Abstract
The purpose of this article is to encourage research investigating the role of measured gene-environment interaction (G × E) in the etiology of posttraumatic stress disorder (PTSD). PTSD is uniquely suited to the study of G × E as the diagnosis requires exposure to a potentially-traumatic life event. PTSD is also moderately heritable; however, the role of genetic factors in PTSD etiology has been largely neglected both by trauma researchers and psychiatric geneticists. First, we summarize evidence for genetic influences on PTSD from family, twin, and molecular genetic studies. Second, we discuss the key challenges in G × E studies of PTSD and offer practical strategies for addressing these challenges and for discovering replicable G × E for PTSD. Finally, we propose some promising new directions for PTSD G × E research. We suggest that G × E research in PTSD is essential to understanding vulnerability and resilience following exposure to a traumatic event.
Keywords: posttraumatic stress disorder, trauma, genetics, gene-environment interaction
Introduction
Posttraumatic stress disorder (PTSD) is unique among the mental disorders in that it requires exposure to a potentially-traumatic life event (Criterion A). PTSD is further defined by the presence of three symptom clusters: reexperiencing, avoidance and numbing, and arousal [3]. Individuals who develop PTSD are at substantially increased risk for unemployment, marital instability, and health problems [67, 82]. However, whereas a majority of individuals are exposed to a traumatic event over the course of their life, only a minority of those exposed to traumatic events develop PTSD [68]. The conditional risk of PTSD ranges from 8–24% depending on the type of trauma exposure [10, 68], with interpersonal traumatic events (e.g., sexual assault) associated with greater risk than accidental events (e.g., motor vehicle accidents).
Gene-environment interaction occurs when the effect of genotype on risk for a disorder differs by the presence or absence of an environmental pathogen or vice versa. The pattern observed in epidemiologic research on PTSD, in which only a subset of individuals exposed to an environmental pathogen display secondary psychiatric morbidity, is one indicator of a potential gene-environment interaction (G × E) [97]. In addition to evidence for heterogeneity in the influence of environmental factors, the potential for G × E is further indicated under conditions where genetic factors do not completely explain the disorder. These conditions are both met in the case of PTSD. Nonetheless, the role of G × E in PTSD etiology has been largely neglected by both trauma researchers and psychiatric geneticists.
We believe the study of PTSD presents both unique challenges and opportunities for researchers interested in measured G × E in psychiatry. For example, genetically similar individuals may fail to show symptoms of PTSD simply because they have not been exposed to a potentially-traumatic event. Thus, unlike genetic studies of other common psychiatric disorders, genetic studies of PTSD must take exposure to an environmental pathogen (i.e., trauma exposure) into account or risk potentially attenuating estimates of genetic effects. On the other hand, unlike in most psychiatric conditions, the defining environmental pathogen is known in PTSD, permitting more precise measurement of the proximal environmental stressor(s). The presence of an identifiable event affords measurement of characteristics of the trauma (e.g., type of event, duration of exposure, severity, intentionality), estimates of prior levels of functioning, and developmental timing of exposure (including both age at exposure and time lapsed between trauma exposure and assessment).
The purpose of this article is to contextualize G × E studies of PTSD within the landscape of extant genetic research and to promote informed, measured G × E research in PTSD. First, we summarize evidence for genetic influences on PTSD from family, twin, and molecular genetic studies. Second, we discuss the key challenges in G × E studies of PTSD and offer practical strategies for addressing these challenges and for discovering replicable G × Es for PTSD. Finally, we propose some promising new directions for PTSD G × E research and suggest that G × E research in PTSD is essential to understanding vulnerability and resilience following trauma exposure.
Evidence for genetic influences on PTSD
Returning to previously described signs of possible G × E, one of the primary indicators is evidence for genetic influences on the disorder. If risk for PTSD is partially explained by genetic factors, biological relatives (family members) of individuals with PTSD should have a higher prevalence of PTSD than non-relatives. Moreover, among biological relatives of individuals with PTSD, the prevalence of the disorder should be higher in first-degree (parents, siblings) than second-degree (grandparents) relatives. The major limitation of family studies is that they cannot tell us whether a disorder, such as PTSD, runs in families for genetic or environmental reasons. Twin studies are needed to disentangle the roles of genetic and environmental influences in disorder etiology. Twins studies are limited in that they cannot specify which genes actually increase risk for the disorder. Molecular genetic studies are needed to accomplish this aim. However, to date there have been strikingly few molecular genetic studies of PTSD as compared to the numerous studies conducted on other common mental disorders such as major depression.
PTSD runs in families
Standard family studies generally seek to examine whether the prevalence of PTSD is higher in relatives of individuals with PTSD (called probands in genetic studies) than in relatives of similarly trauma-exposed individuals who did not develop PTSD. As noted, family studies in PTSD are complicated by the practical reality that PTSD cannot be assessed in relatives who have not experienced a traumatic event. It is unknown whether these relatives would have developed PTSD if they were exposed. Thus, family studies of PTSD have focused on genetically-related dyads who have known (often shared) trauma exposure. Most often these studies have examined the association between parent and child PTSD in trauma-exposed samples.
Extant research suggests relatives of probands with PTSD are at elevated risk of the disorder as compared to relatives of similarly trauma-exposed controls who did not develop PTSD. For example, adult children of Holocaust survivors with PTSD had a higher risk of PTSD following trauma compared to adult children of Holocaust survivors without PTSD [140]. Similarly, Cambodian refugee children whose mother and father both had PTSD were five times more likely to receive the diagnosis than refugee children whose parents did not have PTSD [117].
Increasingly common are family studies that examine the association between child and parent post-traumatic stress symptoms (PTSS) following child traumatic exposure. Parent-child PTSS correlations following child traumatic exposure have been mixed, with some studies reporting a significant correlation between child PTSS and parent PTSS [5, 34, 58, 105] whereas other studies have found no relationship [83, 92, 129]. Attempts to resolve this apparent discrepancy have highlighted the importance of time lapsed between traumatic exposure and assessment [106]. Studies examining acute distress levels soon after the trauma typically find no association between parent and child PTSS [16, 137], while longitudinal studies show increases in parent-child PTSS associations over time [79, 103, 124]. Initial symptoms in the child have been found to impact subsequent symptoms in the parent [79] and initial parent symptoms have been found to predict subsequent child symptoms [31, 93, 100].
Research has further indicated that impact of parental PTSS on subsequent child PTSS is not simply accounted for by overall levels of general parental distress [100]. Thus, parent PTSS appear to influence child PTSS through a more specific mechanism than merely parental availability or child concern for global parental distress. Parents may model poor adjustment to trauma, may directly alter their child’s avoidance behaviors, or may share underlying genetic vulnerabilities to the development of PTSD. Moreover, parent and child posttraumatic adjustment/coping is likely reciprocally influential, particularly when the trauma was shared. These investigations illustrate the challenge inherent in using family study paradigms to examine genetic factors in PTSD, as children share not only genes with their parents, but also many environmental factors.
Familial association between PTSD and other mental disorders
Family studies have also looked more broadly at whether there is a familial association between PTSD and other mental disorders. Family history studies of combat veterans have reported greater prevalence of anxiety disorders in families of individuals with PTSD [28, 29]. Similarly, a civilian epidemiological study found familial anxiety to be a risk factor in the development of PTSD [9] and parental mental disorders are associated with risk for PTSD in male civilians [14]. A family history of depression has been associated with proband PTSD in trauma-exposed veterans, albeit inconsistently [28, 29, 73]. Female rape victims with PTSD and comorbid lifetime depression also evidenced an increased prevalence of familial depression [30]. These findings suggest PTSD has a familial association with anxiety disorders and major depression.
The major limitation of family studies is that they cannot tell us whether a disorder, such as PTSD, runs in families for genetic or environmental reasons. Twin studies are needed to disentangle the roles of genetic and environmental influences in disorder etiology.
PTSD is heritable
Twin studies have made three major contributions to our understanding of the genetic etiology of PTSD. First, they indicate that genetic factors influence exposure to potentially-traumatic events. This is referred to as gene-environment correlation, whereby selection of environment, and subsequently potential for exposure to trauma, is partly determined by genetic factors [66]. For example, twin studies have demonstrated that genetic factors influence exposure to potentially-traumatic events such as combat exposure [90] and assaultive violence [128]. These gene-environmental correlations are likely due in part to individual differences in personality. Personality characteristics are moderately heritable and influence the tendency for individuals to select themselves into potentially harmful environments. For example, longitudinal investigations have found that childhood adjustment and neuroticism predicted subsequent stressful life events in adulthood [134]. Similarly, research has found that childhood externalizing is prospectively associated with both risk of trauma exposure and with PTSD in adulthood [75]. One investigation found that genetic factors partially mediated the association between personality variables (such as antisocial personality traits, psychoticcism, and openness to novelty) and exposure to violent traumatic events [65].
Second, twin studies suggest genetic influences explain a substantial proportion of vulnerability to PTSD even after accounting for genetic influences on trauma exposure. The first twin study to estimate heritability of PTSD was conducted by True et al. on members of the Vietnam Era Twin (VET) Registry [133]. The authors found that approximately 30% of the variance in reported PTSD symptoms was accounted for by genetic factors, even after controlling for combat exposure. Genetic influences on PTSD were similar for twins who did not serve in Southeast Asia, suggesting heritability of PTSD has generalization to traumatic events other than combat exposure. The second twin study of PTSD was conducted on a sample of male and female civilian volunteers [128]. Consistent with True et al., the authors found moderate heritability in PTSD symptoms, with additional variance accounted for by non-shared environmental factors. The findings from these two twin studies support suggest that genetic factors play a substantial role in vulnerability to developing PTSD.
Third, twin studies have demonstrated that genetic influences on PTSD overlap with those for other mental disorders. The extent of the overlap varies with the disorder studied. For example, genetic influences on major depression account for the majority of the genetic variance in PTSD. Genetic influences common to major depression account for the majority of the genetic variation in PTSD [47, 72]. Genetic influences common to generalized anxiety disorder and panic disorder symptoms account for approximately 60% of the genetic variance in PTSD [20] and those common to alcohol and drug dependence [139] and nicotine dependence [74] account for over 40%. Thus, the limited data available suggest that the majority of genes that affect risk for PTSD also influence risk for other psychiatric disorders and vice versa.
Variation in specific genes associated with PTSD
The primary limitation of twin studies is that they cannot tell us which genes are important in PTSD etiology. In contrast, molecular genetic studies have the potential to identify genetic markers of vulnerability or resilience. To date, published molecular genetic studies of PTSD have all used the case-control candidate gene association design. The association method detects genes with small effects on risk and has been, until very recently, the method of choice for molecular genetic studies of complex disorders [114]. Disorders are referred to as “complex” when their etiology is thought to involve a combination of many genes and environmental factors such as is the case in PTSD. Association studies correlate a DNA marker’s alleles, which are different sequences of DNA at a specific position (or locus) on the chromosome, with an outcome.
Table 1 summarizes the findings from the eleven candidate gene studies of PTSD to date. Six of these have focused on dopamine (DA) system genes. Both animal and human studies have implicated the dopaminergic system in the etiology of PTSD. Higher levels of plasma [59] and urinary [32, 88, 127, 141] DA have been associated with PTSD in humans. In animal studies limbic system innervation of DA has been found to be reactive to stress [53, 64].
Table 1.
Review of published case-control candidate gene associations studies of PTSD
| First author | Year | Cases N (% male) |
Controls N (% male) |
Nationality/race or ethnicity | Case ascertainment | Chronic PTSD? |
|---|---|---|---|---|---|---|
| Comings | 1991 | 35 (100) | 314 (100) | United States/Non-Hispanic White | NNRB/VA Clinic | Yes |
| Comings | 1996 | 24 (100) | 9 (100) | United States/Non-Hispanic White | VA Clinic | Yes |
| 1996 | 13 (100) | 11 (100) | United States/Non-Hispanic White | VA Clinic | Yes | |
| Gelernter | 1999 | 52 (100) | 87 (100) | United States/Non-Hispanic White | VA Clinic | Yes |
| Lappalainen | 2002 | 77 (100) | 202 (100) | United States/Non-Hispanic White | VA Clinic | Yes |
| Segman | 2002 | 102 (56) | 104 (47) | Israel/Ashkenazi and Non-Ashkenazi Jews | PTSD Research Studies/Mental Health Clinics | Yes |
| Young | 2002 | 91 (100) | 53 (100) | Australia/Non-Hispanic White | Inpatient Unit | Yes |
| Bachman | 2005 | 118 (100) | 42 (100) | Australia/Non-Hispanic White | PTSD Clinic | Yes |
| Lee | 2005 | 100 (43) | 197 (39) | Korea/Korean | Mental Health Clinics | Yes |
| Zhang | 2006 | 96 (76) | 250 (41) | United States/Non-Hispanic White | VA Clinic | Yes |
| Kilpatrick | 2007 | 19 (32) | 570 (37) | United States/various | Epidemiologic sample of hurricane exposed adults | No |
| First author | Year | Trauma exposed controls? |
Trauma type | Gene name (symbol) | Finding | |
| Comings | 1991 | No | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 Allele in PTSD cases P = 0.007 | |
| Comings | 1996 | Yes | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 Allele in PTSD cases P = 0.041 | |
| 1996 | Yes | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 Allele in PTSD cases P = 0.002 | ||
| Gelernter | 1999 | No | Combat | Dopamine receptor D2 (DRD2) | No significant association between D2A1 allele/DRD2 haplotypes and PTSD | |
| Lappalainen | 2002 | No | Combat | Neuropeptide Y (NPY) | No significant association between Leu7Pro polymorphism and PTSD | |
| Segman | 2002 | Yes | Various | Dopamine transporter (DAT1) | Excess 9-repeat allele in PTSD cases P = 0.012 | |
| Young | 2002 | No | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 allele only in PTSD cases with harmful drinking P < 0.001 | |
| Bachman | 2005 | Yes | Combat | Glococorticoid receptor (GCCR) | No significant association between GCCR polymorphisms and PTSD | |
| Lee | 2005 | No | Various | Serotonin transporter (SLC6A4) | Excess s allele in PTSD cases P = 0.04 | |
| Zhang | 2006 | Not specified | Not specified | Brain derived neurotrophic factor (BDNF) | No significant association between three BDNF variants and PTSD | |
| Kilpatrick | 2007 | Yes | Hurricane | Serotonin transporter (SLC6A4) | Significant association between s/s genotype and PTSD in adults with high hurricane exposure and low social support | |
PTSD posttraumatic stress disorder, NNRB National Neurological Research Bank, Los Angeles, California, VA Veterans Affairs, D2DA1 Al one allele of DRD2 gene, s allele short version (versus long) of the serotonin transporter promoter polymorphism
Five out of the six investigations examining DA system genes studied the association between marker alleles at the D2 dopamine receptor gene (DRD2) and PTSD. Whereas initial investigations found a positive association with the DRD2A1 allele [23, 24], a subsequent investigation found no association with the DRD2A1 allele or with any combination of alleles for the DRD2 locus [51]. However, it is important to note that the investigation conducted by Gelernter et al. [51] did not assess for trauma exposure in the control group. The Comings et al. [24] investigation consisted of a relatively small sample of substance abusers with PTSD (N = 37) compared with substance abusers without PTSD (N = 19); limiting generalizability to a substance abusing population. Comorbid PTSD and substance abuse was also addressed in a subsequent investigation of combat veterans with and without PTSD, with analyses revealing a positive association between DRD2A1 and PTSD only in the subset of PTSD cases who engaged in harmful drinking [142]. The final study examined a slightly different facet of dopaminergic transmission in patients with chronic PTSD and trauma-exposed healthy controls, reporting a positive association between of the dopamine transporter SLC6A3 (DAT1) 3′ polymorphism and chronic PTSD [121].
The five remaining studies explored genetic polymorphisms across alternative neurobiological pathways, with the majority of studies reporting no association between specific genes and chronic PTSD. More specifically, one investigation found no association between polymorphisms in the brain derived neurotrophic factor (BDNF) gene and chronic PTSD [143]. No significant association was found between chronic PTSD and either the Leu7Pro polymorphism in the neuropeptide Y (NPY) gene [84]or two glucocorticoid receptor polymorphisms (N363S and BclI) [4].
Investigations of the serotonergic system have proven slightly more fruitful. One investigation examined an insertion/deletion polymorphism in the promoter region of the serotonin transporter (SLC6A4, locus 5-HTTLPR), reporting an excess of s/s genotypes in Korean PTSD patients compared with normal controls [87]; control participants were not necessarily trauma-exposed. Kilpatrick et al. [69] also documented a significant association between the 5-HTTLPR genotype and PTSD in a sample of hurricane-exposed adults. However, 5-HTTLPR genotype was only associated with increased risk of PTSD among adults with high stress exposure. Since this is the first report of a significant G × E in PTSD, we will discuss this study in more detail below.
Evidence for gene-environment interaction in PTSD
The accumulation of evidence reviewed above proffers support for a possible G × E. Although research has highlighted a role for both environmental and genetic factors in the development of PTSD, studies have also been consistent with moderate levels of variability in each term. To date, only one investigation has specifically examined the role of G × E in the etiology of PTSD [69]. Using epidemiologic sampling strategies, Kilpatrick et al. examined whether 5-HTTLPR variation moderated risk of developing PTSD in 589 adults exposed to the 2004 Florida hurricanes (for details about the sample and design see [1, 2]). To the best of our knowledge, this is the first study to collect genetic samples by mail in the context of a large epidemiological telephone survey. Other studies have collected saliva samples for DNA analyses by mail [45], but this has been in the context of studies in which investigators had ongoing research relationships with participants. Likelihood of returning a saliva sample did not differ in relation to sex, level of hurricane exposure, level of social support, and PTSD and MD status. Additional details regarding response rate and correlates of participation are summarized elsewhere [2, 48]. The success of the Kilpatrick et al. study suggest that it is both feasible and useful to add genetic components to studies using telephone interviews to collect data about exposure to traumatic events as well as PTSD.
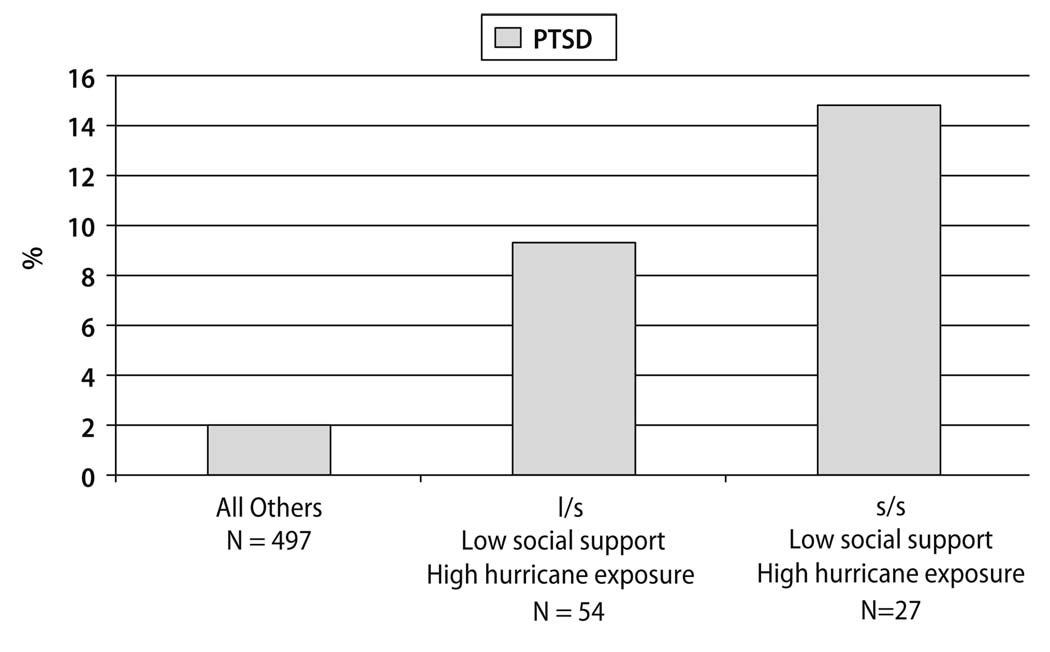
Kilpatrick et al. [69] found the low expression (s) variant of the 5-HTTLPR increased risk of post-hurricane PTSD only under the conditions of high hurricane exposure and low social support. Figure 1 presents the prevalence of post-hurricane PTSD by SLC6A4 genotype, level of social support, and level of hurricane exposure. High risk (n = 27) were those with the s/s genotype, low social support and high hurricane exposure. Medium risk (n = 54) were those with the l/s (long/short) genotype, low social support and high hurricane exposure. Low risk (n = 498) were all others. There was a strong association between risk group and prevalence of PTSD (χ2(2, n = 579) = 19.94, p < 0.001). High-risk individuals (high hurricane exposure, the low expression 5-HTTLPR variant, low social support) had 4.5 times (95% CI = 1.2, 17.9) the risk of developing PTSD as compared to low-risk individuals. Additional research is needed to replicate the Kilpatrick et al. findings. Such studies can add to our knowledge of how genetic and environmental risk and protective factors interact to foster resilience or increase psychopathology following trauma.
Fig. 1.
Prevalence of post-hurricane PTSD by SLC6A4 genotype, level of social support, and level of hurricane exposure in adults exposed to 2004 Florida Hurricanes
Specific genes associated with PTSD and other mental disorders
As noted above, twin studies suggest comorbidity between PTSD and other mental disorders can largely be explained by a shared genetic diathesis. The presence of a common genetic diathesis between major depression and PTSD is further supported by molecular genetic studies, which have implicated the 5-HTTLPR s/s polymorphism in both PTSD and depression [18, 87]. Polymorphisms in FKBP5 have been associated with recurrence of major depressive episodes and response to antidepressant treatment [6] as well as with peri-traumatic dissociation, an acute predictor of PTSD [76]. DA system genes, such as DAT1, which have been associated with PTSD, have also been associated with ADHD (e.g., [15] and alcohol use (e.g., [78].
Gene-phenotype associations in individuals with PTSD
As a direct gene-disorder link is highly unlikely, several researchers have begun to investigate the association between variation in specific genes and a range of phenotypes expressed among individuals diagnosed with PTSD. In a recent study of male Vietnam veterans with PTSD the DRD2 A1 allele was associated with comorbid conditions [86]. Specifically, those with the DRD2A1 allele had higher levels of anxiety/insomnia, social dysfunction, and depression compared to those without the A1 allele. Similarly, in an open label treatment study for PTSD, male veterans with the DRD2 A1 allele had higher baseline levels of anxiety/insomnia, social dysfunction, and depression, compared to PTSD those without the A1 allele [85]. Following an 8-week paroxetine trial, all patients improved in terms of anxiety/insomnia, depression, and social dysfunction regardless of A1 allele status; however, those with the A1 allele improved to a greater degree on social dysfunction than did those without the allele.
In addition to investigations of candidate genes within dopaminergic system, gamma-aminobutyric acid (GABA) neurotransmitter system and Apoliprotein E (APOE) systems have also been targets of genetic studies of comorbid conditions of PTSD. Feusner et al. [44] examined the GABAA receptor β3 subunit gene (GABRB3) in relation to comorbid conditions in male Caucasians with PTSD. The risk genotype for GABRB3 is the heterozygote (G1+G1−). Those with a heterozygote genotype had greater somatic symptoms, anxiety/insomnia, depression, and social dysfunction symptoms compared to those with a homozygote genotype (G1−G1− or G1+G1+) [44].
APOE has been associated with psychiatric conditions marked by cognitive disturbances [50]. Given the association between PTSD and neurocognitive deficits (e.g., [52]), Freeman et al. [46] investigated the association between APOE genotype and PTSD. Patients with the APOE allele had higher PTSD reexperiencing symptoms and performed poorer on several facets of memory function compared to those without the APOE allele [46].
Strategies for identifying replicable G × E in PTSD
The major stumbling blocks to identifying replicable G × E in psychiatry have already been discussed in detail by other authors [97]. Here, we focus on challenges that are more specific to PTSD research. We also suggest strategies to address these challenges that we believe will improve our ability to conduct replicated G × E studies in PTSD.
Trauma assessment
A vital component of G × E research is precise measurement of the environment. In the case of PTSD, the typical environmental variable of interest is exposure to a potentially traumatic event. According to the DSM-IV [3] to diagnose PTSD an individual must be exposed to a potentially-traumatic event that meets the following two criteria: (A1) the person experienced, witnessed, or was confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others; (A2) the person’s response involved intense fear, helplessness, or horror. Diagnosing PTSD relies heavily on conducting a reliable and valid trauma assessment; a wide variety of events satisfy Criterion A, thereby underscoring the need for assessment instruments to capture the breadth of potentially-traumatic events. Reliable and valid trauma exposure assessment is a necessary component to conducting replicable studies of G × E in PTSD. Simulation studies have shown that the sample size needed to detect a given G × E effect will increase by a power of ten if the assessment of the environmental exposure is poor [138]. Thus, researchers interested in detecting G × E in PTSD must prioritize selecting reliable and valid measures of trauma exposure.
There are a wide variety of trauma assessment instruments with strong psychometric properties available. The specific instrument best suited for a particular study will depend on the study population (e.g., children versus adults, civilian versus military), the period of time assessed (e.g., lifetime versus past year), the method of assessment to be used (e.g., self-report questionnaire versus interview), and length of time available for the trauma assessment. Detailed papers as well as entire books have been dedicated to the topic of PTSD assessment (e.g., [136]), and the reader is referred to such sources for more in-depth analysis of these measures. Regularly updated information on trauma assessment instruments is also available via the National Center for PTSD’s website: (http://www.ncptsd.va.gov/ncmain/assessment/trau-ma_exp.jsp).
Risk for PTSD is influenced by a number of trauma characteristics that need to be considered when conducting assessments. First, individuals exposed to multiple traumatic events are at greater risk for PTSD than those exposed to a single stressor [61, 104, 118]. Second, type of traumatic exposure is associated with differential risk for development of PTSD. For example, criminal victimization tends to be associated with higher risk than other traumatic events—particularly those that do not involve intentionality—such as motor vehicle accidents and natural disasters [68, 113]. Third, other variables and event incidents affect risk for PTSD, such as life threat, presence of a weapon, victim-perpetrator relationship, and severity (e.g., [13, 49, 70]. Therefore, a comprehensive trauma assessment must encompass multiple traumatic experiences across the lifespan, determine whether traumatic experiences were single incidents or chronic in nature, and gather data on incident characteristics.
A final consideration when conducting a trauma assessment is the issue of retrospective self-report, which may introduce recall bias related to mood-dependent recall [22]. Evidence suggests trauma-exposure reports are influenced by level of current PTSD symptoms; self-reports of trauma-exposure severity increase as PTSD symptoms increase and vice versa [77, 126]. This recall bias is concerning because it has the potential to inflate the association between trauma-exposure severity and PTSD.
Despite concerns about recall bias, most studies of PTSD will involve retrospective assessment of trauma exposure to some degree. Even prospective studies will often involve retrospective reports of trauma exposure during the baseline assessment. The reliability of retrospective self-reports of trauma exposure can be improved by the use of life history calendar methods [17] and labor-intensive narrative based interview approaches [41]. For specific populations, trauma exposure information may be available via other sources such as military historical records [39–41], court records of childhood abuse and neglect cases [135] and surgeons ratings of the severity of a medical injury or degree of body area burned [119].
Control selection
One of the biggest challenges to G × E PTSD studies is appropriate control selection. According to epidemiologic principles [115], controls should be selected from the same underlying population as the cases, representative of all controls with regard to exposure, and identical to the exposed cases except for the risk factor (in this case the genetic variant) under investigation. One practical implication of this last principle, referred to as “exchangeability” between cases and controls, is that controls must be similar to cases in severity of trauma exposure; as noted previously several PTSD candidate gene studies do not report assessing trauma exposure in controls (Table 1). Violation of the exchangeability principle increases the likelihood that positive associations may be biased due to confounding factors and, in addition to the small sample sizes used in many studies, makes negative associations difficult to interpret.
Two types of study designs commonly used in trauma and PTSD research can facilitate appropriate control selection. The first is the standard epidemiologic study design where a random sample is drawn from an underlying population and assessed for trauma exposure and PTSD. This design was used by Acierno et al. in their study of older adults living in Florida counties affected by the 2004 Hurricanes [1, 2, 69]. Cases are then individuals in the sample who were diagnosed with PTSD; controls are individuals from the same underlying population exposed to similar traumas, who did not develop PTSD. Although this is one of the most feasible designs for trauma researchers, its limitations include inherently lower reliability in assessing trauma exposure and PTSD retrospectively. Lower measurement reliability will reduce power to detect a given G × E effect [138].
The second design is the prospective exposed cohort design commonly used to study individuals who are seen in the emergency room following a physical injury (e.g., car accident). In this design, individuals are enrolled in a study upon exposure to a traumatic event and followed over time to see who develops PTSD (cases) and who does not (controls). Cases and controls are therefore ascertained from the same underlying population. Some of the strengths of this design include prospective assessments of PTSD and the enhanced feasibility of collecting DNA samples from participants in the hospital. However, such designs also have limitations in terms of generalizability and projected sample size as compared to retrospective epidemiologic studies.
Power and sample size
The statistical power of a candidate gene association study refers to the probability of detecting a true genetic effect. Power in a genetic study is determined by factors similar to those that influence power in any research design: significance level, sample size, prevalence of the risk factor (e.g., risk genotype) in controls, and the effect size conferred by the risk factor (e.g., risk genotype). See [130] for a detailed discussion of power issues in candidate gene association studies.
The power to detect a G × E interaction will depend on the frequency of the risk allele and environmental factors (e.g., trauma exposure), as well as the way in which they are measured and the actual nature of the interaction effect itself. As a consequence, the effective magnitude of the interaction effect will vary depending on whether or not continuous or dichotomous measures are used, as well as the frequencies of the genotypes, exposure to environmental stressors, and phenotype prevalence. In general, if the disease, the risk genotype, and the dichotomous environment all are rare, then power will be marginal for most achievable sample sizes; if at least two of these factors are more common, then power exceeds the 80% level much more often. These results reflect the well-known result that more power can be extracted by use of continuous measures whenever appropriate.
Successful G × E studies in other complex disorders suggest several potential study designs investigators can use to ensure adequate numbers of PTSD cases and trauma-exposed controls to conduct well-powered G × E PTSD studies. For example, researchers may capitalize on existing large cohorts using a two-stage sampling strategy involving an initial screen of a cohort for trauma/PTSD and selection of cases and controls based on screening data. Additionally, the Kilpatrick et al. [69] study demonstrates the feasibility of using epidemiologic strategies for research. Additionally, PTSD researchers may consider recruitment from military populations as a majority of military personnel are exposed to potentially-traumatic experiences. Recruitment from samples such as military personnel affords additional unique opportunities, as military records may have a wealth of pre-and peri-trauma data.
Gene-trauma correlations
One well-recognized challenge to G × E research is that risk factors commonly considered to be ‘environmental’ actually have a strong genetic component. As noted above, this is referred to as gene-environment correlation, whereby genetic factors influence exposure to environmental pathogens [109, 116]. Most likely, heritable factors such as personality impact subsequent tendency to select potentially harmful circumstances. For example, longitudinal investigations have found that childhood emotional adjustment and neuroticism predicted subsequent stressful life events in adulthood [19, 134]. One investigation found that genetic factors partially mediated the association between personality variables (such as antisocial personality traits, psychoticcism, and openness to novelty) and violent traumatic events [65]. Similarly, research has found that childhood externalizing is prospectively associated with both risk of trauma exposure and with PTSD in adulthood [75].
A detailed discussion of how to address gene-environment correlation in G × E studies is beyond the scope of this article. However, given evidence that trauma exposure may be influenced by genetic factors, PTSD researchers interested in G × E research need to consider issues related to gene-trauma correlation carefully in their study design and statistical analysis. Concerns about gene-trauma correlation can be addressed in part by careful assessment of trauma exposure characteristics (such as type, severity, frequency, etc.) and selection of controls as we discussed above. Investigators might also consider matching PTSD cases and trauma-exposed controls on important trauma exposure characteristics. Alternatively, the problem of gene-trauma correlation can be addressed by examining the development of PTSD following exposure to potentially-traumatic events whose occurrence is largely independent of the individual victim’s behavior or personality. Such events have been described as “ fateful” whereby “both the prelude to the event and its actual occurrence” are “mostly determined by external circumstances” [38, p. 490]. Determining whether or not a specific potentially-traumatic event is fateful is complex and may require time consuming assessment procedures [38]. One option is to study the development of PTSD in populations recently affected by a natural (e.g., hurricanes, earthquakes) or human-made disasters (e.g., large scale terrorist attacks). Disasters are one type of potentially-traumatic event largely immune from concerns about gene-trauma correlation as they are (almost by definition) outside of the victims’ control but also affect large numbers of people at once. Kilpatrick [69] used this design in their study of G × E in older adults exposed to the 2004 Florida Hurricanes.
Genetic overlap between PTSD and other mental disorders
PTSD is highly comorbid with other psychiatric disorders. As noted above, twin studies suggest this comorbidity can largely be explained by a shared genetic diathesis. The presence of a common genetic diathesis between major depression in PTSD is further supported by molecular genetic studies, which have implicated the serotonin transporter promoter s/s polymorphism in both PTSD and depression [18, 87]. Polymorphisms in FKBP5 have been associated with recurrence of major depressive episodes and response to antidepressant treatment [6] as well as with peri-traumatic dissociation, a major risk factor for PTSD [76].
Thus, the limited data available suggest that the same genes involved in other psychiatric disorders, particularly major depression and other anxiety disorders, may influence risk for PTSD. This has an important implication for PTSD candidate gene studies: the presence of other psychiatric disorders in trauma-exposed controls likely increases the genetic variance shared by cases and controls and attenuates the possibility of finding a positive PTSD-gene association. Psychiatric comorbidity, therefore, needs to be carefully assessed in both cases and controls in PTSD genetic studies. This may be accomplished through identification of coherent patterns of PTSD comorbidity, such as those proposed by Miller et al. in their work on developing a personality-based typology of posttraumatic response [96]. Using cluster-analyses based on personality assessments, Miller has shown that PTSD comorbidity coheres along the dimensions of externalization and internalization, parallel to those found by Krueger et al. for comorbidity among common mental disorders [81]. Externalizers showed the highest rates of alcohol-related and antisocial personality disorders; internalizers, the highest rates of panic and major depressive disorder. Our ability to find genes for PTSD might improve if PTSD internalizing/externalizing subtypes are considered.
Case definition
The PTSD candidate gene association studies presented in Table 1 have included only cases with current PTSD, where current PTSD involves chronic disorder extending over many years or even decades. When considering disorder etiology, it is useful to distinguish between risk factors for onset or development of the disorder and risk factors for course or chronicity of the disorder. Factors that influence who develops the disorder in the first place may differ from those that influence who recovers from the disorder once it develops. For example, members of disadvantaged ethnic groups are not at higher risk for the development of psychiatric disorders. However, once they develop a psychiatric disorder, their disorders are more chronic than those of non-Hispanic whites [7].
The sensitivity of PTSD to timing is captured in the current diagnostic schema (which requires a lapse of at least four weeks post-trauma) and in the literature on PTSD chronicity. Epidemiologic studies of PTSD have made careful distinction in identifying risk factors for onset versus course of the disorder [8, 9]. A recent analysis of the Vietnam Veterans Readjustment Study (NVVRS) revealed that factors affecting the development of PTSD occur prior to, during, or after combat trauma are associated with development of PTSD symptoms, whereas factors affecting the maintenance of PTSD are primarily occurring during and after trauma [120]. Further, numerous investigations have investigated the longitudinal development of PTSD, indicating mean-level decreases in symptoms (e.g., [12, 37, 42, 60, 122]); however, these studies have generally looked at single risk factors or have characterized mean-level changes, limiting interpretations about relationships among symptoms over time. Toward an understanding of the mechanisms underlying development and maintenance of PTSD, investigations have also begun to explore the temporal progression of PTSD symptoms/symptom clusters [25, 43, 89, 94, 99]. Research on the role of stressful life events and major depression provides support for the kindling hypothesis; major stressful life events are needed to induce onset of the disorder but recurrence is more strongly associated with minor events [98].
The degree to which patterns/relationships of symptoms may change over the course of time may have meaningful implications for researchers examining neurobiological factors that may also vary as a function of these changes. For example, a study of pediatric injury found that whereas acute biological measures (heart rate and urinary cortisol) were positively associated with symptoms of emotional numbing at 6-weeks, these factors were negatively associated with emotional numbing at the 6-month time point [99]. If the direction, or even the strength, of neurobiological associations may change over the course of time, genetic studies that sample participants with varying time since trauma may fail to correctly identify true relationships. Similarly, research has shown that the factors that may promote development of PTSD may differ from factors that maintain symptoms. This introduces a significant potential confound, as twin studies have relied almost exclusively on diagnoses of lifetime PTSD and, therefore, heritability estimates from such studies explain the proportion of variation in risk for developing PTSD explained by genetic factors. It is not known whether genetic factors explain as much of the variance in chronicity of PTSD or whether the same genes that influence risk for the development of PTSD affect the maintenance of PTSD.
New directions for G × E research in PTSD
Given these considerations, as well as the state of the larger literatures of research in PTSD, behavioral genetics, and biological risk factors for PTSD, we outline possible avenues for future research. Given the nascent state of G × E literature, as well as numerous indicators that PTSD has the potential for G × E, a wealth of exciting approaches to research is available (e.g., haplotype blocks, whole-genome association studies; see [71]for review). For the present purposes, we will focus on avenues for research that are likely to be unique to PTSD, with the awareness that below proposed frameworks for approaching G × E research in PTSD can be easily adapted for use with alternate genetic methodologies.
PTSD endophenotypes
“Endophenotypes” were first described by Gottesman and Shields [56] as internal measurable biological phenomena that underlie overt phenotypes. A more recent discussion of endophenotypes provided by Gottesman and Gould [55] characterizes endophenotypes as “neurophysiological, biochemical, endocrinological, neuroanatomical, cognitive, or neuropsychological (including configured self-report data)” constructs that mediate the relationship between genetic underpinnings and expressed syndromes.
As expressed syndromes (such as PTSD) are the product of a complex interplay of genetic and environmental factors, endophenotypes may represent a more proximal indicator of genotype. Additionally, the use of endophenotypes in gene association studies may partly circumvent above enumerated concerns with our present approach to diagnosis. As Gottesman and Gould [55] detail, potential endophenotypes can be identified using research findings from both human and animal studies across a variety of scientific arenas, with the extant PTSD literature providing particularly well-developed foundations for neuropsychologically-, endocrinologically-, and neuroanatomically-based endophenotypes.
In PTSD research, we propose that endophenotypes may be viewed as the broad biological processes that underlie an individual’s: (1). underlying neuro-cognitive vulnerabilities, (2). acute response to a potentially-traumatic event, and (3). long term psychobiological adaptations that underlie PTSD. As discussed, underlying neurocognitive vulnerabilities such as above summarized cognitive [11, 80, 91, 108] and neurocognitive [107], likely influence subsequent broad acute and long-term broad biological responses. As such, these factors may be more closely related to genetic factors.
Similarly, we postulate that genetic influences may be most evident in acute biological response relative to chronic PTSD endophenotypes, as development of chronic PTSD may be influenced by a host of environmental factors that may promote resilience (e.g., social support) or factors that may increase risk (e.g., presence of environmental traumatic reminders, subsequent stressors). Examples of potential endophenotypes in acute biological response which have been found to predict PTSD include acute sympathetic response to trauma (see [16] for review) and initial neuroendocrine response (see [35]). Acute peritraumatic responses such as dissociation may represent another promising endophenotype for genetic research. For example, Koenen et al. [76] have found that polymorphisms in FKBP5 are associated with peritraumatic dissociation pediatric injury patients. This approach could be further extended to G × E research through examination of environmental factors that may interact with identified polymorphisms. For example, parental presence during the traumatic stressor or severity of injury could feasibly interact with genetic factors in the prediction of a variety of peritraumatic endophenotypes.
Potential endophenotypes of chronic PTSD include chronic alterations in neuroendocrine and psychophysiological response to stress, see [21] for review. Freeman et al. [46] investigation of the APOE genotype in PTSD is an example of this approach. Findings supported that patients with the APOE allele, relative to those without the allele, showed poorer performance on distinct elements of memory function. Similar methodology could be applied to explore the degree to which environmental factors (such as amount of alcohol imbibed or quality of sleep) may interact with genetic influences (such as the APOE genotype) in the prediction of endophenotypes in chronic PTSD (such as memory functioning).
Developmental trajectories
As our review of the literature highlights, particularly in trauma research, it is important to take developmental factors into account, including both: (1) trajectory of PTSS development following trauma exposure, and (2) developmental level at the time of trauma exposure. Extant research has shown that the pattern of PTSD phenotype as well as associations between PTSD and endophenotypes may change over the course of time. It follows that different genetic factors may confer risk for development of disorder versus risk for chronicity. However, the majority of PTSD candidate gene association studies conducted to date have included only cases with current PTSD, where current PTSD involves chronic disorder extending over many years or even decades. G × E studies are particularly vulnerable to the time lapsed between trauma exposure and assessment as the strength of environmental effects may increase over time. Prospective exposed-cohort designs where individuals are followed over an adequate time period have the potential to address this concern.
Similar to examinations with sensitivity to trajectory over time, accumulating evidence that age at trauma exposure may exact distinct psychiatric outcomes, see [110], opens vast potential for future research. Chronology may be particularly important in neurobiological aspects of PTSD, as research has shown different patterns of acute and chronic cortisol response in children with PTSD relative to adults with PTSD [32, 33, 36]. Although the nascent nature of this area of literature affords a wealth of possible research investigations, relatively little information is available to inform endophenotypes at each stage of development. Furthermore, assessment of psychological constructs such as PTSD is particularly difficult in young children. Nonetheless, numerous recent reviews, see [110] and [95], are available to guide developmentally-informed research. Additionally, as research using child samples necessarily involves parental consent, investigations such as these could be enhanced by obtaining additional information from family members, who may be an important source of environmental influence (e.g., social support) and who may be genetically similar.
Sex differences in PTSD
Researchers are also cautioned to consider the potential for participant sex to play a role in G × E investigations. Epidemiologic investigations have identified a markedly higher prevalence of PTSD in females relative to males in both adult samples [9, 27, 62, 68] and child/adolescent samples [26, 123]. A recent meta-analytic review of more than two decades of research in the area indicated that whereas females were more likely to meet criteria for PTSD, they were less likely to experience traumatic events [132]. Although greater exposure to childhood sexual abuse and sexual assault has been proposed to explain this difference, within-trauma type analyses revealed that women were more likely than men to show PTSD following exposure to accidents, nonsexual assaults, witnessing death/injury, disaster, fire, and war. These findings suggest that the greater prevalence of PTSD in women cannot be completely explained by type of trauma.
Biological differences, particularly related to sex hormones, have been proposed to explain differences in rates of PTSD (i.e., [112], although few studies have directly examined this possibility. Morning cortisol levels of postpubertal girls are about 20% higher than in age-matched boys, although the difference is not apparent in prepubertal children [54]. Research with pediatric injury patients indicated that the relationship between initial urinary stress hormone levels and PTSS was only significant in boys [36]. Sex, phase of menstrual cycle, and developmental stage have all been proposed to exact complicated patterns of biological response to trauma or to traumatic reminders [112].
Relative to men, women have been found to show greater fear-potentiated startle when presented with a predictable aversive event [57]. Female biological response patterns are further complicated by differences in patterns of biological response over the course of the menstrual cycle [131]. Moreover, cortisol circadian release has been shown to be related to developmental stage [54].
Olff et al. [101] provide an integrated framework for understanding neurobiological factors as they may contribute to sex-related differences in PTSD. Central to their model, peritraumatic phenomena such as cognitive appraisals and peritraumatic dissociation, may differ in men and women, with concomitant neurobiological changes potentially accounting for sex-related differences in subsequent development of PTSD. Additionally, DHEA and DHEA-S, which differ in men and women in both healthy [102]and trauma-exposed samples [125], have shown alterations in individuals with PTSD [111, 112]. Pertinent to earlier appeals for the consideration of developmental stage in future research, DHEA levels have been shown to vary across development, with plasma DHEA levels high at birth and then dropping until adrenarche, when levels rapidly increase, peaking in 20s to 30s [63].
Six of the ten published PTSD candidate gene studies are on exclusively male samples, specifically non-Hispanic White combat veterans recruited from clinics. Clearly, genetic studies of PTSD need to include women, other race/ethnic and age groups, and participants exposed to different types of trauma. However, to be truly generalizable, PTSD genetic studies need to be conducted on epidemiologic samples.
Concluding statements
In sum, converging evidence from diverse research designs supports a role for G × E research in PTSD. For example, family studies have laid the foreground for research in this area, indicating increased risk for PTSD in relatives. Twin studies further support the heritability of PTSD, yielding three main findings related to the genetic influences on: the likelihood of exposure to potentially traumatic events, the development of PTSD, and the existence of comorbidity. Although these studies support a role for genetics, little information is provided regarding the specific genetic underpinnings. Partially filling this void are candidate gene studies, which attempt to identify specific genes related to the etiology of PTSD.
Moving beyond the main effect model, innovative research designs have used gene-environment interaction models to afford a more sophisticated approach to examining the interrelationships between genes and environmental risk or resilience factors. Utilizing this novel research design, Kilpatrick et al. [69] found that the s/s geneotype for 5-HTTLPR conferred increased risk for the development of PTSD under conditions of low social support and high hurricane exposure (see Fig. 1).
For future research, investigators are encouraged to be cognizant of posited biological mechanisms of PTSD development and to consider use of endophenotypes. For example, trauma researchers should continue to attend to potential differences in underlying factors related to development and maintenance of PTSD, as the genetic basis may be different. Similarly, developmental considerations, particularly in relation to pubertal stage, are likely to be important in future genetic studies. Moreover, research has identified differences in biological aspects of PTSD in men and women; however, the majority of genetic studies of PTSD have used predominately male samples. In short, research in G × E of PTSD is in its infancy and the areas of possible contribution are immense. Furthermore, the potential impact of genetically informed studies of trauma is substantial.
Acknowledgments
Dr. Koenen is supported in part by US-NIMH K08 MH070627.
Ananda Amstadter is supported by US-NIAAA T32 AA007474.
Nicole Nugent is supported by US-NIMH T32 MH18869.
Contributor Information
Karestan C. Koenen, Department of Society, Human Development, and Health and Epidemiology, Harvard School of Public Health, 677 Huntington Avenue, Kresge 613, Boston, MA 02115, USA, Tel.: 617/4324622. Fax: 617/4323755 E-Mail: kkoenen@hsph.harvard.edu Department of Psychiatry, Boston University School of Medicine, Boston, MA, USA.
Nicole R. Nugent, Department of Psychiatry and Behavioral Science, Medical University of South Carolina, Charleston, SC, USA Department of Psychology, Kent State University, Kent, OH, USA.
Ananda B. Amstadter, Department of Psychiatry and Behavioral Science, Medical University of South Carolina, Charleston, SC, USA Department of Psychology, Auburn University, Auburn, AL, USA.
References
- 1.Acierno R, Ruggiero KJ, Galea S, Resnick HS, Koenen KC, Rotizsch J, De Arellano M, Boyle J, Kilpatrick DG. Psychological sequelae of the 2004 Florida hurricanes: implications for post-disaster intervention. Am J Public Health. 2007:S103–S108. doi: 10.2105/AJPH.2006.087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acierno R, Ruggiero KJ, Kilpatrick D, Resnick H, Galea S. Risk and protective factors for psychopathology among older versus younger adults after the 2004 florida hurricanes. Am J Geriatr Psychiatry. 2006;14:1051–1059. doi: 10.1097/01.JGP.0000221327.97904.b0. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Washington DC: Author; 1994. [Google Scholar]
- 4.Bachmann AW, Sedgley TL, Jackson RV, Gibson JN, Young RM, Torpy DJ. Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology. 2005;30:297–306. doi: 10.1016/j.psyneuen.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Barakat LP, Kazak AE, Meadows AT, Casey R, Meeske K, Stuber ML. Families surviving childhood cancer: a comparison of posttraumatic stress symptoms with families of healthy children. J Pediatr Psychol. 1997;22:843–859. doi: 10.1093/jpepsy/22.6.843. [DOI] [PubMed] [Google Scholar]
- 6.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 7.Breslau J, Kendler KS, Su M, Gaxiola-Aguilar S, Kessler RC. Lifetime risk and persistence of psychiatric disorders across ethnic groups in the United States. Psychol Med. 2005;35:317–327. doi: 10.1017/s0033291704003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149:671–675. doi: 10.1176/ajp.149.5.671. [DOI] [PubMed] [Google Scholar]
- 9.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 10.Breslau N, Kessler R, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 11.Breslau N, Lucia VC, Alvarado GF. Intelligence and other predisposing factors in exposure to trauma and post-traumatic stress disorder: a follow-up study at age 17 years. Arch Gen Psychiatry. 2006;63:1238–1245. doi: 10.1001/archpsyc.63.11.1238. [DOI] [PubMed] [Google Scholar]
- 12.Brewin CR, Andrews B, Rose S, Kirk M. Acute stress disorder and posttraumatic stress disorder in victims of violent crime. Am J Psychiatry. 1999;156:360–366. doi: 10.1176/ajp.156.3.360. [DOI] [PubMed] [Google Scholar]
- 13.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:317–336. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 14.Bromet E, Sonnega A, Kessler RC. Risk factors for DSM-III-R posttraumatic stress disorder: findings from the National Comorbidity Survey. Am J Epidemiol. 1998;147:353–361. doi: 10.1093/oxfordjournals.aje.a009457. [DOI] [PubMed] [Google Scholar]
- 15.Brookes KJ, Guindalini C, Curran S, Xu X, Knight J, et al. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Arch Gen Psychiatry. 2006;63:74–81. doi: 10.1001/archpsyc.63.1.74. [DOI] [PubMed] [Google Scholar]
- 16.Bryant RA. Longitudinal psychophysiological studies of heart rate: mediating effects and implications for treatment. Ann N Y Acad Sci. 2006;1071:19–26. doi: 10.1196/annals.1364.002. [DOI] [PubMed] [Google Scholar]
- 17.Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, Smeijers J, Silva PA. The life history calendar: a research and clinical assessment method for collecting retrospective event-history data. Int J Methods Psychiatr Res. 1996;6:101–104. [Google Scholar]
- 18.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig I, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 19.Champion LA, Goodall G, Rutter M. Behavior problems in children and stressors in early adult life. I. A 20 year follow-up of London school children. Psychol Med. 2005;25:231–246. doi: 10.1017/s003329170003614x. [DOI] [PubMed] [Google Scholar]
- 20.Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103:133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 21.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Cohen LH, Towbes LC, Flocco R. Effects of induced mood on self-reported life events and perceived and received social support. J Pers Soc Psychol. 1988;55:669–674. doi: 10.1037//0022-3514.55.4.669. [DOI] [PubMed] [Google Scholar]
- 23.Comings DE, Comings BG, Muhleman D, Dietz G, Shabbahrami B, Tast D. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorder. JAMA. 1991;266:1793–1800. [PubMed] [Google Scholar]
- 24.Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 25.Creamer M, Burgess P, Pattison P. Reaction to trauma: a cognitive processing model. J Abnorm Psychol. 1992;101:452–459. doi: 10.1037//0021-843x.101.3.452. [DOI] [PubMed] [Google Scholar]
- 26.Cuffe SP, Addy CL, Garrison CZ, Waller JL, Jackson KL, McKeown RE, Chilappagari S. Prevalence of PTSD in a community sample of older adolescents. J Am Acad Child Adolesc Psychiatry. 1998;37:147–154. doi: 10.1097/00004583-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Davidson JR, Hughes D, Blazer DG, George LK. Post-traumatic stress disorder in the community: an epidemiological study. Psychol Med. 1991;21:713–721. doi: 10.1017/s0033291700022352. [DOI] [PubMed] [Google Scholar]
- 28.Davidson JR, Schwartz M, Storch M, Krishnan RR, Hammett E. A diagnostic and family study of posttraumatic stress disorder. Am J Psychiatry. 1985;142:90–93. doi: 10.1176/ajp.142.1.90. [DOI] [PubMed] [Google Scholar]
- 29.Davidson JR, Smith R, Kudler H. Familial psychiatric illness in chronic posttraumatic stress disorder. Comp Psychiatry. 1989;30:339–345. doi: 10.1016/0010-440x(89)90059-x. [DOI] [PubMed] [Google Scholar]
- 30.Davidson JR, Tupler LA, Wilson WH, Connor KM. A family study of chronic post-traumatic stress disorder following rape trauma. J Psychiatr Res. 1998;32:301–309. doi: 10.1016/S0022-3956(98)00016-8. [DOI] [PubMed] [Google Scholar]
- 31.Daviss WB, Mooney D, Racusin R, Ford JD, Fleischer A, McHugo GJ. Predicting posttraumatic stress after hospitalization for pediatric injury. J Am Acad Child Adolesc Psychiatry. 2000;39:573–583. doi: 10.1097/00004583-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 32.De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Dev Psychopathol. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- 33.De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology part I: biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 34.de Vries APJ, Kassam- Adams N, Cnaan A, Sherman- Slate E, Gllagher PR, Winston FK. Looking beyond the physical injury: posttraumatic stress disorder in children and parents after pediatric traffic injury. Pediatrics. 1999;104:1293–1299. doi: 10.1542/peds.104.6.1293. [DOI] [PubMed] [Google Scholar]
- 35.Delahanty DL, Nugent NR. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Ann N Y Acad Sci. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- 36.Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Difede J, Barocas D. Acute intrunsive and avoidant PTSD symptoms as predictors of chronic PTSD following burn injury. J Trauma Stress. 1999;12:363–369. doi: 10.1023/A:1024788812393. [DOI] [PubMed] [Google Scholar]
- 38.Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: toward resolution of the problem of intracategory variability. Psychol Bull. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dohrenwend BP, Neria Y, Turner JB, Turse N, Marshall R, Lewis-Fernandez R, Koenen KC. Positive tertiary appraisals and posttraumatic stress disorder in U.S. male veterans of the war in Vietnam the roles of positive affirmation, positive reformulation, and defensive denial. J Consult Clin Psychol. 2004;72:417–433. doi: 10.1037/0022-006X.72.3.417. [DOI] [PubMed] [Google Scholar]
- 40.Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. Continuing controversy over the psychological risks of Vietnam for U.S. veterans. J Trauma Stress. 2007;20:449–465. doi: 10.1002/jts.20296. [DOI] [PubMed] [Google Scholar]
- 41.Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans a revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epstein RS, Fullerton CS, Ursano RJ. Posttraumatic stress disorder following an air disaster: a prospective study. Am J Psychiatry. 1998;155:934–938. doi: 10.1176/ajp.155.7.934. [DOI] [PubMed] [Google Scholar]
- 43.Feuer CA, Nisith P, Resick PA. Prediction of numbing and effortful avoidance in female rape survivors with chronic PTSD. J Trauma Stress. 2005;18 doi: 10.1002/jts.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feusner J, Ritchie T, Lawford B, Young RM, Kann B, Noble EP. GABA(A) receptor beta 3 subunit gene and psychiatric morbidity in a post-traumatic stress disorder population. Psychiatry Res. 2001;104:109–117. doi: 10.1016/s0165-1781(01)00296-7. [DOI] [PubMed] [Google Scholar]
- 45.Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting samples from widely dispersed populations. Behav Genet. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- 46.Freeman T, Roca V, Guggenheim F, Kimbrell T, Griffin WST. Neuropsychiatric association of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2005;17:541–543. doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- 47.Fu Q, Koenen KC, Miller MW, Heath AC, Bucholz KK, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Differential etiology of posttraumatic stress disorder with conduct disorder and major depression in male veterans. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galea S, Acierno R, Ruggiero KJ, Resnick HS, Kilpatrick DG. Social context and the psychobiology of trauma. Ann N Y Acad Sci. 2006;1071:231–241. doi: 10.1196/annals.1364.018. [DOI] [PubMed] [Google Scholar]
- 49.Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, Vlahov D. Psychological sequelae of the september 11 terrorist attacks in new york city. N Engl J Med. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher-Thompson D, O’Hara R, Simmons A, Kraemer HC, Murphy GM., Jr Apolipoprotein E epsilon4 allele affects the relationship between stress and depression in caregivers of patients with Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2001;14:115–119. doi: 10.1177/089198870101400303. [DOI] [PubMed] [Google Scholar]
- 51.Gelernter J, Southwick S, Goodson S, Morgan A, Nagy L, Charney DS. No association between D2 dopamine receptor (DRD2) ‘A’ system alleles, or DRD2 haplotypes, and posttraumatic stress disorder. Biol Psychiatry. 1999;45:620–625. doi: 10.1016/s0006-3223(98)00087-0. [DOI] [PubMed] [Google Scholar]
- 52.Gilbertson MW, Paulus LA, Williston SK, Gurvits TV, Lasko NB, Pitman RK, Orr SP. Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. Br J Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- 55.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 56.Gottesman II, Shields J. Schizophrenia and genetics: a twin study vantage point. New York: Academic Press; 1972. [Google Scholar]
- 57.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 58.Hall E, Saxe G, Stoddard F, Kaplow J, Koenen K, Chawla N, Lopez C, King L, King D. Posttraumatic stress symptoms in parents of children with acute burns. J Pediatr Psychol. 2006;31:403–412. doi: 10.1093/jpepsy/jsj016. [DOI] [PubMed] [Google Scholar]
- 59.Hamner MB, Diamond BI. Elevated plasma dopamine in posttraumatic stress disorder: a preliminary report. Biol Psychiatry. 1993;33:304–306. doi: 10.1016/0006-3223(93)90302-t. [DOI] [PubMed] [Google Scholar]
- 60.Harvey AG, Bryant RA. The relationship between acute stress disorder and posttraumatic stress disorder: a prospective evaluation of motor vehicle accident survivors. J Consult Clin Psychol. 1998;66:507–512. doi: 10.1037//0022-006x.66.3.507. [DOI] [PubMed] [Google Scholar]
- 61.Hedtke KA, Ruggiero KJ, Saunders BE, Resnick HS, Kilpatrick DG. A longitudinal analysis of the relation between interpersonal violence types and mental health outcomes: results from the National Women’s Study. 2007. Manuscript submitted for publication. [Google Scholar]
- 62.Helzer JE, Robins LN, McEnvoy L. Post-traumatic stress disorder in the general population: findings of the epidemiologic catchment area survey. N Engl J Med. 1987;317:1630–1634. doi: 10.1056/NEJM198712243172604. [DOI] [PubMed] [Google Scholar]
- 63.Hornsby PJ. Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann N Y Acad Sci. 1995;774:29–46. doi: 10.1111/j.1749-6632.1995.tb17370.x. [DOI] [PubMed] [Google Scholar]
- 64.Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- 65.Jang KL, Stein MB, Taylor S, Asmundson GJ, Livesley WJ. Exposure to traumatic events and experiences: aetiological relationships with personality function. Psychiatry Res. 2003;120:61–69. doi: 10.1016/s0165-1781(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 66.Kendler KS, Eaves LJ. Models for the joint effects of genotype and environment on liability to psychiatric illness. Am J Psychiatry. 1986;143:279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- 67.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatr. 2000;61:4–12. [PubMed] [Google Scholar]
- 68.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 69.Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. Serotonin transporter gene and social support moderate PTSD and depression in hurricane-exposed adults. Am J Psychiatry. 2007 doi: 10.1176/appi.ajp.2007.06122007. in press. [DOI] [PubMed] [Google Scholar]
- 70.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J Consult Clin Psychol. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- 71.Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for future studies. J Trauma Stress. 2007 doi: 10.1002/jts.20205. in press. [DOI] [PubMed] [Google Scholar]
- 72.Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord. 2007 doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koenen KC, Harney R, Lyons MJ, Wolfe J, Simpson JC, Goldberg J, Eisen SA, Tsuang M. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. J Nerv Ment Dis. 2002;190:209–218. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Eisen SA, True W, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- 75.Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychol Med. 2007;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- 77.Koenen KC, Stellman SD, Dohrenwend BP, Sommer JF, Jr, Stellman JM. The consistency of combat exposure reporting and course of PTSD in Vietnam War veterans. J Trauma Stress. 2007;20:3–13. doi: 10.1002/jts.20191. [DOI] [PubMed] [Google Scholar]
- 78.Kohnke MD, Batra A, Kolb W, HKohnke AM, Lutz U, Schick S, et al. Association of the dopamine transporter gene with alcoholism. Alcohol Alcohol. 2005;40:339–342. doi: 10.1093/alcalc/agh179. [DOI] [PubMed] [Google Scholar]
- 79.Koplewicz HS, Vogel JM, Solanto MV, Morrissey RF, Alonso CM, Abikoff H, et al. Child and parental response to the 1994 World Trade Center bombing. J Trauma Stress. 2002:77–85. doi: 10.1023/A:1014339513128. [DOI] [PubMed] [Google Scholar]
- 80.Kremen WS, Koenen KC, Boake C, Purcell S, Eisen SA, Franz CE, Tsuang MT, Lyons MJ. Pretrauma cognitive ability and risk for posttraumatic stress disorder: a twin study. Arch Gen Psychiatry. 2007;64:361–368. doi: 10.1001/archpsyc.64.3.361. [DOI] [PubMed] [Google Scholar]
- 81.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 82.Kubzansky LD, Koenen KC, Spiro A, 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the normative aging study. Arch Gen Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- 83.Landolt MA, Vollrath M, Ribi K, Gnehm HE, Sennhauser FH. Incidence and associations of parental and child posttraumatic stress symptoms in pediatric patients. J Child Psychol Psychiatry. 2003;44:1199–1207. doi: 10.1111/1469-7610.00201. [DOI] [PubMed] [Google Scholar]
- 84.Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck D, Krystal JH, Gelernter J. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–831. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- 85.Lawford BR, Mc DYR, Noble EP, Kann B, Arnold L, Rowell J, Ritchie TL. D2 dopamine receptor gene polymorphism: paroxetine and social functioning in posttraumatic stress disorder. Eur Neuropsychopharmacol. 2003;13:313–320. doi: 10.1016/s0924-977x(02)00152-9. [DOI] [PubMed] [Google Scholar]
- 86.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with comorbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 87.Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, Kim YH, Kim YK, Kim JB, Yeon BK, Oh KS, Oh BH, Yoon JS, Lee C, Jung HY, Chee IS, Paik IH. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 88.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendoctrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Litz BT. Emotional numbing in combat-related posttraumatic stress disorder: a critical review and reformulation. Clin Psychol Rev. 1992;12:417–432. [Google Scholar]
- 90.Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM. Do genes influence exposure to trauma? A twin study of combat. Am J Med Genet. 1993;48:22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- 91.Macklin ML, Metzger LJ, Litz BT, McNally RJ, Lasko NB, Orr SP, Pitman RK. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol. 1998;66:323–326. doi: 10.1037//0022-006x.66.2.323. [DOI] [PubMed] [Google Scholar]
- 92.McDermott BM, Cvitanovich A. Posttraumatic stress disorder and emotional problems in children following motor vehicle accidents: an extended case series. Aust N Z J Psychiatry. 2000;34:446–452. doi: 10.1080/j.1440-1614.2000.00753.x. [DOI] [PubMed] [Google Scholar]
- 93.McFarlane AC, Policansky SK, Irwin C. A longitudinal study of the psychological morbidity in children due to natural disaster. Psychol Med. 1987;17:727–738. doi: 10.1017/s0033291700025964. [DOI] [PubMed] [Google Scholar]
- 94.McFarlane J, Parker B, Soeken K, Bullock L. Assessing for abuse during pregnancy. Severity and frequency of injuries and associated entry into prenatal care. Jama. 1992;267:3176–3178. doi: 10.1001/jama.267.23.3176. [DOI] [PubMed] [Google Scholar]
- 95.Miller MM, McEwen BS. Establishing an agenda for translational research on PTSD. Ann N Y Acad Sci. 2006;1071:294–312. doi: 10.1196/annals.1364.023. [DOI] [PubMed] [Google Scholar]
- 96.Miller MW, Kaloupek DG, Dillon AL, Keane TM. Externalizing and internalizing subtypes of combat-related PTSD: a replication and extension using the PSY-5 scales. J Abnorm Psychol. 2004;113:636–645. doi: 10.1037/0021-843X.113.4.636. [DOI] [PubMed] [Google Scholar]
- 97.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 98.Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- 99.Nugent NR, Christopher NC, Delahanty DL. Initial physiological responses and perceived hyperarousal predicts subsequent emotional numbing in child trauma victims. J Trauma Stress. 2006;19:349–359. doi: 10.1002/jts.20130. [DOI] [PubMed] [Google Scholar]
- 100.Nugent NR, Ostrowski S, Christopher NC, Delahanty DL. Parental posttraumatic stress symptoms as a moderator of child’s acute biological response and subsequent posttraumatic stress symptoms in pediatric injury patients. J Pediatr Psychol. 2007;32:309–318. doi: 10.1093/jpepsy/jsl005. [DOI] [PubMed] [Google Scholar]
- 101.Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 102.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 103.Ostrowski SA, Christopher NC, Delahanty DL. Brief report: The impact of maternal posttraumatic stress disorder symptoms and child gender on risk for persistent posttraumatic stress disorder symptoms in child trauma victims. J Pediatr Psychol. 2007;32:338–342. doi: 10.1093/jpepsy/jsl003. [DOI] [PubMed] [Google Scholar]
- 104.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 105.Pelcovitz D, Libov BG, Mandel F, Kaplan S, Weinblatt M, Septimus A. Posttraumatic stress disorder and family functioning in adolescent cancer. J Trauma Stress. 1998;11:205–221. doi: 10.1023/A:1024442802113. [DOI] [PubMed] [Google Scholar]
- 106.Pfefferbaum B, Pfefferbaum RL. Contagion in stress: an infectious disease model for posttraumatic stress in children. Child Adolesc Psychiatr Clin N Am. 1998;7:183–194. [PubMed] [Google Scholar]
- 107.Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitman RK, Orr SP, Lowenhagen MJ, Macklin ML, Altman B. Pre-Vietnam contents of posttraumatic stress disorder veterans’ service medical and personnel records. Compr Psychiatry. 1991;32:416–422. doi: 10.1016/0010-440x(91)90018-8. [DOI] [PubMed] [Google Scholar]
- 109.Plomin R, Bergeman CS. The nature of nurture: genetic influence on ‘environmental’ measures. Behav Brain Sc. 1991;14:373–386. [Google Scholar]
- 110.Pollak SD. Early adversity and mechanisms of plasticity: integrating affective neuroscience with developmental approaches to psychopathology. Dev Psychpathol. 2005;17:735–752. doi: 10.1017/S0954579405050352. [DOI] [PubMed] [Google Scholar]
- 111.Rasmusson AM, Vasek J, Lppschitz DS, Vojvoda D, Mustone ME, Shi Q, Gudmundsen G, Morgan CA, Wolfe J, Charney DS. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1546–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- 112.Rasmusson AM, Vythilingam M, Morgan CA., 3rd The neuroendocrinology of posttraumatic stress disorder: new directions. CNS Spectr. 2003;8:651–656. doi: 10.1017/s1092852900008841. 665–657. [DOI] [PubMed] [Google Scholar]
- 113.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 114.Risch NJ, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 115.Rothman KJ. Epidemiology: an introduction. New York: Oxford University Press; 2002. [Google Scholar]
- 116.Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 117.Sack WH, Clarke GN, Seeley J. Posttraumatic stress disorder across two generations of Cambodian refugees. J Am Acad Child Adolesc Psychiatry. 1995;34:1160–1166. doi: 10.1097/00004583-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 118.Saunders BE. Understanding children exposed to violence: toward an integration of overlapping fields. J Interpers Violence. 2003;18:356–376. [Google Scholar]
- 119.Saxe GN, Miller A, Bartholomew D, Hall E, Lopez C, Kaplow J, Koenen KC, Bosquet M, Allee L, Erikson I, Moulton S. Incidence of and risk factors for acute stress disorder in children with injuries. J Trauma. 2005;59:946–953. doi: 10.1097/01.ta.0000187659.37385.16. [DOI] [PubMed] [Google Scholar]
- 120.Schnurr PP, Lunney CA, Sengupta A. Risk factors for the development versus maintenance of posttraumatic stress disorder. J Trauma Stress. 2004;17:85–95. doi: 10.1023/B:JOTS.0000022614.21794.f4. [DOI] [PubMed] [Google Scholar]
- 121.Segman RH, Cooper-Kazaz R, Macciardi F, Goltser T, Halfon Y, Dobroborski T, Shalev AY. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol Psychiatry. 2002;7:903–907. doi: 10.1038/sj.mp.4001085. [DOI] [PubMed] [Google Scholar]
- 122.Shalev AY, Freedman S, Peri T, Brandes D, Saher T, Orr SP, Pitman RK. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155:630–635. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- 123.Singer MI, Anglin TM, Song LY, Lunghofer L. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA. 1995;273:477–482. [PubMed] [Google Scholar]
- 124.Smith P, Perrin S, Yule W, Rabe-Hesketh S. War exposure and maternal reactions in the psychological adjustment of children from Bosnia–Hercegovina. J Child Psychol Psychiatry. 2001;42:395–404. [PubMed] [Google Scholar]
- 125.Sondergaard HP, Hansson LO, Theorell T. Elevated blood levels of dehydroepiandrosterone sulphate vary with symptom load in posttraumatic stress disorder: findings from a longitudinal study of refugees in Sweden. Psychother Psychosom. 2002;71:298–303. doi: 10.1159/000064806. [DOI] [PubMed] [Google Scholar]
- 126.Southwick SM, Morgan CAI, Nicolaou AL, Charney DS. Consistency of memory for combat-related traumatic events in veterans of Operation Desert Storm. Am J Psychiatry. 1997;154:173–177. doi: 10.1176/ajp.154.2.173. [DOI] [PubMed] [Google Scholar]
- 127.Spivak B, Vered Y, Graff E, Blum I, Mester R, Weizman A. Low platelet-poor plasma concentrations of serotonin in patients with combat-related posttraumatic stress disorder. Biol Psychiatry. 1999;45:840–845. doi: 10.1016/s0006-3223(98)00231-5. [DOI] [PubMed] [Google Scholar]
- 128.Stein MB, Jang KJ, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: a twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 129.Stuber ML, Christakis DA, Houskamp B, Kazak AE. Posttrauma symptoms in childhood leukemia survivors and their parents. Psychosomatics. 1996;37:254–261. doi: 10.1016/S0033-3182(96)71564-5. [DOI] [PubMed] [Google Scholar]
- 130.Sullivan PF, Eaves LJ, Kendler KS, Neale MC. Genetic case-control association studies in neuropsychiatry. Arch Gen Psychiatry. 2001;58:1015–1024. doi: 10.1001/archpsyc.58.11.1015. [DOI] [PubMed] [Google Scholar]
- 131.Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- 132.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder A quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 133.True WJ, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 134.Van Os J, Jones PB. Early risk factors and adult person-environment relationships in affective disorder. Psychol Med. 1999;29:1055–1067. doi: 10.1017/s0033291799001026. [DOI] [PubMed] [Google Scholar]
- 135.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 136.Wilson JP, Keane TM. Assessing psychological trauma and PTSD. New York: Guilford Press; 2004. [Google Scholar]
- 137.Winston FK, Kassam-Adams N, Vivarelli-O’Neill C, Ford J, Newman E, Baxt C, Stafford P, Cnaan A. Acute stress disorder symptoms in children and their parents after pediatric traffic injury. Pediatrics. 2002;109:90–99. doi: 10.1542/peds.109.6.e90. [DOI] [PubMed] [Google Scholar]
- 138.Wong MY, Day NE, Luan JA, Chan KP, Wareham NJ. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int J Epidemiol. 2003;32:51057. doi: 10.1093/ije/dyg002. [DOI] [PubMed] [Google Scholar]
- 139.Xian H, Chantarujikapong SI, Shrerrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True W. Genetic and environmental influences on posttraumatic stress disorder, alcohol, and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 140.Yehuda R, Halligan SL, Bierer LM. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J Psychiatr Res. 2001;35:261–270. doi: 10.1016/s0022-3956(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 141.Yehuda R, Southwick S, Giller EL, Ma X, Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. J Nerv Ment Dis. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 142.Young BR, Lawford BR, Noble EP, Kanin B, Wilkie A, Ritchie T, Arnold L, Shadforth S. Harmful drinking in military veterans with posttraumatic stress disorder: association with the D2 dopamine receptor A1 allele. Alcohol Alcohol. 2002;37:451–456. doi: 10.1093/alcalc/37.5.451. [DOI] [PubMed] [Google Scholar]
- 143.Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, Price LH, Southwick S, Yang BZ, Rasmussen A, Gelernter J. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]