Abstract
Neurosteroids play an important role in the development of the cerebellum. In particular, estradiol and progesterone appear capable of inducing increases in dendritic spine density during development, and there is evidence that both are synthesized de novo in the cerebellum during critical developmental periods. In normal neonates and adults, there are few differences in the cerebellum between the sexes and most studies indicate that hormone and receptor levels also do not differ significantly during development. However, the sexes do differ significantly in risk of neuropsychological diseases associated with cerebellar pathology, and in animal models there are noticeable sex differences in the response to insult and genetic mutation. In both humans and animals, males tend to fare worse. Boys are more at risk for autism and Attention Deficit Hyperactivity Disorder than girls, and schizophrenia manifests at an earlier age in men. In rats males fare worse than females after perinatal exposure to polychlorinated biphenyls, and male mice heterozygous for the staggerer and reeler mutation show a more severe phenotype. Although very recent evidence suggests that differences in neurosteroid levels between the sexes in diseased animals may play a role in generating different disease phenotypes, the reason this hormonal difference occurs in diseased but not normal animals is currently unknown.
Keywords: Neurosteroids, cerebellum, neuropsychological diseases, gender
Introduction
The cerebellum is importantly involved in sensory perception and discrimination in addition to its well known role in motor coordination (1). The visual, auditory, and somatosensory cortices project to the cerebellum via the pontine nuclei, and association cortices project to it via the superior colliculus. It has been proposed that the cerebellum may be responsible for sensory modulation and the integration of multisensory information. Additionally, the cerebellum’s bidirectional connection with the limbic lobe, amygdala, septal nuclei, thalamus and hypothalamus all point to a role for the cerebellum in emotional processing, also suggested by the blunting of emotional affect observed following lesions to the cerebellar vermis (2). The corticothalamic-cerebellar-cortical circuit may coordinate the ‘fluid execution of cortical activity’, suggesting a role in more cognitive tasks (3). Moreover, functional magnetic resonance imaging (fMRI) studies have demonstrated cerebellar activity during attentional tasks in humans, and that cerebellar activation during such tasks differs between healthy controls and autistic patients (4). Animal studies have revealed deficits in working memory in animals with cerebellar lesion (5). Taken together, these findings demonstrate that it is no longer accurate to think of the cerebellum as simply a motor control center, and make less surprising the frequent finding that cerebellar pathology is often associated with complex neuropsychological diseases.
The cerebellum: A sexually dimorphic area?
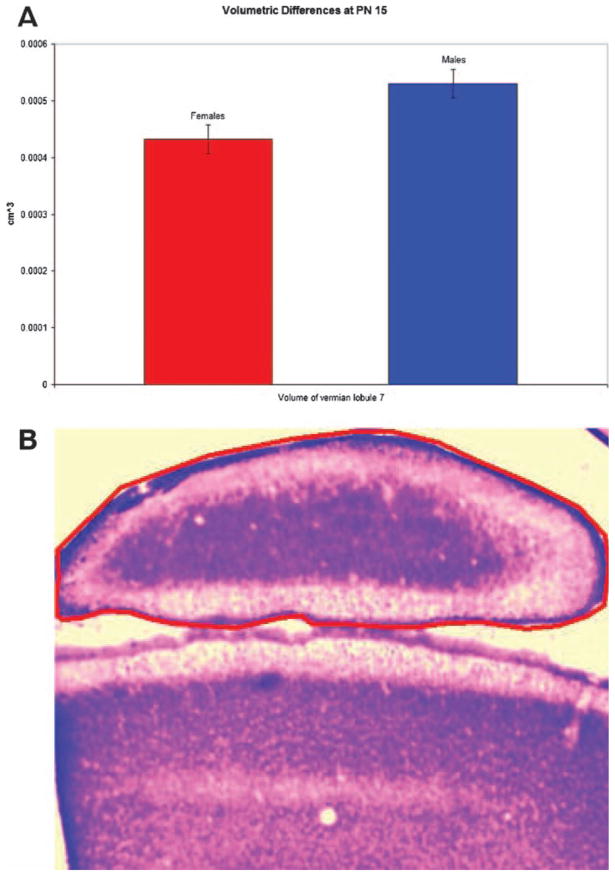
Structurally, the cerebellum has traditionally been considered to be a fairly monomorphic structure with few reliable sex differences. Some magnetic resonance imaging (MRI) studies have found that in humans the cerebellum is larger in adult men than women even when controlling for height; in the hemispheres and anterior vermis the effect size of gender is large and in the posterior vermis it is moderate (6). In children and adolescents MRI reveals an 8% difference in cerebellar volume, with the cerebella of males being larger even when controlling for height and weight (7). In juvenile rats, we have found a trend towards increased volume in lobule seven of the posterior vermis in males versus females (Figure 1). However, in other cases MRI (8) and examination of fixed human tissue (9) have failed to find volumetric differences in the cerebella of women and men, and men and women have an equal number of Purkinje cells (10). Some differences in glial structure may exist. In hamsters, glial fibrillary acidic protein (GFAP) staining is more intense in males, whereas vimentin staining in the Bergman glia is more intense in females both during development and in adulthood (11). Rats show a similar difference in glial staining (12). Although this suggests the possibility of differences in the regulation of glial development between the sexes, the functional significance is not clear. mRNA levels of nerve growth factor (NGF), an important neurotrophic factor, are higher in the cerebella of female rats as compared to males (13).
Figure 1.
(A) Volume of vermian lobule seven in females and males, males exhibited a statistical trend toward slightly greater volume (t-test, p<0.06, n=5). (B) Photomicrograph of a coronal section the cerebellar vermis at low magnification, with lobule seven circled in red.
The cerebellum has also been examined for physiological differences. Adult women have a slightly higher rate of metabolism in the cerebellum as determined by positrom emission tomography (PET) analysis (14). Energy use differences may also exist in animal models; male rats have higher creatinine kinase activity than females, but females display a greater variety of creatinine kinase isoenzymes (15). Behaviorally, inbred mouse strain females show greater improvement in active rotation scores than males after three days (16), but this was not replicated in healthy rats (12). Human females need approximately 50 ms longer to make unexpected 90 degree turns while walking at all ages (17).
In summary while some studies have found basic differences between the sexes in terms of cerebellar anatomy and function, these differences have tended to be small or inconsistent. If one is searching for a robust, reliable sex difference that persists into adulthood and is independent of circulating hormones, looking in the cerebellum is likely to lead to disappointment. Then why examine the cerebellum in the context of sex differences and sex hormones? Similar morphology in adulthood does not preclude differing strategies between the sexes in reaching a similar developmental endpoint (18). Moreover, differences in developmental strategy may lead to differences in susceptibility to early developmental injury. This may provide insight into the sex bias in neurodevelopmental diseases associated with cerebellar pathology, such as autism and schizophrenia.
A brief overview of cerebellar development
The cerebellum develops relatively late, primarily from the metencephalon with a small rostromedial region originating from the mesencephalon (19). In rats the neurons of the deep nuclei of the cerebellum are born between embryonic day 13 and 14 (E13 and 14) (20). In rats the Purkinje cells, which are responsible for all cerebellar cortex output, are born from the ventricular epithelium surrounding the 4th ventricle between embryonic days 14–16 (E14–16). In general, cells in the hemispheres are born before those in the vermis. Starting on E16 the external granule layer (EGL), eventual birth place of the granule cells, spreads across the surface of the developing cerebellum (21). Purkinje cells begin migrating on E17 and settle in the posterior vermis on E19 as the developing lateral cerebellar primoridia fuse (22). Purkinje cells in the anterior portions of the vermis do not reach their final destination until E22. The Golgi cells are born from the same region starting on E19 and continuing on to postnatal day 3 (PN3) (23). Between E20 and E21 fissures appear in the cerebellar cortex, and the white matter of each future lobule begins to organize into discrete trunks (24). By the day of birth (PN0), dendrites from settled Purkinje cells begin to sprout from all aspects of the soma and are innervated by multiple climbing fibers from the cells of the inferior olivary nucleus (25). From approximately PN3 to PN6 these dendrites undergo a pruning process with many dendrites retracting. From PN6 to PN10 a single stem dendrite forms which splits onto many branches. Granule cells are born in the external granule cell layer at the surface of the cerebellar folia, begin to form parallel fibers, and then migrate to the internal granule cell layer during the first two weeks of life (21). The internal granule layer is first visible on PN5. From PN5 to 10 secondary fissures and lobules appear in the cortex of the cerebellum, the Purkinje cells are arranged in a single monolayer (24). Basket cells are primarily born from precursors located in the white matter on postnatal day 6–7 (PN6–7) and begin to take on mature morphology by the middle of the second week of life; stellate cells are born next on PN8–11 and appear in their mature form during the third postnatal week (26). The complex branching of the Purkinje cell dendritic tree reaches its adult width by PN13 and adult height by PN30. By PN15, synaptic contact with multiple climbing fibers has been reduced to contact with a single climbing fiber per Purkinje cell (27). By PN20 the EGL has almost entirely disappeared (24).
Steroid hormones: Not just for sexual differentiation anymore
Introduction to neuroendocrinology
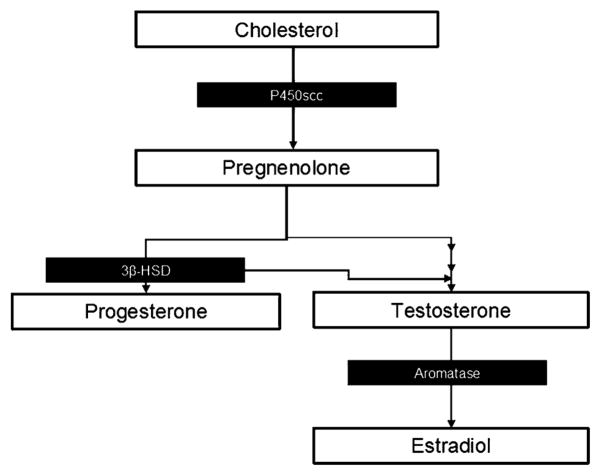
Estradiol, progesterone, and testosterone are all steroid hormones synthesized from cholesterol. Steroidogenesis begins when cholesterol is transferred from the outer mitochondrial membrane to the inner mitochondrial membrane by steroidogenic acute regulatory protein (StAR) and then cleaved to form pregnenolone, precursor of the steroid hormones, by cytochrome P450 side chain cleavage (P450scc) (see Figure 2). These hormones are best known for their role in sexual behavior and fertility in adult humans and animals. However, they are also active players in the normal development of the CNS.
Figure 2.
Simplified diagram of steroid synthesis with pertinent enzymes noted.
In male rodents, there is a surge in testosterone produced by the developing testes during late gestation and again shortly after birth. This results in 2–3 times greater androgen exposure for male compared to female pups. This hormone surge results in significant sexual dimorphisms in several structures important to reproductive behavior, as well as differences in reproductive behavior observed in adult animals in response to circulating hormones in adulthood. For example, as a result of the early hormonal surge the sexually dimorphic nucleus of the preoptic area (POA) is several times larger in males than in females (28), and the density of dendritic spine synapses on POA neurons is greater (29). The POA is a boundary structure that develops from cells migrating from both the classic diencephalic and telencephalic compartments (82). It is treated as a diencephalic structure in this paper due to its similarity in function and hormonal response to other hypothalamic structures. The ventromedial nucleus of the hypothalamus (VMN) is also slightly larger (30) with increased branching of neuronal dendrites (31) in males as compared to females. However, while the hormone secreted by the testes is testosterone, administration of estradiol is just as effective in inducing masculinization in many cases. This is because during development in the CNS testosterone is converted into estradiol by the enzyme aromatase (P450arom). In many cases, it is estradiol that then actually induces these differences.
Estradiol
Estradiol acts primarily via two cytoplasmic receptors, estrogen receptor α(ERα) and estrogen receptor β (ERβ). Once bound, the receptor and ligand can enter the nucleus to modulate gene transcription. In addition to this traditional pathway, estrogen receptor complexes at the membrane allow for more rapid hormone responses (32).
In the diencephalon estradiol acts to produce profound sexual dimorphisms, whereas in telencephalic structures such as the hippocampus, sex differences are far subtler. During adulthood (33) and inconsistently during development (34,35) small volumetric differences have been found, with the hippocampus of males 10–16% larger than in females. However, these differences are significantly smaller than the differences found in diencephalic structures. Further, the functional significance of these small differences is uncertain, particularly in light of increasing evidence that the previous findings of a sex difference in spatial memory may be better explained by a difference in response to anxiety, problem solving strategy, etc. (18). Moreover, in the hippocampus estradiol levels are similar between the sexes during development. This has led to the hypothesis that, rather than relying on gonadally produced steroids, the hippocampus produces its own estradiol de novo resulting in a reduction rather than a production of sex differences (36).
As in the hippocampus, estradiol plays an important role in development of the cerebellum and does not necessarily differ significantly between the sexes. In rats immunoreactivity for ERα is detected as early as PN3 in scattered Purkinje cells in both sexes (37). Cerebellar mRNA levels of ERα are higher during the first three weeks of life than in adulthood, when expression drops to negligible levels (38). In contrast, levels of estrogen receptor beta (ERβ) mRNA are at adult levels on PN2 and then fall throughout the remainder of the first week (39). They begin to rise again, peaking on PN10, and then fall to adult levels. ERβ expression is observed in the external granule cell layer during the first week of life in the posterior midline vermis. Although it is not seen in mature settled glia in the cerebellum, ERβ staining is seen in the immature glia lining the medial portion of the isthmal canal and above the trigonal area, as well as glia migrating through the white matter presumably from these two areas into the cerebellum proper. ERβ staining begins to appear in the Purkinje layer at the end of the second week, appearing first in the cell body and gradually spreading to more distal parts of the maturing dendritic tree. ERβ immunostaining was the most intense in the cytoplasm of the soma but also present in the dendrites of Purkinje cells and dendrite like processes of developing granule cells. In adults, ERβ is expressed in the cytoplasm of most Purkinje cells and scattered cells in the granule cell layer (potentially Golgi cells) (40). No sex differences have yet been reported in estrogen receptor expression levels or distribution in the cerebellum.
There is good evidence that de novo estradiol synthesis occurs in the cerebellum during early development in both sexes. mRNA for StAR and P450scc are both present in the rat cerebellum during early development (41). In males, mRNA for both of these enzymes peaks between PN5 and 10 and then falls thereafter. In females the peak is smaller in magnitude and mRNA levels do not begin to fall until after PN20. In both male and female rats, real-time polymerase chain reaction (RT-PCR) reveals P450arom mRNA expression specifically in Purkinje cells that peaks on PN3 (42). A second, sexually dimorphic peak again occurs at PN10 in males that falls rapidly by PN15 (41). P450arom is low in females at PN10 and remains low until adulthood. The sex difference is not mediated by increased neonatal androgens in males since administration of androgens to newborn female pups does not produce the peak in mRNA observed in males. Estradiol levels have been reported to be higher in the cerebellum than in the plasma during the first week of life (5.92 pmol/g versus 1.97 pmol/g on PN5) but not thereafter and no difference between the sexes has been found, although the PN10 time point was not examined (42).
In both sexes, treatment with the ER antagonist tamoxifen during PN6–9 eliminates dendritic spines on the distal dendrites of Purkinje cells, whereas estradiol administration produces cells with longer dendrites and increased dendritic spine density (42). This is consistent with the developmental role estradiol plays in other areas of the brain. In the POA, for example, estradiol causes an increase in the density of spine like processes on dendrites via induction of PGE2 synthesis (43). PGE2 is a prostaglandin that also mediates fever production by acting in the ventromedial preoptic area. In the VMN estradiol treatment causes an increase in neuronal branching (31), although in the VMN this is not mediated by PGE2 synthesis (44). Neurons in the immediately adjacent arcuate nucleus, in contrast, show reduced density of dendritic spines and axospinous synapses in response to estradiol treatment (31).
Progesterone
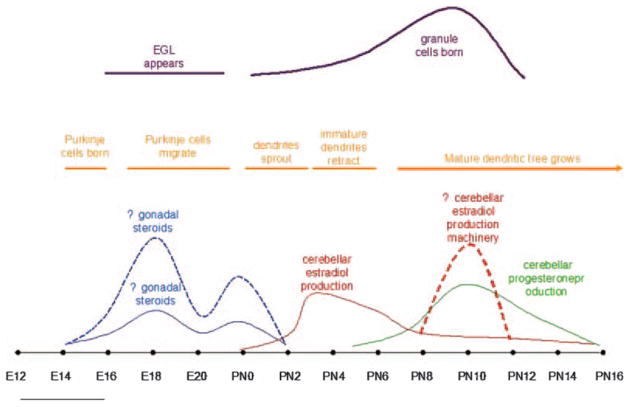
Progesterone is also produced in Purkinje cells and the EGL. In addition to P450scc, Purkinje cells express 3β hydroxysteroid dehydrogenase (3βHSD) (45). In male rats, 3βHSD mRNA expression peaks between PN7 and 14 (female rats were not examined), and enzyme activity assays show that it is most active at this time. Additionally, progesterone concentration measured by RIA is higher in early neonatal life than in adulthood and greatest at 10 days of age, when it is measurably higher in the cerebellum (142 pmol/g) than in the plasma (10 pmol/g). In adulthood, 3βHSD expression is restricted to Purkinje and granule cells near the cerebellar meninges. Additionally, in vivo treatment with progesterone, but not its metabolite allopregnanolone, during PN 3–6 increases maximal Purkinje dendrite outgrowth and differentiation and increases the density of synapses on dendritic spines as measured on day 7. Treatment with the progesterone receptor antagonist, RU 486, during the progesterone peak from day 7 to 10 decreases maximal dendrite length (46). Again, only male rats were examined. In both males and females, progesterone receptor (PR) mRNA is present at low levels at birth and increases until PN7. PR mRNA levels are comparatively decreased on PN14, and then begin to rise again, remaining high up to at least PN60. In the neonate, PR expression is confined to the nuclei of Purkinje cells. This, and the failure of the progesterone metabolite allopregnanolone to replicate the effects of progesterone, suggest that progesterone acts to increase Purkinje cell dendrite length by binding with its receptor and presumably changing gene expression. For schematic representation of hormone levels compared to key developmental time points, see Figure 3.
Figure 3.
Hormonal production overlaps with significant time points in cerebellar development. The surge in estradiol synthesized by the cerebellum corresponds with the retraction of immature dendrites in cerebellar Purkinje cells. Progesterone levels rise at the same time that the mature dendritic tree of Purkinje cells is in the process of developing and the biggest surge in granule cell production is taking place.
Other steroid players
Androgens may also have a role in cerebellar development. Androgen receptors are present in cultured PN 7 cerebellar granule cells. Treatment with androgens reduces susceptibility to oxidative stress, an effect inhibited by the androgen receptor antagonist flutamide (47). However, not all steroid effects on cerebellar development are mediated through traditional steroid receptors. Allopregnanolone, a 5α, 3α reduced metabolite of progesterone, increases proliferation of immature granule cell precursors from PN6–8 rats in culture (48). Allopregnanolone is a positive allosteric modulator of GABAA receptors. At higher concentrations, it functions as GABAA agonist. The GABAA antagonist bicuculline blocks allopregnenolone’s ability to increase granule cell proliferation. GABA is an important neurotrophic factor in many areas of the developing brain (49). Although GABA is traditionally thought of as an inhibitory neurotransmitter in the adult, during early development GABAA receptor stimulation is depolarizing in many cell types, including Purkinje cells (50) and granule cells (51). This is due to high expression of the Na+/K+/2Cl− transporter NKCC1 and low expression of the K+/Cl− transporter KCC2 during development, which leads to high concentrations of Cl− inside the cell and a relatively depolarized reversal potential (around −44 mV in Purkinje cells) (50). Depolarizing GABA stimulation leads to the influx of Ca++ ions through voltage gated Ca++ channels, primarily of the L type. Calcium entry in turn, activates a variety of signaling cascades, including the phosphorylation of CREB (49) which can then modulate gene transcription, leading to responses such as increased survival or neuronal plasticity. In the cerebellum, blockade of GABAA receptors with the selective antagonist bicuculline reduces the number of Purkinje cells in slice cultures of the developing mouse cerebellum (52). Moreover, neurosteroids are known to modulate the GABA response. As has been discussed, allopregnanolone directly modulates GABAA channel opening. Other steroids modulate depolarizing GABA via their traditional receptors. In the hippocampus (53) and hypothalamus (54), estradiol influences both the magnitude of Ca++ influx in response to depolarizing GABA and the developmental time window during which Ca++ influx occurs in response to GABAA receptor stimulation. These effects are receptor dependent as they are inhibited by the ER antagonist ICI182,780. This effect is potentially due to an increase in active phosphorylated NKCC1 (pNKCC1) expression (53).
So far we have discussed normal cerebellum and its development. Most studies show that the traditional sex steroids are key players in cerebellar development but have generally not found differences in steroid or receptor levels between the sexes. The one notable exception is the sex specific peak at PN 10 in mRNA levels of several key proteins in steroid hormone synthesis, but the functional significance of this phenomenon has not yet been explored. However, evidence from both animal studies and human disease demonstrates that when things start to go wrong, the sexes diverge in their ability to recover.
Sex differences in cerebellar pathology
Human studies
Autism is a relatively common neurodevelopmental disease that manifests as deficits in social interaction and communication as well as abnormalities in sensory processing. Males are diagnosed with this disease three to four times more commonly than females (55). Although it is an extremely heterogeneous disease, cerebellar pathology is one of the most frequent findings. Autistic patients have abnormal cerebellar volumes on MRI scans: an MRI study in 3–4 year old children found increased cerebellar volume in autistic patients compared to controls (56), whereas in adults hypoplasia has generally been found (57). These volumetric differences have been most consistently found in the posterior lobe (58,59). In post mortem tissue there are reduced numbers of Purkinje cells (60) and granule cells (61) in autistic patients as compared to controls. In terms of function, fMRI indicates autistic patients have lower activation of the cerebellum during attentional tasks and greater activation during motor tasks than unaffected individuals (62). The sensory abnormalities commonly observed in autism, such as hypersensitivity to touch and sound but reduced sensitivity to normally painful stimuli, has been attributed to disruption of a multisensory feedback loop that normally serves to filter sensory stimuli (1).
Attention-deficit hyperactivity disorder (ADHD) affects approximately four times as many males as females. Impairments include impulsivity, hyperactivity, and attention deficits. ADHD has also been associated with reductions in cerebellar volume in both children and adolescents, and the magnitude of the volume reduction correlates with severity of attentional problems and clinical ADHD ratings (63). Decreased bilateral cerebellar blood flow has also been reported (64).
Schizophrenia is another neuropsychiatric disorder that varies between the sexes. This disease, characterized by social withdrawal, hallucinations and delusions, and disorganized speech or behavior, differs in time of onset rather than prevalence between the sexes. Childhood onset of schizophrenia is more common in boys than girls. Child hood onset schizophrenia is similar in symptomology to adult onset schizophrenia, although the onset tends to be insidious rather than acute and in children younger than seven care must be taken to distinguish between pathological thought disorder and wandering speech normal for this developmental period (65). Although the percentage varies from 91 to 50% in different studies, all studies agree that the majority of children diagnosed with schizophrenia will suffer from a schizophrenia spectrum disorder into adulthood. Adult-onset schizophrenia manifests before the age of 30 in 90% of men with a mean age of 21 for the first psychotic episode (55). In contrast, only 67% of women are diagnosed before the age of 30, with a mean onset of 27. Cerebellar atrophy has been reported in male schizophrenics (66) and in childhood onset schizoprenia (67). Schizophrenic patients show reduced activation of the cerebellum during the Wisconsin Card Sorting Task (68). Genes associated with schizophrenia differ in their expression in the cerebellum in a sex specific manner. Dempster et al. (69) found reduced expression of catelchol-o-methyltransferase (COMT), an enzyme responsible for metabolizing dopamine, in males versus females. Although they did not observe different expression levels of COMT in schizophrenic patients as compared to controls, other groups have observed linkage disequilibrium in the COMT gene in some schizophrenic patients (70).
Cerebellar abnormalities also appear in disorders more common in women; generally these appear later in life. Studies have reported cerebellar atrophy in unipolar depression as well as late onset schizophrenia. Patients with unipolar depression show greater activation of the prefrontal cortex and less activation of the cerebellum when presented with sad stimuli (2). This reduction in activation may actually reflect increased baseline activity rather than decreased response to stimuli, as increased cerebral blood flow has also been observed in depression (71), and successful treatment of both major depression (72) and schizophrenia (73) has been associated with a reduction in cerebral blood flow.
Animal studies
There is a clear gender bias in several developmental human diseases associated with cerebellar pathology. Does this bias reflect some inherent difference in cerebellar development between the sexes that leads to a greater vulnerability in one sex? Animal studies suggest that this could potentially be the case. Males and female rats respond differently to early environmental insults. Although there are no significant differences between healthy male and female rats in performance on the rotorod task, in rat neonates exposed to polychlorinated biphenyls (PCBs) from E11 to PN 21, males fare significantly worse than females in rotorod performance (13). Males exposed to PCBs also show slightly greater reductions in cerebellar mass. PCBs have a variety of industrial applications and are persistent environmental toxins, particularly in areas such as the Hudson River in the northeastern United States. Male rats prenatally exposed to cocaine also show greater motor impairment later in life than their female counterparts, again suggesting greater cerebellar vulnerability in males (74). However, males do not always fare more poorly in response to environmental insults. Female offspring from dams deprived of copper during gestation had reduced levels of protein kinase C gamma (PKCγ) as compared to males, although this effect is reversible with postnatal copper supplements (75). It is known that PKCγ plays a role in cerebellar synaptogenesis and climbing fiber-Purkinje cell synapse maturation, but whether the difference in PKCγ content in females versus males indicates a long lasting functional difference has not yet been investigated.
Mutant studies also hint at intrinsic differences between males and females. Male mice heterozygous for the reeler mutation lose Purkinje cells as they age, but this does not occur in females (76). Reeler mice have a mutation in the reelin gene, an extracellular matrix glycoprotein whose lack in homozygous mutants causes profound pathology in several areas of the CNS, including severe cerebellar hypoplasia. Intriguingly, pilot data has recently linked the mechanism of selective cell loss in male heterozygotes to differences in estradiol (77). Male heterozygotes show reduced levels of aromatase, increased levels of testosterone and a trend towards decreased levels of estradiol. Estradiol treatment decreases Purkinje cell loss in mutant heterozygote males, and treatment with estrogen receptor antagonists such as tamoxifen causes Purkinje cell loss in both heterozygote mutant and wild type female mice.
Another interesting mouse mutant is staggerer. This mutation occurs in the RORα gene, a nuclear receptor unique to Purkinje cells. Homozygotes for this mutation have severely reduced numbers of Purkinje cells with abnormal cytoarchitecture, and lose their granule cells during the first month of life presumably due to the absence of their normal targets. In staggerer heterozygotes, Purkinje cell numbers begin to decline in male mice starting at one month of age. While females do not show decline in Purkinje cell numbers until nine months, by the end of thirteen months the decline is similar between the two sexes (78). This difference in timing of cell loss is particularly intriguing considering that in humans males are more prone to neuropsychiatric diseases that appear developmentally, whereas females are more vulnerable to those that appear later in life.
Discussion and conclusion
The cerebellum is a relatively late developing structure with only subtle sex differences in normal adult humans and animals. However, this relative monomorphism does not preclude a role for the sex steroids in the developing cerebellum. Estradiol is high in the cerebellum during the first week of life and increases the density of dendritic spines in the cerebellum. Progesterone may play a similar role during the second week of development. Moreover, both of these steroids are produced de novo in the developing cerebellum by the Purkinje cells themselves. Most evidence suggests that in normal animals there is no difference between the sexes in terms of hormone or receptor levels.
In contrast with the findings in healthy humans and animals, there is a significant gender bias in the prevalence of human diseases tied to cerebellar malfunction. Generally, males have increased prevalence of developmental disorders. This observation in humans correlates well with the increased vulnerability in males to a variety of environmental insults as well as the more severe phenotype in males seen in some mutant animals.
We can clearly show males and females differ in their response to insult and injury. What remains elusive is the reason why this should be so. If males and females mostly appear the same during normal development and reach the same developmental end point, how can it be that they differ so significantly in their vulnerability to disruption of normal development? Perhaps the most tantalizing piece of evidence brought to light so far is the difference in aromatase seen in male and female heterozygous reeler mutants. Yet we still cannot explain why aromatase should be reduced selectively in heterozygote males when it is the same in normal animals. Uncovering the mechanism behind this difference would be a major step forward in explaining the sex difference in cerebellar vulnerability during development. One major step forward in this regard would be distinguishing whether this sexually specific aromatase reduction is ultimately gonadally driven or due to some effect of the sex chromosomes themselves. Another area that needs to be explored is the partners of the steroid hormones during development. As we have seen, in other brain areas estradiol can modulate depolarizing GABA and upregulate prostaglandins such as PGE2. GABA is depolarizing in Purkinje cells during the developmental time window when estradiol is high in the cerebellum, and irregularities in GABA production have been associated with autism (79). Cycloxogenase-2 (COX2), the rate limiting first step in prostaglandin production, has been observed in some glial cells in the cerebellum (80). In some studies autism has been associated with viral infections (81) which cause an upregulation in prostaglandins during fever production. A satisfying explanation for the gender bias in neurodevelopmental diseases tied to the cerebellum has yet to be reached. We can hypothesize that early insults to the cerebellum disrupt endocrine signaling which in turn prevents normal cerebellar development, and that this may be one of the causes of disease states such as autism and ADHD, but the questions posed above need to be answered before a detailed mechanism for this disruption can be proposed. Studying the role of steroid hormones and their partners in the developing cerebellum gives us many potential puzzle pieces. The trick will be figuring out how they fit together.
References
- 1.Kern JK. The Possible role of the cerebellum in autism/PDD: disruption of a multisensory feedback loop. Med Hypoth. 2002;59:255–60. doi: 10.1016/s0306-9877(02)00212-8. [DOI] [PubMed] [Google Scholar]
- 2.Konarski JK, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–86. [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional pre-frontal–thalamic–cerebellar circuitry. Proc Natl Acad Sci USA. 1996;93(18):9985–90. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry. 2003;160:262–73. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- 5.Mandolesi L, Leggio MG, Graziano A, Neri P, Petrosini L. Cerebellar contribution to spatial event processing: involvement in procedral and working memory components. Eur J Neurosci. 2001;14:2011–22. doi: 10.1046/j.0953-816x.2001.01819.x. [DOI] [PubMed] [Google Scholar]
- 6.Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex difference in the cerebellum and the ventral pons: a prospective MR study of healthy adults. Am J Neuroradiol. 2001;22:1161–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 8.Nopoulos P, Flaum M, O’Leary D, Andreason NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 9.Henery CC, Mayhew TM. The cerebrum and cerebellum of the fixed human brain: efficient and unbiased estimates of volumes and cortical surface areas. J Anat. 1989;167:167–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Mayhew TM, MacLaren R, Henery CC. Fractionator studies on Purkinje cells in the human cerebellum: numbers in right and left halves of male and female brains. J Anat. 1990;169:63–70. [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez I, Bodega G, Rubio M, Fernandez B. Sexual dimorphism in the hamster cerebellum demonstrated by glial fibrillary acidic protein (GFAP) and vimetin immunoreactivity. Glia. 1992;5:10–16. doi: 10.1002/glia.440050103. [DOI] [PubMed] [Google Scholar]
- 12.Nguon K, Ladd B, Baxter MG, Sadjel-Sulkowska EM. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog Brain Res. 2005;148:341–51. doi: 10.1016/S0079-6123(04)48027-3. [DOI] [PubMed] [Google Scholar]
- 13.Kornack DR, Lu B, Black IB. Sexually dimorphic expression of the NGF receptor gene in the developing rat brain. Brain Res. 1991;542:171–4. doi: 10.1016/0006-8993(91)91015-s. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GL, Fowler JS, Hitzemann R, Pappas N, Paskani K, Wong C. Gender differences in cerebellar metabolism: test-retest reproducibility. Am J Psychiatry. 1997;154:119–21. doi: 10.1176/ajp.154.1.119. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez O, Jiminez E. Sexual dimorphism in rat cerebrum and cerebellum: different patterns of catalytically active creatinine kinase isoenzymes during postnatal development and aging. Int J Dev Neurosci. 2002;20:627–29. doi: 10.1016/s0736-5748(02)00102-8. [DOI] [PubMed] [Google Scholar]
- 16.McFayden MP, Kusek G, Bolivar BJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–19. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 17.Cao C, Ashton-Miller JA, Schultz AB, Alexander NB. Abilities to turn suddenly while walking: effect of age, gender and available response time. J Gerentol A Biol Sci Med Sci. 1997;52:M88–93. doi: 10.1093/gerona/52a.2.m88. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Hallonet ME, Teillet MA, Le Douarin NM. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. doi: 10.1242/dev.108.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Altman J, Bayer SA. Prenatal development of the cerebellar system in the rat. I. Cytogenesis and histogenesis of the deep nuclei and the cortex of the cerebellum. J Comp Neurol. 1978;179(1):23–48. doi: 10.1002/cne.901790104. [DOI] [PubMed] [Google Scholar]
- 21.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 22.Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985;231:42–65. doi: 10.1002/cne.902310105. [DOI] [PubMed] [Google Scholar]
- 23.Uzman LL. The histogenesis of the mouse cerebellum as studied by its tritiated thymidine uptake. J Comp Neurol. 1960;114:137–59. doi: 10.1002/cne.901140204. [DOI] [PubMed] [Google Scholar]
- 24.Altman J, Bayer SA. The cerebellar system in relation to its evolution, structure, and function. CRC Press, Inc; New York: 1997. [Google Scholar]
- 25.Crepel F, Delhaye-Bouchaud N, Dupont JL. Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, x-irradiated and hypothyroid rats. Brain Res. 1981;227:59–71. doi: 10.1016/0165-3806(81)90094-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron. 1996;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 27.Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–78. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- 28.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–39. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- 29.Larriva-Sahd J. Ultrastructural evidence of a sexual dimorphism in the neuropil of the medial preoptic nucleus of the rat: a quantitative study. Neuroendocrinology. 1991;54:416–9. doi: 10.1159/000125923. [DOI] [PubMed] [Google Scholar]
- 30.Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432:329–45. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- 31.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–72. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moriarty K, Kim KH, Bender JR. Estrogen receptor-mediated rapid signaling. Endocrinology. 2006 Dec;147(12):5557–63. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 33.Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–98. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- 34.Hilton GD, Nunez JL, McCarthy MM. Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats. Neuroscience. 2003;116:383–91. doi: 10.1016/s0306-4522(02)00716-9. [DOI] [PubMed] [Google Scholar]
- 35.Nunez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003;181:258–69. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–17. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 37.Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–39. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda Y, Nagai A. Differential expression of the estrogen receptors alpha and beta during postnatal development of the rat cerebellum. Brain Res. 2006;1083:39–49. doi: 10.1016/j.brainres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Jakab RL, Wong JK, Belcher SM. Estrogen receptor beta immunoreactivity in differentiating cells of the developing rat cerebellum. J Comp Neurol. 2001;430:396–409. doi: 10.1002/1096-9861(20010212)430:3<396::aid-cne1039>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 40.Price RH, Jr, Handa RJ. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci Lett. 2000;288:115–8. doi: 10.1016/s0304-3940(00)01221-0. [DOI] [PubMed] [Google Scholar]
- 41.Lavaque E, Mayen A, Azcoitia I, Tena-Sempere M, Garcia-Segura LM. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J Neurobiol. 2006;66:308–18. doi: 10.1002/neu.20221. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144:4466–77. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- 43.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–96. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–21. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Ukena K, Kohchi C, Tsutsui K. Expression and activity of 3beta-hydroxysteroid dehydrogenase/delta5-delta4-isomerase in the rat Purkinje neuron during neonatal life. Endocrinology. 1999;140:805–13. doi: 10.1210/endo.140.2.6516. [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto H, Ukena K, Tsutsui K. Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci. 2001;21:6221–32. doi: 10.1523/JNEUROSCI.21-16-06221.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–62. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 48.Keller EA, Zamparini A, Borodinsky LN, Gravielle MC, Fiszman ML. Role of allopregnanolone on cerebellar granule cells neurogenesis. Brain Res Dev Brain Res. 2004;153:13–7. doi: 10.1016/j.devbrainres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy MM, Auger AP, Perrot-Sinal TS. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002;25:307–12. doi: 10.1016/s0166-2236(02)02182-3. [DOI] [PubMed] [Google Scholar]
- 50.Eilers J, Plant TD, Marandi N, Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol. 2001;536(Pt 2):429–37. doi: 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chavas J, Marty A. Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J Neurosci. 2003;23:2019–31. doi: 10.1523/JNEUROSCI.23-06-02019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obata K. Excitatory and trophic action of GABA and related substances in newborn mice and organotypic cerebellar culture. Dev Neurosci. 1997;19:117–9. doi: 10.1159/000111195. [DOI] [PubMed] [Google Scholar]
- 53.Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–61. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- 54.Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JP, McCarthy MM. Estradiol enhances excitatory gamma-aminobutyric [corrected] acid-mediated calcium signaling in neonatal hypothalamic neurons. Endocrinology. 2001;142:2238–43. doi: 10.1210/endo.142.6.8180. [DOI] [PubMed] [Google Scholar]
- 55.Andreasen NC, Black DW. Introductory textbook of psychiatry. 3. Washington, DC: American Psychiatric Publishing, Inc; 2001. [Google Scholar]
- 56.Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–92. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 57.Murakami JW, Courchesne E, Press GA, Yeung-Courchesne R, Hesselink JR. Reduced cerebellar hemisphere size and its relationship to vermal hypoplasia in autism. Arch Neurol. 1989;46:689–94. doi: 10.1001/archneur.1989.00520420111032. [DOI] [PubMed] [Google Scholar]
- 58.Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–54. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- 59.Levitt JG, Blanton R, Capetillo-Cunliffe L, Guthrie D, Toga A, McCracken JT. Cerebellar vermis lobules VIII-X in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:625–33. doi: 10.1016/s0278-5846(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 60.Courchesne E. Neuroanatomic imaging in autism. Pediatrics. 1991;87(5 Pt 2):781–90. [PubMed] [Google Scholar]
- 61.Heh CW, Smith R, Wu J, Hazlett E, Russell A, Asarnow R, Tanguay P, Buchsbaum MS. Positron emission tomography of the cerebellum in autism. Am J Psychiatry. 1989;146:242–5. doi: 10.1176/ajp.146.2.242. [DOI] [PubMed] [Google Scholar]
- 62.Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry. 2003;160:262–73. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- 63.Schneider M, Retz W, Coogan A, Thome J, Rosler M. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD)-a neurological view. Eur Arch Psychiatry Clin Neurosci. 2006;256 (Suppl 1):i32–i41. doi: 10.1007/s00406-006-1005-3. [DOI] [PubMed] [Google Scholar]
- 64.Kim BN, Lee JS, Shin MS, Cho SC, Lee DS. Regional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysis. Eur Arch Psychiatry Clin Neurosci. 2002;252:219–25. doi: 10.1007/s00406-002-0384-3. [DOI] [PubMed] [Google Scholar]
- 65.Asarnow JR, Tompson MC, McGrath EP. Annotation: childhood-onset schizophrenia: clinical and treatment issues. J Child Psychol Psychiatry. 2004;4:180–94. doi: 10.1111/j.1469-7610.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 66.Okugawa G, Sedvall G, Nordstrom M, Andreasen N, Pierson R, Magnotta V, Agartz I. Selective reduction of the posterior superior vermis in men with chronic schizophrenia. Schizophr Res. 2002;55:61–7. doi: 10.1016/s0920-9964(01)00248-1. [DOI] [PubMed] [Google Scholar]
- 67.Keller A, Castellanos FX, Vaituzis AC, Jeffries NO, Giedd JN, Rapoport JL. Progressive loss of cerebellar volume in childhood-onset schizophrenia. Am J Psychiatry. 2003;160:128–33. doi: 10.1176/appi.ajp.160.1.128. [DOI] [PubMed] [Google Scholar]
- 68.Riehemann S, Volz HP, Stutzer P, Smesny S, Gaser C, Sauer H. Hypofrontality in neuroleptic-naive schizophrenic patients during the Wisconsin Card Sorting Test – a fMRI study. Eur Arch Psychiatry Clin Neurosci. 2001;251:66–71. doi: 10.1007/s004060170055. [DOI] [PubMed] [Google Scholar]
- 69.Dempster EL, Mill J, Craig IW, Collier DA. The quantification of COMT mRNA in post mortem cerebellum tissue: diagnosis, genotype, methylation and expression. BMC Med Genet. 2006;7:10. doi: 10.1186/1471-2350-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handoko HY, Nyholt DR, Hayward NK, Nertney DA, Hannah DE, Windus LC, McCormack CM, Smith HJ, Filippich C, James MR, Mowry BJ. Separate and interacting effects within the catechol-O-methyltransferase (COMT) are associated with schizophrenia. Mol Psychiatry. 2005;10:589–97. doi: 10.1038/sj.mp.4001606. [DOI] [PubMed] [Google Scholar]
- 71.Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia – focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–15. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 72.Davies J, Lloyd KR, Jones IK, Barnes A, Pilowsky LS. Changes in regional cerebral blood flow with venlafaxine in the treatment of major depression. Am J Psychiatry. 2003;160:374–6. doi: 10.1176/appi.ajp.160.2.374. [DOI] [PubMed] [Google Scholar]
- 73.Loeber RT, Sherwood AR, Renshaw PF, Cohen BM, Yurgelun-Todd DA. Differences in cerebellar blood volume in schizophrenia and bipolar disorder. Schizophr Res. 1999;37:81–9. doi: 10.1016/s0920-9964(98)00137-6. [DOI] [PubMed] [Google Scholar]
- 74.Markowski VP, Cox C, Weiss B. Prenatal cocaine exposure produces gender-specific motor effects in aged rats. Neurotoxicol Teratol. 1998;20:43–53. doi: 10.1016/s0892-0362(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 75.Johnson WT, Prohaska JR. Gender influences the effect of perinatal copper deficiency on cerebellar PKC gamma content. Biofactors. 2000;11:163–9. doi: 10.1002/biof.5520110302. [DOI] [PubMed] [Google Scholar]
- 76.Hadj-Sahraoui N, Frederic F, Delhaye-Bouchaud N, Mariani J. Gender effect on Purkinje cell loss in the cerebellum of the heterozygous reeler mouse. J Neurogenet. 1996;11:45–58. doi: 10.3109/01677069609107062. [DOI] [PubMed] [Google Scholar]
- 77.Biamonte F, Assenza G, Marino R, Caruso D, Crotti S, Melcangi RC, Cesa R, Strata P, Keller F. Interaction between estrogens and reelin in Purkinje cell development. Program # 322.18/C5 2006; Neuroscience Meeting Planner; Atlanta, GA: Society for Neuroscience; 2006. Online. [Google Scholar]
- 78.Doulazmi M, Frederic F, Lemaigre-Dubreuil Y, Hadj-Sahraoui N, Delhaye-Bouchaud N, Mariani J. Cerebellar Purkinje cell loss during life span of the heterozygous staggerer mouse (Rora(+)/Rora(sg)) is gender-related. J Comp Neurol. 1999;411:267–73. [PubMed] [Google Scholar]
- 79.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDA proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–10. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 80.Hirst WD, Young KA, Newton R, Allport VC, Marriott DR, Wilkin GP. Expression of COX-2 by normal and reactive astrocytes in the adult rat central nervous system. Mol Cell Neurosci. 1999;13:57–68. doi: 10.1006/mcne.1998.0731. [DOI] [PubMed] [Google Scholar]
- 81.Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirol. 2005;11:1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- 82.Henderson RG, Brown AE, Tobet SA. Sex differences in cell migration in the preoptic area/anterior hypothalamus of mice. J Neurobiol. 1999 Nov 5;41(2):252–66. doi: 10.1002/(sici)1097-4695(19991105)41:2<252::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]