Abstract
GnRH neurosecretion is subject to regulation by insulin, IGF-I, leptin, and other neuroendocrine modulators whose effects may be conveyed by activation of phosphoinositide 3-kinase (PI3K)-mediated pathways. It is not known, however, whether any of these regulatory actions are exerted directly, via activation of PI3K in GnRH neurons, or whether they are primarily conveyed via effects on afferent circuitries governing GnRH neurosecretion. To investigate the role of PI3K signaling in GnRH neurons, we used conditional gene targeting to ablate expression of the major PI3K regulatory subunit, p85α, in GnRH neurons. Combined in situ hybridization and immunohistochemistry confirmed reduction of p85α mRNA expression in GnRH neurons of GnRH-p85α knockout (KO) animals. Females of both genotypes exhibited estrous cyclicity and had comparable serum LH, estradiol-17β, and FSH levels. In male GnRH-p85αKO mice, serum LH, testosterone, and sperm counts were significantly reduced compared with wild type. To investigate the role of the other major regulatory subunit, p85β, on the direct control of GnRH neuronal function, we generated mice with a GnRH-neuron-specific p85α deletion on a global βKO background. No additional reproductive effects in male or female mice were found, suggesting that p85β does not substitute p85 activity toward PI3K function in GnRH neurons. Our results suggest that p85α, and thus PI3K activity, participates in the control of GnRH neuronal activity in male mice. The sex-specific phenotype in these mice raises the possibility that PI3K activation during early development may establish sex differences in GnRH neuronal function.
GnRH neuron-specific deletion of the PI3 kinase p85α subunit leads to male-specific reduction in hypothalamic-pituitary-gonadal function.
The central control of the reproductive axis is governed by the pulsatile neurosecretory activity of hypothalamic GnRH neurons. The GnRH neuronal system is, in turn, regulated by a variety of neural and endocrine factors that can exert homeostatic feedback control as well as prompt changes in hypothalamic-pituitary-gonadal function that are appropriate to alterations in the internal and external environment. Among the peripheral factors that control GnRH release are steroid hormones such as estradiol (E2) (1), peripheral metabolic cues such as insulin and leptin (2), and growth factors such as IGF-I (3). The complexity of the hypothalamic circuitries that control GnRH release have made it extremely difficult to discern the cell signaling pathways that mediate the effects of these feedback and metabolic regulators. It is not known, for example, whether any of their actions are exerted via common signaling intermediates. Equally unclear is the extent to which any of their effects are exerted directly on GnRH neurons, or indirectly, on interneurons that ultimately control GnRH neurosecretion.
Many of the factors that regulate GnRH release also have the capacity to activate cell signaling pathways mediated by the enzyme phosphatidylinositol-3-kinase (PI3K) and thereby exert some of their cellular effects in the hypothalamus. In neurons, as in a variety of other cell types, PI3K is a key signaling intermediate shared by peripheral metabolic cues, growth factors, and hormones to regulate a number of biological functions such as metabolism (4,5,6), cell growth (7,8), and immunity (9,10). PI3K catalyzes the conversion of phosphatidylinositol 4,5 biphosphate to phosphatidylinositol 3,4,5-triphosphate at the plasma membrane, which then functions as a lipid second messenger (11). Structurally, class IA PI3Ks exist as heterodimers composed of a regulatory/adapter subunit (p85) tightly associated with a catalytic subunit (p110) (11). Four isoforms of the catalytic subunit have been described (p110α, p110β, p110γ, and p110δ), whereas three mammalian genes encode adapter subunits (p85α, p85β, and p85γ) (11). In the hypothalamus, PI3K activation mediates at least some of the effects of insulin and leptin on energy homeostasis (4,5,6). Both E2 and IGF-I, moreover, can exert facilitating effects on female sexual behavior via PI3K activation (12). Organizational effects of E2 have also recently been shown to be mediated by PI3K activation and consequent dendrite morphogenesis (13).
There is little known, however, of the potential involvement of PI3K signaling in the physiological control of GnRH release or GnRH neuronal development. Receptors for ligands that are known activators of PI3K such as insulin, IGF-I, and leptin are expressed in immortalized GnRH-expressing GT1-7 cells (14,15,16,17,18). However, whereas these ligands activate the PI3K signaling in this in vitro system, a connection between PI3K activity and GnRH release has not been demonstrated. Many of the extracellular factors that activate PI3K have nevertheless been shown to be important regulators of the reproductive axis in vivo. For example, mice lacking receptors for insulin (19) or leptin (20) exhibit altered energy homeostasis as well as deficits at various levels of the reproductive axis. Other studies confirm that these factors and their downstream targets exert their actions in the central nervous system to regulate reproductive activity; brain-specific deletion of the insulin receptor results in animals that are subfertile due to hypogonadism (19). Furthermore, central infusion of leptin (21) and insulin (22,23) restores the reproductive deficits observed using in vivo models in which energy balance is severely compromised such as food restriction and type1 diabetes, respectively. Despite the obvious role of these factors in the central regulation of male and female reproductive function, it is not clear whether they directly regulate GnRH neurons in vivo and whether their actions are mediated via PI3K signaling.
Of all the PI3K adapter subunits, p85α and its two splice variants, p55α and p50α, are the most abundantly expressed in the brain (24). Furthermore, complete loss of Pik3r1, the gene encoding p85α, results in perinatal lethality (25), making it impossible to study its role in the control of the reproductive axis. Therefore, to assess the potential role of PI3K in mediating direct effects of these factors on GnRH neuronal function in vivo, the effect of a GnRH-neuron-specific deletion of p85α [GnRH-p85α knockout (KO)] on the reproductive axis of mice was investigated. The reproductive hormonal phenotypes of these animals were then compared with their wild-type (WT) counterparts. Surprisingly, we found that deletion of this regulatory subunit in GnRH neurons results in a sex-specific decrement in the activity of the reproductive axis in the male, suggesting a role for PI3K activation in the sexual differentiation of GnRH neuronal function.
Materials and Methods
Animals and breeding strategy
Mice were housed in the Northwestern University animal facility under a 12-h light, 12-h dark cycle and had access to water and rodent chow ad libitum. All experimental procedures adhered to guidelines provided in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Northwestern University.
Generation of Pik3r1 GnRH-p85αKO
All animals used were of a 129Sv-C57BL/6FVB mixed genetic background. To generate mice in which the gene for p85α, Pik3r1, is specifically deleted in GnRH neurons, mice bearing loxP sites flanking exon 7 of this gene (26) (provided by Lewis C. Cantley’s laboratory, Division of Signal Transduction and Department of Systems Biology, Harvard Medical School, Boston, MA) were mated with mice carrying the Cre transgene under the control of the murine GnRH promoter (GnRH-Cre) (27). The GnRH-Cre transgene in these animals has been shown to be expressed in virtually all GnRH neurons in the basal forebrain and in a sparse population of septal neurons (27). In addition, Western blot analysis failed to show CRE protein expression in other tissues such as cortex, pituitary, liver, heart, lung, and testis (data not shown). Mice generated by the first breeding were then intercrossed to generate GnRHCre−-p85αwt/wt and GnRHCre+-p85αfl/lfl animals, referred to here as WT and GnRH-p85αKO, respectively (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). For measurements of hormone levels, all genotypes were analyzed and the WT group included all genotypes in which a CRE deletion was not present (GnRHCre−-p85αfl/lfl, GnRHCre−-p85αfl/wt, and GnRHCre+-p85αwt/wt); data from these genotype groups were not significantly different from the GnRHCre−-p85αwt/wt group. Animals were screened for the presence of Cre and floxed p85α by PCR of the genomic DNA isolated from the tails as described previously (28).
Generation of Pik3r1 GnRH-p85αKO Pik3r2−/− mice
Mice homozygous for the Pik3r1 floxed allele and heterozygous for Pik3r2 null allele (Pik3r1flx/flx, Pik3r2wt/−; provided by Lewis C. Cantley’s laboratory, Division of Signal Transduction and Department of Systems Biology, Harvard Medical School) were bred with mice that were also homozygous for the Pik3r1 floxed allele and heterozygous for Pik3r2 null allele and expressing CRE transgene, (GnRH-Pik3r1−/−, Pik3r2wt/−) to produce the groups used in the study (supplemental Fig. 2).
Combined in situ hybridization and immunohistochemistry
Animals and tissue fixation
Control adult mice (Cre negative) and GnRH-p85αKO mice (homozygous for the floxed Pik3r1 and expressing Cre recombinase) were anesthetized with 80 mg/kg, ip, ketamine (Fort Dodge Laboratories, Fort Dodge, IA) and 32 mg/kg, ip, xylazine (Burns Veterinary Supply, Inc., Rockville Center, NY) and perfused intracardially with 0.1 m PBS followed by 4% paraformaldehyde in 0.1 m PBS. Brains were removed and postfixed in 4% paraformaldehyde for 2 h at room temperature followed by immersion in 30% sucrose cryoprotectant. Twenty-five-micrometer floating sections were cut using a cryostat.
Probe preparation
The probes were generously provided by Dr. Jennifer Hill (University of Texas Southwestern Medical Center, Dallas, TX). These were generated by in vitro transcription with 35S-uridine 5-triphosphate. The cDNA was obtained by reverse transcription using total rat brain RNA (Ambion, Inc., Austin, TX) as a template. The cDNA was then amplified by PCR using the 5′ primer: 5′-AGA ACG GCT ATC GAA GCA-3′ corresponding to nt 1997-2015 and the 3′ primer: 5′-GAC GCA ATG CTT GAC TTC-3′ corresponding to nt 2552-2569 of p85α. The region bounded by these primers is 572 bases and corresponds to the COOH-terminal SH2 domain of p85α. The amplification products were gel purified and cloned into pCR4-TOPO vector (Invitrogen, Carlsbad, CA). To generate antisense 35S-labeled cRNA, the plasmids were linearized by digestion with PstI and subjected to in vitro transcription with T7 RNA polymerase (Promega, Madison, WI). For generation of sense 35S-labeled cRNA, the plasmids were linearized by digestion with NotI and subjected to in vitro transcription with T3 RNA polymerase.
Dual-labeling ISH histochemistry
The procedure was a modification of that described previously (29). Tissue was rinsed with diethylpyrocarbonate (DEPC)-treated PBS (pH 7.0) for 1 h and in 0.1% sodium borohydride (Sigma, St. Louis, MO) in DEPC-PBS for 15 min. After rinses in DEPC-PBS, tissue was incubated for 10 min in 0.25% acetic anhydride in 0.1 m tetraethylammonium. The tissue was then rinsed in DEPC-treated 2× sodium chloride/sodium citrate (SSC) before hybridization. cRNA probes were diluted to 106 cpm/ml in 50% formamide, 10 mm Tris-HCl (pH 8.0) (Life Technologies, Inc.-BRL, Bethesda, MD), 5 mg tRNA (Invitrogen), 10 mm dithiothreitol, 10% dextran sulfate, 0.3 m NaCl, 1 mm EDTA (pH 8.0), and 1× Denhardt’s solution (Sigma) and applied to the tissue. Sections were hybridized overnight at 50 C. Tissue was rinsed four times in 4× SSC and incubated in 0.002% ribonuclease A (Roche Applied Bioscience, Indianapolis, IN) diluted in 0.5M NaCl, 10 mm Tris-HCl (pH 8.0), and 1 mm EDTA (ribonuclease buffer) for 30 min at 37 C. After two rinses in 2× SSC, tissue was submitted to stringency wash in 0.1× SSC for 60 min at 55 C, rinsed in 2× SSC, and incubated in anti-GnRH antibody (LR-5, a gift from Dr. R. Benoit, Montréal General Hospital, Montréal, Québec, Canada; 1:20,000), and 3% normal donkey serum diluted in 0.1 m PBS and 0.25% Triton X-100 (Sigma), overnight, at room temperature.
Next, sections were incubated in biotinylated donkey antirabbit IgG (The Jackson Laboratory, Bar Harbor, ME; 1:1000) and avidin-biotin complex (Vector Elite Kit, Vector Laboratories, Burlingame, CA; 1:500 in PBS), both for 1 h at room temperature. After rinsing, sections were incubated in 0.04% diaminobenzidine tetrahydrochloride (Sigma) and 0.01% hydrogen peroxide. The reaction was terminated with successive rinses in PBS. Tissue was mounted onto SuperFrost Plus slides (Fisher, Pittsburgh, PA) and dehydrated in increasing concentrations of ethanol and delipidated in xylene. Slides were placed in x-ray film cassettes with BMR-2 film (Kodak, Rochester, NY) for 2 d. Slides were then dipped in NTB2 photographic emulsion (Kodak), dried, and stored in desiccant-containing, foil-wrapped slide boxes at 4 C for 2 wk. Slides were developed with D-19 developer, dehydrated in increasing concentration of ethanol, cleared in xylenes, and coverslipped with Permaslip. Sections were analyzed with a Axioplan microscope (Zeiss, New York, NY) and scored blindly. Cells stained brown were counted as GnRH-expressing cells and brown-stained cells with black grain density 3 times above background were scored as double-labeled cells. Adobe Photoshop CS was used to adjust brightness and contrast.
Immunohistochemistry and quantification of GnRH neurons
After perfusion, brains were cut into three sets of 30-μm-thick coronal sections. After rinses in PBS, the tissue was blocked in PBS containing 0.3% Triton X-100 (PBST), 1% BSA, and 2% normal goat serum for 1 h at room temperature. After rinses, the tissue was incubated overnight at room temperature in PBST containing an anti-GnRH antibody (LR-5, a gift from Dr. R. Benoit, Montréal General Hospital; 1:10,000). Then sections were washed in PBS and incubated in PBST containing biotinylated goat antirabbit IgG (Vector Laboratories), followed by incubation in Vectastain avidin-biotin complex reagent (Vectors Laboratories) for 1 h. Sections were washed and immunoreactivity was visualized using diaminobenzidine as the chromagen. Sections were washed, mounted on slides, dried overnight, and coverslipped.
GnRH neurons were counted under an Eclipse TE2000-S microscope (Nikon, Melville, NY). Scoring was carried out by visualizing GnRH neurons located between the A-P coordinates (30), extending from the diagonal band of Broca through the retrochiasmatic area. Only cells with darkly stained cytoplasm were included in tallies.
Vaginal opening and estrous cyclicity
After weaning at 21–24 d of age, females were examined for the onset of vaginal opening. To examine the possible effects of genotype on estrous cyclicity, vaginal lavages from female mice (1.5–3 months old) were obtained and viewed under a microscope daily (9000–1000 h) for at least 31 d. A normal estrous cycle was defined as exhibiting vaginal histology that was leukocytic for 2 d followed by 1 d of nucleated and 1–2 d of cornified vaginal histology.
Sperm counts
Vas deferens were dissected from 3-month-old male mice and placed in 1 ml of M2 medium (Sigma). Seminal fluids were removed using a watchmaker’s no. 5 forceps and incubated at 37 C for 20 min in warmed M2 medium. A 1:1 dilution of fluid was used for a hemocytometric count (31).
Hormonal assays
Animals were anesthetized with ketamine/xylazine ip, and blood was withdrawn by cardiac puncture (0900–1100 h). Serum was assayed for LH, E2, testosterone (T), and FSH. Serum LH levels were determined by using RIA reagents obtained from the National Institute of Diabetes and Digestive and Kidney Diseases, including the LH reference (RP-3) and S-11 antibody. The assay had a lower limit of detection of 0.2 ng/ml. The intraassay and interassay coefficients of variance (CVs) were 4.1 and 7.95%, respectively. For experiments using samples from GnRH-p85αKO mice, serum FSH levels were determined using RP-2 standard and S-11 antibody, also provided by the National Institute of Diabetes and Digestive and Kidney Diseases; the sensitivity and intraassay CVs were 0.05 ng/tube, and 11.3%, respectively. For experiments in which GnRH-p85DKO animals were used, an immunoradiometric assay assay (MP Biomedicals, Solon, OH) was used. This assay had a sensitivity of 0.2 ng/ml and intraassay CV of 2.96%. Estradiol RIA was performed using kits purchased from Diagnostic Products Corp. (Los Angeles, CA). The E2 assay had a lower limit of detection of 2 pg/ ml, and the intraassay and interassay CVs were 3.3 and 13.2%, respectively. The T RIA was performed using kits from MP Biomedicals. The sensitivity and intraassay CVs for the T assay were 0.02 ng/ml, and 3.8% respectively.
Statistical analyses
All data are presented as the mean ± sem. For experiments investigating the effect of GnRH-neuron-specific deletion of p85α, data were analyzed with unpaired t test. For experiments in which data from double-KO animals were analyzed, if variances differed significantly, as determined by Barlett’s test of equal variances, the Kruskal-Wallis test and Dunn’s multiple comparisons post hoc test were used. If the variances did not differ significantly, a one-way ANOVA and Newman-Keuls post hoc test were used. Differences were considered significant when P < 0.05.
Results
Generation of Pik3r1 GnRHKO
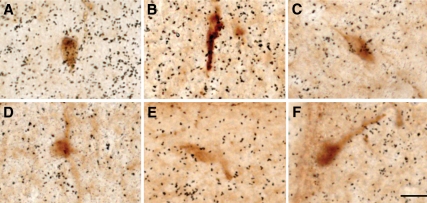
To obtain selective ablation of p85α in GnRH neurons, we crossed mice in which loxP sites flank exon 7 of the p85α gene, Pik3r1, with transgenic mice expressing Cre driven by the murine GnRH promoter. Cre recombinase expression in this GnRH-cre model is identical with the known distribution of GnRH neurons in the brain, such as olfactory tissues, ventral diagonal band of Broca, and medial preoptic area, and at the level of the organum vasculosum of the laminas terminalis (27). To confirm the deletion of p85α in GnRH neurons, dual-labeled in situ hybridization and immunohistochemistry was performed (Fig. 1). As previously reported, p85α mRNA is ubiquitously and widespread expressed in the mouse brain (supplemental Fig. 3). In sections of the brain examined containing GnRH neurons, the lowest signal was observed in the corpus callosum an area devoid of cell bodies, whereas the highest hybridization signal was found in the cortex (supplemental Figs. 3 and 4). Therefore, the corpus callosum and cortex of each individual animal were used as references to classify mRNA levels in GnRH neurons as background or above background, respectively. Accordingly, we found that GnRH neurons with a p85α mRNA signal that clearly exceeded 3 × background were observed in all WT animals tested, which included both males and females; in all of these animals, an average of 20% of GnRH neurons expressed the p85α mRNA (n = 4). By contrast, very few GnRH neurons in any of the GnRHCre+-p85αfl/lfl mice appeared to express the p85α mRNA (less than 5% showed signal above background, n = 3).
Figure 1.
Dual in situ hybridization and immunohistochemistry showing no apparent expression of p85α mRNA in GnRH neurons from GnRH-p85αKO mice. GnRH immunoreactivity (brown) and p85α mRNA expression (black grains) from WT (A–C) and GnRH-p85αKO (D–F) mice. All micrographs were taken at the same magnification. Scale bar, 100 μm.
Reproductive phenotype of female GnRH-p85αKO mice
To examine whether the targeted disruption of p85α in GnRH neurons affected their number or tissue distribution, we performed immunohistochemistry and a GnRH neuronal count on every third section collected was performed. We observed no significant differences in GnRH neuron numbers in male and female GnRH-p85α KO mice (males, 125 ± 16.1, n = 5; females, 113 ± 13.9, n = 4, respectively) compared with their WT counterparts (males, 136 ± 11.5, n = 5; females, 116 ± 15.40, n = 4, respectively). In addition, obvious differences in gross appearance or distribution were not observed (supplemental Fig. 5). These results indicate that the specific deletion of p85α in GnRH neurons did not overtly disrupt the differentiation, migration, or maturation of the GnRH neuronal population.
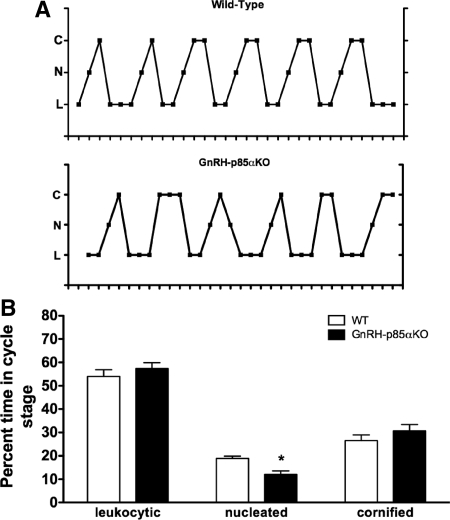
Based on the time at which vaginal opening was observed, there was no difference in pubertal onset between WT and GnRH-p85αKO female mice (28.0 ± 0.6, n = 8 vs. 28.3 ± 0.5, n = 7, respectively). However, the deletion of p85α in GnRH neurons had a mild but significant effect on estrous cyclicity (Fig. 2A); compared with WT mice, GnRH-p85αKO females showed significant fewer days at proestrus (P < 0.05, Fig. 2B; n = 8).
Figure 2.
GnRH-neuron-specific deletion of p85α does not disrupt estrous cyclicity. A, Representative estrous cycles as measured by vaginal cytology in WT (top panel), and GnRH-p85αKO (bottom panel) females. C, Cornified; N, nucleated; L, leukocytic. B, Percent of time per stage of estrous cycle. The only abnormality noted in GnRH-p85αKO females was a slight decrease in days spent in proestrus (nucleated vaginal cytology, *, P = 0.002) compared with WT females.
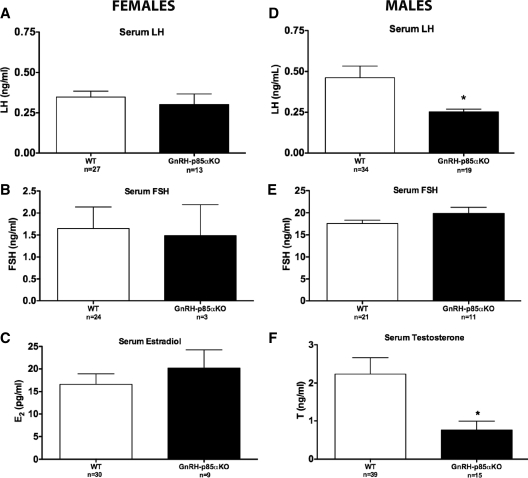
The effects of the p85α GnRH neuron-specific deletion on LH release were assessed in intact adult females. Due to the pulsatile nature of GnRH/LH release, LH levels in both groups were at or below the detectability level of the assay (<0.20 ng/ml) in the majority of animals. LH levels in GnRH-p85αKO females were not significantly different from WT controls (0.35 ± 0.037 vs. 0.30 ± 0.07, respectively; Fig. 3A). Moreover, the GnRH-specific deletion of p85α did not affect E2 or FSH levels in females (Fig. 3, B and C, respectively).
Figure 3.
Serum hormone levels in adult intact female and male animals. There was no effect of genotype on serum LH (A), FSH (B), or E2 (C) levels in female animals. In contrast, in males the GnRH-neuron-specific deletion of p85α resulted in a significant decrease in serum LH (D) and T (F) levels compared with WT animals, with no effect on serum FSH (E) levels. *, P < 0.05. Bars, mean ± sem.
Histological analysis of ovarian tissue did not show gross morphological abnormalities. Ovaries obtained from sexually mature mice contained populations of follicles at all stages of development. An analysis of follicle distribution did not reveal significant differences in the number of secondary, preantral, antral, or corpus luteum per section between WT and GnRH-p85αKO mice (supplemental Fig. 6). Fertility in both female and younger adult male GnRH-p85aKO mice was not overtly impaired because they could produce offspring.
Reproductive phenotype of male GnRH-p85αKO mice
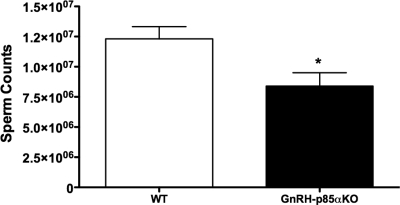
Serum LH levels were low in WT animals (0.46 ± 0.07 ng/ml), similar to values previously noted in testes-intact male mice (32). The LH values for the GnRH-p85αKO mice were even lower (0.25 ± 0.02 ng/ml), with most values falling below the level of detectability of the LH RIA. Statistical analysis revealed that this difference was significant (P = 0.03; Fig. 3D). Serum FSH levels were comparable in the two genotypes (Fig. 3E). Mean serum T levels were significantly decreased in GnRH-p85αKO compared with WT (P < 0.05, Fig. 3F). Furthermore, a significantly lower sperm count was observed GnRH-p85αKO compared with WT (P < 0.05, Fig. 4). However, histological examination did not reveal any grossly observable alteration in testicular morphology (supplemental Fig. S7). Lastly, p85α mRNA levels in the testis of GnRH-p85αKO animals were not different from WT (supplemental Fig. 8), suggesting that the observed phenotype is due to the specific Cre-mediated deletion of p85α in GnRH neurons.
Figure 4.
Sperm counts were also significantly reduced in GnRH-p85αKO adult males, compared with WT animals. *, P < 0.05 (n = 9–11). Bars, mean ± sem.
Reproductive phenotype of GnRH-p85DKO mice
To eliminate the possibility that PI3K activity in GnRH neurons of GnRH-p85αKO mice is functional due to a compensatory role of p85β, we generated mice that lack both regulatory subunits in GnRH neurons. This was achieved by crossing GnRH-p85αKO mice with Pik3r2 KO mice, which lack p85β in all tissues. For these experiments mice in which Pik3r1 was specifically deleted in GnRH neurons on a global p85β knockout background (double knockout of Pik3r1 and Pik3r2 in GnRH neurons, GnRH-p85DKO) and mice not expressing GnRH-cre (βKO) were compared with Pik3r1 homozygous floxed controls on a WT background. A similar strategy has been shown to impair PI3K signaling in response to leptin and insulin in other neurons and tissues (28,33).
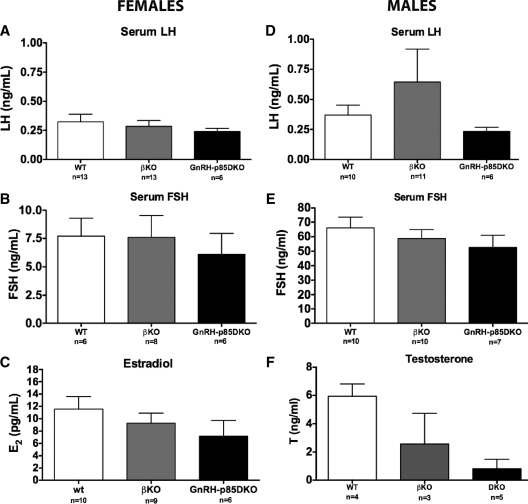
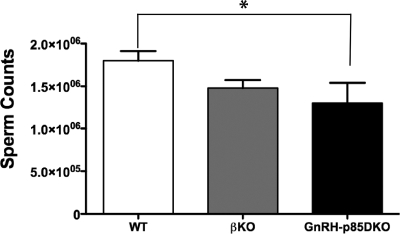
Compared with WT, the elimination of p85α in GnRH neurons on a global p85β KO did not result in significant differences in total GnRH cell counts of male or female animals (108 ± 8.8, n = 3 vs. 136 ± 11.5, n = 5, p85DKO and WT males, respectively, and 116 ± 15.40, n = 4 vs. 110 ± 12.0, n = 4, p85DKO and WT females, respectively). Serum LH, FSH, or E2 levels were not significantly different between WT and p85DKO females (Fig. 5, A–C, respectively). A genotype effect was observed in males, in which there was a significant reduction in sperm concentrations in GnRHp85DKO vs. WT males (Fig. 6, ANOVA, P < 0.05). Similarly, T levels in GnRH-p85DKO males were 83% lower than corresponding hormone levels in WT mice (Fig. 5F). As observed for the GnRH-p85αKO males, the reduction in T levels in the GnRH-p85DKO males reached significance (P < 0.05) in a two-way comparison with T values in WT mice; when values for p85β KO animals were also included in the analysis, this difference did not reach significance, likely due to reduced number of samples available. Serum LH levels in the GnRH-p85DKO males were almost all below the detectable limits of the LH assay, mirroring the results obtained in GnRH-p85αKO males, whereas LH levels in the p85βKO males were similar to those in WT mice. Finally, serum FSH levels were not significantly different among the groups (Fig. 5E).
Figure 5.
The generation of a GnRH-neuron-specific deletion of p85α on a global p85β KO background did not affect serum hormone levels in adult intact female mice (A–C) and did not produce any additional effects in male (D–F) animals. Values shown are means ± sem.
Figure 6.
Sperm counts were significantly reduced in GnRH-p85DKO, compared with WT mice. *, P < 0.05 (n = 9–12). Bars, mean ± sem.
Discussion
In the present study, we assessed the reproductive effects of eliminating the PI3K regulatory subunit p85α in GnRH neurons in both male and female mice. Our findings revealed that GnRH neuron-specific p85α gene deletion reduces reproductive hormone secretions and spermatogenesis in males but has little impact on the reproductive axis of the female. In addition, the GnRH-specific deletion of p85α superimposed on a global p85β knockout did not affect serum hormone levels in females or result in further disruption of the reproductive axis in males. Therefore, the p85α regulatory subunit of PI3K in GnRH neurons appears to participate in sustaining normal GnRH neurosecretory activity in the male mouse. It remains unknown whether p85α and PI3K activity in GnRH neurons is required in this regard during development, in adulthood, or throughout the life span.
Given the importance of insulin, leptin, and IGF-I in the normal functioning of GnRH neurons (3,19,34), and their capacities for signaling through PI3K-mediated transduction pathways (11), we had hypothesized that the disruption of PI3K signaling in GnRH neurons would eliminate any direct stimulatory effects by these peripheral metabolic cues. This scenario, however, fails to explain the male-specific decrement in reproductive hormone secretions in the conditional GnRH-p85αKO mice. Indeed, the female reproductive axis has long been considered to be more sensitive to the suppressive effects of nutritional and metabolic stress (35), a view that is not consistent with the idea that the loss of PI3K and hence a portion of insulin and leptin signaling in GnRH neurons would impact GnRH neurosecretion to a greater extent in the male than the female.
It is also unlikely that the neuroendocrine effects observed in GnRH-p85αKO male mice are a consequence of the disruption of GnRH neuronal migration. Whereas a role of PI3K in GnRH neuronal migration and survival has been suggested (36,37), the absence of p85α in GnRH neurons did not lead to a diminishment of the GnRH neuronal population or a disruption of the normal GnRH neuronal topography. We additionally found that the GnRH neuronal distribution is similarly unperturbed in combined p85αKO GnRH-complete p85β null mutant mice. These findings do not rule out an important role of PI3K during GnRH migration and survival in vivo because it is likely that additional parallel signaling pathways are also involved (37).
The male-specific GnRH-p85αKO phenotype could instead reflect the absence of a PI3K-mediated, stimulatory effect of androgens on GnRH release. Whereas we (38,39) and others (40) demonstrated that testicular hormones exert predominantly negative feedback actions on GnRH pulse frequency, paradoxical stimulatory effects of testosterone on GnRH tissue content and release rate have also been documented (41). Specifically, rapid stimulatory effects of testosterone on GnRH release from immortalized GT1-7 cells have been reported that are dissociable from inhibitory effects of testosterone on GnRH gene expression (42). Because androgens can activate PI3K in a variety of cell types through a membrane-initiated mechanism (43,44), it is possible that such a transduction process normally operates in GnRH neurons. Disruption of this pathway in the GnRH-p85αKO mice would therefore produce a male-specific decrement in GnRH release and hence in the overall activity of the reproductive axis.
We consider it even more likely that androgens exert direct PI3K-mediated effects on GnRH neurons during the prenatal/neonatal surges of testosterone production that occur in male fetuses/neonates. A recent study (13) demonstrated that organizational effects of testosterone in the hypothalamus can be mediated by aromatization to estrogen and activation of estrogen receptors coupled to rapid activation of PI3K. The subsequent enhancement of glutamate release leads to activation of ionotropic glutamate receptors, MAPKs, and dendritic spine formation. Because fetal GnRH neurons may express estrogen receptors (45), it is possible that this mechanism may be activated in the GnRH neurons of males by prenatal/perinatal testosterone secretions. In this way, neuronal connectivity may be induced that supports either a higher level of GnRH neurosecretory activity in adulthood or increased responsiveness to synaptic inputs. Such a mechanism might explain the observations that the GnRH pulse generator operates at a higher frequency in males than females in many species (46) and that prenatal androgen exposure leads to an acceleration of GnRH pulsatility in female mice (47), rats (48), sheep (49), and monkeys (50).
Recent studies suggest that the elimination of both p85α and p85β regulatory subunits is necessary to affect neuronal responsiveness to upstream regulators of the PI3K pathway such as insulin and leptin (33). Hence, we hypothesized that the combined deletion of both regulatory subunits in GnRH neurons would significantly affect the function of the hypothalamic-pituitary-gonadal axis. However, we found no additional effects caused by GnRH-p85DKO on serum hormone levels, nor did it further decrease sperm counts in males. These findings suggest that in GnRH neurons the disruption of p85α is enough to decrease PI3K function. Alternatively, p85 regulatory subunits have been shown to have intracellular and biological functions that are independent of their role as regulators of PI3K activity. For example, in the liver (28) and brown adipocytes (51), whereas elimination of one p85 isoform reduces PI3K activity, phosphatidylinositol 4,5 biphosphate to phosphatidylinositol 3,4,5-triphosphate levels can stay unchanged or actually increase in response to insulin The regulatory subunits by themselves are also important in certain physiological functions, such as the regulation of actin cytoskeleton (52,53) and cell cycle progression (54) that might be independent of the catalytic activity of the p110 subunits. Therefore, p85α independent of the p110 catalytic subunit might have a role in an as yet unknown aspect in the regulation of GnRH neuronal function.
In the present study, we used combined immunohistochemistry/in situ hybridization procedures to confirm the absence of the Pik3r1 gene in GnRH neurons of GnRH-p85αKO animals. This technical approach proved to be a challenging one because the mRNA for p85α is abundantly and widely expressed throughout the mouse brain. An average of 20% of GnRH neurons in WT animals showed p85α mRNA hybridization signal that clearly exceeded background levels, whereas less than 5% of GnRH neurons in the GnRH-p85aKO mice appeared to express p85α mRNA. It is possible that a higher expression of p85α in GnRH neurons might occur at earlier developmental stages, contributing to the male-biased phenotype. Thus, we may underestimate the presence of p85α in the GnRH neuronal population at its maximum extent, and we may therefore underestimate the degree to which it has been deleted. We also note that the residual p85α hybridization signal observed in GnRH neurons of GnRH-p85αKO mice might be due to incomplete deletion of the p85α message. Nevertheless, the reduction in the number of GnRH neurons expressing p85α mRNA was significant and nearly complete.
The present studies support the hypothesis that direct regulation of GnRH neurons in vivo by modulators of PI3K activity is an important mechanism used for the regulation of the hypothalamic-pituitary-gonadal axis. Interestingly, this effect was most pronounced in males in which a significant reduction in serum T levels and sperm concentrations was observed. Our findings suggest that PI3K signaling in GnRH neurons comprises a component of the mechanisms regulating sex differences in neuroendocrine regulation of GnRH neurosecretion. Future studies will address the role of PI3K as a potential mediator of the organizational effects of steroid hormones on the GnRH neuronal system.
Supplementary Material
Acknowledgments
We appreciate the excellent technical assistance of Brigitte Mann, Kate Leitner, Claire Capshew, and Charlotte Lee.
Footnotes
This work was supported by National Institutes of Health Grants RO1 HD20677, P50HD44405, and K99 HDO55446- 01A1.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 18, 2009
Abbreviations: CV, Coefficient of variance; DEPC, diethylpyrocarbonate; E2, estradiol; KO, knockout; PBST, PBS containing Triton X-100; PI3K, phosphatidylinositol-3-kinase; SSC, sodium chloride/sodium citrate; T, testosterone; WT, wild type.
References
- Moenter SM, Defazio RA, Straume M, Nunemaker CS 2003 Steroid regulation of GnRH neurons. Ann NY Acad Sci 1007:143–152 [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF 2008 Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294:E827–E832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftary SS, Gore AC 2005 IGF-I in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med (Maywood) 230:292–306 [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW 2005 Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289:E1051–E1057 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW 2005 Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2:411–420 [DOI] [PubMed] [Google Scholar]
- Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers Jr MG, Rhodes CJ, Schwartz MW 2006 Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 3:67–73 [DOI] [PubMed] [Google Scholar]
- Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J 2008 NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 68:8022–8030 [DOI] [PubMed] [Google Scholar]
- Hallmann D, Trümper K, Trusheim H, Ueki K, Kahn CR, Cantley LC, Fruman DA, Hörsch D 2003 Altered signaling and cell cycle regulation in embryonal stem cells with a disruption of the gene for phosphoinositide 3-kinase regulatory subunit p85α. J Biol Chem 278:5099–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC 1999 Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science 283:393–397 [DOI] [PubMed] [Google Scholar]
- Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA 2004 Enhanced T cell proliferation in mice lacking the p85β subunit of phosphoinositide 3-kinase. J Immunol 172:6615–6625 [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC 2005 Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci 30:194–204 [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M 2003 Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology 144:3828–3835 [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM 2008 Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron 58:584–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, DiVall SA, Deneau RM, Wolfe A 2005 Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Mol Cell Endocrinol 242:42–49 [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhou LB, Liu SQ, Tang JF, Li FY, Li RY, Song HD, Chen MD 2005 Expression of feeding-related peptide receptors mRNA in GT1-7 cell line and roles of leptin and orexins in control of GnRH secretion. Acta Pharmacol Sin 26:976–981 [DOI] [PubMed] [Google Scholar]
- Olson BR, Scott DC, Wetsel WC, Elliot SJ, Tomic M, Stojilkovic S, Nieman LK, Wray S 1995 Effects of insulin-like growth factors I and II and insulin on the immortalized hypothalamic GTI-7 cell line. Neuroendocrinology 62:155–165 [DOI] [PubMed] [Google Scholar]
- Longo KM, Sun Y, Gore AC 1998 Insulin-like growth factor-I effects on gonadotropin-releasing hormone biosynthesis in GT1-7 cells. Endocrinology 139:1125–1132 [DOI] [PubMed] [Google Scholar]
- Magni P, Vettor R, Pagano C, Calcagno A, Beretta E, Messi E, Zanisi M, Martini L, Motta M 1999 Expression of a leptin receptor in immortalized gonadotropin-releasing hormone-secreting neurons. Endocrinology 140:1581–1585 [DOI] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Johnson LM, Sidman RL 1979 A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol Reprod 20:552–559 [DOI] [PubMed] [Google Scholar]
- Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL 1998 Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67:370–376 [DOI] [PubMed] [Google Scholar]
- Kovacs P, Morales JC, Karkanias GB 2003 Central insulin administration maintains reproductive behavior in diabetic female rats. Neuroendocrinology 78:90–95 [DOI] [PubMed] [Google Scholar]
- Kovacs P, Parlow AF, Karkanias GB 2002 Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology 76:357–365 [DOI] [PubMed] [Google Scholar]
- Hörsch D, Kahn CR 1999 Region-specific mRNA expression of phosphatidylinositol 3-kinase regulatory isoforms in the central nervous system of C57BL/6J mice. J Comp Neurol 415:105–120 [PubMed] [Google Scholar]
- Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC 2000 Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85α. Nat Genet 26:379–382 [DOI] [PubMed] [Google Scholar]
- Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC 2005 Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol 25:9491–9502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A, Divall S, Singh SP, Nikrodhanond AA, Baria AT, Le WW, Hoffman GE, Radovick S 2008 Temporal and spatial regulation of CRE recombinase expression in gonadotrophin-releasing hormone neurones in the mouse. J Neuroendocrinol 20:909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR 2006 Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKCλ/ζ. Cell Metab 3:343–353 [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK 2003 Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23:7143–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GaF, KBJ 2001 The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O'Malley B, Levine JE 2005 Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology 146:4340–4348 [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE 2007 Estrogen response element-independent estrogen receptor (ER)-α signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERα knockout mice. Endocrinology 148:5288–5294 [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK 2008 Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118:1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA 1996 Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147 [DOI] [PubMed] [Google Scholar]
- Schneider JE 2004 Energy balance and reproduction. Physiol Behav 81:289–317 [DOI] [PubMed] [Google Scholar]
- Allen MP, Zeng C, Schneider K, Xiong X, Meintzer MK, Bellosta P, Basilico C, Varnum B, Heidenreich KA, Wierman ME 1999 Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol Endocrinol 13:191–201 [DOI] [PubMed] [Google Scholar]
- Nielsen-Preiss SM, Allen MP, Xu M, Linseman DA, Pawlowski JE, Bouchard RJ, Varnum BC, Heidenreich KA, Wierman ME 2007 Adhesion-related kinase induction of migration requires phosphatidylinositol-3-kinase and ras stimulation of rac activity in immortalized gonadotropin-releasing hormone neuronal cells. Endocrinology 148:2806–2814 [DOI] [PubMed] [Google Scholar]
- Levine JE, Duffy MT 1988 Simultaneous measurement of luteinizing hormone (LH)-releasing hormone, LH, and follicle-stimulating hormone release in intact and short-term castrate rats. Endocrinology 122:2211–2221 [DOI] [PubMed] [Google Scholar]
- Meredith JM, Levine JE 1992 Effects of castration on LH-RH patterns in intrahypophysial microdialysates. Brain Res 571:181–188 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A 1988 Effect of time after castration on secretion of LHRH and LH in the ram. J Reprod Fertil 82:263–269 [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS 1989 Do testosterone and estradiol-17β enforce inhibition or stimulation of luteinizing hormone-releasing hormone secretion? Biol Reprod 41:559–570 [DOI] [PubMed] [Google Scholar]
- Shakil T, Hoque AN, Husain M, Belsham DD 2002 Differential regulation of gonadotropin-releasing hormone secretion and gene expression by androgen: membrane versus nuclear receptor activation. Mol Endocrinol 16:2592–2602 [DOI] [PubMed] [Google Scholar]
- Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ 2003 Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85α, androgen receptor, and Src. J Biol Chem 278:42992–43000 [DOI] [PubMed] [Google Scholar]
- Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L 2004 Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem 279:14579–14586 [DOI] [PubMed] [Google Scholar]
- Sharifi N, Reuss AE, Wray S 2002 Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology 143:2503–2507 [DOI] [PubMed] [Google Scholar]
- Foecking EM, McDevitt MA, Acosta-Martínez M, Horton TH, Levine JE 2008 Neuroendocrine consequences of androgen excess in female rodents. Horm Behav 53:673–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM 2004 Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking EM, Szabo M, Schwartz NB, Levine JE 2005 Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod 72:1475–1483 [DOI] [PubMed] [Google Scholar]
- Robinson JE, Forsdike RA, Taylor JA 1999 In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology 140:5797–5805 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Eisner JR, Goy RW 1997 Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril 67:155–163 [DOI] [PubMed] [Google Scholar]
- Ueki K, Fruman DA, Yballe CM, Fasshauer M, Klein J, Asano T, Cantley LC, Kahn CR 2003 Positive and negative roles of p85α and p85β regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J Biol Chem 278:48453–48466 [DOI] [PubMed] [Google Scholar]
- Jiménez C, Portela RA, Mellado M, Rodríguez-Frade JM, Collard J, Serrano A, Martínez-A C, Avila J, Carrera AC 2000 Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J Cell Biol 151:249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KM, Huang Y, Yip SC, Yu J, Segall JE, Backer JM 2001 N-terminal domains of the class ia phosphoinositide 3-kinase regulatory subunit play a role in cytoskeletal but not mitogenic signaling. J Biol Chem 276:16374–16378 [DOI] [PubMed] [Google Scholar]
- Xia X, Cheng A, Akinmade D, Hamburger AW 2003 The N-terminal 24 amino acids of the p55γ regulatory subunit of phosphoinositide 3-kinase binds Rb and induces cell cycle arrest. Mol Cell Biol 23:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.