Abstract
Mutations of the thyroid hormone (TH) cell membrane transporter MCT8, on chromosome-X, produce severe mental and neurological impairment in men. We generated a Mct8-deficient mouse (Mct8KO) manifesting the human thyroid phenotype. Although these mice have no neurological manifestations, they have decreased brain T3 content and high deiodinase 2 (D2) activity, reflecting TH deprivation. In contrast and as in serum, liver T3 content is high, resulting in increased deiodinase 1 (D1), suggesting that in this tissue TH entry is Mct8 independent. We tested the effect of 3,5-diiodothyropropionic acid (DITPA), a TH receptor agonist, for its dependence on Mct8 in Mct8KO and wild-type (Wt) mice tissues. After depletion of endogenous TH, mice were given three different doses of DITPA. Effects were compared with treatment with two doses of l-T4. As expected, physiological doses of l-T4 normalized serum TSH, brain D2, and liver D1 in Wt mice but not the Mct8KO mice. The higher dose of T4 suppressed TSH in the Wt mice, normalized TSH and brain D2 in Mct8KO mice, but produced a thyrotoxic effect on liver D1 in both genotypes. In contrast DITPA produced similar effects on TSH, D2, and D1 in both Wt and Mct8KO mice. The higher dose fully normalized all measurements and other parameters of TH action. Thus, DITPA is relatively MCT8 independent for entry into the brain and corrects the TH deficit in Mct8KO mice without causing thyrotoxic effect in liver. The potential clinical utility of this analog to patients with MCT8 mutations requires further studies.
The thyroid hormone agonist, 3,5-diiodothyropropionic acid, is relatively independent of monocarboxylate transporter 8 for entering tissues.
In 2003 Friesema et al. (1) demonstrated that the monocarboxylate transporter 8 (MCT8) is an active and specific membrane transporter of thyroid hormone (TH). However, the crucial role played by MCT8 on thyroid function and the development of the central nervous system became apparent only 1 yr later when two different laboratories identified patients with mutations in this gene (also known as SLC16A2) located on the X-chromosome (2,3). Since this first description, 120 individuals belonging to 41 families have been identified (Ref. 4 and our personal observations). The clinical presentation of patients with MCT8 defects is very similar, having two components: thyroid and neuropsychiatric abnormalities (4,5). Characteristic thyroid function test abnormalities are increased serum T3 and decrease of serum rT3 concentrations. T4 is reduced in most cases and TSH is normal or slightly elevated. These thyroid abnormalities were faithfully reproduced in MCT8 knockout (Mct8KO) mice (6,7). In contrast, the MCT8 deficient mice do not manifest the severe psychomotor retardation observed in hemizygous affected males and in one case of homozygous female (8). These include truncal hypotonia, spastic quadriplegia, absent speech, severe mental retardation, and in some cases paroxysmal dyskinesia (8,9,10). The latter is often triggered by tactile or emotional stimuli but seizures, although not uncommon, do not dominate. Only a few patients have been able to walk with ataxic gait and have a limited dysarthric speech (10). Nevertheless, studies of Mct8KO mice have been helpful in understanding some of the abnormalities observed in humans. We have learned from the mice that the tissue-specific increases in deiodinase (D) types 1 and 2 and decreases in D3 activities together with reduced tissue uptake of T3 are responsible for the high serum T3 concentration, low rT3, and the consumptive reduction in T4 observed in both mice and humans (6,7,11). The high liver T3 content in Mct8KO mice, reflecting the serum concentration, was associated with changes in markers indicating increased TH action. These included increase in D1 and serum alkaline phosphatase and decrease in serum cholesterol and glutathione S transferase (Gst)-α2 mRNA (6,7). These findings are also congruent with hypermetabolic state, failure to thrive, and inability to gain weight observed in humans with MCT8 defects (12,13).
It remains unclear why Mct8KO mice do not manifest obvious neurological impairment, despite the fact that they have significant reduction in brain T4 and, particularly, T3 content (6,7). Whereas the possibility that MCT8 may transport another, yet unidentified, substance has not been excluded, it is also possible that the degree of thyroid hormone deficiency in mouse brain is less severe than that in humans. In support of this hypothesis is the recent finding that mice, but not humans, express high levels of the transporter organic anion-transporting polypeptide 14 (SLCO1C1), important in the blood-brain barrier transport of TH (14).
From the foregoing it is obvious that the therapeutic options for the patients with MCT8 mutations are limited. In fact, contrary to other forms of TH deficiency, the apparent tissue-specific hypothyroidism due to this cell transport defect cannot be corrected with physiological doses of TH (2,13,15,16,17). High doses produce symptoms of thyrotoxicosis and aggravate the weight loss (Ref. 18 and our personal observations). This stimulated us to explore the possibility that available analogs of TH may be less dependent on MCT8 for their transfer from blood to tissues.
The TH analog 3,5-diiodothyropropionic acid (DITPA) is a TH receptor (TR)-α and TRβ agonist that binds with almost the same affinity to both TRs, although that for TRβ is 350-fold less than T3 (19). Compared with TH, DITPA has low metabolic activity as shown by the reduced stimulation of hepatic α-glycerolphosphate dehydrogenase activity when given to rats in equivalent doses as T4 (19). DITPA can also act by a nongenomic mechanism, as demonstrated by its angiogenic effect (20). In humans, DITPA underwent phase II clinical trials for treatment of heart failure. Four weeks of treatments with two incremental doses of DITPA did not increase the heart rate or blood pressure but did reduce the serum TSH, T4, cholesterol, triglycerides, lipoproteins, and body weight (21,22).
In the present work, we examined the effects of DITPA in Mct8KO and wild-type (Wt) mice in comparison with those of TH. Even if these mice are not comparable in all respect to humans lacking MCT8, they provide the best available in vivo approximation.
Materials and Methods
Experimental animals
Procedures carried out in mice and described below were approved by the University of Chicago Institutional Animal Care and Use Committee. Animals were housed in temperature (22 ± 2 C)- and light (12 h light,12 h dark cycle; lights on at 0700 h)-controlled conditions and had free access to food and water. Mct8KO mice were generated as described previously (6). Experiments were carried out on 14- to 15-wk-old male Wt (Mct8+/y) and knockout (Mct8−/y) littermates derived from more than 10 back-crossing of heterozygous females (Mct8−/+) with Wt males (Mct8+/y) of the C57BL/6J strain. The genotype was confirmed by PCR of tail DNA (38 cycles at 55 C annealing temperature) using the following primers: forward common, 5′-ACAACAAAA AGCCAAGCATT-3′; reverse Wt specific, 5′-GAGAGCAGCGTAAGGACAAA-3′; reverse knockout specific, 5′-CTCCCA AGCCTGATTTCTAT-3′. Using this procedure the Wt allele generated a 476-bp products and the null allele a 239-bp PCR product.
Induction of hypothyroidism and treatment with TH and DITPA
The protocols for treatment with TH after the suppression of endogenous TH are those used in our laboratory for mice of various genotypes since 1996 (6,23,24,25,26,27,28,29). l-T3 and l-T4 are given as a single injection ip and the duration of treatment (4 d for l-T3 and 7 d for l-T4) was determined by the achievement of at least 90% maximal suppression of TSH in the TH-deprived animals. These treatment regimens also reverted the effects of hormone deprivation on markers reflecting TH action on heart and liver (25,27,28). The same criterion was used to establish that 4 d are required for treatment with DITPA. The doses of DITPA are in the range used in hypothyroid rats and are in agreement with the potency of this agonist relative to l-T3 (19).
A baseline blood sample was obtained from the tail vein of each mouse before initiation of experiments. Endogenous production of TH was suppressed with low iodine diet (Harlan Teklad Co., Madison, WI) and the addition of 0.5% perchlorate and 0.02% methimazole in the drinking water (LoI/MMI/ClO4). After 3 wk, another blood sample was obtained, and separate groups of animals were given ip two different doses of l-T4 (4 and 10 μg/100 g body weight (BW) · d) each for 7 d and three different doses of DITPA (0.3, 0.6, and 1.0 mg/100 g BW · d) each for 4 d. Blood samples were obtained again after the termination of treatment with each dose of l-T4 and DITPA. Changes in body weight after the period of treatment with DITPA were not significant. In particular, compared with weights (grams) before the administration of 0.6 mg DITPA/100 g BW · d: WT, 28.1 ± 1.2 and 27.1 ± 1.0; Mct8KO, 28.1 ± 0.8 and 27.3 ± 0.7. Weights for mice given 1.0 mg DITPA/100 g BW · d were: WT, 28.6 ± 0.9 and 27.9 ± 0.7; Mct8KO, 27.6 ± 1.2 and 26.5 ± 0.5. In a separate but similarly designed experiment, l-T3 (0.8, 2.0, and 5 μg/100 g BW · d) was given, each dose for 4 d. All ip-injected substances were dissolved in PBS containing 0.002% albumin.
Ten and 11 Wt and Mct8KO male littermates, respectively, were used in the baseline groups and five to seven animals in the various treatment groups. The experiment was terminated 16 h after the last injection of l-T4 and l-T3 and 6 h after the last injection of DITPA. Timing coincided with the maximal suppressive effect of each compound on serum TSH determined in a preliminary study. Mice were anesthetized and after obtaining the last blood sample were perfused with heparinized saline to remove blood from tissues before their collection. Mct8KO mice have no increased prenatal or postnatal mortality (6), and no deaths occurred during the course of the experiments. All animals were humanely euthanized.
For the treatment with DITPA without prior suppression of endogenous TH, separate groups of untreated mice were given two doses of DITPA (0.6 and 1.0 mg/100 g BW · d). Five Wt and five Mct8KO male mice for each group were bled at baseline and after 4 d of DITPA administration and tissues were collected, as described above.
l-T4, l-T3 were obtained from Sigma (St. Louis, MO). DITPA was a gift from Dr. Eugene Morkin (University of Arizona, Tuczon, AZ).
Measurements in serum
Serum total T4 and T3 concentration were measured by coated tube RIAs (Diagnostic Products, Los Angeles, CA) adapted for mouse serum using 25 and 50 μl serum, respectively. TSH was measured in 50 μl serum using a sensitive, heterologous, disequilibrium, double-antibody precipitation RIA (30). Cholesterol was measured on 10 μl serum using a clinical chemistry autoanalyzer.
Measurement of serum and tissue DITPA content
We exploited the cross-reactivity of T3 antibody with DITPA to measure the latter in the serum of animals deprived of endogenous TH by treatment with LoI/MMI/ClO4 and then treated with DITPA. The serum of those animals with no T4 or T3 was set to zero and was used as diluent to construct a standard curve with addition of incremental amount of DITPA. The amount of DITPA measured in serum of TH-deprived mice was confirmed by HPLC. A serum pool from mice injected with DITPA determined to have 2.01 μg/ml of DITPA in the T3 RIA, measured 1.85 and 2.37 μg/ml by HPLC.
Measurement of specific mRNA content in tissues
Total RNA was extracted using phenol/guanidine isothiocyanate (TRIZOL; Invitrogen, Carlsbad, CA), and 2 μg total RNA was reverse transcribed using Superscript III ribonuclease H reverse transcriptase kit (Invitrogen) in the presence of 100 ng random hexamers. Reactions for the quantification of mRNAs by real-time quantitative PCR were performed in an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA), using SYBR Green I as detector dye. The oligonucleotide primers were designed to cross introns. Primers used for the quantitative PCR of Gstα2, iodothyronine deiodinases (Dio; Dio1 and Dio3) and hairless (Hr) mRNAs are available on request. Amplification of the housekeeping gene RNA polymerase II was used as internal control (31).
D2 and D1 enzymatic activities
D2 enzymatic activity was performed as described (32) with the following modifications: 100 μg tissue homogenates in 100 μl reaction mixture containing 0.1 m phosphate buffer (pH 7), 1 mm EDTA, 20 mm dithiothreitol, 1 mm propylthiouracil, 100,000 cpm [125 I]T4, and 2 nm unlabeled T4 were incubated at 37 C for 1 h. Saturating levels of unlabeled T3 (1 μm) were added to the reaction mixture to inhibit the D3 enzyme. D1 enzymatic activity in liver was measured using [125 I]T4 as previously described (33). It was modified as described above for D2 except 20 μg tissue and 10 mm dithiothreitol, 1 μm unlabeled T4 were used; no propylthiouracil was added and incubation time was 30 min. The enzymatic activities were expressed in femtomoles (for D2) and picomoles (for D1) per hour and milligram of protein and were corrected for nonenzymatic deiodination observed in the tissue-free controls.
Statistics
Statistic analysis was performed using ANOVA with Fisher’s protected least significant differences. Significant differences were confirmed by the Tukey-Kramer method of single-step multiple comparison. Data are represented as mean ± se. Logarithmic transformation of data were performed when sds for different groups varied by more than 20-fold and sometimes by 1000-fold (see TSH). P > 0.05 was considered not to be significant (NS).
Results
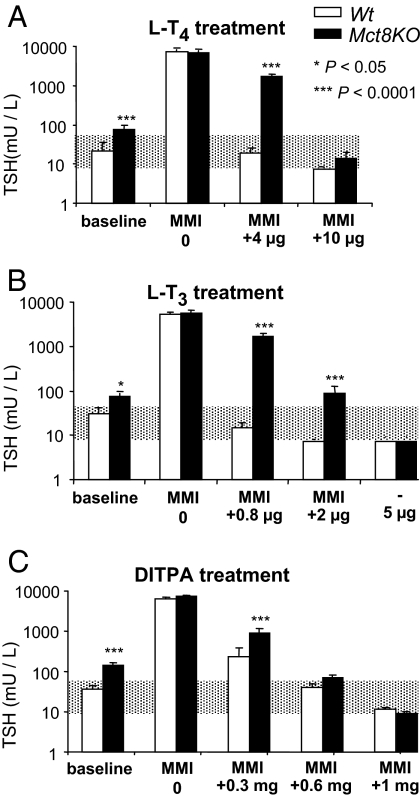
Effects of TH and treatments with DITPA on serum TSH concentrations (Fig. 1)
Figure 1.
Comparison of the effect of T4, T3, and DITPA on serum TSH of Wt and Mct8KO mice. Doses are indicated in the abscissae. Data are expressed as mean ± se. The shaded area represents the normal range for Wt animals. Statistical differences between Wt and Mct8KO mice for each treatment group are indicated above the Mct8KO bars. Bars just below the range of normal and not showing se indicate TSH values suppressed bellow limits of the assay sensitivity. Treatment with LoI/MMI/ClO4 is simply indicated as methimazole (MMI) on the abscissa.
The suppression of endogenous TH after 3 wk of LoI/MMI/ClO4 (serum T4 < 0.2 μg/dl in all groups of mice) increased the serum TSH levels to the same extent in all animal groups, irrespective of their genotype. Mean serum TSH concentrations ranged from 6500 to 7350 mU/liter and were not significantly different.
Compared with Wt mice, physiological dose of l-T4 (4 μg/100 g BW · d) and l-T3 (0.8 μg/100 g BW · d) failed to normalize the TSH in Mct8KO animals. However, 2.5-fold more l-T4 (10 μg/100 g BW · d) did normalize the serum TSH concentration of Mct8KO mice, and for this reason a third incremental dose was not given. In contrast, a third incremental dose of l-T3 or 6-fold the physiological dose (5 μg/100 g BW · d) was required to reduce the elevated serum TSH level of Mct8KO animals. These results demonstrate the relatively more severe resistance of the hypothalamo-pituitary axis to l-T3 than l-T4. Although a DITPA dose of 0.3 mg/100 g BW · d had only modest effect on the suppression of serum TSH in both genotypes, the mean TSH level of the Wt mice was significantly lower. In contrast, serum TSH was normalized in both genotypes with the same dose of DITPA (0.6 mg/100 g BW · d). It declined further to the same extent in both genotypes, and without being fully suppressed, with 1 mg DITPA/100 g BW · d. These results indicate that Mct8 plays a lesser role on the feedback regulation of TSH by DITPA.
Because of these results on serum TSH and those of recent studies showing that systemic administration of T4 to Mct8KO mice has a less restrictive passage into neuronal target cells (34), we compared DITPA to the more accessible T4 rather than T3. The doses of l-T4 and DITPA that normalized the TSH levels in the Wt and Mct8KO animals were used in the more detailed tissue analyses.
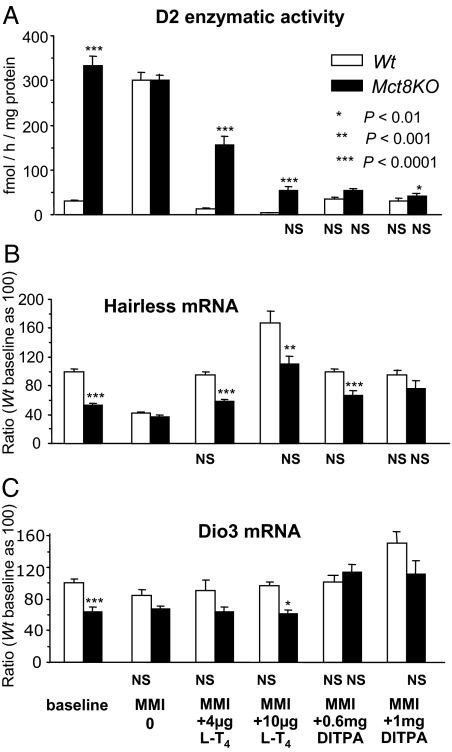
Effects of DITPA on brain (Fig. 2)
Figure 2.
Brain D2, Hr, and Dio3 at baseline and during treatment with l-T4 and DITPA. Data are expressed as mean ± se. Statistical differences between Wt and Mct8KO mice for each treatment group are indicated above the Mct8KO bars. Lack of statistical difference from the values in Wt mice at baseline are indicated below each value bar as NS (not significant). Treatment with LoI/MMI/ClO4 is simply indicated as methimazole (MMI) on the abscissa.
The thyromimetic activity of DITPA on brain (cerebrum) was assessed by its suppressive effect on D2 enzymatic activity and stimulation of Hr and Dio3 mRNAs (35,36). At baseline, compatible with reduced TH in brain of Mct8KO mice (6), D2 enzymatic activity was 10.6-fold higher, whereas Hr and Dio3 mRNAs were lower by 47 and 36%, respectively, compared with the Wt animals. LoI/MMI/ClO4-induced TH deprivation produced the expected stimulation of brain D2 enzymatic activity and decreased Hr mRNA in the Wt mice. Although the decline in Dio3 mRNA was not significant compared with baseline the differences between genotypes for all three markers of TH action observed at baseline were obliterated. Whereas treatment with both l-T4 doses reversed the D2 and Hr changes induced by TH deprivation in Wt mice, only the high l-T4 dose normalized these two parameters in the Mct8KO mice. The effect was overshot (below baseline for D2 and above for Hr; P < 0.0001) in the Wt mice, indicating that the high l-T4 dose was thyrotoxic to these animals. Both doses of DITPA brought the D2 enzymatic activity of Mct8KO and Wt mice to those observed in the Wt at baseline. This was achieved for Hr mRNA of the Mct8KO mice only with the high DITPA dose. It is of note that whereas treatment with either l-T4 dose failed to normalize the Dio3 expression in the Mct8KO mice, both DITPA doses did so. In the case of the Wt mice, the high dose of DITPA increased Dio3 mRNA above baseline. This suggests a different distribution of DITPA in brain cells or an uncharacterized effect of this TH agonist that may be distinct of that of TH.
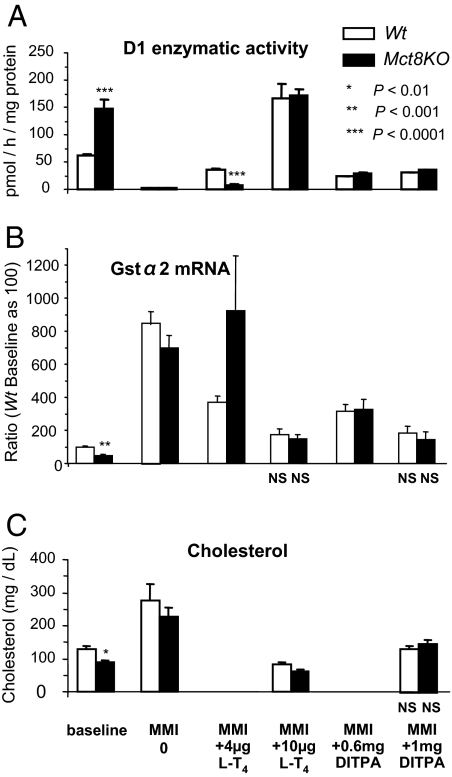
Effects of DITPA on liver (Fig. 3)
Figure 3.
Liver D1 and Gstα2 and serum cholesterol at baseline and during treatment with l-T4 and DITPA. Data are expressed as mean ± se. Statistical differences between Wt and Mct8KO mice for each treatment group are indicated above the Mct8KO bars. Lack of statistical difference from the values in Wt mice at baseline are indicated below each value bar as NS (not significant). Treatment with LoI/MMI/ClO4 is simply indicated as minimum maintenance dose of thiamazole (MMI) on the abscissa. Serum cholesterol was measured only in animals that received the higher dose of l-T4 and DITPA. Note that the higher dose of l-T4, which partially corrected the TH deprivation of brain in Mct8KO mice, caused thyrotoxic effects in the liver of mice of both genotypes. DITPA, however, corrected the effects of TH deprivation in brain of Mct8KO mice without causing thyrotoxicity.
As markers of thyromimetic activity on liver, we measured D1 enzymatic activity and mRNA, Gstα2 mRNA and serum cholesterol (25,29). The relative TH excess in liver of Mct8KO mice, due to lesser dependence on MCT8 for TH transport (6), was confirmed by the significantly higher D1 and lower Gstα2 mRNA content and serum cholesterol concentration compared with the Wt animals mice at baseline. As expected, TH deprivation reduced significantly the enzymatic activity of D1 (P < 0.0001) and increased the Gstα2 mRNA (P < 0.0001) and serum cholesterol (P < 0.001) concentrations to the same extent in both genotypes. Whereas treatment with the lower dose of l-T4 nearly corrected (D1 and to lesser degree Gstα2 mRNA) the changes induced by TH deprivation in the Wt mice, it did not do so in the Mct8KO animals. In contrast, the high l-T4 dose increases the D1 activity by 2.8-fold (P < 0.0001) and reduced cholesterol concentrations (P < 0.001) relative to the Wt baseline in both mouse genotypes, compatible with the induction of thyrotoxicosis in liver. Gstα2 mRNA returned only to the baseline value of Wt mice probably because of longer half-life of Gstα2 mRNA. These results show that the dose of l-T4 required to partially improve the TH deprivation in brain, produced thyrotoxic effects in liver.
Both DITPA doses increased by 150- to 300-fold the D1 activities observed in both mouse genotypes during TH deprivation and brought them to near Wt baseline levels. Serum cholesterol and Gstα2 mRNA values in mice of both genotypes also reached those of the Wt baseline on the high dose of DITPA. Results of Dio1 mRNA liver content were in agreement with the enzymatic activity and are thus not shown. From these results it can be concluded that DITPA was able to correct in the brains of Mct8KO mice all the tested parameters of TH action that indicated hormone deprivation without producing toxic effects in liver. The apparent lower potency of DITPA on the liver compared with cerebrum and pituitary may be due to a different mode of action.
DITPA concentration in serum and content in liver and brain (Table 1)
Table 1.
DITPA concentration in serum and tissues of mice receiving LoI/MMI/ClO4
| DITPA dosea | 0.3
|
0.6
|
1.0
|
|||
|---|---|---|---|---|---|---|
| Genotype | Wt | Mct8KO | Wt | Mct8KO | Wt | Mct8KO |
| Serum (μg/dl) | 58.5 ± 6.5 (4)b | 76.0 ± 7.5 (4)b | 155 ± 14 (7) | 133 ± 19 (7) | 163 ± 13 (7) | 196 ± 10 (4)c |
| Brain (ng/mg)d | ND | ND | 2.66 ± 0.39 (7) | 3.53 ± 1.07 (6) | 5.22 ± 1.34 (5) | 3.54 ± 0.45 (4) |
| Liver (ng/mg)d | ND | ND | 30.4 ± 5.3 (6) | 19.6 ± 3.5 (7) | 27.7 ± 3.5 (6) | 40.6 ± 8.7 (5)c |
Values are mean ± se. The number of animals used for each determination is in parentheses.
DITPA dose is in milligrams DITPA per 100 g BW. DITPA was measured 6 h after the last injection;
P < 0.001 compared with values on the two higher doses of DITPA;
P < 0.01 compared with Mct8KO treated with 0.6 mg DITPA;
Tissue concentrations are expressed per milligrams of protein.
Because T3 antibodies were used for the measurement of DITPA, the latter could be only assessed in serum and tissues deprived of endogenous iodothyronines, that is only in experiments using LoI/MMI/ClO4-treated animals. Their serum, before treatment with DITPA, was set as zero and used as diluent in the construction of the standard curve (for details see Materials and Methods). As shown in Table 1, increasing amounts of DITPA were detected in serum with the higher doses of the compound given. Whereas the concentration on the average doubled (from 67 to 144 μg/dl, mean for both genotypes) when the DITPA dose was increased from 0.3 to 0.6 mg/100 g BW · d, a much smaller further increase (mean 180 μg/dl) was observed with the administration of 1.0 mg of DITPA/100 g BW · d. This suggests increase in the metabolism or excretion, and less likely decreased absorption, of DITPA. Concentrations were not significantly different between mouse genotypes at all doses. The content of DITPA in liver was 6- to 10-fold that of brain when expressed per milligram tissue protein. Although mean values in Mct8KO and Wt animals were different by up to 35%, the trend was not in the same direction with the two DITPA doses and the differences were not statistically significant.
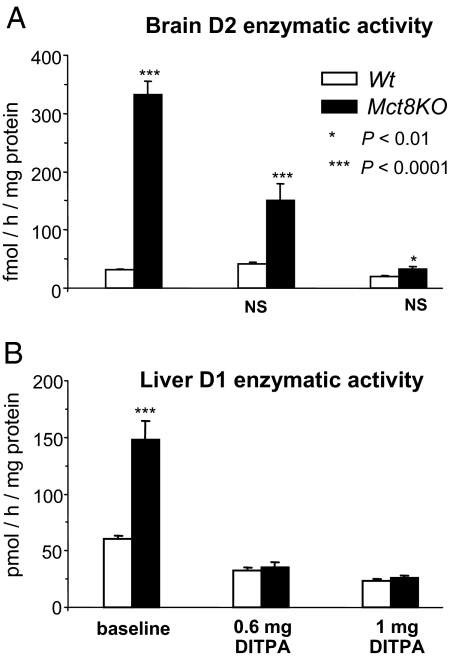
Thyromimetic effects of DITPA in untreated mice (Fig. 4)
Figure 4.
Effect of DITPA in mice without prior depletion of endogenous TH assessed by measurement of D2 and D1 enzymatic activity in brain (A) and liver (B), respectively. Data are expressed as mean ± SE. Lack of statistical difference from the values in Wt mice at baseline are indicated below each value bar as NS (not significant). The higher dose corrected the effect of TH deficiency on D2 in brain of Mct8KO mice and corrected the thyrotoxic effect on D1 in Mct8KO mice.
We also gave DITPA to mice without pretreatment with LoI/MMI/ClO4. Two doses of DITPA, 0.6 and 1.0 mg/100 g BW · d were given for the same period of time. Responses of brain and liver were assessed as in experiments described above.
With 0.6 mg/100 g BW · d, brain D2 enzymatic activity was lower by 55% compared with the baseline value in the Mct8KO mice, whereas in the Wt there was no change. With 1.0 mg/100 g BW · d D2 activity normalized in the Mct8KO mice and decreased below baseline in the Wt mice.
In liver, similar to the group of mice treated with DITPA after LoI/MMI/ClO4 (see Fig. 2A), no thyrotoxic effect was observed in either mouse genotypes. In fact, both doses failed to increase D1 above the baseline level of Wt animals, suggesting a lower thyromimetic activity of the two doses of DITPA in liver and the partial suppression of endogenous TH, particularly in the Mct8KO mice (see below). These results provide further evidence that DITPA can act in brain without producing thyrotoxic effects in a peripheral tissue, such as the liver.
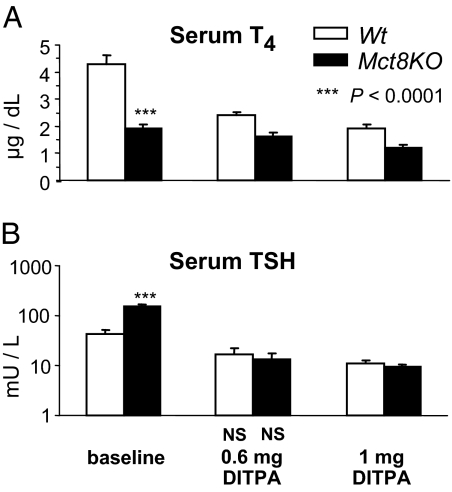
Effect of DITPA on suppression of endogenous TH (Fig. 5)
Figure 5.
Effect of DITPA on suppression of endogenous T4 and TSH in untreated mice. Data are expressed as mean ± se. Statistical differences between Wt and Mct8KO mice for each treatment group are indicated above the Mct8KO bars. Lack of statistical difference from the values in Wt mice at baseline are indicated below each value bar as NS (not significant). At baseline serum T4 was lower in Mct8KO than Wt mice. T4 and TSH declined with the administration of DITPA in both genotypes. The absolute value of TSH reached was not statistically different between the two genotypes.
We measured serum T4 concentrations in sera of mice given DITPA without LoI/MMI/ClO4 pretreatment. As previously shown (6), at baseline serum T4 was lower in Mct8KO than Wt animals. It declined after the administration of DITPA. With the highest DITPA dose, this decline was from 4.3 ± 0.31 to 1.9 ± 0.15 μg/dl (56%) in Wt mice and from 1.9 ± 0.17 to 1.2 ± 0.13 μg/dl (37%) in the Mct8KO mice. Corresponding TSH values for the Wt mice declined from 43.1 ± 7.9 to 11.2 ± 1.2 mU/liter (74%) and for the Mct8KO mice from 153.9 ± 16.0 to 9.8 ± 0.0.8 mU/liter (94%). The absolute value of TSH reached was not statistically different between the two genotypes, indicating that at these two doses, DITPA has equipotent effect on the hypothalamo-pituitary axis of both mouse types. Although on both DITPA doses, T4 levels were not significantly different between the two genotypes, and it is unknown whether a more prolonged treatment would have brought the TH to lower levels, maintaining the same lack of difference.
Discussion
This work tests the ability of the TH analog DITPA to produce a thyromimetic activity in tissues in the absence of Mct8. We carried out our experiments in Mct8KO and Wt control mice. After more than 10 back-crossings in the C57BL/J6 strain, the Mct8KO mice continued to reproduce and express the thyroid phenotype of patients with MCT8 mutations already observed early after their generation by us and another group of investigators (6,7). Similarly, baseline differences between the Mct8KO and Wt animals in tissue D1 and D2 enzymatic activities, abundance of Dio1, Dio3, Gstα2, and Hr mRNA and serum cholesterol concentrations were as previously reported (6).
One drawback of using such mice as a model to predict the effect of DITPA in humans could be the lack of a readily measurable neurological deficit in the Mct8KO mice. This raises the possibility that the psychomotor defect in humans may not be solely due to the TH deficiency in brain at a crucial time of development but also to other factors, such as the transport by MCT8 of other ligands essential for the normal human development. However, studies to date failed to demonstrate that MCT8 transports substances other than TH (1,37). On the other hand, a recent work suggested that the absence on neurological abnormalities in Mct8KO mice is due to the presence of an alternative TH transporter (14), providing further evidence for the importance of TH availability for the brain development. This transporter, organic anion-transporting polypeptide 14, expressed in a high level at the blood brain barrier (cerebral microvessels) of mice, is present at very low levels in the humans. This alternative route of TH transport in mice partially correcting the TH deficiency in animals lacking functional Mct8 might be sufficient to prevent the manifestations of the neurological phenotype observed in humans. A species-specific constitutive effect of Mct8 cannot be excluded (38). Still, the partial TH deficiency in Mct8KO mice, demonstrable by measurement of tissue levels of TH and by markers of TH action in brain (6,7), renders this animal model useful to study the potential usefulness of DITPA in the treatment of humans.
Our results show that the transport of DITPA into the tissues was less dependent of Mct8, and its levels in brain and liver of Mct8KO compared with Wt animals were not significantly different. This appears to be in contradiction with in vitro work using cardiomyocytes, which showed that DITPA partially inhibited T3 uptake (39), although the precise transporter involved is not known. However, our in vivo study has the advantage of involving other transporters not present in isolated cell types in culture. We observed 6- to 10-fold difference in DITPA levels between brain and liver. This is in agreement with our previous data showing 4 times higher T3 content in liver than brain after l-T3 administration in Wt mice (6). This might due to the differences in specific transporters in these two tissues.
The same dose of DITPA (0.6 mg/100 g BW per d) normalized the serum TSH of TH-deprived Mct8KO and Wt animals. This was not the case when l-T4 and l-T3 were used separately. Compared with Wt mice, 2.5-fold more l-T4 and nearly 6-fold more l-T3 were required to reduce the serum TSH of Mct8KO animals below the upper limit of normal, confirming the relatively more severe resistance of the hypothalamo-pituitary axis to l-T3 than l-T4 (34). This can be due to the presence of other transporters of TH in brain that can compensate more easily for the reduced Mct8-mediated transport of T4 thanT3.
The larger dose of l-T4 (10 μg/100 g BW·d), which normalized the serum TSH of Mct8KO mice, only partially compensated the TH deficiency in brain as evidenced by the normalization of cerebral D2 activity and Hr but not Dio3 mRNA. However, that same dose was thyrotoxic to liver, as shown by the marked increase in D1 activity and reduction in serum cholesterol. Therefore, in MCT8-deficient humans already having failure to thrive and nutritional deficiency (12,13), the administration of supraphysiological doses of l-T4 is not a viable option because it will further aggravate their hypermetabolic state resulting from tissue selective effect of the high serum T3 levels.
DITPA, on the other hand, normalized the basal state of brain hypothyroidism in the Mct8KO mice, and it did so with the same doses that corrected the hypothyroidism in the TH-deprived Wt mice. The one exception was the Hr mRNA with remained below normal with the 0.6 mg DITPA dose. It is of note that contrary to l-T4, DITPA could normalize Dio3 expression in the Mct8KO mice, indicating that it can enter different brain cell types to exert its thyromimetic effect. DITPA also normalized the peripheral tissue hyperthyroidism in the Mct8KO mice. In fact, serum cholesterol and Gstα2 mRNA values reached those of the Wt baseline on the high dose of DITPA, and D1 was brought to near Wt baseline levels in mice of both genotypes. This occurred even when the endogenous TH was not suppressed by LoI/MMI/ClO4. Evidently the suppressive effect of DITPA on TSH was able to diminish sufficiently the endogenous TH in Mct8KO mice so that T3 was reduced to nontoxic levels. Unfortunately, the latter could not be documented as DITPA interferes with the T3 assay. It should be noted that in intact animals, not receiving LoI/MMI/ClO4, DITPA reduced but did not suppress the serum TSH. Thus, the observed thyromimetic effects are complex because they represent the action of DITPA as well as the endogenous T4 and T3.
This study shows that DITPA is less dependent on MCT8 for entering tissues and that the quantitative differences in T4 and T3 effects on liver compared with brain in Mct8KO mice are reduced, resulting in the achievement of near euthyroidism. Whether this effect could be sustained without untoward effect during long-term DITPA treatment needs to be determined. The species differences in DITPA effect and that on target tissues not studied will help determine the application of DITPA to MCT8-deficient humans.
Acknowledgments
We thank Dr. Eugene Morkin for the provision of DITPA and Dr. Neal Scherberg and Theresa Marcinkowski for the analysis of DITPA by HPLC.
Footnotes
This work was supported by the National Institutes of Health Grants 4R37-DK15070 and 2P60-DK020595 and the Esformes Endowment.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 4, 2009
Abbreviations: BW, Body weight; D, deiodinase enzyme; Dio, deiodinase; DITPA, diiodothyropropionic acid; Gst, glutathione S transferase; Hr, hairless; MCT8, monocarboxylate transporter 8; MMI, methimazole; TH, thyroid hormone; TR, TH receptor; Wt, wild type.
References
- Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ 2003 Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135 [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S 2004 A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ 2004 Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Stevenson RE 2007 The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab 21:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S, Dumitrescu AM 2007 Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab 21:277–305 [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S 2006 Tissue specific thyroid hormone deprivation and excess in Mct8 deficient mice. Endocrinology 147:4036–4043 [DOI] [PubMed] [Google Scholar]
- Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H 2007 Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frints SG, Lenzner S, Bauters M, Jensen LR, Van Esch H, des Portes V, Moog U, Macville MV, van Roozendaal K, Schrander-Stumpel CT, Tzschach A, Marynen P, Fryns JP, Hamel B, van Bokhoven H, Chelly J, Beldjord C, Turner G, Gecz J, Moraine C, Raynaud M, Ropers HH, Froyen G, Kuss AW 2008 MCT8 mutation analysis and identification of the first female with Allan-Herndon-Dudley syndrome due to loss of MCT8 expression. Eur J Hum Genet 16:1029–1037 [DOI] [PubMed] [Google Scholar]
- Brockmann K, Dumitrescu AM, Best TT, Hanefeld F, Refetoff S 2005 X-linked paroxysmal dyskinesia and severe global retardation caused by defective MCT8 gene. J Neurol 252:663–666 [DOI] [PubMed] [Google Scholar]
- Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE 2005 Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 77:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J 2006 Role of monocarboxylate anion transporter 8 (MCT8) in thyroid hormone transport: answers from mice. Endocrinology 147:4034–4035 [DOI] [PubMed] [Google Scholar]
- Wémeau JL, Pigeyre M, Proust-Lemoine E, d'Herbomez M, Gottrand F, Jansen J, Visser TJ, Ladsous M 2008 Beneficial effects of propylthiouracil plus l-thyroxine treatment in a patient with a mutation in MCT8. J Clin Endocrinol Metab 93:2084–2088 [DOI] [PubMed] [Google Scholar]
- Kakinuma H, Itoh M, Takahashi H 2005 A novel mutation in the monocarboxylate transporter 8 gene in a boy with putamen lesions and low free T4 levels in cerebrospinal fluid. J Pediatr 147:552–554 [DOI] [PubMed] [Google Scholar]
- Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N 2008 Expression of the thyroid hormone transporters MCT8 (SLC16A2) and OATP14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149:6251–6261 [DOI] [PubMed] [Google Scholar]
- Herzovich V, Vaiani E, Marino R, Dratler G, Lazzati JM, Tilitzky S, Ramirez P, Iorcansky S, Rivarola MA, Belgorosky A 2007 Unexpected peripheral markers of thyroid function in a patient with a novel mutation of the MCT8 thyroid hormone transporter gene. Horm Res 67:1–6 [DOI] [PubMed] [Google Scholar]
- Papadimitriou A, Dumitrescu AM, Papavasiliou A, Fretzayas A, Nicolaidou P, Refetoff S 2008 A novel monocarboxylate transporter 8 gene mutation as a cause of severe neonatal hypotonia and developmental delay. Pediatrics 121:e199–e202 [DOI] [PubMed] [Google Scholar]
- Namba N, Etani Y, Kitaoka T, Nakamoto Y, Nakacho M, Bessho K, Miyoshi Y, Mushiake S, Mohri I, Arai H, Taniike M, Ozono K 2008 Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr 167:785–791 [DOI] [PubMed] [Google Scholar]
- Biebermann H, Ambrugger P, Tarnow P, von Moers A, Schweizer U, Grueters A 2005 Extended clinical phenotype, endocrine investigations and functional studies of a loss-of-function mutation A150V in the thyroid hormone specific transporter MCT8. Eur J Endocrinol 153:359–366 [DOI] [PubMed] [Google Scholar]
- Pennock GD, Raya TE, Bahl JJ, Goldman S, Morkin E 1992 Cardiac effects of 3,5-diiodothyropropionic acid, a thyroid hormone analog with inotropic selectivity. J Pharmacol Exp Ther 263:163–169 [PubMed] [Google Scholar]
- Mousa SA, O'Connor L, Davis FB, Davis PJ 2006 Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated. Endocrinology 147:1602–1607 [DOI] [PubMed] [Google Scholar]
- Morkin E, Pennock GD, Spooner PH, Bahl JJ, Goldman S 2002 Clinical and experimental studies on the use of 3,5-diiodothyropropionic acid, a thyroid hormone analogue, in heart failure. Thyroid 12:527–533 [DOI] [PubMed] [Google Scholar]
- Ladenson PW, McCarren M, Morkin E, Edson R, Warren S, Thai T, O'Brien T, Anand I, Warner A, Dunlap M, Hattler B, Erikson J, Goldman S, Effects of thyromimetic agent 3,5-diiodothyropropionic acid (DITPA) on body weight, BMI, and serum lipoproteins: a prospective randomized, controlled, double-blind VA cooperative study. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008, p 68 (Abstract S68-2) [Google Scholar]
- Hayashi Y, Mangoura D, Refetoff S 1996 A mouse model of resistance to thyroid hormone produced by somatic gene transfer of a mutant thyroid hormone receptor. Mol Endocrinol 10:100–106 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Forrest D, Pohlenz J, Cua K, Curran T, Refetoff S 1997 Thyrotropin regulation by thyroid hormone in thyroid hormone receptor ß-deficient mice. Endocrinology 138:3624–3629 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S 1998 Thyroid hormone action on liver, heart and energy expenditure in thyroid hormone receptor ß deficient mice. Endocrinology [Erratum (2000) 141:4767] 139:4945–4952 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S 1999 Mice deficient in the steroid receptor coactivator-1 (SRC-1) are resistant to thyroid hormone. EMBO J 18:1900–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchia PE, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, Seo H, Hayashi Y, Samarut J, Murata Y, Weiss RE, Refetoff S 2001 Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor α. Proc Natl Acad Sci USA 98:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Murata Y, Sadow P, Hayashi Y, Seo H, Xu J, O'Malley BW, Weiss RE, Refetoff S 2002 Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of T3-regulated transcription of genes in vivo. Endocrinology 143:1346–1352 [DOI] [PubMed] [Google Scholar]
- Sadow PM, Chassande O, Gauthier K, Samarut J, Xu J, O'Malley BW, Weiss RE 2003 Specificity of thyroid hormone receptor subtype and steroid receptor coactivator-1 on thyroid hormone action. Am J Physiol Endocrinol Metab 284:E36–E46 [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S 1999 Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A 2004 Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313:856–862 [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S 2005 Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252 [DOI] [PubMed] [Google Scholar]
- Balzano S, Bergmann BM, Gilliland MA, Silva JE, Rechtschaffen A, Refetoff S 1990 Effect of total sleep deprivation on 5′-deiodinase activity of rat brown adipose tissue. Endocrinology 127:882–890 [DOI] [PubMed] [Google Scholar]
- Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, Morte B, Bernal J 2009 Importance of monocarboxylate transporter 8 (Mct8) for the blood-brain barrier dependent availability of 3,5,3′-triiodo-l-thyronine (T3). Endocrinology 150:2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- Potter GB, Zarach JM, Sisk JM, Thompson CC 2002 The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol 16:2547- 2560 [DOI] [PubMed] [Google Scholar]
- Friesema EC, Kuiper GG, Jansen J, Visser TJ, Kester MH 2006 Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20:2761–2772 [DOI] [PubMed] [Google Scholar]
- James SR, Franklyn JA, Reaves BJ, Smith VE, Chan SY, Barrett TG, Kilby MD, McCabe CJ 2009 Monocarboxylate transporter 8 in neuronal cell growth. Endocrinology 150:1961–1969 [DOI] [PubMed] [Google Scholar]
- Verhoeven FA, Van der Putten HH, Hennemann G, Lamers JM, Visser TJ, Everts ME 2002 Uptake of triiodothyronine and triiodothyroacetic acid in neonatal rat cardiomyocytes: effects of metabolites and analogs. J Endocrinol 173:247–255 [DOI] [PubMed] [Google Scholar]