Abstract
TNF-α signals through two receptors, TNFR1 and TNFR2. Our goals were: 1) determine the role of TNFRs in obesity and metabolic disease and 2) investigate whether TNFRs contribute to the link between obesity and adipose tissue macrophage infiltration and polarization. R1−/−R2−/− (RKO) and wild-type (WT) mice were fed standard chow or a high-fat/high-sucrose diet (HFHS) over 14 wk. Body composition, food intake, and energy expenditure were measured. Oral glucose tolerance and insulin sensitivity tests assessed glucose homeostasis. Adipose tissue and systemic inflammatory status were evaluated by quantifying plasma adipokine levels and macrophage-specific gene expression in fat. RKO mice were heavier (10%) and fatter (18%) than WT controls at 4 wk of age and were 26% heavier and 50% fatter than WT after 14 wk of HFHS diet feeding. Age- and diet-adjusted 24-h oxygen consumption, activity, and respiratory exchange ratio were significantly reduced in RKO mice. Obese RKO mice were markedly insulin resistant, suggesting that intact TNFR signaling is not required for the effect of obesity to impair glucose metabolism. Adipose tissue from HFHS-fed RKO mice exhibited increased macrophage infiltration, but compared with WT mice, macrophage phenotypic markers featured a predominance of antiinflammatory M2 over proinflammatory M1 cells. TNFRs play a physiological role to limit body weight and adiposity by modestly increasing metabolic rate and fatty acid oxidation, and they are required for obesity-induced activation of adipose tissue macrophages. Despite these effects, TNFRs are not required for obesity-induced insulin resistance.
In humans and rodents, obesity is associated with low-grade systemic and tissue inflammation, yet ablation of cytokines and their receptors, such as the two TNF receptors studied herein, predispose mice to obesity and do not protect mice from insulin resistance.
Type 2 diabetes mellitus (T2D) is prevalent in the United States, affecting more than 18 million people. Obesity predisposes individuals to insulin resistance and T2D and is also associated with other comorbidities including cancer and cardiovascular disease (1,2,3). In obese individuals, proinflammatory cytokines produced as a result of increasing adiposity can influence both energy balance and glucose homeostasis. Unexpectedly, genetic ablation of IL-1 receptor (IL-1R), inducible nitric oxide synthase (iNOS), IL-18, or IL-6 predisposes mice to maturity-onset obesity and also limits the associated systemic inflammation and insulin resistance (4,5,6,7,8). In humans, increased fat mass (FM) is associated with elevations in the expression of multiple proteins implicated in impaired insulin signaling (9,10), and genetic studies have linked polymorphisms in several cytokine genes to body mass index and obesity (11,12). Yet little is known about the cellular and molecular targets of cytokine action in the control of energy balance or predisposition to diabetes.
TNF-α is the prototypical inflammatory cytokine discovered initially as a mediator of cancer cachexia (13). TNF is produced by macrophages, T cells, endothelial cells, adipocytes, and a variety of other cell types. TNF signals through two transmembrane receptors, TNFR1 (R1) and TNFR2 (R2) (14,15,16), which are also expressed in multiple cell types (17). Whereas interventions that reduce circulating TNF levels [e.g. by immunodepletion or inactivation of genes encoding either TNF or TNF-α converting enzyme (TACE)] have beneficial effects on glucose metabolism, their consequences with respect to obesity are less clear (18,19,20,21). These considerations prompted us to consider the role of TNFRs in obesity and glucose homeostasis.
Obesity and insulin resistance are accompanied by a low-grade inflammatory state characterized in part by elevated levels of circulating cytokines, adipokines, and chemokines and an accumulation of macrophages in adipose tissue (22,23,24,25,26,27). Although adipose tissue macrophages (ATMs) exhibit a variety of phenotypes, in obese states, they are characterized by a predominance of classically activated M1 cells expressing proinflammatory proteins including IL1-α, TNF, IL-6, IL-18, and iNOS (26,28). In contrast, ATMs in lean mice express primarily antiinflammatory proteins associated with the M2 phenotype, such as Chi3l3 (also known as Ym1), Arginase1 (Arg1), and IL-10 (26,28,29). An altered balance between M1 and M2 macrophages is thought to mediate, at least in part, the effect of obesity to reduce insulin sensitivity (26,28). Because TNF and its receptors are central to macrophage function, another goal of this study was to investigate whether TNFRs contribute to the link between obesity and ATM infiltration and polarization. Our data indicate that whereas the absence of both TNFR subtypes predisposes to obesity by modulating metabolism in mice, it does not protect against obesity-induced insulin resistance. Additionally, TNFRs are not required for macrophage infiltration into adipose tissue but do contribute to the obesity-induced shift in ATM activation phenotype.
Materials and Methods
Mice
Male mice deficient in both TNFR1 and TNFR2 (RKO) and isogenic control C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME; strains 03234 and 0064, respectively) and housed four per cage unless otherwise noted. At 4 wk of age, mice were randomly assigned to two diet groups and fed either pelleted rodent chow or a high-fat/high-sucrose (HFHS) (30,31,32) diet for up to 14 wk unless noted otherwise (see Figs. 1 and 3 for additional details). At necropsy, mice were fasted for 4 h in the morning, bled from the retroorbital sinus into tubes containing 1 mm EDTA, killed by cervical dislocation, and tissues collected and stored at −80 C until analyses. Mice were maintained in a specific pathogen-free animal facility at the University of Washington at 25 C with a fixed 12-h light, 12-h dark cycle. All procedures were done in accordance with current National Institutes of Health guidelines and approved by the Animal Care and Use Committee of the University of Washington.
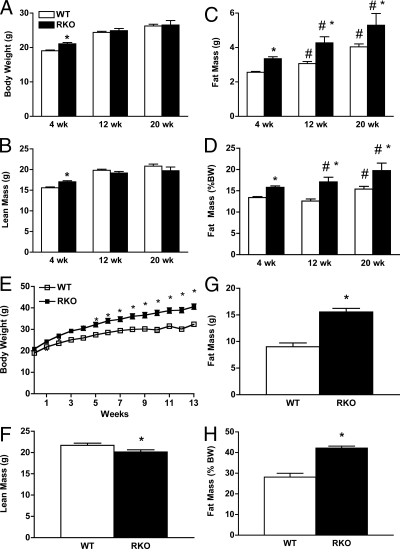
Figure 1.
Body weight and composition for WT and RKO mice. Mice were maintained on rodent chow [4% fat (wt/wt), 24% protein, and 4.5% crude fiber] and aged for 4, 12, and 20 wk and body weight (A), lean mass (B), FM (C), and FM as percent of total body weight (D) were determined. Body composition was obtained using quantitative magnetic resonance imaging as described in the text (n = 10–12 per strain per group). *, P < 0.0001 between genotypes; #, P < 0.05 between ages. A separate set of mice were fed the HFHS diet for 13 wk, and body weight (E), lean mass (F), FM (G), and FM as percent of total body weight (H) were determined. The HFHS diet (no. F1850; Bioserve, Frenchtown, NJ) contained 35.5% fat (primarily lard), 20% protein, and 36.6% carbohydrate (primarily sucrose) (n = 10–12 per group). *, P < 0.05.
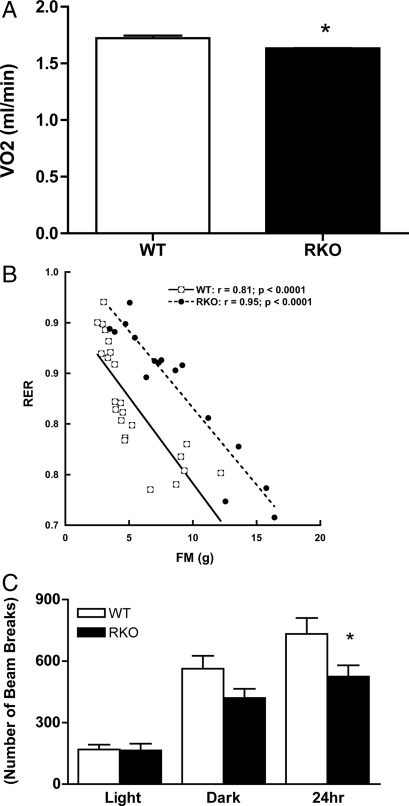
Figure 3.
Adjusted VO2 and RER vs. FM relationship for WT and RKO mice. A, Mean 24-h VO2 values for WT (white bar) and RKO (black bar) mice after adjustment for body weight and age group. RKO mice show a significant reduction in VO2 compared with WT mice (P < 0.05). B, Scatterplot showing strong negative associations between RER and FM for WT (white squares) and RKO (black circles) mice. The significant upward shift in RER for RKO relative to WT mice indicates that the RKO genotype causes a relative defect in fat oxidation. Data used in A and B are for mice fed rodent chow or HFHS diet for 4 and 9 wk with n = 23 for WT and n = 16 for RKO. *, P < 0.05. C, Ambulatory activity measured as beam breaks for WT (white bar) and RKO (black bar) for mice fed diets for 9 wk (n = 8). *, P < 0.01. For calorimetric measurements, mice were individually housed and acclimated to calorimeter cages for 1 d before 2–3 d of data collection for gas exchanges. Indirect calorimetry was performed with a computer-controlled open circuit calorimetry system (Oxymax; Columbus Instruments) comprised of four respiratory chambers equipped with a stainless steel elevated wire floor, water bottle, and food tray connected to a balance. Rates of VO2 and VCO2 were measured for each mouse at 6-min intervals, and room air reference values were determined after every 10 measurements. Gas sensors were calibrated daily with primary gas standards containing known concentrations of O2, CO2, and N2 (Tech Air, White Plains). Statistical analyses involved multiple regression control for the influence of body size (and other covariates) on metabolic rate. This approach is well established in metabolic analysis (56,57,58,59), particularly in human obesity research (e.g. Ref. 40,41), and avoids the spurious conclusions that can result when metabolic rate is simply divided by body weight or by LBM (58). For each mouse, VO2, VCO2, and RER were averaged over the 24-h period encompassing the second light cycle and second dark cycle (n = 23 for WT and n = 16 for RKO). *, P < 0.05.
Plasma lipid measurements
Plasma total cholesterol and triglyceride levels were determined using colorimetric kits as described previously (33).
Glucose and insulin tolerance tests
After a 16-h overnight fast, mice were orally gavaged or ip injected with 1 mg glucose per gram body weight or injected ip with 1 mU Humulin R insulin per kilogram body weight (Elli Lilly, Indianapolis, IN). Blood glucose was monitored (OneTouch Ultra; LifeScan Inc., Milpitas, CA) before and serially after glucose or insulin administration.
Plasma insulin and adipokine measurements
Plasma insulin levels were measured using the Linco insulin ELISA (Millipore, St. Charles, MO; catalog EZRMI-13K). Plasma levels of TNF, IL-6, leptin, plasminogen activator inhibitor (PAI)-1, resistin, and macrophage chemoattractant protein (MCP)-1 were determined using the Lincoplex adipokine panel (Millipore; catalog MADPK-71K) with plate reading done on a Luminex 100 (Luminex Corp., Austin TX).
Body composition analysis
Body composition was performed on conscious immobilized mice using quantitative magnetic resonance (EchoMRI whole body composition analyzer; Echo Medical Systems, Houston, TX) (34).
Energy balance
Food Intake was measured over 20 d for HFHS-fed and over 4 d for chow-fed, singly housed mice. Evaluation of energy metabolism was done by indirect calorimetry [measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2)], as described previously (18) (also see Fig. 3). The respiratory exchange ratio (RER; an indicator of substrate use) was computed as VCO2 divided by VO2.
Locomotor activity
Ambulatory activity was measured using an Opto-Varimetrix-3 sensor system (Columbus Instruments, Columbus, OH). Consecutive adjacent infrared beam breaks were scored on individual mice as an activity count. Counts were recorded each hour for 24 h.
Real-time quantitative RT-PCR
Total RNA samples were isolated from epididymal adipose tissue using the RNeasy minikit (Qiagen, Valencia, CA) and quantified by spectrometry. First-strand cDNAs were synthesized from 2.0–4.0 μg of total RNA with Moloney murine leukemia virus reverse transcriptase. The primers and probes were purchased from IDT (Coralville, IA). The sequences of primers/probes are given in supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Results were analyzed using the δ-δ cycle threshold method (35).
Statistical analysis
Data are presented as mean ± se and statistical significance was established at α < 0.05, two tailed. The t tests were done using the Prism statistical programming package (La Jolla, CA) and a two-way ANOVA was used to evaluate main effects and interactions involving genotype and diet status. For calorimetry studies and associated FM analyses, statistical associations between dependent and independent variables with adjustment for variation in body size were assessed with multiple regression analyses (see Fig. 3 legend) using the general linear model option in SPSS (version 16; SPSS Inc., Chicago, IL).
Results
RKO mice have greater adiposity than wild-type (WT) controls
Body weight and composition are shown for chow-fed WT and RKO mice at 4, 12, and 20 wk of age (Fig. 1). RKO mice were 10% heavier than WT mice at 4 wk of age, and RKO mice had a significantly greater proportion of body weight as fat than WT mice at all time points (Fig. 1D). Thus, when fed a standard chow diet, RKO mice exhibit a modest excess FM from a very young age, which is maintained through at least the first 4 months of life.
Feeding the HFHS diet for 13 wk increased body weight gain in both genotypes compared with rodent chow, but the effect was greater for RKO than WT mice (Fig. 1E). At 13 wk, RKO mice were 35% heavier than WT controls, and this effect was due to increased FM because lean body mass was 15% reduced for RKO vs. WT mice (Fig. 1F). RKO FM was 77% greater than that of WT mice (Fig. 1G), and the proportion of body weight as fat was 50% greater than WT mice fed the HFHS diet (Fig. 1H). ANOVA revealed both a significant effect of diet alone (P < 0.0001) and an unambiguous genotype by diet interaction (P < 0.001). Thus, RKO mice have increased susceptibility to diet-induced obesity (DIO).
Further evidence for the importance of genotype on FM was revealed using multiple regression analysis of mice fed chow and HFHS diets for 4 and 9 wk. After adjustment for lean body mass (LBM), age group, locomotor activity, and diet, the effect on FM of the RKO genotype was 4.08 ± 0.58 g (P < 0.0001), and again there was a significant genotype by diet interaction (P = 0.003), indicating an enhanced susceptibility to DIO in the RKO mice. Overall, the RKO genotype was associated with a significant and substantial effect to increase FM in comparison with mice of the same LBM, age, and diet history.
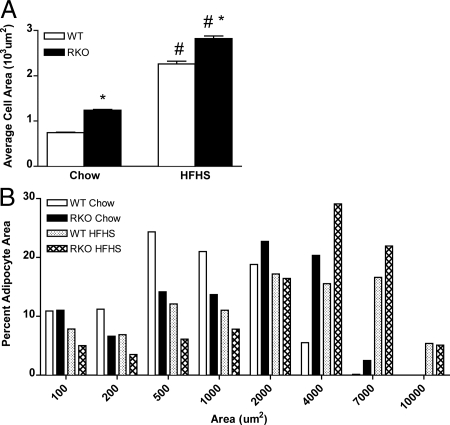
Adipocyte size is a marker related to both FM and fat distribution in humans (36) and rodents (37). The mean cell area of epididymal adipocytes increased for WT (300%) and RKO mice (230%) fed the HFHS diet compared with control chow (Fig. 2). For both diets, adipocyte area was significantly greater for RKO than WT mice (66 and 25% for chow and HFHS diets, respectively; P < 0.01). Taken together, these data demonstrate that the absence of both TNFR subtypes leads to excessive FM on standard chow and predisposes to obesity induced by a HFHS diet.
Figure 2.
Adipocyte areas for WT and RKO mice. A, Mean adipocyte cell areas for WT (white bars) and RKO (black bars) mice fed control rodent chow or the HFHS diet for 14 wk. B, Adipocyte cell area as percent of total counted cells for chow (n = 4000 cells) and HFHS (n = 2000 cells) diets. Epididymal fat pads were collected and fixed overnight for paraffin embedding. Three consecutive 5-μm sections were taken 100 μm apart and mounted on glass slides. The sections were dewaxed in xylene (2 × 5 min) and cleared in petroleum ether for 5 min (Fluka, St. Louis, MO). The sections were then stained with hematoxylin and eosin and dehydrated through graded alcohol and coverslips applied with Permount (Fisher Scientific, Pittsburgh, PA). Computer-assisted morphometry was used to determine adipocyte areas [Image J software (shareware); National Institutes of Health, Bethesda, MD]. In total, 4000 and 2000 cells were included for the chow and HFHS, groups, respectively (n = 4 per group). *, P < 0.0001. For both diets, adipocyte area was significantly greater for RKO than WT mice (66 and 25% for chow and HFHS diets, respectively; P < 0.01). The distribution of adipocyte areas also differed between strains (B). For control chow-fed WT and RKO mice, about 50% of adipocytes were between 500 and 1,000 μm2 and 1,000 and 2,000 μm2, respectively, and for mice fed the HFHS diet, adipocyte sizes ranged from 2,000 to 7,000 μm2 and 4,000 to 10,000 μm2 for WT and RKO mice, respectively.
RKO mice are hypometabolic and hypoactive with reduced lipid oxidation
Food intake was evaluated both during metabolic cage studies and by direct measures of food eaten over 20 d by animals housed singly in their home cage. No differences in food intake between genotypes (∼5 g/d per mouse for control chow and ∼4 g/d per mouse for HFHS diet) were detected with either intake measurement on either diet.
We used indirect calorimetry to account for differences in adiposity between RKO and WT mice fed either chow or HFHS diets for 4 or 9 wk. Based on an analysis that accounted for genotype, diet, and age group but did not account for variation in body size, VO2 did not differ significantly between RKO and WT mice (P = 0.60). However, after adjustment for total body mass and age group, RKO mice exhibited a significantly lower 24-h average VO2 in comparison with WT animals (−0.091 ± 0.038 ml/min; P = 0.022) (Fig. 3A). There was no evidence of a genotype by body weight interaction when this term was included in the analysis (P = 0.58). When the analysis was adjusted for LBM, FM, and age group, a similar effect of genotype on VO2 was observed (RKO minus WT difference: −0.081 ± 0.041 ml/min; P = 0.054). In the latter model, both LBM and FM were significant determinants of VO2, with FM being the more reliable of the two. Specifically, the estimated contribution to VO2 of LBM was 35 ± 17 ml/kg · min (P = 0.047), whereas that for FM was 30 ± 6 ml/kg · min (P < 0.0001), indicating that FM can be an important determinant of metabolic rate in mice. When the statistical analysis included diet in addition to the body size and age variables, genotype was not significant (P = 0.99). However, this may reflect multicollinearity (genotype was collinear with the variate comprised of diet, LBM, FM, and age group; r = 0.76; P < 0.0001) and the modest sample sizes in this analysis. Nonetheless, our analysis did indicate that RKO mice exhibit a reduced body size- and age-adjusted VO2 in comparison with WT animals, an effect that could help explain the obese phenotype of the RKO mutants.
Based on multiple regression analysis with RER as the dependent variable and genotype, diet, age, LBM, and FM simultaneously included as independent variables, each of the latter with the exception of LBM (P = 0.409) were significantly associated with RER. Of particular note was that the RKO genotype conferred an adjusted increase in RER of 0.035 ± 0.011 relative to WT mice (P = 0.004), indicating a significant decrease of fat oxidation in mutant animals (legend for Fig. 3B). In both genotypes, each per-gram increase of FM independently yielded an adjusted change in RER of −0.006 ± 0.002 (P = 0.021), consistent with an effect of increased of fat storage to promote a shift to increased lipid oxidation. Similarly, the HFHS diet was associated with a substantial adjusted change in RER equal to −0.097 ± 0.019 (P < 0.0001), consistent with the known effect of high-fat feeding to favor increased lipid oxidation. The younger mice exhibited an adjusted RER that was lower (by −0.008 ± 0.003) in comparison with older animals (P = 0.016). Thus, the obese phenotype of the RKO mice may stem, in part, from reduced fat oxidation relative WT animals of similar body composition, diet history, and age.
Compared with WT mice, 24-h ambulatory activity was about 25% lower for RKO mice (P < 0.01) (Fig. 3C). In an unadjusted analysis of locomotor activity (Fig. 2C), RKO mice exhibited significantly lower activity in comparison with WT mice (−143.71 ± 50.81 counts/min; P = 0.008). This hypoactivity phenotype remained significant after adjustment for body weight (−114.96 ± 52.65 counts/min; P = 0.036) or body weight and diet simultaneously (−115.43 ± 51.13 counts/min; P = 0.030). Thus, in comparison with WT animals, the RKO mice were less active, an effect that could also contribute to their obesity.
Overall, these data indicate that the increased adiposity exhibited by RKO mice reflects subtle decreases in oxygen consumption, lipid oxidation, and activity.
RKO mice exhibit features of the metabolic syndrome
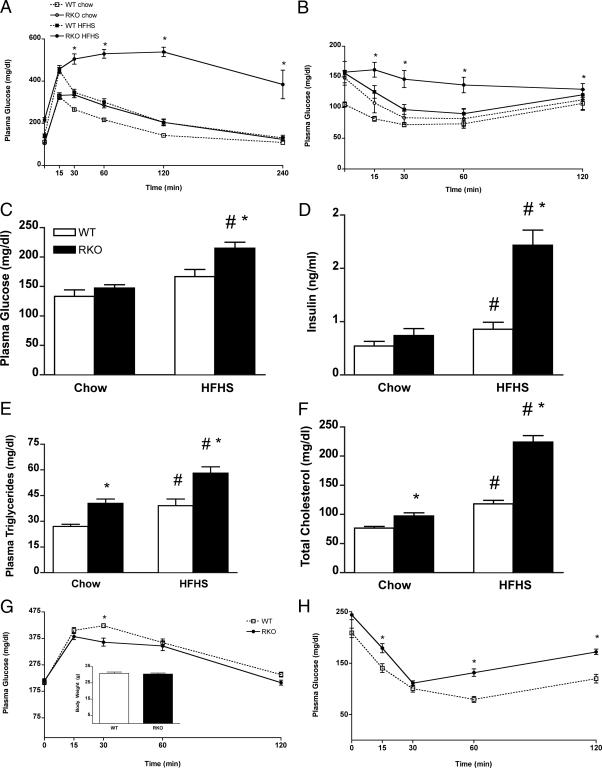
In humans, the metabolic syndrome is characterized the combination of obesity, insulin resistance, dyslipidemia, and hypertension, and several of these features were seen for RKO mice. RKO mice exhibited aberrant glucose homeostasis compared with WT mice (Fig. 4, A–D). After an oral glucose challenge, the returns to pregavage glucose values for RKO mice were significantly (control chow) and severely (HFHS) delayed compared with WT mice (Fig. 4A), indicating impaired oral glucose tolerance. RKO mice also exhibited features of insulin resistance, as indicated by a 50% elevation of plasma insulin at t = 30 min of the oral glucose tolerance test compared with WT mice. Moreover, the rate of glucose lowering after ip insulin injection was significantly reduced in RKO mice (Fig. 4B). Finally, plasma glucose and insulin levels after a 4-h fast were 30 and 330% higher, respectively, in RKO vs. WT mice fed the HFHS diet (Fig. 4, C and D).
Figure 4.
Glucose homeostasis and plasma lipid quantification for WT and RKO mice. A, Oral glucose tolerance test performed at 12 wk of diet feeding. B, Insulin sensitivity test performed at 13 wk of diet feeding. Blood glucose (C) and plasma insulin (D) levels measured after a 4-h fast at 13 wk of diet challenge. Plasma total TG (E) and cholesterol (F) levels after a 16-h fast at 14 wk of diet challenge (n = 9–12 per group). *, P < 0.05 between genotypes; #, P < 0.05 between diets. Glucose homeostasis was also assessed among mice matched for body weight. Intraperitoneal glucose tolerance test (G) and insulin sensitivity test (H) for weight-matched RKO (solid line) and WT (dotted line) mice that were fed the HFHS diet for 4 and 8 wk, respectively, are shown. WT (white bar) and RKO (black bar) mice were approximately 30 g at the time of these tests (n = 9–12). *, P < 0.05.
Plasma total cholesterol and triglyceride (TG) levels were increased by 27 and 50%, respectively, for chow-fed RKO compared with WT mice (Fig. 4, E and F). Although both genotypes showed the expected increase of plasma total cholesterol and TG levels with HFHS feeding (31), the increase of TG in RKO mice (48%), was greater than in WT controls (44%), likely reflecting greater insulin resistance and consequent increased production of hepatic TG-rich lipoproteins (38). Collectively, these results indicate that TNFR deficiency does not attenuate obesity-induced deterioration of glucose and lipid homeostasis.
Insulin resistance in RKO mice is secondary to excess body fat
To determine whether the increased insulin resistance of RKO mice is a direct result of the loss of TNFRs or is secondary to their greater obesity, we compared measures of glucose homeostasis between WT and RKO mice after matching them for body weight. Mice were fed the HFHS diet for 4 wk (RKO) or 7 wk (WT), and glucose and insulin tolerance tests were conducted for mice of each genotype with a mean body weight of 30 g (Fig. 4G, inset). Although differences between genotypes were seen for glucose levels, the overall glucose excursion (Fig. 4G) and clearance (Fig. 4H) responses were comparable between strains and did not show the wide disparity as seen in Fig. 4, A and B. These data suggest that for RKO mice, increased insulin resistance is secondary to greater body fat accumulation rather than a direct consequence of reduced TNF signaling.
Leptin and systemic inflammatory markers
For mice fed chow, plasma leptin levels were 5-fold higher for RKO than WT mice (Fig. 5A). After 14 wk of HFHS feeding, leptin levels were 2-fold higher for RKO than WT mice. The increased leptin levels are consistent with the greater adiposity of RKO mice.
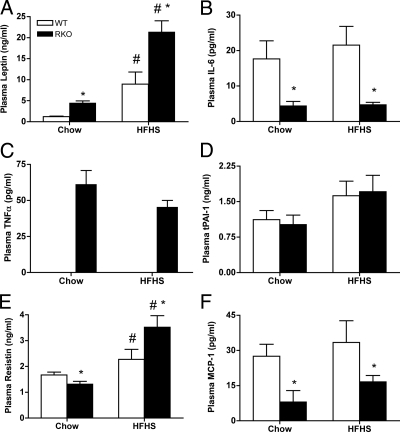
Figure 5.
Plasma adipokine panel. At 14 wk of control chow and HFHS diet challenge, plasma from 4-h fasted animals was evaluated for levels of leptin (A), IL-6 (B), TNF (C), tissue plasminogen activator inhibitor (D), resistin (E), and MCP-1 (F), and data are presented as picograms per milliliter for n = 9–12 per group. *, P < 0.05 between genotypes; #, P < 0.05 between diets.
Obesity in mice and humans is associated with increased circulating levels of inflammatory adipokines (23,24). With ablation of TNFRs, we expected reduced inflammatory adipokine responses in plasma of RKO mice. Whereas plasma TNF levels were below the assay detection level (<12 pg/ml) in WT mice (Fig. 5C), they were markedly elevated (40–55 pg/ml) in RKO mice to levels comparable with those seen in mice with severe obesity and diabetes (e.g. db/db mice). Resistin levels, associated with insulin resistance in mice (39,40), were also elevated in RKO vs. WT controls fed the HFHS diet (Fig. 5E), whereas circulating levels of IL-6 and MCP-1 were reduced (Fig. 5, B and F), suggesting a tendency toward reduced systemic inflammation compared with WT mice.
Adipose tissue inflammation and macrophage phenotypic markers are altered in RKO mice
Obesity is accompanied by macrophage infiltration into adipose tissue and increased expression of proinflammatory adipokines and cytokines (24,26,41). We hypothesized that loss of TNFRs would influence ATM phenotype and diminish the general inflammatory state of adipose tissue. This was tested by quantifying molecular markers expressed in epididymal fat depots of mice fed the HFHS or chow diet for 13 wk (Fig. 6).
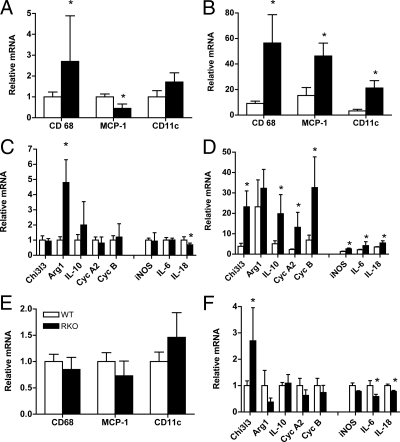
Figure 6.
Real-time PCR determination of mRNA levels for a panel of macrophage, inflammatory, and antiinflammatory markers in adipose tissue. Epididymal fat pads were collected and processed for total RNA, and quantitative RT-PCR was performed as described in the text. Data are presented as relative fold changes as normalized to WT mice. The accumulation and proinflammatory status of ATMs correlate with insulin resistance in humans and mice (22,26,27). Although ATMs exhibit a wide range of functions and gene expression profiles (29), M1 macrophages are associated with elevated expression of iNOS, and M2 macrophages express higher relative levels of Arg1 and Chi3l3 (26,29). CD11c expression is indicative of M1 macrophages and dendritic and T cells and is associated with insulin resistance (60). Other inflammatory (IL-6, IL-18) or antiinflammatory/remodeling [IL-10, cyclin A2 (Cyc A2), cyclin B (Cyc B)] genes are expressed in multiple cell types. CD68 mRNA levels were used as an indicator of relative macrophage content. CD-68, MCP-1, and CD11c mRNA levels for WT (white bars) and RKO (black bars) mice fed control rodent chow (A) or the HFHS diet (B) for 13 wk. Macrophage and adipose tissue mRNA markers for proinflammatory (iNOS IL-18, and IL-6) and antiinflammatory (Chi3l3, Arg1, IL-10, cyclin A2, cyclin B) states for mice fed control rodent chow (C) and the HFHS (D) diet for 13 wk. E and F, Levels of mRNA for inflammatory and antiinflammatory markers among WT and RKO mice matched for body weight (∼30 g) (n = 4–10 per group). *, P < 0.05.
Consumption of the HFHS diet was generally associated with an increase in macrophage marker expression, with the most dramatic elevations seen for RKO mice (Fig. 6, A and B). CD68 expression was 3- and 6-fold higher for RKO as compared with WT mice fed chow and HFHS, respectively, demonstrating a greater accumulation of ATMs in the fatter mouse strain. Levels of MCP-1 mRNA were also markedly increased in HFHS-fed RKO mouse tissue (3-fold; Fig. 6B), contrasting with the reduction of plasma MCP-1 protein (Fig. 5F). MCP-1 attracts macrophages and dendritic cells that are CD11c positive, and CD11c mRNA levels were elevated by 7-fold in RKO mice (Fig. 6B). Thus, local expression of MCP-1 may play a role in autocrine signaling involved in macrophage recruitment without affecting circulating levels of the protein. Overall, the loss of TNFRs potentiated the obesity-induced recruitment of macrophages and dendritic cells into adipose tissue.
Although we observed dramatic increases in expression of several antiinflammatory genes with HFHS feeding in both mouse strains (Fig. 6D), the relative increases in these M2 phenotype markers were greatest for RKO mice. For example, Chi3l3 mRNA levels were increased 7-fold over levels for WT mice. For IL-10, an important antiinflammatory cytokine directing ATMs toward M2 status, levels were 4-fold higher for RKO than WT mice. For cyclin A2 and cyclin B, genes involved in tissue remodeling, levels for RKO tissue were 6-fold greater than seen for WT. By comparison, adipose tissue expression of M1 markers including iNOS and proinflammatory cytokines IL-18 and IL-6 were more modestly increased in RKO mice over that of WT (2-fold) (Fig. 6D), consistent with their increased FM.
To determine the extent to which differences in macrophage recruitment and activation were due to ablation of TNFRs vs. increased adiposity, gene expression measurements were done in epididymal adipose tissue taken from weight-matched mice of both genotypes (Fig. 4, E and F). CD68, MCP-1, and CD11c mRNA levels were comparable between the weight-matched groups (Fig. 6E), suggesting that increased ATM recruitment in RKO mice is driven primarily by their increased body FM. By comparison, Chi3l3 mRNA levels were significantly increased and IL-6 and IL-18 levels were significantly lower in RKO than WT mice (Fig. 6F), suggesting that for RKO, ATM phenotype is weighted toward M2 over M1 status.
Discussion
Obesity remains an important medical problem in this country because it is both highly prevalent and predisposes to insulin resistance, T2D, and comorbidities including cancer and cardiovascular disease. Here we show that loss of signaling pathways driven by a major inflammatory cytokine, TNF, results in obesity and offers no protection against obesity-associated insulin resistance.
TNF is a major inhibitor of insulin sensitivity by way of promoting serine phosphorylation of key proteins involved in insulin signal transduction including insulin receptor substrate-1 and the insulin receptor (42,43). TNF decreases insulin receptor mRNA transcription and stimulates proteosome-mediated degradation and phosphatase-mediated inactivation of the insulin receptor (44). Because RKO mice are unable to transmit TNF signaling due to lack of TNFRs and because obese RKO mice are severely insulin resistant, our findings demonstrate that intact TNF signaling is not required for the development of insulin resistance in this setting, confirming our earlier observation (30). Further work is needed to identify pathways mediating aberrant glucose homeostasis in these mutant mice.
The insulin resistance phenotype appears to be a secondary feature of the greater obesity of RKO vs. WT mice. Once RKO mice were weight matched to WT animals, glucose responses to injected glucose or insulin became comparable with those of WT mice. Thus, increased FM, but not insulin resistance, is the primary consequence of deficient TNF signaling, and these mice provide an important model to clarify mechanisms involved in fat accumulation.
Unlike the obese phenotypes of IL-18 knockout and double IL-6−/−IL-1−/− mice (4,7), RKO mice were not hyperphagic. Our analysis, based on a single prolonged period in which VO2 was measured, provides evidence that the obese phenotype of the RKO mice arises in part, from a relatively reduced metabolic rate compared with WT animals of similar body size and age. This analysis indicates that the relative decrease in metabolic rate of the mutant mice was subtle, amounting to approximately 5% when measured, and unmasked only after statistical adjustment for body size and age variation. This is perhaps not surprising because the weight gain phenotype of RKO mice on a chow diet was mild and because differences in energy expenditure contributing to relatively modest differences in fat mass may not be detectable with current calorimetric technology (5). However, small differences in food intake and/or energy expenditure between RKO and WT mice must account for the difference in fat mass. Such is also the case for human obesity, which often arises from a state of positive energy balance that amounts to only a very small fraction of the total yearly caloric budget.
In both humans and rodents, obesity is associated with low-grade systemic inflammation and insulin resistance (24,26). Thus, we expected that genetic ablation of proinflammatory cytokines or receptors would prevent the sequelae of obesity and/or insulin resistance. In contrast to these expectations, mice lacking IL-1R1, IL-1Rα, IL-6, IL-18, or granulocyte macrophage colony-stimulating factor each show maturity-onset increases of fat mass and aberrant glucose homeostasis (4,5,6,7,45,46). Combined IL-6 and IL-1 deficiency in mice and polymorphisms in these genes in humans are linked to severe early-onset and late onset obesity, respectively (4,46). Our findings with respect to the RKO mice add to evidence that deletion of genes involved in proinflammatory immune responses can predispose to obesity.
An exception to this pattern is mice lacking the TNF ligand (47). Unlike RKO mice, these animals are lean, obesity resistant, and more insulin sensitive than WT controls. One possible explanation for this observation is that in the absence of TNF ligand, TNFRs remain able to generate signals that limit weight gain and its metabolic sequelae. Lymphotoxin-α is secreted primarily by lymphocytes and is able to bind and promote signaling activity through both R1 and R2 (48). T cells are present in adipose tissue (49) and T cell-derived lymphotoxin-α may be a candidate for modulating TNFR activity in the absence of TNF itself. We are currently testing this concept.
Pertinent to this discussion are recent findings from mice deficient in TACE (ADAM17) (18,21). TNF and its receptors are produced initially as membrane bound proteins that interact with one another only after they are shed through the activity of TACE. Because TACE deficiency protects against DIO, juxtacrine activities exerted by the TNF system that are independent of TNF itself may be protective against obesity. These observations suggest a role in energy homeostasis played by TNFR signal transduction that is independent of TNF ligand binding.
Of note is the observation that genetic ablation of both R1 and R2 results in phenotypes that are distinct from mice lacking only one receptor or TNF itself. This observation holds in both obesity (30) and models of myocardial infarction, in which RKO mice experience a marked increased in infarct sizes (50) compared with mice lacking TNF (51), R1 (52), or R2 (50). Mechanisms by which loss of both TNFRs lead to aberrant responses are unclear but may involve important synergistic interactions between their signaling pathways (53,54).
The lack of TNF signaling altered the overall inflammatory status of the circulation and adipose tissue. We hypothesized that in RKO mice, levels of circulating inflammatory markers would be reduced compared with WTs due to the loss of a key proinflammatory signaling pathway. This was the case for IL-6, tissue plasminogen activator inhibitor, and MCP-1. In contrast, plasma leptin and resistin levels were significantly elevated for RKO mice over WT for mice fed the HFHS diet, suggesting that TNF signaling is not required for their synthesis and release and that their levels are reflective of the greater adiposity of RKO mice. Of note were the dramatically elevated plasma TNF levels in RKO mice. This could reflect an important role for TNFRs in TNF clearance (55), or alternatively, signaling downstream of one or both TNFRs may serve to inhibit TNF release. Overall, however, systemic markers of inflammation associated with obesity were diminished by TNFR deficiency.
Although the macrophage content of RKO adipose tissue was about 6-fold higher than for WT adipose tissue, the expression of iNOS, IL-6, and IL-18 were each 2-fold lower in RKO than WT mice, shifting the overall balance to an antiinflammatory state. Among mice matched for body weight, RKO mice exhibited increased levels of Chi3l3 and decreased levels of IL-6 and IL-18 mRNA, indicative of an antiinflammatory M2 status. Yet despite the reduced mRNA levels of inflammatory genes and the increased levels of antiinflammatory genes such as IL-10, RKO mice developed severe insulin resistance with the obesigenic diet. Overall, therefore, the presence of TNFRs is required to maintain an inflammatory activation phenotype for ATMs particularly in DIO, but not for the development of obesity-insulin resistance.
In summary, we report that TNFRs play a physiological role to limit body weight and adiposity but are not required for obesity-induced impairment of glucose metabolism. Although TNFRs are also not required for macrophage infiltration into adipose tissue, they influence the activation phenotype of adipose tissue macrophages during DIO.
Supplementary Material
Acknowledgments
We thank Dr. Gregory Morton and Dr. Kayoko Ogimoto for guidance and performing the body composition and calorimetry studies, Barbara Houston and Phuong-Oanh Mai for animal care, and Mark Caldwell for his technical expertise with the PCR studies. We also acknowledge the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases/National Heart, Lung, and Blood Institute) Grant U24 DK076126 for their support of the Seattle Mouse Metabolic Phenotyping Center at the University of Washington.
Footnotes
This work was supported by National Institutes of Health Grants HL079382 (to R.C.L.) and DK068384 (to M.W.S.) with assistance from Grant U24 DK076126 for their fee-for-service activities (Mouse Metabolic Phenotyping Center, Seattle, WA).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 28, 2009
Abbreviations: ATM, Adipose tissue macrophage; DIO, diet-induced obesity; FM, fat mass; HFHS, high-fat/high-sucrose; IL-1R, IL-1 receptor; iNOS, inducible nitric oxide synthase; LBM, lean body mass; MCP, macrophage chemoattractant protein; PAI, plasminogen activator inhibitor; RER, respiratory exchange ratio; RKO, TNFR1 and TNFR2 deficient; TACE, TNF-α converting enzyme; T2D, type 2 diabetes mellitus; TG, triglyceride; TNFR, TNF receptor; VCO2, carbon dioxide production; VO2, oxygen consumption; WT, wild type.
References
- Misra A, Khurana L 2008 Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 93:S9–S30 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- Wisse BE, Kim F, Schwartz MW 2007 Physiology. An integrative view of obesity. Science 318:928–929 [DOI] [PubMed] [Google Scholar]
- Chida D, Osaka T, Hashimoto O, Iwakura Y 2006 Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes 55:971–977 [DOI] [PubMed] [Google Scholar]
- García MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahrén B, Enerback S, Ohlsson C, Wallenius V, Jansson JO 2006 Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes 55:1205–1213 [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO 2002 Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8:75–79 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B 2007 Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA 104:11097–11102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire P, Bellmann K, Laplante M, Gélinas S, Centeno-Baez C, Penfornis P, Peyot ML, Latour MG, Lamontagne J, Trujillo ME, Scherer PE, Prentki M, Deshaies Y, Marette A 2008 Obese mice lacking inducible nitric oxide synthase are sensitized to the metabolic actions of peroxisome proliferator-activated receptor-γ agonism. Diabetes 57:1999–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli N, Kern PA 2008 Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93:S64–S73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Flier JS 2000 Obesity and insulin resistance. J Clin Invest 106:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Zhang C, van Dam RM, Hu FB 2007 Interleukin-6 genetic variability and adiposity: associations in two prospective cohorts and systematic review in 26,944 individuals. J Clin Endocrinol Metab 92:3618–3625 [DOI] [PubMed] [Google Scholar]
- Perrier S, Darakhshan F, Hajduch E 2006 IL-1 receptor antagonist in metabolic diseases: Dr. Jekyll or Mr. Hyde? FEBS Lett 580:6289–6294 [DOI] [PubMed] [Google Scholar]
- Kriegler M, Perez C, DeFay K, Albert I, Lu SD 1988 A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell 53:45–53 [DOI] [PubMed] [Google Scholar]
- Aggarwal BB 2003 Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev 3:745–756 [DOI] [PubMed] [Google Scholar]
- Ashkenazi A 2002 Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev 2:420–430 [DOI] [PubMed] [Google Scholar]
- LeBoeuf RC, Schreyer SA 1998 The role of tumor necrosis factor α receptors in atherosclerosis. Trends Cardivasc Med 8:131–138 [DOI] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG 1994 The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76:959–962 [DOI] [PubMed] [Google Scholar]
- Gelling RW, Yan W, Al-Noori S, Pardini A, Morton GJ, Ogimoto K, Schwartz MW, Dempsey PJ 2008 Deficiency of TNFα converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology 149:6053–6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voros G, Maquoi E, Collen D, Lijnen HR 2004 Influence of membrane-bound tumor necrosis factor (TNF)-α on obesity and glucose metabolism. J Thromb Haemost 2:507–513 [DOI] [PubMed] [Google Scholar]
- Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS 2002 Exclusive action of transmembrane TNF α in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology 143:1502–1511 [DOI] [PubMed] [Google Scholar]
- Serino M, Menghini R, Fiorentino L, Amoruso R, Mauriello A, Lauro D, Sbraccia P, Hribal ML, Lauro R, Federici M 2007 Mice heterozygous for tumor necrosis factor-α converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes 56:2541–2546 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS 2003 Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS 2006 Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- Neels JG, Olefsky JM 2006 Inflamed fat: what starts the fire? J Clin Invest 116:33–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR 2007 Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56:16–23 [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H 2003 Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR 2007 Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S 2003 Alternative activation of macrophages. Nat Rev Immunol 3:23–35 [DOI] [PubMed] [Google Scholar]
- Schreyer SA, Chua Jr SC, LeBoeuf RC 1998 Obesity and diabetes in TNF-α receptor-deficient mice. J Clin Invest 102:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC 2003 Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis 171:49–55 [DOI] [PubMed] [Google Scholar]
- Black BL, Croom J, Eisen EJ, Petro AE, Edwards CL, Surwit RS 1998 Differential effects of fat and sucrose on body composition in A/J and C57BL/6 mice. Metabolism 47:1354–1359 [DOI] [PubMed] [Google Scholar]
- Pamir N, McMillen TS, Li YI, Lai CM, Wong H, LeBoeuf RC 2009 Overexpression of apolipoprotein A5 in mice is not protective against body weight gain and aberrant glucose homeostasis. Metabolism 58:560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystkowski P, Shankland E, Schreyer SA, LeBoeuf RC, Schwartz RS, Cummings DE, Kushmerick M, Schwartz MW 2000 Validation of whole-body magnetic resonance spectroscopy as a tool to assess murine body composition. Int J Obes Relat Metab Disord 24:719–724 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔ C(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD 2008 Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 87:56–63 [DOI] [PubMed] [Google Scholar]
- Wueest S, Rapold RA, Rytka JM, Schoenle EJ, Konrad D 2009 Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia 52:541–546 [DOI] [PubMed] [Google Scholar]
- den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA 2004 Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol 24:644–649 [DOI] [PubMed] [Google Scholar]
- Kim KH, Lee K, Moon YS, Sul HS 2001 A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem 276:11252–11256 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA 2001 The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB 2006 Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM 1993 Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Spiegelman BM 1994 Tumor necrosis factor α: a key component of the obesity-diabetes link. Diabetes 43:1271–1278 [DOI] [PubMed] [Google Scholar]
- Björnholm M, Zierath JR 2005 Insulin signal transduction in human skeletal muscle: identifying the defects in type II diabetes. Biochem Soc Trans 33:354–357 [DOI] [PubMed] [Google Scholar]
- Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, Seeley RJ 2005 GM-CSF action in the CNS decreases food intake and body weight. J Clin Invest 115:3035–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg L, Mellstrom D, Ljunggren O, Grundberg E, Karlsson MK, Holmberg AH, Orwoll ES, Eriksson AL, Svedberg J, Bengtsson M, Ohlsson C, Jansson JO 2008 IL6 and IL1B polymorphisms are associated with fat mass in older men: the MrOS Study Sweden. Obesity (Silver Spring) 16:710–713 [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS 1997 Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610–614 [DOI] [PubMed] [Google Scholar]
- Gommerman JL, Browning JL 2003 Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat Rev Immunol 3:642–655 [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P 2008 Interferon-γ, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 103:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monden Y, Kubota T, Inoue T, Tsutsumi T, Kawano S, Ide T, Tsutsui H, Sunagawa K 2007 Tumor necrosis factor-α is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol 293:H743–H753 [DOI] [PubMed] [Google Scholar]
- Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP 2004 Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation 110:3221–3228 [DOI] [PubMed] [Google Scholar]
- Ramani R, Mathier M, Wang P, Gibson G, Tögel S, Dawson J, Bauer A, Alber S, Watkins SC, McTiernan CF, Feldman AM 2004 Inhibition of tumor necrosis factor receptor-1-mediated pathways has beneficial effects in a murine model of postischemic remodeling. Am J Physiol Heart Circ Physiol 287:H1369–H1377 [DOI] [PubMed] [Google Scholar]
- Kavurma MM, Tan NY, Bennett MR 2008 Death receptors and their ligands in atherosclerosis. Arterioscler Thromb Vasc Biol 28:1694–1702 [DOI] [PubMed] [Google Scholar]
- Lee NK, Lee SY 2002 Modulation of life and death by the tumor necrosis factor receptor-associated factors (TRAFs). J Biochem Mol Biol 35:61–66 [DOI] [PubMed] [Google Scholar]
- Bemelmans MH, van Tits LJ, Buurman WA 1996 Tumor necrosis factor: function, release and clearance. Crit Rev Immunol 16:1–11 [DOI] [PubMed] [Google Scholar]
- Arch JR, Hislop D, Wang SJ, Speakman JR 2006 Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30:1322–1331 [DOI] [PubMed] [Google Scholar]
- Toth MJ 2001 Comparing energy expenditure data among individuals differing in body size and composition: statistical and physiological considerations. Curr Opin Clin Nutr Metab Care 4:391–397 [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Toth MJ 1995 Mathematical ratios lead to spurious conclusions regarding age- and sex-related differences in resting metabolic rate. Am J Clin Nutr 61:482–485 [DOI] [PubMed] [Google Scholar]
- Lighton JRB 2008 Measuring metabolic rates: a manual for scientists. New York: Oxford University Press; 201 [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG 2008 Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.