactor form of thiamin, TPP, was by far the most abundant under stressed and control conditions (i.e. approximately 20 times higher than free thiamin and approximately 10 times higher than TMP), it is possible that TPP levels increase in response to increased cofactor demand. This makes sense in light of the fact that TPP plays an important role in providing NADH and NAPDH to combat ROS-induced damage. In animals and yeast, it has been shown that oxidative stress increases the demand for pentose phosphate pathway (PPP)-derived NADPH by ROS-detoxifying enzymes (Palmer, 1999; Larochelle et al., 2006). Because a key PPP enzyme, transketolase, requires TPP for enzymatic function, PPP flux and NADPH production are highly dependent on TPP availability (Shangari et al., 2007). Transketolase plays an important role in the stress-induced production of cytosolic NADPH in animals and fungi, and recently a similar role was identified in plants. A moderate increase in the activity of transketolase was detectable under conditions of salt and oxidative stress in maize (Zea mays; Rapala-Kozik et al., 2008). Furthermore, in vitro studies have shown that spinach (Spinacia oleracea) transketolase may function in an alternative stress-protecting role in plants by its ability to quench superoxide radicals (Kaiser, 1976; Takabe et al., 1980).
An alternative explanation for the stress accumulation of thiamin, TMP, and TPP is that one or all of these compounds function as chemical antioxidants. In vitro studies showed that thiamin is a potent scavenger of hydroxyl radicals (Hu et al., 1995; Jung and Kim, 2003) and superoxide (Jung and Kim, 2003) and could protect membranes from lipid peroxidation (Lukienko et al., 2000). However, because our analyses of stressed seedlings failed to show the accumulation of thiochrome (data not shown), the primary thiamin oxidation product (Lukienko et al. 2000), it is unclear to what degree thiamin and its phosphate ester function as chemical antioxidants in plants. Furthermore, our studies showed that when supplemented with the same amount of exogenous thiamin, the total thiamin content did not differ significantly between paraquat-treated or untreated plants (Fig. 4B). If thiamin or one of its phosphate esters were functioning directly as chemical antioxidants, one would expect the thiamin content to decrease upon antioxidant treatment. While it could be argued that this observation fails to support an antioxidant role for thiamin, it is also possible that mechanisms exist to recycle oxidized thiamin, in a manner similar to oxidized glutathione or ascorbate, to maintain steady-state cellular levels. More research is needed to address thiamin's chemical antioxidant potential in vivo.
Our studies showed that the stress-induced accumulation of thiamin was at least in part due to increased expression of thiamin biosynthetic genes. Semiquantitative RT-PCR analyses showed that transcript levels of four major thiamin biosynthetic genes, HMP-P synthase, HET-P synthase, TMPPase/HMP-P kinase, and TPK, all increased in response to paraquat-induced oxidative stress (Fig. 2). These results are consistent with those from microarray and proteomic studies that also showed increased HMP-P synthase and HET-P synthase transcript accumulation in response to cold, heat, and drought stresses (Rizhsky et al., 2004; Ferreira et al., 2006; Wong et al., 2006). While our results clearly showed a relationship between increased thiamin biosynthetic gene expression and thiamin accumulation, our studies were not sufficient to determine if the stress induction of thiamin biosynthetic genes was being regulated at the transcriptional or posttranscriptional level. However, analyses of the Arabidopsis HET-P synthase gene showed that its promoter was sufficient to drive a 2- to 3-fold increase in GUS reporter gene expression during salt stress (Ribeiro et al., 2005). Closer analyses of this promoter revealed the presence of an abscisic acid response element (Ribeiro et al., 2005) that may function to promote stress induction in the HET-P synthase gene. While the HMP-P synthase gene has been reported to be feedback regulated through a riboswitch-mediated alternative splicing mechanism (Wachter et al., 2007), in silico analyses (Higo et al., 1999) revealed the presence of putative stress-related promoter elements, including abscisic acid response elements and drought response elements (Supplemental Table S1), in the HMP-P synthase promoter. This finding suggests that like the HET-P synthase gene, the rate of HMP-P synthase gene transcription may play a role in regulating its stress-induced expression.
In addition to showing that plants accumulated thiamin in response to various stress treatments, we were also able to show that the application of exogenous thiamin helped Arabidopsis seedlings better tolerate the oxidizing effects of the herbicide paraquat. The degree of tolerance observed in the thiamin-supplemented plants was directly related to the amount of thiamin added to the medium (Fig. 4A) and was independent from thiamin's reported growth-promoting effect (Ohira et al., 1976; Oertli, 1987). Although thiamin did not absolutely protect plants from the growth-inhibitory effects of paraquat, it did significantly increase the ability of plants to survive the herbicide treatment. This observation indicates that thiamin alone is not sufficient to combat paraquat-induced oxidative stress and that the plant uses other protective mechanisms in conjunction with thiamin to battle stress. Because paraquat was shown to primarily affect the chloroplast (Mano et al., 2001), the only partial ability of thiamin and TPP to protect plants from paraquat stress could suggest that they primarily function through a cytosolic mechanism.
Surprisingly, while we were able to achieve an approximately 8-fold increase in thiamin incorporation through medium supplementation (Fig. 4B), only a 2-fold increase in paraquat tolerance was observed in these plants (Fig. 4A). One possible explanation for this apparent discrepancy is that thiamin must first be converted to TPP before it can confer oxidative protection. This hypothesis is consistent with the observation that free thiamin accumulates to much higher levels than those observed for TPP or TMP in thiamin-supplemented plants (Supplemental Fig. S3). This would suggest that thiamin pyrophosphokinase or thiamin kinase activity is not sufficient to convert all of the incorporated free thiamin to TPP or TMP, respectively. An alternative explanation is that thiamin or one of its phosphate esters is inefficiently delivered to the specific subcellular locale required for oxidative protection. While this would not be likely if the site of action was the cytosol, it would be problematic if these compounds were required in the mitochondria or plastid compartments for oxidative protection. Unfortunately, the intracellular transport of thiamin and its phosphate esters is very poorly understood in plants, and further investigation is needed to address this possibility.
Thiamin supplementation was also shown to confer increased paraquat tolerance to the Arabidopsis mutant apx1, which is highly susceptible to oxidative damage. apx1 lacks cytosolic ascorbate peroxidase activity, which is central to ROS detoxification in plants, and accumulates hydrogen peroxide when subjected to a moderate level of light stress (Davletova et al., 2005; Miller et al., 2007). The fact that thiamin was able to increase apx1's tolerance to paraquat (Fig. 5) suggests that thiamin's function is complementary to that of APX1 and that thiamin has a direct role in alleviating oxidative stress in plants. Furthermore, the apx1-1 mutant was found to up-regulate HET-P synthase expression, which indicates that the thiamin pathway is induced in a plant persistently experiencing oxidative stress (Supplemental Fig. S4). In support of the suggested antioxidative role for thiamin, as demonstrated with the apx1 mutant (Fig. 5), supplementation of plants with thiamin was shown to suppress ROS accumulation and protein oxidation (Figs. 6 and 7), providing additional evidence for a role for thiamin in mitigating oxidative stress in plants.
Studies were also performed to examine the linkage between thiamin and SA signaling during stress. SA was shown to induce thermotolerance and protection against plant pathogens and was reported to regulate the redox homeostasis of the cell (Raskin, 1992; Dat et al., 1998; Mateo et al., 2006). Additionally, exogenous treatment with SA leads to increased antioxidant capacity in leaves of barley (Hordeum vulgare) plants exposed to paraquat (Ananieva et al., 2004). SA also appears to play a central role in thiamin-induced defense of plants against pathogens (Ahn et al., 2005, 2007). Nevertheless, in our hands, thiamin protected the sid2 mutant, which does not accumulate SA (Fig. 8), suggesting that SA is not involved in the pathway(s) triggered in cells by thiamin to enhance oxidative stress tolerance. This result could also suggest that thiamin has a direct antioxidative activity.
Thiamin accumulation in the plant resulted in enhanced tolerance to oxidative stress, implying that thiamin deficiency could cause increased sensitivity to oxidative stress. Nevertheless, two thiamin-deficient mutants, py and tz, were more tolerant to oxidative stress compared with control wild-type plants (Fig. 9). We propose, based on our analysis of thiamin function in plants during stress (Figs. 1–9), that thiamin deficiencies in py and tz mutants cause a continuous state of oxidative stress under controlled growth conditions and subsequently the activation of different antioxidative mechanisms. Results showing increased expression of the CSD1, FSD1, CAT1, and AOX1 transcripts in untreated py and tz plants were consistent with this hypothesis (Fig. 10). Interestingly, although supplemental thiamin could increase the oxidative tolerance in the apx1 mutant (Fig. 5), APX1 transcript levels were not elevated in unstressed thiamin auxotrophs (Fig. 10). It is possible that the preactivation of the other antioxidative enzymes in the auxotrophs provided sufficient protection and negated the need to increase ascorbate peroxidase activity. The py and tz mutants, therefore, are primed to tolerate oxidative stress and grow better than controls when challenged with paraquat. Similar results were previously reported for the csd2 mutant that is deficient in chloroplastic copper/zinc-superoxide dismutase grown on paraquat (Rizhsky et al., 2003) and for the apx1 mutant subjected to salinity stress (Ciftci-Yilmaz et al., 2007). The tolerance of the py and tz mutants to paraquat (Fig. 9A) was augmented by thiamin supplementation (Fig. 9B), further supporting our interpretation that in the absence of thiamin (i.e. in py and tz mutants), additional antioxidative mechanisms are activated and these could be complemented by thiamin addition.
In conclusion, our results suggest an important role for thiamin in the protection of cells against oxidative stress in plants. The fact that thiamin accumulation is induced upon stress treatment and that exogenous thiamin confers oxidative protection to plants strongly supports this hypothesis. Thiamin can now be added to the list of metabolites utilized by plants to combat oxidative damage.
MATERIALS AND METHODS
Plant Material
Wild-type Arabidopsis (Arabidopsis thaliana ecotype Columbia), tz-1 (CS3375), and py (Li and Rédei, 1969) seeds were obtained from the Arabidopsis Biological Resource Center. The apx1-1 (Pnueli et al., 2003), apx1-2 (SALK_000249), and sid2 seeds were obtained as described previously by Miller et al. (2007) and Suzuki et al. (2008). Plants were grown under controlled conditions: 21°C and 100 μmol m−2 s−1 constant light as described by Suzuki et al. (2005).
Stress Assays
Stress assays were performed according to Ciftci-Yilmaz et al. (2007). All stress experiments were performed with three to five technical replications, each containing 15 to 30 seedlings per line, and repeated at least three times. For thiamin HPLC analysis, seedlings were germinated on 1× Murashige and Skoog (MS) medium supplemented with the following compounds for 9 d: 0.25 μm paraquat (methyl viologen), 100 mm NaCl, or 50 mm sorbitol. For heat and cold stress treatments, 8-d-old seedlings grown on 1× MS agar plates were subjected to 38°C or 4°C, respectively, for 24 h in 100 μmol m−2 s−1 constant light.
For thiamin protection experiments, seeds were germinated on 1× MS medium containing thiamin at 0 to 200 μm with or without 0.25 μm paraquat. The seedlings were grown vertically, and the increase in root length was measured over 7 d in 100 μmol m−2 s−1 constant light. apx1-1, sid2, and their related wild-type controls were grown on plates containing 0.1 or 0.25 μm paraquat along with 100 μm thiamin for 7 d. Root length of each line was measured and normalized to the corresponding untreated seedlings on control plates. The relative root elongation of the different lines in response to each treatment was compared with that of the wild-type control. Seedlings of py and tz mutants were also evaluated for their resistance to oxidative stress on agar plates prepared with varying concentrations of paraquat ranging from 0 to 0.5 μm at 7 d after germination. The position of the root tip was marked on the back of the dishes with a marker every 2 d. Root elongation rates were scored at day 7 by measuring the portions between the marked points and the root tip.
Thiamin accumulation in response to high-light conditions was measured in wild-type plants that were grown on soil for 10 d under constant low-light conditions (40–50 μmol m−2 s−1) and transferred to high light (850–900 μmol m−2 s−1) for 8 d. Plants were recovered thereafter under low light for 24 h. Leaf tissue was collected and frozen in liquid nitrogen and kept at −80°C until extraction.
HPLC Analysis
Frozen leaf tissue (75–100 mg) was ground in 300 μL of 2% (w/v) trichloroacetic acid. Samples were incubated at 100°C for 30 min, left on ice for 2 min, and subsequently centrifuged at 14,000g for 5 min. The supernatant was centrifuged through Nanosep Centrifugal Filter Device (0.2 μm) columns (Pall Life Sciences) for 3 min. The acid precipitate was used for protein quantification with the EZQ Protein Quantification Kit (Invitrogen). Thiamin and its phosphate esters in 2% (w/v) trichloroacetic acid were then converted into thiochrome using cyanogen bromide. Thiochrome peaks were identified by fluorescence at 365-nm excitation and 433-nm emission and were quantified by integrating peak areas relative to a standard curve (Bettendorff et al., 1986).
RNA Isolation and Real-Time Quantitative RT-PCR
Total RNA was isolated from 50 to 60 seedlings grown on 0.25 μm paraquat or paraquat-free medium using the RNeasy plant mini kit (Qiagen). First-strand cDNA was synthesized using 2 μg of total RNA, GeneRacer Oligo dT Primer, and SuperScript II RNase H− reverse transcriptase (Invitrogen). The PCR thermal cycling conditions were as follows: an initial step at 65°C for 5 min, then 1 min at 4°C, and a last cycle consisting of 60 min at 50°C and 15 min at 70°C. A fraction (1.4 μg) of the cDNA was used as the template in 25 μL of reaction mixture per well in real-time PCR. Amplification and detection were performed with an ABI7000 real-time PCR system (PE Biosystems). The reaction conditions were 55°C for 2 min, followed by 95°C for 15 min and then 50 cycles of 76°C for 45 s, 94°C for 45 s, and 56°C for 45 s. The eEF-1α gene tagged with VIC dye (Qiagen) was included in every well as an internal control.
The sequences of the forward primer, reverse primer, and probe for HMP-P synthase were 5′-TAGCTTACCACAAGGAGAA-3′, 5′-CCAATGGAAAGAGCCACATCA-3′, and 5′-ACGAGCACTGGNGATGA-3′, respectively. The sequences of the forward primer, reverse primer, and probe for Arabidopsis HET synthase were 5′-CTGCTGAGGATTTGATTGTG-3′, 5′-CGATTGTGTGTGGTGGTT-3′, and 5′-GTTGGTGGTGTGGTGA-3′, respectively. The sequences of the forward primer, reverse primer, and probe for Arabidopsis TMP-PPase/HMP-P kinase were 5′-GTGATCTTCCTGACTCAT-3′, 5′-CCAAAGTGCAACCAGTCC-3′, and 5′-CTCCGTTCTCCTCGCAT-3′, respectively. The sequences of the forward primer, reverse primer, and probe for Arabidopsis TPK were 5′-CACGATTCACTCCTCTTCTC-3′, 5′-AATTCGTNCGTAGATGCGATTAG-3′, and 5′-AACTTCGTCTCTGTGCT-3′, respectively. The sequences of the forward primer, reverse primer, and probe for Arabidopsis eEF-1α (internal control) were 5′-GAGCCCAAGTTTTTGAAGA-3′, 5′-CTAACAGCGAAACGTCCCA-3′, and 5′-CCCCAACCAAGCCCAT-3′, respectively.
In all experiments, appropriate negative controls containing RNA or no template cDNA were subjected to the same procedure to exclude genomic DNA contamination or carryover. Relative fold changes in expression levels were calculated using the 2−ΔΔCT method as described in Applied Biosystems User Bulletin No. 2 (P/N 4303859). PCR for each sample was repeated at least in duplicate. Values were compared using Student's t test and are considered significant at P < 0.05 in comparison with the untreated controls.
Northern Blot
RNA from the wild type and the py and tz mutants grown under controlled conditions (21°C, 100 μmol m−2 s−1 constant light) was isolated and analyzed by gel-blot analysis (Rizhsky et al., 2002; Luhua et al., 2008). cDNA probes corresponding to the following genes were used for the RNA gel blots shown in Figure 10: APX1, CAT1, FSD1, CSD1, AOX1, and 18S ribosomal RNA (Rizhsky et al., 2002; Luhua et al., 2008).
Protein Oxidation Assay
Seedlings (10 d old) grown on MS agar plates containing 0.25 μm paraquat supplemented with increased concentrations of thiamin were harvested and immediately homogenized in protein extraction buffer (100 mm Tris, pH 8.3, 50 mm NaCl, 5 mm EDTA, 10% glycerol, 40 μg mL−1 phenylmethylsulfonyl fluoride, 50 mm dithiothreitol, and 0.1% Triton X-100). Protein oxidation was determined in extracts from whole paraquat-treated and control plants by measuring the degree of protein carbonylation present using the Oxyblot kit as recommended by the manufacturer (Chemicon International; Rizhsky et al., 2004). Protein extracts were reacted with 2,4-dinitrophenylhydrazone to label carbonylated amino acid residues on oxidized proteins. Ten micrograms of each of the resulting 2,4-dinitrophenyl (DNP)-labeled protein extracts were fractionated by SDS-PAGE and blotted to nitrocellulose. DNP-modified carbonylated proteins were identified by immunoblot analyses using rabbit anti-DNP antibodies (1:150 dilution). The large subunit of Rubisco (RbcL) protein loading control was detected in subsequent immunoblot analyses of the same blot using rabbit anti-pea RbcL polycolonal antibodies (1:4,000 dilution). Cross-reacting protein bands to rabbit anti-DNP and anti-RbcL antibodies were visualized using goat anti-rabbit IgG/horseradish peroxidase-conjugated secondary antibodies and chemiluminescence detection (1:300 and 1:12,000 dilution, respectively).
Hydrogen Peroxide Measurements
Seedlings that were grown vertically on MS plates containing paraquat and increased levels of thiamin were immersed for 30 min in 5 μm DCF solution. The plates were washed with double-distilled water five times, and the DCF fluorescence was imaged on Kodak Image Station 2000MM using 465-nm and 535-nm excitation and emission filters, respectively. Root fluorescence intensity was quantified by the Kodak MI imaging software (Luhua et al., 2008).
Data Analysis
The significance of differences between data sets was evaluated by Student's t test (Suzuki et al., 2008). Microsoft Excel software was used for calculations.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers BT020417.1 (At1g22940), AK317288.1 (At1g02880), AY128756 (At2g29630), AK221070 (At5g54770), BT010373 (At1g20630), AK318790 (At1g07890), DQ492232 (At3g22370), AY091168 (At1g08830), and AF326862 (At4g25100).
Supplemental Data
Supplemental Figure S1. Effects of various environmental stresses on thiamin composition.
Supplemental Figure S2. Thiamin improves root growth of apx1-2 seedlings subjected to oxidative stress.
Supplemental Figure S3. When plants are grown in the presence of high levels of thiamin, they accumulate free thiamin more than TMP and TPP.
Supplemental Figure S4. Identification of TH1 in a proteomic analysis of the response of apx1-1 to high light.
Supplemental Table S1. In silico analysis of HMP-P synthase promoter.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Harper for his constructive criticism on the thiamin mutant experiments.
This work was supported by the National Science Foundation (grant nos. MCB–0236210, IBN–0420033, NSF–0431327, and IOS–0743954), the Nevada Agricultural Experimental Station, the National Institutes of Health IDeA Network of Biomedical Research Excellence (grant no. RR–03–008), Israeli Science Foundation 214/08 and European Union FP7-Marie Curie 447 (to R.M.), and the Department of Biotechnology, India (grant nos. BT/INT/BTOA/22/2006 and BT/BI/04/055/2001 to A.N.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David Shintani (shintani@unr.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ahn IP, Kim S, Lee YH (2005) Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol 138 1505–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn IP, Kim S, Lee YH, Suh SC (2007) Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in Arabidopsis. Plant Physiol 143 838–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajjawi I, Rodriguez Milla MA, Cushman J, Shintani DK (2007. a) Thiamin pyrophosphokinase is required for thiamin cofactor activation in Arabidopsis. Plant Mol Biol 65 151–162 [DOI] [PubMed] [Google Scholar]
- Ajjawi I, Tsegaye Y, Shintani D (2007. b) Determination of the genetic, molecular, and biochemical basis of the Arabidopsis thaliana thiamin auxotroph th1. Arch Biochem Biophys 459 107–114 [DOI] [PubMed] [Google Scholar]
- Ananieva EA, Christov KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol 161 319–328 [DOI] [PubMed] [Google Scholar]
- Bettendorff L, Grandfils C, De Rycker C, Schoffeniels E (1986) Determination of thiamin and its phosphate esters in human blood serum at femtomole levels. J Chromatogr 382 297–302 [DOI] [PubMed] [Google Scholar]
- Bogdanović J, Mojović M, Milosavić N, Mitrović A, Vučinić Z, Spasojević I (2008) Role of fructose in the adaptation of plants to cold-induced oxidative stress. Eur Biophys J 37 1241–1246 [DOI] [PubMed] [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, Van Montagu M, Inze D (1983) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 10 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Jurgenson CT, Schroeder FC, Ealick SE, Begley TP (2007) Biosynthesis of thiamin thiazole in eukaryotes: conversion of NAD to an advanced intermediate. J Am Chem Soc 129 2914–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciftci-Yilmaz S, Morsy MR, Song L, Coutu A, Krizek BA, Lewis MW, Warren D, Cushman J, Connolly EL, Mittler R (2007) The EAR-motif of the Cys2/His2-type zinc finger protein Zat7 plays a key role in the defense response of Arabidopsis to salinity stress. J Biol Chem 282 9260–9280 [DOI] [PubMed] [Google Scholar]
- Dat JF, Foyer CH, Scott IM (1998) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 118 1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmuş N, Kadioğlu A (2005) Reduction of paraquat toxicity in maize leaves by benzyladenine. Acta Biol Hung 56 97–107 [DOI] [PubMed] [Google Scholar]
- Ferreira S, Hjernø K, Larsen M, Wingsle G, Larsen P, Fey S, Roepstorff P, Salomé Pais M (2006) Proteome profiling of Populus euphratica Oliv. upon heat stress. Ann Bot (Lond) 98 361–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett DR, Banks SW, Millhollon EP, Lucas MC (1996) Antioxidant response to NaCl stress in a control and an NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiol 112 803–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JA, Corpas FJ, Gomez M, Del Rio LA, Sevilla F (1993) Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant 89 103–110 [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S, Meacock PA (1998) Thiamin metabolism and thiamin diphosphate-dependent enzymes in the yeast Saccharomyces cerevisiae: genetic regulation. Biochim Biophys Acta 1385 201–219 [DOI] [PubMed] [Google Scholar]
- Hu ML, Chen YK, Lin YF (1995) The antioxidant and prooxidant activity of some B vitamins and vitamin-like compounds. Chem Biol Interact 97 63–73 [DOI] [PubMed] [Google Scholar]
- Iriti M, Faoro F (2007) Review of innate and specific immunity in plants and animals. Mycopathologia 164 57–64 [DOI] [PubMed] [Google Scholar]
- Jung IL, Kim IG (2003) Thiamin protects against paraquat-induced damage: scavenging activity of reactive oxygen species. Environ Toxicol Pharmacol 15 19–26 [DOI] [PubMed] [Google Scholar]
- Kaiser W (1976) The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim Biophys Acta 440 476–482 [DOI] [PubMed] [Google Scholar]
- Kim YS, Nosaka K, Downs DM, Kwak JM, Park D, Chung IK, Nam HG (1998) A Brassica cDNA clone encoding a bifunctional hydroxymethylpyrimidine kinase/thiamin-phosphate pyrophosphorylase involved in thiamin biosynthesis. Plant Mol Biol 37 955–966 [DOI] [PubMed] [Google Scholar]
- Kong D, Zhu Y, Wu H, Cheng X, Liang H, Ling HQ (2008) AtTHIC, a gene involved in thiamine biosynthesis in Arabidopsis thaliana. Cell Res 18 566–576 [DOI] [PubMed] [Google Scholar]
- Larochelle M, Drouin S, Robert F, Turcotte B (2006) Oxidative stress-activated zinc cluster protein Stb5 has dual activator/repressor functions required for pentose phosphate pathway regulation and NADPH production. Mol Cell Biol 26 6690–6701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhorn BG, Mehl RA, Begley TP (2004) Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction. Org Biomol Chem 2 2538–2546 [DOI] [PubMed] [Google Scholar]
- Li SL, Rédei GP (1969) Thiamine mutants of the crucifer, Arabidopsis. Biochem Genet 3 163–170 [DOI] [PubMed] [Google Scholar]
- Luhua S, Ciftci-Yilmaz S, Harper J, Cushman J, Mittler R (2008) Enhanced tolerance to oxidative stress in transgenic Arabidopsis plants expressing proteins of unknown function. Plant Physiol 148 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukienko PI, Mel'nichenko NG, Zverinskii IV, Zabrodskaya SV (2000) Antioxidant properties of thiamin. Bull Exp Biol Med 130 874–876 [PubMed] [Google Scholar]
- Machado CR, de Oliveira RL, Boiteux S, Praekelt UM, Meacock PA, Menck CF (1996) Thi1, a thiamin biosynthetic gene in Arabidopsis thaliana, complements bacterial defects in DNA repair. Plant Mol Biol 31 585–593 [DOI] [PubMed] [Google Scholar]
- Mano J, Ohno C, Domae Y, Asada K (2001) Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochim Biophys Acta 1504 275–287 [DOI] [PubMed] [Google Scholar]
- Mateo A, Funck D, Mühlenbock P, Kular B, Mullineaux PM, Karpinski S (2006) Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J Exp Bot 57 1795–1807 [DOI] [PubMed] [Google Scholar]
- Mehta R, Shangari N, O'Brien PJ (2008) Preventing cell death induced by carbonyl stress, oxidative stress or mitochondrial toxins with vitamin B anti-AGE agents. Mol Nutr Food Res 52 379–385 [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R (2007) Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol 144 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertli JJ (1987) Exogenous application of vitamins as regulators for growth and development of plants. Z Pflanzenernaher Bodenk 150 375–391 [Google Scholar]
- Ohira K, Ikeda M, Ojima K (1976) Thiamin requirements of various plant cells in suspension culture. Plant Cell Physiol 17 583–590 [Google Scholar]
- Palmer AM (1999) The activity of pentose phosphate pathway is increased in response to oxidative stress in Alzheimer's disease. J Neural Transm 106 317–328 [DOI] [PubMed] [Google Scholar]
- Papini-Terzi FS, da Silva Galhardo R, Farias LP, Menck CFM, Van Sluys MA (2003) Point mutation is responsible for Arabidopsis tz-201 mutant phenotype affecting thiamin biosynthesis. Plant Cell Physiol 44 856–860 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Liang H, Rozenberg M, Mittler R (2003) Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J 34 187–203 [DOI] [PubMed] [Google Scholar]
- Prassad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapala-Kozik M, Kowalska E, Ostrowska K (2008) Modulation of thiamine metabolism in Zea mays seedlings under conditions of abiotic stress. J Exp Bot 59 4133–4143 [DOI] [PubMed] [Google Scholar]
- Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43 439–463 [Google Scholar]
- Ribeiro DT, Farias LP, de Almeida JD, Kashiwabara PM, Ribeiro AF, Silva-Filho MC, Menck CF, Van Sluys MA (2005) Functional characterization of the thi1 promoter region from Arabidopsis thaliana. J Exp Bot 56 1797–1804 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Rodermel S, Inzé D, Mittler R (2002) Double antisense plants with suppressed expression of ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants with suppressed expression of ascorbate peroxidase or catalase. Plant J 32 329–342 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R (2003) The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem 278 38921–38925 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide: the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed SA, Gadallah MAA (2002) Effects of shoot and root application of thiamin on salt-stressed sunflower plants. Plant Growth Regul 36 71–80 [Google Scholar]
- Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52 2207–2211 [DOI] [PubMed] [Google Scholar]
- Shangari N, Mehta R, O'Brien PJ (2007) Hepatocyte susceptibility to glyoxal is dependent on cell thiamin content. Chem Biol Interact 165 146–154 [DOI] [PubMed] [Google Scholar]
- Sheline CT, Choi DW (2004) Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann Neurol 55 645–653 [DOI] [PubMed] [Google Scholar]
- Silverman FP, Petracek PD, Fledderman CM, Ju Z, Heiman DF, Warrior P (2005) Salicylate activity. 1. Protection of plants from paraquat injury. J Agric Food Chem 53 9764–9768 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R (2008) The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem 283 9269–9275 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Rizhsky L, Liang H, Shuman J, Shulaev V, Mittler R (2005) Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiol 139 1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T, Asami S, Akazawa T (1980) Glycolate formation catalyzed by spinach leaf transketolase utilizing the superoxide radical. Biochemistry 19 3985–3989 [DOI] [PubMed] [Google Scholar]
- Wachter A, Tunc-Ozdemir M, Grove BC, Green PJ, Shintani DK, Breaker RR (2007) Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant Cell 19 3437–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liang J, Zhang C, Bi Y, Shi X, Shi Q (2007) Effect of ascorbic acid and thiamin supplementation at different concentrations on lead toxicity in liver. Ann Occup Hyg 51 563–569 [DOI] [PubMed] [Google Scholar]
- Wang G, Ding X, Yuan M, Qiu D, Li X, Xu C, Wang S (2006) Dual function of rice OsDR8 gene in disease resistance and thiamin accumulation. Plant Mol Biol 60 437–449 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414 562–565 [DOI] [PubMed] [Google Scholar]
- Wong CE, Li Y, Labbe A, Guevara D, Nuin P, Whitty B, Diaz C, Golding GB, Gray GR, Weretilnyk EA, et al (2006) Transcriptional profiling implicates novel interactions between abiotic stress and hormonal responses in Thellungiella, a close relative of Arabidopsis. Plant Physiol 140 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.