Abstract
Malignant transformation of epithelial cells is frequently associated with the alteration of glycosylation pathways. Tn is a common tumor-associated carbohydrate antigen present in 90% of human carcinomas and its expression correlates with metastatic potential and poor prognosis. Despite its relevance, the functional role of Tn in tumor biology has not been firmly established probably for the lack of appropriate experimental tools. Our aims were to produce highly reactive monoclonal antibodies against Tn making use of synthetically produced Tn and to test their usefulness for in vivo imaging as well as to define their potential functional activity in tumor cell spread. We immunized mice with Tn clustered on cationized BSA and screened the positive hybridomas with Tn-biotinylated alginate. Enzyme-linked immuno sorbent assay and immunofluorescence assays revealed that the most reactive anti-Tn IgM mAb (2154F12A4) selectively recognized Tn on the MCF7 breast cancer cell line since its binding to the cell membrane was completely abolished by preincubation with purified Tn. Importantly, QDot 800-conjugated mAb injected in MCF7-tumor bearing mice specifically bound to primary tumor lesions as well as to metastases in lymph nodes. In addition, this mAb was able to inhibit cancer cell adhesion to lymphatic endothelium suggesting a novel involvement of Tn in the lymphatic dissemination of cancer cells and hypothesizing future applications in inhibiting lymphatic metastases.
Keywords: in vivo imaging, lymphatic endothelial cells, metastasis, monoclonal antibody, Tn antigen
Introduction
Altered glycosylation is a hallmark of cancer cells (Brooks et al. 2008). The so-called tumor-associated carbohydrate antigens (TACAs) are structures originated during neoplastic transformation from changes in multiple genes within the glycosylation pathways. They greatly contribute to the malignant phenotype of cancer cells (Escrevente et al. 2006; Serpa et al. 2006; Sewell et al. 2006), influencing functional processes such as cell adhesion, migration, and metastasis (Baldus et al. 2004). The most common TACAs are Tn and TF antigens as well as their sialylated forms. They derive from an incomplete elongation of O-glycan saccharide chains leading to the expression of shorter carbohydrate structures in multiple glycoproteins and in mucins (Springer 1984; Slovin et al. 2005; Galonic and Gin 2007). Both Tn and TF have been used clinically as prognostic indicators of cancer and have been detected immunologically in primary breast cancer tissues, lymph nodes, and distant metastases (Kumar et al. 2005). Whereas the function of Tn remains unclear, TF has been shown to be involved in cancer cell proliferation, adhesion, and migration and recent evidences suggest that TF may be actively involved in tumor metastasis promoting several key cell–cell interactions (Yu 2007). Tn (GalNAc-α-O-Ser/Thr: the innermost O-linked structure), an early biomarker of cancer, both in humans (Li et al. 2009) and in animal models (Babino et al. 2000), which is usually masked by additional sugar residues in normal tissues, was characterized as one of the most specific human cancer-associated structures, and it was detected in 90% of human carcinomas (Springer 1997). Tn and its sialylated derivate STn correlated with metastatic potential and poor prognosis in many cancers (Kawaguchi 2005) including cervical (Terasawa et al. 1996), lung (Laack et al. 2002), colorectal (Konno et al. 2002), breast (Fernandez Madrid et al. 2005), and gastric carcinomas (Pinho et al. 2007). Moreover, Tn induced an effective immune response against cancer cells (Lo-Man et al. 2004; Ingale et al. 2007; Li et al. 2009).
The adhesion of cancer cells to the vascular endothelium is an essential rate-limiting step in cancer metastasis. The current knowledge of the molecular mechanism of cancer cell adhesion to endothelium is largely derived from studies performed on blood endothelial cells (BECs) highlighting the involvement of a wide array of molecules, such as cadherins, integrins, Ig class of cell adhesion molecules, selectins, carbohydrates, and their lectin receptors (Miles et al. 2008). In particular, the interaction between TF and Galectin-3, a galactoside-binding lectin (Liu and Rabinovich 2005), expressed on BECs has been shown to promote the adhesion of breast cancer cells to blood endothelium (Glinsky et al. 2000, 2001, 2003; Khaldoyanidi et al. 2003; Zou et al. 2005; Yu et al. 2007). Consequently, an anti-TF mAb inhibited in vivo breast cancer metastasis (Heimburg et al. 2006). In contrast, the molecular mechanisms underlying cancer cells and lymphatic endothelium adhesion are still poorly understood. It was demonstrated that changes in the expression of cell adhesion molecules by lymphatic endothelial cells (LECs) in inflammation may promote tumor metastasis via lymphatics. Several studies suggest that CLEVER-1 and macrophage mannose receptor I (Irjala et al. 2003; Marttila-Ichihara et al. 2008), as well as ICAM-1 (Kawai et al. 2009), may be important mediators of cancer cell adhesion to the lymphatic endothelium. Moreover, the expression of Vicia villosa agglutinin-binding carbohydrates correlated with the aggressiveness of breast cancer hypothesizing that Tn was involved in lymphatic metastasis (Kawaguchi et al. 2006).
Altogether the above-mentioned evidences suggest that Tn is a valuable target for early diagnosis and for the development of novel therapeutic approaches of neoplastic diseases. Active immunotherapy, using vaccines targeted toward Tn antigen, has already been evaluated with promising results (Slovin et al. 2003; Lo-Man et al. 2004; Freire et al. 2006; O’Boyle et al. 2006; Ingale et al. 2007; Gilewski et al. 2007; Li et al. 2009). A number of anti-Tn IgG and IgM antibodies have been generated (Hirohashi et al. 1985; Takahashi et al. 1988; Numata et al. 1990; O’Boyle and Wright 1994; Avichezer et al. 1997; Oppezzo et al. 2000; Kannagi and Hakomori 2001; Ando et al. 2008) and the anti-tumor activities of some of them already reported (Takahashi et al. 1988; Avichezer et al. 1997; Oppezzo et al. 2000; Ando et al. 2008).
The first aim of the present investigation was to produce highly reactive mAbs against Tn making use of novel conjugated compounds of Tn antigen. The second aim of the study was to determine if the mAbs produced were capable to detect tumor cells in vivo and if they display any functional activity. We succeeded in isolating a specific mAb against Tn. This antibody reacted in vitro and in vivo with a Tn-expressing breast cancer cell line, MCF7 (Valentiner et al. 2005). Importantly, when injected in MCF7-tumor bearing mice, this mAb specifically bound to primary tumor lesions as well as to lymph node metastases. Moreover, it was able to inhibit cancer cell adhesion to LECs pointing out a novel involvement of Tn in this interaction and suggesting its future potential application in inhibiting lymphatic metastases.
Results
Experimental strategy
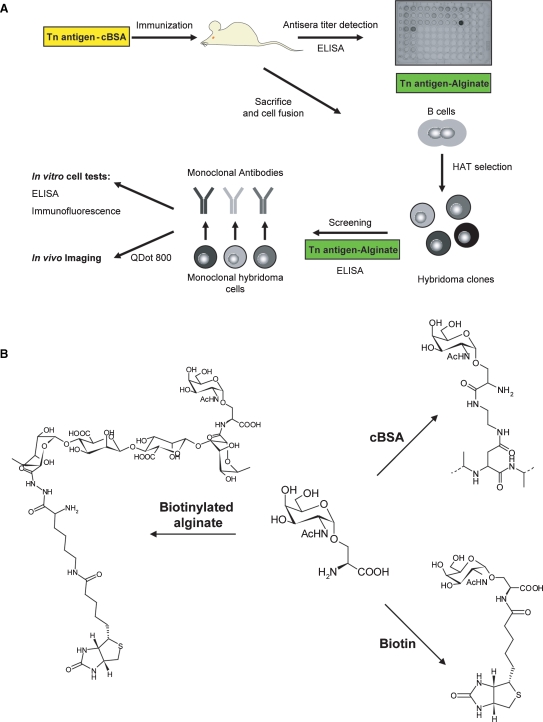
Considering the low immunogenicity and the consequent difficulties in obtaining efficient antibodies targeting small carbohydrate antigens, we based our strategy on Tn-antigen clusterization using different polymeric scaffolds and on a stepwise selection of the most reactive antibody producing clones. Briefly, the glycoaminoacid GalNAc-α-O-Ser (Tn) was synthesized, conjugated with cBSA for mice immunization and with biotinylated alginate for serum titration and anti-Tn clone screening. The selected hybridomas were further tested by enzyme-linked immuno sorbent assay (ELISA) and immunofluorescence on a highly expressing Tn breast cancer cell line and, after conjugation with QDot, were used for in vivo imaging (Figure 1A).
Fig. 1.
Experimental strategy. (A) The approach followed for the selection of specific anti-Tn mAbs is schematically illustrated. Tn conjugated with cBSA was used for mice immunization, whereas Tn conjugated with biotinylated alginate was used for mouse serum titration and for the screening of anti-Tn clones. The selected hybridomas were further tested by ELISA and immunofluorescence on a highly expressing Tn breast cancer cell line (MCF7) and finally, after Qdot conjugation, were used for in vivo imaging. (B) Schematic representation of Tn-conjugates. The three compounds were obtained starting from pure Tn antigen, synthesized in seven steps from 2-azido-2-deoxy-1,3,4,6-tetra-O-acetyl galactose, exploiting the trichloroacetimidate strategy. All the derivatives were obtained applying the same carbodiimide chemistry: EDC and NHS were used as activating agents for the carboxylic group in the MES buffer, pH 6.0. The products were fully characterized before further utilization.
Synthesis of Tn-conjugates
Briefly, for the synthesis of Tn antigen (unpublished data), 2-azido-2-deoxy-1,3,4,6-tetra-O-acetyl galactose was selectively deacetylated at the anomeric position with hydrazine hydrate (2h, RT), which was further converted into trichloroacetimidate (CCl3CN/CH2Cl2). The activated sugar was conjugated to N-benzyloxycarbonyl-l-serine benzylester (TMSOTf, −20°C). Successively, the azido group was reduced (NaBH4) and acetylated (1/1 pyridine/Ac2O), and finally all the protecting groups were removed, giving rise to the product as the anomeric mixture with a global yield equal to 20%. The α-form of Tn, which represents 65% of the mixture, was separated with HPLC-UV (amino-derivatized column, Luna NH2, 250 × 10 mm, 10 μm Phenomenex, eluent 30/70 water/acetonitrile) and recovered as pure anomer. The glycoaminoacid was then further modified through conjugation with cBSA or biotinylated alginate; alternatively, it was directly linked to biotin. The three reactions were performed exploiting the carbodiimide chemistry, which leads to the formation of a new amidic bond (Figure 1B). The derivatized polymers were purified through extensive dialysis against doubly distilled water, while the purification conditions of the biotinylated antigen were first optimized with LC-MS. Indeed, notwithstanding the presence of the biotin derivative, the compound was only slightly detectable by UV; in order to verify peak separation and elution times, it was necessary to monitor them with MS. After assessment of the separation conditions, the purification was carried out on preparative scale using an HPLC-UV setup.
Immunization, serological responses, and antibody selection
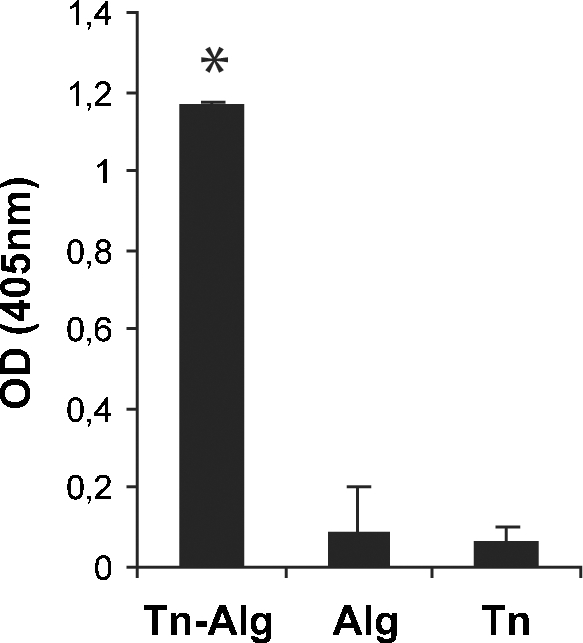
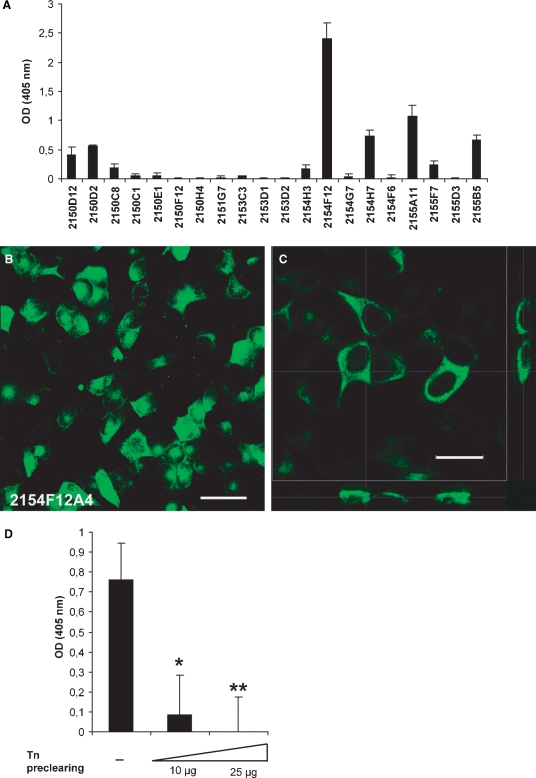
Three series of immunization were performed on a group of BALB/c mice (n = 2, each) by weekly intraperitoneal injections of 200 μg of Tn-cBSA. The levels of anti-Tn immunoglobulins in the mouse sera were monitored by ELISA. As shown in Figure 2, Tn-alginate conjugation significantly improved the binding of specific antibodies to the target antigen, thus proving the efficacy of antigen clusterization. The most reactive clones in a solid-phase ELISA on Tn-alginate (data not shown) resulted to be IgM and were then assayed for their ability to bind Tn expressed on the surface of tumor cells. For this purpose, an ELISA assay was performed on 96-well-plated MCF7 cells and clone 2154F12 showed the highest reactivity (Figure 3A). The latter was subcloned and the resulting subclones were tested by immunofluorescence on MCF7 cells. mAb 2154F12A4 was the most efficient in labeling the breast cancer cell line (Figure 3B) in a membrane-specific manner, as evidenced by xz and yz sections of confocal images (Figure 3C). The specific binding of the antibody to the MCF7-expressed Tn was further assessed following a solid-phase preclearing test. For this purpose, the 2154F12A4 supernatant was first incubated with different amounts of plastic adsorbed-Tn and then it was assayed on MCF7 cells. The dose-dependent abrogation of binding to MCF7 cells demonstrated the specificity of mAb 2154F12A4 (Figure 3D).
Fig. 2.
Efficacy of clusterization and antibody response. BALB/c mouse (n = 6) sera were assayed after the third Tn-cBSA immunization. The histogram reports the mean values ± standard deviation (SD) of a comparative ELISA performed on Tn clusterized on alginate (Tn-Alg), alginate (Alg), and Tn (GalNAc-α-O-Ser). Mouse serum was used at a dilution of 1:100. Tn-alginate conjugation significantly improved the binding of specific antibodies to the target antigen compared with unclusterized Tn (*P = 0.01). For these experiments, all the compounds were biotinylated to assure their immobilization on a streptavidin-coated 96-well plate and a polyvalent IgG/A/M-HRP secondary antibody was used. OD, optical density.
Fig. 3.
Selection of specific anti-Tn antibodies. (A) Mean values ± SD of optical density (OD) obtained from an ELISA screening of anti-Tn clones on MCF7 cells grown on a 96-well plate. (B) Representative immunofluorescence analysis performed on MCF7 cells grown on a glass coverslip. The green staining indicates cell surface binding of mAb 2154F12A4. Bar: 50 μm. (C) A higher magnification image of MCF7 cells stained with mAb 2154F12A4 and its xz (lower panel) and yz (right panel) sections evidenced the binding of mAb to the cell membrane. Bar: 25 μm. (D) Inhibition of mAb 2154F12A4 binding to the surface of MCF7 cells after solid-phase preclearing with Tn. The values report the mean values ± SD of three ELISA assays performed in duplicate, indicating the reactivity of the mAb on a monolayer of MCF7 cells after pre-incubation with scalar doses of Tn (10 and 25 μg) adsorbed onto a plastic microtiter plate. *P = 0.006; **P = 0.003.
In vivo imaging
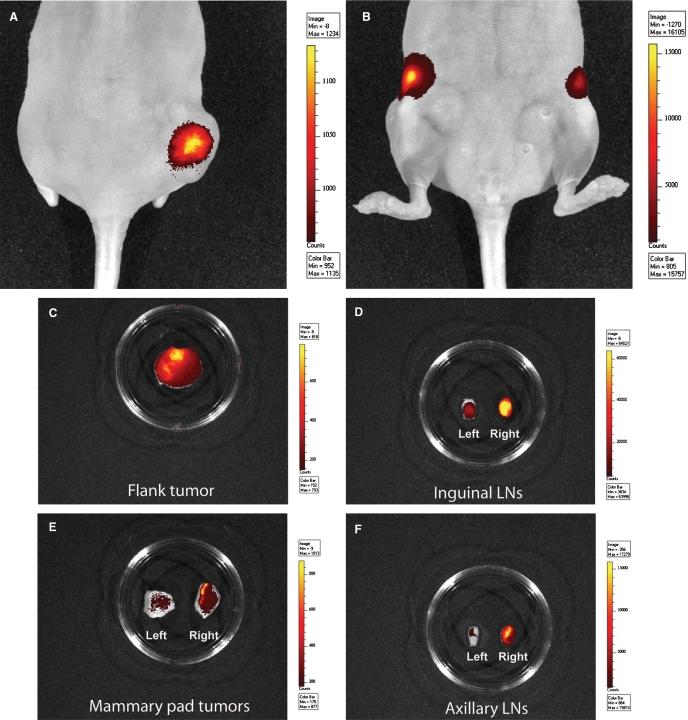
To evaluate the ability of mAb 2154F12A4 to bind Tn-positive cells in vivo, MCF7 cells were injected subcutaneously in the right flank and in the mammary fat pad of nude mice and stimulated by weekly injections of β-estradiol. At 90 days, when the tumor masses reached 5–7 mm, the QDot800-conjugated 2154F12A4 was injected into the tail vein and the fluorescent signal was recorded with the IVIS Lumina Imaging System. After 72 h, the mAb strongly stained the flank tumor mass (Figure 4A) and the inguinal lymph nodes (Figure 4B). Since the limitations of the instrument sensitivity are related to the depth from which the signal is emitted, the mAb localization was further investigated by excising the tissues of interest. The flank tumor mass and the inguinal lymph nodes still demonstrated a high antibody binding (Figure 4C and D). In addition, the mammary pad as well as the axillary lymph nodes, which both were negative at the in vivo analysis, resulted positive (Figure 4E and F). Notably, the ex vivo analysis evidenced an higher antibody binding in the homolateral right inguinal and axillary lymph nodes, suggesting the presence of a larger number of MCF7 metastatic cells in the draining lymph nodes from the tumor implanted in the right flank. The signal emitted by lung, liver, kidney, and spleen were negligible (data not shown) confirming the binding specificity of the antibody.
Fig. 4.
In vivo imaging of MCF7 tumor bearing mice. Fluorescent signal captured by IVIS Lumina Imaging System in tumor bearing mice after injection with anti-Tn QDot800-conjugated mAb 2154F12A4. (A and B) In vivo acquired images after 72 h from the antibody injection. (C–F) Ex vivo images acquired after the excision of the tumor masses and lymph nodes. The color scale indicates the intensity of the signal recorded.
Immunohistochemical analysis of MCF7 tumors and metastatic lymph nodes
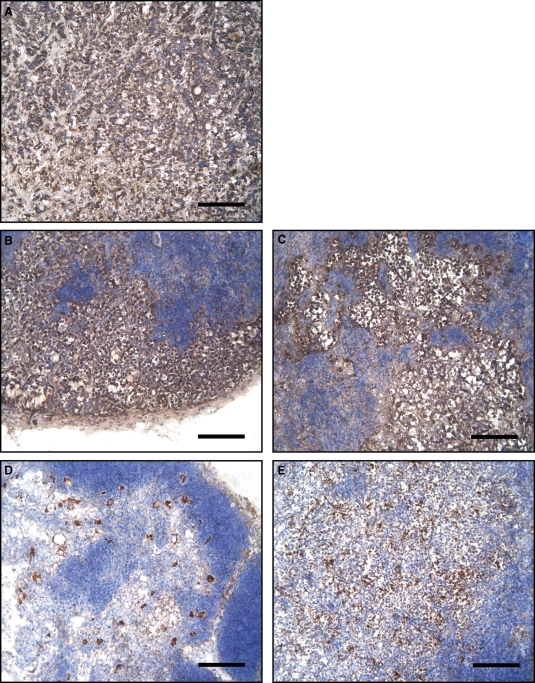
To assess whether the QDot 800-conjugated mAb had identified real metastatic cells in vivo, cryostat sections of the primary tumors as well as those of inguinal and axillary lymph nodes were analyzed by immunohistochemistry. For this purpose, an anti-pan cytokeratin antibody, representing a specific marker of epithelial as well as of carcinoma cells such as MCF7 (Gusterson et al. 2005), was used. As expected, the cryostat sections of the tumors were strongly positive for the anti-cytokeratin antibody (Figure 5A, brown staining). In addition, an intense staining was present in the right inguinal and axillary lymph nodes, demonstrating the presence of metastatic MCF7 cells in these tissues (Figure 5B and C). The cytokeratin staining was weaker in the controlateral lymph nodes (Figure 5D and E), in accordance with the lower fluorescence signal recorded by in vivo imaging (Figure 4D and F).
Fig. 5.
Cytokeratin staining of MCF7-derived tumors and mouse lymph nodes. (A–E) Immunoperoxidase staining of mouse cryostat sections. (A) MCF7 flank tumor; (B and C) right inguinal and axillary lymph nodes; (D and E) left inguinal and axillary lymph nodes. Brown staining indicates the pan-cytokeratin positive cells, a marker for human carcinoma cells. Notice the large metastatic nests in the right lymph nodes, ipsolateral to the flank tumor. Nuclei are evidenced in blue by hemallume staining. Bars: 120 μm.
Inhibition of cancer cell adhesion to lymphatic endothelium
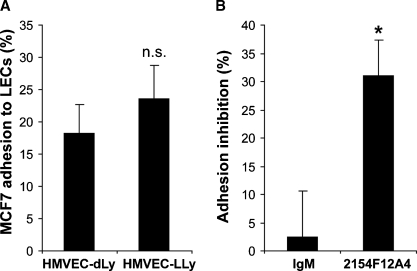
Based on the suggestion formulated by Kawaguchi and colleagues (2006) that Tn is implicated in the molecular mechanism of lymphatic metastasis, we next investigated the functional significance of the interaction of tumor cells expressing high levels of Tn with LECs. Considering the phenotypic heterogeneity of LECs isolated from different organs (Garrafa et al. 2005), as first approach, we assayed the adhesion of MCF7 cells on Human Microvascular Endothelial Cells Dermal Lymphatic-Neonatal (HMVEC-dLyNeo) and Human Microvascular Endothelial Cells Lung Lymphatic (HMVEC-LLy). As shown in Figure 6A, MCF7 cells displayed comparable levels of adhesion to dermal and lung LECs. A higher, even if not significant, adhesion to HMVEC-LLy was observed and for this reason they were used for the subsequent experiments. To assess the involvement of Tn antigen in this interaction, we performed MCF7 adhesion assays in the presence of mAb 2154F12A4 and an isotype control antibody (IgM). Interestingly, mAb 2154F12A4 significantly (P = 0.002) inhibited breast cancer cell adhesion to LECs up the 30% (Figure 6B), demonstrating that this oligosaccharidic antigen is implicated in the cancer cell lymphatic endothelium interaction, and thus it is a potential molecular player in the process of lymph node metastasis.
Fig. 6.
Inhibition of cancer cell adhesion to LECs. (A) MCF7 cell adhesion to Human Microvascular Endothelial Cells Dermal Lymphatic-Neonatal (HMVEC-dLy) and Human Microvascular Endothelial Cells Lung Lymphatic (HMVEC-LLy). Mean values ± SE were obtained from three independent experiments performed in triplicate. (B) Inhibition of MCF7 adhesion to HMVEC-LLy in the presence of 10 μg/mL of mAb 2154F12A4 or 10 μg/mL of control IgM. The mean values ± SE refer to six experiments performed in triplicate and express the percentage of inhibition versus the isotype control antibody. *P = 0.002.
Discussion
Tn is expressed by 90% of human carcinomas (Springer 1997) and has been proposed as diagnostic and prognostic tumor biomarker for breast carcinoma (Kumar et al. 2005). Despite its importance, the functional role of Tn has not been established yet, probably because of the lack of appropriate experimental tools. Here, we report the generation of a specific mouse mAb against Tn (2154F12A4) obtained by using novel conjugated Tn compounds. Importantly, when injected in MCF7-tumor bearing mice 2154F12A4 specifically bound to primary tumor lesions as well as to metastatic lymph nodes. Moreover, it was able to inhibit cancer cell adhesion to lymphatic endothelium, unveiling a new molecular function of this widespread tumor marker.
A number of anti-Tn antibodies have been already generated by using mucins purified from cancer cell culture supernatant (Takahashi et al. 1988; Numata et al. 1990; Ando et al. 2008), asialo ovine sumaxillar mucin, (Kjeldsen et al. 1988; O’Boyle and Wright 1994), tumor extracts (Nuti et al. 1982; Hirohashi et al. 1985; Siddiki et al. 1993), and synthetic Tn conjugated with different molecular scaffolds such as KLH (keyhole limpet hemocyanine; O’Boyle et al. 1996; Reddish et al. 1997). Our approach differs from the previous ones since we used a purified synthetic Tn antigen in all the steps of mAb selection. In detail, a synthetic Tn conjugated to cBSA was used as immunogen, whereas a synthetic Tn conjugated to biotinylated-alginate was used to define the specificity of anti-Tn antibodies. For the preparation of the samples, we explored a new conjugation strategy. Kuduk and co-workers (1998) reported an elegant procedure for the preparation of a synthetic vaccine obtained by conjugating a glycopeptide containing the Tn or TF motive with KLH, through a linker. This method provided a high density of Tn on the glycopeptidic side-chain of the protein. Differently, we succeeded in directly clusterizing Tn on the immunogenic protein in a more convenient single step procedure. Our approach did not necessitate the pre-clusterization of Tn on a short peptide, or the use of a linker such as mercaptoacetamide or tripalmitoyl-S-glycerylcysteinylserine, and the subsequent protection–deprotection steps. We synthesized only the core of the recognized motif, namely N-acetylgalactosamine alpha linked to the amminoacid serine. Since Tn is not immunogenic, it must be linked to a carrier molecule (biotin or a polymeric chain); we decided to leave the functional groups of the aminoacidic moiety unprotected for the following conjugation step. Thus, in its final form, the glycoconjugate has always been a functional group (amino or carboxylic) that is involved in an amidic bond (mimicking the native structure) while the other is free. This approach provided high clusterization efficacy able to generate a considerable mouse immune response against the target antigen.
Our principal aim was to produce a mAb for in vivo imaging and the purified oligosaccaridic antigen assured us the successful selection of highly specific mAbs. mAb 215F12A4 (IgM) in vitro selectively recognized Tn on the MCF7 surface, since its binding to cell membrane was completely abolished by preincubation with purified Tn. Importantly, QDot 800-conjugated 2154F12A4 was able to specifically detect MCF7 primary tumors and their lymph node metastases in nude mice. As expected, the signal emitted by the tumor cell-bound antibody was correlated to the extent of metastasis, as confirmed by the ex vivo acquisition data. The subsequent immunohistochemical cytokeratin staining confirmed that a larger number of MCF7 tumor cells corresponded to higher fluorescence signal. Altogether, these results support the specificity and selectivity of mAb 2154F12A4 in binding Tn in vivo. Accordingly, 72 h from mAb injection, a negligible signal was observed in organs such as lung, liver, kidney, and spleen. It is interesting to note that the present in vivo signals were obtained with soluble mAb 2154F12A4; however, we are aware that high-quality mAb-targeted tumor imaging in mice bearing human xenografts is quite different from what may be achieved clinically. In view of a translational application of mAb 2154F12A4 as a diagnostic and therapeutic tool, we are trying to ameliorate the sensitivity for detecting as small as possible cancer lesions by generating probes constituted by clustered mAbs on a labeled delivery system such as a suitable polymeric scaffold.
Lymph node metastasis is a major complication associated with many carcinomas, in particular with breast carcinoma (Achen and Stacker 2008). A broad array of adhesion molecules, such as carbohydrates, lectins, selectins, cadherins, integrins, have been implicated in the adhesion of tumor cells to the BECs (Miles et al. 2008). Much less is known about the mechanism and the molecules involved in tumor cells adhesion to LECs. Cell surface carbohydrate structures may indeed play a role in the dissemination of cancer cells to the draining as well as distal lymph nodes. The selective spread of MCF7 breast cancer cells to the lymph nodes that was evidenced by in vivo imaging after the injection of the labeled mAb 2154F12A4 in tumor-bearing mice suggested that Tn may be directly involved in the process of lymphatic metastasis. This hypothesis was supported also by a previous study demonstrating that the expression of Vicia villosa agglutinin (VVA)-binding carbohydrates, such as Tn, correlates with tumor stage, lymphatic invasion, and lymph node metastasis (Kawaguchi et al. 2006). In the present investigation, we showed that MCF7 cells adhered to the same extent to LECs isolated from human derma or lung and mAb 2154F12A4 partially interfered with this static adhesion. Similarly to the interaction of galectin-3 and TF that occurs in BEC-cancer cell adhesion (Khaldoyanidi et al. 2003), it is tempting to speculate that galectin-8, recently described to play a role in LECs function (Cueni and Detmar 2009), could be the counter-receptor of Tn and this pair could represent a possible target for an anti-metastatic activity of mAb 2154F12A4. While tumor cells have been shown in different studies to adhere to LECs (Irjala et al. 2003; Kawai et al. 2009), there is no direct experimental evidence to date demonstrating that adhesive interactions with LECs are indeed required for tumor cell entry into the lymphatics (Das and Skobe 2008). However, the active role of Tn played in lymphatic metastasis is strongly supported by clinical data evidencing that lymphatic metastasis is related to the expression of simple mucin-type carbohydrates such as Tn and Tn-like antigens (Kawaguchi et al. 2006). These present novel results directly demonstrate the involvement of Tn in the tumor cell–LEC interaction and suggest its functional role in lymphatic metastasis. Further studies will be addressed to assess if mAb 2154F12A4 will prevent or reduce cancer cell spread to lymph nodes in vivo, building the basis for a new approach for lymphatic metastasis treatment.
Material and methods
Alginate biotinylation
Alginate was modified through the introduction of a biotin derivative, (N-(+)-biotinyl-l-lysine-hydrazide), which is much more water soluble than biotin and has amino groups capable to react with the carboxylic functions on the polymer. Alginate, isolated from Laminaria hyperborea, was provided by Protan A/S (Norway) and purified by precipitation in isopropanol. For the biotinylation, 30 mg (0.15 mmol – repetitive unit) of alginate was dissolved in 10 mL of 0.2 M 2-morpholinoethanesulphonic acid buffer (MES, Fluka, Buchs, Switzerland) at pH 6. After solubilization, 72 mg of 1,1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDC, Sigma-Aldrich, St. Louis, MO) and 8.5 mg of N-hydroxy-succinimide (NHS, Sigma-Aldrich) were added and, after 10 min, 3.2 mg of Nε-(+)-biotinyl-l-lysinehydrazide (Fluka) was further added. The solution was stirred for 6–8 h, and then dialyzed and freeze-dried.
Tn-biotinylation
4.0 mg (0.016 mmol) of biotin was dissolved in 2.5 mL of 0.2 M MES buffer at pH 6.0. 7.64 mg of EDC and 1.0 mg of NHS were dissolved in 0.5 mL of the same buffer and after few minutes added to the solution. After 10 min, 1.0 mg (0.0032 mmol) of GalNAcα-O-Ser was added and the solution was stirred overnight. The product was purified with HPLC-UV. The yield was equal to 89%.
Clusterization of Tn
Fourteen milligrams (0.071 mmol) of biotinylated alginate was dissolved in 5.0 mL of 0.2 M MES buffer, pH 6.0. After solubilization, EDC (33.9 mg) and NHS (4.7 mg) were added and, after 10 min, 0.6 mg (1.95 × 10−3 mmol, 0.027 eq.) of GalNAc-α-O-Ser was further added. The solution was stirred for 8 h, dialyzed, and freeze-dried. Elemental analysis gives a molar degree of substitution of Tn-Alg equal to 30%. Cationized BSA (cBSA) was prepared according to the procedure reported by Domen et al. (1987); the number of amino groups was equal to the number of native amino residues of BSA plus the number of carboxylic groups (glutamate and aspartate) which were derivatized with ethylenediamine. GalNAc-α-O-Ser (1 mg) and cBSA (2 mg) were dissolved in a solution containing the MES buffer (0.2 M), pH 6; EDC and NHS were added, and the mixture was stirred for 2 h. The solution was then dialyzed and freeze-dried. The molar degree of substitution was evaluated by enzymatic hydrolysis of N-acetylgalactosamine (GalNAc) deriving from Tn using α-N-acetylgalactosaminidase from Acremonium spp. (Seikagaku Tokyo, Japan; 50 mM acetate buffer, pH 4.5, 37°C, 2.5 h) and subsequent evaluation of the amount of GalNAc by capillary electrophoresis. The molar degree of substitution of Tn-cBSA was equal to 3%.
Capillary electrophoresis
The high-performance capillary electrophoresis system was from Hewlett-Packard (Agilent, Waldbronn, Germany), Model HP3DCE, with HP Chemstation software. Derivatization of GalNAc, either standard (Sigma-Aldrich) or arising from hydrolysis of Tn-cBSA, with 2-aminobenzoic acid (2-AA) was carried out by mixing an aqueous solution of the sugar with the derivatizing solution (0.25 M 2-AA, 0.159 M sodium cyanoborohydride in 99% methanol and 1% acetic acid). The solution was heated at 60°C for 5 h. Before capillary electrophoresis analysis, the samples were diluted five times in water. Before each run, the fused silica capillary (56 cm total length, 50 μm i.d.) was washed with sodium hydroxide (2 min, 985 mbar) and with 25 mM sodium phosphate buffer, pH 3.0 (4 min, 985 mbar).
HPLC-MS
Instrument: HPLC SURVEYOR Thermo Finnigan system equipped with MS Pump, Autosampler and PDA Detector, coupled with a LCQ DECA XP Plus Thermo Finnigan mass spectrometer with electrospray ionization (ESI). The Xcalibur software was used for managing system and data. HPLC-MS experimental conditions were the following: a Synergi Polar – RP 80A column (2.0 × 250 mm; 4 μm) from Phenomenex was used at 45°C at a flow rate of 200 μL/min and a run time of 45 min with a PDA detector. Detection with an MS detector was performed with a run time of 45 min and capillary temperature of 250°C and a mass range of 50–2000. HPLC-UV Merck-Hitachi equipped with a L-6200. Pump, an AS-200A Autosampler, and a L-4500A Diode Array Detector was used for UV analysis with a run time of 30 min. Data acquisition and analysis were performed by D-700 Chromatography Data Station software (Merck-Hitachi).
Production of mouse monoclonal antibodies
Procedures involving animals and their care were conducted according to the institutional guidelines in compliance with national laws (D.Lgs. n° 116/92). Anti-Tn mAbs were produced according to a modified version of the protocol previously described by Galfre et al. (1977). Briefly, 8-week-old female BALB/c mice (Harlan Italy S.r.L., Udine, Italy) were immunized by intraperitoneal injections of 200 μg of Tn-cBSA and properly emulsified in Ribi's (MPL® + TDM Adjuvant System; Sigma-Aldrich, St. Louis, MO). Injection was done once a week for 3 weeks, and after 1 month, the mice were boosted by three daily additional intravenous injections of 20 μg of antigen properly diluted in phosphate-buffered saline (PBS; Cambrex Bio Science; Verviers, Belgium). The animals were then sacrificed, and spleen cells were fused with mouse myeloma NS1 cells (American Type Culture Collection, ATCC; Manassas, VA). Hybrid cells were grown in 4.5 g/L glucose modified Dulbecco's medium (DMEM; Lonza Milano S.r.l., Milan, Italy) supplemented with 20% fetal calf serum (FCS; Lonza Milano S.r.l.), and then selected in Hypoxanthine Aminopterin Thymidine medium (HAT; Sigma). The resulting hybridoma clones were then grown in DMEM with 20% FCS and further subcloned. The isotype characterization and the purification of the antibodies were performed following the manufacturer's procedures of Mouse MonoAB ID KIT (Zymed Laboratories, Inc.; San Francisco, CA) and HiTrap IgM Purification HP (GE Healthcare Italia, Milan, Italy), respectively.
Immobilized-antigen enzyme-linked immuno sorbent assay
Mouse serum and hybridoma supernatants were screened for the presence of specific anti-Tn mAbs with the following procedure. To immobilize the antigen to a solid phase, microtiter plate wells were coated with 10 μg/mL streptavidin (Sigma-Aldrich) in the carbonate buffer 100 mM, pH 9.6, overnight at 4°C; 100 μg/mL of Tn-biotinylated alginate conjugate was then added to the wells and incubated for 1 h at 37°C. 1% ovalbumin (Sigma-Aldrich) in PBS for 1 h at 37°C was used to block unspecific sites; then, mouse serum or hybridoma supernatants were added for 2 h at 37°C followed by a polyvalent anti-mouse IgG-, IgA-, and IgM-peroxidase-conjugated secondary antibody (MP Biomedicals; Aurora, OH) for 1 h at 37°C. The antibody binding was visualized by 1 mg/mL of 2,2′-azino-bis-3-ethyl-benzthiazoline-6-sulphonic acid (ABTS) peroxidase substrate (Sigma-Aldrich) diluted in the 100 mM citrate buffer, pH 4.5. Absorbance was measured at 405 nm by a computer-interfaced GENios Plus microplate reader (TECAN Italia S.r.l., Milan, Italy).
Cell surface ELISA
The selected subclones were assayed by ELISA on the Tn-expressing mammary carcinoma cell line, MCF7 provided by ATCC and maintained in DMEM (Lonza Milano S.r.l) supplemented with antibiotics, 20% FCS, 1 mM sodium pyruvate (Sigma), 2 mg/mL glutamine (Sigma), 0.1 mM non-essential aminoacids (Sigma), 10 μg/mL insulin (Humulin R, Eli Lilly, Italy). Briefly, cells were grown in a 96-well plate for 3–5 days until confluence and fixed with 4% paraformaldehyde (PFA) in PBS for 15 min at room temperature. After blocking with 1% ovalbumin in PBS for 1 h at 37°C and incubating the hybridoma supernatants for 2 h at 37°C, the binding of the specific mAbs against Tn antigen was detected with a goat anti-mouse IgM-HRP-conjugated antibody (MP Biomedicals). The colorimetric reaction developed with the ABTS substrate was measured spectrophotometrically at 405 nm by a computer-interfaced GENios Plus microplate reader (TECAN Italia S.r.L.).
Solid-phase preclearing
To evaluate the specificity of the subclones to the MCF7-expressed Tn antigen, a solid-phase preclearing test was performed. Briefly, microtiter plate wells were coated with 10 μg/mL of streptavidin (Sigma-Aldrich) in the carbonate buffer 100 mM, pH 9.6, overnight at 4°C; serial dilutions of Tn-biotinylated alginate conjugate were added to the wells and incubated for 1 h at 37°C. After blocking with 1% ovalbumin (Sigma-Aldrich) in PBS for 1 h at 37°C, mAbs were added for 2 h at 37°C. The precleared supernatants were subsequently collected and tested on MCF7 cells following the cell surface ELISA procedure.
Immunofluorescence staining
MCF7 cells were grown on acid-washed coverslips for 3 days, fixed in PBS 4% PFA for 15 min, and blocked with 2% goat serum and 1% ovalbumin in PBS for 1 h at 37°C. The cells were then incubated with hybridoma supernatants for 2 h at 37°C and further with the anti IgM-Alexa Fluor® 488 (Molecular Probes, Eugene, OR) secondary antibody. After extensive washes, coverslips were mounted in Mowiol 4–88 (Calbiochem-Novabiochem) containing 2.5% (w/v) DABCO (Sigma). Images were acquired with a Leica TCS SP2 confocal system (Leica Microsystems Heidelberg, Mannheim, Germany) using the Leica Confocal Software (LCS).
Immunohistochemistry
Mouse tissues were excised, embedded in OCT (Kaltek, Padova, Italy), snap-frozen and stored at −80°C. Cryostat sections of 7 μm were air-dried at room temperature and kept at −80°C wrapped in aluminum foils. Before using, the sections were equilibrated at room temperature, hydrated, and permeabilized with 1% Triton X-100 in PBS for 15 min. After inactivation of endogenous peroxidase activity with 3% of hydrogen peroxide, tissue sections were fixed with 4% PFA in PBS for 15 min and saturated with the blocking buffer (PBS, 1% BSA and 2% FCS) for 30 min. The rabbit anti-pan-cytokeratin antibody (DakoCytomation, CA) was then added at room temperature for 1 h, followed by three 5 min washes in PBS. Tissue sections were then incubated with the anti-rabbit-peroxidase-conjugated secondary antibody for 1 h. The enzymatic reaction was developed with diaminobenzidine (DAB; Vector Laboratories, Inc, CA), and nuclei were visualized with Mayer Hemallume (Kaltek srl, Padova, Italy) staining. Images were acquired with a camera equipped microscope (Leica Microsystems).
In vivo imaging
8-week-old female nude mice (Harlan Italy S.r.L) were injected subcutaneously in the right flank and in the mammary fat pad with 5 × 106 MCF7 cells resuspended in 100 μL of PBS. Hundred micrograms of 17 β-estradiol (Sigma) diluted in sesame oil (Sigma) was weekly subcutaneously administered to stimulate cell growth in vivo. All tumors were allowed to grow to approximately 5–7 mm in diameter before imaging studies were performed. The hybridoma clone that demonstrated the highest reactivity against Tn antigen (clone 2154F12A4) was purified and conjugated with QDot 800 according to the manufacturer's instructions (Molecular Probes). Fifty micrograms of the labeled antibody was injected into the tail vein of anesthetized tumor-bearing mice, and the fluorescent images were recorded by IVIS Lumina Imaging System (Xenogen Corporation, CA).
Tumor cell adhesion to LECs
HMVEC-dLyNeo or HMVEC-LLy (Lonza Milano S.r.l.) were seeded in a 96-well plate and grown in EGM-2 MV (Lonza Milano S.r.l.) for 3 days until confluence. MCF7 cells were detached with 5 mM ethylenediaminetetraacetic acid (EDTA) in PBS, labeled with the vital fluorochrome calcein AM (Molecular Probes) for 15 min at 37°C, resuspended in PBS with the addition of 1 mM CaCl2 and MgCl2 and incubated with 10 μg/mL of anti-Tn antigen mAb (clone 2154F12A4) or with the isotype control antibody (IgM, Sigma) for 20 min at 37°C. 1 × 105 MCF7 per well were seeded into the LEC-coated plates and incubated for 30 min at 37°C. Unbound cells were removed by three washes with PBS, and cancer cell adhesion to lymphatic endothelium was quantified by a computer-interfaced GENios Plus microplate reader (TECAN Italia S.r.L.).
Statistical analysis
Statistical significance of the results was determined by using the unpaired Student's t-test. A value of P < 0.05 was considered significant.
Funding
The Italian Ministry of Education funding “Programma di Ricerca Scientifico di Rilevante Interesse Nazionale” (to Al.C.); the “Associazione Italiana per la Ricerca sul Cancro” (AIRC to Al.C.); the Government of the Regione Friuli-Venezia Giulia (“Contributi a favore della ricerca applicata e l’innovazione tecnologica”, L.R. 30/84) through Bracco Imaging S.p.A. (to F.U., Al.C. and S.P.). Funding to pay the Open Access publication charges for this article was provided by the Government of the Regione Friuli-Venezia Giulia (“Contributi a favore della ricerca applicata e l'innovazione tecnologica”, L.R. 30/84) through Bracco Imaging S.p.A.
Acknowledgments
We thank Maurizio Mongiat for the in vivo imaging analysis assistance. Part of the present study was performed while C.D. was holding a “Piano Operativo Regionale Obiettivo 3 FVG 2000 – 2006 – Asse D – Misura D4” fellowship. C.D. presently holds a “Federazione Italiana Ricerca sul Cancro” fellowship. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact by department grants to Al.C.
Conflict of interest statement
None declared.
Abbreviations
- ABTS
2,2′-azino-bis-3-ethyl-benzthiazoline-6-sulphonic acid
- BEC
blood endothelial cell
- BSA
bovine serum albumin
- cBSA
cationized BSA
- DMEM
modified Dulbecco's medium
- EDC
1,1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide
- ELISA
enzyme-linked immuno sorbent assay
- ESI
electrospray ionization
- FCS
fetal calf serum
- GalNAc
N-acetylgalactosamine
- HAT
aminopterin thymidine medium
- HMVEC-dLyNeo
Human Microvascular Endothelial Cells Dermal Lymphatic-Neonatal
- HMVEC-LLy
Human Microvascular Endothelial Cells Lung Lymphatic
- KLH
keyhole limpet hemocyanine
- LEC
lymphatic endothelial cell
- mAb
monoclonal antibody
- MES
morpholinoethanesulphonic acid buffer
- NHS
N-hydroxy-succinimide
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- TACA
tumor-associated carbohydrate antigen
References
- Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci. 2008;1131:225–234. doi: 10.1196/annals.1413.020. [DOI] [PubMed] [Google Scholar]
- Ando H, Matsushita T, Wakitani M, Sato T, Kodama-Nishida S, Shibata K, Shitara K, Ohta S. Mouse-human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biol Pharm Bull. 2008;31:1739–1744. doi: 10.1248/bpb.31.1739. [DOI] [PubMed] [Google Scholar]
- Avichezer D, Springer GF, Schechter B, Arnon R. Immunoreactivities of polyclonal and monoclonal anti-T and anti-Tn antibodies with human carcinoma cells, grown in vitro and in a xenograft model. Int J Cancer. 1997;72:119–127. doi: 10.1002/(sici)1097-0215(19970703)72:1<119::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Babino A, Oppezzo P, Bianco S, Barrios E, Berois N, Navarrete H, Osinaga E. Tn antigen is a pre-cancerous biomarker in breast tissue and serum in N-nitrosomethylurea-induced rat mammary carcinogenesis. Int J Cancer. 2000;86:753–759. doi: 10.1002/(sici)1097-0215(20000615)86:6<753::aid-ijc1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: A family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41:189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- Brooks SA, Carter TM, Royle L, Harvey DJ, Fry SA, Kinch C, Dwek RA, Rudd PM. Altered glycosylation of proteins in cancer: What is the potential for new anti-tumour strategies. Anticancer Agents Med Chem. 2008;8:2–21. doi: 10.2174/187152008783330860. [DOI] [PubMed] [Google Scholar]
- Cueni LN, Detmar M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp Cell Res. 2009;315:1715–1723. doi: 10.1016/j.yexcr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- Domen PL, Muckerheide A, Michael JG. Cationization of protein antigens: III. Abrogation of oral tolerance. J Immunol. 1987;13:3195–3198. [PubMed] [Google Scholar]
- Escrevente C, Machado E, Brito C, Reis CA, Stoeck A, Runz S, Marmé A, Altevogt P, Costa J. Different expression levels of a3/4 fucosyltransferases and Lewis determinants in ovarian carcinoma tissues and cell lines. Int J Oncol. 2006;29:557–566. [PubMed] [Google Scholar]
- Fernandez Madrid F, Tang N, Alansari H, Karvonen RL, Tomkiel JE. Improved approach to identify cancerassociated autoantigens. Autoimmun Rev. 2005;4:230–235. doi: 10.1016/j.autrev.2004.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire T, Lo-Man R, Piller F, Piller V, Leclerc C, Bay S. Enzymatic large-scale synthesis of MUC6-Tn glycoconjugates for antitumor vaccination. Glycobiology. 2006;16:390–401. doi: 10.1093/glycob/cwj082. [DOI] [PubMed] [Google Scholar]
- Galfre G, Howe SC, Milstein C, Butcher GW, Howard JC. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977;266:550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Galonic DP, Gin DY. Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines. Nature. 2007;446:1000–1007. doi: 10.1038/nature05813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrafa E, Trainini L, Benetti A, Saba E, Fezzardi L, Lorusso B, Borghetti P, Bottio T, Ceri E, Portolani N, et al. Isolation, purification, and heterogeneity of human lymphatic endothelial cells from different tissues. Lymphology. 2005;38:159–166. [PubMed] [Google Scholar]
- Gilewski TA, Ragupathi G, Dickler M, Powell S, Bhuta S, Panageas K, Koganty RR, Chin-Eng J, Hudis C, Norton L, et al. Immunization of high-risk breast cancer patients with clustered sTn-KLH conjugate plus the immunologic adjuvant QS-21. Clin Cancer Res. 2007;13:2977–2985. doi: 10.1158/1078-0432.CCR-06-2189. [DOI] [PubMed] [Google Scholar]
- Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- Glinsky VV, Huflejt ME, Glinsky GV, Deutscher SL, Quinn TP. Effects of Thomsen-Friedenreich antigen-specific peptide P-30 on beta-galactoside-mediated homotypic aggregation and adhesion to the endothelium of MDA-MB-435 human breast carcinoma cells. Cancer Res. 2000;60:2584–2588. [PubMed] [Google Scholar]
- Gusterson BA, Ross DT, Heath VJ, Stein T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7:143–148. doi: 10.1186/bcr1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg J, Yan J, Morey S, Glinskii OV, Huxley VH, Wild L, Klick R, Roy R, Glinsky VV, Rittenhouse-Olson K. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia. 2006;8:939–948. doi: 10.1593/neo.06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi S, Clausen H, Yamada T, Shimosato Y, Hakomori S. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and -81 expressed in cancer of blood group O or B individuals: Its identification as Tn antigen. Proc Natl Acad Sci USA. 1985;82:7039–7043. doi: 10.1073/pnas.82.20.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irjala H, Alanen K, Grénman R, Heikkilä P, Joensuu H, Jalkanen S. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)-1 direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res. 2003;63:4671–4676. [PubMed] [Google Scholar]
- Kannagi R, Hakomori S. A guide to monoclonal antibodies directed to glycotopes. Adv Exp Med Biol. 2001;491:587–630. doi: 10.1007/978-1-4615-1267-7_38. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T. Cancer metastasis: Characterization and identification of the behavior of metastatic tumor cells and the cell adhesion molecules, including carbohydrates. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:39–64. doi: 10.2174/1568006053005038. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Takazawa H, Imai S, Morimoto J, Watanabe T, Kanno M, Igarashi S. Expression of Vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast cancer cells in relation to lymphatic metastasis: Is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast Cancer Res Treat. 2006;98:31–43. doi: 10.1007/s10549-005-9115-6. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Kaidoh M, Yokoyama Y, Sano K, Ohhashi T. Chemokine CCL2 facilitates ICAM-1-mediated interactions of cancer cells and lymphatic endothelial cells in sentinel lymph nodes. Cancer Sci. 2009;100:419–428. doi: 10.1111/j.1349-7006.2008.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaldoyanidi SK, Glinsky VV, Sikora L, Glinskii AB, Mossine VV, Quinn TP, Glinsky GV, Sriramarao P. MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen–galectin-3 interactions. J Biol Chem. 2003;278:4127–4134. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- Kjeldsen T, Clausen H, Hirohashi S, Ogawa T, Iijima H, Hakomori S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2-6 alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 1988;48:2214–2220. [PubMed] [Google Scholar]
- Konno A, Hoshino Y, Terashima S, Motoki R, Kawaguchi T. Carbohydrate expression profile of colorectal cancer cells is relevant to metastatic pattern and prognosis. Clin Exp Metastasis. 2002;19:61–70. doi: 10.1023/a:1013879702702. [DOI] [PubMed] [Google Scholar]
- Kuduk SD, Schwarz JB, Chen XT, Glunz PW, Sames D, Ragupathi G, Livingstone PO, Danishefsky D. Synthetic and immunological studies on clustered modes of mucin-related Tn and TF O-linked antigens: The preparation of a glycopeptide-based vaccine for clinical trials against prostate cancer. J Am Chem Soc. 1998;120:12474–12485. [Google Scholar]
- Kumar SR, Sauter ER, Quinn TP, Deutscher SL. Thomsen-Friedenreich and Tn antigens in nipple fluid: Carbohydrate biomarkers for breast cancer detection. Clin Cancer Res. 2005;11:6868–6871. doi: 10.1158/1078-0432.CCR-05-0146. [DOI] [PubMed] [Google Scholar]
- Laack E, Nikbakht H, Peters A, Kugler C, Jasiewicz Y, Edler L, Hossfeld DK, Schumacher U. Lectin histochemistry of resected adenocarcinoma of the lung: Helix pomatia agglutinin binding is an independent prognostic factor. Am J Pathos. 2002;160:1001–1008. doi: 10.1016/S0002-9440(10)64921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Anver MR, Butcher DO, Gildersleeve JC. Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther. 2009;8:971–979. doi: 10.1158/1535-7163.MCT-08-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Lo-Man R, Vichier-Guerre S, Perraut R, Dériaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- Marttila-Ichihara F, Turja R, Miiluniemi M, Karikoski M, Maksimow M, Niemelä J, Martinez-Pomares L, Salmi M, Jalkanen S. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood. 2008;112:64–72. doi: 10.1182/blood-2007-10-118984. [DOI] [PubMed] [Google Scholar]
- Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: Capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25:305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- Numata Y, Nakada H, Fukui S, Kitagawa H, Ozaki K, Inoue M, Kawasaki T, Funakoshi I, Yamashina I. A monoclonal antibody directed to Tn antigen. Biochem Biophys Res Commun. 1990;170:981–985. doi: 10.1016/0006-291x(90)90488-9. [DOI] [PubMed] [Google Scholar]
- Nuti M, Teramoto YA, Mariani-Costantini R, Hand PH, Colcher D, Schlom J. A monoclonal antibody (B72.3) defines patterns of distribution of a novel tumor-associated antigen in human mammary carcinoma cell populations. Int J Cancer. 1982;29:539–545. doi: 10.1002/ijc.2910290509. [DOI] [PubMed] [Google Scholar]
- O’Boyle KP, Coatsworth S, Anthony G, Ramirez M, Greenwald E, Kaleya R, Steinberg JJ, Dutcher JP, Wiernik PH. Effects of desialylation of ovine submaxillary gland mucin (OSM) on humoral and cellular immune responses to Tn and sialylated Tn. Cancer Immun. 2006;6:5–14. [PubMed] [Google Scholar]
- O’Boyle KP, Markowitz AL, Khorshidi M, Lalezari P, Longenecker BM, Lloyd KO, Welt S, Wright KE. Specificity analysis of murine monoclonal antibodies reactive with Tn, sialylated Tn, T, and monosialylated (2→6) T antigens. Hybridoma. 1996;15:401–408. doi: 10.1089/hyb.1996.15.401. [DOI] [PubMed] [Google Scholar]
- O’Boyle KP, Wright KE. Anti-Tn human monoclonal antibodies generated following active immunization with partially desialylated ovine submaxillary mucin. Hum Antibodies Hybridomas. 1994;5:25–31. [PubMed] [Google Scholar]
- Oppezzo P, Osinaga E, Tello D, Bay S, Cantacuzene D, Irigoin F, Ferreira A, Roseto A, Cayota A, Alzari P, et al. Production and functional characterization of two mouse/human chimeric antibodies with specificity for the tumor-associated Tn-antigen. Hybridoma. 2000;19:229–239. doi: 10.1089/02724570050109620. [DOI] [PubMed] [Google Scholar]
- Pinho S, Marcos NT, Ferreira B, Carvalho AS, Oliveira MJ, Santos-Silva F, Harduin-Lepers A, Reis CA. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007;249:157–170. doi: 10.1016/j.canlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Reddish MA, Jackson L, Koganty RR, Qiu D, Hong W, Longenecker BM. Specificities of anti-sialyl-Tn and anti-Tn monoclonal antibodies generated using novel clustered synthetic glycopeptide epitopes. Glycoconj J. 1997;14:549–560. doi: 10.1023/a:1018576224062. [DOI] [PubMed] [Google Scholar]
- Serpa J, Mesquita P, Mendes N, Oliveira C, Almeida R, Santos-Silva F, Reis CA, LePendu J, David L. Expression of Lea in gastric cancer cell lines depends on FUT3 expression regulated by promoter methylation. Cancer Lett. 2006;242:191–197. doi: 10.1016/j.canlet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Sewell R, Bäckström M, Dalziel M, Gschmeissner S, Karlsson H, Noll T, Gätgens J, Clausen H, Hansson GC, Burchell J, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 2006;281:3586–3594. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- Siddiki B, Ho JJ, Huang J, Byrd JC, Lau E, Yuan M, Kim YS. Monoclonal antibody directed against colon cancer mucin has high specificity for malignancy. Int J Cancer. 1993;54:467–474. doi: 10.1002/ijc.2910540319. [DOI] [PubMed] [Google Scholar]
- Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky S, Livingston PO, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: Clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Metoki R, Hakomori S. Immunoglobulin G3 monoclonal antibody directed to Tn antigen (tumor-associated alpha-N-acetylgalactosaminyl epitope) that does not cross-react with blood group A antigen. Cancer Res. 1988;48:4361–4367. [PubMed] [Google Scholar]
- Terasawa K, Furumoto H, Kamada M, Aono T. Expression of Tn and sialyl-Tn antigens in the neoplastic transformation of uterine cervical epithelial cells. Cancer Res. 1996;56:2229–2232. [PubMed] [Google Scholar]
- Valentiner U, Hall DM, Brooks SA, Schumacher U. HPA binding and metastasis formation of human breast cancer cell lines transplanted into severe combined immunodeficient (scid) mice. Cancer Lett. 2005;219:233–242. doi: 10.1016/j.canlet.2004.07.046. [DOI] [PubMed] [Google Scholar]
- Yu LG. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconj J. 2007;24:411–420. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- Zou J, Glinsky VV, Landon LA, Matthews L, Deutscher SL. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis. 2005;6:309–318. doi: 10.1093/carcin/bgh329. [DOI] [PubMed] [Google Scholar]