Abstract
The principal nucleus of the bed nucleus of the stria terminalis (BNSTp) is larger in volume and contains more cells in male than female mice. These sex differences depend on testosterone and arise from a higher rate of cell death during early postnatal life in females. There is a delay of several days between the testosterone surge at birth and sexually dimorphic cell death in the BNSTp, suggesting that epigenetic mechanisms may be involved. We tested the hypothesis that chromatin remodeling plays a role in sexual differentiation of the BNSTp by manipulating the balance between histone acetylation and deacetylation using a histone deacetylase inhibitor. In the first experiment, a single injection of valproic acid (VPA) on the day of birth increased acetylation of histone H3 in the brain 24 h later. Next, males, females, and females treated neonatally with testosterone were administered VPA or saline on postnatal d 1 and 2 and killed at 21 d of age. VPA treatment did not influence volume or cell number of the BNSTp in control females but significantly reduced both parameters in males and testosterone-treated females. As a result, the sex differences were eliminated. VPA did not affect volume or cell number in the suprachiasmatic nucleus or the anterodorsal nucleus of the thalamus, which also did not differ between males and females. These findings suggest that a disruption in histone deacetylation may lead to long-term alterations in gene expression that block the masculinizing actions of testosterone in the BNSTp.
Changing histone acetylation blocks masculinization of the brain.
The bed nucleus of the stria terminalis is a sexually dimorphic limbic forebrain structure involved in the control of male sex behavior, gonadotropin release, and the modulation of stress and anxiety (1,2,3,4,5). The principal nucleus of the bed nucleus of the stria terminalis (BNSTp) is larger and contains more cells in males than females of several species, including humans (6,7,8,9,10). In rats and mice, sexually dimorphic cell death during the first week of life can account for the sex difference in cell number in the BNSTp seen in adulthood (7,11,12), and this sex difference can be eliminated by neonatal castration of males or treating females with testosterone propionate (TP) at birth (6,13).
A single neonatal injection of TP leads to a reduction of cell death in females about 5 d later (12). This suggests a cellular memory for the early hormone exposure consistent with changes in the epigenome. A number of epigenetic processes have been identified, including DNA methylation and covalent modifications of histones (14). Steroid hormones can induce such chromatin modifications to bring about long-lasting changes in gene expression in cancer cell lines, peripheral tissues, and the brain (15,16,17,18).
Of the many possible histone modifications, acetylation is currently the best understood in terms of effects on gene transcription. Addition of acetyl groups to lysine residues on histone tails is catalyzed by histone acetyltransferases (HATs) and is most commonly associated with transcriptional activation. Conversely, histone deacetylation is generally associated with repression of transcription (19,20,21), although there are some important exceptions (22,23,24). Several of the best-known steroid hormone receptor coactivators have HAT activity or recruit HATs to the transcription complex (25,26,27), whereas corepressors often have histone deacetylase (HDAC) activity. Thus, changes in histone acetylation are likely to be important for many effects of steroid hormones.
We hypothesized that the effects of testosterone on sexual differentiation of the brain requires orchestrated changes in histone acetylation. To test this, we administered an HDAC inhibitor, valproic acid (VPA), to neonatal mice during the critical period for sexual differentiation and examined the volume and cell number of the BNSTp and two control regions at 3 wk of age. Our results suggest that neonatal disruption of histone deacetylation blocks masculinization of the BNSTp.
Materials and Methods
Animals
Wild-type C57BL/6 mice from our breeding colony were housed under 14-h light, 10-h dark conditions at 22 C. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts (Amherst, MA).
Tissue preparation for immunoblotting
Mice were injected sc with 50 mg/kg VPA on the day of birth and killed by rapid decapitation at 6, 12, 24, 48, 72, or 96 h after injection. Five males and five females were collected at each time point. Brains were removed and rapidly frozen with 2-methylbutane kept at −80 C. One hemisphere of each brain was homogenized over ice in 30 volumes of radioimmunoprecipitation assay buffer [0.05 m Tris, 1% Igepal CA-630, 0.1% sodium dodecyl sulfate, 0.5% deoxycholate, 1 mm sodium orthovanadate, and protease inhibitor cocktail (Sigma, St. Louis, MO)]. Protein was extracted at 4 C for 30 min followed by centrifugation at the same temperature for 20 min at 15,000 × g. The resulting supernatant was aliquoted and stored at −80 C before SDS-PAGE analysis. Total protein concentration was determined with the BCA protein assay (Pierce, Rockford, IL) using BSA as the standard.
SDS-PAGE
Equal amounts of protein (15 μg/lane) from each sample were combined with loading buffer and boiled for 5 min. Samples were run on a 12% precast Tris-HCl polyacrylamide gel (Bio-Rad, Hercules, CA), followed by protein electrotransfer at 100 V for 25 min onto a polyvinylidine difluoride membrane (Bio-Rad). Membranes were then rinsed in 0.05 m Tris-buffered saline (TBS), followed by blocking in 5% nonfat dry-milk in TBS for 1 h. Acetylation of histone H3 is often used as a measure of HDAC inhibition (28). Membranes were therefore probed with an antibody directed against acetylated histone H3 (AcH3; Upstate, Temecula, CA) overnight at 4 C. This antibody recognizes acetylated Lys-9 and Lys-14 on histone 3 and has previously been used to detect AcH3 in the mouse brain (29). Membranes were rinsed with TBS containing 0.2% Tween 20 and incubated with a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. After washing in TBS containing 0.2% Tween 20, membranes were immersed in enhanced chemiluminescent reagent (ECL-Plus; Amersham Biosciences, GE Healthcare, UK), and the resulting chemiluminescent reaction was exposed to film (Biomax MR; Kodak, Rochester, NY). Membranes were subsequently stripped and reprobed with an antibody against β-actin (Sigma) to verify equal loading of proteins.
OD of protein bands was determined using Image J densitometry software (National Institutes of Health, Bethesda, MD). The OD of the AcH3 band was divided by the OD of the β-actin band for each sample. Equal numbers of saline and VPA-treated animals were run on each gel, and data were expressed as percent of saline control.
Testosterone and VPA treatments
We first conducted a pilot study to determine an appropriate dose of VPA for neonatal mice. Doses used routinely in adult mice (100–500 mg/kg) often caused pup mortality. In contrast, 50 mg/kg VPA was well tolerated by pups and led to reliable changes in histone acetylation in the brain (see below).
Females received TP (100 μg in 25 μl peanut oil) or an equal volume of oil on the day of birth; males were treated with oil. Half of the animals in each of the three hormonal conditions also were treated with VPA (50 mg/kg in 0.9% saline) or saline on postnatal d 1 and 2. Two daily doses of VPA were administered to overlap with the expected period of elevated testosterone after TP injection [>24 h (30)]. Animals were weighed and killed by CO2 inhalation at 3 wk of age, after the period of sexually dimorphic cell death (11). Brains were removed, postfixed in 5% acrolein in 0.1 m phosphate buffer for 4 h, and immersed in 30% sucrose until sectioning. Brains were cut (40 μm) in the coronal plane on a freezing microtome and alternate sections were mounted, dehydrated, and stained with thionin.
BNSTp volume and cell counts
All measurements were made on slides coded to conceal the sex and treatment of the animals. StereoInvestigator software (MicroBrightfield, Williston, VT) was used to measure nuclear volume and perform stereological cell counts as previously described (7). The outline of the BNSTp was traced bilaterally in every section in which it appeared, and volume was calculated by multiplying the summed area by the sampling ratio and section thickness. The optical dissector method was used to perform stereological counts. A counting frame of 16 × 16 μm was systematically moved throughout the traced region of the BNSTp, with counts performed every 75 μm (i.e. a sampling grid of 75 × 75 μm). Upper and lower guard zones of 1 μm were used to avoid edge artifacts. Cells with a visible nucleus that fell within the counting frame and had the morphological characteristics of neurons were counted. Volume measurements and cell counts were also carried out for two control regions, the suprachiasmatic nucleus of the hypothalamus (SCN) and the anterodorsal nucleus of the thalamus (AD). These regions were chosen because they have clear boundaries, occur in the same sections as the BNSTp, and were not expected to be sexually dimorphic.
Statistical analysis
There was no effect of sex on histone acetylation in the brain after VPA treatment in a three-way ANOVA (sex by time by treatment). Therefore, data from males and females were combined in subsequent analyses. Effects of VPA on histone acetylation were tested with a two-way ANOVA (treatment by time), followed by planned comparisons of individual time points. Volume and cell number in the BNSTp, SCN, and AD were also analyzed using two-way ANOVAs (group by drug treatment). Planned comparisons used Fisher’s least significant differences.
Results
VPA increases AcH3 in the brain
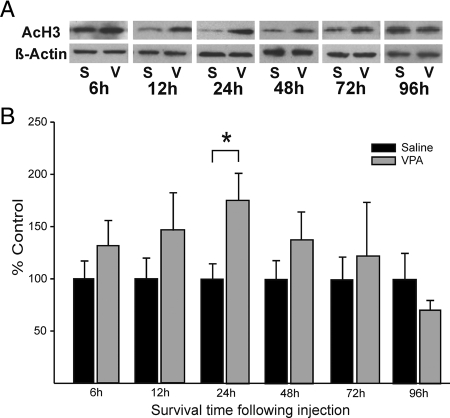
Levels of AcH3 in the brain were measured at six time points after a single injection of 50 mg/kg VPA on the day of birth (Fig. 1). ANOVA revealed a significant overall effect (P < 0.05), with higher levels of AcH3 in VPA-treated animals. Although AcH3 levels appeared elevated at 6, 12, 24, and 48 h after injection, planned comparisons at individual time points indicated that the difference reached significance only at 24 h (P < 0.05; Fig. 1). Thus, a single injection of VPA on the day of birth causes a transient increase in AcH3.
Figure 1.
VPA increases AcH3 in the mouse brain. A, Western blots showing levels of AcH3 and β-actin 6, 12, 24, 48, 72, or 96 h after saline (S) or VPA (V) treatment. Data from males and females were combined for this analysis. B, Mean ± sem level of AcH3 corrected for β-actin and expressed as percent of saline control for each time-point (n = 8–10/group). VPA significantly increased AcH3 24 h after injection. *, P < 0.05 and across all time points combined (main effect of treatment, P < 0.05).
VPA blocks masculinization of BNSTp volume and cell number
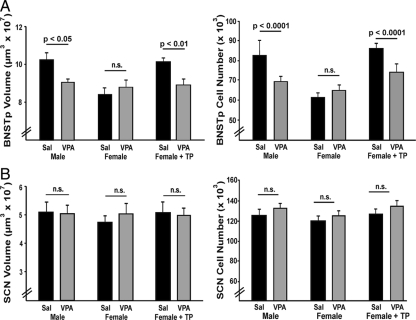
As predicted, volume and cell number in the BNSTp were significantly greater in oil-treated males than oil-treated females on postnatal d 21 (volume: P < 0.0001; cell number: P < 0.0001, Figs. 2 and 3). In females treated with TP at birth, BNSTp volume and cell number were increased relative to oil-treated females (volume: P < 0.0001; cell number: P < 0.0001, Fig. 3) and did not differ from males on either measure (P > 0.6).
Figure 2.
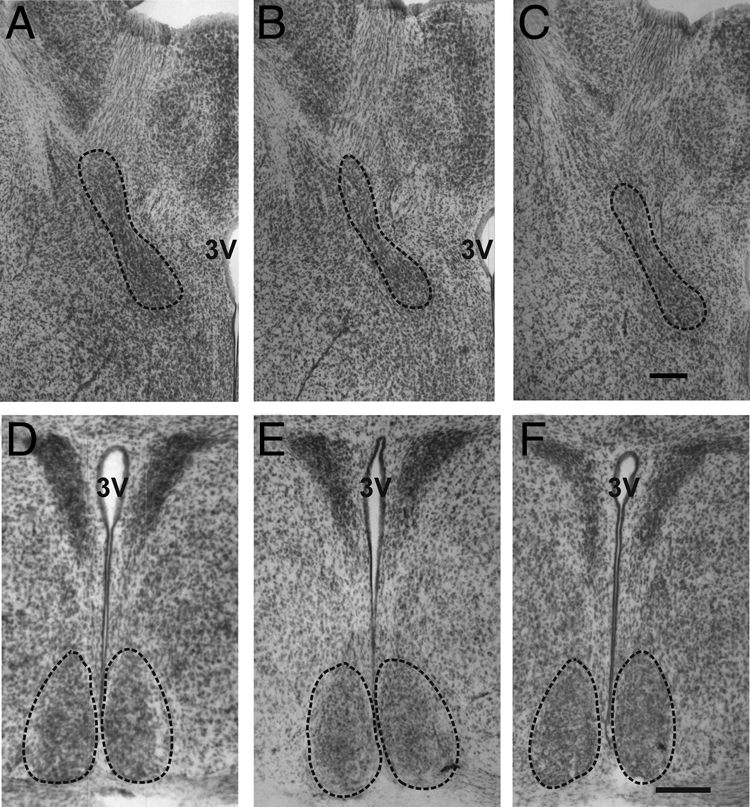
Representative photomicrographs through the BNSTp (dotted line, A–C) and SCN (dotted line, D–F) of a control male (A and D), control female (B and E), and VPA-treated male (C and F). Volume of the BNSTp was larger in males than females. Masculinization of BNSTp volume was blocked by VPA treatment. There was no effect of sex or treatment on the volume of the SCN. Scale bar in C, 100 μm for A–C; in F, 200 μm for D–F. 3V, Third ventricle.
Figure 3.
Effect of VPA treatment on volume and cell number in the BNSTp and SCN of males, females, and TP-treated females treated with saline (Sal) or VPA on postnatal d 1 and 2 (n = 9–11/group). A, Volume and cell number of the BNSTp were greater in males and TP-treated females than oil-treated females. VPA blocked masculinization of the BNSTp in males and TP-treated females but did not affect BNSTp volume or cell number in control females. B, VPA had no effect on volume or cell number in the SCN in any group. n.s., Not significant.
Treatment with VPA reduced overall volume and cell number in the BNSTp of males (volume: P < 0.05; cell number: P < 0.0001) and TP-treated females (volume: P = 0.01; cell number: P < 0.0001; Figs. 2 and 3) but had no effect on these measures in oil-treated females (P > 0.3). Males and TP females treated with VPA did not differ from control females on any measure. Thus, masculinization of the BNSTp was blocked by VPA treatment. These findings were reflected in significant group-by-treatment interactions in the two-way ANOVAs (volume: P < 0.005; cell number: P < 0.0001).
There were no sex differences and no effect of VPA treatment in the SCN (Figs. 2 and 3) or AD (data not shown). VPA also had no effect on body weight; treated animals, if anything, were slightly (nonsignificantly) heavier than controls (saline: 9.57 ± 0.36 g; VPA: 9.93 ± 0.19 g; P > 0.25).
Discussion
Several recent studies provided evidence for sex differences in epigenetic alterations in the brain. In rats, levels of the DNA methyl binding protein, MeCP2, are transiently elevated in the amygdala and ventromedial hypothalamus of females at birth (31), and decreasing MeCP2 expression in the amygdala prevents sex differences in juvenile social play (32). In addition, levels of acetylated and trimethylated histone H3 are sexually dimorphic (greater in males) in the cortex and hippocampus of perinatal mice (33). The functional consequences of these sex differences are unknown, and to date, no study has manipulated epigenetic processes and subsequently examined the effect on sexual differentiation of brain morphology. Here we report that treatment with a histone deacetylase inhibitor during the critical period for sexual differentiation prevents masculinization of the BNSTp in mice. Males and androgenized females treated neonatally with VPA had female-like BNSTp volume and cell number. Importantly, effects of VPA depended on hormonal status because VPA had no effect on the BNSTp of females in the absence of testosterone. Taken together, our data are consistent with the hypothesis that testosterone acts through epigenetic processes, in particular the regulation of histone acetylation, to direct sexual differentiation of the brain.
A growing number of histone deacetylase inhibitors are currently available. VPA was chosen in this study primarily for its long half-life in vivo: approximately 12 h in adults and longer in newborns (34). By contrast the commonly used HDAC inhibitor trichostatin A has a half-life less than 10 min in mice (35). We find that a single injection of VPA at birth increases levels of acetylated histone H3 in the brain 24 h later, suggesting that systemic VPA treatment of pups can disrupt brain histone acetylation patterns during the critical period for sexual differentiation.
However, several alternative explanations for our results must be considered. Like all HDAC inhibitors, VPA has activities independent of HDAC inhibition (36). For example, long-term administration of VPA increases γ-aminobutyric acid (GABA) levels in the brain (37), and GABA has been implicated in sexual differentiation (38). Male rats have higher levels of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD) than do females in some regions of the hypothalamus and hippocampus neonatally (39), and a blockade of GAD reduces male sexual behavior in androgenized females (39,40). As far as we know, sex differences in GAD or GABA have not been reported in the bed nucleus of the stria terminalis. Moreover, if our findings were due to effects on GABA, one would predict masculinization in response to VPA treatment, whereas the opposite was found.
VPA also may have teratogenic effects on developing rodents (41,42). The studies reporting such effects use doses of VPA approximately 10-fold higher than that used here and administer VPA much earlier in development. In addition, although VPA can be cytotoxic to cancer cell lines (43,44,45), it is often protective to neurons (44,46,47,48,49). Importantly, BNSTp volume and cell number were unchanged in females treated with VPA in the current study, and there was no effect of treatment on volume or cell number in two control regions. These observations argue against a nonspecific or toxic effect of VPA and instead suggest a specific interaction between VPA treatment and hormone status.
It is not yet known how VPA blocks masculinization of the BNSTp. A generalized disruption of histone deacetylation could potentially exert widespread effects on the epigenome, although in normal cell populations, treatment with an HDAC inhibitor alters the expression of a relatively small number of genes. For example, less than 2% of more than 30,000 genes surveyed in the hippocampus were changed by HDAC inhibition in vivo (50). Interestingly, several investigators have noted that genes regulated by HDAC inhibitors fall predominantly into two categories: those related to cell cycle and apoptosis (51,52). This suggests that cells undergoing active regulation may be particularly sensitive to HDAC inhibition.
It is difficult to predict the direction of changes in gene expression after VPA treatment. Although HDAC inhibition might be expected to increase the expression of genes, in fact, treatment with an HDAC inhibitor leads to nearly equal numbers of genes with increased vs. decreased expression (53,54,55). This is perhaps not surprising because the protein products of some genes will suppress the expression of other genes. In addition, for some genes an increase in histone acetylation directly inhibits expression (22,23,56). The sex difference in cell number in the BNSTp is determined by hormone-dependent sexually dimorphic cell death during development and requires bax, a prodeath member of the bcl-2 family of apoptosis-related genes (7,11). In the neonatal rat preoptic area, testosterone decreases Bax but increases Bcl-2 protein expression (57). Therefore, a disruption of the hormonal regulation of bax or other bcl-2 family genes is a potential mechanism of VPA action in the current study; future studies will identify direct and indirect gene targets of neonatal VPA administration.
Understanding the epigenetic regulation of cell number in the BNSTp in response to hormones may have important clinical implications. The BNSTp plays a vital role in modulating emotional and stress responses, with lesions increasing the hypothalamic-pituitary-adrenal response to acute stress (58,59). This indicates that the BNSTp normally reduces stress responsivity and suggests that the larger BNSTp in males may contribute to the known sex difference in stress responsiveness (60). Because VPA eliminated sex differences in the BNSTp in the current study, it would be interesting to test the effects of neonatal VPA treatment on sex differences in the hypothalamic-pituitary-adrenal axis and anxiety-related behaviors. In humans, women are more susceptible to mood disorders than men (60,61), and there are sex differences in the prevalence of most psychiatric illnesses. Interestingly, gender-specific epigenetic modifications have recently been reported in the brains of patients with schizophrenia and bipolar disorder (62). This suggests that sex differences in the epigenome may underlie sex biases in the incidence of neurological disorders.
Acknowledgments
We thank Jill McCutcheon and Lynn Bengston for technical assistance and Benjamin Rood for helpful comments on the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants RO1-MH047538 (to G.J.d.V.), RO1-MH068482 and KO2-MH072825 (to N.G.F.).
Disclosure Summary: E.K.M. and A.H. have nothing to disclose. G.J.d.V. is a co-principal investigator on a grant proposal related to this project. N.G.F. is principal investigator on a grant proposal related to this project.
First Published Online June 4, 2009
For article see page 3980
Abbreviations: AcH3, Acetylated histone H3; AD, anterodorsal nucleus of the thalamus; BNSTp, principal nucleus of the bed nucleus of the stria terminalis; GABA, γ-aminobutyric acid; GAD, glutamic acid decarboxylase; HAT, histone acetyltransferase; HDAC, histone deacetylase; SCN, suprachiasmatic nucleus of the hypothalamus; TBS, Tris-buffered saline; TP, testosterone propionate; VPA, valproic acid.
References
- Emery DE, Sachs BD 1976 Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav 17:803–806 [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S 1980 Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinology 30:238–242 [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M 2003 Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463:199–216 [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF 2004 Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci 118:443–448 [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ 2005 The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci 119:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón A, Segovia S, del Abril A 1988 Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res 44:281–290 [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ 2004 Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA 101:13666–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA 1985 Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci 5:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA 1992 Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res 579:321–326 [DOI] [PubMed] [Google Scholar]
- Allen LS, Gorski RA 1990 Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol 302:697–706 [DOI] [PubMed] [Google Scholar]
- Gotsiridze T, Kang N, Jacob D, Forger NG 2007 Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol 67:355–362 [DOI] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, De Vries GJ 2000 Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol 43:234–243 [PubMed] [Google Scholar]
- Hisasue S, Seney ML, Immerman E, Forger NG 2007 Cell death and the development of sex differences in the bed nucleus of the stria terminalis in mice. Society for Neuroscience Meeting Planner Abstract 294:23 [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E 2007 Epigenetics: a landscape takes shape. Cell 128:635–638 [DOI] [PubMed] [Google Scholar]
- Ruiz-Cortés ZT, Kimmins S, Monaco L, Burns KH, Sassone-Corsi P, Murphy BD 2005 Estrogen mediates phosphorylation of histone H3 in ovarian follicle and mammary epithelial tumor cells via the mitotic kinase, Aurora B. Mol Endocrinol 19:2991–3000 [DOI] [PubMed] [Google Scholar]
- Zhu X, Asa SL, Ezzat S 2008 Fibroblast growth factor 2 and estrogen control the balance of histone 3 modifications targeting MAGE-A3 in pituitary neoplasia. Clin Cancer Res 14:1984–1996 [DOI] [PubMed] [Google Scholar]
- Guo JZ, Gorski J 1989 Estrogen effects on modifications of chromatin proteins in the rat uterus. J Steroid Biochem 32:13–20 [DOI] [PubMed] [Google Scholar]
- Mani ST, Thakur MK 2006 In the cerebral cortex of female and male mice, amyloid precursor protein (APP) promoter methylation is higher in females and differentially regulated by sex steroids. Brain Res 1067:43–47 [DOI] [PubMed] [Google Scholar]
- Grunstein M 1997 Histone acetylation in chromatin structure and transcription. Nature 389:349–352 [DOI] [PubMed] [Google Scholar]
- Marmorstein R 2004 Structural and chemical basis of histone acetylation. Novartis Found Symp 259:78–98; discussion 98–101, 163–169 [PubMed] [Google Scholar]
- Cosgrove MS, Wolberger C 2005 How does the histone code work? Biochem Cell Biol 83:468–476 [DOI] [PubMed] [Google Scholar]
- Nusinzon I, Horvath CM 2003 Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc Natl Acad Sci USA 100:14742–14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfer L, Huang J, Swaby LA, Augenlicht L 2004 Requirement of histone deacetylase activity for signaling by STAT1. J Biol Chem 279:30358–30368 [DOI] [PubMed] [Google Scholar]
- Wilson MA, Ricci AR, Deroo BJ, Archer TK 2002 The histone deacetylase inhibitor trichostatin A blocks progesterone receptor-mediated transactivation of the mouse mammary tumor virus promoter in vivo. J Biol Chem 277:15171–15181 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW 1997 Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194–198 [DOI] [PubMed] [Google Scholar]
- Kishimoto M, Fujiki R, Takezawa S, Sasaki Y, Nakamura T, Yamaoka K, Kitagawa H, Kato S 2006 Nuclear receptor mediated gene regulation through chromatin remodeling and histone modifications. Endocr J 53:157–172 [DOI] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL 2007 Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, Kim JM, Park DK, Kun Lee S, Kim M, Roh JK 2007 Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis 26:464–472 [DOI] [PubMed] [Google Scholar]
- Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich JB, Aunis D, Zwiller J 2006 Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol 70:487–492 [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM 1977 Plasma testosterone and sexual behavior following intracerebral implantation of testosterone propionate in the castrated male rat. Horm Behav 8:77–87 [DOI] [PubMed] [Google Scholar]
- Kurian JR, Forbes-Lorman RM, Auger AP 2007 Sex difference in MeCP2 expression during a critical period of rat brain development. Epigenetics 2:173–178 [DOI] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP 2008 MeCP2 organizes juvenile social behavior in a sex-specific manner. J Neurosci 28:7137–7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HW, Grant PA, Rissman EF 2009 Sex differences in histone modifications in the neonatal mouse brain. Epigenetics 4:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau H, Rating D, Koch S, Häuser I, Helge H 1981 Valproic acid and its metabolites: placental transfer, neonatal pharmacokinetics, transfer via mother’s milk and clinical status in neonates of epileptic mothers. J Pharmacol Exp Ther 219:768–777 [PubMed] [Google Scholar]
- Sanderson L, Taylor GW, Aboagye EO, Alao JP, Latigo JR, Coombes RC, Vigushin DM 2004 Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin A after intraperitoneal administration to mice. Drug Metab Dispos 32:1132–1138 [DOI] [PubMed] [Google Scholar]
- Blaheta RA, Michaelis M, Driever PH, Cinatl Jr J 2005 Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev 25:383–397 [DOI] [PubMed] [Google Scholar]
- Löscher W 1993 Effects of the antiepileptic drug valproate on metabolism and function of inhibitory and excitatory amino acids in the brain. Neurochem Res 18:485–502 [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Perrot-Sinal TS 2002 Getting excited about GABA and sex differences in the brain. Trends Neurosci 25:307–312 [DOI] [PubMed] [Google Scholar]
- Davis AM, Grattan DR, Selmanoff M, McCarthy MM 1996 Sex differences in glutamic acid decarboxylase mRNA in neonatal rat brain: implications for sexual differentiation. Horm Behav 30:538–552 [DOI] [PubMed] [Google Scholar]
- Davis AM, Grattan DR, McCarthy MM 2000 Decreasing GAD neonatally attenuates steroid-induced sexual differentiation of the rat brain. Behav Neurosci 114:923–933 [PubMed] [Google Scholar]
- Alsdorf R, Wyszynski DF 2005 Teratogenicity of sodium valproate. Expert Opin Drug Saf 4:345–353 [DOI] [PubMed] [Google Scholar]
- Gravemann U, Volland J, Nau H 2008 Hydroxamic acid and fluorinated derivatives of valproic acid: anticonvulsant activity, neurotoxicity and teratogenicity. Neurotoxicol Teratol 30:390–394 [DOI] [PubMed] [Google Scholar]
- Gao D, Xia Q, Lv J, Zhang H 2007 Chronic administration of valproic acid inhibits PC3 cell growth by suppressing tumor angiogenesis in vivo. Int J Urol 14:838–845 [DOI] [PubMed] [Google Scholar]
- Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, Lu RB, Gean PW, Chuang DM, Hong JS 2007 Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 149:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DY, Cayo MA, Adler JT, Ning L, Haymart MR, Kunnimalaiyaan M, Chen H 2008 Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg 247:1036–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragancokova D, Jahn K, Kotsiari A, Schlesinger F, Haastert K, Stangel M, Petri S, Krampfl K 28 Mar 2009 Analysis of neuroprotective effects of valproic acid on primary motor neurons in monoculture or co-cultures with astrocytes or Schwann cells. Cell Mol Neurobiol 10.1007/s10571-009-9393-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM 2008 Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci 28:2576–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM 2008 Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 155:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai F, Yamamoto Y, Miyaguchi K, Zhou Z, Sumi H, Hamasaki T, Goto M, Sakoda S 2004 Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur J Neurosci 20:3179–3183 [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M 2006 Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA 103:3480–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK 2003 Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther 2:151–163 [PubMed] [Google Scholar]
- Menegola E, Di Renzo F, Broccia ML, Giavini E 2006 Inhibition of histone deacetylose as a new mechanism for teratogenesis. Birth Defects Res C Embryo Today 78:345–353 [DOI] [PubMed] [Google Scholar]
- Mariadason JM, Corner GA, Augenlicht LH 2000 Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res 60:4561–4572 [PubMed] [Google Scholar]
- Nusinzon I, Horvath CM 2005 Unexpected roles for deacetylation in interferon- and cytokine-induced transcription. J Interferon Cytokine Res 25:745–748 [DOI] [PubMed] [Google Scholar]
- Chen B, Cepko CL 2007 Requirement of histone deacetylase activity for the expression of critical photoreceptor genes. BMC Dev Biol 7:78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HM, Paulson M, Holko M, Rice CM, Williams BR, Marié I, Levy DE 2004 Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci USA 101:9578–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Kakeyama M, Toyofuku Y 2006 Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol 66:1411–1419 [DOI] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP 2008 The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology 149:818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP 2007 Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27:2025–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL 2006 Stress sensitivity and the development of affective disorders. Horm Behav 50:529–533 [DOI] [PubMed] [Google Scholar]
- Altemus M 2006 Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav 50:534–538 [DOI] [PubMed] [Google Scholar]
- Connor CM, Akbarian S 2008 DNA methylation changes in schizophrenia and bipolar disorder. Epigenetics 3:55–58 [DOI] [PubMed] [Google Scholar]