Abstract
Objective:
In 2004, results from The Texas Medication Algorithm Project (TMAP) showed better clinical outcomes for patients whose physicians adhered to a paper-and-pencil algorithm compared to patients who received standard clinical treatment for major depressive disorder (MDD). However, implementation of and fidelity to the treatment algorithm among various providers was observed to be inadequate. A computerized decision support system (CDSS) for the implementation of the TMAP algorithm for depression has since been developed to improve fidelity and adherence to the algorithm.
Method:
This was a 2-group, parallel design, clinical trial (one patient group receiving MDD treatment from physicians using the CDSS and the other patient group receiving usual care) conducted at 2 separate primary care clinics in Texas from March 2005 through June 2006. Fifty-five patients with MDD (DSM-IV criteria) with no significant difference in disease characteristics were enrolled, 32 of whom were treated by physicians using CDSS and 23 were treated by physicians using usual care. The study's objective was to evaluate the feasibility and efficacy of implementing a CDSS to assist physicians acutely treating patients with MDD compared to usual care in primary care. Primary efficacy outcomes for depression symptom severity were based on the 17-item Hamilton Depression Rating Scale (HDRS17) evaluated by an independent rater.
Results:
Patients treated by physicians employing CDSS had significantly greater symptom reduction, based on the HDRS17, than patients treated with usual care (P < .001).
Conclusions:
The CDSS algorithm, utilizing measurement-based care, was superior to usual care for patients with MDD in primary care settings. Larger randomized controlled trials are needed to confirm these findings.
Trial Registration:
clinicaltrials.gov Identifier: NCT00551083
Major depressive disorder (MDD) is a common and debilitating psychiatric illness, with lifetime prevalence rates of up to 16.2%.1 Furthermore, it is well known that MDD is a chronic, recurring illness. According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), 50%–60% of patients who suffer a first-time major depressive episode will develop a second episode, and those with 2 or more prior episodes can almost be assured of having further episodes.2 Additionally, the presence of residual symptoms of depression is a strong predictor for relapse of major depression,3 and for this reason, full remission of symptoms is the goal of acute-phase treatment.4 However, based on recent “real-world” effectiveness trials in routine outpatient psychiatric and primary care clinics, remission rates following up to 14 weeks of standard citalopram antidepressant treatment are modest at 28%.5
The issue of inadequate remission rates for patients with MDD is compounded in primary care, where some reports suggest that 50%–70% of patients with MDD are unrecognized.6 In 2001, Young and colleagues7 also reported that only 1 in 5 patients with either a depressive or anxiety disorder received adequate treatment in primary care practice. To ensure more aggressive identification and treatment of MDD in primary care settings, the US Preventive Services Task Force recommended that all adult patients be screened for MDD in clinical practices that have systems in place to ensure accurate diagnosis of and effective treatment for MDD.8
The development of guidelines and algorithms has been the traditional solution to improve MDD treatment in primary care. Schulberg9 called for the use of treatment guidelines in regard to the psychopharmacologic treatment of depression in primary care. In recent years, a number of studies have consistently depicted the superiority of clearly defined, multifaceted treatment protocols in primary care settings over usual care models.10–13 The Texas Medication Algorithm Project (TMAP) was developed with the purpose of disseminating “real-world” pharmacologic treatment guidelines, founded on current evidence-based medicine (where available) and consensus expert opinion, for the treatment of psychiatric illnesses. The first iteration of the TMAP consensus guideline for nonpsychotic and psychotic MDD was developed in 1999 and was provided in a paper-and-pencil format.14 Trivedi and colleagues15 compared clinical outcomes of patients with MDD whose providers (psychiatrists in the public mental health sector) used TMAP guidelines to those of patients provided with usual care and found a statistically significant benefit for the guideline group. Even with these findings, research on the efficacy of guidelines suggests that development and simple dissemination alone are not sufficient to improve the quality of primary care.16 There has been considerable effort in promoting consensus guidelines created for multiple fields, but finding an effective implementation strategy continues to be a challenge. Furthermore, studies have consistently shown that the initial benefits of algorithm implementation in primary care are not sustained once the implementation support is withdrawn.10,11 For this reason, we decided to develop a computerized decision support system (CDSS) for implementation of the TMAP guidelines for depression (CompTMAP) to provide ongoing, sustained, point-of-care assistance to primary care clinicians.16
The current study was designed to assess the impact that a CDSS aligned with the TMAP MDD algorithm would have in aiding primary care physicians treating patients with major depression compared to colleagues providing usual care. The benefits of computerized decision support systems are far-reaching in other primary care settings, from improving clinical endpoints (ie, antibiotic use) to reducing unnecessary costs and procedures.17,18
METHOD
Design Overview
The objectives for this preliminary study were to establish feasibility and efficacy for implementing a CDSS to treat MDD in primary care. This was a prospective, proof-of-concept efficacy trial conducted from March 2005 through June 2006 comparing depressive symptom outcomes in 2 distinct outpatient groups treated by primary care physicians. Treatment groups were unblinded (ie, physicians and patients were aware of which intervention they belonged to); however, the depression symptom severity assessments were conducted by an independent rater separate from treatment teams/assignments.
Settings and Participants
Primary care physicians were assigned to treat MDD with either a CDSS or usual care. Four physicians from 3 private practice, primary care clinics in Texas agreed to participate in the study and provided informed consent; 2 physicians were assigned to usual care and 2 to CDSS. Outpatients aged 18 years and older were initially identified by their primary care physicians as having nonpsychotic MDD (DSM-IV criteria) on the basis of a routine clinical interview. Identified subjects then underwent a rigorous diagnostic interview conducted by an independent rater,19 who also interviewed the subjects using the 17-item Hamilton Depression Rating Scale20 (HDRS17). Patients with an HDRS17 score ≥ 14 who did not have a condition excluding them from the study were then consented and enrolled. Criteria excluding patients from participating in this study included (1) a current Axis I diagnosis of somatization disorder, anorexia nervosa, bulimia, or obsessive-compulsive disorder; (2) current alcohol or substance dependence; (3) women with a positive pregnancy test or who were lactating; (4) women of child-bearing potential who were not practicing a clinically accepted method of contraception; (5) general medical conditions that contraindicated antidepressant medications; and (6) a clinical status requiring inpatient or day hospital treatment.
Initially, 60 patients were approached to provide informed consent as approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, Dallas, Texas. Five patients were deemed ineligible for the study because their primary diagnosis was not MDD. Of the remaining 55 patients enrolled in the study, 32 were treated by physicians using the CDSS, and 23 were treated by physicians using usual care.
Interventions
Description of CDSS.
An in-depth explanation of the rationale and design of the CompTMAP program is provided elsewhere.21 In general, the CDSS for depression for the present study was based on an up-to-date model of CompTMAP that employs the principles of measurement-based care,5,22,23 while at the same time having a user interface for providers that is easy to use. Measurement-based care refers to the systematic use of measuring clinical outcomes at routine visits to guide treatment management. These outcomes may include symptoms, side effects, and medication adherence. Recent efforts from the large, multisite effectiveness study, Sequenced Treatment Alternatives to Relieve Depression (STAR*D),24,25 show that a treatment plan guided by measurement-based care is integral in implementing algorithm-based care.5 The initial treatment step in the CDSS group for this study was treatment with sertraline, and the initial treatment step in the usual care group was treatment with a selective serotonin reuptake inhibitor (SSRI) other than sertraline. Thereafter, treatment for patients in the CDSS group was guided by the TMAP algorithm.
Clinician training.
Before the study commenced, all 4 participating clinicians received a 1-hour lecture reviewing current guidelines for the pharmacologic treatment for depression (see Table 1). The CDSS group then received another 2-hour introductory teleconference followed by a 2-hour on-site training session focusing on the TMAP algorithm for MDD. The on-site session for the 2 clinicians assigned to the CDSS group included education on the program and hands-on practice with the CDSS. Simulated visits were created to illustrate how the CDSS would be used in routine practice. In addition, the CDSS clinicians received software support through the first few visits with actual study patients. The overall goals of training were (1) to assist physicians with becoming familiar with both the depression treatment algorithm and the CDSS, as well as (2) to emphasize the importance of measuring depressive symptoms at each visit. Each clinician assigned to CDSS was given a copy of the TMAP Manual for MDD.14 Protocol details, as well as how to access these materials, were also discussed during training. Regular teleconferences were set up to provide support and to assist in any further training on using the CDSS. We have extensive experience in training and monitoring adherence and fidelity to algorithm implementation through our ongoing R01 MH-164062-01A1 grant for examining the efficacy of the implementation of a computerized algorithm in tertiary care psychiatric outpatient clinics compared to a paper-and-pencil algorithm.26
Table 1.
Characteristics of the Treatment Interventions Used by Physicians in the Study of a CDSS for Depression
| Characteristic | Usual Care | CDSS for Depression |
| Initial 1-hour training on current depression guidelines | X | X |
| Independent assessment to confirm primary care patients with MDD | X | X |
| Independent rater assesses depression severity throughout study | X | X |
| Initial 2-hour training on TMAP manual for depression | X | |
| 2-Hour patient visit simulation integrating CDSS into routine practice | X | |
| Clinicians given a copy of TMAP manual for depression | X | |
| Regular follow-up teleconferences to technically assist CDSS | X |
Abbreviations: CDSS = computerized decision support system, MDD = major depressive disorder, TMAP = Texas Medication Algorithm Project.14
Outcomes
Subjects were followed longitudinally, rated at baseline and then every 6 weeks for a total of up to 24 weeks. The primary outcome, depression symptom severity, was measured using the HDRS17. In addition to the HDRS17, depressive symptomatology was also assessed using the 16-item Quick Inventory of Depressive Symptomatology–Self-Report27,28 (QIDS-SR16) and the 30-item Inventory of Depressive Symptomatology–Clinician-Rated28,29 (IDS-C30). Aside from the QIDS-SR16, which was based on patient self-assessment, all rating assessments were performed by an independent, blinded rater not associated with the treatment team.
Statistics
Baseline demographic and clinical characteristics between the 2 groups were tested using χ2 tests (or Fisher exact test when smallest expected cell count was < 5) for dichotomous measures and the Wilcoxon rank sum test for continuous measures (see Table 2). Similarly, treatment patterns were assessed using Fisher exact test for dichotomous variables and the Wilcoxon rank sum test for continuous measures (see Table 3). All significant tests reported are 2-tailed analyses with α set at .05.
Table 2.
Baseline Demographic Characteristics of Patients With Major Depressive Disorder (MDD)
| Variable | Usual Care (n = 23) | CDSS for Depression (n = 32) |
| Gender, female, n (%) | 22 (96) | 26 (81) |
| Ethnicity, n (%) | ||
| White | 20 (87) | 26 (81) |
| African American | 1 (4.3) | 2 (6.2) |
| Hispanic | 1 (4.3) | 1 (3.1) |
| Asian/other | 1 (4.3) | 0 (0) |
| Unknown | 0 (0) | 3 (9.4) |
| Age, mean ± SD, y | 52.6 ± 16.5 | 47.9 ± 13.5 |
| Age at onset of MDD, mean ± SD, y | 21.0 ± 16.9 | 28.1 ± 17.5 |
| No. of episodes, mean ± SD | 2.8 ± 1.3 | 2.4 ± 1.6 |
| No. with depression > 2 years, n (%) | 17 (74) | 23 (72) |
| Length of current episode, mean ± SD, mo | 88.0 ± 84.4 | 75.6 ± 59.1 |
| Baseline symptom severity scores, mean ± SD | ||
| HDRS17 | 18.6 ± 4.0 | 18.5 ± 3.0 |
| IDS-C30 | 34.3 ± 7.6 | 31.2 ± 5.9 |
| QIDS-SR16 | 12.7 ± 4.8 | 12.6 ± 4.1 |
Abbreviations: CDSS = computerized decision support system, HDRS17 = 17-item Hamilton Depression Rating Scale, IDS-C30 = 30-item Inventory of Depressive Symptomatology–Clinician-Rated, QIDS-SR16 = 16-item Quick Inventory of Depressive Symptomatology–Self-Report.
Table 3.
Treatment Patterns Among Intervention Groups
| Treatment Characteristic | Usual Care (n = 23) | CDSS for Depression (n = 32) | P |
| Received an adequate antidepressant dose, n (%)ab | 15 (68.2) | 22 (71.0) | .99c |
| Treatment switch (new antidepressant), n (%) | 2 (8.7) | 7 (21.9) | .27c |
| Treatment augmentation (algorithm approved), n (%) | 3 (13.0) | 4 (12.5) | .99c |
| No. of treatment visits, mean ± SD | 3.7 ± 1.7 | 5.0 ± 2.1 | .02d |
Adequate dose based on recommendations in the Texas Medication Algorithm Project for depression.14
Dosage data missing on 2 subjects (1 in the usual care group, 1 in the CDSS group).
P values for categorical variables were calculated using Fisher exact test; not significant (P > .05).
P value for mean treatment visits was calculated using Wilcoxon rank sum test.
Abbreviation: CDSS = computerized decision support system.
Analysis of the primary outcome measure, the change in symptom severity based on the HDRS17, was conducted using a hierarchical random regression model with a first-order autoregressive AR(1) covariate structure. For this model, patients were nested within physicians, and physicians were nested within the treatment group (ie, CDSS vs usual care). All analyses were conducted using the SAS (SAS Institute, Inc, Cary, North Carolina) MIXED procedure.30,31 The hierarchical random regression model was used to test for group differences on the secondary symptom outcomes (IDS-C30, QIDS-SR16) as well.
Response on the primary outcome was defined as a 50% decrease in symptom severity from baseline on the HDRS17. Remission on the primary outcome was defined as a score of 7 or less on the HDRS17.27 For secondary outcomes, response was defined as a ≥ 50% decrease in depressive symptom severity from baseline visit, with remission defined as a QIDS-SR16 score ≤ 5 and/or an IDS-C30 score ≤ 12.
RESULTS
Baseline Sample Demographics and Disease Characteristics
Of the 55 patients, 48 were female (87%), and based on the demographics of the practice populations, the vast majority were white. Table 2 provides a clinical summary of the demographic features of the cohort. The majority of participants also had a history of recurrent MDD (mean number of prior episodes for CDSS and usual care, 2.4 and 2.8, respectively). There were no statistically significant differences in baseline demographic and disease characteristics between the 2 groups in the sample.
Main Outcome Measures
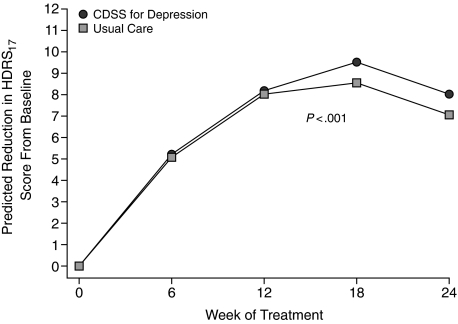
The primary outcome, change in mean HDRS17 score, as predicted by the random regression model, was superior among patients receiving CDSS vs usual care (P < .0001). Figure 1 illustrates the predicted mean HDRS17 scores over time for the 2 groups. Similarly, the secondary outcomes predicted by the random regression model, change in mean QIDS-SR16 (P < .0001) and IDS-C30 (P < .0001) scores, were significantly lower for the CDSS group than for usual care.
Figure 1.
Predicted Reduction in Mean HDRS17 Scores From Baseline for Patients Treated With CDSS and Usual Carea
aThe circles represent predicted values from the random regression model (which allows for computation of missing data) for patients treated with CDSS, and the squares represent the predicted values for patients treated with usual care.
Abbreviations: CDSS = computerized decision support system, HDRS17 = 17-item Hamilton Depression Rating Scale.
Rates of Response and Remission
The overall rates of response and remission were calculated for both the primary and secondary outcomes. The rates of response, based on the HDRS17, were comparable among the 2 groups, 59% for CDSS and 61% for usual care. In contrast, rates of remission based on the HDRS17 were slightly better for CDSS (44%) than for usual care (39%). The most striking differences in rates of response were seen in the QIDS-SR16, in which patients used a brief self-report measure to document depressive symptomatology. CDSS patients reported a 41% response rate based on the QIDS-SR16 compared to 26% reported by the usual care patients. Response and remission rates for the IDC-C30 displayed no differences between treatment groups.
Evaluation of Patterns of Treatment
Table 3 represents treatment patterns among the 2 interventions. The groups did not differ significantly with regards to the number of subjects attaining an adequate dose of antidepressant medication. The use of either switching or augmentation also did not significantly differ between the 2 groups.
The one significant difference between treatment groups came in relation to the number of treatment visits. On average, the CDSS group had a significantly higher mean number of treatment visits with their physicians during the study period than their usual care counterparts (5.0 vs 3.7, P = .02). The proportion of dropouts at study week 12 was roughly equivalent between CDSS and usual care (19% vs 17%, respectively).
DISCUSSION
On the basis of the primary outcome, it appears that computerized decision support systems employing the TMAP algorithm for MDD can be an effective tool for primary care physicians treating MDD. Furthermore, physician self-report indicated that CDSS was easy to use and preferable over usual care. In spite of the small sample size, patients treated by primary care physicians employing CDSS had significantly greater mean symptom reduction on all 3 depressive symptomatology measures compared with usual care. This was true on the basis of clinician-rated as well as self-reported symptom rating scales. While both CDSS and usual care appear to have an increase in symptom severity at study end (Figure 1), this is likely the result of dropouts in both sample groups who had achieved a positive response to treatment prior to week 24. Thus, those remaining in the study at week 24 were more likely to have higher depressive symptom severity scores. A post hoc analysis revealed that of the 36 total persons still enrolled in the study at week 18, half (18) had a treatment response, and 5 of these responders did not return for their week 24 visit, whereas only 2 of the 18 who did not have a treatment response did not return for their week 24 visit.
To establish a measure of clinical significance, we have also calculated the number needed to treat (NNT), ie, the number of patients needed to treat using the CDSS for depression intervention to gain a clinically meaningful outcome (remission). To make these results interpretable, they were rounded to the nearest integer. For most instances, including the HDRS17, this NNT was negligible (HDRS17 remission-based NNT = 21). Further, given the small initial sample size of the cohort coupled with the number of dropouts at study end, all estimates and conclusions from response rates, remission rates, and NNT must be tempered.
In general, the gross measures of quality of care did not show differences between the 2 groups. No significant between-group difference existed in the number of patients that were prescribed an “adequate dose” of their antidepressant medication, with both groups being well above expected rates.32,33 While differences between groups regarding augmentation and switch strategies were not statistically significant, it should be noted that group differences in categorical variables are difficult to capture due to the small sample size, and a larger sample is necessary to make this distinction. However, the mean number of study visits did significantly differ between the 2 groups. Since visit frequency is an integral piece of the computerized TMAP algorithm recommendations, we feel this is a measure of the CDSS clinicians’ adherence to the algorithm guidelines. This information supports prior findings indicating that structured and more frequent visits that are tailored using measurement-based care account for improved symptom efficacy.5 Additionally, visit frequency, particularly early in the course of treatment, is the standard for pharmacologic therapy for depression based on the National Committee on Quality Assurance's Health Plan Employer Data and Information Set (HEDIS) standards.34
The primary objective of this study was to assess the feasibility of introducing a point-of-care decision support tool to assist physicians with the treatment and management of major depression. The results of this study demonstrate that CDSS may be a successful tool for primary care physicians in real-world clinical practice. Recent studies have also shown that, when adhered to, treatment algorithms vastly improve clinical depression outcomes, reducing the occurrence of future depressive episodes.35
Study Limitations
The study was limited, however, by the sample size and the number of participants who dropped out prior to week 24. Week 24 was chosen as the endpoint for the primary outcome in order to give the CDSS physicians enough time to employ at least 2 treatment steps, including the first SSRI treatment, based on the TMAP algorithm. On the other hand, given the small sample size and the equal proportion of dropouts in each treatment arm, the treatment effect we see in this preliminary study is all the more encouraging. Additional study limitations include potential bias posed by treatment groups that were unblinded to study physicians, different starting antidepressants in each group (sertraline in CDSS and other SSRIs in usual care), and the CDSS physicians’ receiving additional training utilizing the computer algorithm. Of note, however, both groups were given copies of the TMAP algorithm, and both received an initial depression guideline training session. Larger randomized controlled trials are needed to definitively confirm the results of this study in primary care and to test their validity in other practice settings.
Our results are consistent with some other studies assessing depression interventions in primary care settings,10,11,36 but differ from a recently published report from primary care clinics in a US Department of Veterans Affairs medical center.37 Of note, however, is that prior studies assessing depression in primary care have included multifaceted treatment interventions in addition to a treatment algorithm (ie, patient education, trained nurses assisting follow-up, and psychiatric consultations).10–13 Our results are unique in that this is the first study to assess the impact of a computerized decision support system utilized directly by primary care physicians in the treatment visit (without other care components). While prior studies have shown that multifaceted collaborative depression interventions in primary care settings are vitally important, they have also shown a propensity toward a slight increase in outpatient costs.10,11,36 These studies, however, included other treatment components and were not computerized decision support systems, which in other fields of medicine have been shown to reduce medical and prescription costs.17,18 Incorporating a CDSS integrated with tools of measurement-based care and the TMAP algorithm could provide a lower-cost, more efficacious depression treatment option for primary care settings.
Future Direction
Future studies to explore the feasibility of implementing a CDSS in large primary care and mental health settings are necessary to further discern the impact of measurement-based care in routine clinical practice. Our goal is to develop dissemination approaches to easily incorporate adaptive guidelines while minimizing user burden.
Drug names: citalopram (Celexa and others), sertraline (Zoloft and others).
Financial disclosure: Dr Kurian and Mr Grannemann have received grant support from the National Institute of Mental Health (NIMH). Dr Trivedi has been a consultant for Abbott, Akzo (Organon), AstraZeneca, Bayer, Bristol-Myers Squibb, Cephalon, Cyberonics, Fabre-Kramer, Forest, GlaxoSmithKline, Janssen, Johnson & Johnson PRD, Eli Lilly, Meade Johnson, Neuronetics, Parke-Davis, Pfizer, Pharmacia & Upjohn, Sepracor, Solvay, VantagePoint, and Wyeth-Ayerst; has served on speakers’ bureaus for Abdi Brahim, Akzo (Organon), Bristol-Myers Squibb, Cephalon, Cyberonics, Forest, GlaxoSmithKline, Janssen, Eli Lilly, Pharmacia & Upjohn, Solvay, and Wyeth-Ayerst; and has received grant support from Bristol-Myers Squibb, Cephalon, Corcept Therapeutics, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Merck, NIMH, National Alliance for Research in Schizophrenia and Depression, Novartis, Pfizer, Pharmacia & Upjohn, Predix, Solvay, and Wyeth-Ayerst. Dr Daly has received research grant support from Eli Lilly. Dr Sunderajan has received research grant support from Eli Lilly, Bristol-Myers Squibb, Takeda, Forest, and Wyeth. Dr Claassen reports no financial disclosure.
Funding/support: This work is supported by R01 MH-164062-01A1, Computerized Decision Support System for Depression, awarded through the NIMH to Dr Trivedi, Principal Investigator; T32 MH067543-05; and a grant from Pfizer Pharmaceuticals. Neither NIMH nor Pfizer performed any role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; nor the preparation, review, or approval of the manuscript.
Previous presentation: Initial results from this study were also presented at the 47th annual meeting of the New Clinical Drug Evaluation Unit, June 2007, Boca Raton, Florida.
Acknowledgments
The authors thank the medical and clinic staff at the Iron Mountain Medical Center in Madisonville, Texas; the Wendover Family Medicine Clinic in Odessa, Texas; and the Hillcrest Family Health Center in Waco, Texas.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Press, Inc; 2000. Text Revision. [Google Scholar]
- 3.Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Kraemer HC, Sackeim HA, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 6.Coyne JC, Schwenk TL, Fechner-Bates S. Nondetection of depression by primary care physicians reconsidered. Gen Hosp Psychiatry. 1995;17(1):3–12. doi: 10.1016/0163-8343(94)00056-j. [DOI] [PubMed] [Google Scholar]
- 7.Young AS, Klap R, Sherbourne CD, et al. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58(1):55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]
- 8.US Preventive Services Task Force (USPSTF) Screening for depression: recommendations and rationale. Ann Intern Med. 2002;136:760–764. doi: 10.7326/0003-4819-136-10-200205210-00012. [DOI] [PubMed] [Google Scholar]
- 9.Schulberg HC. Treating depression in primary care practice: applications of research findings. J Fam Pract. 2001;50(6):535–537. [PubMed] [Google Scholar]
- 10.Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56(12):1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 11.Katon W, Von Korff M, Lin E, et al. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273(13):1026–1031. [PubMed] [Google Scholar]
- 12.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 13.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 14.Crismon ML, Trivedi M, Pigott TA, et al. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry. 1999;60(3):142–156. [PubMed] [Google Scholar]
- 15.Trivedi MH, Rush AJ, Crismon ML, et al. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch Gen Psychiatry. 2004;61(7):669–680. doi: 10.1001/archpsyc.61.7.669. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi MH, Kern JK, Marcee A, et al. Development and implementation of computerized clinical guidelines: barriers and solutions. Methods Inf Med. 2002;41(5):435–442. [PubMed] [Google Scholar]
- 17.Evans RS, Pestotnik SL, Classen DC, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338(4):232–238. doi: 10.1056/NEJM199801223380406. [DOI] [PubMed] [Google Scholar]
- 18.McMullin ST, Lonergan TP, Rynearson CS, et al. Impact of an evidence-based computerized decision support system on primary care prescription costs. Ann Fam Med. 2004;2(5):494–498. doi: 10.1370/afm.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer RL, Williams JB, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID), pt 1: history, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 21.Trivedi MH, Kern JK, Grannemann BD, et al. A computerized clinical decision support system as a means of implementing depression guidelines. Psychiatr Serv. 2004;55(8):879–885. doi: 10.1176/appi.ps.55.8.879. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi MH, Daly EJ. Measurement-based care for refractory depression: A clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(suppl 2):S61–S71. doi: 10.1016/j.drugalcdep.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trivedi MH, Rush AJ, Gaynes BN, et al. Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR(*)D measurement-based care. Neuropsychopharmacology. 2007;32(12):2479–2489. doi: 10.1038/sj.npp.1301390. [DOI] [PubMed] [Google Scholar]
- 24.Fava M, Rush AJ, Trivedi MH, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 25.Rush AJ, Fava M, Wisniewski SR, et al. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi MH, Claassen CA, Grannemann BD, et al. Assessing physicians’ use of treatment algorithms: project IMPACTS study design and rationale. Contemp Clin Trials. 2007;28(2):192–212. doi: 10.1016/j.cct.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 29.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 30.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19(13):1793–1819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 31.Singer J. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–355. [Google Scholar]
- 32.Simon GE, Von Korff M, Rutter CM, et al. Treatment process and outcomes for managed care patients receiving new antidepressant prescriptions from psychiatrists and primary care physicians. Arch Gen Psychiatry. 2001;58(4):395–401. doi: 10.1001/archpsyc.58.4.395. [DOI] [PubMed] [Google Scholar]
- 33.Simon GE, VonKorff M, Wagner EH, et al. Patterns of antidepressant use in community practice. Gen Hosp Psychiatry. 1993;15(6):399–408. doi: 10.1016/0163-8343(93)90009-d. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Quality Assurance The state of health care quality: 2006. Antidepressant Medication Management. Available at: http://www.ncqa.org/communications/SOHC2006/SOHC_2006.pdf. Accessed September 8, 2008
- 35.Hepner KA, Rowe M, Rost K, et al. The effect of adherence to practice guidelines on depression outcomes. Ann Intern Med. 2007;147(5):320–329. doi: 10.7326/0003-4819-147-5-200709040-00007. [DOI] [PubMed] [Google Scholar]
- 36.Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53(10):924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 37.Dobscha SK, Corson K, Hickam DH, et al. Depression decision support in primary care: a cluster randomized trial. Ann Intern Med. 2006;145(7):477–487. doi: 10.7326/0003-4819-145-7-200610030-00005. [DOI] [PubMed] [Google Scholar]