Abstract
Increasing evidence suggests that the renin-angiotensin-system contributes to the etiology of obesity. To evaluate the role of the renin-angiotensin-system in energy and glucose homeostasis, we examined body weight and composition, food intake, and glucose tolerance in rats given the angiotensin-converting enzyme inhibitor, captopril (∼40 mg/kg · d). Rats given captopril weighed less than controls when fed a high-fat diet (369.3 ± 8.0 vs. 441.7 ± 8.5 g after 35 d; P < 0.001) or low-fat chow (320.1 ± 4.9 vs. 339.8 ± 5.1 g after 21 d; P < 0.0001). This difference was attributable to reductions in adipose mass gained on high-fat (23.8 ± 2.0 vs. 65.12 ± 8.4 g after 35 d; P < 0.0001) and low-fat diets (12.2 ± 0.7 vs. 17.3 ± 1.3 g after 21 d; P < 0.001). Rats given captopril ate significantly less [3110.3 ± 57.8 vs. 3592.4 ± 88.8 kcal (cumulative 35 d high fat diet intake); P < 0.001] despite increased in neuropeptide-Y mRNA expression in the arcuate nucleus of the hypothalamus and had improved glucose tolerance compared with free-fed controls. Comparisons with pair-fed controls indicated that decreases in diet-induced weight gain and adiposity and improved glucose tolerance were due, primarily, to decreased food intake. To determine whether captopril caused animals to defend a lower body weight, animals in both groups were fasted for 24 h and subsequently restricted to 20% of their intake for 2 d. When free food was returned, captopril and control rats returned to their respective body weights and elicited comparable hyperphagic responses. These results suggest that angiotensin-converting enzyme inhibition protects against the development of diet-induced obesity and glucose intolerance.
Angiotensin-converting enzyme inhibition using captopril prevents diet-induced weight gain and glucose intolerance in rats.
Despite the presence of a rigorous homeostatic system that regulates energy balance with great precision, the incidence of obesity and obesity-related disorders continues to grow (1,2,3). Consequently, determining effective strategies to treat obesity and its comorbidities is a critical problem facing medical science. The renin-angiotensin system (RAS) has emerged as an important target system in this regard (4,5,6,7,8). The RAS is best known for the regulation of hydromineral balance and cardiovascular function and most physiological actions of the RAS are exerted by angiotensin-II (A-II), which is formed from angiotensinogen via cleavage first by renin and then by angiotensin-converting enzyme (ACE). A-II binds to angiotensin type 1 or type 2 receptors in diverse target tissues including adrenal cortex, kidney, vascular smooth muscle, adipose tissue, and brain. Its actions include the release of aldosterone, sodium reabsorption, vasoconstriction, adipocyte hypertrophy, activation of the hypothalamus-pituitary-adrenal axis, and increased drinking (9,10). All of the critical components of the RAS also exist in adipose tissue and brain and A-II generated by these particular tissue-specific RASs has critical roles in adipocyte growth and as a neurotransmitter, respectively (11,12).
Hyperactivity of systemic and adipose tissue-specific RASs is associated with obesity, and the RAS is implicated in the control of glucose homeostasis, providing a potential causal link among obesity, diabetes, and hypertension (13,14,15). Consistent with this, drugs that reduce A-II synthesis (ACE inhibitors) or action (angiotensin receptor blockers) alleviate many symptoms associated with obesity (16,17,18,19), and genetic interference with any critical component of the RAS prevents excessive weight gain in rodent models of obesity (4,6,20,21,22). However, clinical studies directly examining effects of RAS inhibition on energy balance have produced mixed results (23,24,25). There are reports of decreased body weight and adiposity as well as reports no effect of pharmacological RAS interference on energy balance in humans. Nonetheless, even in humans the correlation between RAS activity and adiposity is well documented, whereas the mechanism(s) underlying the contribution of the RAS to obesity and glucose intolerance are unknown (26,27,28,29).
Whereas systemic RAS interference consistently decreases weight gain in rodents, changes of food intake are contradictory (17,19,30). These discrepancies may be attributed to the use of compounds that comparably reduce systemic RAS activity but differentially penetrate the blood-brain barrier to influence central angiotensin receptors. Systemic administration of ACE inhibitors that do not access the brain actually results in elevated central A-II due to increased circulating substrate [angiotensin I (A-I)] and enhanced conversion of A-I to A-II locally within the brain (31,32,33). This is of importance because central A-II inhibition increases food intake, whereas central A-II administration decreases food intake (34,35).
We have reconsidered the role of the RAS in energy and glucose homeostasis by using captopril, an ACE inhibitor that does not effectively cross the blood-brain barrier (31), and assessing food intake, body weight, body fat, and glucose tolerance. We hypothesized that a change in hypothalamic leptin sensitivity could contribute to the alterations in energy balance seen in rats treated with captopril, and we therefore also assessed hypothalamic arcuate nucleus (ARC) molecular targets of leptin, agouti-related peptide (AgRP), neuropeptide-Y (NPY), and proopiomelanocortin C (POMC). However, we found that systemic captopril reduces weight gain and adiposity and improves glucose tolerance in rats primarily by decreasing food intake despite augmented expression of NPY in the ARC, suggesting that hypothalamic leptin sensitivity is not altered. Administration of the ACE inhibitor into the lateral cerebral ventricle [intracerebroventricular (icv)] blunted the anorexic response to peripherally administered ACE, implying that active conversion of A-I to A-II, locally within the brain, contributes to the anorexia of rats given captopril systemically.
Materials and Methods
Animals
Adult male Long Evans rats (Harlan, Indianapolis, IN), weighing 250–300 g on arrival were individually housed and maintained on a 12-h light, 12-h dark cycle (lights on at 0100 h). Unless otherwise noted, rats were given free access to water and food. Rats were fed either high-fat diet (HFD; 40% fat by calories at a density of 4.54 kcal/g; Research Diets, New Brunswick, NJ) or Purina rodent chow (∼5% calories by fat; 3.4 kcal/g). All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Systemic captopril administration
In most experiments, rats were given captopril (Sigma-Aldrich, St. Louis, MO) in their drinking water (∼40 mg/kg body weight · d), allowing more drug to be available when rats were most active and consuming more water. The dose is based on previous studies of captopril and blood pressure and hydromineral balance in rats (31,36). Water intake was monitored daily to ensure that rats received the appropriate dose of captopril. In one experiment, captopril (or saline vehicle) was administered sc via osmotic minipump (ALZET 2ML2; Durect, Cupertino, CA) for 14 d at the same average dose as received in the other experiments. Primed minipumps loaded with captopril or saline were implanted sc in isofluorine-anesthetized rats. A 1.5-cm incision was made in the rostral midscapular region, and osmotic minipumps were inserted through the incision, such that the minipump rested between the scapulae. The wound was closed using wound clips and assessment of food and water intake began immediately.
Assessment of systemic ACE activity
To verify that the dose of captopril used was sufficient to inhibit systemic ACE (i.e. the conversion of A-I to A-II), we assessed the acute drinking response to sc administered A-I (75 μg) in rats administered captopril (∼40 mg/kg · d for 5 d) and free-fed controls.
Plasma renin activity (PRA) and A-I levels
PRA and plasma A-I levels were assessed using 125I RIA kits from DiaSorin (Vercelli, Italy) as previously described (10).
Body composition and fat distribution
Body composition was determined using nuclear magnetic resonance (NMR) technology (Echo NMR, Waco, TX) on unanesthetized rats as previously described (37). Distribution of adipose tissue in the sc and visceral depots was determined using the pelting method in euthanized rats (38). The skin with attached sc layer of adipose tissue was carefully removed from the carcass. The pelt portion and remaining carcass were then assessed separately via NMR to determine the adipose tissue content of each compartment.
Food restriction
Rats fed HFD were matched by body weight (n = 16/group) and received unlaced water or captopril-laced water (∼40 mg/kg · d). After 20 d, rats in each group were subdivided such that half of each group was fasted for 24 h, restricted to 20% of their baseline daily caloric intake for 2 d, and then returned to free food. The other two subgroups had free access to food throughout. Food intake was assessed 1, 2, and 24 h after returning food ad libitum in the restricted groups.
Glucose tolerance tests
Oral (oGTTs) and ip glucose tolerance tests (GTTs) were conducted at selected time points. Both routes of administration were used to ascertain whether the intestinal component (present during oGTTs but not ip GTTs) was of importance to our results. Overnight fasted rats were moved to the procedure room (0700 h), and after 2 h, baseline blood glucose was obtained in duplicate from the tail vein using glucometers and glucose strips (FreeStyle, Alameda, CA) and 250 μl of blood were collected into heparin-containing tubes and placed on ice. Rats then received a 1.5 mg/kg bolus of 50% dextrose via ip injection (ip GTT) or oral-gastric gavage (oGTT). Blood glucose was assessed after 15, 30, 45, 60, and 120 min, and blood samples for plasma insulin analyses were collected after 15, 30, and 60 min. Blood was cold centrifuged and plasma was frozen at −80 C until insulin was assessed using an ELISA (CrystalChem, Inc., Downers Grove, IL), as previously described (39).
RNA isolation and cDNA synthesis
Fasted rats were euthanized and whole brains were removed and placed in ice-cold saline. The ARC-enriched area was quickly dissected, placed in RNAlater (Ambion, Austin, TX; 1.5 ml) and held at −80 C as previously described (39). RNAeasy columns (QIAGEN, Valencia, CA) were used to isolate RNA according to the manufacturer’s instructions. iScript (Bio-Rad, Hercules, CA) was used to synthesize cDNA from 1 μg total RNA.
Semiquantitative real-time PCR
Primer sequences were as follows: L32, forward 5′-CAG-ACG-CAC-CAT-CGA-AGT-TA and reverse 5′-AGC-CAC-AAA-GGA-CGT-GTT-TC at 61.2 C; NPY, forward 5′-CTC-TGC-GAC-ACT-ACA-TCA-A and reverse 5′-GGG-GCA-TTT-TCT-GTG-CTT-T at 61.2 C; AgRP, forward 5′-TTC-CCA-GAG-TTC-TCA-GGT-CTA and reverse 5′-ATC-TAG-CAC-CTC-TGC-CAA-A at 55 C; and POMC, forward 5′-TCC-ATA-GAC-GTG-TGG-AGC-TG and reverse 5′-ACT-TCC-GGG-GAT-TTT-CAG-TC at 57.1 C (IDT, Coralville, IA). Primers were optimized as previously described (40). Samples were run in triplicate using an iCycler (Bio-Rad) and the iQ SYBR Green Supermix (Bio-Rad). Expression patterns of genes of interest were normalized to constitutively expressed ribosomal protein L32 and relative expression was quantified as previously described (40).
Sterotaxic surgery
While under ketamine and xylazine anesthesia, rats were placed in a stereotaxic device and implanted with 22-gauge microinjection cannulae (Plastics One Inc., Roanoke, VA) in the lateral cerebral ventricle (icv) with lambda and bregma at the same vertical coordinate. The coordinates from bregma were as follows: anterior 0.9 mm, lateral 1.4 mm, ventral 3.5 mm. The cannulae were fixed to the skull using anchor screws and dental acrylic. One week of recovery was allowed for body weight to return to presurgical levels before verification of cannula placement by administration of 10 ng A-II (in 1 μl saline). Rats that consumed more than 5 ml water in 1 h were considered hits and included in further studies.
Data analysis
Data were analyzed using GraphPad (Prism, San Diego, CA). Food intake, body weight, body composition, blood glucose, and plasma insulin were assessed using the appropriate ANOVA. Post hoc analyses of main effects and interactions were assessed using the Bonferroni test and area under the curve (AUC) was analyzed using a one-way ANOVA or a t test.
Experimental design
Experiment 1
Rats fed low-fat chow received plain water (controls) or captopril-laced water for 21 d (40 mg/kg · d). One control group had free access to food and another was pair-fed (two rations of food per day) to match the daily intake of the captopril group on the previous day. Body weight, food intake, and body composition were monitored throughout. At the end of the study, rats were fasted for 16 h and euthanized, and brains were collected.
Experiment 2
Rats had plain water or captopril-laced water for 35 d. After 4 d, low-fat chow was replaced with ad libitum HFD for the remainder of the study. Food intakes, body weights, and body composition were monitored throughout. After 35 d, captopril was discontinued and body weights and body composition were monitored for 28 additional days.
Experiment 3
Rats were on the same protocol as in experiment 2 except that they received captopril for 42 d. Glucose tolerance (after 5 and 35 d of captopril), the response to food restriction (after 20 d of captopril) and adipose tissue distribution (after 42 d of captopril) were assessed.
Experiment 4
Rats were maintained on HFD for 10 wk and subsequently assigned to two weight-matched groups receiving plain water or captopril-laced water for 12 d. Intraperitoneal GTTs were performed after 5 d of captopril administration.
Experiment 5
Rats fed HFD received osmotic minipumps (Durect) containing captopril or saline. This route of delivery is referred to as sc administration. Saline controls had free access to food or else were pair fed to captopril rats. Body weight, body composition, and food intake were monitored throughout, and ip GTTs were performed before and after 11 d of captopril administration.
Experiment 6
Rats fed low-fat chow and implanted with icv cannulae were given plain water or captopril-laced water for 5 d. Rats then received an icv infusion of captopril (10 μg in 2 μl saline) or vehicle immediately before the onset of the dark phase, and 2-h food intake was monitored. Infusions were performed using a Hamilton syringe and injector that projects 1-mm past the termination of the cannula.
Results
Chronic oral captopril administration reduces systemic ACE activity and increases PRA and plasma A-I levels
Administration of captopril via the drinking water blunted the increase in water intake after sc administered A-I, implying that systemic ACE activity was reduced (supplemental Fig. S1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Consistent with the captopril causing a decrease of plasma A-II, PRA, and plasma A-I levels were increased in captopril-treated rats relative to free-fed and pair-fed controls (supplemental Fig. S1, B and C).
Chronic oral captopril reduces food intake, body mass gain, and adipose mass gain in rats fed low-fat chow
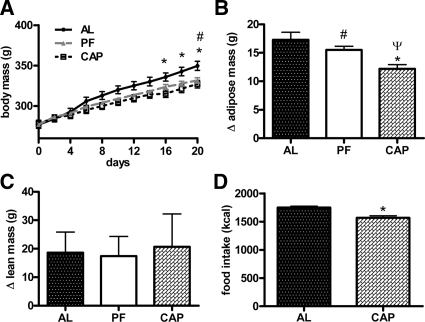
At the start of the experiment, rats weighed 277.8 ± 2.1 g and had a mean adipose mass of 15.2 ± 0.8 g. Administration of captopril via the drinking water resulted in decreased body mass relative to ad libitum-fed controls (Fig. 1A). This difference became significant on d 16 and persisted throughout (320.1 ± 4.9 vs. 339.8 ± 5.1 g; P < 0.0001). There was no difference in weight between the captopril and the pair-fed control groups (320.1 ± 4.9 vs. 328.3 ± 2.8 g; P > 0.05). After 21 d of captopril, rats gained significantly less fat mass than free-fed and pair-fed controls [12.2 ± 0.7 vs. 17.3 ± 1.3 g (P < 0.001) and 15.5 ± 0.6 g (P < 0.05), respectively; Fig. 1B]. There were no differences of lean mass (Fig. 1C) or body water content among the groups.
Figure 1.
Mean body mass (A), change in adipose mass (B), and change in lean mass (C) of low-fat chow-fed rats given captopril in their drinking water (∼40 mg/kg · d) for 21 d and controls. D, Mean cumulative food intake over 21 d. AL, Free-fed controls; PF, pair-fed controls; CAP, captopril-treated rats. *, CAP significantly different from AL (P < 0.05); ψ, CAP significantly different from PF (P < 0.05); #, PF significantly different from AL (P < 0.05) (n = 10/group). Bars, 1 sem.
Consistent with previous reports (31,32,33), captopril increased water intake (37.3 ± 1.9 vs. 29.4 ± 1.1 ml/d; P < 0.0001). Mean 21-d cumulative food intake was reduced in the captopril group relative to the ad libitum-fed controls (1568.7 ± 37.8 vs. 1749.0 ± 25.9 kcal; P < 0.001; Fig. 1D).
Oral captopril protects against diet-induced obesity
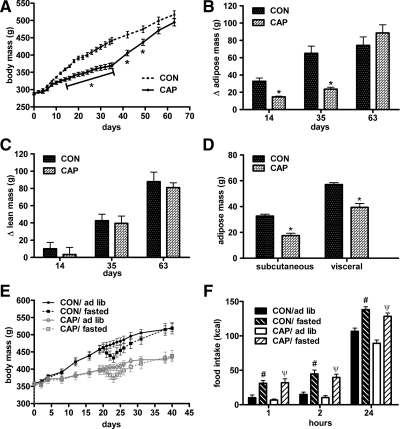
Captopril caused a decrease in weight gain in rats given free access to HFD (Fig. 2A). Captopril rats weighed significantly less than controls by d 14. On d 35 captopril rats weighed 16% less than controls (369.3 ± 8.0 vs. 441.7 ± 8.5 g; P < 0.001) and had gained significantly less fat mass than controls (23.8 ± 2.0 vs. 65.12 ± 8.4 g; P < 0.0001; Fig. 2B). Captopril rats had less sc (Fig. 2D) as well as visceral fat. The relative reduction of adipose mass gain was greater in captopril rats fed the HFD than those fed chow (18.2 ± 0.88 vs. 5.5 ± 0.76 g; P < 0.0001; Figs. 1B and 2B). Lean mass was not altered (Fig. 2C). When captopril was discontinued, rats quickly regained body weight and adipose mass to control levels (Fig. 2, A and B).
Figure 2.
Mean body mass (A), change in adipose mass (B), and change in lean mass (C) of rats given captopril (CAP) and controls (CON) during and after captopril administration. D, The distribution of adipose mass in the sc vs. visceral depots in rats given captopril for 42 d and controls. E, Mean body mass in rats given captopril and controls fed the HFD ad libitum or subjected to a food-restriction paradigm starting on d 20 of captopril administration. F, Food intakes of these rats given captopril after the food restriction paradigm when food was returned ad libitum. *, Significantly different from controls (P < 0.05); #, CON/fasted group is significantly different from CON/ad libitum-fed group (P < 0.05); ψ, CAP/fasted group is significantly different from CAP/ad libitum-fed group (P < 0.05) (n = 8/group). Bars, 1 sem.
Captopril rats consumed more water than controls (36.4 ± 1.74 vs. 25.9 ± 0.58 ml/d; P < 0.0001). Cumulative food intake (35 d) was decreased in the captopril rats (3110.3 ± 57.8 vs. 3592.4 ± 88.8 kcal; P < 0.001). The difference in calories consumed was comparable with the difference in calories gained as adipose tissue between captopril and control rats, suggesting that attenuation of adipose mass gained was attributable to decreased food intake in captopril rats.
Both control and captopril rats defended their respective body weights in response to food restriction (Fig. 2E), i.e. both groups had a hyperphagic response when returned to ad libitum food (Fig. 2F). Mean daily water intake did not change during restriction, indicating that the captopril dose received was not altered (37.2 ± 3.5 vs. 35.6 ± 3.2 ml/d; P = 0.74).
Subcutaneous captopril administration prevents diet-induced body and adipose mass gain
Subcutaneous captopril (40 mg/kg · d via minipump) led to reduced body weight relative to free-fed controls over 14 d (416.7 ± 6.9 vs. 462.7 ± 13.5 g; P < 0.0001). Pair-fed controls (432.8 ± 6.0 g) had intermediate weights, indicating that the decreased body weight was, in part, a result of decreased food intake. Captopril rats lost more body fat (−7.3 ± 1.9 g) than pair-fed controls (0.83 ± 1.8 g; P < 0.05) and free-fed controls (15.1 ± 1.8 g; P < 0.001), and pair-fed rats gained significantly less fat than free-feeding controls (P < 0.001). Lean mass was not significantly altered (6.6 ± 10.0 vs. 29.3 ± 11.4 and −1.4 ± 7.9 g for free fed and pair fed controls, respectively).
Water intake was increased and 14-d cumulative food intake was reduced in rats given sc captopril (1195 ± 37.2 vs. 1495 ± 46.1 g; P < 0.001).
Oral captopril reduces body weight and fat mass in diet-induced obese rats
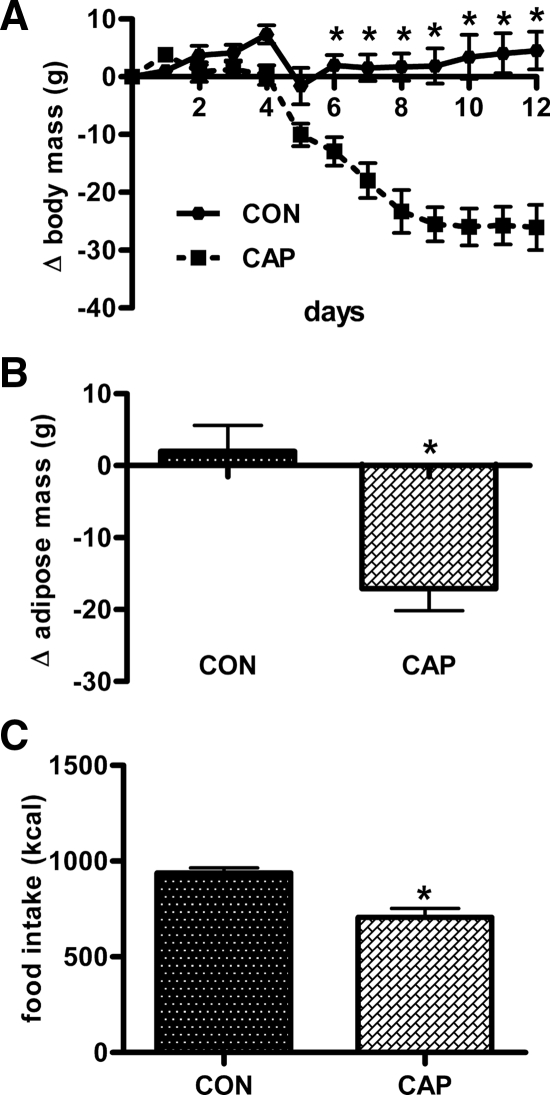
After 10 wk on HFD, rats weighed 565.0 ± 13.6 g and had an adipose mass of 140.8 ± 11.5 g, compared with rats on chow (see body weight of chow rats in experiment 1). When subsequently receiving captopril in their water for 12 d, these rats lost more weight (−26.1 ± 3.9 vs. 4.5 ± 3.3 g; P < 0.0001; Fig. 3A) and adipose mass (−17.1 ± 3.1 vs. 2.0 ± 3.6 g; P < 0.01; Fig. 3B) than controls. Lean mass was not altered (4.14 ± 4.11 vs. 12.83 ± 4.07 g; P > 0.05). Captopril reduced 12-d cumulative food intake (937.1 ± 27.5 vs. 706.1 ± 45.8 kcal; P < 0.01; Fig. 3C) and increased water intake.
Figure 3.
Mean body mass (A), change in adipose mass (B), and cumulative food intake (C) in rats given captopril (CAP; ∼40 mg/kg · d in their drinking water) and controls (CON). Rats were previously rendered obese by the consumption of HFD. *, significantly different from controls (P < 0.05) (n = 8/group). Bars, 1 sem.
Captopril protects against diet-induced impairment of glucose tolerance
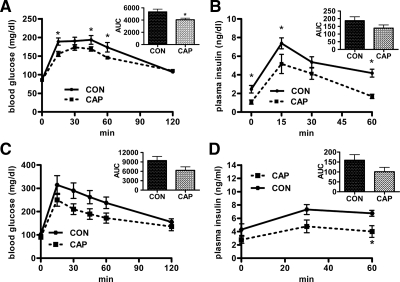
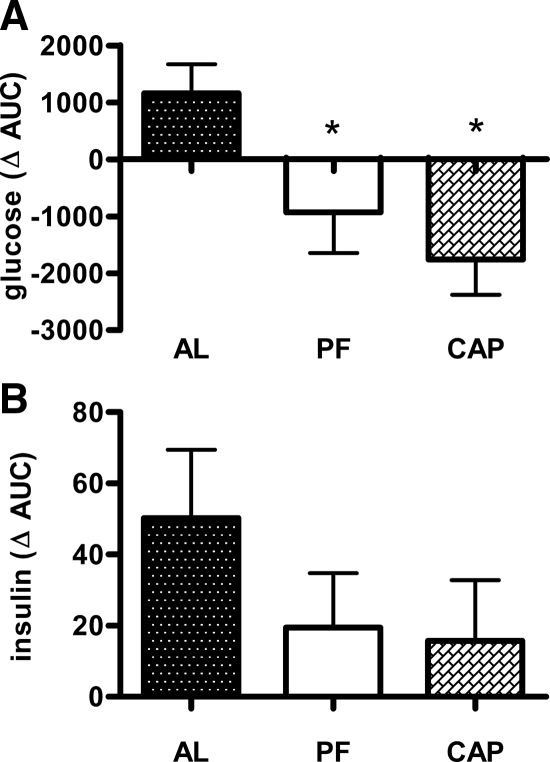
An ip GTT was conducted after 5 d of oral captopril at a time when body weights were not different. The glucose AUC did not differ between the groups (supplemental Fig. S2A). However, the water intake and thus the approximate amount of captopril consumed varied among rats over the 24-h period before the test. When the approximate dose received was plotted against peak glucose achieved, a negative correlation was observed (supplemental Fig. S2B; r2 = 0.53; P < 0.05).
After 5 wk captopril rats weighed less and had less fat than controls; and they had significantly improved glucose tolerance in an oGTT, i.e. captopril rats had a smaller AUC and lower absolute glucose levels at 15, 45, and 60 min after glucose administration (Fig. 4A). Plasma insulin during the oGTT was significantly lower in the captopril rats, implying that they were more insulin sensitive (Fig. 4B).
Figure 4.
The effect of captopril (CAP) administration (∼40 mg/kg · d in their drinking water) on glucose tolerance in rats fed the HFD. In A and B rats were given access to HFD starting on d 4 of captopril administration, whereas in C and D rats were previously rendered obese via the consumption of HFD and were maintained on HFD throughout. Blood glucose levels (A) and plasma insulin levels (B) of rats during an oGTT in rats given captopril for 35 d and controls (CON) and the AUC of these blood glucose (A; inset) and plasma insulin (B; inset) responses (n = 10/group); the blood glucose (C) and plasma insulin (D) response during the ip GTT of rats given captopril for 5d and controls. (n = 8/group) The AUC of the blood glucose (E; inset) and plasma insulin (F; inset) response to the ip GTT. *, Significantly different from controls (P < 0.05). Bars, 1 sem.
In rats already rendered obese by 10 wk on HFD, oral captopril (5 d) improved glucose tolerance (Figs. 4, C and D). This was at a time when food intake but not body weight was reduced. Although blood glucose was not reliably different between groups (Fig. 4C), significantly less insulin was necessary to clear the glucose in captopril-treated rats (Fig. 4D).
Glucose tolerance was also assessed in rats given captopril sc. After 11 d, captopril rats had a reduced blood glucose excursion relative to free-fed controls (Fig. 5A). Pair-fed controls had a comparably reduced glucose AUC, implying that the improved glucose tolerance was secondary to decreased food intake. Plasma insulin did not differ among groups (ΔAUC; Fig. 5B).
Figure 5.
The effect of sc captopril administration (∼40 mg/kg · d sc) on glucose tolerance in rats fed the HFD. Change in AUC of the blood glucose response (A) and the plasma insulin response (B) to the ip GTT from d 0 to d 11 in rats given captopril and controls. AL, Free-fed controls; PF, pair-fed controls; CAP, captopril-treated rats. *, Significantly different from the AL (P < 0.05) (n = 10/group). Bars, 1 sem.
Captopril increases NPY mRNA expression in the ARC
Captopril rats had elevated NPY mRNA in the ARC relative to free-fed and pair-fed controls (supplemental Fig. S3A). There were no differences of AgRP or POMC mRNA levels among the groups (supplemental Fig. S3, B and C).
A reduction in brain ACE activity using icv captopril blunts the anorexic effect of systemically administered captopril
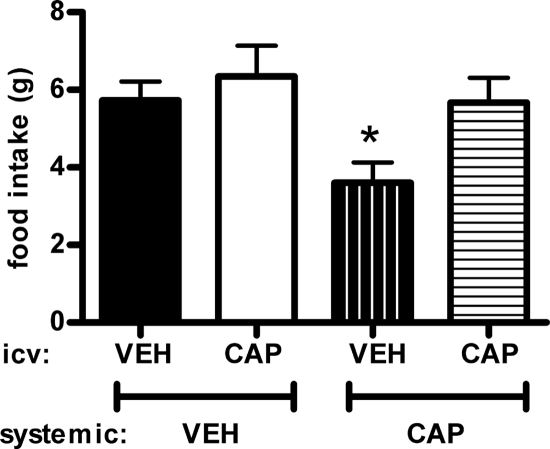
Consistent with the previous experiments, when saline was administered icv, rats receiving captopril-laced drinking water had reduced food intake during the first 2 h of the dark compared with controls (Fig. 6), However, when rats drinking captopril-laced water additionally received captopril icv (10 μg), the anorexia elicited by systemically administered captopril was blunted. This icv dose of captopril had no significant effect on food intake in rats given plain drinking water.
Figure 6.
The effect of icv captopril administration on food intake in rats administered captopril-laced drinking water (∼40 mg/kg · d) for 5 d or plain drinking water. Rats were fed low-fat chow. Immediately before the onset of dark, rats were additionally given icv captopril (CAP) or saline vehicle (VEH), and 2-h food intake was monitored. *, Significantly different from all other groups (P < 0.05) (n = 12/group). Bars, 1 sem.
Discussion
The key findings of this study are that systemic reduction of RAS activity through chronic inhibition of ACE results in decreased body weight and fat mass gain, as well as improved glucose tolerance, in rats without impairing lean tissue growth. The decreases in body mass and adiposity (relative to controls) are more pronounced in rats given free access to HFD than standard low-fat chow, and they occur with concomitant decreases in energy consumption. Accordingly, decreases in food intake primarily explain the protection against diet-induced weight gain in this model. Systemic ACE inhibition by captopril resulted in an actual loss of body weight in rats previously rendered obese by the consumption of HFD, implying that targeting the RAS may be a viable approach to treat already-established obesity. Moreover, the present data suggest that protection against diet-induced glucose intolerance in rats given captopril is also dependent on food intake. These data support a role for the RAS in energy balance and glucose homeostasis.
Several recent studies have examined effects of pharmacological RAS interference on energy balance and glucose homeostasis in rats. Although the age and strain of rats, as well as the compound used to target the RAS varied, results concerning body weight and adiposity were consistent with those presented here (17,19,30). Conversely, experiments evaluating the effect of RAS inhibition on food intake have produced mixed results, which may be attributed to the differential ability of the ACE inhibitors at the various doses to access brain nuclei involved in energy balance. For example, administration of the ACE inhibitor, enalapril, resulted in reduced body weight and body adiposity in young Wistar rats, and this occurred with a concomitant decrease in caloric intake (17). However, in Sprague Dawley rats, the administration of another ACE inhibitor, perindopril, resulted in decreased energy consumption when given the ACE inhibitor from birth but had no effect in 10-wk-old rats (19,30).
In all of these experiments, body weight was reduced in rats given systemic captopril, relative to free-fed controls, and this was primarily due to a profound decrease in adipose mass in both the sc and visceral compartments (relative to controls). The reduction in body weight and fat was most pronounced in rats fed a HFD and was likely due to both direct effects on adipose tissue and decreased energy consumption. As highlighted in recent studies and reviews, adipocytes express all critical components of the RAS and A-II is believed to promote adipose tissue growth (7,11,41). Moreover, adipocyte expression of angiotensinogen (AGT) is elevated in obese rodents and humans (28,29,42). Consistent with A-II’s trophic role in adipose tissue, mice lacking any critical component of the RAS are lean, whereas rodents selectively overexpressing AGT in adipose tissue have increased adiposity (4,20,21,22,43,44). Specifically, AGT-deficient (AGT−/−) mice exhibit profound adipocyte hypotrophy and decreased fatty acid synthase activity (4), whereas targeted overexpression of AGT in adipocytes in AGT−/− and wild-type mice produces the opposite effect (43). A-II has also been implicated as a proangiogenic factor, and pharmacological interference with the RAS decreases tumor angiogenesis (45,46). This is of importance because for adipose tissue to expand, angiogenesis must occur within the tissue to provide adequate oxygen and nutrients. Factors that inhibit angiogenesis also inhibit adipose tissue expansion (47,48), and it is possible that this may play a role in the decreased adiposity in our model.
Given all of the studies that highlight a role for A-II in adipose tissue expansion and the fact that rats given captopril have significantly less adipose mass than their pair-fed controls, it is likely that the reduced fat mass that we observed is due, in part, to decreased adipose A-II. It is important to note, however, that ACE also cleaves bradykinin into inactive degradation products, and we cannot rule out a role for this process in our results. Nonetheless, the administration of angiotensin receptor blockers also results in the prevention of diet-induced obesity (16,49), suggesting that the effects of ACE inhibitors on metabolism are due to antagonism of the RAS. These results are consistent with studies examining effects of pharmacological or genetic RAS interference on metabolism and support a role for ACE in the growth of adipose tissue (17,19,30).
In addition to previously explored peripheral mechanisms of the captopril-induced decreased body weight and adiposity, the central nervous system, either directly or indirectly, must be involved in the potent effects of captopril administration on food intake. Consistent with previous studies, water intake was significantly increased in rats given captopril (31,32,33). This seemingly paradoxical increase in water consumption in rats given captopril has been attributed to increased formation of A-II in the brain, specifically in circumventricular organs (31,32,33). Systemic captopril inhibits the conversion of A-I to A-II in the periphery, such that plasma A-I increases. Furthermore, due to its hydrophilic properties, captopril does not readily cross the blood-brain barrier, and the increase in circulating substrate (A-I) combined with active central ACE then leads to elevated central A-II and, consequently, augmented water intake. Despite profound increases in water intake, cumulative food intake was decreased in rats given captopril. As with the increase in water intake, the decreased food intake may also be a result of increased central A-II. Direct central administration of A-II reportedly suppresses food intake and increases water intake (35). Conversely, inhibition of the RAS in the brain via transgenic expression of AGT antisense oligonucleotides in glial cells results in increased energy consumption (34). Interestingly, in our study, the icv captopril-induced reduction of central conversion of A-I to A-II in rats given systemic captopril does indeed attenuate the anorectic effect of systemic captopril. The inability of captopril to readily access the brain, coupled with the chronic access to HFD, may underlie the more pronounced effects seen in our model relative to some previous studies using other ACE inhibitors to examine the RAS involvement in energy homeostasis (17,19,30).
Although numerous circuits in the brain influence energy homeostasis, several peptides in the ARC are thought to have a key role (1,2). Specifically, ARC NPY and AgRP are anabolic, eliciting increased food intake and body weight, whereas ARC POMC and its product α-MSH are catabolic, reducing food intake and body weight. The reduction in food intake elicited by systemic captopril occurred despite elevated NPY mRNA expression in the ARC. The elevated ARC NPY is consistent with a centrally mediated compensatory response to negative energy balance, i.e. it might be expected that rats given captopril would consume more rather than less energy relative to free-fed controls due to the elevated ARC NPY. Rather, rats given captopril systemically actually consumed less energy than controls, and therefore, it is unlikely that an alteration in this system underlies the negative energy balance in this model.
Humans and animals have a rigorous homeostatic system that balances energy consumption with energy expenditure such that in any given environment, a specific body weight (or amount of body fat) tends to be defended. The present data suggest that rats given captopril maintain this ability, although the amount of body fat they defend is significantly below that of controls. Upon return of free access to HFD after food restriction, both captopril-treated and control rats had comparable hyperphagic responses and quickly returned to the body weight of their free-fed counterparts.
Another finding of this report is that ACE inhibition via systemic captopril administration leads to improved glucose tolerance that appears to be secondary to reduced food intake and reduced body weight. Improvements in glucose tolerance were seen regardless of whether glucose was administered ip or via oral gastric-gavage. Consistent with the improved glucose tolerance being secondary to alterations in food intake, the impairment of glucose tolerance prevalent in rats after 11 d of free access to HFD was comparably blunted in captopril-treated rats and pair-fed controls. This is of interest because several recent studies examined the use of ACE inhibitors and angiotensin receptor blockers on glucose tolerance and insulin sensitivity and have reported mixed results (18,50,51,52,53,54). From previous studies, proposed mechanisms underlying improvements in glucose homeostasis are plentiful and include improving muscle and/or islet blood flow through vasodilation, decreasing sympathetic nerve activity, enhancing insulin signaling, partial peroxisomal proliferator-activated receptor-γ activity of some RAS inhibitors, and direct effects on adipose tissue (18,51,52,53,54).
The associations between obesity, hypertension, and diabetes are well established, and the RAS may provide a link among them (13,14,15). In humans, positive correlations have been observed between PRA, as well as plasma and adipose tissue angiotensinogen levels, with body mass index and blood pressure (26,55,56). Moreover, RAS inhibition improves many deleterious consequences of obesity in a clinical setting (51,52,57,58). Interfering with the RAS using angiotensin receptor blockers or ACE inhibitors is a common therapeutic option for hypertensive patients and, consequently, these agents are currently approved for clinical use. More recently, the administration of these agents has also been recognized as an effective strategy for improving insulin sensitivity (51,52,57,58). Analogously, interfering with the RAS reduces the incidence of type 2 diabetes in patients with cardiovascular disorders (51,52). There are a number of reports of body weight and composition alterations in hypertensive patients during ACE inhibition; however, the mechanism(s) for these changes have not been unequivocally discerned, nor are these results constant among clinical reports and the different pharmacological agents used (23,24,25). Nevertheless, consistent with several recent reports (17,19,30), the present data provide additional support for a role for the RAS in the control of energy balance and the potential for beneficial effects of captopril as a therapeutic strategy for patients with obesity and concomitant hypertension.
Supplementary Material
Footnotes
This work was supported by the following National Institutes of Health and National Science Foundation Grants/Fellowships NSF-DGE-IGERT-0333377 (to A.D.d.K.), DK79710 (to E.G.K.), DK68273 (to R.R.S.), DK66596 (to R.R.S.), DK56863 (to R.J.S.), DK073505 (to R.J.S.), and DK078201 (to S.C.W.).
Disclosure Summary: A.D.d.K., E.G.K., D.-H.K., R.R.S., and S.C.W. have nothing to declare. R.J.S. has received grant support from Zafgen and Ethicon Endo-Surgery and also currently has stock/stock options with Zafgen and consults for Zafgen, Eli Lilly, and Ethicon Endo-Surgeries.
First Published Online June 4, 2009
Abbreviations: ACE, Angiotensin-converting enzyme; AgRP, agouti-related peptide; AGT, angiotensinogen; A-I, angiotensin I; A-II, angiotensin-II; ARC, arcuate nucleus; AUC, area under the curve; GTT, glucose tolerance test; HFD, high-fat diet; icv, intracerebroventricular; NMR, nuclear magnetic resonance; NPY, neuropeptide-Y; oGTT, oral glucose tolerance test; POMC, proopiomelanocortin C; PRA, plasma renin activity; RAS, renin-angiotensin system.
References
- Woods SC, Seeley RJ, Porte Jr D, Schwartz MW 1998 Signals that regulate food intake and energy homeostasis. Science 280:1378–1383 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, Fukamizu A, Negrel R, Ailhaud G, Teboul M 2001 Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology 142:5220–5225 [DOI] [PubMed] [Google Scholar]
- Weisinger RS, Begg DP, Chen N, Jois M, Mathai ML, Sinclair AJ 2007 The problem of obesity: is there a role for antagonists of the renin-angiotensin system? Asia Pac J Clin Nutr 16:359–367 [PubMed] [Google Scholar]
- Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, Egan GF, McKinley MJ, Rodger PD, Sinclair AJ, Wark JD, Weisinger HS, Jois M, Weisinger RS 2008 Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci USA 105:6531–6536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiéra F, Sharma AM 2003 The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol 35:807–825 [DOI] [PubMed] [Google Scholar]
- Cassis LA, Marshall DE, Fettinger MJ, Rosenbluth B, Lodder RA 1998 Mechanisms contributing to angiotensin II regulation of body weight. Am J Physiol Endocrinol Metab 37:E867–E876 [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT 1998 Angiotensin, thirst, and sodium appetite. Physiol Rev 78:583–686 [DOI] [PubMed] [Google Scholar]
- Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR 2008 Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology 149:6416–6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassis LA, Police SB, Yiannikouris F, Thatcher SE 2008 Local adipose tissue renin-angiotensin system. Curr Hypertens Rep 10:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C 1997 Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18:383–439 [DOI] [PubMed] [Google Scholar]
- Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA 2004 Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol 287:R943–R949 [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BMW 2007 Renin-angiotensin system and cardiovascular risk. Lancet 369:1208–1219 [DOI] [PubMed] [Google Scholar]
- Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH 1991 Association of the renin sodium profile with the risk of myocardial-infarction in patients with hypertension. N Engl J Med 324:1098–1104 [DOI] [PubMed] [Google Scholar]
- Mori Y, Itoh Y, Tajima N 2007 Angiotensin II receptor blockers downsize adipocytes in spontaneously type 2 diabetic rats with visceral fat obesity. Am J Hypertens 20:431–436 [DOI] [PubMed] [Google Scholar]
- Santos EL, de Picoli Souza K, Guimarães PB, Reis FCG, Silva SMA, Costa-Neto CM, Luz J, Pesquero JB 2008 Effect of angiotensin converting enzyme inhibitor enalapril on body weight and composition in young rats. Int Immunopharmacol 8:247–253 [DOI] [PubMed] [Google Scholar]
- Shiuchi T, Iwai M, Li H-S, Wu L, Min L-J, Li J-M, Okumura M, Cui T-X, Horiuchi M 2004 Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension 43:1003–1010 [DOI] [PubMed] [Google Scholar]
- Weisinger HS, Begg DP, Egan GF, Jayasooriya AP, Lie F, Mathai ML, Sinclair AJ, Wark JD, Weisinger RS 2008 Angiotensin converting enzyme inhibition from birth reduces body weight and body fat in Sprague-Dawley rats. Physiol Behav 93:820–825 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Li F, Hua K, Deng J, Wang C-H, Bowers RR, Bartness TJ, Kim H-S, Harp JB 2007 Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab 6:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y 2005 Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology 146:3481–3489 [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A 2005 Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes 54:991–999 [DOI] [PubMed] [Google Scholar]
- Berne C 1991 Metabolic effects of ACE inhibitors. J Intern Med Suppl 735:119–125 [PubMed] [Google Scholar]
- Dominguez JR, de la Calle H, Hurtado A, Robles RG, Sancho-Rof J 1986 Effect of converting enzyme inhibitors in hypertensive patients with non-insulin-dependent diabetes mellitus. Postgrad Med J 62(Suppl 1):66–68 [PubMed] [Google Scholar]
- Shimabukuro M, Tanaka H, Shimabukuro T 2007 Effects of telmisartan on fat distribution in individuals with the metabolic syndrome. J Hypertens 25:841–848 [DOI] [PubMed] [Google Scholar]
- Cooper R, Forrester T, Ogunbiyi O, Muffinda J 1998 Angiotensinogen levels and obesity in four black populations. ICSHIB Investigators. J Hypertens 16:571–575 [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM 2005 Weight loss and the renin-angiotensin-aldosterone system. Hypertension 45:356–362 [DOI] [PubMed] [Google Scholar]
- Hainault I, Nebout G, Turban S, Ardouin B, Ferre P, Quignard-Boulange A 2002 Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese (fa/fa) Zucker rat. Am J Physiol Endocrinol Metab 282:E59–E66 [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Mark AL, Haynes WG, Sigmund CD 2004 Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab 286:E891–E895 [DOI] [PubMed] [Google Scholar]
- Mathai ML, Naik S, Sinclair AJ, Weisinger HS, Weisinger RS 2008 Selective reduction in body fat mass and plasma leptin induced by angiotensin-converting enzyme inhibition in rats. Int J Obes (Lond) 32:1576–1584 [DOI] [PubMed] [Google Scholar]
- Rowland NE, Fregly MJ 1988 Comparison of the effects of the dipeptidyl peptidase inhibitors captopril, ramipril, and enalapril on water-intake and sodium appetite of Sprague-Dawley rats. Behav Neurosci 102:953–960 [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Genest J 1982 Mechanism of captopril-induced drinking. Am J Physiol Regul Integr Comp Physiol 242:R136–R140 [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, Fitts DA, Simpson JB 1989 Angiotensin-converting enzyme in subfornical organ mediates captopril-induced drinking. Behav Neurosci 103:1302–1310 [DOI] [PubMed] [Google Scholar]
- Kasper SO, Carter CS, Ferrario CM, Ganten D, Ferder LF, Sonntag WE, Gallagher PE, Diz DI 2005 Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin-angiotensin systems. Physiol Genomics 23:311–317 [DOI] [PubMed] [Google Scholar]
- Porter JP, Potratz KR 2004 Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol 287:R422–R428 [DOI] [PubMed] [Google Scholar]
- Sun Y, Mendelsohn FA 1991 Angiotensin converting enzyme inhibition in heart, kidney, and serum studied ex vivo after administration of zofenopril, captopril, and lisinopril. J Cardiovasc Pharmacol 18:478–486 [DOI] [PubMed] [Google Scholar]
- Taicher GZ, Tinsley FC, Reiderman A, Heiman ML 2003 Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem 377:990–1002 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC 2006 Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55:978–987 [DOI] [PubMed] [Google Scholar]
- Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ 2008 Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab 294:E630–E639 [DOI] [PubMed] [Google Scholar]
- Kim DH, Sandoval D, Reed JA, Matter EK, Tolod EG, Woods SC, Seeley RJ 2008 The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab 295:E1038–E1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saye JA, Cassis LA, Sturgill TW, Lynch KR, Peach MJ 1989 Angiotensinogen gene expression in 3T3-L1 cells. Am J Physiol 256:C448–C451 [DOI] [PubMed] [Google Scholar]
- Safonova I, Aubert J, Negrel R, Ailhaud G 1997 Regulation by fatty acids of angiotensinogen gene expression in preadipose cells. Biochem J 322:235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Urs S, Massiera F, Wortmann P, Joshi R, Heo YR, Andersen B, Kobayashi H, Teboul M, Ailhaud G, Quignard-Boulangé A, Fukamizu A, Jones BH, Kim JH, Moustaid-Moussa N 2002 Effects of high-fat diet, angiotensinogen (agt) gene inactivation, and targeted expression to adipose tissue on lipid metabolism and renal gene expression. Horm Metab Res 34:721–725 [DOI] [PubMed] [Google Scholar]
- Massiéra F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M 2001 Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 15:2727–2729 [DOI] [PubMed] [Google Scholar]
- Anandanadesan R, Gong Q, Chipitsyna G, Witkiewicz A, Yeo CJ, Arafat HA 2008 Angiotensin II induces vascular endothelial growth factor in pancreatic cancer cells through an angiotensin II type 1 receptor and ERK1/2 signaling. J Gastrointest Surg 12:57–66 [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Sidman RL, Pasqualini R, Arap W 2008 Does the renin-angiotensin system participate in regulation of human vasculogenesis and angiogenesis? Cancer Res 68:9112–9115 [DOI] [PubMed] [Google Scholar]
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W 2004 Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10:625–632 [DOI] [PubMed] [Google Scholar]
- Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ 2002 Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 99:10730–10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H 2006 Telmisartan prevents obesity and increases the expression of uncoupling protein 1 in diet-induced obese mice. Hypertension 48:51–57 [DOI] [PubMed] [Google Scholar]
- Tabbi-Anneni I, Buchanan J, Cooksey RC, Abel ED 2008 Captopril normalizes insulin signaling and insulin-regulated substrate metabolism in obese (ob/ob) mouse hearts. Endocrinology 149:4043–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ 2004 Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 2. Overview of physiological and biochemical mechanisms. Diabetes Metab 30:498–505 [DOI] [PubMed] [Google Scholar]
- Scheen AJ 2004 Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta-analysis of randomised clinical trials. Diabetes Metab 30:487–496 [DOI] [PubMed] [Google Scholar]
- Iwai M, Chen R, Imura Y, Horiuchi M 2007 TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens 20:579–586 [DOI] [PubMed] [Google Scholar]
- Erbe DV, Gartrell K, Zhang YL, Suri V, Kirincich SJ, Will S, Perreault M, Wang S, Tobin JF 2006 Molecular activation of PPARγ by angiotensin II type 1-receptor antagonists. Vascul Pharmacol 45:154–162 [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, Corvol P 1992 Molecular basis of human hypertension: role of angiotensinogen. Cell 71:169–180 [DOI] [PubMed] [Google Scholar]
- Licata G, Scaglione R, Ganguzza A, Corrao S, Donatelli M, Parrinello G, Dichiara MA, Merlino G, Cecala MG 1994 Central obesity and hypertension. Relationship between fasting serum insulin, plasma renin activity, and diastolic blood pressure in young obese subjects. Am J Hypertens 7:314–320 [DOI] [PubMed] [Google Scholar]
- Parhofer KG, Münzel F, Krekler M 2007 Effect of the angiotensin receptor blocker irbesartan on metabolic parameters in clinical practice: the DO-IT prospective observational study. Cardiovasc Diabetol 6:36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie EL, White CM, Kardas M, Lindberg M, Coleman CI 2005 The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 28:2261–2266 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.