Abstract
Understanding the factors that regulate the induction, quality and longevity of anti-viral T cell responses is essential for devising rational strategies to prevent or combat infections. In this study we show that interleukin-21 (IL-21), likely produced by CD4+ T cells, directly influences the generation of poly-functional CD8+ T cells and that the number of CD4+ T cells that produce IL-21 differs markedly between acute and chronic infections. Strikingly, IL-21 regulates the development of CD8+ T cell exhaustion and the ability to contain chronic lymphocytic choriomeningitis virus infection. Thus, IL-21 serves as a critical helper factor that shapes the functional quality of anti-viral CD8+ T cells and is required for viral control.
A hallmark of robust anti-viral immunity is the induction of CD4+ and CD8+ T cell responses, which act cooperatively and in conjunction with other immune system components, to control infection. The consequences of an ineffective immune response can be catastrophic, favoring viral persistence or the erosion of long-lived immunological memory. During the initial phases of many chronic infections, including hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections, CD8+ T cell responses are induced but can fail to attain or lose the ability to elaborate key effector functions (1–9). Although the cellular and molecular cues that dictate the development of robust CD8+ T cells are not fully elucidated, in the absence of CD4+ T cell help, CD8+ T cell responses are compromised (2, 5, 6, 10–18). CD4+ T cells are the primary producers of interleukin-21 (IL-21), a member of the common-γ chain family of cytokines (19–21). Functions of IL-21 are wide-ranging and include promoting B cell and antibody responses and inducing the development of Th17 and follicular helper CD4+ (Tfh) lineages (19–24). Given that CD4+ T cells are necessary for optimal anti-viral CD8+ T cell responses, we investigated the role of IL-21 in CD8+ T cell responses to viral infections.
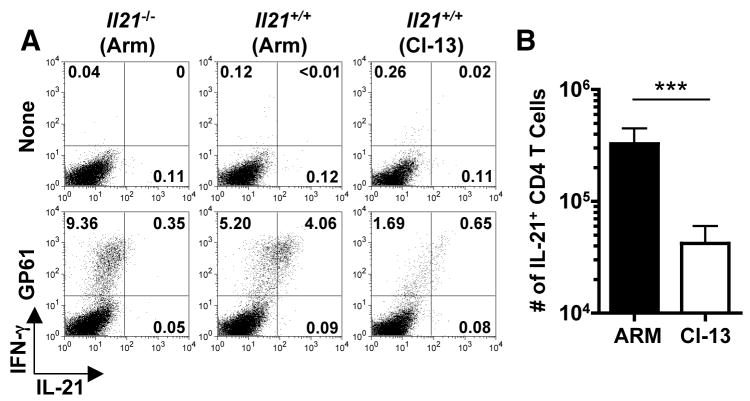
A comparative analysis of lymphocytic choriomeningitis virus (LCMV)-specific CD4+ T cells revealed marked differences in the induction of IL-21+ CD4+ T cells following acute LCMV-Armstrong (Arm) and chronic LCMV-clone 13 (Cl 13) infections of C57BL/6 (B6) mice (Fig. 1) (25). By eight days both infections elicited polyclonal virus-specific IL-21+ CD4+ T cells; however, this response was 7.8 fold lower in the LCMV-Cl 13 infected cohort (Fig. 1B; P <0.001). Since CD4+ T cells are obligatory for the control of LCMV-Cl 13 these results suggest that the CD4+ T cell response to this infection, albeit weak, is capable of providing some help (2, 5, 6, 10).
Fig. 1.
Diminished IL-21+ CD4+ T cell responses during the initial phase of LCMV-Cl 13 infection. IL-21 and IFN-γ production by LCMV GP61-80 CD4+ T cells was determined eight days following LCMV-Arm or Cl 13 infections of B6 mice. (A) Flow cytometric analysis of intracellular staining for IL-21 and IFN-γ in splenocytes from LCMV infected Il21−/− and Il21+/+ mice after stimulation with GP61-80 peptide. Gated total CD4+ T cells are shown. (B) Enumeration of IL-21-producing CD4+ T cells at eight days after LCMV-Arm or Cl 13 infection. Graphs represent mean ± SD; ***P < 0.001. Representative results are shown from two independent experiments (n = 8–9 for Il21+/+ cohorts and n = 2 for Il21−/− mice).
The pronounced IL-21+CD4+ T cell response following acute LCMV-Arm infection promoted us to analyze whether IL-21 influenced the generation of germinal center (GC) B cells, Tfh cells and antibody responses (fig. S1). The percentage and number of GC B cells and Tfh (ICOS+, CXCR5+) T cells as well as LCMV-specific antibody titers were similar between eight to nine days following LCMV-Arm infection of Il21+/+ and −/− mice. Thus, acute LCMV infection can trigger the development of these responses even in the absence of IL-21. Moreover, all cohorts successfully controlled LCMV-Arm infection as serum viral titers were below the limits of detection (<50pfu/ml) by this time point. Notably, although appreciable antibody levels were detectable between days 44–120 following infection, endpoint titers were three fold lower in Il21−/− mice.
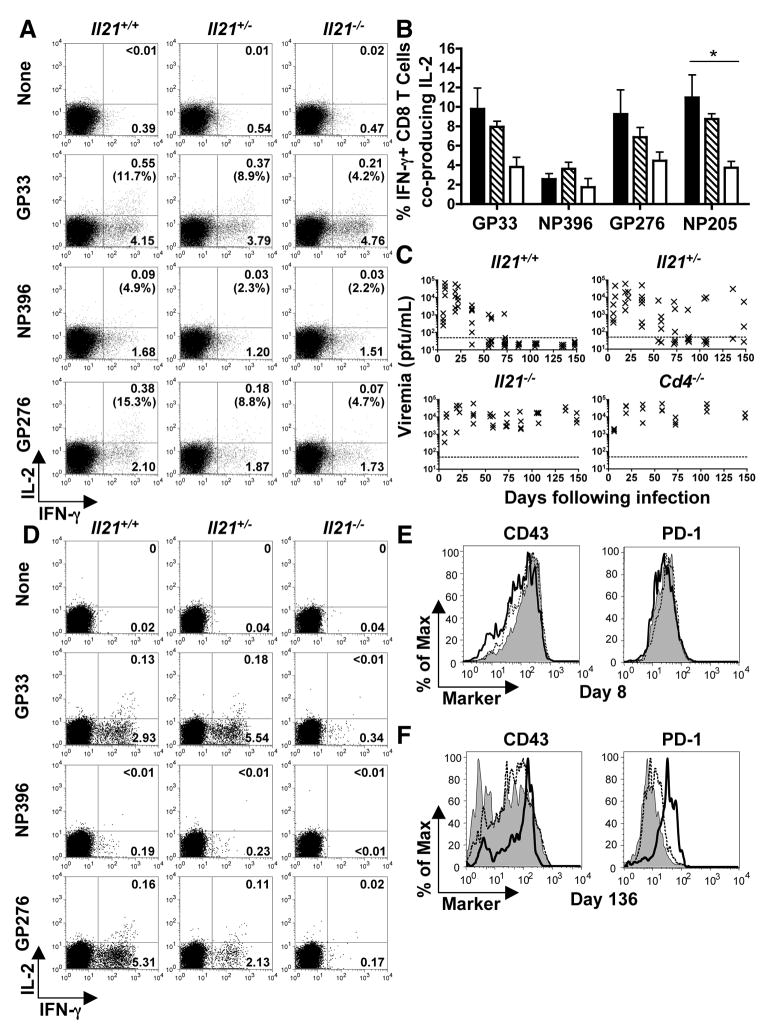
To further analyze the role of IL-21 in promoting anti-viral immunity we evaluated the responses of Il21+/+, +/−, and −/− mice to LCMV-Cl 13 infection. Mice infected with LCMV-Cl 13 typically exhibit high-grade viremia and progressive reductions in the functional capacity of anti-viral CD8+ T cells, termed “exhaustion” (1, 2, 5, 6, 9, 26). Eight days after LCMV-Cl 13 infection, during the effector phase, the magnitudes of the anti-viral CD4+ and CD8+ T cell responses and the frequency of interferon-γ (IFN-γ)producing anti-viral CD8+ T cells were similar between the Il21+/+, +/−, and −/− cohorts (fig. S2 and S3A and B); however, we observed differences in their functional quality (Fig. 2, A and B; fig. S3B). Both the percentage and absolute numbers of polyfunctional, IL-2-producing CD8+ T cells were reduced in Il21−/− mice, with Il21+/− mice exhibiting an intermediate phenotype (Fig. 2, A and B; fig. S3B). We observed similar trends for tumor necrosis factor-α (TNF-α) production (fig. S4A). Thus, IL-21 deficiency results in impaired polyfunctional effector CD8+ T cell responses during the initial phases of LCMV infection.
Fig. 2.
Severe CD8+ T cell exhaustion and viral persistence in the absence of IL-21. Splenic CD8+ T cell responses and viral titers were evaluated following LCMV-Cl 13 infection of Il21+/+, +/−, and −/− mice. (A) Flow cytometric analysis of intracellular cytokine staining for IFN-γ and IL-2 production by CD8+ T cells at eight days following infection after restimulation without or with the indicated peptide epitopes. Gated CD8+ T cells are shown and the percentages of CD8+, IFN-γ+ cells that co-produce IL-2 are reported in parentheses. (B) Percentages of epitope-specific CD8+, IFN-γ+ cells that coproduce IL-2 at eight days following infection. Error bars are SEM; * P<0.05 by comparison with Il21+/+ group. (C) Serum viral titers over time following LCMV-Cl 13 infection of Il21+/+, +/−, −/−, and Cd4−/− mice. Results from individual mice are shown; the dotted line represents the limit of detection. (D) IFN-γ and IL-2 production by LCMV-specific CD8+ T cells at 136 days following infection. Gated CD8+ T cells are shown. (E and F) CD43 and PD-1 expression by GP33 tetramer+ CD8+ T cells from Il21+/+ (shaded), +/− (dashed line), and −/− (bold line) mice at eight (E) and 136 days (F) post-infection. The Il21+/− data shown in (D) and (F) are from mice that were aviremic at the time of analysis. Representative or composite data are shown from two independent experiments (n=3–6).
Given the altered cytokine responses by anti-viral CD8+ T cells following LCMV-Cl 13 infection of IL21−/− mice, we next tracked viral loads over time to determine whether the absence of IL-21 compromised the containment of the infection (Fig. 2C; fig. S5). As expected, Il21+/+ hosts slowly controlled LCMV-Cl 13 infection (1, 2, 5, 9, 26). By contrast, viral titers in the serum, livers, and lungs of Il21−/− mice remained high, a phenotype similar to that observed in mice lacking CD4+ T cells (Fig. 2C; fig. S5). Il21+/− mice showed an intermediate pattern of clearance, with viral loads decreasing more slowly than in Il21+/+ mice, and the infection persisting at high levels or breaking through in three of the five Il21+/− mice tested between days 136 and 148 following infection. These data suggest that IL-21 is critical for control of chronic viral infection.
Evaluation of anti-viral CD8+ T cell responses at later stages after LCMV-Cl 13 infection revealed severe functional exhaustion in Il21−/− mice (Fig. 2D; fig. S3, C and D; fig. S4). By 136 days, Il21+/+ mice had brought the infection under control and IFN-γ, IL-2, (Fig. 2D) and TNF-α (fig. S4B) production was detectable by anti-viral, primarily GP-specific, CD8+ T cells. Moreover, these anti-viral CD8+ T were CD43int, programmed death-1 (PD-1)low, a phenotype more similar to resting memory T cells (Fig. 2E and F). In Il21+/− mice the phenotypic and functional properties of the anti-viral CD8+ T cells diverged depending upon their virological status. CD8+ T cells from mice able to contain the infection resembled anti-viral CD8+ T cells from Il21+/+; however, in mice with high viral titers, CD8+ T cell responses were more similar to those observed in Il21−/− hosts (fig. S4B). Strikingly, IL-2, IFN-γ and TNF-α production by Il21−/− virus-specific CD8+ T cells was greatly reduced or absent (Fig. 2D; fig. S3D; fig. S4B); but these T cells were CD43high, PD-1high (Fig. 2, E and F), a hallmark of the exhaustion that develops in chronically infected hosts (6, 9). Interestingly, the persistence of exhausted virus-specific CD8+ T cells in Il21−/− mice parallels what is observed following LCMV-Cl 13 infection of CD4-deficent hosts (2, 5, 6, 10). Thus, the absence of IL-21 production results in a failure to contain the infection and a silencing of anti-viral CD8+ T cell functions.
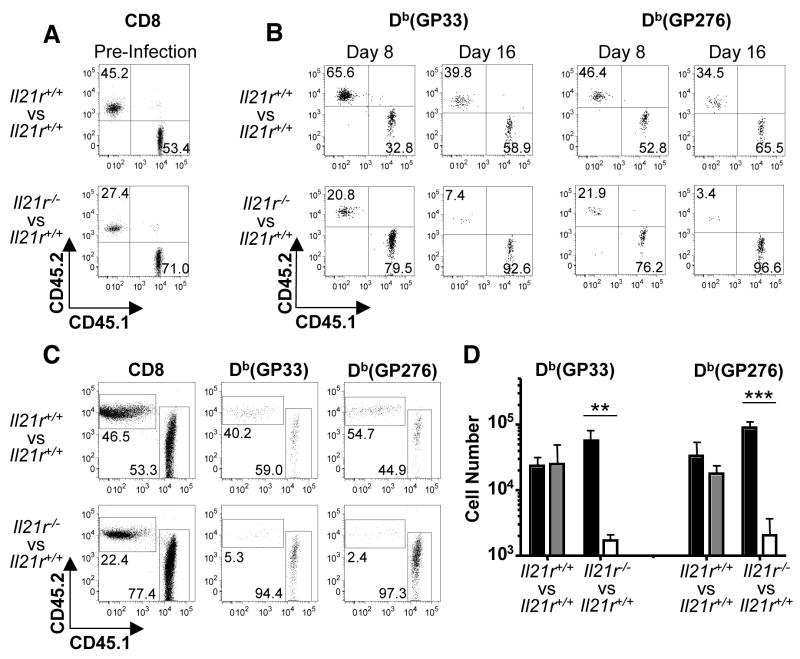
We next examined whether IL-21 was acting directly to promote and sustain CD8+ T cell responses. To directly compare, within the same host, the responses and fates of cells of the immune system that can and cannot perceive IL-21 derived-signals, we generated bone-marrow chimeras using a mixture of allelically marked Il21r+/+ (CD45.1) and Il21r−/− (CD45.2) donor cells. As a control, we also generated mixed bone-marrow chimeric mice that were reconstituted with Il21r+/+ (CD45.1) bone marrow and Il21r+/+ (CD45.2) bone marrow prepared from the littermates of Il21r−/− mice. Prior to infection the ratio of CD45.1:CD45.2 CD8+ T cells was 52:48 and 70:30 in the control and experimental cohorts, respectively. Nevertheless, both Il21r+/+ and Il21r−/− virus-specific CD8+ T cells were detectable in the circulation by eight days following LCMV-Cl 13 infection (Fig. 3). Thus the elaboration of primary virus-specific CD8+ T cell responses can occur independently of IL-21. Further tracking of virus-specific CD8+ T cells in the circulation at 16 days after infection revealed a preferential and rapid loss of Il21r−/− anti-viral CD8+ T cells. This was confirmed by enumeration of splenic responses three weeks after infection, which revealed a 40 fold lower number of Il21r−/− virus-specific CD8+ T cells by comparison with Il21r+/+ counterparts in the same host (Fig. 3, C and D). These data illustrate the direct requirement of IL-21 for supporting and maintaining anti-viral CD8+ T cells during chronic viral infections.
Fig. 3.
IL-21 acts directly to sustain virus-specific CD8+ T cells during an ongoing infection. Cohorts of control Il21r+/+/IL21r+/+ (CD45.1/CD45.2) and experimental IL21r+/+/IL21r−/− (CD45.1/CD45.2) mixed bone-marrow chimeras were infected with LCMV-Cl 13 and CD8+ T cell responses evaluated over time. (A) PBMCs were evaluated by flow cytometry to check reconstitution of CD8+ T cells in Il21r+/+/Il21r+/+ or Il21r+/+/Il21r−/− mixed bone-marrow chimeras prior to infection. Gated CD8+ T cells are shown. (B) Flow cytometric analysis of GP33- and GP276-specific CD8+ T cell responses in the circulation at days eight and 16 after infection. Gated tetramer+ CD8+ T cells are shown. (C) Flow cytometric analysis of splenic CD8+ T cells and GP33- and GP276-specific responses at three weeks following infection. Gated CD8+ (left panel) or CD8+ tetramer+ (right panels) cells are shown. (D) Absolute numbers of GP33- and GP276-specific CD8+ T cells in mixed bone-marrow chimeras three weeks following infection. Graphs represent average + SD of Il21r+/+ CD45.1 CD8+ T cells (black), Il21r+/+ CD45.2 CD8+ T cells (gray) and Il21r−/− CD45.2 CD8 T cells (white). **P < 0.01, ***P < 0.001. Representative results are shown from one of two similar experiments (n= 7 and 8 for the Il21r+/+/IL21r+/+ and IL21r+/+/IL21r−/− cohorts, respectively)
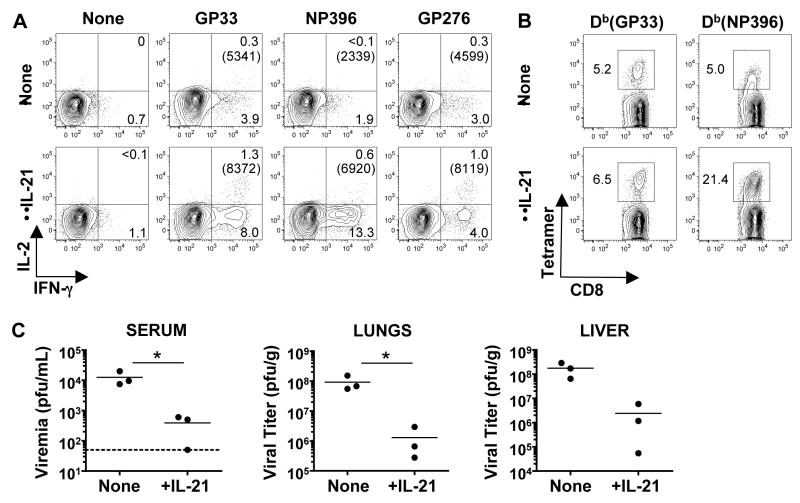
We next evaluated whether the addition of IL-21 enhanced anti-viral CD8+ T cells because the absence of IL-21-dependent signaling impaired the these responses. We investigated whether administration of IL-21 could improve responses and viral control in Cd4−/− mice because CD4+ T cells are a principle source of this cytokine. These “helpless” mice do not usually control LCMV-Cl 13 infection and develop severe CD8+ T cell exhaustion (2, 5, 6, 10). Daily injections of recombinant IL-21 to LCMV-Cl 13 infected Cd4−/− mice enhanced the functional quality of virus-specific CD8+ T cells, particularly the NP396 epitope-specific population, which usually rapidly succumbs to exhaustion (Fig 4). Importantly, IL-21 treatment resulted in lower viral titers (Fig. 4C). The delicate balance between the quality and size of the anti-viral immune response and the hosts’ viral burden became apparent as 70% of the treated mice became moribund, reaching experimental endpoints requiring euthanasia. Illness and death following LCMV infection is classically associated with immunopathology (27). Thus, although IL-21 administration can improve anti-viral CD8+ T cell responses and viral clearance, and has been safely used in the context of tumor immunotherapy in mice and humans, care will need to be taken before applying this treatment strategy to chronic viral infections (21, 28, 29).
Fig. 4.
IL-21 treatment enhances CD8+ T cell responses and reduces viral titers in Cd4−/− mice. LCMV-Cl 13 infected Cd4−/− mice were either left untreated or administered daily doses of 10μg recombinant IL-21 for eight days. At day nine after infection CD8+ T cell responses and viral loads were analyzed. (A) Flow cytometric analysis of intracellular staining of IFN-γ and IL-2 production in splenic virus-specific CD8+ T cells from control or treated cohorts. Gated CD8+ T cells are shown. The mean-fluorescence-intensity (MFI) of IFN-γ producing CD8+ T cells are reported in parentheses. (B) Flow cytometric analysis of GP33 and NP396 tetramer+ CD8+ T cells. Plots show gated CD8+ T cells. (C) Viral titers were assessed in the serum, lungs, and liver of control and IL-21-treated mice. Dotted line indicates the limit of detection (50 pfu/mL) for serum samples. *P < 0.05. Representative results from one of two independent experiments are shown (n= 7 and 6 for control and treated groups, respectively).
Collectively, our findings provide new insights into the determinants of the functional quality of CD8+ T cell responses and the role of IL-21 in ensuring the successful control of infection. The results implicate IL-21 as a critical helper factor which couples the requirement for CD4+ T cells which produce this cytokine, to the elaboration and maintenance of polyfunctional CD8+ T cells capable of clearing virus infected cells. We observed that induction of IL-21-producing CD4+ T cells is markedly reduced during the initial phase of chronic viral infection, suggesting that this could be a general feature of infections that elicit non-protective adaptive immune responses, such as hepatitis B virus, HCV, and HIV (8, 30). This poor induction of IL-21 may result from the failure to initiate robust CD4+ T cell responses as well as the exhaustion or viral-induced depletion of these cells which may occur during certain chronic infections. Therefore, we anticipate that the cautious development of approaches to modulate the levels of IL-21, or regulate the induction of cellular subsets that generate IL-21, will provide new therapeutic opportunities to improve immunity to diseases that require CD8+ T cell responses to be controlled, such as chronic viral infection and tumors.
Supplementary Material
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Nature. 1993;362:758. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 2.Matloubian M, Concepcion RJ, Ahmed R. J Virol. 1994;68:8056. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oxenius A, Zinkernagel RM, Hengartner H. Immunity. 1998;9:449. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 4.Gallimore A, et al. J Exp Med. 1998;187:1383. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zajac AJ, et al. J Exp Med. 1998;188:2205. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. J Immunol. 2004;172:4204. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 7.Brooks DG, Teyton L, Oldstone MB, McGavern DB. J Virol. 2005;79:10514. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letvin NL, Walker BD. Nat Med. 2003;9:861. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. J Virol. 2003;77:4911. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battegay M, et al. J Virol. 1994;68:4700. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Herrath MG, Yokoyama M, Dockter J, Oldstone MB, Whitton JL. J Virol. 1996;70:1072. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. J Virol. 2002;76:12388. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourgeois C, Veiga-Fernandes H, Joret AM, Rocha B, Tanchot C. Eur J Immunol. 2002;32:2199. doi: 10.1002/1521-4141(200208)32:8<2199::AID-IMMU2199>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Janssen EM, et al. Nature. 2003;421:852. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 15.Shedlock DJ, Shen H. Science. 2003;300:337. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 16.Sun JC, Bevan MJ. Science. 2003;300:339. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CM, et al. Nat Immunol. 2004;5:1143. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 18.Khanolkar A, Fuller MJ, Zajac AJ. J Immunol. 2004;172:2834. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- 19.Parrish-Novak J, et al. Nature. 2000;408:57. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 20.Brandt K, Singh PB, Bulfone-Paus S, Ruckert R. Cytokine Growth Factor Rev. 2007;18:223. doi: 10.1016/j.cytogfr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Spolski R, Leonard WJ. Annu Rev Immunol. 2008;26:57. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki K, et al. Science. 2002;298:1630. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 23.Korn T, et al. Nature. 2007;448:484. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Immunity. 2009;30:324. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Materials and methods are available as supporting material on Science Online.
- 26.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. J Exp Med. 1984;160:521. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchmeier MJ, Zajac AJ. In: Persistent viral infections. Ahmed R, Chen ISY, editors. John Wiley and Sons Ltd; West Sussex, England: 1999. pp. 575–605. [Google Scholar]
- 28.Zeng R, et al. J Exp Med. 2005;201:139. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodds MG, et al. Cancer Immunol Immunother. 2009;58:843. doi: 10.1007/s00262-008-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boni C, et al. J Virol. 2007;81:4215. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.We thank Laurie Harrington, Paul Goepfert, Beatrice Hahn, George Shaw, and the members of the Zajac laboratory for discussions and assistance. This work was supported by grants AI049360 and AI067993 (to AJZ), and T32 AI007051 (to JSY) from the National Institutes of Health.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.