Abstract
Clinical research suggests that type of ovarian hormone loss at menopause influences cognition. Until recently ovariectomy (OVX) has been the primary rodent model to examine effects of ovarian hormone loss on cognition. This model limits evaluations to abrupt and complete ovarian hormone loss, modeling less than 13% of women who receive surgical menopause. The majority of women do not have their ovaries surgically removed and undergo transitional hormone loss via ovarian follicular depletion. 4-Vinylcyclohexene-diepoxide (VCD) produces gradual ovarian follicular depletion in the rodent, with hormone profiles more similar to naturally menopausal women vs. OVX. We directly compared VCD and OVX models to examine whether type of hormone loss (transitional vs. surgical) impacted cognition as assessed on a maze battery as well as the cholinergic system tested via scopolamine mnemonic challenge and brain acetylcholinesterase activity. Middle-aged rats received either sham surgery, OVX surgery, VCD, or VCD then OVX to assess effects of removal of residual ovarian output after transitional menopause and follicular depletion. VCD-induced transitional menopause impaired learning of a spatial recent memory task; surgical removal of residual ovarian hormones by OVX abolished this negative effect of transitional menopause. Furthermore, transitional menopause before OVX was better for memory than an abrupt loss of hormones via OVX only. Surgical ovarian hormone loss, regardless of menopause history, increased hippocampal acetylcholinesterase activity. Circulating gonadotropin and androstenedione levels were related to cognitive competence. Collectively, findings suggest that in the rat, initiation of transitional menopause before surgical ovary removal can benefit mnemonic function and could obviate some negative cognitive consequences of surgical menopause alone.
The type of menopause etiology influences cognition and the cholinergic system.
There is accumulating clinical and basic science evidence that ovarian hormone loss contributes to cognitive decline. Clinical findings show women exhibited cognitive decline after surgical menopause (1,2,3), which negatively impacted performance on global cognitive function tests by 3 months, and persisted 6 months, after surgery (3). Preclinical rodent model evaluations have also found that ovarian hormone loss can induce cognitive changes, an effect, depending on many factors, including age (4,5,6,7). Decrements after surgical ovarian hormone loss [ovariectomy (OVX)] have been observed in female rats that were young adult (4,8,9,10,11,12), whereas enhancements after OVX have been observed in old age (13,14). To date, there have only been four studies evaluating OVX effects on maze learning and memory in middle-aged rats (4,5,6,7). In middle-aged females 12–16 months old, OVX did not impact spatial reference memory (RM) (4,5,7) or working memory (WM) (5,6). However, in a study using a repeated testing of longitudinal design, Markowska and Savonenko (5) found OVX-induced deficits in 17-month-old rats on a WM delayed-nonmatching-to-position task, an effect observed only after extended delays. Thus, it is possible that OVX-related memory changes in middle age are evident only when WM demands become more challenging. In this regard, we previously demonstrated that OVX-induced memory decrements in young rats were more pronounced as number of items of information to be remembered, the WM load, increased (8). Thus, elevating WM demand either by extending time delays or increasing number of items to remember may allow a broader scope of evaluations allowing realization of OVX-induced memory changes in middle-aged animals.
Clinical evidence suggests that the cognitive consequences of surgical vs. transitional hormone loss in women may have differential effects. Surgically menopausal women exhibited lower memory scores relative to naturally menopausal women, and age of oophorectomy and greater years since surgery correlated with poorer performance (15). Thus, surgical menopause may have greater negative consequences for cognitive function than natural menopause. Whether type of hormone loss impacts cognition has not been tested in a rodent model.
Surgical menopause models only less than 13% of women (see the web site http://www.menopause.org). The majority of women undergo menopause not from oophorectomy, but as a transitional hormone loss resulting from age-related alterations of the hypothalamus, pituitary, and ovary, ultimately resulting in follicular depletion (17). It is hypothesized that neuronal changes in the hypothalamus initiate transition into reproductive decline early in the aging process, leading to reproductive senescence (18). There are differences in the ultimate mechanism of age-related reproductive senescence of female rats and women, however, that preclude use of ovary-intact female rats as an optimal model of human menopause. In women, as aging ensues, estrogen and progesterone decline due to decreased ovarian follicular reserves (17). Thus, hormone loss during natural, nonsurgical menopause is ultimately due to ovarian follicle depletion. In contrast, the aging rat undergoes estropause, a persistent estrus state due to chronic anovulation rendering intermediate estrogen levels or a pseudopregnant/persistent diestrus state characterized by high progesterone levels due to increased ovulation and corpora lutea (19). These changes in ovarian-derived hormone release are primarily due to hypothalamic/pituitary axis alterations (19). Thus, the primary mechanism that ultimately results in reproductive senescence in the woman is ovarian follicle depletion, whereas in the rat it is the hypothalamic-pituitary axis.
Recently, it was shown that the industrial chemical 4-vinylcyclohexene diepoxide (VCD) produces follicular depletion in rodents (20,21,22,23,24,25,26,27,28,29,30,31). VCD selectively destroys primordial and primary follicles via acceleration of the natural process of atresia (21,29). Because the pool of primordial follicles is finite and cannot proliferate, destruction of these follicles results in ovarian failure (32). Similar to women, interstitial ovarian tissue then yields decreased progesterone and androstenedione, and because estrogen becomes deplete, this results in an androgen-rich milieu. In rodents, including VCD regimens similar to that used in the current study, VCD treatment results in undetectable estradiol levels (29,30). Consequently, VCD-treated follicle-deplete rodents cease to cycle and plasma FSH, and to a lesser extent LH levels, increase (29). This is a profile resembling natural, nonsurgically menopausal women (17). Due to absence of nonovarian side effects, studies have used the VCD-induced transitional ovarian-failure model to investigate disease progression in cardiovascular, osteoporosis, and diabetes rodent models (30,33,34).
The discovery that VCD induces transitional follicular depletion affords the tools to evaluate consequences of this type of ovarian loss in an animal model, thereby allowing methodical experimental evaluation and controlling for or obviating factors possibly influencing outcome in clinical studies such as age, socioeconomic status, and education. The purpose of the current study was 2-fold: 1) to compare transitional (via VCD) vs. abrupt (via surgical manipulation) rodent models of ovarian hormone loss and 2) to further define the effects of ovarian hormone loss in middle-aged rats by assessing memory changes resulting from increasing WM load. After menopause induction, rats were given a cognitive test battery tapping spatial WM and RM. Tests included the water radial-arm maze (WRAM), which incorporated memory load increases across trials, the Morris maze (MM) to test spatial reference memory, and the delayed-match-to-sample place learning task (DMS), including delays spanning hours, to evaluate high-demand memory retention. These tasks are sensitive to OVX in ages ranging from young to aged (4,5,8,9,11,13,14,35). Based on human literature (15), we hypothesized that abrupt hormone loss incurred by OVX would be more detrimental to cognition relative to transitional hormone loss in our rodent evaluations. Based on the animal literature showing greatest OVX-induced changes with higher working memory demands, we hypothesized that menopause effects would be most pronounced when memory demand was greatest. We also hypothesized that cognitive effects of ovarian hormone loss would be related to alterations in cholinergic system function because this system is intimately involved in cognition, and OVX increased sensitivity to challenge with the muscarinic antagonist scopolamine in the rat (5,6). We measured acetylcholinesterase (AChE) levels to yield insight into cholinergic function after hormone loss, as others have shown OVX-induced increases in AChE (36), and higher AChE levels have been associated with poorer memory (37,38,39). Measurements were taken in brain regions intimately involved in WM and spatial memory, the frontal cortex, and the hippocampus. As shown in numerous rodent studies (29,30,31), VCD treatment results in complete follicular depletion and thereby undetectable circulating estradiol levels as well as elevated gonadotropins and present progesterone and androstenedione. Thus, to assess which specific hormones might modulate cognitive function after surgical vs. transitional menopause, circulating levels of LH, FSH, androstenedione, and progesterone were measured and correlated with maze scores.
Materials and Methods
Subjects
Eleven- to 12-month-old Fischer-344 female rats raised at the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN) were used. After arrival, rats were pair housed, had food and water ad libitum, and had a 12-h light, 12-h dark cycle. Animals were 14 months old at maze initiation. Procedures were Institutional Animal Care and Use Committee approved and adhered to NIH standards.
Hormone manipulations
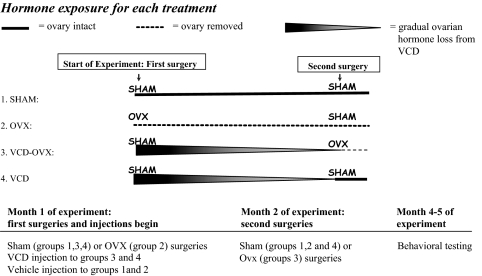
Rats were randomly divided into four groups (n at testing conclusion): SHAM (7), OVX (9), VCD-OVX (10), and VCD (8) (Fig. 1). After isoflurane anesthetization (Baxter HealthCare, Deerfield, IL), for the first surgery, rats received either OVX (OVX group) or sham surgery (SHAM, VCD-OVX, and VCD groups). Bilateral-dorsolateral incisions were made in skin and peritoneum, and ovaries and uterine tips were removed. Muscle and skin were sutured. Sham surgery consisted of muscle/skin incision and suture only. VCD procedures were adapted from elsewhere (29,31). Two days after the first surgery, animals received either VCD (160 mg/kg diluted in dimethyl sulfoxide at a volume of 2.5 μl/g body weight, ip; Sigma-Aldrich, St. Louis, MO), or vehicle, injections for 15 consecutive days. The second surgery (sham surgery for SHAM, OVX, VCD groups; OVX surgery for VCD-OVX group) occurred 55 d after the first VCD injection.
Figure 1.
Schematic representation of the experimental design of the study.
Time-dependent changes in follicular depletion after VCD injections have been demonstrated; gradual depletion started 15 d after injection, continuing thereafter (29,31). To confirm follicular depletion in VCD animals, complete OVX, and SHAM animal cyclicity, vaginal smears were taken for 10 d, 99 d after the first injection. Smears were classified as proestrus, estrus, metestrus, or diestrus (40).
Water radial-arm maze
The eight-arm WRAM tested spatial working and reference memory, including performance as working load increased, as described previously (8,41,42). Working memory correct (WMC) errors were first and repeat entries into arms from which a platform was removed during that day. RM errors were first entries into arms that never contained a platform. Working memory incorrect errors (WMI) were repeat entries into RM arms. On d 13, a 4-h delay between trials 2 and 3 was given. For detailed WRAM procedures, see supplemental Methods, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Morris water maze
The MM was a water-filled tub (188 cm diameter) with a hidden platform (10 cm wide), remaining in a fixed location, testing spatial RM (7,43). For detailed MM procedures, see supplemental Methods.
DMS plus maze
Spatial working memory and short-term retention was tested using a win-stay water-escape DMS place learning task. There was a hidden platform in one arm that remained in the same location within a day but changed location across days. After initial testing, a 6-h delay was instilled after the information trial. On subsequent separate days, rats were given two scopolamine challenges (0.2 or 0.4 mg/kg, ip, scopolamine; Sigma-Aldrich). For detailed DMS procedures, see supplemental Methods.
Decapitations
All decapitations occurred under isoflurane anesthesia on the same day, with researchers blinded to group. Brains were rapidly dissected by one researcher, another collected trunk blood, and a third weighed uteri.
Ovarian histology and uterine weights
The uterus was cut above the cervical junction and visible fat removed and weighed (44). For animals with ovaries at the time the animals were euthanized (SHAM and VCD groups), ovaries were removed, trimmed of connective tissue/fat, and fixed in 10% formalin for 48 h. All ovarian tissues were processed for paraffin embedding, sectioned at 5 μm, mounted, and stained with hematoxylin and eosin. Corpus lutea were counted (Aristoplan compound microscope; Leitz, Charlotte, VT) and a representative picture taken (AXIOcam MrC5, ×2.5 objective; Zeiss, Thornwood, NY).
Hormone assays
Serum levels of LH, FSH, androstenedione, and progesterone were determined by liquid chromatography-tandem mass spectrometry according to procedures published elsewhere (45). After dansyl-chloride derivatization, samples were separated by fast-gradient chromatography and injected in a tandem mass spectrometer after formation of positive ions with atmospheric pressure chemical ionization.
Brain acetylcholinesterase assays
Frontal cortex and hippocampal CA1/CA2 regions were assayed for AChE levels. AChE was analyzed using an AChE assay kit (Invitrogen, Carlsbad, CA). Values were interpolated off a standard curve and normalized to tissue weight (U-AChE per gram wet tissue weight). For detailed procedures, see supplemental Methods.
Statistical analyses
Uterine weight, hormone, and AChE comparisons were done using ANOVA followed by Fisher’s least significant differences post hoc tests. The corpus lutea analysis comparing VCD with SHAM groups (groups maintaining ovaries until study conclusion) used a t test. For behavior, data were analyzed separately for each maze with an omnibus ANOVA with treatment as the between variable and block, days, and/or trials as repeated measures. Follow-up two-group planned comparisons were conducted to allow interpretation of blocks, days, and/or trials repeated-measures effects in the context of potentially complex group interactions. Analyses were two tailed (α < 0.05). For detailed statistical procedures, see supplemental Methods.
Regression examined relationships between brain neurochemistry, hormones, and memory. AChE, LH, FSH, androstenedione, and progesterone were predictor variables in the model, and the following were outcome variables: MM distance collapsed across all trials/days; DMS WM errors for both blocks; DMS WM postdelay and postscopolamine errors; WRAM WMC, WMI, and RM errors block 2 for all trials; or trial 4 alone; and WRAM WMC, WMI, RM errors for postdelay trials.
Results
Water radial arm maze
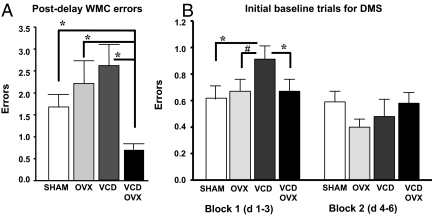
There were no treatment effects for WMC, WMI, or RM for blocks 1 or 2 (data not shown). For postdelay trials, there was a treatment effect for WMC errors [F3,33 = 4.91; P < 0.01] (Fig. 2A). The VCD-OVX group made the fewest WMC errors [VCD-OVX vs. SHAM: F1,17 = 9.90; P < 0.005; OVX: F1,17 = 8.24; P < 0.01; and VCD: F1,17 = 18.10; P < 0.0005].
Figure 2.
A, Mean ± se WM errors for each group for postdelay trials on the WRAM. The VCD-OVX group made fewer errors relative to all groups, suggesting that transitional ovarian hormone loss before OVX aids memory retention and that removal of residual ovarian tissue after transitional ovarian hormone loss improves memory retention relative to retaining residual ovarian tissue. *, P < 0.0005. B, Mean ± se for each group for blocks 1 and 2 for trials 2–6. SHAM and VCD-OVX groups showed fewer errors during initial learning in block 1, relative to the VCD group. *, P < 0.05. The OVX group was marginally different from the VCD group. #, P = 0.06.
Morris water maze
For distance, there was no treatment main effect or interaction. For the probe, a higher percent distance was spent in the platform vs. opposite quadrant [quadrant effect: F1,33 = 101.21; P < 0.0001; data not shown]. All groups localized the platform location by the end of testing [null quadrant × treatment interaction: P > 0.14].
DMS plus maze testing
For block 1 there was a treatment effect [F3,32 = 2.91; P < 0.05; Fig. 2B]. Transitional menopause increased errors [SHAM vs. VCD: F1,15 = 6.21; P < 0.05], and OVX reversed the negative effect of transitional menopause [VCD vs. VCD-OVX: F1,17 = 4.44; P < 0.05], whereas the transitional menopause group made marginally more errors than the surgical menopause group [VCD vs. OVX: F1,16 = 1.26; P = 0.06]. There were no group differences for block 2. Scopolamine marginally impaired performance at the 0.2 mg/kg dose [day effect: F1,3 = 3.55; P = 0.07] and the 0.4 mg/kg dose [day effect: F1,3 = 3.90; P = 0.06], with no treatment interactions. There were no treatment main effects or interactions for the 6-h delay.
Vaginal smears, ovarian histology, and uterine weights
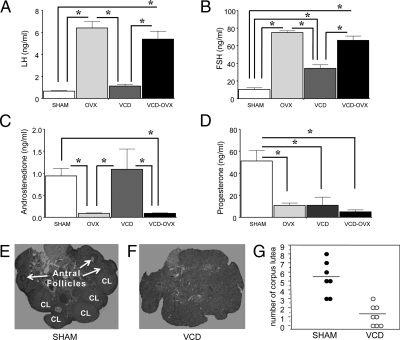
The OVX and VCD-OVX groups showed diestrus vaginal smears, with few, primarily leukocyte, cells. VCD rats showed persistent estrous or diestrous with either cornified or leukocyte cells, respectively. For SHAM rats, 44.4% had regular cyclicity of proestrous, estrous, metestrous, and diestrous; 33.3% showed extended diestrous; and 22.2% extended estrous. All groups showed the expected vaginal smear profile. Ovarian histology indicated all SHAM rats were cycling, with presence of follicles of all stages and corpora lutea (Fig. 3E). Ovarian sections from VCD animals indicated complete follicle depletion and ovarian failure (Fig. 3F). Corpus lutea in SHAM animals (mean = 5.29 ± 0.71, n = 7) were greater than VCD-treated animals (mean = 1.13 ± 0.39 n = 8) [t13 = 5.27; P < 0.0002 (Fig. 3G)].
Figure 3.
Mean ± se serum LH (A), FSH (B), androstenedione (C), and progesterone (D) levels for each group. For LH, ovary intact animals (SHAM and VCD) had lower levels relative to OVX animals (OVX and VCD-OVX). *, P < 0.0001. For FSH, ovary-intact animals had lower levels relative to OVX animals, and SHAM animals showed lower levels relative to VCD animals. *, P < 0.0001. For androstenedione, ovary-intact animals showed increased levels relative to OVX animals. *, P < 0.005. For progesterone, the SHAM group exhibited higher levels relative to all other groups. *, P < 0.0001. Representative samples of ovaries from rats given vehicle (E) or VCD (160 mg/kg, ip) (F) are shown. Number of corpus lutea (CL) in SHAM animals was greater than VCD animals (P < 0.0002), and (G) there were no antral follicles in the VCD animals.
There were treatment differences in uterine weight [F3,30 = 5.51; P < .01], with mean ± ses for SHAM (0.61 ± 0.06), OVX (0.14 ± 0.01), VCD (0.99 ± 0.36), and VCD-OVX (0.18 ± 0.03). Uterine weights of VCD animals did not differ from SHAMs (P = 0.15) and were elevated compared with OVXs (P < 0.005). VCD animals had higher uterine weights compared with VCD-OVX animals (P < 0.005).
Serum hormone levels
Each omnibus ANOVA demonstrated treatment differences for LH [F3,30 = 29.37; P < 0.0001], FSH [F3,27 = 63.02; P < 0.0001], androstenedione [F3,27 = 6.22; P < 0.01], and progesterone [F3,27 = 14.30; P < 0.0001] (Fig. 3). For LH, ovary-intact animals (SHAM and VCD) had decreased levels relative to animals without ovaries [OVX and VCD-OVX, P values (Ps) < 0.0001]. For FSH, SHAM and VCD groups had lower levels than OVX and VCD-OVX groups, and SHAM animals showed lower levels than VCD animals that retained their ovaries (Ps < 0.0001). As expected (30), SHAM and VCD animals (ovary intact animals) showed increased androstenedione relative to OVX and VCD-OVX animals (Ps ≤ 0.01). OVX, VCD, and VCD-OVX treatments each decreased levels relative to the SHAM group (Ps < 0.0001).
Serum hormone levels and relationships with memory
Figures 4 and 5 represent scatterplots for each significant regression. For regression analyses, animals without ovaries (OVX and VCD-OVX) were combined because their hormone status did not differ at the time the animals were killed, whereas animals with intact ovaries (VCD and SHAM) were initially analyzed separately because their hormone status differed at the time the animals were killed.
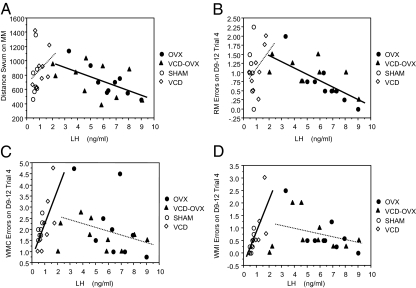
Figure 4.
Regression analysis indicated that in animals without ovaries (OVX and VCD-OVX), higher LH was associated with better reference memory for the MM (A) and WRAM (B). In animals with ovaries (SHAM and VCD), higher LH was associated with worse WM for multiple measures; higher LH was associated with more radial-arm maze WMC (C), and WMI (D) errors. Graphically, the significant regression coefficients for these analyses are shown as solid lines; the dashed lines of the other groups are shown for comparison purposes to aid interpretation.
Figure 5.
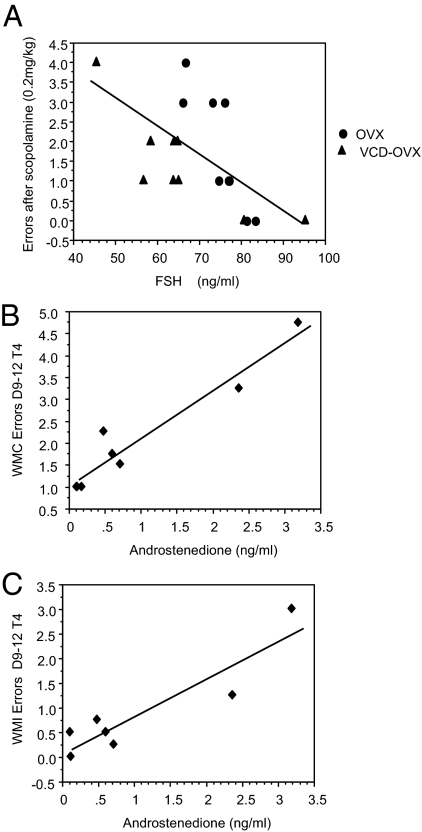
Regression analysis indicated that in OVX animals (OVX and VCD-OVX), higher FSH was associated with fewer WM errors after scopolamine (0.2 mg/kg) challenge (A) and that higher androstenedione in VCD animals was associated with more radial-arm maze WMC and WMI errors on the trial with the highest memory load (B and C).
For animals without ovaries, higher gonadotropin levels were associated with better maze scores. In OVX/VCD-OVX animals, higher LH levels were associated with better RM performance on two different mazes; higher LH levels were associated with lower MM distance scores [B = −64.48, se = 21.04, P = 0.007, R = −0.60] and fewer WRAM RM errors on the highest memory load trial for block 2 [B = −0.16, se = 0.04, P = 0.001, R = −0.68]. Graphically, the regression coefficients (i.e. the Bs) for these significant analyses are the solid lines in Fig. 4, A and B, respectively. Our ANOVA analyses for these RM maze scores as well as the corresponding scatterplots in Fig. 4 indicate that, for the OVX and VCD-OVX groups, the association between LH and memory errors were statistically equivalent (i.e. there were no treatment × LH interactions). Consequently, pooling these two conditions into a single group allows us to obtain a more accurate estimate of the association between LH and memory errors by increasing power. In addition, in OVX/VCD-OVX animals, higher FSH levels were associated with fewer WM errors after scopolamine (0.2 mg/kg) challenge [B = 0.07, se = 0.02, P = 0.006, R = −0.62] (Fig. 5A). Again, there was no treatment × FSH interaction, confirming that it is statistically appropriate to combine these groups for this assessment.
As reported above, androstenedione was still present in physiologically relevant levels in VCD animals. This is especially noteworthy, given that, in VCD animals, higher androstenedione levels were associated with poorer WRAM WM scores on the highest memory load trial for block 2, as seen for WMC errors [B = 1.1, se = 0.14, P = 0.0005, R = 0.96] and WMI errors [B = 0.77, se = 0.15, P = 0.004, R = 0.91]. This relationship was not seen in SHAM animals. Furthermore, although higher LH levels were associated with better RM performance in animals without ovaries, higher LH levels were associated with more WMC errors on the highest memory load trial for block 2 for VCD animals [B = 2.03, se = 0.74, P = 0.033, R = 0.75] and marginally associated in this same direction for SHAM animals [B = 2.39, se = 0.99, P = 0.059, R = 0.74]. This same pattern was also realized for WMI, with higher LH significantly associated with more WMI errors for SHAM animals [B = 2.5, se = 0.72, P = 0.017, R = 0.84] and marginally associated for VCD animals [B = 1.42, se = 0.59, P = 0.052, R = 0.70]. Because the direction and magnitude of the associations were similar (regressions coefficients of 3.96 and 1.08 for WMC and WMI, respectively), we combined the VCD and SHAM animals into one group for further assessment. Using the procedure similar to that supporting combination of animals without ovaries, treatment × LH interactions were not significant for the regression analyses with both VCD and SHAM groups included. This indicated that the regression lines, and thus the relationship between the independent and dependent variables, did not differ depending on group membership. This was true for both WMC and WMI. For the combined group of animals with ovaries, higher LH levels were related to more WRAM WMC [B = 1.52, se = 0.46, P = 0.006, R = 0.67] and WMI [B = 1.31, se = 0.34, P = 0.002, R = 0.73] errors. Graphically, the regression coefficients for these significant analyses are the solid lines in Fig. 4, C and D, respectively. VCD and SHAM groups showed no association between LH levels and the MM and WRAM RM outcomes. For consistency and further discussion, we displayed the data for all groups in the regression scatterplots in Fig. 4.
AChE levels
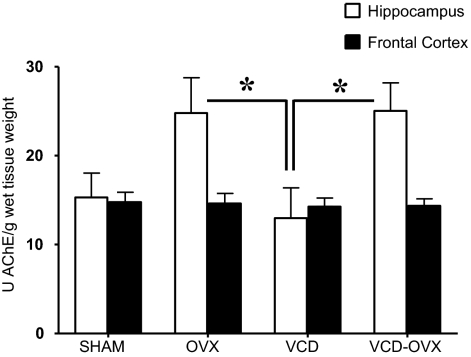
Figure 6 shows mean AChE activity ± se for each brain region and treatment group. For hippocampus, there was a treatment effect for AChE activity [F3,28 = 3.28; P < 0.05]. The OVX and VCD-OVX groups each had higher hippocampal AChE levels relative to the VCD group (Ps = 0.02).
Figure 6.
Mean ± se. AChE activity in units per gram for hippocampus and frontal cortex. The VCD group had higher levels of AChE in the hippocampus relative to OVX animals (OVX and VCD-OVX). *, P < 0.05.
Discussion
The primary finding of the current study is that type of ovarian hormone loss in middle age impacts memory retention and learning, correlating with circulating gonadotropin and androstenedione levels. Results suggest that transitional ovarian hormone loss before OVX is better for cognition than an abrupt loss due to OVX, and that removal of residual ovaries after transitional ovarian hormone loss improves cognition relative to retention of residual ovaries. The present study is the first to compare the cognitive effects of surgical and transitional models of menopause in the rodent.
Consistent with previous findings (30), uterine weights of VCD-treated animals did not differ from SHAM animals and were elevated compared with animals that had their ovaries removed (OVX and VCD-OVX). This was likely due to the presence of androgens in the VCD animals. Indeed, androgens can induce uterine weight increases via direct actions; androgen-mediated increases were not reversed by estrogen antagonists, suggesting the effect was not due to aromatized estrogens (46). Importantly, vaginal smears and ovarian histology verified SHAM animals were cycling, as evidenced by presence of follicles of all stages and corpus lutea. In the current study, VCD animals showed complete follicle depletion and premature ovarian failure (31), effects that have been reliably associated with undetectable estradiol levels in rodents (29,30). Ovary-intact animals (SHAM and VCD animals) had lower LH and FSH levels, and higher androstenedione levels, compared with animals without ovaries (OVX and VCD-OVX animals). Only the SHAM group exhibited high progesterone levels relative to all other groups. This is important because it also supports the histology data showing that VCD animals had no corpus lutea, which is the primary derivation of ovarian-derived progesterone. The elevated FSH levels, presence of androstenedione, lack of corpus lutea, lack of follicles, and negative vaginal smears support that VCD animals had complete follicle depletion and premature ovarian failure. These data correspond with VCD-induced primordial and primary ovarian follicle depletion (20,21,22,23,24,25,26,27,28,29,30,31), cessation of cyclicity, reduced estrogen, and elevated gonadotropin levels (29,30), a hormone profile that resembles naturally menopausal women (17).
Tasks sensitive to ovarian hormone manipulation in the current study were limited to acquisition of WM maze learning and memory retention. The hypothesis that abrupt hormone loss incurred by OVX would be more detrimental to cognition relative to transitional hormone loss was supported, but only when ovarian-derived hormones after transitional loss were removed. Indeed, unexpectedly, the VCD-OVX group, which experienced gradual follicular depletion followed by surgical removal of residual ovarian tissue, had enhanced WM performance on the WRAM after a 4-h delay relative to the SHAM, OVX, and VCD groups. Because the delay was given after trial 2, after two platform locations had to be remembered, removal of residual ovarian hormones after follicular depletion may enhance memory retention for numerous items of information. It is also noteworthy that the VCD-OVX group had better performance than the SHAM group, suggesting that transitional loss followed by OVX results in better memory retention than that afforded by the ovary-intact status of the middle-aged female rat.
DMS place task acquisition was altered by type of reproductive senescence. Transitional menopause impaired learning, which was shown when the VCD group made more errors on the initial testing block relative to the SHAM group. This poorer acquisition after transitional menopause may be due to an initial maze-solving strategy that impaired learning to solve the task spatially; indeed, ovarian hormone manipulations have been shown to impact maze solving strategy in rats (47). Removal of residual ovarian hormones abolished this impaired acquisition due to transitional menopause; the VCD-OVX group made fewer errors than the VCD group, and did not differ from the SHAM group, during this testing block. No mode of ovarian hormone loss impacted performance after the DMS 6-h delay, scopolamine challenges, or MM performance. Thus, cognitive effects of ovarian hormone loss may be task dependent, possibly limited to learning of a WM task and memory retention when the task is made more challenging such as required by numerous items of information across a delay (as tested on the WRAM).
Research testing the cognitive effects of OVX in middle-aged rats has not been extensive. In Markowska and Savonenko (5), although OVX had no impact on memory at 15 or 16 months old, by 17 months old, OVX-induced WM deficits were observed on a delayed-nonmatching-to-position task after delays of 1–30 min (5). Based on these findings, as well as our prior work that a higher WM load exacerbates the negative effects of OVX in young animals (8), we hypothesized that OVX deficits would be evident in middle-age if WM demands became more challenging. Although the current study did find that a higher WM demand yielded changes induced by ovarian hormone loss, the effects were in the opposite direction to that hypothesized. Indeed, the VCD-OVX group exhibited the best performance of all the groups when the WM demand was highest on the RAM, indicating that OVX after transitional hormone loss enhanced performance during this challenge. Effects in the study by Markowska and Savonenko (5) could have been accelerated by aging because rats in that study were 17 months of age when OVX effects were realized; there were no effects of OVX in that study at 15–16 months of age, the age animals were in the current study. In fact, if we take age into consideration, the herein lack of effects in surgical-only ovarian hormone loss (OVX) correspond with lack of OVX effects in the study by Markowska and Savonenko (5) testing WM, as well as the lack of OVX effects in our recent study testing RM (4). In addition, multiple maze testing across age in the study by Markowska and Savonenko (5), and using a maze battery in the current study, could have differentially impacted performance and hormone-induced memory changes by altering stress responses due to procedure acclimation, hippocampal neuroregeneration, or other neurophysiological effects (48).
Cognitive evaluations directly comparing surgical vs. transitional menopause have been examined infrequently in the clinical literature and, previous to the current study, were nonexistent in the rodent model. In contrast to lack of OVX effects, and beneficial effects of removal of residual hormones after follicular depletion (VCD-OVX) in the current study, surgically menopausal women exhibited lower memory scores relative to naturally menopausal women, an effect correlating with oophorectomy age and years since surgery (15). Furthermore, surgically menopausal women exhibited decreased global cognitive function and Wechsler Memory Scale scores relative to premenopausal age-matched women (3). However, not all studies find differences between surgical and natural menopausal women on cognitive measures (49,50). Similar to cognitive effects of estrogen replacement, cognitive effects of ovarian hormone loss in women may be impacted by type of test used to evaluate cognitive efficacy (51,52). In fact, surgically and naturally menopausal women differed significantly only on tests of recency from a word list but not on several other cognitive measures (15).
The present findings suggest that transitional ovarian hormone loss followed by removal of residual hormones (VCD-OVX) provides cognitive benefit compared with transitional ovarian hormone loss without removal of residual hormones (VCD). Mayer et al. (29) showed that the hormonal milieu of VCD follicle-deplete rodents became androgen rich, and we found elevated androstenedione levels in VCD-treated animals; there is also a greater androgen to estrogen ratio in postmenopausal women who still have their ovaries (53). The VCD-OVX group showed enhanced performance on some maze measures relative to the OVX and VCD groups, suggesting that although transitional ovarian hormone loss may provide cognitive benefit relative to abrupt hormone loss, this may be true only if residual androgens are removed. Residual androgens in a follicle deplete hormone environment may be detrimental to cognition. Consistent with this idea, we also found that higher androstenedione levels were correlated with more WRAM WMC and WMI errors for VCD animals. Other research has shown that androgens impact maze performance in the rodent (54,55,56,57,58). However, not all androgens are associated with cognitive impairment, and there are different mnemonic effects depending on the specific androgen tested (55,59).
Nappi et al. (15) found that in women poorer cognitive scores corresponded with longer durations of oophorectomy-induced hormone deprivation. Importantly, in that study, surgically menopausal women were younger at menopause than naturally menopausal women. It is therefore possible that the surgically menopausal women did not undergo an extended period of transitional menopause before surgical menopause, and thus they did not benefit from transitional hormone decline before oophorectomy. Indeed, Rocca et al. (60) found that women who had undergone oophorectomy before menopause onset had elevated cognitive impairment risk compared with age-matched women without oophorectomy. Furthermore, cognitive impairment risk increased as oophorectomy age decreased, presumably limiting transitional menopause. Further evaluations of women who received oophorectomy before, vs. after, transitional menopause will better discern whether history of transitional menopause before surgical ovarian removal affects trajectory of subsequent cognitive change. It may be that an as-yet-undetermined duration of transitional menopause before surgical ovarian removal is optimal for cognitive outcome, a tenet supported by the current experiment; this would obviate the abrupt ovarian hormone loss associated with oophorectomy.
In the present study, LH was related to maze scores. Associations between LH and memory scores revealed an inverted U-shaped function, with highest and lowest levels associated with best performance. This relationship became apparent in scatterplots including all groups so that the range of values across groups could be noted. When LH levels ranged from approximately 0 to 2 (SHAM+VCD groups), the relationship was positive with higher LH levels associated with worse maze performance. When LH levels ranged from approximately 2 to 10 (OVX+VCD-OVX groups), the relationship was negative, with higher LH levels associated with better maze performance. Thus, higher LH levels were associated with memory enhancement in animals without ovaries and memory impairment in animals with ovaries, although the relative value of high LH differed dramatically, depending on hormone manipulation. This pattern was seen for multiple measures, including WM (WRAM WMC and WMI errors) and RM (MM distance and WRAM RM errors). Although we are limited in interpreting this relationship between LH and memory scores in the present study because LH levels were confounded by group membership, this inverted U function is nonetheless striking, especially given the increasing evidence that LH levels are linked to cognition and pathologies associated with neurodegenerative disorders. Other studies reported that higher LH levels are related to better cognitive performance, as seen in our OVX subjects. Tonic treatment with LHRH, elevating LH concentrations to OVX levels, enhanced performance on visual-discrimination in young rats (61), and enhanced nonspatial WM in aged rats (62). That higher LH levels were associated with better memory in these studies is likely related to LH levels being increased to that of OVX animals. On the other hand, corresponding with our findings in ovary-intact animals that higher LH levels correlated with worse cognitive performance, in ovary-intact aged female mice, experimentally induced LH reductions decreased amyloid-β concentrations and enhanced cognition, whereas LH increases promoted biochemical brain changes consistent with Alzheimer’s disease (AD), although none of these studies correlated circulating LH levels with memory scores in individual animals (63,64,65). Also, men and women with AD had higher circulating LH levels than controls (66,67). Thus, both high and low LH levels have been associated with enhanced cognition, although collective interpretations are limited to levels obtained by the respective LH manipulations. Moreover, supporting plausibility of LH effects on the brain and cognition, the highest density of LH receptors in the brain are found in the hippocampus (68,69), a region involved in cognition and affected by aging and AD, and LH can cross the blood-brain-barrier (70).
The current findings, taken with growing literature showing relations between LH and cognition, suggest this relationship may subserve a U-shaped function, with an intermediate level resulting in optimal cognition. This is an important area that will require further study, with results possibly revealing important mediators of cognitive function. Many biological systems fit a curvilinear function, including relationships between amyloid precursor protein and memory in aged female rats (71) and potential associations between estrogen levels and memory in the human and the rodent (discussed in Ref. 4). A complementary explanation that must also be considered is that this relationship is in part resulting from other secondary, as-yet-unknown factors including balance of hormone/gonadotropin levels in the brain vs. the serum. Future studies should help clarify these likely complex and multidimensional effects.
Surgical ovarian hormone loss, regardless of transition history, impacted the cholinergic system. Animals without ovaries, both OVX and VCD-OVX groups, exhibited higher hippocampal AChE levels relative to ovary-intact VCD rats, in accordance with findings in young rats showing increased cortical AChE after OVX (36). Not all studies, however, found OVX-induced AChE increases in cognitive brain regions, including the hippocampus (12,72,73). We found no significant AChE changes in frontal cortex due to any form of ovarian hormone loss. Notably, recent research found a dissociation between the hippocampus and frontal cortex for the acetylcholine synthesizing enzyme, choline acetyltransferase, after hormone manipulation. Specifically, temporal parameters of estradiol administration influenced not only cognition (74), but also the brain region in which effects were seen and the direction of the effects (75). Choline acetyltransferase was increased in hippocampus, but not frontal cortex, when estradiol was given immediately after OVX, yet the opposite pattern was seen when estradiol was given 5 months after OVX (75). It is therefore plausible that we may have seen a different pattern of effects on the cholinergic system had we evaluated multiple time points as follicular depletion ensued.
Although AChE activity did not correlate with cognitive scores, VCD-OVX animals showed enhanced memory retention, and previous findings have associated lower, not higher, AChE activity with better cognitive performance (37,38,39). Notably, recent research indicates that AChE subtypes could play different roles in normal and diseased brains (for a review see Ref. 16). Future studies evaluating neurobiological function of these subtypes, and resulting changes due to hormone modulation, may yield valuable insight into interactions between hormones, cognition, and the cholinergic system.
In conclusion, the current study suggests that in the middle-aged rodent, transitional ovarian hormone loss before OVX aids cognition compared with OVX without a transitional period before surgery. Findings also indicate that removal of residual follicle-deplete ovaries after transitional hormone loss improves cognition, relative to retaining residual follicle-deplete ovarian tissue. These findings could have implications in women, pending clinical research evaluating whether a transitional menopause before oophorectomy improves cognitive outcome, compared with oophorectomy without a prior transition. Studies evaluating menopause status relative to timing of oophorectomy, in the context of circulating levels of LH and androstenedione, may reveal valuable information toward optimization of factors that could influence women’s health. That these findings might translate to brain health in women provides exciting new avenues for research and intervention.
Supplementary Material
Acknowledgments
We thank Dr. Lotta Granholm and Dr. Cheryl Dyer for invaluable discussion of this novel animal model, Dr. Laurence Demers and the Core Endocrine Laboratory at Penn State for performing the hormone assays and for discussion, and the Discovery Research Laboratories at Northern Arizona University for performing the AChE assays. We also thank B. Blair Braden, Elizabeth Engler, Cynthia Zay, Ian Crain, Bronson Bowman, Sean Nonnenmacher, Robert Audet, and Heidi Miers for excellent technical assistance.
Footnotes
This work was supported by Grant AG028084 from the National Institute on Aging and grants from the Institute for Mental Health Research, Evelyn F. McKnight Brain Research Foundation (Tucson, AZ), state of Arizona, Arizona Department of Health Services, Alzheimer’s Disease Core Center Pilot grant program (to H.A.B.-N.), and the American Psychological Association Diversity Program in Neuroscience Predoctoral Fellowship (to J.I.A.) as well as Grant AG028084, a Diversity Supplement from the National Institute on Aging.
Disclosure Summary: J.I.A., J.S.T., C.W.S.T., C.J.S., C.K.E., and H.A.B.-N. have nothing to declare. L.M. has equity interests in Senestech, Inc.
First Published Online May 21, 2009
Abbreviations: AChE, Acetylcholinesterase; AD, Alzheimer’s disease; DMS, delayed-match-to-sample place learning task; MM, Morris maze; OVX, ovariectomy; Ps, P values; RM, reference memory; VCD, 4-vinylcyclohexene diepoxide; WM, working memory; WMC, WM correct; WMI, WM incorrect errors; WRAM, water radial-arm maze.
References
- Phillips SM, Sherwin BB 1992 Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 17:485–495 [DOI] [PubMed] [Google Scholar]
- Sherwin BB 1988 Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 13:345–357 [DOI] [PubMed] [Google Scholar]
- Farrag AK, Khedr EM, Abdel-Aleem H, Rageh TA 2002 Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord 13:193–198 [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA 2008 Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem 90:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV 2002 Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci 22:10985–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL 2003 The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience 119:821–830 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC 2006 Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci 24:229–242 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH 1999 Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24:161–173 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP 1999 Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav 66:11–20 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA 2008 Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149:3176–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Suliman I, Lindgren U, Winblad B, Adem A 2004 Ovariectomy and gonadal hormone treatment: effects on insulin-like growth factor-1 receptors in the rat brain. Growth Horm IGF Res 14:388–393 [DOI] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang JT 2004 Long-term effects of melatonin or 17β-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res 37:198–206 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC 2003 Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci 117:1395–1406 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC 2004 Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci 118:707–714 [DOI] [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G 1999 Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest 47:29–36 [DOI] [PubMed] [Google Scholar]
- García-Ayllón MS, Silveyra MX, Sáez-Valero J 2008 Association between acetylcholinesterase and β-amyloid peptide in Alzheimer’s cerebrospinal fluid. Chem Biol Interact 175:209–215 [DOI] [PubMed] [Google Scholar]
- Timaras P, Quay W, Vernadakis A, eds. 1995 Hormones and aging. New York: CRC Press [Google Scholar]
- Downs JL, Wise PM 2009 The role of the brain in female reproductive aging. Mol Cell Endocrinol 299:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J, Lu JKH 1994 Reproductive aging and neuroendocrine function. In: Charlton HM, ed. Oxford review of reproductive biology. New York: Oxford Press; 215–239 [Google Scholar]
- Springer LN, Tilly JL, Sipes IG, Hoyer PB 1996 Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol 139:402–410 [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB 1996 Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol 139:394–401 [DOI] [PubMed] [Google Scholar]
- Springer LN, Flaws JA, Sipes IG, Hoyer PB 1996 Follicular mechanisms associated with 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Reprod Toxicol 10:137–143 [DOI] [PubMed] [Google Scholar]
- Kao SW, Sipes IG, Hoyer PB 1999 Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol 13:67–75 [DOI] [PubMed] [Google Scholar]
- Borman SM, VanDePol BJ, Kao S, Thompson KE, Sipes IG, Hoyer PB 1999 A single dose of the ovotoxicant 4-vinylcyclohexene diepoxide is protective in rat primary ovarian follicles. Toxicol Appl Pharmacol 158:244–252 [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB 1994 Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol 8:509–514 [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Sipes IG, Hoyer PB 2001 Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod 65:1489–1495 [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB 2001 Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod 65:87–93 [DOI] [PubMed] [Google Scholar]
- Hu X, Flaws JA, Sipes IG, Hoyer PB 2002 Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod 67:718–724 [DOI] [PubMed] [Google Scholar]
- Mayer LP, Devine PJ, Dyer CA, Hoyer PB 2004 The follicle-deplete mouse ovary produces androgen. Biol Reprod 71:130–138 [DOI] [PubMed] [Google Scholar]
- Mayer LP, Dyer CA, Eastgard RL, Hoyer PB, Banka CL 2005 Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler Thromb Vasc Biol 25:1910–1916 [DOI] [PubMed] [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB 2002 Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol 16:775–781 [DOI] [PubMed] [Google Scholar]
- Hirshfield AN 1991 Development of follicles in the mammalian ovary. Int Rev Cytol 124:43–101 [DOI] [PubMed] [Google Scholar]
- Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, Hoyer PB 2008 Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res 23:1296–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL 2007 Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol 293:F193–F199 [DOI] [PubMed] [Google Scholar]
- Feng Z, Chang Y, Cheng Y, Zhang BL, Qu ZW, Qin C, Zhang JT 2004 Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J Pineal Res 37:129–136 [DOI] [PubMed] [Google Scholar]
- Monteiro SC, Stefanello FM, Vianna LP, Matte C, Barp J, Belló-Klein A, Trindade VM, Wyse AT 2005 Ovariectomy enhances acetylcholinesterase activity but does not alter ganglioside content in cerebral cortex of female adult rats. Metab Brain Dis 20:35–44 [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim M, Kim S, Kim M, Chung JH 2004 Effects of green tea polyphenol on cognitive and acetylcholinesterase activities. Biosci Biotechnol Biochem 68:1977–1979 [DOI] [PubMed] [Google Scholar]
- Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN 2008 Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res 198:352–358 [DOI] [PubMed] [Google Scholar]
- Sudha S, Lakshmana MK, Pradhan N 1995 Changes in learning and memory, acetylcholinesterase activity and monoamines in brain after chronic carbamazepine administration in rats. Epilepsia 36:416–422 [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL 2007 The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH 2000 Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav 68:495–499 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH 2000 In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav 70:311–317 [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J 1982 Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683 [DOI] [PubMed] [Google Scholar]
- Ashby J, Odum J, Foster JR 1997 Activity of raloxifene in immature and ovariectomized rat uterotrophic assays. Regul Toxicol Pharmacol 25:226–231 [DOI] [PubMed] [Google Scholar]
- Nelson RE, Grebe SK, OKane DJ, Singh RJ 2004 Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem 50:373–384 [DOI] [PubMed] [Google Scholar]
- Schmidt WN, Katzenellenbogen BS 1979 Androgen-uterine interactions: an assessment of androgen interaction with the testosterone- and estrogen-receptor systems and stimulation of uterine growth and progesterone-receptor synthesis. Mol Cell Endocrinol 15:91–108 [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL 2002 Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci 116:411–420 [DOI] [PubMed] [Google Scholar]
- Carrasco C, Vicens P, Redolat R 2006 Neuroprotective effects of behavioural training and nicotine on age-related deficits in spatial learning. Behav Pharmacol 17:441–452 [DOI] [PubMed] [Google Scholar]
- Kok HS, Kuh D, Cooper R, van der Schouw YT, Grobbee DE, Wadsworth ME, Richards M 2006 Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause 13:19–27 [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E 2002 Hysterectomy, oophorectomy, and cognitive function in older women. J Am Geriatr Soc 50:55–61 [DOI] [PubMed] [Google Scholar]
- Sherwin BB 1998 Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med 217:17–22 [DOI] [PubMed] [Google Scholar]
- Henderson VW, Sherwin BB 2007 Surgical versus natural menopause: cognitive issues. Menopause 14:572–579 [DOI] [PubMed] [Google Scholar]
- Simpson ER 2002 Aromatization of androgens in women: current concepts and findings. Fertil Steril 77(Suppl 4):S6–S10 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK 2001 Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav 39:167–174 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC 2003 Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol 181:301–312 [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH 2001 Posttraining androgens’ enhancement of cognitive performance is temporally distinct from androgens’ increases in affective behavior. Cogn Affect Behav Neurosci 1:172–182 [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA 2006 Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav 50:18–26 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 2005 Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav 48:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA 2008 Androgens with activity at estrogen receptor β have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm Behav 54:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton 3rd LJ 2007 Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69:1074–1083 [DOI] [PubMed] [Google Scholar]
- Nauton P, Giry N, Bruhat MA, Alliot J 1992 Effect of administration of an analog of LHRH on appetitive learning in young and middle-aged female rats. Pharmacol Biochem Behav 43:1005–1013 [DOI] [PubMed] [Google Scholar]
- Alliot J, Nauton P, Bruhat MA 1993 Administration of LHRH analog can improve working memory in aged female rats. Psychoneuroendocrinology 18:543–550 [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS 2004 Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-β precursor protein and amyloid-β deposition. J Biol Chem 279:20539–20545 [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA 2007 Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol 269:107–111 [DOI] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA 2006 Luteinizing hormone modulates cognition and amyloid-β deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta 1762:447–452 [DOI] [PubMed] [Google Scholar]
- Short RA, Bowen RL, O'Brien PC, Graff-Radford NR 2001 Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc 76:906–909 [DOI] [PubMed] [Google Scholar]
- Bowen RL, Isley JP, Atkinson RL 2000 An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol 12:351–354 [DOI] [PubMed] [Google Scholar]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES 1993 Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology 132:2262–2270 [DOI] [PubMed] [Google Scholar]
- Zhang W, Lei ZM, Rao CV 1999 Immortalized hippocampal cells contain functional luteinizing hormone/human chorionic gonadotropin receptors. Life Sci 65:2083–2098 [DOI] [PubMed] [Google Scholar]
- Lukacs H, Hiatt ES, Lei ZM, Rao CV 1995 Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav 29:42–58 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Granholm AC, Seo H, Isacson O 2002 Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neurosci Lett 328:50–54 [DOI] [PubMed] [Google Scholar]
- Pereira RT, Porto CS, Godinho RO, Abdalla FM 2008 Effects of estrogen on intracellular signaling pathways linked to activation of muscarinic acetylcholine receptors and on acetylcholinesterase activity in rat hippocampus. Biochem Pharmacol 75:1827–1834 [DOI] [PubMed] [Google Scholar]
- Das A, Dikshit M, Srivastava SR, Srivastava UK, Nath C 2002 Effect of ovariectomy and estrogen supplementation on brain acetylcholinesterase activity and passive-avoidance learning in rats. Can J Physiol Pharmacol 80:907–914 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL 2006 Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147:607–614 [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM 2008 Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol 20:1023–1027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.