Abstract
In Arabidopsis (Arabidopsis thaliana), the carotenoid cleavage dioxygenases MORE AXILLARY GROWTH3 (MAX3) and MAX4 act together with MAX1 to produce a strigolactone signaling molecule required for the inhibition of axillary bud outgrowth. We show that both MAX3 and MAX4 transcripts are positively auxin regulated in a manner similar to the orthologous genes from pea (Pisum sativum) and rice (Oryza sativa), supporting evolutionary conservation of this regulation in plants. This regulation is important for branching control because large auxin-related reductions in these transcripts are associated with increased axillary branching. Both transcripts are up-regulated in max mutants, and consistent with max mutants having increased auxin in the polar auxin transport stream, this feedback regulation involves auxin signaling. We suggest that both auxin and strigolactone have the capacity to modulate each other's levels and distribution in a dynamic feedback loop required for the coordinated control of axillary branching.
Shoot branching is dependent on both the formation of axillary meristems in the axils of leaves and the precise control of their outgrowth by genetically, hormonally, and environmentally regulated signals (for review, see Schmitz and Theres, 2005; Dun et al., 2006; Ongaro and Leyser, 2007). Mutations in the Arabidopsis (Arabidopsis thaliana) MORE AXILLARY GROWTH (MAX) genes and orthologous genes in garden pea (Pisum sativum), rice (Oryza sativa), and petunia (Petunia hybrida) all lead to increased branching (Beveridge, 2000; Stirnberg et al., 2002; Sorefan et al., 2003; Ishikawa et al., 2005; Snowden et al., 2005; Zou et al., 2005, 2006; Johnson et al., 2006; Arite et al., 2007; Simons et al., 2007). Two of these genes, MAX3 (AtCCD7) and MAX4 (AtCCD8), encode plastid-targeted carotenoid cleavage dioxygenases (CCDs) that together with the cytochrome P450 family member, MAX1, control the production of an upwardly mobile signal that inhibits axillary bud outgrowth (Turnbull et al., 2002; Sorefan et al., 2003; Booker et al., 2004, 2005; Auldridge et al., 2006).
This mobile signal appears to be a strigolactone or a derivative (Gomez-Roldan et al., 2008; Umehara et al., 2008). Strigolactones are carotenoid-derived terpenoid lactones previously shown to stimulate mycorrhizal hyphal branching and seed germination of the plant parasitic weeds Striga and Orobanche (Cook et al., 1972; Akiyama et al., 2005; Humphrey and Beale, 2006). ccd7 and ccd8 mutants of rice and pea are deficient in strigolactones, and the strigolactone analog, GR24 (Johnson et al., 1981), can restore branching inhibition in these mutants as well as in max1, max3, and max4 (Gomez-Roldan et al., 2008; Umehara et al., 2008). In the shoot, strigolactone signal transduction probably involves the F-box Leu-rich repeat protein, MAX2 (Stirnberg et al., 2002, 2007; Booker et al., 2005). Accordingly, branching in max2 plants is not repressed by GR24. F-box proteins function in Skp1-Cul1/Cdc53-F-box (SCF) E3 ubiquitin ligase complexes, which target proteins for ubiquitination, often triggering subsequent degradation by the 26S proteosome (for review, see Petroski and Deshaies, 2005).
A second hormone central to branching control is auxin, which was first shown to inhibit branching over seven decades ago (Thimann and Skoog, 1934). Auxin also signals via SCF-mediated targeted protein degradation. The F-box protein, TRANSPORT INHIBITOR RESPONSE1 (TIR1), and closely related family members (AFBs) act as auxin receptors (Dharmasiri et al., 2005a, 2005b; Kepinski and Leyser, 2005). Auxin binding stabilizes the interaction between TIR1/AFBs and members of the Aux/IAA family of transcriptional repressors (Tan et al., 2007). This induces Aux/IAA degradation, allowing auxin-mediated up-regulation of transcription (Gray et al., 2001; Tiwari et al., 2001; Zenser et al., 2001; Dharmasiri and Estelle, 2002). As a homeostatic mechanism, many Aux/IAA genes are rapidly transcriptionally induced by auxin in an SCFTIR1/AFB-dependent manner (Park et al., 2002). Another protein, AUXIN RESISTANT1 (AXR1), is necessary for proper SCF function by facilitating the conjugation of the ubiquitin-like protein, RUB1, to the Cullin subunit (Leyser et al., 1993; Wu et al., 2000; Schwechheimer et al., 2001; del Pozo et al., 2002). Mutations in AXR1 cause changes in SCFTIR1/AFB-dependent auxin-responsive gene expression and subsequent defects in downstream auxin responses. AXR1 also appears to regulate SCF signaling in photomorphogenesis and jasmonate responses (Schwechheimer et al., 2002; Tiryaki and Staswick, 2002). Phenotypes conferred by axr1 include increased branching, agravitropic root growth, fewer lateral roots and root hairs, and reduced fertility due to poor stamen elongation (Lincoln et al., 1990).
Buds of strigolactone pathway mutants are resistant to inhibition by apically applied auxin, suggesting that strigolactones are required for this inhibition (Beveridge, 2000; Sorefan et al., 2003; Bennett et al., 2006). Additionally, GR24 can inhibit branching in auxin-depleted decapitated pea plants and in auxin signaling mutants, including axr1 and the tir1afb1afb2afb3 quadruple mutant (Brewer et al., 2009). In pea, the genes orthologous to MAX3 and MAX4, RAMOSUS5 (RMS5) and RMS1, are highly auxin regulated, with transcripts greatly diminished or slightly increased by treatments shown to deplete or enhance auxin, respectively (Foo et al., 2005; Johnson et al., 2006). Auxin regulation was also shown for the rice orthologs, HIGH TILLERING DWARF1 and DWARF10 (D10; Zou et al., 2006; Arite et al., 2007). These data suggest that the auxin regulation of strigolactone synthesis genes could represent a conserved and important mode of auxin action in branching control. In Arabidopsis, the response of MAX3 and MAX4 transcripts to auxin depletion has not been assessed in detail. A MAX4 promoter:GUS fusion was responsive to auxin treatments, particularly in the root elongation zone and in hypocotyls, and this was dependent on AXR1 (Bainbridge et al., 2005). In contrast, up-regulation of GUS by auxin was not detected in shoot tissues.
In strigolactone pathway mutants of pea, RMS1 and RMS5 transcription is feedback up-regulated (Foo et al., 2005; Johnson et al., 2006). This was also shown for the MAX4 ortholog in rice and petunia (Snowden et al., 2005; Arite et al., 2007; Simons et al., 2007). A comparatively minor increase in MAX4 transcription has been reported in Arabidopsis max2 hypocotyls (Bainbridge et al., 2005). In pea, grafting experiments indicate that feedback up-regulation of RMS1 and RMS5 occurs both locally and systemically (Foo et al., 2005; Johnson et al., 2006; for review, see Dun et al., 2006). Local feedback regulation could be mediated by a direct effect of strigolactone signaling on RMS gene expression in a manner typical of many hormone biosynthetic pathways (Wang et al., 2002; Yamaguchi, 2008). However, systemic feedback regulation requires a signal in addition to strigolactone, since strigolactones move upward while systemic feedback signaling has been shown to move down the plant (Foo et al., 2005; Johnson et al., 2006). In pea and Arabidopsis, feedback signaling from the shoot also down-regulates the levels of the branching promoter, cytokinin, in the root xylem sap (Foo et al., 2007).
In Arabidopsis, mutations in the MAX genes result in increased stem conductivity for auxin and increased expression of several auxin transporters, including some of the PIN family of auxin exporters (Bennett et al., 2006; Lazar and Goodman, 2006; Brewer et al., 2009). The stems of max mutants have increased expression from the synthetic auxin-responsive promoter DR5, suggestive of increased auxin content. Similarly, increased auxin levels have been observed in strigolactone mutants of rice (Arite et al., 2007) and sometimes, but not always, in pea (for review, see Dun et al., 2006). Therefore, high auxin content in the stems of max mutants is a good candidate for mediating feedback regulation of the MAX pathway. Low strigolactone would lead to increased auxin content, which in turn would up-regulate strigolactone synthesis. In pea, both auxin and another long-distance feedback signal are suggested to be involved in feedback (for review, see Dun et al., 2006). Therefore, auxin and strigolactone signaling could interact in interlocking feedback loops, with each hormone having the potential to regulate the levels of the other. Here, we describe the results of experiments aimed at further investigating the auxin-strigolactone interaction in Arabidopsis and exploring its functional significance.
RESULTS
MAX3 and MAX4 Expression in the Shoot Is Up-Regulated by Auxin in an AXR1-Dependent Manner
Auxin regulation of RMS1/MAX4 and RMS5/MAX3 in stems has been detected in pea but not in Arabidopsis (Bainbridge et al., 2005; Foo et al., 2005; Johnson et al., 2006). To determine if auxin up-regulation of MAX3 and MAX4 can be detected in the shoot using the more sensitive and quantitative quantitative real-time PCR (qRT-PCR), and whether this effect is AXR1 dependent, a dose response of MAX3 and MAX4 expression to the natural auxin indole-3-acetic acid (IAA) was undertaken in the basal cauline internode of intact axr1-3 and wild-type plants. The INDOLE-3-ACETIC ACID INDUCIBLE1 (IAA1) gene known to be up-regulated by auxin via the AXR1/TIR1 pathway (Park et al., 2002; Yang et al., 2004) was analyzed as a control.
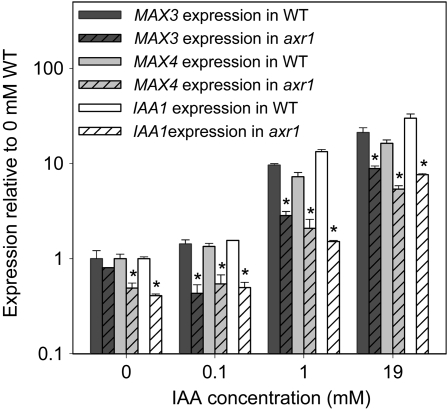
In pea, 3 and 19 mm IAA applied in lanolin to decapitated plants can substantially enhance total stem auxin content and slightly but significantly up-regulate RMS1 and RMS5 expression (Foo et al., 2005; Johnson et al., 2006). When tested here, 1 and 19 mm IAA, but not 100 μm or below, significantly increased MAX3 and MAX4 transcript levels in the basal cauline internode of intact plants by at least 10-fold at 3 h (t test P < 0.01; Fig. 1). These genes behaved similarly to IAA1, although IAA1 was slightly more responsive to IAA addition. For all genes, the response to IAA was clearly diminished in the axr1-3 mutant (P < 0.01–0.05). In untreated plants, expression levels were slightly reduced in axr1-3 relative to the wild type for MAX4 (P < 0.05) and IAA1 (P < 0.01), while this was not significant for MAX3. Together, these data suggest that, like IAA1, MAX3 and MAX4 transcripts are induced by auxin in internode tissue in an AXR1-dependent manner. As is typical with auxin responses in axr1 mutants, the auxin dose-response curve is shifted such that high auxin doses successfully induce a response similar to that observed with lower doses in the wild type.
Figure 1.
Auxin dose response of MAX3, MAX4, and IAA1 expression in wild-type (WT) and axr1-3 basal cauline internodes. IAA was applied in a lanolin ring around the primary bolt of 5-week-old plants 15 mm above the base, and the cauline tissue below the application site was collected 3 h post application. Expression is shown relative to wild-type controls for each gene; data are means of two biological pools of six plants ± se. Asterisks indicate significant differences between axr1 and the wild type.
MAX3 and MAX4 Expression Is Reduced by Auxin Depletion Treatments
In pea, the largest changes in RMS5 and RMS1 expression occur following treatments that cause auxin depletion (Foo et al., 2005; Johnson et al., 2006). Thus, to investigate this for MAX3 and MAX4, transcript levels were quantified following treatments predicted to deplete auxin in a number of tissues.
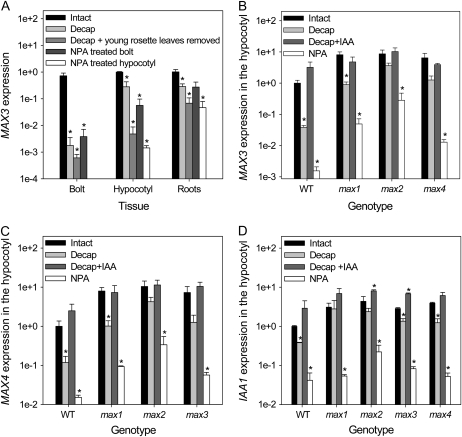
Young expanding leaves at the shoot apex are important sources of auxin (Thimann and Skoog, 1934; Ljung et al., 2001). In 5-week-old wild-type plants, removing these sources by decapitation, or applying the auxin polar transport inhibitor 1-N-naphthylphthalamic acid (NPA) in lanolin, significantly depleted MAX3 transcripts in tissues below (Fig. 2A; all P < 0.05). Specifically, these treatments reduced MAX3 transcripts in the primary bolting stem by 200- to 400-fold after 24 h. In hypocotyls and roots, the greatest reductions in MAX3 transcript occurred when plants were decapitated, including removal of young rosette leaves, or when NPA was applied below these leaves around the top of the hypocotyls (all P < 0.05). Qualitatively similar, but generally quantitatively smaller, changes were seen for MAX4 transcripts (data not shown).
Figure 2.
A, MAX3 expression in response to auxin-depleting treatments in various tissues of 5-week-old wild-type (WT) plants relative to intact hypocotyls. Plants were intact, treated with 3 mg mL−1 NPA in a lanolin ring around the primary bolt 15 mm above the base (NPA-treated bolt) or around the top of the hypocotyl (NPA-treated hypocotyl), or decapitated (Decap) 15 mm above the base of the primary inflorescence with or without young rosette leaves removed. “Bolt” refers to the basal 1 cm of primary cauline stem below the treatment site. B to D, MAX3 (B), MAX4 (C), and IAA1 (D) expression in response to auxin-depleting treatments in the hypocotyls of 3-week-old wild-type and max mutant plants. Plants were intact, treated with 3 mg mL−1 NPA in a lanolin ring around the top of the hypocotyl, or decapitated by removing the entire primary bolt and youngest leaves with or without 19 mm IAA applied to the decapitation site. Tissues were collected 24 h after treatments. Data are means of two to three biological pools of seven to 25 plants ± se. Asterisks indicate significant differences from intact plants per genotype/tissue.
The reductions in MAX3 and MAX4 transcript levels in hypocotyls following NPA treatment and decapitation were generally not as great in max mutants as in wild-type plants (Fig. 2, B and C). This was particularly obvious for max2, the most severe max mutant, where there was no significant difference in transcript levels between intact and decapitated plants (P ≥ 0.1). A similar effect was seen for IAA1, which while relatively less responsive to decapitation in general shows a significant reduction in transcript levels (P < 0.05) except in max1 and max2 (Fig. 2D), possibly reflecting the increased auxin content/signaling properties of these mutants (Bennett et al., 2006). In decapitated plants of all genotypes, transcript loss was prevented by the application of 19 mm IAA to the decapitation site.
Auxin-Related Down-Regulation of MAX3 and MAX4 Expression Can Activate Branching
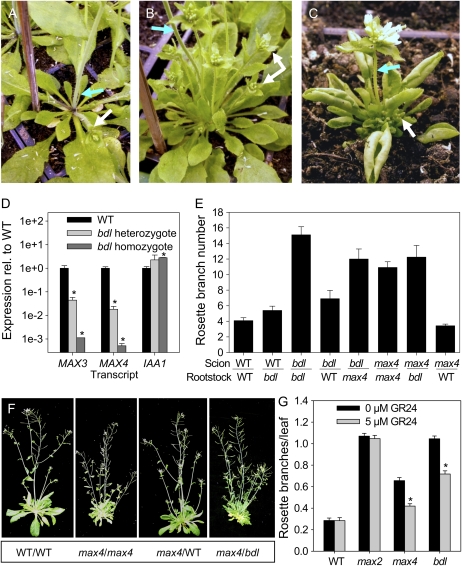
Grafting studies revealed that wild-type roots can restore the branching in max3 and max4 shoots to wild-type levels while max3 roots cannot rescue max4 shoots and visa versa (Turnbull et al., 2002; Sorefan et al., 2003; Booker et al., 2005). This indicates that MAX3 and MAX4 are required in the same tissue for strigolactone production and that their product can move from the root to the shoot to inhibit branching. Previously, roots of the axr1-3 mutant were also shown to restore the branching in max4 shoots to wild-type levels, suggesting that the axr1-3 roots of these grafts are not strigolactone deficient (Sorefan et al., 2003; Bainbridge et al., 2005). These and other data (Bennett et al., 2006) suggest a MAX-independent role for auxin signaling in branching suppression, but they do not rule out a role for AXR1/TIR1-mediated regulation of MAX3 and MAX4 transcription in modulating branching levels. To investigate this possibility, we turned to the bodenlos-2 (bdl-2) mutant. This is a semidominant auxin response mutant that confers defective primary root formation in the embryo and greatly increased shoot branching in the adult plant, particularly obvious in heterozygous plants (Fig. 3). The bdl-2 allele was isolated from an ethyl methanesulfonate-mutagenized population in the ecotype Columbia (Col-0) background (Stirnberg et al., 2002). It has a Pro-74-to-Ser amino acid substitution in domain II of the Aux/IAA transcriptional repressor IAA12 identical to that described for bdl-1, which is in the Landsberg erecta genetic background (Hamann et al., 1999, 2002). This substitution results in auxin-resistant stabilization of the IAA12/BDL protein and thus likely constitutive repression of target auxin-up-regulated genes (Hamann et al., 2002; Dharmasiri et al., 2005b). The bdl-2 mutation caused increased bud outgrowth from the rosette in both homozygotes and heterozygotes. Shoot height was far more reduced in homozygotes, which were severely dwarfed, than in heterozygotes, which with moderately shorter stature and increased branching somewhat resembled max mutants (Fig. 3B).
Figure 3.
Rosette branching and gene expression in bdl mutants. A to C, Representative rosette phenotypes of 5-week-old wild-type plants (WT; A), bdl-2 heterozygotes (B), and bdl-2 homozygotes (C). Blue arrows indicate the primary inflorescence stem; white arrows indicate example axillary rosette branches/buds. D, Relative expression levels of MAX3, MAX4, and IAA1 in the basal cauline internodes of 5-week-old bdl-2 homozygotes and heterozygotes. Data are means of two biological pools of eight to 10 plants ± se; asterisks indicate significant differences from the wild type. E, Number of rosette branches 5 mm or longer in reciprocally grafted wild-type plants, max4-1 mutants, and bdl-2 heterozygotes ± se; n = 4 to 10. F, Photographs of representative max4-1 grafted plants (scion/rootstock). G, Number of rosette branches 5 mm or longer per rosette leaf in wild-type plants, max2-1 and max4-1 mutants, and bdl-2 heterozygotes with or without GR24; n = 13 to 21. Data are means ± se; asterisks indicate significant inhibition of branching by GR24. [See online article for color version of this figure.]
Transcript abundance of MAX3 and MAX4 was reduced by approximately 3 orders of magnitude in bdl-2 homozygotes and also to a lesser extent in heterozygotes (Fig. 3D). IAA1 expression was equal to, or higher than, wild-type expression in bdl-2, suggesting distinct Aux/IAA regulation of these genes. To examine this hypothesis further, we searched for coexpression of IAA1, IAA12, MAX3, and MAX4 using the Genevestigator V3 microarray database (Zimmermann et al., 2004; Hruz et al., 2008). IAA12 and IAA1 were both corepresented with MAX3 and MAX4 in the hypocotyls and inflorescence stems (data not shown); however, IAA12, MAX3, and MAX4 all showed highest differential expression in the hypocotyl xylem, while IAA1 was expressed most highly in callus tissue and the lateral root cap.
To investigate whether low MAX3 and MAX4 expression in bdl-2 mutants correlates with insufficient graft-transmissible branching suppression, as expected if strigolactone levels have been affected, we carried out grafting between bdl-2 heterozygotes, max4-1, and the wild type. If bdl-2 mutants are strigolactone deficient, two results were expected. First, bdl-2 mutant roots should not rescue branching in max4-1 shoots; second, branching in bdl-2 shoots should be rescued by wild-type but not max4-1 rootstocks. Self-grafted bdl-2 heterozygous mutants produced significantly more rosette branches than self-grafted max4-1 mutants (P < 0.01; Fig. 3E), and both mutants branched more than self-grafted wild-type plants (P < 0.001). As expected, branching in max4-1 scions was completely restored to wild-type levels by grafting to wild-type rootstocks (Sorefan et al., 2003; Fig. 3, E and F). However, bdl-2 heterozygote rootstocks did not restore the branching in max4-1 shoots, suggesting that BDL affects root strigolactone production. Furthermore, wild-type rootstocks inhibited branching in heterozygote bdl-2 scions by over 50% relative to bdl-2 heterozygote self grafts (P < 0.001) and compared with only 20% inhibition by max4-1 rootstocks (P < 0.05). These data support the hypothesis that increased branching in bdl-2 mutants is partly due to insufficient strigolactones but also that there is a strigolactone-independent component to the increased branching observed in bdl-2 heterozygous plants.
We also determined the ability of exogenous strigolactone to inhibit branching in bdl-2. The synthetic strigolactone analog, GR24, was applied to rosette axils every 2 to 3 d as described by Gomez-Roldan et al. (2008). This reduced rosette branching in max4-1 and bdl-2 heterozygotes by a similar degree (39% and 34%, respectively; P < 0.001; Fig. 3G), but branching remained increased relative to the wild type for both genotypes, particularly in bdl-2. Branching was unchanged in both the wild type and, as expected for a putative strigolactone signal transduction mutant, in max2-1 (Booker et al., 2005; Stirnberg et al., 2007; Gomez-Roldan et al., 2008; Umehara et al., 2008).
Auxin Signaling Contributes to Feedback Up-Regulation of MAX3 and MAX4 Expression in max Mutants
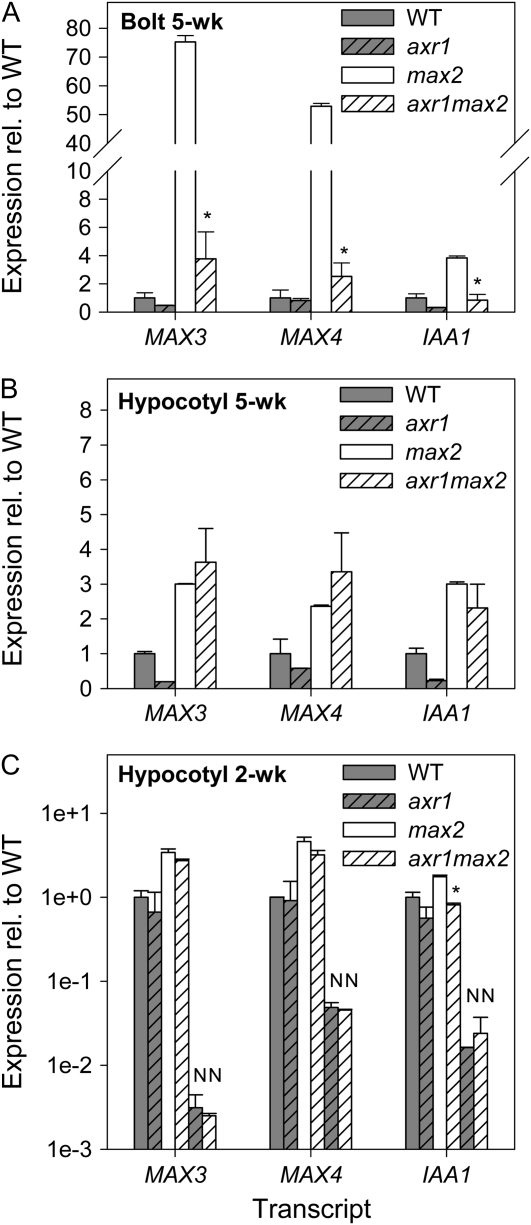
Sixfold to 10-fold increases in MAX3 and MAX4 expression were observed in hypocotyls of all max mutants relative to the wild type, indicative of feedback up-regulation (Fig. 2, B and C). Given that auxin levels and/or signaling are increased in max mutant stems (Bennett et al., 2006), MAX3 and MAX4 transcription is auxin regulated in an AXR1-dependent manner (Bainbridge et al., 2005; this study), and auxin is downwardly mobile, it was hypothesized that this feedback up-regulation may be in part mediated by auxin. To investigate this possibility, we determined the role of AXR1 in feedback signaling using the axr1-3max2-1 double mutant (Bennett et al., 2006).
In the basal cauline internodes of 5-week-old plants, MAX3, MAX4, and IAA1 transcripts accumulated to significantly higher levels in max2-1 mutants than in wild-type plants (P < 0.01; Fig. 4A). In the axr1-3max2-1 background, the expression of all genes in this tissue was reduced to levels not statistically significantly different from the wild type or the single axr1-3 mutant. Thus, axr1 is required for feedback up-regulation in the shoot. In the hypocotyls of the same plants, the up-regulation of MAX3 and MAX4 in max2-1 was less than in cauline tissue (significant at P < 0.05; Fig. 4B), and this was not abolished in the axr1-3 background. This might indicate that the observed elevated expression in max2-1 hypocotyls is not related to auxin; however, like MAX3 and MAX4, IAA1 and additional auxin-responsive Aux/IAA genes (IAA5, IAA19, and IAA29; data not shown) showed increased expression in max2-1 that was not significantly affected by the axr1-3 mutation.
Figure 4.
Expression of MAX3, MAX4, and IAA1 in wild-type (WT), axr1-3, max2-1, and axr1-3max2-1 plants. A and B, Expression in the basal cauline internodes (A) and hypocotyls (B) of 5-week-old plants. C, Expression in the hypocotyls of vegetative 2-week-old plants; samples marked with “N” were treated with 3 mg mL−1 NPA around the top of the hypocotyls (note log scale). Data are means of two biological pools of five to 10 plants ± se. Asterisks indicate a significant effect of axr1 on feedback in max2.
In the hypocotyls of vegetative 2-week-old max2-1 plants, MAX3, MAX4, and IAA1 transcripts were again slightly but significantly up-regulated (P < 0.05; Fig. 4C). At this developmental stage, the axr1-3 background prevented the up-regulation of IAA1, but not MAX3 or MAX4, in max2-1 hypocotyls. This could suggest auxin-independent feedback specific to MAX3 and MAX4. However, we cannot rule out an auxin-dependent relationship, because NPA treatment of these plants abolished the up-regulation of MAX3 and MAX4 in axr1-3max2-1 relative to axr1-3 (Fig. 4D), and our results with bdl-2 above suggest that differences in the auxin responsiveness of these genes could be due to different Aux/IAAs involved in their regulation.
Graft Transmissibility of Feedback Signals
To test if feedback regulation of MAX3 and MAX4 expression can occur over long distances, as shown in pea (Foo et al., 2005; Johnson et al., 2006), we reciprocally grafted max2-1 and wild-type plants.
Feedback in the Shoot
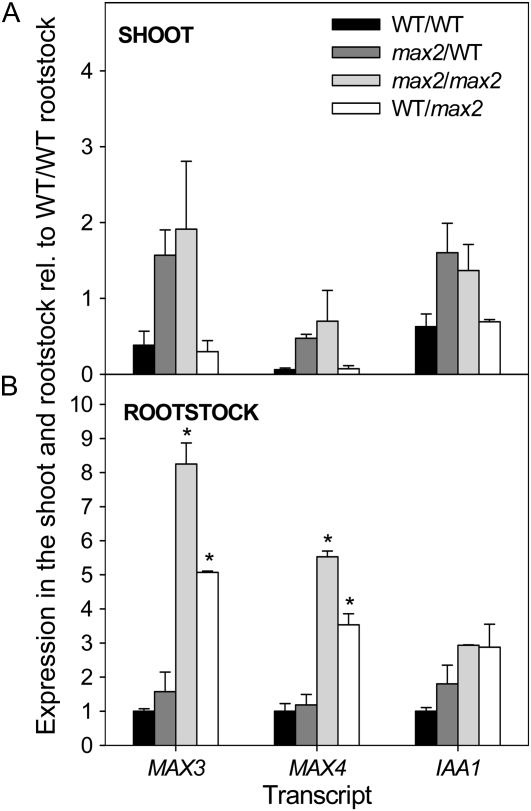
As shown for pea, a local feedback effect was noted in the shoot, as evidenced by the increased expression of MAX3, MAX4, and IAA1 in the shoots of max2/wild-type (scion/rootstock) grafts compared with wild-type/wild-type grafts (Fig. 5A). For all grafts, the expression of all genes in the shoot was dependent only on the shoot genotype; therefore, similar to results in pea, no upwardly mobile feedback signaling was detected (Foo et al., 2005; Johnson et al., 2006).
Figure 5.
Expression of MAX3, MAX4, and IAA1 in the shoot (A) and the rootstock (B) of grafted wild-type (WT) and max2-1 plants. Expression was measured in vegetative plants 2 weeks after transfer to soil; values are shown relative to wild-type/wild-type (scion/rootstock) rootstocks. Data are means of two biological pools of five to eight plants ± se. Bars with asterisks are significantly different from each other due to a long-distance effect.
Feedback in the Root
The expression of MAX3, MAX4, and IAA1 was higher in the rootstocks of wild-type/max2 grafts relative to wild-type/wild-type grafts, supporting a local feedback effect also in the root (Fig. 5B). However, MAX3, MAX4, and IAA1 expression was not increased in the rootstocks of max2/wild-type grafts relative to wild-type/wild-type grafts. Therefore, in contrast with results in pea (Foo et al., 2005), a downwardly mobile feedback signal from max2-1 shoots, able to up-regulate gene expression in wild-type roots, was not detected. Nonetheless, the increased expression of MAX3 and MAX4, but not IAA1, in max2 rootstocks was reduced by approximately 40% when grafted to a wild-type shoot compared with a max2 shoot (Fig. 5B; P < 0.05). This is suggestive of some long-distance effect on MAX3 and MAX4 expression in the rootstock depending on the shoot genotype.
As growing shoot apices export auxin, it was considered that auxin, or additional, downwardly mobile feedback signals may be enhanced by the presence of actively growing branches in max mutants. Thus, grafts were repeated and grown until plants had primary bolting stems 15 to 30 cm in length and rosette branches. MAX3, MAX4, and IAA1 were up-regulated in the rootstocks of branching max2 self-grafts by a similar degree as in vegetative max2 self-grafts and again were not up-regulated in wild-type rootstocks grafted to max2 shoots (data not shown). Therefore, while there appears to be both a local and a long-distance feedback effect of strigolactone signaling on MAX3 and MAX4 transcript abundance in Arabidopsis, it appears that, in contrast to pea, this long-distance effect is relatively minor.
DISCUSSION
Auxin Can Affect Strigolactone-Mediated Branch Inhibition via MAX3 and MAX4 Regulation
Previous analyses have shown that auxin may interact with the strigolactone pathway in both monocots and dicots by regulating the transcription of MAX3 (CCD7) and MAX4 (CCD8) orthologs (Sorefan et al., 2003; Foo et al., 2005; Johnson et al., 2006; Zou et al., 2006; Arite et al., 2007). Here, we reveal that auxin positively regulates both MAX3 and MAX4 expression in Arabidopsis more extensively than previously thought. This further supports conservation, and hence possible functional significance, of this regulation throughout evolution.
In the shoot, auxin enhanced MAX3 and MAX4 transcript levels within 3 h, and this was AXR1 dependent (Fig. 1). However, high concentrations (>1 mm) of IAA in lanolin were required to elicit this response, even for the IAA1 gene, which in etiolated seedlings can be induced by as little as 1 μm IAA in solution over a similar time frame (Abel et al., 1995). This may suggest reduced permeability or sensitivity of intact light-grown cauline tissue to IAA applied in lanolin. Similarly to pea, rapid and large reductions in MAX3 and MAX4 transcript levels were observed following treatments predicted to deplete endogenous auxin, and these reductions were prevented by auxin addition (Fig. 2). Together, these data imply that the basal levels of MAX3 and MAX4 transcripts in intact wild-type plants are substantially maintained by auxin. Consistent with this idea, the levels of MAX3 and MAX4 transcript in the auxin response mutant axr1-3 were in general lower than in the wild type, although these differences were not always statistically significant (Figs. 1 and 4). This is likely due in part to reduced feedback inhibition on auxin synthesis in the axr1-3 mutant, resulting in increased auxin levels (Romano et al., 1995), which may partially compensate for reduced auxin sensitivity. Furthermore, AXR1 acts partly redundantly with an additional AXR1-like protein, AXL1 (Dharmasiri et al., 2007), and the axr1-3 allele used in this work is not a null allele. AXR1-dependent auxin regulation of MAX3 and MAX4 is consistent with the existence of auxin-response cis-elements in the promoter regions of these genes, including four and two copies, respectively, of the TGTCTC ARF-binding motif (Ulmasov et al., 1999) within 3 kb upstream.
In contrast to the mild effect of the axr1-3 mutant on MAX3 and MAX4 transcript levels, the highly branched bdl-2 mutant (Fig. 3), expressing the IAA12 transcriptional repressor that is resistant to AXR1/TIR1-mediated destabilization (Hamann et al., 2002; Dharmasiri et al., 2005b), had transcript levels similar to those of plants treated to deplete endogenous auxin (Figs. 2 and 3D). Thus, an attractive hypothesis, also suggested by Brewer et al. (2009), is that substantial auxin depletion results in increased branching at least in part by reducing strigolactone synthesis. Consistent with this, branching in bdl-2 shoots appears to involve strigolactone deficiency. First, shoot branching in max4 mutants can be restored to wild-type levels by grafting to wild-type rootstocks but not bdl-2 rootstocks (Sorefan et al., 2003; Bainbridge et al., 2005; Fig. 3E), suggesting that bdl-2 rootstocks do not produce sufficient strigolactone. Second, wild-type rootstocks rescued over half of the branching in bdl-2 shoots, while strigolactone-deficient max4-1 rootstocks did not, suggesting this strigolactone deficiency may be at least partly responsible for the increased shoot branching in bdl (Fig. 3, E–G). Consistent with this idea, GR24 suppressed branching by a similar degree in bdl-2 and max4-1. An interaction between BDL/IAA12 and MAX3 and MAX4 is consistent with their preferential expression in the xylem and the localization of a BDL:GUS fusion protein in vascular tissues (Zimmermann et al., 2004; Dharmasiri et al., 2005b; Hruz et al., 2008). Although strigolactone appears to be involved in the shoot-branching phenotype of bdl-2 mutants, it is unlikely to play a role in the embryonic phenotype (Hamann et al., 1999, 2002; Hardtke et al., 2004), since mutants for the strigolactone pathway do not show similar pleiotropic abnormalities.
Taken together, our results suggest that auxin-regulated MAX expression can be blocked by stabilization of BDL/IAA12, and this can modulate the degree of branching. Conversely, consistent with max4 shoot branching being rescued by grafting to axr1 roots (Bainbridge et al., 2005), the mild down-regulation of MAX3 and MAX4 transcripts observed in axr1-3 mutant roots may not be sufficient for strigolactone-dependent modulation of branching. The increased branching observed in axr1-3 mutants, the additive bud auxin-insensitivity and branching phenotype of axr1-3max double mutants relative to the single mutants, and the normal levels of auxin transport in axr1-3 mutants also point to mutually independent roles for MAX and auxin signaling in suppressing branching (Bainbridge et al., 2005; Bennett et al., 2006). Although some effects of axr1 mutants may not be related solely to auxin signaling (Schwechheimer et al., 2002; Tiryaki and Staswick, 2002), an additional role for auxin signaling in branching is supported here by the fact that branching in bdl-2 shoots was partially suppressed by max4-1 roots compared with bdl self-grafts, suggesting that some of the branching in bdl-2 cannot be explained by strigolactone deficiency (Fig. 3).
An additional mode of auxin action is likely to involve down-regulated synthesis of the branching promoter cytokinin (Wickson and Thimann, 1958, Sachs and Thimann, 1967; Bangerth, 1994; Li et al., 1995; Nordström et al., 2004; Ferguson and Beveridge, 2009). Apically derived auxin has been shown to down-regulate cytokinin levels in the xylem exudate of pea and bean (Phaseolus vulgaris), and in pea it can inhibit the expression of ISOPENTYL-TRANSFERASE cytokinin biosynthesis genes in the stem (Bangerth, 1994; Li et al., 1995; Tanaka et al., 2006; Ferguson and Beveridge, 2009). In Arabidopsis, the auxin-mediated repression of cytokinin synthesis requires AXR1 (Nordström et al., 2004). Thus, differences in branching between bdl-2 self-grafts and bdl-2 shoots grafted with max4 roots could involve differences in cytokinin levels, caused by auxin insensitivity of bdl-2 root and shoot tissues. However, while local production of cytokinin in shoot tissues can affect branching, the importance of root-derived cytokinin to axillary branching remains controversial (Chaudhury et al., 1993; Beveridge et al., 1997a; Faiss et al., 1997; McKenzie et al., 1998; Catterou et al., 2002; Schmülling, 2002; Sakamoto et al., 2006).
The Mechanisms of Strigolactone Feedback Regulation in Arabidopsis
We show that both MAX3 and MAX4 transcripts are up-regulated in all max mutants, suggesting feedback control (Fig. 2, B and C). The degree of up-regulation varies between tissues and is of a similar magnitude to that for orthologous genes in rice and petunia, being substantially less than that for RMS1 in pea, where transcripts can accumulate to more than 1,000-fold in rms4 mutants (Foo et al., 2005; Snowden et al., 2005; Arite et al., 2007; Simons et al., 2007; Umehara et al., 2008). There are at least three not mutually exclusive potential mechanisms for the up-regulation of MAX3 and MAX4 gene expression in max mutants: (1) it is a direct effect of the strigolactone signaling pathway; (2) it is associated with the demonstrated effect of auxin on MAX3 and MAX4 expression, coupled with the effect of the MAX pathway on auxin transport/levels and thus auxin distribution; and (3) it is associated with an additional downwardly mobile strigolactone-regulated signal, as proposed for pea (Foo et al., 2005; Dun et al., 2006).
Direct Effects of Strigolactone Signaling on Strigolactone Biosynthesis Gene Expression
The best evidence for a direct feedback effect of strigolactone signaling comes from studies of rice roots, where up-regulated D10 expression in strigolactone pathway mutants can be restored to wild-type levels by strigolactone application in the biosynthesis mutant but not the signaling mutant (Umehara et al., 2008). However, this was determined 24 h after strigolactone application and thus remains of limited value in distinguishing a direct effect from one mediated by another signal such as auxin, considering that auxin alters gene expression within 3 h (Foo et al., 2005; Fig. 1).
In this study, we included parallel observations of MAX gene expression and the auxin-responsive gene IAA1. We have also analyzed the axr1-3 mutant, which has known defects in auxin-responsive gene expression. This allows some discrimination between a direct effect of strigolactone on MAX3 and MAX4 expression and an indirect effect mediated by changes in auxin levels in the mutants. As discussed below, our findings suggest that the major portion of feedback regulation in Arabidopsis is indirectly mediated by auxin signaling.
Involvement of Auxin in the Feedback Regulation of MAX3 and MAX4
Where there were large differences in MAX3 and MAX4 transcript accumulation between the wild type and the max2 mutant, these were substantially reduced in the axr1-3 mutant background. In contrast, where the differences were small, these appeared to be unaffected by the axr1-3 mutation (Fig. 4). Thus, it seems likely that a major contributor to the elevated levels of MAX3 and MAX4 transcript in max mutant backgrounds is increased auxin in some tissues, particularly cauline nodes. This is consistent with the very high DR5:GUS levels observed in the vasculature of max mutant stems and the expression of MAX genes in vascular-associated cells where polar auxin transport is occurring (Sorefan et al., 2003; Booker et al., 2005; Bennett et al., 2006; Stirnberg et al., 2007).
A role for auxin in feedback is further suggested by the strong correlation between IAA1 expression and MAX3 and MAX4 expression across the feedback assays used in this study (Figs. 2, 4, and 5), even in cases where axr1 had no effect. For example, axr1 did not suppress the elevated levels of MAX3 and MAX4 transcripts in mature max2 hypocotyls, yet the same was observed for IAA1 transcripts and additional Aux/IAAs (Fig. 4B; data not shown). A case for direct or auxin-independent feedback on MAX3 and MAX4 expression (as in mechanisms 1 or 3 above) is better suggested where the response of their transcripts to feedback differs from IAA1. We found only two such instances. First, in young hypocotyls, the up-regulation of IAA1 expression in max2 was suppressed in the axr1-3 background (axr1max2), as expected if auxin was responsible for its up-regulation, while MAX3 and MAX4 transcripts were expressed at equally high levels in max2 and axr1max2 (Fig. 4C). In this case, however, the enhanced MAX3 and MAX4 expression in axr1max2 relative to axr1 was abolished by NPA treatment (Fig. 4D). This could again mean a function for increased auxin in the polar auxin transport stream (PATS) of max2 plants in this feedback (Bennett et al., 2006) or that transcript levels are at their lowest after NPA treatment, preventing an additional effect of MAX2. The other case where IAA1 expression levels did not reflect MAX3 and MAX4 expression was in the rootstock of grafted plants, as discussed below (Fig. 5B).
Systemic Feedback Regulation of MAX3 and MAX4
The data described above demonstrate that auxin, likely that traveling in the PATS, is a major component of feedback signaling on MAX3 and MAX4 transcript abundance, especially in cauline nodes. Therefore, at least in the shoot, auxin could act as a long-range feedback signal for MAX action.
In this study, similar to those for pea (Foo et al., 2005; Johnson et al., 2006), the expression of MAX3 and MAX4 in the rootstocks of wild-type/max2 (scion/rootstock) grafts was intermediate between wild-type/wild-type and max2/max2 grafts (Fig. 5). This suggests that MAX2 activity in either the shoot or the root can affect MAX3 and MAX4 expression in the rootstock. Although wild-type shoots suppressed MAX3 and MAX4 gene expression in max2 roots by about one-third compared with max2 self-grafts, they had no effect on IAA1 gene expression. Thus, one possibility is a non-auxin long-distance signaling effect. However, the extent of this long-distance regulation is considerably less than that in pea, where 140-fold differences in RMS1 transcripts were observed for the comparable graft combinations (Foo et al., 2005). Also unlike comparable grafts of pea, we did not detect a systemic feedback signal from max2 shoots that could up-regulate MAX3 and MAX4 expression in wild-type rootstocks (Fig. 5; Foo et al., 2005). Interestingly, in petunia, the up-regulation of the MAX4 ortholog, DECREASED APICAL DOMINANCE1, was not found to be graft transmissible to the rootstock (Simons et al., 2007).
The relatively minor effect of the shoot genotype, wild type or max2, on MAX3 and MAX4 gene expression in the roots contrasts with clear evidence of the long-distance regulation of xylem sap cytokinin by strigolactone signaling in Arabidopsis shoots (Foo et al., 2007). Auxin regulation of cytokinin content is well documented (Nordström et al., 2004), and decapitation leads to depletion of xylem sap cytokinins, which can be restored with exogenous auxin (Bangerth, 1994; Li et al., 1995). However, a feedback signal in addition to auxin has been proposed for pea, because feedback down-regulated xylem cytokinin is not significantly increased by decapitation in the rms1, rms3, and rms4 mutants over 24 h, and the up to 2,000-fold increases in RMS1 expression observed in the rms4 (Psmax2) mutant have not been achieved by auxin addition (Foo et al., 2005, 2007; for review, see Dun et al., 2009). More direct studies of auxin involvement in shoot-to-root regulation of xylem sap cytokinin levels in Arabidopsis are required to determine whether a signal in addition to auxin affects xylem cytokinin content.
Sources of Differences
In this and previous studies, similar experiments have given quantitatively and sometimes qualitatively different results. Our data suggest that some of the variability is likely technical, such as the method of hormone application or expression analysis, the hormone concentrations used, and the tissue types included in the analyses. Different phenotypes of species dictate the use of different tissues for grafting and gene expression analysis, epicotyl in pea versus hypocotyl in Arabidopsis and petunia, and perhaps this could influence the movement of, or degree of response to, long-distance signals. Importantly, some interesting biological differences are also emerging. In pea but not Arabidopsis, mutant rms4 (max2) rootstocks are more able than wild-type rootstocks to suppress branching in wild-type and various rms mutant shoots, and this has been associated with the small local feedback up-regulation of strigolactone biosynthesis genes in mutant rootstocks (Beveridge et al., 1996, 1997b; Morris et al., 2001; Booker et al., 2005; Foo et al., 2005; Johnson et al., 2006). While this could involve differences in the strength of feedback regulation, perhaps strigolactone levels could be more rate limiting in pea than in Arabidopsis in standard conditions. As discussed above, the balance of auxin and auxin-independent feedback regulation on strigolactone synthesis genes may also differ, the latter component appearing to be greater in pea than in Arabidopsis (Foo et al., 2005, 2007). Although many similarities are apparent between species, differences in strigolactone pathways could potentially reflect differences in developmental programs and shoot architectures.
CONCLUSION
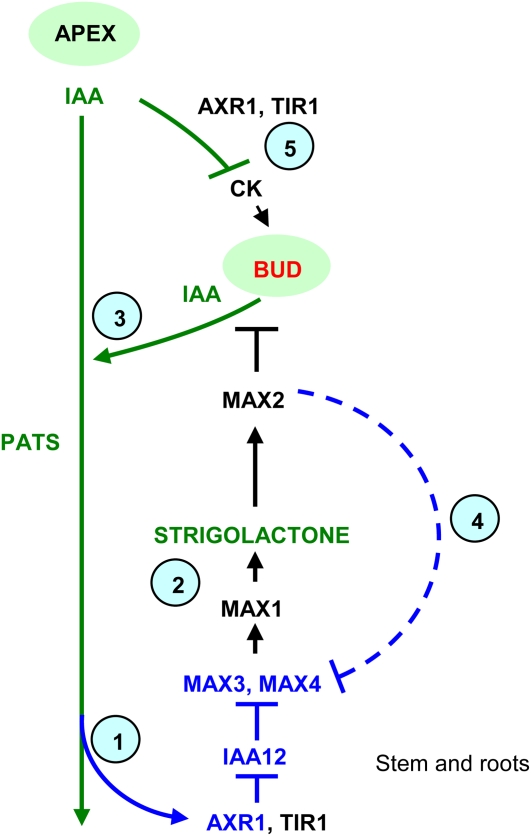
We demonstrate that the auxin regulation of strigolactone biosynthetic genes is conserved in Arabidopsis. This involves AXR1/TIR1-regulated Aux/IAA stability and contributes to branching inhibition. Furthermore, this auxin regulation also acts as a feedback mechanism, with increased auxin content in conditions of low strigolactone acting as a downstream communicator to increase strigolactone biosynthesis. We suggest that auxin-regulated strigolactone biosynthesis may form a conserved component of auxin-mediated branching inhibition and that auxin and strigolactone signaling may participate in an interlocking feedback loop, involving interplay with additional stimuli, to precisely control branching in plants. A diagrammatic representation of the interactions between auxin and the MAX pathway built upon by this study is shown in Figure 6.
Figure 6.
Interactions between auxin and the MAX pathway. Regulatory signals represented in blue were investigated in this study. Step 1, Auxin traveling in the PATS promotes MAX3 and MAX4 expression in an AXR1-dependent manner, prevented if IAA12 is stabilized. Step 2, Auxin-promoted MAX3 and MAX4 transcript levels lead to increased strigolactone production, which leads to reduced bud outgrowth and reduced auxin export from buds into the PATS. Step 3, If strigolactones are low, branching is increased and auxin level increases, feedback up-regulating MAX3 and MAX4 to increase strigolactone levels as in step 1. Step 4, Strigolactone signaling may feedback down-regulate transcript levels independent of auxin; the dashed line indicates weak/putative interaction. Step 5, Auxin also down-regulates cytokinin synthesis, and cytokinin promotes branching. The environment/genotype/developmental program may influence each of these regulatory stages tissue specifically. [See online article for color version of this figure.]
MATERIALS AND METHODS
Plant Growth and Materials
For Figures 1, 2, 3E to 3G, 4C, 4D, and 5, Arabidopsis (Arabidopsis thaliana) seeds were sown on a mixture of California potting mix type C and vermiculite (3:2, v/v) at a density of one to two per 5 cm2 or one per 1 cm2 (Fig. 2) and stratified for 2 to 3 d at 4°C. Trays were transferred either to a temperature-controlled growth room at 22°C ± 2°C/18°C ± 2°C for 18 h of light/6 h of dark with fluorescent lighting (Philips 36 W/840) supplying approximately 120 μmol m−2 s−1 light or to a temperature-controlled glasshouse at 24°C ± 2°C/18°C ± 2°C for 18 h of light/6 h of dark with the natural daylength extended by incandescent light. For Figures 3A to 3D, 4A, and 4B, seeds were cold treated and grown at a density of one per 4 cm2 in a temperature-controlled glasshouse according to Bennett et al. (2006). All plant lines used were in the Col-0 background as follows: Col-0 wild type, max1-1, max2-1, max3-11, max4-1, axr1-3, axr1-3max2-1, and bdl-2 (see below). Vegetative 2-week-old plants had approximately six to eight mature leaves expanded, and bolting plants were grown until the primary bolt was 2 to 5 cm (3 weeks) or 15 to 30 cm (approximately 5 weeks) as indicated in the text.
The bdl-2 Allele
The bdl-2 mutant allele was found in a screen for branching mutants of the ethyl methanesulfonate-mutagenized Col-0 population described by Stirnberg et al. (2002). Genetic analysis of the mutant individual recovered showed that its max-like phenotype was caused by a semidominant mutation in a heterozygous state. Homozygotes were severely dwarfed. The mutation mapped between PVV4 and nga63 on chromosome I, an interval containing four IAA genes: IAA3/SHY2, IAA17/AXR3, IAA10, and IAA12/BDL. As mutations with semidominant inheritance were known for this class of genes but homozygous or heterozygous shy2 or axr3 were unlike our mutant (Leyser et al., 1996; Tian and Reed, 1999), IAA10 and IAA12 were considered as possible candidates and their coding regions were amplified from genomic DNA of homozygous mutant plants. Sequencing revealed no change in IAA10 but a CCA-to-TCA change in codon 74 of IAA12, predicting a Pro-to-Ser substitution identical to the bdl-1 allele, which is in the Landsberg erecta genetic background (Hamann et al., 1999, 2002).
Decapitation and Hormone Treatments
Three-week-old plants were decapitated by removing the entire primary inflorescence (2–5 cm) and the youngest three to four expanding rosette leaves using a pair of fine forceps. For 5-week-old plants, primary bolts (15–30 cm) were decapitated 15 mm above the stem base using a sterile scalpel, with or without the youngest rosette leaves removed as indicated in the figure legends. For hormone treatments, hormones were dissolved in ethanol and mixed with lanolin to give the various concentrations indicated with a final ethanol concentration of less than 10%. NPA was applied in a ring either around the top of the hypocotyls or around the primary bolt 15 mm above the base as indicated. IAA was applied to the decapitation site or, for the dose-response assay, in a ring around the primary bolt of intact plants 15 mm above the base. Control treatments were as above minus hormone. For expression analyses, tissues as indicated (approximately 2–5 mm of hypocotyl, the basal 10 mm of the primary inflorescence stem, and the first 5 cm of root tissue) were harvested after the times indicated. Statistical P values for all phenotypic and expression data were calculated using the t test.
Plants were treated with GR24 (http://www.chiralix.com/) as described by Gomez-Roldan et al. (2008) with modifications. For 5 μm-treated and 0 μm control plants, solutions containing 0.05% Silwet and 0.05% acetone with or without GR24 were applied to plants every 3 d starting at 18 d after transfer to the growth room. At 24 d, the GR24 dose was doubled to 10 μm applied every 2 d for two treatments followed by an additional four treatments of 5 μm every 3 d. Rosette leaf numbers (minus cotyledons) were scored 1 week after bolting, and branch numbers of 5 mm or greater were scored at 47 to 50 d.
Grafting
Transverse grafts were performed using a protocol adapted from Turnbull et al. (2002). Sterilized cold-treated seeds were germinated vertically on plates containing half-strength Murashige and Skoog basal salts, 1% Suc, and 0.8% agar in an incubator at 24°C with an 18-h photoperiod. Seedlings were grown for 4 to 5 d before careful transfer to sterilized petri dishes containing a premoistened layer of Millipore nitrocellulose filter (type HA; pore, 0.45 μm) on a single layer of Whatman No. 1 filter paper for grafting. Grafts were returned to the incubator, and successful grafts were transferred to soil by 7 d and grown in a temperature-controlled growth room as above. Grafts were visually assessed, and phenotypic analyses were carried out at the cessation of primary meristem activity. For expression analyses, hypocotyl and root tissues below the graft union (approximately 2 cm) were collected as rootstock tissue and rosette stem tissue (rosette stem plus approximately 0.5 mm of petiole tissue) was collected as shoot tissue. For vegetative-stage plants, tissues were collected 2 weeks after transfer to soil. For mature plants, tissues were collected following phenotypic analysis.
qRT-PCR
Total RNA was isolated from pools of five to 25 plants using NucleoSpin RNA plant kits (Machery-Nagel) and quantified using a NanoDrop 1000. cDNA was synthesized in 20 μL with 100 ng to 1 μg of total RNA, 250 ng of random primers (Promega), 250 ng of oligo(dT)15 (Promega), 0.5 mm deoxyribonucleotide triphosphates, 5 mm dithiothreitol (Invitrogen), 1× first-strand buffer, and 100 units of SuperScript III reverse transcriptase (Invitrogen) as described in the Invitrogen SSIII RT protocol. Reverse transcriptase-minus control reactions were performed for each RNA sample to assess DNA contamination during qRT-PCR. Each qRT-PCR was performed in duplicate using SYBR Green PCR Master Mix, 200 nm of each primer, and 2 to 15 ng of cDNA in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) with melt-curve analysis. No-template control reactions were performed for each primer. Primer efficiencies (PE) of each gene were calculated per run by LinRegPCR (Ramakers et al., 2003). Relative quantitation was determined using the comparative cycle threshold (CT) method; CT values for technical replicates for each gene were averaged (AvCT), and MAX3 (At2g44990), MAX4 (At4g32810), and IAA1 (At4g14560) expression was normalized against 18S (At2g01010; Figs. 1, 2, B–D, and 3–5) or three β-actin reference genes (At3g18780, At5g09810, and At1g49240; Fig. 2A) according to Brown et al. (2003) using the formula PEgene−AvCTgene/PEreference−AvCTreference. Resulting values were averaged for biological replicates and plotted with se values relative to a chosen sample (the calibrator), as indicated on the y axis. Primers were designed using PrimerExpress 1.5 (Applied Biosystems) with one primer across intron junctions where possible. The qRT-PCR for Figure 3 was performed using Power SYBR Green PCR Master Mix in an ABI Prism 7300 Sequence Detection System (Applied Biosystems) with melt-curve analysis. Gene expression was normalized to 18S using the comparative CT method with the formula 2−CTgene/2−CT18S. Primer sequences are as follows: 18SF (5′-TTCCTAGTAAGCGCGAGTCATCA-3′), 18SR (5′-GAACACTTCACCGGATCATTCAAT-3′), QRTMAX4F (5′-GAAAGATACCCACTTGGCTGAATG-3′) QRTMAX4R (5′-TGTGGAGTAGCCGTCGAAGAG-3′), QRTMAX3F (5′-GATTCGTTGGTGAGCCCATG-3′), QRTMAX3R (5′-CACCGAAACCGCATACTCGA-3′), QRTIAA1F (5′-GCTCCTCCTCCTGCAAAAACAC-3′), QRTIAA1R (5′-ACGGTTAGATCTCACTGGAGGC-3′); β-actin primers were as described by Brown et al. (2003). The Genevestigator V3 Classic Metaprofile analysis tool (available at https://www.genevestigator.ethz.ch/) was used to generate an anatomy heat map of IAA1 (245397_at), MAX3 (266129_at), MAX4 (253398_at), and IAA12 (264605_at; data not shown).
Acknowledgments
At the University of Queensland, we thank Julia Cremer and the Beveridge laboratory for assistance with the experiment in Figure 2, Dr. Philip Brewer for assistance with the strigolactone application experiment, Dr. Elizabeth Dun and Tanya Brcich for helpful discussions, Mr. Bob Simpson for qRT-PCR advice, and Kerry Condon for technical assistance. At the University of York, we thank horticultural staff for plant care, Dr. Karin van de Sande for initial isolation of the bdl-2 mutant, and University of York Technology Facility staff for qRT-PCR advice. We are also indebted to Dr. Catherine Rameau (INRA, France), who provided the GR24.
This work was supported by the Australian Research Council Centre of Excellence for Integrative Legume Research, by the Biotechnology and Biological Sciences Research Council, and by an Australian Postgraduate Award, a Travelling Fellowship from the Company of Biologists (Development), and a University of Queensland Graduate School Research Travel Award to A.H.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christine Beveridge (c.beveridge@uq.edu.au).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Abel S, Nguyen MD, Theologis A (1995) The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251 533–549 [DOI] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435 824–827 [DOI] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51 1019–1029 [DOI] [PubMed] [Google Scholar]
- Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ (2006) Characterisation of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45 982–993 [DOI] [PubMed] [Google Scholar]
- Bainbridge K, Sorefan K, Ward S, Leyser O (2005) Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J 44 569–580 [DOI] [PubMed] [Google Scholar]
- Bangerth F (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment and relationship to apical dominance. Planta 194 439–442 [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Opin Plant Biol 16 553–563 [DOI] [PubMed] [Google Scholar]
- Beveridge CA (2000) Long-distance signalling and mutational analysis of branching in pea. Plant Growth Regul 32 193–203 [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C (1997. a) The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J 11 339–345 [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC (1996) Branching in pea: action of genes Rms3 and Rms4. Plant Physiol 110 859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C (1997. b) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signals. Plant Physiol 115 1251–1258 [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signalling molecule. Curr Biol 14 1232–1238 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8 443–449 [DOI] [PubMed] [Google Scholar]
- Brewer P, Dun E, Ferguson B, Rameau C, Beveridge C (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM (2003) A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol 132 1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterou M, Dubois F, Smets R, Vaniet S, Kichey T, Van Onckelen H, Sangwan-Norreel BS, Sangwan RS (2002) hoc: an Arabidopsis mutant overproducing cytokinins and expressing high in vitro organogenic capacity. Plant J 30 273–287 [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Letham S, Dennis ES (1993) amp1: a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J 4 907–916 [Google Scholar]
- Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA, McPhail AT (1972) Germination stimulants. II. The structure of strigol: a potent seed germination stimulant for witchweed (Striga lutea Lour.). J Am Chem Soc 94 6198–6199 [Google Scholar]
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M (2002) AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis cullin AtCUL1 is required for auxin response. Plant Cell 14 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005. a) The F-box protein TIR1 is an auxin receptor. Nature 435 441–445 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Karunarathna N, Jurgens G, Estelle M (2007) AXL and AXR1 have redundant functions in RUB conjugation and growth and development in Arabidopsis. Plant J 52 114–123 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M (2005)b Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9 109–119 [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Estelle M (2002) The role of regulated protein degradation in auxin response. Plant Mol Biol 9 401–409 [PubMed] [Google Scholar]
- Dun EA, Brewer PB, Beveridge CA (2009) Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci 14 364–372 [DOI] [PubMed] [Google Scholar]
- Dun EA, Ferguson BJ, Beveridge CA (2006) Apical dominance and shoot branching: divergent opinions or divergent mechanisms. Plant Physiol 142 812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss M, Zalubilova J, Strnad M, Schmülling T (1997) Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signalling in whole tobacco plants. Plant J 12 401–415 [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Beveridge CA (2009) Roles for auxin, cytokinin and strigolactone in regulating shoot branching. Plant Physiol 149 1929–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA (2005) The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Morris SE, Parmenter K, Young N, Wang H, Jones A, Rameau C, Turnbull CGN, Beveridge CA (2007) Feedback regulation of xylem cytokinin content is conserved in pea and Arabidopsis. Plant Physiol 143 1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al (2008) Strigolactone inhibition of shoot branching. Nature 455 189–194 [DOI] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414 271–276 [DOI] [PubMed] [Google Scholar]
- Hamann T, Benkova E, Baurle I, Kientz M, Juergens G (2002) The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev 16 1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jurgens G (1999) The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 126 1387–1395 [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T (2004) Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131 1089–1100 [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendrop F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Advances in Bioinformatics 2008 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AJ, Beale MH (2006) Strigol: biogenesis and physiological activity. Phytochemistry 67 636–640 [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46 79–86 [DOI] [PubMed] [Google Scholar]
- Johnson AW, Gowda G, Hassanali A, Knox J, Monaco S, Razavi Z, Rosebery G (1981) The preparation of synthetic analogs of strigol. J Chem Soc Perkin Trans 1 1981 1734–1743 [Google Scholar]
- Johnson X, Brcich T, Dun EA, Goussot M, Haurogné K, Beveridge CA, Rameau C (2006) Branching genes are conserved across species: Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol 142 1014–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435 446–451 [DOI] [PubMed] [Google Scholar]
- Lazar G, Goodman HM (2006) MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proc Natl Acad Sci USA 103 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HM, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M (1993) Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364 161–164 [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle M (1996) Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J 10 403–413 [DOI] [PubMed] [Google Scholar]
- Li CJ, Herrera GJ, Bangerth F (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant 94 465–469 [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axrl mutants of Arabidopsis. Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28 465–474 [DOI] [PubMed] [Google Scholar]
- McKenzie MJ, Mett VV, Stewart Reynolds PH, Jameson PE (1998) Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter. Plant Physiol 116 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Turnbull CGN, Murfet IC, Beveridge CA (2001) Mutational analysis of branching in pea: evidence that Rms1 and Rms5 regulate the same novel signal. Plant Physiol 126 1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101 8039–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongaro V, Leyser O (2007) Hormonal control of shoot branching. J Exp Bot 59 67–74 [DOI] [PubMed] [Google Scholar]
- Park JY, Kim HJ, Kim J (2002) Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J 32 669–683 [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ (2005) Function and regulation of cullin-ring ubiquitin ligases. Nat Rev Mol Cell Biol 6 9–20 [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Lekanne Deprez LH, Moorman AFM (2003) Assumption-free analysis of quantitative real-time PCR data. Neurosci Lett 339 62–66 [DOI] [PubMed] [Google Scholar]
- Romano CP, Robson PR, Smith H, Estelle M, Klee H (1995) Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol 27 1071–1083 [DOI] [PubMed] [Google Scholar]
- Sachs T, Thimann K (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54 136–144 [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, Yamamoto Y, Nagasaki H, Inukai Y, Sato Y, Matsuoka M (2006) Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol 142 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G, Theres K (2005) Shoot and inflorescence branching. Curr Opin Plant Biol 8 506–511 [DOI] [PubMed] [Google Scholar]
- Schmülling T (2002) New insights into the functions of cytokinins in plant development. J Plant Growth Regul 21 40–49 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, Gray WM, Estelle M, Deng XW (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292 1379–1382 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Deng X (2002) Multiple ubiquitin-mediated processes require COP9 signalosome and AXR1 function. Plant Cell 14 2553–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JL, Napoli CA, Janssen BJ, Plummer KM, Snowden KC (2007) Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol 143 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ (2005) The Dad1/PhCCD8 gene affects branch production and has a role in leaf senescence, root growth and flower development. Plant Cell 17 746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogne K, Goussot M, Bainbridge K, Foo E, Chatfield SP, Ward S, Beveridge CA, Rameau C, et al (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Leyser HMO (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50 80–94 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, van de Sande K, Leyser HMO (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129 1131–1141 [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446 640–645 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45 1028–1036 [DOI] [PubMed] [Google Scholar]
- Thimann KV, Skoog F (1934) On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc R Soc Lond B Biol Sci 114 317–339 [Google Scholar]
- Tian Q, Reed JW (1999) Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126 711–721 [DOI] [PubMed] [Google Scholar]
- Tiryaki I, Staswick PE (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ (2001) AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13 2809–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull CG, Booker JP, Leyser O (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32 255–262 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1999) Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci USA 96 5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455 195–200 [DOI] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR (2002) Ethylene biosynthesis and signalling networks. Plant Cell (Suppl) 14 S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickson ME, Thimann KV (1958) The antagonism of auxin and kinetin in apical dominance. Physiol Plant 11 62–74 [Google Scholar]
- Wu K, Chen A, Pan ZQ (2000) Conjugation of Nedd8 to CUL1 enhances the ability of the ROC1-CUL1 complex to promote ubiquitin polymerization. J Biol Chem 275 32317–32324 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59 225–251 [DOI] [PubMed] [Google Scholar]
- Yang X, Lee S, So JH, Dharmasiri S, Dharmasiri N, Ge L, Jensen C, Hangarter R, Hobbie L, Estelle M (2004) The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant J 40 772–782 [DOI] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J (2001) Auxin modulates the degradation rate of AUX/IAA proteins. Proc Natl Acad Sci USA 98 11795–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Chen Z, Zhang S, Zhang W, Jiang G, Zhao X, Zhai W (2005) Characterizations and fine mapping of a mutant gene for high tillering and dwarf in rice (Oryza sativa L.). Planta 222 604–612 [DOI] [PubMed] [Google Scholar]
- Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L (2006) The rice HIGH-TILLERING DWARF1 encoding an orthologue of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J 48 687–696 [DOI] [PubMed] [Google Scholar]