Abstract
Fibroblast growth factor 21 (FGF21) is a novel metabolic regulator shown to improve glycemic control. However, the molecular and functional mechanisms underlying FGF21-mediated improvements in glycemic control are not completely understood. We examined FGF21 effects on insulin sensitivity and glucose fluxes upon chronic (daily injection for 8 d) and acute (6 h infusion) administration in ob/+ and ob/ob mice. Results show that chronic FGF21 ameliorated fasting hyperglycemia in ob/ob mice via increased glucose disposal and improved hepatic insulin sensitivity. Acute FGF21 suppressed hepatic glucose production, increased liver glycogen, lowered glucagon, and improved glucose clearance in ob/+ mice. These effects were blunted in ob/ob mice. Neither chronic nor acute FGF21 altered skeletal muscle or adipose tissue glucose uptake in either genotype. In conclusion, FGF21 has potent glycemic effects caused by hepatic changes in glucose flux and improved insulin sensitivity. Thus, these studies define mechanisms underlying anti-hyperglycemic actions of FGF21 and support its therapeutic potential.
The current data detail the mechanism of action for FGF21 to lower blood glucose and improve insulin sensitivity using state-of-the-art techniques in the conscious mouse.
Fibroblast growth factor 21 (FGF21) is a potent metabolic regulator shown to improve glucose and lipid metabolism as well as to reduce overall body weight and adipose mass (1,2,3,4,5,6,7,8). Importantly, beneficial effects associated with therapeutic administration and/or transgenic overexpression of FGF21 occurred without concomitant hypoglycemia or mitogenicity characteristic of several current antidiabetic drugs (9). Accordingly, FGF21 is now considered a potential therapeutic agent to treat a variety of metabolic diseases including hyperglycemia and dyslipidemia (9).
Based on evidence that chronic FGF21 administration lowers plasma insulin and improves glucose tolerance in diabetic rodents and nonhuman primates (2,4,5,6,8), it is likely that FGF21 ameliorates insulin resistance, and this outcome contributes to the striking anti-hyperglycemic effects of FGF21. This concept may be important considering that insulin resistance is a hallmark of numerous metabolic diseases and critically associated with defects in glucose flux. Indeed, several current therapies aim to treat hyperglycemia through modulation of insulin signaling (10). The link between FGF21 treatment and insulin sensitivity has been recently strengthened by findings that insulin sensitivity is improved in chronically treated high-fat-fed mice (11). This notion is further supported by recent evidence of insulin-FGF21 cross talk as well as a functional interplay between FGF21- and peroxisome proliferator-activated receptor (PPAR)-dependent mechanisms because PPAR agonists, which are potent insulin sensitizers, to a certain extent signal through changes in either FGF21 expression or action (8,12,13,14).
In the present studies, FGF21 was administered sc to obese, diabetic ob/ob mice for 8 d and compared with vehicle-treated ob/ob and ob/+ mice to test whether, and to what extent, FGF21 improves insulin sensitivity and glucose flux in a mouse model of severe insulin resistance. This was examined in catheterized, conscious animals using hyperinsulinemic-euglycemic clamp techniques. In subsequent experiments, ob/+ and ob/ob mice were infused with FGF21 for 6 h, and blood glucose was clamped to assess the direct metabolic effects of FGF21 on glucose fluxes. In both experiments, isotopic tracer techniques were used to quantify whole-body and tissue-specific glucose fluxes. The present studies clearly demonstrate that FGF21 treatment improves insulin sensitivity in the liver.
Materials and Methods
Animals
Animal procedures were approved by the Vanderbilt University Animal Care and Use Committee. Male ob/ob and ob/+ mice on a C57BL/6 background (Harlan, Indianapolis, IN) at 7 wk of age were acclimated for 1 wk before surgery in an environmentally controlled facility with a 12-h light, 12-h dark cycle and free access to food and water.
Surgical techniques
A jugular vein catheter was surgically implanted 5 d before study using previously described techniques (15). Body weight was monitored daily, and only mice returning to within about 10% of presurgical body weight were studied.
Hyperinsulinemic-euglycemic clamp
Insulin sensitivity was assessed using previously described clamp techniques (15) in ob/ob mice treated daily via sc injection for 8 d with FGF21 (1 mg · kg−1) or vehicle. Vehicle-treated ob/+ mice were studied as an insulin-sensitive control. Body composition was assessed 24 h before study using NMR (Bruker-Optics). On the day of study, mice were placed in plastic restraint tube designed for rats at approximately 0800 h to begin a 5 h fast. At t = −90 min, a primed continuous infusion of HPLC-purified [3-3H]glucose (5 μCi bolus + 0.05 μCi · min−1) was started to measure glucose turnover. Basal blood samples from the cut tail for blood glucose, insulin, and glucose turnover were taken at t = −15 and −5 min. At t = 0 min, a continuous infusion of insulin (10mU · kg−1 · min−1) was started to induce hyperinsulinemia, and the tracer infusion was increased to 0.1 μCi · min−1 to minimize changes in specific activity. Blood glucose was measured at t = 5, 10, 15, and 20 min and every 10 min thereafter, and a variable glucose infusion rate (GIR) was adjusted as needed to maintain blood glucose at about 8.0 mmol · liter−1. At t = 78 min, a 12-μCi bolus of HPLC-purified 2-[14C]deoxyglucose (2-DG) was given to assess tissue-specific glucose uptake. The steady-state clamp period was t = 80–120 min. Samples to determine plasma [3-3H]glucose and 2-DG were taken every 10 min from t = 80–120 min. Samples to measure plasma insulin were taken at t = 100 and 120 min. After the final blood samples, mice were anesthetized using a bolus of sodium pentobarbital and tissues were removed and frozen in liquid nitrogen.
Acute effects of FGF21
Ob/ob and ob/+ mice were placed in a rat restraint tube at approximately 0600 h to begin a 5-h fast. At t = −90 min, a primed continuous infusion of HPLC-purified [3-3H]glucose (5 μCi bolus + 0.05 μCi · min−1) was started to assess glucose turnover. Basal samples for blood glucose, insulin, glucagon, and glucose turnover were taken at t = −15 and −5 min from the cut tail. At t = 0 min, a continuous infusion of FGF21 (1 μg · kg−1 · min−1) was started and [3-3H]glucose was increased to 0.1 μCi · min−1 to minimize changes in specific activity. Samples to measure plasma glucose and determine glucose turnover were taken every 60 min for 5 h. A variable GIR was used to prevent a fall in glucose below about 8.0 mmol · liter−1. Samples for insulin and glucagon were taken at t = 150 and 300 min. At t = 318 min, a 12-μCi bolus of HPLC-purified 2-DG was given to assess tissue-specific glucose uptake, and blood samples were taken every 10 min from 320–360 min. At t = 360 min, mice were anesthetized using a bolus of pentobarbital, and a terminal blood sample was taken to measure plasma FGF21. Tissues were removed and frozen in liquid nitrogen.
Blood and plasma analyses
Blood glucose during each clamp was measured using an Accu-Chek meter (Roche, Indianapolis, IN). Plasma nonesterified fatty acids (NEFA) were measured using a kit (Wako Diagnostics, Osaka, Japan). Insulin and glucagon were determined by the Vanderbilt Mouse Metabolic Phenotyping Center Analytical Resources Core (16). FGF21 was measured using a previously described ELISA (6). Plasma [3-3H]glucose and 2-DG radioactivity were measured using liquid scintillation counting (17).
Tissue analyses
Hepatic glycogen was measured enzymatically (18). The percentage of glucose disappearance in glycogen was determined by incorporation of [3-3H]glucose in glycogen. Hepatic triglycerides were measured using a commercial assay (Pointe Scientific Inc., Canton, MI). Liver glucokinase and glucose-6-phosphatase (G-6-Pase) activity assays were performed as previously described (19). Tissue 2-DG was measured as previously described (17). Quantitative real-time PCR was performed using TaqMan Assay-on-Demand primers (Applied Biosystems, Foster City, CA) and normalized to 18S or 364B. Genes of interest are listed in Table 1.
Table 1.
mRNA for genes of interest in ob/+ and ob/ob mice after hyperinsulinemic-euglycemic clamps or infusion with FGF21 or vehicle
| Genes of interest | Insulin clamp
|
FGF21 clamp
|
|||||
|---|---|---|---|---|---|---|---|
| ob/+ | ob/ob | ob/ob | ob/+ | ob/+ | ob/ob | ob/ob | |
| FGF21 | − | − | + | − | + | − | + |
| Liver | |||||||
| AMPKα1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 2.0 ± 0.5a,b | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| AMPKα2 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| βKlotho | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.2 |
| CD36 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.5 ± 0.3 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| CPT1α | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.8 ± 0.2a,b | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.5 ± 0.2 | 1.2 ± 0.2 |
| CPT1β | 1.0 ± 0.4 | 2.1 ± 0.2 | 2.2 ± 0.2a | 1.0 ± 0.1 | 1.4 ± 0.1b | 12.2 ± 0.5 | 11.6 ± 0.8 |
| FOXA2 | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 1.0 ± 0.1 | 1.7 ± 0.2b | 1.0 ± 0.1 | 0.9 ± 0.1 |
| G6Pase | 1.0 ± 0.4 | 0.5 ± 0.1 | 2.1 ± 0.5a,b | 1.0 ± 0.1 | 0.6 ± 0.1b | 2.0 ± 0.9 | 0.9 ± 0.3b |
| Glucokinase | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 |
| Glycogen synthase 1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.2 | 1.4 ± 0.2b |
| Glycogen synthase 2 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Insulin receptor | 1.0 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1b | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Leptin receptor | 1.0 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.2 | 1.0 ± 0.2 | 2.3 ± 0.6b | 1.0 ± 0.1 | 1.0 ± 0.2 |
| MCD | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1b |
| PEPCK | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 |
| PPARα | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Heart | |||||||
| Hexokinase II | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| Glut4 | 1.0 ± 0.1 | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.1 |
| βKlotho | ND | ND | ND | ND | ND | ND | ND |
| Gastrocnemius | |||||||
| βKlotho | ND | ND | ND | ND | ND | ND | ND |
| Adipose | |||||||
| βKlotho | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.1 |
Mice were subjected to hyperinsulinemic (10 mU · kg−1 · min−1), euglycemic (∼8.0 mmol · liter−1) clamps (insulin clamp; n = 8–10 in each group) or infused with FGF21 (1 ng · kg−1 · min−1) or vehicle for 6 h (FGF21 clamp; n = 7–9 in each group). All mice were male, studied at 9–10 wk of age, and fasted for 5 h before study. Mice were euthanized using a bolus of sodium pentobarbital. Samples were run in duplicate using 18S or 36B4 as a reference gene and normalized to ob/+ mice infused with vehicle. Data are presented as mean ± se, and statistical significance is established at P < 0.05. ND, No message detected.
Differences compared with vehicle-infused ob/+ mice.
Differences within a genotype.
Calculations and statistics
Whole-body glucose appearance (Ra), disappearance (Rd), and clearance were calculated as previously described (20). Tissue glucose uptake (Rg) was also calculated as previously described (17). Statistical comparisons were made using t tests or one-way ANOVA followed by the Fisher least significant difference test for post hoc comparisons. Data are presented as means ± se. Statistical significance was defined as P < 0.05.
Results
FGF21 treatment for 8 d reduces fasting blood glucose and improves insulin sensitivity in ob/ob mice
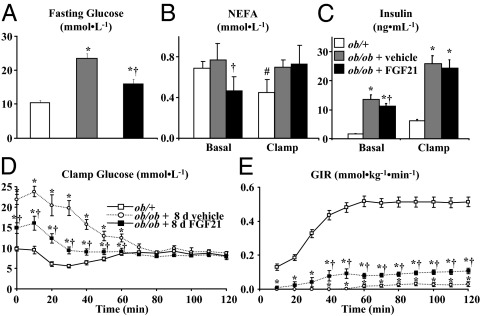
The elevated fasting blood glucose, insulin, and NEFA levels characteristic of ob/ob mice were reduced by 8.0 mmol · liter−1, 1.8 ng · ml−1, and 0.3 mmol · liter−1, respectively, with FGF21 treatment (Fig. 1, A–C). Absolute glucose and insulin levels in fasted ob/ob mice treated with FGF21 were, however, still higher compared with ob/+ mice (Fig. 1, A and C). Body weight in ob/ob mice was not affected by FGF21 treatment (FGF21- and vehicle-treated ob/ob mice were 45.6 ± 0.7 and 45.4 ± 0.7 g, respectively). Body weight was higher in ob/ob mice compared with ob/+ mice (26.5 ± 0.3 g) due to higher fat mass (22.7 ± 0.5 g in both FGF21- and vehicle-treated ob/ob mice vs. 2.7 ± 0.1 g in vehicle-treated ob/+ mice).
Figure 1.
Blood glucose (A), insulin (B), and NEFA (C) from hyperinsulinemic (10 mU · kg−1 · min−1)-euglycemic (∼8.0 mmol · liter−1) clamps in conscious, 5-h fasted ob/+ and ob/ob mice (n = 11–13 in each group). Ob/ob mice were sc injected with vehicle or FGF21 (1 mg · kg−1 · d−1) for 8 d, and jugular vein catheters were implanted 5 d before study. Vehicle-treated ob/+ were studied as an insulin-sensitive control. Blood samples were taken at indicated time points from the cut tail to measure blood glucose (D). The GIR (E) was adjusted as needed to maintain euglycemia. Mice were euthanized using a bolus of sodium pentobarbital at t = 120 min, and tissues were dissected and quickly frozen. All mice were male and studied at 9–10 wk of age. Data are presented as mean ± se, and the steady-state period was defined as t = 80–120 min during the clamp. Statistical significance was established at P < 0.05. *, Comparison to ob/+ mice; †, comparison between ob/ob mice treated with vehicle or FGF21; #, comparison within a group between basal and clamp conditions.
Hyperinsulinemic-euglycemic clamps were done to test the effect of 8 d FGF21 treatment on insulin sensitivity in ob/ob mice. As shown in Fig. 1C, plasma insulin during the clamp was similarly elevated in vehicle- and FGF21-treated ob/ob mice and remained significantly higher than levels in ob/+ mice. NEFA levels were suppressed by hyperinsulinemia in ob/+ mice but were unchanged in ob/ob mice (Fig. 1B). Blood glucose was clamped in all groups during the steady-state period (Fig. 1D). The GIR required to clamp blood glucose was 4-fold greater in FGF21-treated ob/ob mice, indicating improved insulin sensitivity (Fig. 1E). The increased GIR in FGF21-treated ob/ob mice was about 20% of vehicle-treated ob/+ mice indicating a modest improvement in insulin sensitivity (Fig. 1E).
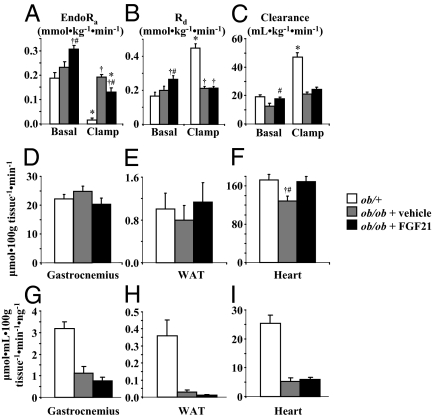
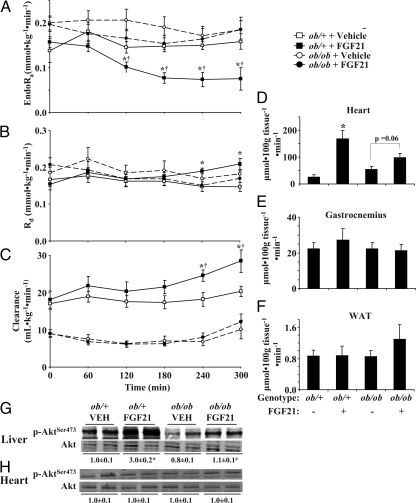
Fasting endogenous Ra (endoRa) and Rd were increased by FGF21 treatment in ob/ob mice (Fig. 2, A and B) and fasting whole-body glucose clearance was fully restored to rates found in ob/+ mice (Fig. 2C). As expected, suppression of endoRa by hyperinsulinemia was nearly complete in ob/+ mice (Fig. 2A). EndoRa was not suppressed in ob/ob mice treated with vehicle (Fig. 2A). FGF21 treatment in ob/ob mice restored the magnitude in suppression of endoRa to equal that of ob/+ mice, indicating improved hepatic insulin sensitivity (Fig. 2A). Clamp Rd (Fig. 2B) and glucose clearance (Fig. 2C) were similar in vehicle- and FGF21-treated ob/ob mice but remained well below rates of ob/+ mice. The index of skeletal muscle and adipose tissue glucose uptake (Rg) after the clamp was unchanged in ob/ob mice treated with FGF21 (Fig. 2, D and E). Absolute Rg rates in ob/ob mice were similar to ob/+ mice but were reduced when normalized for clamp insulin (Fig. 2, G and H). Cardiac Rg was increased in ob/ob mice treated with FGF21 and restored to absolute levels seen in ob/+ mice (Fig. 2F). Cardiac Rg was, however, not different when normalized to clamp insulin (Fig. 2I).
Figure 2.
EndoRa (A), Rd (B), and glucose clearance (C) during a 120-min hyperinsulinemic (10 mU · kg−1 · min−1)-euglycemic (∼8.0 mmol · liter−1) clamp in conscious, 5-h fasted ob/+ and ob/ob mice (n = 11–13 in each group). Ob/ob mice were sc injected with vehicle or FGF21 (1 mg · kg−1 · d−1) for 8 d, and jugular vein catheters were implanted 5 d before study. Vehicle-treated ob/+ were studied as an insulin-sensitive control. [3-3H]Glucose (0.1 μCi · min−1) was infused starting at t = −90 min, and plasma samples were taken from the cut tail at time points indicated in each panel. Mice were euthanized at t = 120 min using sodium pentobarbital. D–F, Rg in the gastrocnemius (D), heart (E), and white adipose tissue (WAT; F) was assessed using 2-DG at t = 78 min; G–I, Rg values normalized to clamp insulin, respectively. Mice were male, studied at 9–10 wk of age, and fasted 5 h before study. *, Differences between basal and clamp; †, differences compared with ob/+ mice; #, differences compared with vehicle-treated ob/ob mice.
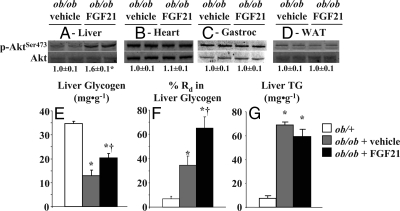
The effect of FGF21 treatment to improve hepatic insulin sensitivity in ob/ob mice, based on increased insulin-mediated suppression of endoRa, corresponded with increased hepatic Akt activation (p-Akt/Akt) (Fig. 3A). Consistent with normalized Rg data, Akt activation was not different in heart, gastrocnemius, or adipose tissue of FGF21-treated ob/ob mice (Fig. 3, B–D). FGF21 treatment also increased hepatic glycogen (Fig. 3E) and the percentage of Rd accounted for in glycogen (Fig. 3F). FGF21 treatment had no significant effect on liver triglyceride in ob/ob mice, although there was a trend for reduced levels (P = 0.07) (Fig. 3G). Hepatic glycogen and triglyceride concentrations remained lower and higher, respectively, compared with ob/+ mice (Fig. 3, F and G). Hepatic glucokinase activity was increased in FGF21-treated ob/ob mice by about 15% (4.5 ± 0.1 vs. 5.5 ± 0.3 μmol · min−1 · g−1, P = 0.02, in vehicle- and FGF21-treated animals, respectively), whereas G-6-Pase activity was unaffected (7.6 ± 0.9 vs. 7.0 ± 0.4 μmol · min−1 · g−1, P = 0.55, in vehicle- and FGF21-treated mice, respectively). Expression of key genes involved in hepatic metabolic regulation was investigated based on evidence of a liver phenotype and were indeed altered in FGF21-treated ob/ob mice. As shown in Table 1, mRNA levels for G-6-Pase, AMPKα1, CPT1α, and CPT1β were increased in FGF21-treated ob/ob mice. There were no changes in the cardiac expression of hexokinase II or glucose transporter 4 (Glut4) (Table 1) associated with FGF21 treatment consistent with Rg normalized to insulin. Expression of βKlotho, the cofactor needed for FGF21 activity, in liver and adipose tissue was unaffected by FGF21 treatment and was undetectable in heart and gastrocnemius (Table 1).
Figure 3.
Hepatic Akt phosphorylation at serine 473 normalized to total Akt protein content in liver (A), heart (B), gastrocnemius (C), and adipose (D) after 120 min hyperinsulinemic (10 mU · kg−1 · min−1)-euglycemic (∼8.0 mmol · liter−1) clamps in conscious, 5-h fasted ob/+ and ob/ob mice (n = 11–13 in each group). Mice were sc injected with either vehicle or FGF21 (1 mg · kg−1 · d−1) for 8 d before study. Liver glycogen (E), percentage of Rd in liver glycogen (F), and triglyceride (TG; G) were measured after the clamp using enzymatic techniques and incorporation of [3-3H]glucose into glycogen. Mice were euthanized at t = 120 min using sodium pentobarbital. Mice were male, studied at 9–10 wk of age, and fasted 5 h before study. *, Differences compared with ob/+ mice; #, differences compared with vehicle-treated ob/ob mice. WAT, White adipose tissue.
Acute effects of FGF21 on glucose fluxes in ob/+ and ob/ob mice
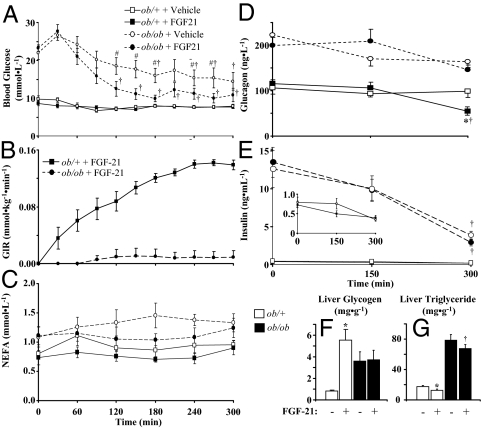
To test the acute effects of FGF21 on glucose fluxes, FGF21 was infused in ob/+ and ob/ob mice for 6 h while GIR was adjusted to maintain steady-state conditions. Steady-state blood glucose and GIR were obtained within 3 h (Fig. 4, A and B). Although endogenous FGF21 levels were below limits of detection in vehicle-infused mice, continuous infusion led to pharmacological FGF21 levels of 53 ± 3 and 46 ± 2 ng · ml−1, respectively, in ob/+ and ob/ob mice. FGF21 had a potent glycemic effect in ob/+ mice requiring a GIR of approximately 0.14 mmol · kg−1 · min−1 to maintain steady-state blood glucose (Fig. 4B). The blood glucose response in ob/ob mice to FGF21 was comparatively modest, requiring a GIR that was only about 20% of that in ob/+ mice (Fig. 4B). NEFA levels were not changed by FGF21 in either genotype (Fig. 4C). Glucagon was decreased after 5 h in ob/+ mice but not in ob/ob mice infused with FGF21 (Fig. 4D). Although insulin fell during the clamp in both ob/+ and ob/ob mice, this was due to the length of the fast and was not affected by FGF21 (Fig. 4E). Liver glycogen was increased in ob/+ mice infused with FGF21, but this effect was absent in ob/ob mice (Fig. 4F). Liver triglycerides were reduced by a similar decrement in ob/+ and ob/ob mice infused with FGF21 (Fig. 4G).
Figure 4.
Whole blood glucose (A), GIR (B), glucagon (C), and insulin (D) in ob/+ and ob/ob mice infused with FGF21 (1 ng · kg−1 · min−1) or vehicle for a total of 6 h (n = 7–9 in each group). Inset in E shows rescaled insulin levels for ob/+ mice. Samples were taken at time points indicated in each panel, and t = 0 is the mean of t = −15 and −5 min samples. Figures show data for the first 5 h before the bolus infusion of 2-DG at t = 318 min to measure tissue-specific glucose uptake. Glycogen (F) and triglyceride (G) were measured after euthanasia via a bolus of pentobarbital. Jugular vein catheters used for infusion purposes were surgically implanted in all mice 5 d before study, and blood samples were taken from the cut tail. All mice were male, studied at 9–10 wk of age, and fasted for 5 h before study. Data are presented as mean ± se, and statistical significance is established at P < 0.05. *, Differences between vehicle- and FGF21-infused ob/+ mice; #, differences between vehicle- and FGF21-infused ob/ob mice; †, differences compared with basal values.
EndoRa in ob/+ mice was reduced by FGF21 infusion but was unchanged in ob/ob mice (Fig. 5A). Rd and glucose clearance were increased in ob/+ mice infused with FGF21 (Fig. 5, B and C). These rates were unchanged in ob/ob mice at steady state (Fig. 5, B and C). Cardiac Rg in ob/+ mice infused with FGF21 was increased about 7-fold compared with about 2-fold in ob/ob mice (Fig. 5D). FGF21 infusion had no effect on skeletal muscle or adipose tissue Rg in ob/+ and ob/ob mice (Fig. 5, E and F). FGF21-mediated suppression of endoRa corresponded to increased hepatic Akt phosphorylation in ob/+ mice (Fig. 5G). FGF21 also increased hepatic Akt activation in ob/ob mice, but this increase remained well below that of ob/+ mice (Fig. 5G). Expression of the insulin receptor, leptin receptor, and Foxa2 in the liver of ob/+ mice was increased by FGF21 infusion (Table 1). In ob/ob mice, hepatic MCD and glycogen synthase mRNA levels were decreased and increased, respectively, by FGF21 infusion, but other genes were unaffected (Table 1). Infusion of FGF21 had no effect on cardiac expression of hexokinase II or Glut4. Expression of βKlotho was unchanged by FGF21 treatment in liver and adipose and was undetectable in heart and gastrocnemius (Table 1).
Figure 5.
EndoRa (A), Rd (B), and glucose clearance (C) in ob/+ and ob/ob mice infused with FGF21 (1 ng · kg−1 · min−1) or vehicle for 6 h (n = 7–9 in each group). Time points are data from t = 0–300 min before the bolus infusion of 2-DG at t = 318 min. [3-3H]Glucose (0.1 μCi · min−1) was infused starting at t = −90 min, and plasma samples were taken from the cut tail at time points indicated in each panel where t = 0 min is the mean of t = −15 and −5 min samples. Rg in the gastrocnemius (D), heart (E), and white adipose tissue (WAT; F) was assessed using 2-DG. Hepatic Akt phosphorylation at serine 473 (p-Akt for 473) normalized to total Akt protein content in liver and heart is shown in G and H, respectively. Mice were euthanized at t = 360 min using sodium pentobarbital. Mice were male, studied at 9–10 wk of age, and fasted 5 h before study. *, Differences between vehicle (VEH)- and FGF21-infused ob/+ mice; †, differences compared with basal values.
Discussion
Metabolic diseases such as type 2 diabetes, obesity, and metabolic syndrome are characterized by impairments in insulin sensitivity and glucose flux. Amelioration of these symptoms is considered to be beneficial in treating metabolic abnormalities. Recent studies have shown that FGF21 reduces hyperglycemia and insulin resistance (9,11). However, the mechanisms by which FGF21 propagates its function to control glucose and its role, if any, in regulating insulin sensitivity and glucose flux are not well defined. Thus, we examined the chronic and acute mechanisms underlying FGF21 anti-hyperglycemic activities using in vivo clamp techniques in ob/ob and ob/+ mice. We hypothesized that the mode of action for FGF21 includes regulation of glucose flux through improvements in hepatic insulin sensitivity.
These results demonstrate that chronic FGF21 treatment (daily sc injection of 1 mg · kg−1 for 8 d) reduced fasting hyperglycemia, hyperinsulinemia, and NEFA levels in ob/ob mice. Such improvements in glycemic control and lipid metabolism are consistent with previous studies in obese and diabetic animals chronically treated with FGF21 (1,2,5,6,8,11). We show that reduced fasting blood glucose associated with FGF21 treatment was due to improved basal glucose disposal (Fig. 2B). Considering the marked decrease in blood glucose, it was surprising that FGF21 treatment increased endoRa (Fig. 2A). Additional analyses of livers from ob/ob mice treated with FGF21 reveal increased glycogen content (accounting for ∼65% of whole-body Rd; Fig. 3F), increased glucokinase activity, and unaffected G-6-Pase activity indicating a potential futile cycling situation favoring glucose uptake. A net influx of glucose into the liver could explain how blood glucose is lowered despite higher endoRa.
A critical finding was that chronic FGF21 administration improved insulin sensitivity in ob/ob mice based on increased GIR needed to maintain euglycemia during hyperinsulinemic-euglycemic clamps. This technique is the gold standard for assessing insulin sensitivity because it is possible to normalize differences in blood glucose, which often complicate interpretation of glucose and insulin tolerance tests. The present improvements in insulin sensitivity are largely ascribed to hepatic FGF21 effects because suppression of endoRa was increased (Fig. 2A), but Rg was unchanged in skeletal muscle, heart, and adipose tissue when normalized for clamp insulin levels. Hepatic effects, at least in part, may be due to insulin-sensitizing effects of FGF21 treatment at a molecular level. Akt phosphorylation, a key step in hepatic insulin and FGF21 signaling (8,21), was increased during the clamp in FGF21-treated ob/ob mice (Fig. 4G). In this context, this likely reflects improved insulin sensitivity considering the approximately 1-h half-life of FGF21 (6). mRNA levels for key mediators of hepatic fuel flux such as AMP-activated protein kinase-α (AMPKα), carnitine palmitoyl-transferase-1α (CPT1α), CD36, and G-6-Pase were also elevated by chronic FGF21 treatment in ob/ob mice (Table 1). These changes may contribute to a FGF21-dependent mechanism to regulate hepatic insulin sensitivity (22,23,24,25).
FGF21 is previously shown to inhibit adipose tissue lipolysis and postulated as a potential mechanism to explain improvements in insulin sensitivity (1). This result, however, contrasts findings that FGF21 stimulates adipose tissue lipolysis (4). The contribution of reduced basal NEFA levels (Fig. 1B) in FGF21-treated ob/ob mice to improvements in insulin sensitivity is difficult to discern in the current study because lipid flux was not assessed. These studies do demonstrate that FGF21 treatment did not improve insulin-mediated suppression of lipolysis in ob/ob mice (Fig. 1C).
It is important to note that there were no differences in steady-state insulin levels in ob/ob mice regardless of treatment (Fig. 1B). This is critical to consider when interpreting insulin clamp data (26). Moreover, there were no differences in body weight or composition due to FGF21 treatment in ob/ob mice. Although previous studies in high-fat-fed and ob/ob mice have shown dose-dependent effects of FGF21 on body weight and adipose mass (3,11), we did not observe a FGF21 weight-lowering effect. The latter finding is consistent with the fact that higher doses (>1 mg · kg−1 · d−1), longer duration of administration, and/or alternative administration routes are needed to reveal the ability of FGF21 to lower body weight/adipose tissue mass (3,6). Thus, the FGF21 insulin-sensitizing effects observed here are not secondary to changes in adiposity or differences in insulin levels but are rather a direct result of FGF21 action.
The current finding that chronic FGF21 treatment improves insulin sensitivity is generally consistent with data in FGF21-treated high-fat-fed mice (11). In agreement with our results, this study found that FGF21 lowered fasting blood glucose and insulin and improved hepatic insulin sensitivity. In contrast, Xu et al. (11) found that FGF21 treatment improved insulin-stimulated glucose uptake in heart, adipose tissue, and skeletal muscle in a dose-dependent manner. It is worthwhile to note that interpreting these data are difficult because details concerning fast duration and specific muscles used to assess glucose uptake are not provided (11). These and other methodological concerns about clamp techniques used in Xu et al. (11) have been previously discussed (15). Additionally, clamp glucose and insulin levels were significantly different between groups in Xu et al. (11). For example, clamp glucose in high-fat-fed mice treated with 0.1 and 10 mg · kg−1 · d−1 FGF21 were about 20 and 25% lower, respectively, than vehicle-treated control mice. Clamp insulin levels were also about 23 and 51% lower, respectively, in FGF21-treated high-fat-fed mice compared with vehicle-treated controls. These differences in clamp conditions complicate comparing between groups. It is noteworthy that normalizing for clamp insulin would augment only effects ascribed to FGF21, resulting in profound effects on muscle glucose uptake. This is curious considering that βKlotho, the cofactor required for FGF21 activity, is not known to be expressed in skeletal muscle. Nevertheless, our studies and those of Xu et al. (11) do generally agree that FGF21 treatment improves glycemic control and has potent hepatic effects. An additional consistent finding was that the lower dose of FGF21 did not stimulate adipose tissue glucose uptake (11). βKlotho is expressed in this tissue, and FGF21 is reported to increase glucose flux into adipose tissue (5,11). Our results, however, do not support this effect in vivo.
To understand the actions of FGF21 in the fasted state and further define sites of action, we next tested whether an acute 6-h constant infusion of FGF21 directly impacts glucose flux. In this novel protocol, FGF21 had rapid and potent metabolic effects in healthy ob/+ mice. FGF21 lowered endoRa and increased whole-body glucose clearance in ob/+ mice (Fig. 5, A and C). There were no changes in plasma NEFA (Fig. 4C) or adipose tissue Rg (Fig. 5F) associated with infusion of FGF21, suggesting that metabolic effects are not secondary to direct actions on adipose tissue. Remarkably, the GIR required to maintain steady-state euglycemia was comparable to exogenous glucose requirements during a 2.5 mU · kg−1 · min−1 insulin clamp in C57BL/6 mice (15). Consistent with the chronic FGF21 treatment results, the acute studies demonstrate a potent effect on the liver. FGF21 reduced liver triglyceride in both ob/ob and ob/+ mice by about 25% (Fig. 4G), consistent with a role for FGF21 in amelioration of hepatosteatosis (2,3,11). FGF21 also led to a remarkable increase in liver glycogen (Fig. 4F), accounting for 72 ± 2% of whole-body Rd in ob/+ mice. FGF21 also increased hepatic Akt activation (Fig. 4G) and expression of the insulin receptor, leptin receptor, and FoxA2 in ob/+ mice consistent with improvements in insulin signaling (Table 1).
The current data indicate that the actions of acutely infused FGF21 on the liver are predominantly direct rather than secondary to effects in other tissues. This conclusion is based on evidence that FGF21 did not alter insulin levels (Fig. 4E), NEFA (Fig. 4C), or Rg in skeletal muscle or adipose tissue (Fig. 5, E and F). It is important to note that the acute infusion of FGF21 in ob/+ mice was associated with effects in tissues other than the liver based on increased cardiac Rg (Fig. 5D) and decreased plasma glucagon (Fig. 4D). This effect in the heart is consistent with absolute Rg rates in chronically treated ob/ob mice (Fig. 2F) and previous studies (11). This finding is somewhat surprising given that βKlotho is not known to be expressed in the heart. To test whether FGF21 directly impacts the heart, we assessed Akt activation and βKlotho transcript in acutely infused and chronically treated mice. Cardiac Akt activation was unchanged, and βKlotho expression was undetectable in the heart (Table 1) despite increased Rg associated with FGF21. These findings suggest that the changes in cardiac glucose flux linked to FGF21 are indirect and likely due to the fact that the heart is constantly active and sensitive to changes in substrate availability. Subtle changes in NEFA availability, for example, may cause the heart to rely on more glucose as energetic substrate. This notion of indirect FGF21 effects on cardiac glucose flux is supported by recent evidence (11). Despite the apparent indirect nature of the effect, this is potentially an important observation considering that individuals with diabetes are at a higher risk for cardiovascular disease (27). Additional studies are needed to explore metabolic changes responsible for this effect and to test the role, if any, of FGF21 in the treatment of cardiovascular disease.
The action of FGF21 to lower glucagon in ob/+ mice is consistent with previous findings and expression of the required receptor complex in the pancreas (5). In the present studies, it is unclear whether this is a direct effect on the α-cell, an indirect effect, or both. It is, however, unlikely that the glucagon-lowering effect is mediated by the β-cell because FGF21 did not change insulin levels during the acute infusion studies (Fig. 4E). FGF21 has also been shown in vitro not to influence insulin secretion in healthy islets (8). Nevertheless, we cannot rule out that additional factors contribute to glucagon-lowering effects. Although it is possible that the fall in glucagon contributed to hepatic changes, the fact that the fall in endoRa preceded changes in the hormone further indicate liver-specific effects of FGF21.
An additional important finding from the acute infusion studies was that, with the exception of lowered hepatic triglycerides, all effects linked to FGF21 in ob/+ mice were blunted or absent in ob/ob mice. Importantly, steady-state plasma FGF21 levels were increased to similar pharmacological levels in both genotypes. FGF did have a modest glucose-lowering effect in ob/ob mice (Fig. 4A), but there were no significant acute effects on steady-state endoRa or whole-body glucose clearance (Fig. 5, A and C). Blood glucose also fell during the experiment in vehicle-infused ob/ob mice (Fig. 4A), but this was likely due to the progressive length of the fast, which was 11 h at the final time point. FGF21 did increase hepatic Akt activation in ob/ob mice, although this was blunted compared with ob/+ mice (Fig. 5G). Acute FGF21 infusion was also associated with increased cardiac Rg in ob/ob mice (Fig. 5D), although this was not statistically significant (P = 0.06).
Taken together, these experiments using chronic treatments and acute infusions provide important and novel information about tissue-specific sites of action and functional outcomes of FGF21 treatment. In both settings, FGF21 is shown to regulate hepatic glucose flux primarily to stimulate Akt activation. These effects are consistent with studies demonstrating a powerful hepatic phenotype associated with changes in FGF21 (3,4,5,6,8,11) and support a direct mechanism of FGF21 action on the liver. These results are unique because they are based on in vivo studies performed using state-of-the-art clamp techniques under well-controlled conditions.
The effect of FGF21 to induce effects in the liver, and possibly the α-cell, also aligns with expression of FGF receptors and the required cofactor, βKlotho, in liver and pancreas (28,29,30). Moreover, absence of βKlotho in skeletal muscle and heart is consistent with findings that neither chronic treatment nor acute infusion of FGF21 directly altered Rg in gastrocnemius or heart. Expression of βKlotho in adipose tissue was, however, not linked to FGF21-mediated metabolic effects. As previously mentioned, this conflicts with in vitro data showing that FGF21 potently increases glucose uptake in adipocytes and in vivo results in white adipose tissue using 10-fold higher treatment doses (5,11). It is possible that subtle changes were undetectable because adipose tissue glucose uptake is at least an order of magnitude lower than other tissues (Figs. 2 and 5) (11). Notwithstanding these methodological concerns, the current results indicate that FGF21 does not alter adipose tissue glucose uptake despite efforts to increase sensitivity (e.g. analyzing more tissue to achieve higher counts). In regard to expression of βKlotho, an additional finding was that FGF21 did not alter expression of βKlotho in liver or adipose tissue and was undetectable in heart and skeletal muscle, consistent with prior evidence (28,29,30).
The striking contrast between marked metabolic improvements in ob/ob mice treated with FGF21 for 8 d compared with blunted pharmacological response in the 6-h FGF21 infusion study suggests that FGF21 may induce chronic and acute effects via different mechanisms. In a chronic setting, FGF21 appears to regulate glycemia via improvements in insulin sensitivity. This conclusion is based on FGF21-mediated effects to lower insulin (Fig. 1C), increase basal glucose clearance (Fig. 2C), and increase GIR needed during an insulin clamp (Fig. 1E). This is supported at a molecular level by increased hepatic Akt phosphorylation (Fig. 2G) and may have contributions from elevations of the insulin receptor, an effect that has been previously reported after chronic FGF21 treatment (3). Consistent with these previous findings, acute infusion of FGF21 induced marked increases in the hepatic expression of transcripts for insulin receptor, Foxa2, and leptin receptor (Table 1) as well as hepatic Akt phosphorylation (Fig. 5G). Overall, these results support the concept that FGF21 functions primarily through transcriptional activation (9).
Differences between genotypes in acute infusion studies also show that ob/ob mice are resistant to acute FGF21 actions. Recent evidence shows that plasma FGF21 protein is elevated in obese and/or diabetic individuals (31,32), and FGF21 tissue transcript is increased in mouse models of endocrine disease (7,28,33). These findings have led to suggestions of FGF21 resistance in the metabolically compromised state. Our data investigating the effects of FGF21 in ob/ob mice compared with healthy ob/+ mice support this concept and provide the first experimental evidence of an attenuated functional response to FGF21 in a disease state.
In summary, this report directly links the glucose-lowering function of FGF21 with improvements in insulin resistance and changes in glucose flux. These studies show that the liver is the primary target organ associated with FGF21 action. Furthermore, although our evidence suggests a partial resistance to acute effects of FGF21 in metabolically abnormal ob/ob mice vs. healthy ob/+ mice, repeated administration of FGF21 resulted in vast metabolic improvements in ob/ob mice indicating that FGF21 resistance can be overcome by FGF21 treatment. Together, these results are critical to better understand the FGF21 mode of action and add significantly to the concept of FGF21 as a viable and promising therapeutic agent to treat metabolic disease.
Acknowledgments
We thank Dr. Julio Ayala for technical assistance and review of the manuscript. We also thank Wanda Snead, Bakula Trevedi, and Greg Poffenberger in the Vanderbilt University Mouse Metabolic Phenotypic Center (MMPC) Hormone and Analytical Core for measuring glucagon and insulin.
Footnotes
This work was supported by grants from the National Institutes of Health (RO1 DK-50277 to D.H.W., U24 DK-59637 to the Vanderbilt MMPC, and T32 DK-07563 to E.D.B.).
Disclosure Summary: All of the authors have nothing to disclose.
First Published Online May 21, 2009
Abbreviations: 2-DG, 2-[14C]Deoxyglucose; endoRa, endogenous Ra; FGF21, fibroblast growth factor 21; GIR, glucose infusion rate; G-6-Pase, glucose-6-phosphatase; NEFA, nonesterified fatty acids; PPAR, peroxisome proliferator-activated receptor; Ra, glucose appearance; Rd, glucose disappearance; Rg, glucose uptake.
References
- Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M 2008 FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett 582:1725–1730 [DOI] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E 2007 Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 [DOI] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A 2008 FGF21 corrects obesity in mice. Endocrinology 12:6018–6027 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA 2007 Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB 2005 FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ 2007 The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–781 [DOI] [PubMed] [Google Scholar]
- Lundåsen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M 2007 PPARα is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 360:437–440 [DOI] [PubMed] [Google Scholar]
- Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J 2006 Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55:2470–2478 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shanafelt AB 2008 Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs 22:37–44 [DOI] [PubMed] [Google Scholar]
- Zhang BB, Moller DE 2000 New approaches in the treatment of type 2 diabetes. Curr Opin Chem Biol 4:461–467 [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM 2008 FGF21 reverses hepatic steatosis, increases energy expenditure and improves insulin sensitivity in diet-induced obese mice. Diabetes 58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K 2008 FGF21 is an Akt-regulated myokine. FEBS Lett 582:3805–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A 2007 Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARγ signaling. J Cell Physiol 210:1–6 [DOI] [PubMed] [Google Scholar]
- Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK 2008 Adipose fibroblast growth factor 21 is up-regulated by PPARγ and altered metabolic states. Mol Pharmacol 74:403–412 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH 2006 Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55:390–397 [DOI] [PubMed] [Google Scholar]
- Morgan CR, Lazarow A 1962 Immunoassay of insulin using a two-antibody system. Proc Soc Exp Biol Med Society 110:29–32 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH 2007 Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56:1025–1033 [DOI] [PubMed] [Google Scholar]
- Chan TM, Exton JH 1976 A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem 71:96–105 [DOI] [PubMed] [Google Scholar]
- Barzilai N, Rossetti L 1993 Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem 268:25019–25025 [PubMed] [Google Scholar]
- Altszuler N, De Bodo RC, Steele R, Wall JS 1956 Carbohydrate metabolism of hypophysectomized dogs as studied with radioactive glucose. Am J Physiol 187:25–31 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shanafelt AB 2009 FGF21: a novel prospect for the treatment of metabolic diseases. Curr Opin Investig Drugs 10:359–364 [PubMed] [Google Scholar]
- Ferre T, Pujol A, Riu E, Bosch F, Valera A 1996 Correction of diabetic alterations by glucokinase. Proc Natl Acad Sci USA 93:7225–7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajri T, Han XX, Bonen A, Abumrad NA 2002 Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 109:1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EA, Mynatt RL, Cook GA, Kashfi K 1995 Insulin regulates enzyme activity, malonyl-CoA sensitivity and mRNA abundance of hepatic carnitine palmitoyltransferase-I. Biochem J 310(Pt 3):853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F 2006 Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol 574:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, Jewell MM, Powers AC, Wasserman DH 2008 Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 57:1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Kahn R, Robertson RM, Rizza RA 2006 Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Diabetes Care 29:1697–1699 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB 2008 FGF-21/FGF-21 receptor interaction and activation is determined by βKlotho. J Cell Physiol 215:1–7 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M 2007 βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 104:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T 2008 βKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol 22:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G 2008 Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 116:65–68 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A 2008 Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57:1246–1253 [DOI] [PubMed] [Google Scholar]
- Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, Kliewer SA, Browning JD, Burgess SC 2008 Partial resistance to PPARα agonists in Zucker diabetic fatty (ZDF) rats is associated with defective hepatic mitochondrial metabolism. Diabetes 57:2012–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]