Abstract
Learning-correlated plasticity at CA1 hippocampal excitatory synapses is dependent on neuronal activity and NMDA receptor (NMDAR) activation. However, the molecular mechanisms that transduce plasticity stimuli to postsynaptic potentiation are poorly understood. Here, we report that neurogranin (Ng), a neuron-specific and postsynaptic protein, enhances postsynaptic sensitivity and increases synaptic strength in an activity- and NMDAR-dependent manner. In addition, Ng-mediated potentiation of synaptic transmission mimics and occludes long-term potentiation (LTP). Expression of Ng mutants that lack the ability to bind to, or dissociate from, calmodulin (CaM) fails to potentiate synaptic transmission, strongly suggesting that regulated Ng–CaM binding is necessary for Ng-mediated potentiation. Moreover, knocking-down Ng blocked LTP induction. Thus, Ng–CaM interaction can provide a mechanistic link between induction and expression of postsynaptic potentiation.
Keywords: calmodulin, hippocampus, long-term potentiation, synaptic plasticity
Introduction
Synaptic plasticity is the ability of synapses to change their strength and is widely thought to underlie learning and memory. One of the best-characterized forms of synaptic plasticity is long-term potentiation (LTP) (Alkon and Nelson, 1990; Bliss and Collingridge, 1993; Chen and Tonegawa, 1997; Baudry, 1998; Elgersma and Silva, 1999; Kandel et al, 2000; Martin et al, 2000; Maren, 2001; Benfenati, 2007). At excitatory synapses in CA1 region of the hippocampus, LTP is NMDA receptor (NMDAR) dependent. During normal synaptic transmission, released glutamate from the presynaptic terminal activates primarily AMPA receptors (AMPARs). During the induction of LTP, depolarization of the postsynaptic cell takes place, resulting in the dissociation of Mg2+ from its binding site within NMDARs and allowing Ca2+ to enter the spine. The local increase in Ca2+ within the spine allows calmodulin (CaM) to be in its Ca2+-binding conformation and activates subsequent targets. A relatively large increase (a few micromolars) in Ca2+ concentration occurring over a short period of time (a few seconds) activates Ca2+/CaM-dependent protein kinase II (CaMKII), leading to the induction of LTP. Thus, CaM availability has a significant function in determining whether LTP can be induced.
It is now appreciated that CaM-binding proteins buffer CaM (whose concentration ranges from 10 to 100 μM in different areas of the brain, Biber et al, 1984), controlling its availability (Persechini and Stemmer, 2002; Tran et al, 2003; Sanabria et al, 2008). One of the most abundant (its concentration was estimated to be about 20 μM in the hippocampus) postsynaptic CaM-binding proteins is neurogranin (Ng) (Represa et al, 1990; Watson et al, 1994; Gerendasy et al, 1994a, 1994b; Alvarez-Bolado et al, 1996; Zhabotinsky et al, 2006). In the non-phosphorylated state and through its IQ motif, Ng binds to CaM. An increase in intracellular Ca2+ activates PKCγ, which phosphorylates a serine residue (S36) within the IQ motif of Ng, rendering it incapable of binding to CaM (Ramakers et al, 1995). Moreover, the increase in local Ca2+ per se can also dissociate CaM from Ng (Baudier et al, 1991; Huang et al, 1993, 2000; Gerendasy et al, 1995). Thus, through its ability to regulate the availability of CaM within the spine, Ng can be an essential element in LTP induction.
Two main views exist regarding the function of Ng in neurons. According to one view, Ng concentrates and targets CaM within the spine and enhances the probability of inducing LTP (Gerendasy, 1999; Prichard et al, 1999; Zhabotinsky et al, 2006). The other view, however, is that Ng negatively modulates CaM and constrains Ca2+/CaM-mediated signalling (Martzen and Slemmon, 1995; Krucker et al, 2002).
In this study, we explored the role of Ng in synaptic function and plasticity in CA1 hippocampal neurons. Using a combination of molecular biology, electrophysiology, and confocal and electron microscopy, we have found that Ng potentiates synaptic transmission in a regulated manner. This potentiation mimics LTP in that it is activity dependent, NMDAR dependent, and CaMKII dependent. We also show that Ng–CaM interaction is required for Ng-mediated potentiation. Therefore, our data suggest that CaM targeting within dendritic spines is essential for enhancing the sensitivity of the postsynaptic terminal. This work provides a mechanistic link between the stimuli of neuronal plasticity and postsynaptic potentiation.
Results
Ng potentiates synaptic transmission in an activity-dependent manner
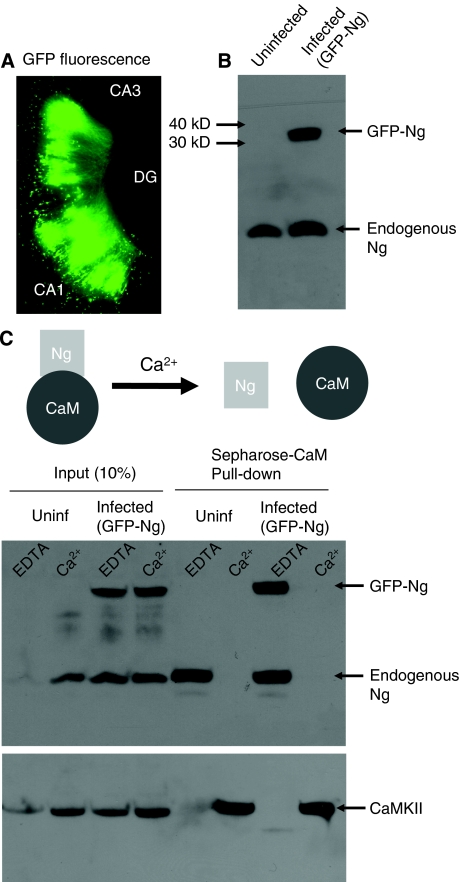
It has been shown that Ng concentrates in the dendritic spines (Watson et al, 1992; Neuner-Jehle et al, 1996). A computational study has also supported the need for a high protein concentration in the spines for the ability to induce LTP (Zhabotinsky et al, 2006). However, the exact molecular mechanism of Ng function is unclear. In particular, the significance of having more Ng in neurons has never been evaluated. As a first step to explore the role of Ng in synaptic function, we expressed GFP-tagged Ng using Sindbis virus in CA1 hippocampal neurons. GFP-Ng was expressed efficiently in hippocampal slice cultures (Figure 1A), as assayed by western blot analysis (Figure 1B). As the only known functional interaction for Ng is that to CaM, we wanted to test whether GFP-Ng binds to CaM in a Ca2+-dependent manner. To do so, we expressed GFP-Ng in CA1 hippocampal neurons. After expression, tissues were homogenized and incubated with CaM-sepharose beads in the presence of 2 mM EDTA or 2 mM Ca2+. The pull-down assay shows that GFP-Ng was able to bind to CaM only in the absence of Ca2+, similar to endogenous Ng (Figure 1C). This is consistent with earlier studies showing that Ng binds to CaM in a Ca2+-dependent manner (Baudier et al, 1991; Huang et al, 1993, 2000; Gerendasy et al, 1995). To further investigate whether there is a Ca2+-independent binding between Ng and CaM, a second elution step was performed in which the beads were boiled in an SDS-containing buffer. This elution step did not show any significant Ng–CaM binding that is Ca2+ independent (Supplementary Figure 1).
Figure 1.
GFP-tagged Ng binds CaM only in the absence of Ca2+. (A) Image of GFP-fluorescence signal from a hippocampal slice infected with a Sindbis virus expressing GFP-tagged Ng. The expression is limited to the CA1 area, in which the virus was delivered by extracellular injection. (B) Western blot analysis of the expression of Ng from slices injected with recombinant Ng (infected) and control (uninfected) slices. Recombinant Ng has the expected molecular weight (about 34 kDa) and is detected by anti-Ng antibody. (C) Western blot analysis of CaM ‘pull-down' assay from hippocampal extracts of uninfected (uninf) or GFP-Ng infected slices in the presence of EDTA or Ca2+. Note that both endogenous and recombinant Ng were pulled down only in the absence of Ca2+. As a control, the membrane was stripped and re-probed for CaMKII, which is pulled down with CaM, only in the presence of Ca2+ as expected.
The effect of GFP-Ng on synaptic transmission was evaluated by simultaneous double whole-cell recordings from pairs of nearby infected and uninfected neurons under voltage-clamp configuration. As shown in Figure 2A, Ng significantly increased AMPAR-mediated responses, without affecting NMDARs. To note, there is no correlation between the age of the slice culture, at the time of recording, and the ability of Ng to enhance synaptic transmission within the time frame used (Supplementary Figure 2).
Figure 2.
Ng enhances AMPAR-mediated synaptic transmission in an activity-dependent manner. (A–C) Left panels, sample traces of evoked AMPAR- and NMDAR-mediated synaptic responses recorded at −60 mV (the peak amplitude) and +40 mV (the amplitude at 60 ms latency, when AMPAR responses are decayed), respectively, as indicated with arrows. Scale bars, 40 pA, 40 ms. Data represent average evoked EPSCs recorded at −60 mV (left graph) or +40 mV (right graph) simultaneously from pairs of nearby uninfected (control) and Ng-infected (Ng) CA1 neurons without TTX or AP5 (A), in the presence of TTX (B) or in the presence of AP5 (C). (D) Left panel, sample traces of spontaneous mEPSCs recorded at −70 mV from uninfected neurons (control) or neurons expressing Ng (Ng) in the absence of AP5. Scale bars, 20 pA, 5 s. Data represent average mEPSC in the absence of AP5 (left graph) or in the presence of overnight (o.n.) AP5 added shortly after the viral injection (right graph), *indicates significance (P<0.05).
To test whether Ng-mediated potentiation in synaptic transmission is dependent on spontaneous activity, we carried out double whole-cell recordings from slices in which spontaneous activity was blocked with the sodium channel blocker tetrodotoxin (TTX, 1 μM) during GFP-Ng expression. Under these conditions, Ng failed to potentiate AMPAR-mediated responses (Figure 2B), indicating that activity is required for Ng-mediated potentiation.
We then tested whether Ng-mediated potentiation is dependent on NMDAR activation. Thus, we carried out simultaneous whole-cell double recordings from infected and uninfected neurons as described above. In these experiments, however, AP5 was added shortly after the local injection of Ng to block NMDARs. Under these conditions, Ng was unable to potentiate synaptic transmission (Figure 2C), indicating that such potentiation is NMDAR dependent.
As an independent method to test the function of Ng on synaptic transmission, we measured miniature excitatory postsynaptic currents (mEPSCs) in uninfected and Ng-expressing neurons. In agreement with the results shown in Figure 2A, Ng overexpression increases the mEPSC amplitude (Figure 2D). This increase in the mEPSC amplitude is also NMDAR dependent (Figure 2D, right panel).
Although Ng was expressed in postsynaptic CA1 neurons, we wished to test whether Ng indirectly interferes with presynaptic mechanisms. To this end, we measured paired pulse facilitation (PPF), a form of short-term synaptic plasticity and an indicator of presynaptic function, from control and Ng-expressing neurons. PPF is unaltered by Ng expression (Supplementary Figure 3). This result confirms that Ng-mediated potentiation is not because of a presynaptic effect.
Ca2+/CaMK signalling is required for Ng-mediated GluR1 synaptic insertion
In hippocampal CA1 neurons, expression of LTP is mediated by synaptic insertion of GluR1-containing AMPARs into the synapses (Hayashi et al, 2000). As Ng-mediated synaptic potentiation shares many of the characteristics of LTP (e.g. potentiates transmission in an activity- and NMDAR-dependent manner) and occludes LTP (Figure 7A), we wished to test whether Ng expression is capable of adding GluR1-containing AMPARs into the synapses. To do so, we used the biolistic method to cotransfect CA1 neurons with GFP-GluR1 and Ng. Delivery of GFP-GluR1 receptors to synapses is monitored using the inward rectification properties of the homomeric recombinant receptor (electrophysiological tagging) (Hayashi et al, 2000; Gerges et al, 2005). Synaptic delivery was then quantified as an increase in the ratio of the evoked postsynaptic current at −60 mV relative to the current at +40 mV (rectification index=I−60/I+40). As shown in Figure 3A, there was a significant increase in the rectification index, indicating that expression of Ng resulted in GluR1 synaptic delivery. These results further support the hypothesis that Ng, similar to CaMKII, mimics LTP. It is worth mentioning that Ng expression did not produce any change in rectification when GluR1 was not co-expressed (Figure 3A). Also, the expression of GluR1 alone does not change rectification (Figure 3A; Hayashi et al, 2000).
Figure 3.
CaM-dependent activation of CaMKII is required for Ng-mediated GluR1 synaptic insertion. (A) The rectification index was calculated as the ratio of the amplitude of the AMPAR-mediated responses at −60 mV over that at +40 mV and normalized to that of untransfected neurons (unt). Endogenous receptors conduct current at −60 and +40 mV, whereas recombinant receptors conduct only at negative potentials. Therefore, delivery of the recombinant GluR1 homomeric receptors is accompanied by the increase in the rectification index; n represents the number of cells; P is the probability value according to Student's t-test. Inset, sample traces of evoked AMPAR-mediated synaptic responses recorded at −60 and +40 mV from control or transfected cells as indicated. Scale bar, 20 pA and 20 ms. (B) Western blot analyses of synaptosomes and homogenates prepared from the CA1 area of control (uninfected) or Ng-infected hippocampal cultured slices. P is the probability value according to Student's t-test.
During synaptic potentiation, CaM is a key for the activation of CaMKII. It is thus not unreasonable to hypothesize that Ng targets more CaM within the spine, making the postsynaptic terminal more sensitive to local Ca2+ changes and leading to the activation of CaMKII, which, in turn, results in AMPAR insertion. To test whether CaM-dependent activation of CaMKII is required for the Ng-mediated insertion of GluR1, we incubated slices with KN-62, a potent inhibitor of CaM–CaMKII interaction that is needed for CaMKII activation, during the co-expression of GluR1 and Ng. As shown in Figure 3A, KN-62 completely abolished Ng-mediated GluR1 delivery. Similarly, KN-93, another potent inhibitor for CaM–CaMKII interaction, also blocked Ng-mediated GluR1 delivery. As a control, KN-92, a structurally similar compound to KN-93, but unable to block CaM–CaMKII interaction, did not interfere with Ng-induced GluR1 delivery. Although both KN-62 and KN-93 are potent inhibitors for CaMKII, it is important to note that they also inhibit CaMKI and CaMKIV equally well. However, based on the literature and the well-established Ca2+–CaM–CaMKII cascade in GluR1 insertion, CaMKII is the most likely CaMK involved. Thus, these results suggest that CaM-mediated activation of CaMKII is necessary for Ng-mediated GluR1 delivery.
Next, we tested whether Ng overexpression results in a higher population of constitutively active CaMKII. To test this possibility, CA1 regions were separated (15–24 h after Ng local injection in the CA1 area), homogenized, and processed for synaptosomal fractionation. Overnight expression of GFP-tagged Ng was sufficient to significantly increase the phosphorylated fraction of CaMKII in synaptosomes (Figure 3B), indicating higher levels of constitutively active CaMKII, than in control (uninfected) conditions. Interestingly, the increase in phospho-CaMKII was much smaller (and not significant) in total slice homogenates (Figure 3B), suggesting that Ng overexpression causes the increase in CaMKII activation preferentially in the synapses.
Ng–CaM interaction is required for Ng-mediated potentiation
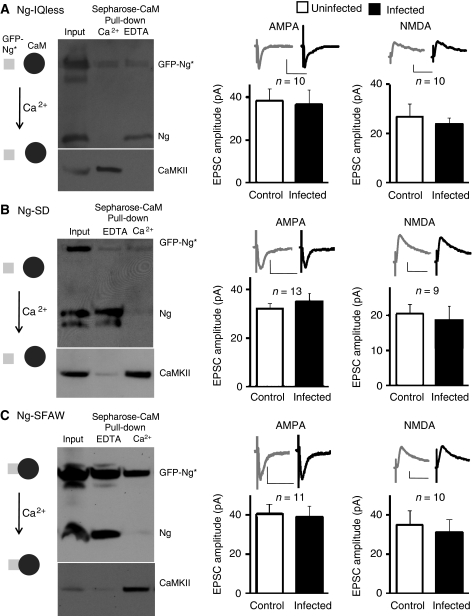
To directly test whether the binding of CaM to Ng is necessary for Ng-mediated potentiation of synaptic transmission, we generated a mutant of Ng that lacks the IQ motif (Ng-IQless). Removal of the IQ motif renders Ng incapable of binding to CaM (Figure 4A; see also Dominguez-Gonzalez et al, 2007). The effect of this mutant on AMPAR and NMDAR functions was evaluated by recording simultaneously from nearby infected and uninfected CA1 neurons (similar to those presented in Figure 2). As shown in Figure 4A, Ng-IQless failed to potentiate synaptic transmission, suggesting that Ng–CaM interaction is required for Ng-mediated potentiation.
Figure 4.
Ng–CaM interaction is required for synaptic potentiation. (A–C) Left panels, western blot analysis of CaM ‘pull-down' assay from hippocampal extracts of slices infected with Ng-IQless (A), Ng-SD (B), or Ng-SFAW (C) in the presence of EDTA or Ca2+, as indicated. Note that neither Ng-IQless nor Ng-SD was pulled down even in the absence of Ca2+, whereas Ng-SFAW was pulled down even in the presence of 2 mM Ca2+. Endogenous Ng was pulled down only in the absence of Ca2+ (similar to Figure 1C). As a control, the membranes were re-probed for CaMKII, which is pulled down only in presence of Ca2+. Data represent average evoked EPSCs recorded at −60 mV (left graphs) or +40 mV (right graphs) simultaneously from pairs of nearby uninfected (control) and infected neurons expressing Ng-IQless (A), Ng-SD (B), or Ng-SFAW (C). Insets, sample traces of evoked AMPAR- and NMDAR-mediated synaptic responses, as indicated.
It has been shown that the phosphorylation of the serine residue (S36) within the IQ motif prevents Ng from binding to CaM (Huang et al, 1993). Therefore, a single point mutation of this serine to aspartate (S36D) renders Ng to a mutant with a very low affinity to CaM (Gerendasy et al, 1994a). To further test the significance of CaM binding to Ng, we generated this single point mutation of Ng (Ng-SD). This Ng mutant is incapable of binding to CaM even in the absence of Ca2+ (Figure 4B). As shown in Figure 4B, Ng-SD failed to potentiate synaptic transmission, further supporting the need of Ng–CaM binding for Ng-mediated potentiation.
These data suggest that Ng may be targeting CaM within the dendritic spines and enhancing the postsynaptic sensitivity. Increasing postsynaptic local Ca2+ will thus dissociate CaM from Ng, allowing it to activate CaMKII. To directly test whether Ng's ability to release CaM on demand (i.e. with the increase of Ca2+ concentration) is required for Ng-mediated potentiation, we developed an Ng mutant that constitutively binds to CaM even in the presence of Ca2+. We developed this mutant based on some published observations: first, single amino-acid mutation in Ng in the phenylalanine residue (F37) to tryptophan rendered Ng to a mutant with high affinity to CaM in the presence of Ca2+ (Gerendasy et al, 1994a). In addition, we mutated serine 36 to alanine (S36A); thus preventing its phosphorylation-induced CaM dissociation. Figure 4C shows that unlike the endogenous Ng, GFP-tagged Ng-SFAW is pulled down with CaM even in the presence of high Ca2+ concentration (2 mM). Ng-SFAW was unable to enhance synaptic transmission, suggesting that Ng-mediated potentiation requires the ability of Ng not only to bind to CaM, but also to release it on demand. (To note, none of these Ng mutants or Ng wild type affected passive membrane properties of the infected cells, such as input resistance and holding current; see Supplementary Figure 4.)
CaM overexpression does not enhance synaptic transmission
To better understand the function of CaM in synaptic transmission, we wished to test the effect of CaM overexpression on AMPA and NMDAR function. In contrast to GFP-Ng expression and to our surprise, CaM overexpression was not able to potentiate synaptic transmission (Figure 5). This result indicates that increasing CaM per se does not increase synaptic transmission. This suggests that Ng may not be randomly distributed within the postsynaptic terminal and thus targeting CaM within the spine may be critical for Ng-mediated potentiation. To note, the expression level of GFP-CaM was comparable to that of GFP-Ng (Supplementary Figure 5).
Figure 5.
CaM does not change synaptic transmission. Inset, sample traces of evoked AMPAR- (left) and NMDAR(right)-mediated synaptic responses recorded at −60 and +40 mV, respectively. Scale bars, 40 pA, 40 ms. Left graph, comparisons of evoked AMPAR-mediated responses from CaM-infected and control neurons. Right graph, simultaneous recordings of evoked NMDAR-mediated responses from CaM-infected and control neurons.
Ultrastructural localization of Ng in dendritic spines
If Ng is acting as a recruiting or targeting factor for CaM, its location within the spine may impact its function. To this end, we wished to understand the precise ultrastructural localization of Ng. Therefore, we carried out post-embedding anti-Ng immunogold labelling on the synaptic CA1 region of the hippocampus. Most of the synapses examined contained Ng labelling (only 13% did not show immunogold labelling), with an abundant labelling in the postsynaptic terminal (Supplementary Figure 6A). To quantitatively determine the localization of Ng within the postsynaptic terminal, we used a method for quantification similar to the one described earlier (Racz and Weinberg, 2006). Briefly, we measured the shortest distance between each gold particle and the plasma membrane (within the spine) next to the postsynaptic density (PSD). This distance was normalized to the radial distance (the radius of the spine). As shown in Figure 6, the distribution of Ng within the spine is significantly different from that of a random distribution. Moreover, as shown in Figure 6, Ng is preferentially localized close to the plasma membrane. Further analysis revealed that 31.3% of Ng labelling was at the extrasynaptic plasma membrane next to the PSD (within 20 nm of the plasma membrane) and only 5.7% at the PSD (Supplementary Figure 6B). Interestingly, Ng localization at the extrasynaptic membrane (within the spine) reveals a non-uniform distribution, in which there is a minimal concentration midway between the PSD edge and the furthest tangential point (Supplementary Figure 6C), an area within the spine that is well characterized as the endocytic zone (Blanpied et al, 2002; Racz et al, 2004; Lu et al, 2007). Our data support a model in which Ng is not randomly distributed within the spine, strongly suggesting that it has a specific function in the localization or targeting of CaM within the spine.
Figure 6.
Ultrastructural organization of Ng in dendritic spines. Left, representative electron micrographs of post-embedding immunogold labelling. Gold particles were found specifically at the postsynaptic terminal and preferentially closer to the plasma membrane. (A) Cumulative probabilities of normalized radial distance. The distance of each gold particle from the plasma membrane (within the spine) has been normalized (x axis) to the radius of the spine. Thus, 0 corresponds to a particle lying on the membrane and 1 to a particle lying at the centre of the spine. The random distribution has been generated by using a random number generator macro in a spine-shaped surface. Ng distribution is significantly different from that of a random distribution (50% of randomly distributed particles are located within 0.45 of normalized lateral distance; unlike in the Ng case, in which 50% of the gold particles exist within 0.2 of the normalized lateral distance). P-value was calculated using Kolmogorov–Smirnov test. (B) Frequency histogram of the same data showing the highest peak for Ng distribution close to the plasma membrane. * in the electron micrographs denotes the presynaptic terminal; PSD, postsynaptic density.
Ng occludes LTP
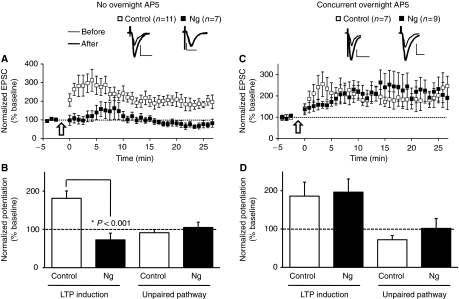
Our observation that Ng-mediated potentiation of synaptic transmission is dependent on activity and NMDAR activation suggests that overexpression of Ng may be redistributing or targeting CaM within the spine, making the spine more sensitive to local Ca2+ changes because of spontaneous activity and, therefore, enhancing AMPAR transmission in an LTP-like manner. This is also supported by the observation that Ng expression results in GluR1 delivery (Figure 3A). To investigate more directly the function of Ng in LTP induction, we evaluated the effect of GFP-Ng expression on LTP in CA1 hippocampal neurons. LTP was induced in infected (GFP-Ng expressing) and uninfected CA1 neurons by pairing presynaptic stimulation (3 Hz, 1.5 min) with postsynaptic depolarization (0 mV). As shown in Figure 7A and B, uninfected neurons exhibited robust LTP. In contrast, neurons expressing Ng did not exhibit LTP (Figure 7A and B). This resembles the effect of PSD95, whose expression enhanced synaptic transmission and occluded LTP (Stein et al, 2003). Similarly, expression of a constitutively active form of CaMKII also enhanced synaptic transmission and occluded LTP (Bach et al, 1995). Nonetheless, in the case of Ng expression, the synaptic potentiation is NMDAR dependent as evidenced by the lack of potentiation when AP5 was incubated during Ng expression (Figure 2C and D). We thus wished to test whether neurons expressing Ng will be able to display LTP if Ng-mediated potentiation is prevented by blocking NMDARs. We carried out LTP experiments similar to those described above. However, in these experiments, hippocampal slices were incubated with AP5, an NMDAR blocker, shortly after GFP-Ng viral delivery to CA1 neurons (slices were returned to the standard ACSF solution during the recordings, i.e. there was no AP5 in the ACSF). As shown in Figure 7C and D, neurons expressing Ng are able to induce LTP comparable to that in control-uninfected neurons. This strongly suggests that the lack of LTP induction because of Ng expression is an occlusion of LTP resulting from the Ng-mediated potentiation in synaptic transmission and that both Ng-mediated potentiation and LTP share a common mechanism. Nonetheless, because of the ability of Ng-expressing cells to produce LTP under these conditions (presence of overnight AP5), it is unlikely that the lack of LTP (in Figure 7A) is secondary to non-specific effects related to the viral gene delivery.
Figure 7.
Ng occludes LTP. (A, C) LTP was induced by pairing 3 Hz presynaptic stimulation (300 pulses) with 0 mV postsynaptic depolarization (indicated with an arrow) in CA1 neurons expressing Ng (black squares) or uninfected neurons (white squares). (C, D) Slices were incubated in AP5 shortly after the local viral delivery to prevent the potentiation of basal AMPAR responses in Ng-expressing neurons. Insets, sample traces of evoked AMPAR-mediated synaptic responses recorded at −60 mV before pairing (thin line) and 20 min after pairing (thick line) from control or infected cells as indicated. (B, D) Normalized average steady-state AMPAR-mediated responses in paired (LTP induction) and control (unpaired pathway) pathways in the absence (B) or presence (D) of overnight AP5. (A, B) Pairing significantly increased AMPAR-mediated responses in uninfected neurons (P<0.01), but not in those expressing Ng. (C, D) When slices were incubated with AP5 during expression, pairing significantly increased AMPAR-mediated responses in control as well as Ng-expressing neurons. AMPAR responses from control pathways were not statistically different from baseline responses.
Ng is required for LTP induction
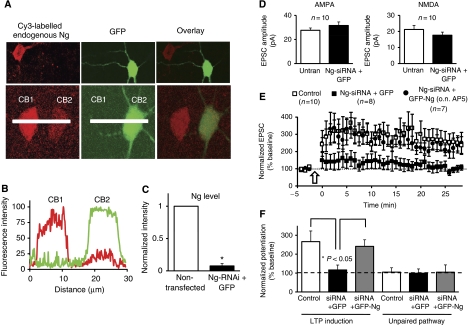
As an alternative method for testing the function of Ng in LTP, we used an RNA interference (RNAi) approach. We designed a unique 19-nucleotide sequence for Ng and used the pSuper RNAi system, which uses a mammalian expression vector that directs intracellular synthesis of small-interfering RNA (siRNA-)-like transcripts. This siRNA effectively down-regulates the expression of endogenous Ng in hippocampal neurons (Figure 8A–C). First, to test whether acutely knocking-down Ng alters synaptic transmission, we carried out simultaneous whole-cell double recordings from neurons co-expressing Ng-siRNA and GFP, and untransfected neurons. As shown in Figure 8D, Ng-siRNA did not significantly change AMPAR- or NMDAR-mediated responses.
Figure 8.
Ng is required for LTP induction. (A) Two representative confocal images from organotypic hippocampal slices. pSuper plasmid (containing Ng-siRNA) was co-expressed with GFP (to visualize the transfected cells) and 14 h later was immunostained for Ng (red signal) under permeabilized conditions. Note, cells transfected with the Ng-siRNA (green cells) have very low levels of endogenous Ng (red signal). (B) Representative line plot analysis of Ng (red signal) and GFP (green signal). CB1 is a cell body of non-transfected neuron; CB2 is that of a transfected neuron. (C) Normalized intensity from the line plots for Ng from 14 different pairs of cells; RNAi dramatically (>90%) and significantly (P=0.001) decreased Ng levels. (D) Simultaneous whole-cell double recordings from nearby pairs of untransfected (untran) neurons and those co-transfected with Ng-siRNA and GFP. Left graph, comparisons of evoked AMPAR-mediated responses (P=0.34). Right graph, simultaneous recordings of evoked NMDAR-mediated responses (P=0.21). (E) LTP was induced (as described in Figure 7) in CA1 neurons co-expressing Ng-siRNA and GFP (black squares), co-expressing Ng-siRNA and GFP-Ng in the presence of AP5 during the expression (black circles) or untranfected neurons (white squares; similar results were obtained in the presence or absence of overnight AP5 and the data were pooled together). Slices were returned to normal solution during recording (ACSF did not contain AP5 in any condition during recordings). (F) Normalized average steady-state AMPAR-mediated responses in paired (LTP induction) and control (unpaired pathway) pathways for the different conditions shown in (E).
To test the function of endogenous Ng on LTP, we co-expressed GFP and Ng-siRNA in organotypic hippocampal slices. LTP was induced as described above (see Figure 7). Knockdown of Ng completely abolished LTP induction (Figure 8E and F). Importantly, neurons co-expressing this Ng-siRNA and GFP-Ng produced robust LTP comparable to that of control untransfected neurons, ruling out off-target effects. It is important to note that Ng-siRNA is targeted against the 5′ untranslated region of Ng; thus, it knocks down the endogenous Ng, but not the recombinant GFP-Ng (in which the plasmid contains only the open reading frame). In this rescue experiment, we also blocked overnight activity to prevent any possible occlusion of LTP because of Ng overexpression. Therefore, for this experiment, AP5 was added shortly after the biolistic delivery of Ng and Ng-siRNA (similar to those in Figure 7C). The combined data, from the overexpression of Ng and that of Ng knockdown, strongly suggest that Ng is required and rate-limiting for LTP induction.
Discussion
CaM, which is a ubiquitous protein, participates in many signalling pathways within all eukaryotic cells when bound to Ca2+. In CA1 hippocampal neurons, synaptic NMDAR activation causes a local, fast Ca2+ increase in dendritic spines allowing CaM to activate downstream effectors and resulting in LTP. In this study, we examined the function of Ng, a postysynaptic CaM-binding protein, in synaptic transmission and plasticity.
Here, we show that the ability of Ng to interact with CaM within the dendritic spines is rate limiting for synaptic potentiation and is required for LTP induction. We also show that Ng-mediated potentiation mimics LTP. The former conclusion is based on three main experimental observations. First, although Ng expression potentiates synaptic transmission, mutants of Ng that are incapable of CaM binding (Ng-IQless and Ng-SD) were not able to enhance synaptic transmission. Second, a mutant of Ng that is incapable of releasing CaM with the increase in Ca2+ concentration (Ng-SFAW) lacks the ability to potentiate synaptic transmission. Third, acute knockdown of Ng, which is essential for CaM targeting within the spine, blocks LTP induction.
The conclusion that Ng-mediated potentiation mimics LTP is supported by six main experimental observations. (1) It is activity dependent as evidenced by its blockade by TTX. (2) It is NMDAR dependent. (3) Ng expression results in CaMKII activation specifically at synaptosomes. (4) Ng expression results in GluR1 insertion into the synapses. (5) Ng-mediated insertion of GluR1 is dependent on CaM–CaMKII interaction, which results in CaMKII activation. (6) Ng-mediated potentiation occludes LTP induction.
CaM is an abundant protein that has been under intensive study, as it contributes to the activation of a diverse array of signalling cascades. Interestingly, many of these interactions reveal an apparent paradox of requiring CaM to activate opposing targets. For example, in hippocampal neurons, two Ca2+/CaM-dependent enzymes are essential for the bidirectional balance between LTP and long-term depression (LTD). Ca2+/CaM-dependent protein phosphatase calcineurin is required for LTD (Klee et al, 1979; Hubbard and Klee, 1987; Mulkey et al, 1994; Torii et al, 1995; Zeng et al, 2001; Yasuda et al, 2003). On the other hand, CaMKII is required for LTP (Miller and Kennedy, 1985; Meyer et al, 1992; Silva et al, 1992; Giese et al, 1998; Hudmon and Schulman, 2002; Lisman et al, 2002; Kennedy et al, 2005; Shifman et al, 2006). Therefore, it has been postulated that the cells may regulate CaM-mediated signalling through the regulation of the availability of its targets or CaM itself. There is experimental evidence suggesting that the cell can regulate CaM signalling through regulating local CaM pools (Toutenhoofd and Strehler, 2000). Here, we propose that Ng spatially regulates the availability of CaM within the dendritic spine and thus favouring synaptic potentiation.
The requirement of Ng–CaM binding for Ng-mediated potentiation may suggest that Ng is enhancing transmission through a concomitant increase in the overall levels of CaM within the spine, thus increasing the sensitivity of dendritic spines to local Ca2+ changes. However, overexpression of CaM does not potentiate synaptic transmission. This is consistent with an earlier report showing that intracellular injection of 20 μM CaM did not change synaptic transmission (Wang and Kelly, 1995). Given the high levels of CaM in neurons, it is possible that the exogenous CaM in both cases was not high enough in dendritic spines to produce synaptic potentiation. However, the same concentration of CaM (20 μM) was able to produce potentiation when co-injected with 80 μM Ca2+ (Wang and Kelly, 1995), suggesting that the lack of effect on synaptic transmission when CaM was injected alone is unlikely to be due to the lack of enough exogenous CaM. Taken together, it is possible that Ng may be targeting CaM within the spine. Indeed, the ultrastructural localization of Ng shows that it is not randomly distributed and it is mainly localized close to the plasma membrane. This spatial localization may allow for preferential activation of targets necessary for LTP induction (e.g. CaMKII). On the other hand, an overall increase in CaM levels may not change the balance in the activities of the Ca2+/CaM-dependent enzymes that are essential in determining the synaptic plasticity balance (e.g. CaMKII and calcineurin). Therefore, we propose that changing Ng levels within the spine may provide a tool to spatially regulate the preferential localization of CaM within the spine and thus change subsequent signalling. Interestingly, there is a close correlation between Ng levels, calcineurin, and CaMKII, that is low Ng levels are correlated with high calcineurin and low CaMKII activity (Krazem et al, 2003a, 2003b; Alzoubi et al, 2005, 2006; Norris et al, 2005). Thus, a decrease in Ng in the spine may decrease CaM localized close to/at the plasma membrane within the dendritic spine and shifting the balance towards easier activation of calcineurin at the expense of CaMKII. On the other hand, increasing Ng at the dendritic spine shifts CaM localization close to the plasma membrane allowing higher localized concentration of CaM and enhancing the chance of CaMKII activation, whose affinity is several folds lower than that of calcineurin towards Ca2+/CaM (Miller and Kennedy, 1985; Hubbard and Klee, 1987; Meyer et al, 1992; Hudmon and Schulman, 2002). Further studies are warranted to explore these possibilities. Moreover, such targeting of CaM could happen in one of two ways: Ng may be recruiting more CaM into the spine, thus concentrating CaM within the dendritic spines. Alternatively, Ng may be redistributing CaM within the spine and targeting it close to or at the plasma membrane.
The findings that Ng-mediated potentiation is dependent on neuronal activity, NMDAR, and CaM binding suggest that Ng may act as a sensor to the Ca2+ signal, and increasing its levels within the spine may enhance the spine sensitivity. Thus, we have originally hypothesized that for Ng to be an effective sensor of local Ca2+ changes arising from NMDAR activation, its localization within the spine might be at or directly below the PSD. However, our immuno-EM data reveal a surprising distribution in which Ng is mainly localized extrasynaptically at the plasma membrane adjacent to, but not at, the PSD (Supplementary Figure 6B). This may suggest a functional role of extrasynaptic NMDARs in LTP induction. Interestingly, it has been suggested that extrasynaptic NMDARs can be activated after synaptic release (Hires et al, 2008). Further studies are needed to dissect out the function of extrasynaptic NMDARs in synaptic plasticity.
Two independent loss-of-function studies of Ng in mice argue for an important function of Ng in LTP induction (Pak et al, 2000; Krucker et al, 2002). However, these studies gave opposite results. One study showed an enhanced LTP by high-frequency stimuli, although the other knockout mice showed deficits in LTP induction. In both studies, the CaM-binding domain was completely deleted. The conflicting results with the Ng knockout studies may reflect different developmental problems and global changes in calcium buffering in neurons. For example, a computational study strongly suggests that the lack of Ng increases the probabilities of all Ca2+/CaM-dependent enzymes to be activated at low Ca2+ concentration (Kubota et al, 2007). Indeed, chronically eliminating Ng resulted in global changes in the activity of several enzymes and substrates (Wu et al, 2002). In this study, we have combined acute knockdown of Ng using siRNA and overexpression techniques to elucidate the role of Ng in synaptic function and plasticity. Our results indicate that Ng is required for LTP induction, and sufficient to produce potentiation that mimics LTP. Our results also show that Ng–CaM interaction, although critical for Ng-mediated potentiation, is not essential in maintaining synaptic transmission.
Our data support a model in which Ng targets CaM within the dendritic spine, and acts as a sensor to local Ca2+ changes. Under normal conditions, overnight spontaneous activity is not sufficient to produce synaptic potentiation. However, there is enough targeted CaM to respond to the high increase in the local Ca2+ induced by the LTP induction protocols, resulting in potentiation (see Figure 9 for illustration). In the absence of Ng, however, the same induction protocols are not able to induce LTP, as there is not enough CaM targeted (spatially regulated) within the spine to allow the proper activation of subsequent targets necessary for LTP induction. In cases in which there is increased local Ng in the spine, more CaM is targeted enhancing the spine sensitivity to spontaneous overnight activity. Under these circumstances, overnight activity is sufficient to produce potentiation that mimics LTP. This model focuses on the Ng–CaM interaction, which is clearly required for Ng-mediated effects. However, this does not exclude the importance or synergism of other possible CaM-independent effects of Ng. For example, earlier studies suggest that Ng can influence the free Ca2+ concentration (Krucker et al, 2002; Huang et al, 2004; Kubota et al, 2008). Further studies are warranted to test whether other mechanisms are involved in Ng-mediated potentiation in synaptic transmission.
Figure 9.
Ng enhances the sensitivity of the postsynaptic terminal. (A) Under normal condition, there is enough targeted CaM that LTP induction protocols result in an increase in the synaptic strength. (B) The lack of Ng, however, results in the loss of the spatial targeting of CaM; hence, LTP cannot be induced. (C) Increasing Ng within the spine redistributes CaM within the spine and/or recruits more CaM from the dendrite, enhancing the postsynaptic sensitivity. Under these conditions, overnight activity enhances synaptic transmission in an LTP-like manner.
This study also raises more questions regarding the details of Ng function in neurons. First, it is not clear how Ng concentrates in the dendritic spines. Earlier studies show that Ng concentrates in the dendritic spines (Watson et al, 1992; Neuner-Jehle et al, 1996). Moreover, an elegant computational study has also supported such a need for a higher concentration in the spines to be able to induce LTP (Zhabotinsky et al, 2006). As mRNA for Ng does exist locally at the dendrites and several studies have supported local protein synthesis on demand, for example local CaMKII synthesis after NMDAR activation (Scheetz et al, 2000; Aakalu et al, 2001), the concentration of Ng at the spine could be due to local synthesis. However, GFP-tagged Ng is also concentrated in the dendritic spines relative to the dendrites (Supplementary Figure 7). As the recombinant GFP-tagged Ng lacked the signal for the RNA to traffic to the dendrite, it is likely that Ng is anchored or retained within spines by an anchoring protein. Further studies are needed to elucidate the identity of such anchoring protein.
Once in the dendritic spine, Ng awaits for the Ca2+ increase, which is enough to release CaM to activate subsequent targets. What then is the function of Ng phosphorylation? Through its IQ motif, Ng binds to CaM (Deloulme et al, 1991; Gerendasy et al, 1994b). The phosphorylation of the serine residue (S36) within this IQ motif prevents Ng binding to CaM (Huang et al, 1993). Thus, it has been hypothesized that Ng phosphorylation is the event that controls CaM availability, as phosphorylated Ng does not bind to CaM. Therefore, on Ng phosphorylation, CaM is released and the synapse can be potentiated. This hypothesis has to be revisited.
As mentioned above, the increase in the local Ca2+ is the first event. Thus, it is most likely that CaM will be released from Ng even before Ng phosphorylation. Interestingly, a recent study showed that Ng binds to phosphatidic acid (PA) in a phosphorylation-dependent manner, that is Ng binds to PA only when it is unphosphorylated (Dominguez-Gonzalez et al, 2007). This may explain, at least partly, our finding that Ng accumulates near the plasma membrane. It is not unreasonable then to hypothesize that, although the local increase in Ca2+ triggers CaM release, the Ng phosphorylation event is important to recycle Ng to and from the plasma membrane. Further studies are needed to elucidate the function of Ng phosphorylation in synapses.
A third question that surfaces from this study is what function Ng has in synaptic plasticity as well as learning and memory deficits associated with ageing, Alzheimer's disease, schizophrenia, or hypothyroidism. Ng knockout mice exhibit spatial memory deficits. Moreover, low Ng levels are correlated with poor performance in the Morris water maze (Huang et al, 2004). In addition, many conditions that are associated with memory and synaptic plasticity deficits are also accompanied with decreased levels of Ng in pyramidal neurons, for example ageing and hypothyroidism (Iniguez et al, 1993; Piosik et al, 1995; Chang et al, 1997; Zoeller et al, 2000; Mons et al, 2001). Thus, there is a close correlation between decreased Ng levels and synaptic plasticity and memory deficits. Interestingly, a recent study shows that Ng is one of the major variants that correlates with schizophrenia, which may explain the cognitive deficits associated with schizophrenia (Stefansson et al, 2009). In instances in which a condition has been corrected (e.g. hypothyroidism by levothyroxin replacement therapy), Ng level and synaptic plasticity were normalized (Alzoubi et al, 2005). Nonetheless, agents that increase Ng levels (e.g. vitamin A) were capable of partially alleviating the ageing-induced deficits in synaptic plasticity and memory (Etchamendy et al, 2001). Our data also show that overexpression of Ng enhances synaptic transmission in an LTP-like manner, whereas knocking it down blocks LTP. Taken together, it is thus possible that reduced Ng levels may be having a function in the induced synaptic plasticity deficits in ageing and disease. Further studies will be needed to investigate any possible function of Ng in the induced plasticity deficits.
In conclusion, our results shed light into the important function of Ng in LTP induction and provide a direct link between CaM targeting within the dendritic spines, through Ng, and the postsynaptic potentiation.
Materials and methods
DNA constructs and expression
Ng and CaM were cloned by PCR from a commercial rat brain cDNA (Clontech). Appropriate in-frame GFP-fusion proteins were made with pEGFP plasmid (GFP was placed at the N-terminus). Ng-IQless mutant was cloned from the pEGFP-Ng plasmid by PCR in which amino-acids 30–45 were removed. For Ng mutants (S36D and S36A-F37W), we used the gene-tailor site-directed mutagenesis system from Invitrogen. Constructs were re-cloned into pSinRep5 for Sindbis virus preparation (Malinow et al, 1999). All recombinant plasmids have been verified by sequencing. Hippocampal slices were prepared from young rats (postnatal day 5 or 6) and cultured as described earlier (Gahwiler et al, 1997). After 2–7 days in culture, the recombinant gene was delivered into the slices. For the experiments shown in Figures 3A and 8, we used the biolistic delivery method (Lo et al, 1994), which allowed us to deliver two plasmids bearing mammalian promoters. For expression of single proteins, we used the Sindbis virus expression system, which is a replication-deficient, low-toxicity, and neuron-specific system (Malinow et al, 1999). The recombinant proteins were expressed for 36 h when GluR1 subunits were expressed (Figure 3A) or for 14 h in the rest of the cases. All biosafety procedures and animal care protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
RNA interference
Five different unique 19-nucleotide sequences targeting rat Ng were designed and pSuper RNAi system, which uses a mammalian expression vector that directs intracellular synthesis of siRNA-like transcripts, was used. The most potent siRNA sequence (CGCCCACCCTACAGAAAGT) was used to assay the effect of acute (14 h) Ng knockdown on LTP induction.
Biochemistry
Hippocampal extracts were prepared in homogenization buffer containing 4 mM HEPES, pH 7.4, 320 mM sucrose, 2 mM DTT, 2 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml chemostatin, 1 μg/ml antipain, and 1 μg/ml pepstatin. The homogenates were processed for synaptosomal fractionation as described earlier (Gerges et al, 2006). Antibodies used for western blot analysis were anti-Ng (Chemicon) and anti-CaM (Epitomics). Phosphorylation of CaM–CaMKII at Thr286 was analysed using phospho-specific anti-CaMKII and regular anti-α CaMKII antibodies (Chemicon) using the homogenization buffer described above supplemented with phosphatase inhibitors (10 mM NaF, 1 μM microcystin LR, and 0.5 μM calyculin A) and 50 mM EDTA.
CaM pull-down
Hippocampal extracts were prepared in homogenization buffer (150 mM NaCl, 20 mM Tris pH 7.5, 1 mM DTT, 1 μg/ml leupeptin, 1 μg/ml chemostatin, 1 μg/ml antipain, 1 μg/ml pepstatin, and 1% Triton X-100) containing either 2 mM EDTA (to chelate any Ca2+) or 2 mM Ca2+. These extracts were then incubated with CaM-sepharose beads (GE Healthcare) for 3 h at 4°C followed by three washes in homogenization buffer. Elution buffer contained either 10 mM CaCl2 (to elute Ca2+-sensitive CaM-binding proteins, e.g. Ng) or 10 mM EDTA (to elute Ca2+-dependent CaM-binding proteins, e.g. CaMKII).
Electrophysiology
Simultaneous double whole-cell recordings were obtained for nearby pairs of infected (fluorescent) and uninfected (non-fluorescent) neurons under visual guidance using differential interference contrast illumination. Synaptic responses were evoked with two bipolar electrodes placed on the Schaffer collateral (presynaptic axonal) fibres between 300 and 500 mm of the recorded cells. The responses obtained from the two stimulating electrodes were averaged for each cell and counted as an ‘n' of 1. As the expression is always in CA1 hippocampal pyramidal neurons and the stimulation is applied on Schaffer collateral fibres from CA3 neurons, this experimental configuration ensures that the potential effects of the expressed recombinant proteins are postsynaptic. The amplitude of the evoked excitatory postsynaptic current (EPSC) measured at −60 mV is a measure of the AMPAR-mediated responses. NMDAR-mediated responses, on the other hand, are measured at +40 mV at a latency when the AMPAR responses have fully decayed (60 ms after stimulation). The recording chamber was perfused with 119 mM NaCl, 2.5 mM KCl, 4 mM CaCl2, 4 mM MgCl2, 26 mM NaHCO3, 1 mM NaH2PO4, 11 mM glucose, 0.1 mM picrotoxin, and 2 μM 2-chloroadenosine at pH 7.4 and gassed with 5% CO2, 95% O2. Patch recording pipettes (3–6 MΩ) were filled with 115 mM cesium methanesulfonate, 20 mM CsCl, 10 mM HEPES, 2.5 mM MgCl2, 4 mM Na2ATP, 0.4 mM Na3GTP, 10 mM sodium phosphocreatine, and 0.6 mM EGTA at pH 7.25. Miniature EPSCs (Figure 2D) were recorded in the presence of 1 μM TTX and no adenosine. For rectification experiments (Figure 4A), 0.1 mM spermine was added in the intracellular solution, and 0.1 mM DL-2-amino-5-phosphonopentanoate (AP5) was present in the bath solution. LTP was induced by pairing 3 Hz presynaptic stimulation (300 pulses) with 0 mV postsynaptic depolarization. Voltage-clamp whole-cell recordings were acquired with a Multiclamp 700A amplifier (Axon Instruments).
Immunohistochemistry and confocal imaging
Confocal images of neurons co-transfected with Ng-siRNA and GFP (Figure 8A) or with GFP-Ng and RFP (Supplementary Figure 7) were taken with a Leica laser-scanning confocal microscope. ImageJ was used for three-dimensional reconstruction and fluorescence intensity quantification. Immunostaining for Ng (Figure 8A) was carried out in permeabilized conditions using anti-Ng and Cy3 coupled anti-rabbit (Jackson Immuno) antibodies. The fluorescence peak at the cell bodies was calculated after background substraction (Gerges et al, 2004, 2005).
Immunogold electron microscopy
Hippocampal slices were fixed and processed for osmium-free post-embedding immunogold labelling essentially as described earlier (Phend et al, 1995). Ng was labelled with anti-Ng antibody (Chemicon) and an anti-rabbit antibody coupled to 10-nm gold particles (electron microscopy sciences). Electron micrographs were obtained with a Joel EM-2100 transmission electron microscopy and an Orius SC 1000 CCD camera.
Statistical analysis
Comparison of electrophysiological responses between pairs of infected and uninfected neurons (Figures 2A–C, 4, 5 and 8D) was carried out using the paired non-parametric Wilcoxon test. Mean values of mEPSC (Figure 2D), red fluorescence in cell bodies (Figure 8C), rectification index of AMPAR synaptic responses (Figure 3A), and protein levels at synaptosomes or homogenates fractions (Figure 3B) were compared using two-side unpaired t-tests. Comparison of cumulative distributions (Figure 6A) was carried out with the Kolmogorov–Smirnov test. Error bars represent standard error of the mean in all figures.
Supplementary Material
Supplementary Figure 1–7
Review Process File
Acknowledgments
We thank Murtaza Khuzema, Christopher Stark, and Clive Wells for excellent technical assistance. We thank Joseph Besharse, Stephen Duncan, José Esteban, Cecelia Hillard, Brian Link, Qing-Song Liu, Margaret Wong-Riley, and members of Gerges laboratory for critical discussions of the data and review of the manuscript. We thank José Esteban for generating the macro used for the random distribution (Figure 6B). This work was supported by grants from US National Institute on Aging (AG032320), MCW Research Affairs Committee, Extendicare Foundation Inc., Alzheimer's Association and American Thyroid Association to NZG.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM (2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489–502 [DOI] [PubMed] [Google Scholar]
- Alkon DL, Nelson TJ (1990) Specificity of molecular changes in neurons involved in memory storage. FASEB J 4: 1567–1576 [DOI] [PubMed] [Google Scholar]
- Alvarez-Bolado G, Rodriguez-Sanchez P, Tejero-Diez P, Fairen A, Diez-Guerra FJ (1996) Neurogranin in the development of the rat telencephalon. Neuroscience 73: 565–580 [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Aleisa AM, Alkadhi KA (2006) Molecular studies on the protective effect of nicotine in adult-onset hypothyroidism-induced impairment of long-term potentiation. Hippocampus 16: 861–874 [DOI] [PubMed] [Google Scholar]
- Alzoubi KH, Gerges NZ, Alkadhi KA (2005) Levothyroxin restores hypothyroidism-induced impairment of LTP of hippocampal CA1: electrophysiological and molecular studies. Exp Neurol 195: 330–341 [DOI] [PubMed] [Google Scholar]
- Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M (1995) Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell 81: 905–915 [DOI] [PubMed] [Google Scholar]
- Baudier J, Deloulme JC, Van Dorsselaer A, Black D, Matthes HW (1991) Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem 266: 229–237 [PubMed] [Google Scholar]
- Baudry M (1998) Synaptic plasticity and learning and memory: 15 years of progress. Neurobiol Learn Mem 70: 113–118 [DOI] [PubMed] [Google Scholar]
- Benfenati F (2007) Synaptic plasticity and the neurobiology of learning and memory. Acta Biomed 78(Suppl 1): 58–66 [PubMed] [Google Scholar]
- Biber A, Schmid G, Hempel K (1984) Calmodulin content in specific brain areas. Exp Brain Res 56: 323–326 [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD (2002) Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36: 435–449 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Chang JW, Schumacher E, Coulter PM II, Vinters HV, Watson JB (1997) Dendritic translocation of RC3/neurogranin mRNA in normal aging, Alzheimer disease and fronto-temporal dementia. J Neuropathol Exp Neurol 56: 1105–1118 [DOI] [PubMed] [Google Scholar]
- Chen C, Tonegawa S (1997) Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Annu Rev Neurosci 20: 157–184 [DOI] [PubMed] [Google Scholar]
- Deloulme JC, Sensenbrenner M, Baudier J (1991) A rapid purification method for neurogranin, a brain specific calmodulin-binding protein kinase C substrate. FEBS Lett 282: 183–188 [DOI] [PubMed] [Google Scholar]
- Dominguez-Gonzalez I, Vazquez-Cuesta SN, Algaba A, Diez-Guerra FJ (2007) Neurogranin binds to phosphatidic acid and associates to cellular membranes. Biochem J 404: 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Silva AJ (1999) Molecular mechanisms of synaptic plasticity and memory. Curr Opin Neurobiol 9: 209–213 [DOI] [PubMed] [Google Scholar]
- Etchamendy N, Enderlin V, Marighetto A, Vouimba RM, Pallet V, Jaffard R, Higueret P (2001) Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J Neurosci 21: 6423–6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM (1997) Organotypic slice cultures: a technique has come of age. Trends Neurosci 20: 471–477 [DOI] [PubMed] [Google Scholar]
- Gerendasy D (1999) Homeostatic tuning of Ca2+ signal transduction by members of the calpacitin protein family. J Neurosci Res 58: 107–119 [PubMed] [Google Scholar]
- Gerendasy DD, Herron SR, Jennings PA, Sutcliffe JG (1995) Calmodulin stabilizes an amphiphilic alpha-helix within RC3/neurogranin and GAP-43/neuromodulin only when Ca2+ is absent. J Biol Chem 270: 6741–6750 [DOI] [PubMed] [Google Scholar]
- Gerendasy DD, Herron SR, Watson JB, Sutcliffe JG (1994a) Mutational and biophysical studies suggest RC3/neurogranin regulates calmodulin availability. J Biol Chem 269: 22420–22426 [PubMed] [Google Scholar]
- Gerendasy DD, Herron SR, Wong KK, Watson JB, Sutcliffe JG (1994b) Rapid purification, site-directed mutagenesis, and initial characterization of recombinant RC3/neurogranin. J Mol Neurosci 5: 133–148 [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Esteban JA (2004) Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem 279: 43870–43878 [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA (2006) Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J 25: 1623–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges NZ, Brown TC, Correia SS, Esteban JA (2005) Analysis of Rab protein function in neurotransmitter receptor trafficking at hippocampal synapses. Methods Enzymol 403: 153–166 [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ (1998) Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279: 870–873 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267 [DOI] [PubMed] [Google Scholar]
- Hires SA, Zhu Y, Tsien RY (2008) Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc Natl Acad Sci USA 105: 4411–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Chen HC (1993) Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch Biochem Biophys 305: 570–580 [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Jager T, Li J, Reymann KG, Balschun D (2004) Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J Neurosci 24: 10660–10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Li J, Schuck P, McPhie P (2000) Calcium-sensitive interaction between calmodulin and modified forms of rat brain neurogranin/RC3. Biochemistry 39: 7291–7299 [DOI] [PubMed] [Google Scholar]
- Hubbard MJ, Klee CB (1987) Calmodulin binding by calcineurin. Ligand-induced renaturation of protein immobilized on nitrocellulose. J Biol Chem 262: 15062–15070 [PubMed] [Google Scholar]
- Hudmon A, Schulman H (2002) Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71: 473–510 [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Rodriguez-Pena A, Ibarrola N, Aguilera M, Munoz A, Bernal J (1993) Thyroid hormone regulation of RC3, a brain-specific gene encoding a protein kinase-C substrate. Endocrinology 133: 467–473 [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM (2000) Principles of Neural Science. McGraw-Hill, New York: Appleton & Lange [Google Scholar]
- Kennedy MB, Beale HC, Carlisle HJ, Washburn LR (2005) Integration of biochemical signalling in spines. Nat Rev Neurosci 6: 423–434 [DOI] [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Krinks MH (1979) Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci USA 76: 6270–6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krazem A, Marighetto A, Higueret P, Jaffard R (2003a) Age-dependent effects of moderate chronic ethanol administration on different forms of memory expression in mice. Behav Brain Res 147: 17–29 [DOI] [PubMed] [Google Scholar]
- Krazem A, Mons N, Higueret P, Jaffard R (2003b) Chronic ethanol consumption restores the age-related decrease in neurogranin mRNA level in the hippocampus of mice. Neurosci Lett 338: 62–66 [DOI] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, McNamara RK, Lindsley KA, Dao A, Allison DW, De Lecea L, Lovenberg TW, Sutcliffe JG, Gerendasy DD (2002) Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus. J Neurosci 22: 5525–5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Putkey JA, Shouval HZ, Waxham MN (2008) IQ-motif proteins influence intracellular free Ca2+ in hippocampal neurons through their interactions with calmodulin. J Neurophysiol 99: 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Putkey JA, Waxham NM (2007) Neurogranin controls the spatiotemporal pattern of postsynaptic Ca2+/CaM signaling. Biophys J 93: 3848–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190 [DOI] [PubMed] [Google Scholar]
- Lo DC, McAllister AK, Katz LC (1994) Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 13: 1263–1268 [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD (2007) Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron 55: 874–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Hayashi Y, Maletic-Savatic M, Zaman S, Poncer J-C, Shi S-H, Esteban JA (1999) Introduction of Green Fluorescent Protein into Hippocampal Neurons through Viral Infection. In Imaging Neurons, Yuste R, Lanni F, Konnerth A (eds). Cold Spring Harbor, NY: Cold Spring Harbor Press [DOI] [PMC free article] [PubMed]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24: 897–931 [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23: 649–711 [DOI] [PubMed] [Google Scholar]
- Martzen MR, Slemmon JR (1995) The dendritic peptide neurogranin can regulate a calmodulin-dependent target. J Neurochem 64: 92–100 [DOI] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H (1992) Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science 256: 1199–1202 [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB (1985) Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem 260: 9039–9046 [PubMed] [Google Scholar]
- Mons N, Enderlin V, Jaffard R, Higueret P (2001) Selective age-related changes in the PKC-sensitive, calmodulin-binding protein, neurogranin, in the mouse brain. J Neurochem 79: 859–867 [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC (1994) Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369: 486–488 [DOI] [PubMed] [Google Scholar]
- Neuner-Jehle M, Denizot JP, Mallet J (1996) Neurogranin is locally concentrated in rat cortical and hippocampal neurons. Brain Res 733: 149–154 [PubMed] [Google Scholar]
- Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, Landfield PW, Kraner SD (2005) Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer′s models. J Neurosci 25: 4649–4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak JH, Huang FL, Li J, Balschun D, Reymann KG, Chiang C, Westphal H, Huang KP (2000) Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci USA 97: 11232–11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A, Stemmer PM (2002) Calmodulin is a limiting factor in the cell. Trends Cardiovasc Med 12: 32–37 [DOI] [PubMed] [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ (1995) An osmium-free method of epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem 43: 283–292 [DOI] [PubMed] [Google Scholar]
- Piosik PA, van Groenigen M, Ponne NJ, Bolhuis PA, Baas F (1995) RC3/neurogranin structure and expression in the caprine brain in relation to congenital hypothyroidism. Brain Res Mol Brain Res 29: 119–130 [DOI] [PubMed] [Google Scholar]
- Prichard L, Deloulme JC, Storm DR (1999) Interactions between neurogranin and calmodulin in vivo. J Biol Chem 274: 7689–7694 [DOI] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ (2004) Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci 7: 917–918 [DOI] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ (2006) Spatial organization of cofilin in dendritic spines. Neuroscience 138: 447–456 [DOI] [PubMed] [Google Scholar]
- Ramakers GM, De Graan PN, Urban IJ, Kraay D, Tang T, Pasinelli P, Oestreicher AB, Gispen WH (1995) Temporal differences in the phosphorylation state of pre- and postsynaptic protein kinase C substrates B-50/GAP-43 and neurogranin during long-term potentiation. J Biol Chem 270: 13892–13898 [DOI] [PubMed] [Google Scholar]
- Represa A, Deloulme JC, Sensenbrenner M, Ben-Ari Y, Baudier J (1990) Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci 10: 3782–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria H, Digman MA, Gratton E, Waxham MN (2008) Spatial diffusivity and availability of intracellular calmodulin. Biophys J 95: 6002–6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz AJ, Nairn AC, Constantine-Paton M (2000) NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci 3: 211–216 [DOI] [PubMed] [Google Scholar]
- Shifman JM, Choi MH, Mihalas S, Mayo SL, Kennedy MB (2006) Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated by calmodulin with two bound calciums. Proc Natl Acad Sci USA 103: 13968–13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Wang Y, Paylor R, Wehner JM, Stevens CF, Tonegawa S (1992) Alpha calcium/calmodulin kinase II mutant mice: deficient long-term potentiation and impaired spatial learning. Cold Spring Harb Symp Quant Biol 57: 527–539 [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD et al. (2009) Common variants conferring risk of schizophrenia. Nature 460: 744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V, House DR, Bredt DS, Nicoll RA (2003) Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci 23: 5503–5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii N, Kamishita T, Otsu Y, Tsumoto T (1995) An inhibitor for calcineurin, FK506, blocks induction of long-term depression in rat visual cortex. Neurosci Lett 185: 1–4 [DOI] [PubMed] [Google Scholar]
- Toutenhoofd SL, Strehler EE (2000) The calmodulin multigene family as a unique case of genetic redundancy: multiple levels of regulation to provide spatial and temporal control of calmodulin pools? Cell Calcium 28: 83–96 [DOI] [PubMed] [Google Scholar]
- Tran QK, Black DJ, Persechini A (2003) Intracellular coupling via limiting calmodulin. J Biol Chem 278: 24247–24250 [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT (1995) Postsynaptic injection of CA2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron 15: 443–452 [DOI] [PubMed] [Google Scholar]
- Watson JB, Sutcliffe JG, Fisher RS (1992) Localization of the protein kinase C phosphorylation/calmodulin-binding substrate RC3 in dendritic spines of neostriatal neurons. Proc Natl Acad Sci USA 89: 8581–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB, Szijan I, Coulter PM II (1994) Localization of RC3 (neurogranin) in rat brain subcellular fractions. Brain Res Mol Brain Res 27: 323–328 [DOI] [PubMed] [Google Scholar]
- Wu J, Li J, Huang KP, Huang FL (2002) Attenuation of protein kinase C and cAMP-dependent protein kinase signal transduction in the neurogranin knockout mouse. J Biol Chem 277: 19498–19505 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Higashi H, Kudo Y, Inoue T, Hata Y, Mikoshiba K, Tsumoto T (2003) Imaging of calcineurin activated by long-term depression-inducing synaptic inputs in living neurons of rat visual cortex. Eur J Neurosci 17: 287–297 [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S (2001) Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 107: 617–629 [DOI] [PubMed] [Google Scholar]
- Zhabotinsky AM, Camp RN, Epstein IR, Lisman JE (2006) Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J Neurosci 26: 7337–7347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Dowling AL, Vas AA (2000) Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 141: 181–189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1–7
Review Process File