Abstract
Asthma is a complex genetic disease with multiple genetic and environmental determinants contributing to the observed variability in response to common anti-asthma therapies. Asthma pharmacogenetic research has focused on multiple candidate genes including the β2-adrenergic receptor gene (ADRβ2) and its effect on individual responses to beta agonist therapy. At present, knowledge about the effects of ADRβ2 variation on therapeutic responses is evolving and should not alter current Asthma Guideline approaches consisting of the use of short acting beta agonists for as-needed symptom based therapy and the use of a regular long-acting beta agonist in combination with inhaled corticosteroid therapy for optimal control of asthma symptoms in those asthmatics who are not controlled on inhaled corticosteroid alone. This approach is based upon studies showing a consistent pharmacogenetic response to regular use of short acting beta agonists (SABA) and less consistent findings in studies evaluating long acting beta agonist (LABA). While emerging pharmacogenetic studies are provocative and should lead to functional approaches, conflicting data with responses to LABA therapy may be caused by factors that include small sample sizes of study populations and differences in experimental design that may limit the conclusions that may be drawn from these clinical trials at the present time.
Introduction
Asthma is a common disease that affects greater than 300 million people worldwide.1 It is a complex, chronic disease with multiple genetic and environmental determinants contributing to its heterogeneous phenotypic expression. Patients with asthma can present with varying degrees of airway inflammation and airflow obstruction that can be treated with different combinations of short-term relief and long-term controller medications including beta-2 adrenergic receptor agonists, corticosteroids (usually inhaled), leukotriene modifiers, cromones, and theophylline.
Asthma is heterogeneous in its responsiveness to common pharmacological therapies. Between 70 to 80% of patients with asthma have a variable response to common anti-asthma therapies, whether beneficial or detrimental.2,3,4 This variability in drug responses cannot be simply attributed to differences in patient adherence, suggesting that a proportion of this variance may be caused by pharmacogenetic factors.4-6 Pharmacogenetics represents a gene by environment interaction where the environment is the drug exposure and the result is the variation in individual response to pharmacologic therapy. (Figure 1) While additional factors such as interactions with other medications, co-morbid conditions, and age also effect the variability in response to anti-asthma therapies, it has been estimated that 20 to 95% of the variation in drug response may be attributed to heritable differences.4,5,6
Figure 1.
No legend self explanatory
Background Issues: β2-adrenergic Agonist Controversy
Among the anti-asthma therapies available today, β2-adrenergic receptor agonists (e.g., beta agonists) are the most commonly prescribed therapeutic agents for the management of asthma and other obstructive pulmonary diseases.7 Two classes of inhaled β2-adrenergic receptor agonists include the long-acting beta agonists [LABA: salmeterol and formoterol] and short-acting beta agonists [SABA: (albuterol)]. LABA therapy is used in conjunction with inhaled corticosteroid therapy (ICS) for the management of asthma during long-term maintenance therapy and SABA therapy is used for acute, as-needed bronchodilator symptom relief. 7
While beta agonists are the most commonly used therapeutic agents in asthma, this drug class has been associated with adverse therapeutic events that have been primarily related to dosing regimens and potency of the specific agent. In the United Kingdom and other countries in which higher dose isoproterenol inhalers (a potent non-specific beta short acting beta agonist) were marketed, there was an increased asthma mortality that disappeared with the withdrawal of this preparation8. In the period between 1976 and 1989 in New Zealand fenoterol (a potent non-specific SABA) was marketed in a high dose and regular dose inhalers. Asthma mortality increased with this agents’ release and decreased when it was taken off the market9-11. Additional data showed that increased numbers of prescriptions of this drug and other SABA’s increased the risk of mortality implying either a cause and effect relationship or that increased SABA use was a marker of poor asthma control12-14. Subsequently, Sears and colleagues showed that asthma control was worse with regular use of fenoterol12 and the NIH Asthma Clinical Research Network Trial (ACRN) BAGS reported that asthma control did not improve with regular compared to as needed symptomatic use of the SABA albuterol15. Finally, two more recent studies have reported an increase for asthma related mortality (SNS)16 and respiratory deaths primarily in patients receiving LABA mono-therapy (SMART Study)17. While some meta analysis have reported increased risk with LABA18 therapy other meta analysis including a Cochrene analysis and a large case-control analysis have not shown adverse effects of LABA on major asthma exacerbations and life threatening events19-21. In addition a large number of clinical trials have shown that the addition of a LABA to an inhaled corticosteroid reduces exacerbations and improves asthma control. 22-27. This background information on the potential adverse effects of beta agonist therapy is important because it emphasizes the heterogeneity in therapeutic responses to beta agonist therapy and the importance of understanding pharmacogenetic responses with beta agonist therapy in asthma.
Pharmacogenetics of the ADRβ2 Gene
Pharmacogenetics is the study of the role of genetic variability in determining individual responses to pharmacological therapies, such as beta agonists. (Figure 1) The goal of pharmacogenetic research to asthma is to characterize genetic determinants that will predict the likelihood that an individual with asthma will respond favorably or adversely to a given pharmacological agent such as beta-agonists. To date, most of asthma pharmacogenetic research has focused on SABA and LABA therapy, the β2-adrenergic receptor and the gene encoding for the receptor, the β2-adrenergic receptor gene or ADRβ2. Over the last 20 years genomic investigators have characterized genetic variants or polymorphisms throughout ADRβ2 and the contribution of those variants to asthma susceptibility and susceptibility to the adverse effects of LABA and SABA therapy. In this review, we will discuss the pharmacogenetics of ADRβ2 genetic polymorphisms from the molecular level to the clinical findings and the role that genomic research has played in determining the role of ADRβ2 genetic variation in the management of asthma with SABA and LABA therapy.
ADRβ2: The Gene
ADRβ2 was first sequenced approximately 20 years ago. ADRβ2 is a small, intronless gene with one exon that encodes for a 413-amino acid, G-protein coupled receptor, the β2-adrenergic receptor.28,29 ADRβ2 is an ideal candidate gene for the study of asthma genetics due to its location on chromosome 5q31: a region within the human genome consistently linked to asthma and related phenotypes (bronchial hyperresponsiveness (BHR), and serum IgE levels) through large family-based linkage studies in the Dutch, Caucasians, African Americans, and U.S. Hispanics.30-33 The linkage to asthma susceptibility within this region of the human genome has also been shown to have a gene-environment interaction with passive smoking exposure.30
In 1992, a detailed mutational analysis of ADRβ2 was reported by Reihaus et al who identified nine genetic variants or polymorphisms along the coding region of ADRβ2.34 Four of these polymorphisms are single nucleotide changes in the genetic code referred to as single nucleotide polymorphisms (SNPs). These SNPs cause coding changes of the amino acid (AA) product at each codon or amino acid position relative to the start codon. Denoted as AA1codonAA2 (AA1 and AA2 referring to the amino acid products of the two differing alleles) these SNPs include Gly16Arg, Gln27Glu, Val34Met, and Thr164Ile. By definition, a single SNP and its resulting amino acid product determines an individual’s genotype (e.g., Arg16 versus Gly16). However, more than one SNP evaluated as a group determines an individual’s haplotype (e.g., Arg16/Gln27). Gly16Arg and Gln27Glu are two common ADRβ2 SNPs in the general population that have been identified in all ethnic and racial groups screened to date with frequencies that vary between ethnic groups such as Caucasians and African-Americans, independent of asthma disease status. The Val34Met and Thr164Ile polymorphisms are exceedingly rare. No individuals homozygote for the less common allele at codon 164, Ile164, have been identified to date due to the rare frequency of this allele.35
ADRβ2 Pharmacogenetics at the Molecular Level
In vitro or cell-based genetic studies of ADRβ2 have characterized the functional role of genetic variation within ADRβ2 in the regulation of beta agonist-induced downregulation and desensitization of the β2-adrenergic receptor. Two ADRβ2 polymorphisms, Gly16Arg and Gln27Glu, have both been shown to influence the downregulation of the β2-adrenergic receptor in Chinese hamster fibroblasts and human airway smooth muscle cells.36, 37 In Chinese hamster fibroblasts, substitution of the amino acid combination or haplotype Arg16/Gln27 with Gly16/Gln27 or Gly16/Gln27 (basically, changing Arg16 to Gly16) results in enhanced isoproterenol-induced downregulation of the β2-adrenergic receptor from 29% to 41% and 39%, respectively. Conversely, substitution of Arg16/Gln27 with Arg16/Gln27 (changing Gln27 to Gln27) results in resistance to isoproterenol-induced downregulaton of the receptor.36 In human airway smooth muscle cells the findings were similar: receptors with the Gly16 genotype underwent enhanced agonist-promoted downregulaton while the Gln27 genotype showed attenuation of agonist-promoted desensitization with isoproterenol.37
In human airway smooth muscle cells, the effect of ADRβ2 polymorphisms on beta agonist-induced acute and long term β2-adrenergic receptor desensitization were studied by measuring the effects of isoproterenol treatment on muscle cell relaxation and cyclic adenosine monophosphate (cAMP) levels. The Gln27 allele (assumed to be Gly16/Gln27) was associated with greater acute and long-term desensitization. Haplotype analysis of ADRβ2 indicates that the Arg16 allele is almost always coinherited with the Gln27 allele or that these polymorphisms are in “linkage disequilibrium” (in other words, Arg16/Gln27, Gly16/Gln27, and Gly16/Gln27 are common haplotypes while Arg16/Gln27 is rare).38 In the human airway smooth muscle cell model, the Gly16/Gln27 haplotype exhibited less acute and long-term desensitization than Gly16/Gln27 virtually eliminating the effect of the Gly16 genotype.39,40
Arg19(BUP)Cys is a SNP located in the promoter region of ADRβ2 (position -47 relative to the start codon) in the 5’ leader cistron or the Beta Upstream Peptide (BUP), a 19 amino acid peptide that modulates receptor translation (Figure 1). The Gln27Glu polymorphism’s association with beta agonist-induced receptor desensitization may be explained, at least in part, by its association through linkage disequilibrium with Arg19(BUP)Cys since Gln27 is coinherited with Arg19(BUP) and Gln27 is coinherited with Cys19(BUP).34, 35 Studies of COS-7 cells transfected with Arg19Cys genotypes show that cells with the Cys19(BUP) allele exhibit increased β2-adrenergic receptor translation. Interestingly, levels of the mRNA transcripts between genotypes were similar indicating that Arg19(BUP)Cys only regulates receptor translation, not transcription.41
Drysdale and co-workers investigated the interaction of multiple ADRβ2 polymorphisms on transcription and translation after sequencing the gene in a subset of Caucasians, African-Americans, Asians, and Hispanics.38 Drysdale identified 13 polymorphisms and organized them into 12 haplotype groups with varying frequencies between ethnic groups (Table 1). The four most common haplotypes identified were haplotypes 1, 2, 4, and 6, representing Cys19(BUP)/Arg16/Gln27, Arg19(BUP)/Gly16/Gln27, Cys19(BUP)/Arg16/Gln27, and Cys19(BUP)/Gly16/Gln27, respectively (Table 1). HEK293 cells that were homozygotes for haplotype 2 exhibited a higher level of receptor expression and gene transcription compared to haplotype 4 homozygotes. The in vitro findings were consistent with in vivo findings of albuterol-induced FEV1 reversibility in 121 Caucasians: FEV1 reversibility was greatest in haplotype 2 homozygotes and lowest in haplotype 4 homozygotes.38
Table 1.
Drysdale haplotypes and haplotype frequencies in different ethnic groups per Drysdale et al Hawkins et al.*ˆ31,34
| Drysdale Haplotype Groups | Polymorphism allele by nucleotide | Haplotype frequencies (%) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -1023 (5’UTR) |
-709 (5’UTR) |
-654 (5’UTR) |
-468 (5’UTR) |
-406 (5’UTR) |
-367 (5’UTR) |
-47 BUP (Cys/Arg19) |
-20 (5’UTR) |
+46 (Gly/Arg16) |
+79 (Gln/Glu27) |
+252 (Leu/Leu84) |
+491 (Thr/Ile164) |
+523 (Arg/Arg175) |
CA per Hawkins et al | AA per Hawkins et al | CA per Drysdale et al | AA per Drysdale et al | HIS per Drysdale et al | AS per Drysdale et al | |
| 1 | A | C | G | C | C | T | T | T | A | C | G | C | C | 0 | 17 | 0.7 | 25 | 10 | 12.5 |
| 2 | A | C | G | G | C | C | C | C | G | G | G | C | C | 38 | 12 | 48.3 | 6.3 | 26.7 | 10 |
| 3 | G | A | A | C | C | T | T | T | A | C | G | C | C | 0 | 0 | 0.7 | 0 | 0 | 0 |
| 4 | G | C | A | C | C | T | T | T | A | C | G | C | C | 37 | 21 | 33 | 29.7 | 40 | 45 |
| 5 | G | C | A | C | C | T | T | T | G | C | G | C | C | 0 | 0 | 1.4 | 0 | 0 | 0 |
| 6 | G | C | G | C | C | T | T | T | G | C | A | C | A | 12 | 28 | 13.2 | 31.3 | 13.3 | 30 |
| 7 | A | C | G | G | C | C | C | C | G | G | G | C | C | 2 | 0 | 1 | 1.6 | 3.3 | 0 |
| 8 | G | C | A | C | C | T | T | T | A | C | A | C | A | 0 | 0 | 0.7 | 0 | 0 | 0 |
| 9 | A | C | G | C | T | T | T | T | A | C | G | C | C | 0 | 3 | 0 | 4.7 | 0 | 0 |
| 10 | G | C | G | C | C | T | T | T | G | C | A | C | C | 2 | 0 | 0.7 | 0 | 0 | 0 |
| 11 | G | C | G | C | C | T | T | T | G | C | G | C | C | 0 | 0 | 0.3 | 0 | 0 | 2.5 |
| 12 | G | C | G | C | C | T | T | T | G | C | A | C | C | 0 | 0 | 0 | 1.6 | 3.3 | 0 |
5’UTR=5’ Untranslated Region, BUP=Beta Upstream Peptide.
CA=Caucasians, AA=African Americans, Hispanics=HIS, AS=Asians.
The effect of the Thr164Ile polymorphism on β2-adrenergic receptor binding affinity and coupling to Gs has been studied in CHW-1102 cells. Cells with the rare variant, Ile164, exhibited decreased receptor binding affinity with epinephrine, isoproterenol, and norepinephrine. Furthermore, cells with the Ile164 variant showed diminished receptor coupling to the Gs protein as determined by depression of adenylyl cyclase activity.40
ADRβ2 Pharmacogenetics, Asthma Susceptibility, and Responsiveness to Acute SABA Therapy
In vitro studies suggest that two ADRβ2 polymorphisms, Gly16Arg and Gln27Glu, play a significant role in receptor down-regulation and desensitization and, in theory, may predict susceptibility to asthma phenotypes and the adverse effects of SABA therapy. These polymorphisms have served as the fundamental basis of interpreting in vivo, candidate gene analyses of ADRβ2. Several genetic association studies of ADRβ2 have found Gly16Arg and Gln27Glu genotypes to be associated with susceptibility to asthma and asthma-related phenotypes such as pulmonary function studies, bronchial hyperresponsiveness (BHR), and dependence on corticosteroid therapy, nocturnal symptoms, and atopy. However, later studies with similar or larger sample sizes and meta-analyses have not been able to replicate these findings, likely due to differences in subject race or ethnicity, insufficient sample size to detect a potentially weak genetic effect, and the exclusion of other ADRβ2 polymorphisms from the analysis.34,42-57
Despite the divergent results of ADRβ2 genetic association studies, pharmacogenetic studies have been more consistent in describing the role ADRβ2 polymorphisms in the pharmacological response to acute and regular SABA therapy. In 1997, Martinez and co-workers described one of the earliest pharmacogenetic relationships between Gly16Arg genotypes and response to a single administration of albuterol in a group of 269 children participating in a longitudinal study of asthma.58 In this study, Arg16 homozygotes and Gly16Arg heterozygotes were 5.3 times and 2.3 times more likely than Gly16 homozygotes to show a positive response to albuterol (FEV1 increase greater 15.3% predicted), respectively. The study did not find a relationship between Gln27Glu genotypes and albuterol response.58 Another, study based on the NHLBI Childhood Asthma Management Program (CAMP) was a family-based genetic association analysis by Silverman and co-workers who analyzed the effect of eight ADRβ2 polymorphisms on albuterol responsiveness in 707 asthmatic children.56 In the CAMP cohort, Gly16Arg was significantly associated with post-bronchodilator FEV1 with Arg16 homozygotes having higher FEV1 percent of predicted values. In addition, a promoter polymorphism, Glu112Lys, and a noncoding SNP, Arg175Arg, were also associated with post-bronchodilator FEV1 and beta agonist responsiveness, respectively.56 Several other smaller studies have observed similar effects of Arg16Gly genotypes on response to acute SABA therapy with the notable exception of a study by Taylor and co-workers.47, 58-63 Taylor and co-workers selected 176 patients based solely on a physician’s diagnosis of asthma and found no relationship between Gly16Arg genotypes or ADRβ2 haplotypes and responsiveness to a single dose of albuterol.60
In the Genetics of Asthma in Latino Americans (GALA) study, Choudhry et al investigated the pharmacogenetic relationship of Gly16Arg genotypes with albuterol-induced FEV1 reversibility in two ethnic groups (365 Puerto Rican asthmatics and 294 Mexican-Americans) through family-based and cross-sectional analyses.59 In Puerto Ricans, the number of Arg16 alleles was associated with increasing FEV1 reversibility and showed an additive effect that was more pronounced among those with a baseline FEV1 less than 80% predicted. The association was not found in Mexican-Americans indicating a pharmacogenetic difference in the response to acute bronchodilator reversibility between these two ethnic groups.59 Admixed ethnic groups such as African-Americans, Puerto Ricans, and Mexican-Americans have different ADRβ2 polymorphism and haplotype frequencies.35,38,59 These genetic differences have the potential to cause ethnic group-specific pharmacogenetic differences in response to beta agonists, such as those observed by Choudhry and co-workers.59
The haplotype-based findings of Drysdale indicate that albuterol-induced FEV1 reversibility is greatest in haplotype 2 (Gly16) homozygotes and lowest in haplotype 4 (Arg16) homozygotes, an inconsistent observation based on other studies.38 These conflicting findings may be better understood in the context of a haplotype effect from other adjacent ADRβ2 genetic polymorphisms. In a haplotype, smaller genotypic effects of multiple, functional genetic polymorphisms, such as Arg19(BUP)Cys, may also determine receptor agonist response. Taylor and co-workers reported that the Drysdale haplotypes were not associated with albuterol-induced FEV1 reversibility in 176 patients selected based on a physician’s diagnosis of asthma, not airflow reversibility.60 Similiar findings were also observed by Hawkins35. In contrast, Drysdale and coworkers selected patients based on a spirometric diagnosis of asthma selection criteria that may explain some of the inconsistent findings of these haplotype-based studies.38 Airflow reversibility is primarily determined by baseline airflow obstruction (e.g., lower baseline FEV1 values64); therefore a requirement for bronchodilator reversibility selects for more subjects with lower baseline FEV1 for analysis and tends to exclude those with milder disease.
To further elucidate the complexity of ADRβ2 haplotypes and their effects on airflow obstruction, Hawkins and co-workers sequenced a 5.3kB region of ADRβ2, including the promoter and untranslated regions, in 669 Caucasians and 240 African Americans identifying a total of 49 polymorphisms, which were used to construct extended, complex haplotypes (Figure 2). One of these polymorphisms includes a poly-cytosine repeat region of 10 to 14 cytosine repeats at nucleotide position +1069, 20 nucleotides adjacent to an AU-rich region that determines mRNA stability (Figure 3). This region shows differences in length in different ethnic groups. (Figure 4) Poly-cytosine repeat lengths greater than 12 have been associated with baseline airflow obstruction in African Americans.35 In a complex genetic disease such as asthma, it is possible that the smaller genetic effects of many polymorphisms throughout the ADRβ2 gene and its untranslated regions may play a role in determining responses to beta agonist therapy.
Figure 2.
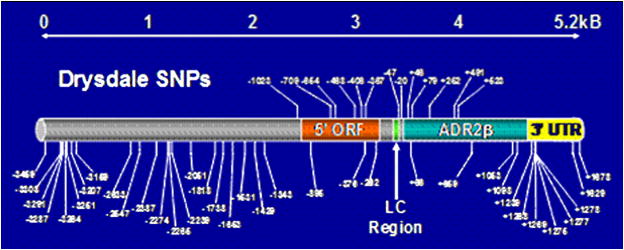
Single Nucleotide Polymorphisms (SNPs) in the ADRβ2 gene. SNPs above the gene diagram were those reported by Drysdale at el38 while more extensive resequencing of the gene by Hawkins et al35 show significantly more variation in this one exon gene. (ORF=open reading frame, ADRβ2=exon region, IC=leader cystrom, 31 UTR= 31 untranslated region)
Figure 3.
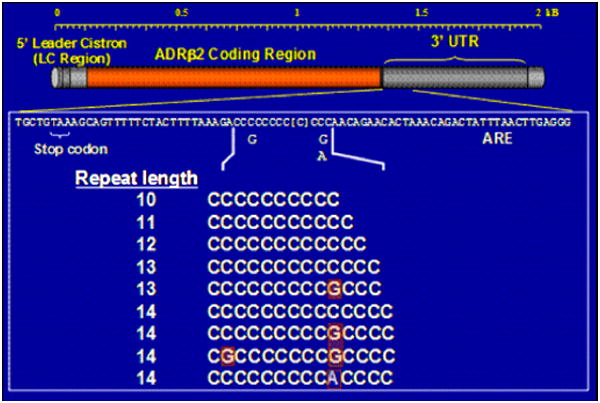
Illustrates poly C region with G/C variation in longer Poly C regions35 (13,14 repeat lengths)
Figure 4.
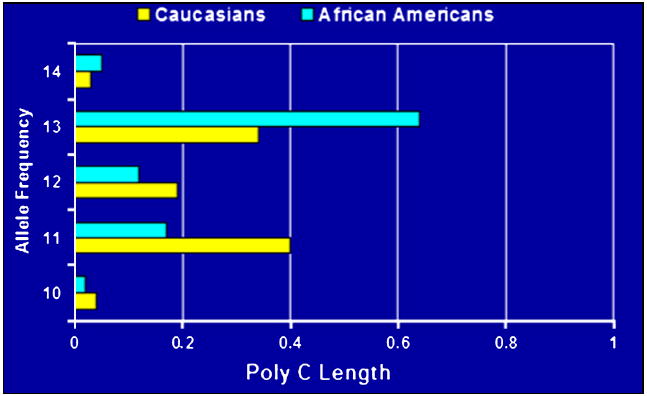
Length of Poly C region in Caucasian and African Americans35
ADRβ2 Pharmacogenetics and the Response to Chronic, Regular SABA Therapy
Pharmacogenetic studies of regular SABA therapy have focused on the effects of Gly16Arg genotypes on beta agonist response, the most important area of research in asthma pharmacogenetics surrently. Hancox and co-workers were among the first investigators to describe the genotypic effects of Gly16Arg during regular SABA therapy. This study was a retrospective genetic analysis of a placebo-controlled, cross-over trial comparing asthma control during regular versus as-needed fenoterol during a 24-week period. The analysis reported that Gly16 homozygotes using regular therapy experienced no increase in BHR while Gly16Arg heterozygotes experienced a significant increase in BHR during regular therapy.45 Taylor and co-workers also analyzed the genotypic effects of Gly16Arg during regular LABA and SABA therapy using a retrospective analysis of a placebo-controlled, crossover trial of regularly scheduled albuterol and salmeterol therapy in 115 patients with asthma. Within the subset of Arg16 homozygotes, exacerbations occurred at a higher frequency during regular albuterol therapy compared to placebo but no adverse effects were noted during the use intermittent albuterol or during LABA (salmeterol) therapy. Furthermore, the Arg16 homozygotes experienced a decline in PEFR during albuterol therapy.65 This finding was found in two different Asthma Clinical Research Network (ACRN) trials.66-67
The Beta-agonists study (BAGS) retrospective analysis of the ACRN BAGS trial evaluated the effects of Gly16Arg genotypes on daily peakflow during regular SABA therapy. In the BAGS trial, Isreal and co-workers retrospectively examined the effects of Gly16Arg genotypes in 190 patients with mild asthma treated with regular or as-needed receptor albuterol therapy over a 16-week period.66 (Figure 5) At the end of the 16-week period, there was a decline in AM PEFR among Arg16 homozygotes using regular beta agonist therapy compared to those using as-needed therapy that was accentuated during the 4-week run-out period. (Figure 5) The evening PEFR also declined among Arg16 homozygotes on regular agonist therapy while there was no effect in the Gly16 homozygotes. Interestingly, Arg16 and Gly16 homozygotes on as-needed beta agonist therapy showed no effects on PEFR.66
Figure 5.
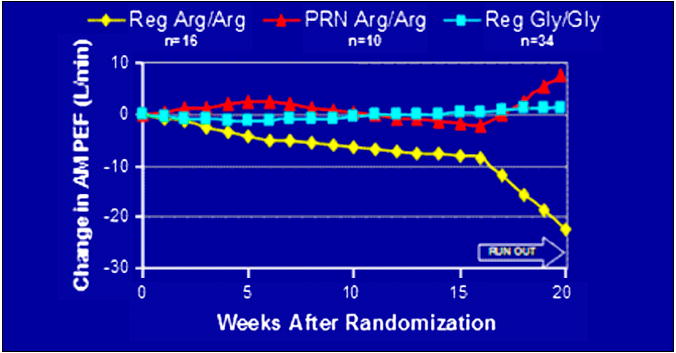
Responses to as needed albuterol therapy by genotype in the ACRN BAGS Study66. Arg16 homozygotes on regular albuterol therapy showed a decrease in AM PEFR during the clinical trial and the run out period.
One of the first The BARGE trial was prospective pharmacogenetic studies (The BARGE Trial) was designed to examine the effects of Gly16Arg genotypes on asthma control during regular SABA therapy in asthma subjects selected and randomized based on individual genotypes.67 Thus, the BARGE trial was a genotype-stratified, placebo-controlled, cross-over trial in which 37 Arg16 homozygotes and 41 Gly16 homozygotes with mild asthma were treated with regular or as-needed albuterol therapy over a 16-week period then crossed over to the alternative therapy. Additionally, rescue therapy with beta agonists was minimized by using ipratropium bromide as the primary reliever medication and albuterol being used only if ipratropium was found to be ineffective. Arg16 homozygotes showed an increased AM PEFR during treatment with placebo therapy, while Gly16 homozygotes had an increase in AM PEFR during regular albuterol therapy. (Figure 6) Furthermore, Arg16 homozygotes had significant adverse effects on FEV1, forced vital capacity (FVC), asthma symptom scores, and rescue inhaler use in comparison with regular albuterol therapy and placebo therapy. In contrast, Gly16 homozygotes showed significant improvements in forced vital capacity (FVC), asthma symptom scores, and rescue inhaler use during regular beta agonist therapy. Interestingly, during the run-in period, an interval when albuterol use was minimized since ipratropium was used as the rescue medication, Arg16 homozygotes showed a significant increase in AM PEFR.67
Figure 6.
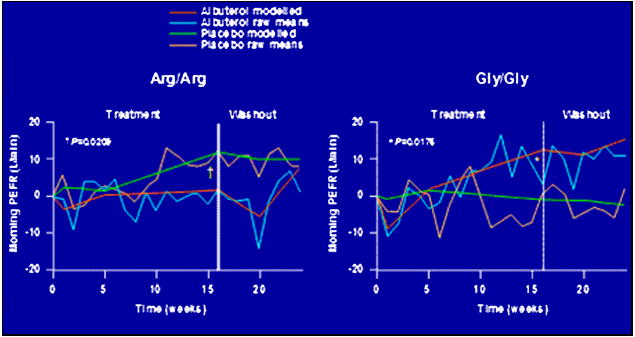
Responses to regular albuterol therapy and placebo (protroprium)in the ACRN BARGE study in Arg16 and Gly16 homozygotes67.
The contrasting genotypic effects of Gly16Arg genotypes on indices of asthma control (e.g., PEFR, BHR, exacerbations rates, etc.) during acute and chronic beta agonist therapy may have multiple explanations. One of these explanations takes into account both in vivo and in vitro observations: the so-called “dynamic model” of receptor kinetics. In this model, endogenous catecholamine activity results in receptor downregulation in Gly16 homozygotes, receptors known to have enhanced downregulatory activity in vitro. The tachyphylaxis observed during regular beta agonist therapy would, therefore, become more apparent in Arg16 homozygotes whose receptors are less likely to have downregulated prior to beta agonist therapy. Based on this model, the initial response to beta agonists among the beta-agonist-naïve would be lower in Gly16 homozygotes and be less likely to worsen over time. In contrast, Arg16 homozygotes would show a greater initial response to therapy but then undergo receptor downregulation during regular beta agonist therapy and, therefore, exhibit tachyphylaxis.68
ADRβ2 Pharmacogenetics and the Response to LABA Therapy
LABA’s are effective anti-asthma medications that are indicated in the long-term management of chronic asthma in conjunction with inhaled corticosteroids. Pharmacogenetic studies have evaluated the genotypic effects of ADRβ2 polymorphisms on LABA response during chronic therapy. To determine whether genetic variants contribute to variation in response to LABA therapy. In the previously described retrospective analysis of a LABA and SABA crossover trial by Taylor and co-workers, Arg16 homozygotes experienced no adverse effects during LABA (salmeterol) in comparison to placebo (as needed SABA therapy) and regular SABA therapy.65
A retrospective genetic analysis of two randomized, placebo-controlled salmeterol trials, the Salmeterol or Corticosteroids (SOCS) trial and Salmeterol +/-Inhaled Corticosteroid (SLIC) trial, was performed by the ACRN to determine a genotypic-difference in LABA response.69 The SOCS trial was a 28-week trial comparing LABA monotherapy with inhaled corticosteroid (ICS) monotherapy in subjects with persistent asthma.70 In contrast, the SLIC trial was a 24-week trial that examined the effects of adding LABA therapy to a stable or declining dose of ICS therapy.71 The SLIC trial concluded that persistent asthmatics who are optimally controlled by LABA and ICS combination therapy are able to have a 50% reduction in ICS dose without loss of asthma control; however, the elimination of ICS therapy results in a deterioration of asthma control.71
Ninety-six subjects from the SOCS trial and 74 subjects from the SLIC trial were genotyped based on eight ADRβ2 polymorphisms, including Gly16Arg.69 Genotypic analysis of the SOCS and SLIC cohorts revealed that Arg16 homozygotes had a lower AM PEFR than Gly16 homozygotes during salmeterol therapy, particularly after week 10 of the trial. In SLIC, Arg16 homozygotes on LABA and ICS combination therapy also experienced a significantly lower FEV1, higher asthma symptom scores, and increased rescue inhaler use when compared to Gly16 homozygotes on similar therapy.69 These results supported a ADβR2 genotype effect on responses to therapy with the LABA’s salmeterol.
In an attempt to further characterize the pharmacogenetic relationship between ADRβ2 polymorphisms and response to chronic LABA therapy in a larger sample size, Bleecker and co-workers genotyped five ADRβ2 SNP’s in 183 subjects with persistent asthma who were randomized to regular salmeterol and ICS combination therapy or montelukast for a 12-week period followed by a 2 to 4 day run-out period. During the 12 week period, all subjects randomized to salmeterol therapy experienced sustained and significant improvements in AM PEFR regardless of Gly16Arg genotype. (Figure 7) During the run-out period, all subjects exhibited a similar and predictable decline in asthma control.72 Issues with this clinical trial include the shorter 12-week duration of the study which may not have been long enough to detect the pharmacogenetic effects reported in the SOCS and SLIC trial. However, the findings reported by Bleecker and co-workers suggest that Gly16Arg genotypes may not be a genetic determinant of reduced responses to chronic LABA therapy in the presence of ICS therapy.72 More recently there have been two reports that do not show an effect of variation at the Arg16Gly locus on the response to LABA Therapy. 73,74 In addition, the NHLBI ACRN network is currently investigating variation at the Arg16Gly locus in the Long Acting Beta Agoinst response by genotype (LARGE) study.
Figure 7.
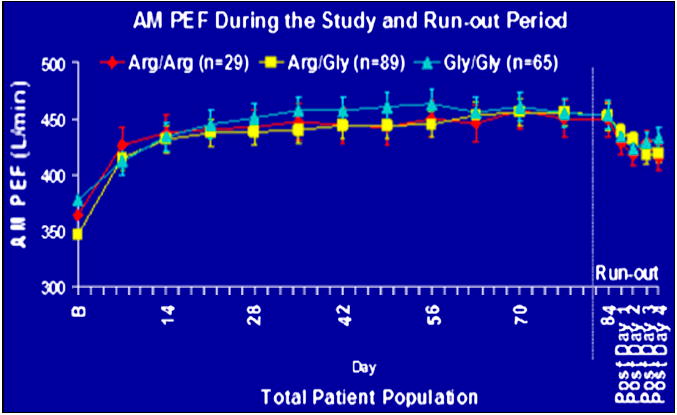
Responses to combination therapy with fluticasone and salmeterol in the Arg16 homozygotes. There was no difference in the response to therapy when stratified by variation of the Arg16Gly locus.72
Pharmacogenetics of LABA and SABA therapies: A Clinician’s Perspective
The divergent effects observed in Arg16Gly genotype-specific responses to acute SABA therapy, chronic SABA therapy, and LABA therapy during most of these trials likely result from multiple issues. Important pharmacological areas include the presence of concomitant therapies such as ICS, the different intrinsic activities of the study agents (beta agonists), and the use of beta agonist rescue therapy in the majority of the trials. In vitro, corticosteroid therapy upregulates the β2-adrenergic receptor, thereby, generating a drug-drug interaction with beta agoinsts. This interaction has the potential to mask the genotypic effects of ADRβ2 polymorphisms during concomitant LABA and ICS therapy. The use of other concomitant anti-asthma therapies may also have the potential of masking these genotypic effects through their clinical effects on asthma control. In addition, beta agonists with lower intrinsic activities (e.g. salmeterol) may be less likely to induce receptor downregulation and generate a detectable genetic effect at the clinical level.
A majority of pharmacogenetic studies to date are retrospective analyses of previous clinical trials; therefore, rescue bronchodilator therapy consisting of beta agonist therapy was a appropriate option. A problem is that the use of beta agonists as an as-needed, rescue therapy during a pharmacogenetic trial based on beta agonist response may effect genotype-specific responses to regular beta agonist therapy. As an example, it is possible that the observed beneficial effects of placebo among Arg16 homozygotes in the BARGE trial may have been attributed to the absence of beta agonist rescue therapy and its substitution with ipratropium rescue therapy. The substitution of beta agonist rescue therapy with ipratropium in the BARGE trial eliminates all beta agoinst exposure during placebo therapy and alter receptor downregulation resulting in the observed benefit among Arg16 homozygotes in the BARGE trial. In contrast, the use of beta agonist rescue therapy throughout the BAGS trial possibly resulted in receptor downregulation.
Two common statistical limitations in the majority of these pharmacogenetic trials include insufficient sample sizes to detect the weak genetic effect of a single polymorphism and a limited number of polymorphisms reported in the genetic analysis. To date, most pharmacogenetic studies do not take into account the effects of other functionally relevant polymorphisms such as Arg19Cys or other polymorphisms in the 5’ promoter and 3’ untranslated region of ADRβ2 that may or may not be in linkage disequilibrium with the commonly analyzed Gly16Arg polymorphism.35,38 It is also possible that another polymorphism in linkage disequilibrium with Gly16Arg may be directly responsible for the observed genetic effects. Furthermore, since asthma is a complex genetic disease, individual polymorphisms likely exhibit a small genetic effect that may not be detectable in small sample size studies. This limitation is further complicated by the fact that ADRβ2 haplotype effects accounting for the combined effects of several polymorphisms are even more difficult to detect in small sample sizes.35,38 ADRβ2 polymorphisms may also interact with polymorphisms on other pathway-related genes, thereby causing gene-gene interactions that further modulate beta agonist responses.75-77 Other forms of genetic variation such as insertion-deletions, base-repeats, and epigenetic changes have not been investigated as determinants of receptor downregulation or desensitization and beta agonist response.35
Finally, there are differences in the study design of these pharmacogenetic studies that may account for their divergent results including differences in asthma severity of the study participants and a lack of recognition or representation from other ethnic or racial groups.35,59 As discussed, criteria such as bronchodilator reversibility tends to select more severe patients with lower baseline FEV1 values, excluding those with milder asthma. Furthermore, studies such as BAGS and BARGE selected participants with mild asthma to minimize the use of concomitant medications, such as ICS, in the study design. In contrast, the study by Bleecker and co-workers and the retrospective analysis of SOCS and SLIC selected participants with persistent asthma and included concomitant ICS therapy in the study design. Variations in the genotypic effect of Gly16Arg observed in these studies could possibly be attributed to the differences in severity of the participants in each study or the use of concomitant ICS therapy.
Some of these studies fail to take into account individual ethnicity by either not stratifying study populations by ethnicity or excluding other ethnic or racial groups in the genetic analysis. Ethnic group-specific pharmacogenetic differences in response to beta agonist therapy, such as those observed between Mexican-Americans and Puerto Ricans, may be overlooked if ethnic groups are excluded from genetic analyses or if within a study population these ethnic groups are not stratified or analyzed seperately.56 Furthermore, failure to account for individual ethnicity or ancestry in a study population contributes to confounding by population stratification. Other important differences in study design between these pharmacogenetic trials include stratification by genotype with a prospective analysis versus stratification by drug-placebo status with a retrospective genotypic analysis, the inclusion of a cross-over design, varying duration of the studies, and the use of beta agonist or anti-cholinergic as rescue therapy.
Conclusions
The results of published studies clearly supports experimental evidence that variation in the ADBR2 gene at the Arg16Gly locus effects therapeutic responses during regular SABA therapy. 45, 65-67 During regular therapy with LABA, reduced responses in Arg16 homozygotes have been reported in two small studies69 but have not been found in several other larger studies. 65,72-74 It is unclear why there are differences in pharmacogenetic responses to short and long acting beta except that they may be related to the longer duration of action of LABAs. In view of the overall safety and efficacy of LABA therapy and the lack of a therapeutic benefit of regular SABA therapy, these pharmacogenetic observations should not alter the current standard of care which encourages the use of as-needed SABA therapy or regular LABA and ICS combination therapy to achieve optimal control of asthma symptoms.6 Understanding the effects of ADRβ2 gene and pathway variation on individual responses to beta agonist therapy will improve as prospective pharmacogenetic studies analyze the genetic effects of a larger number of ADRβ2 SNPs and haplotypes in larger, more ethnically diverse populations taking into account interactions with other genes and the environment.
Acknowledgments
This work was supported by: NIH HL 76285 and NIH/NHLBI HL 69167
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 3.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Oral Montelukast, unhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med. 1999;130:487–95. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins GA, Weiss ST, Bleecker ER. Asthma Pharmacogenetics. In: Meyers Deborah., editor. Immunology and Allergy Clinics of North America. 2005. pp. 723–742. [DOI] [PubMed] [Google Scholar]
- 5.Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 6.Hall IP. Pharmacogenetics of asthma. Eur Respir J. 2000;15:449–451. doi: 10.1034/j.1399-3003.2000.15.04.x. [DOI] [PubMed] [Google Scholar]
- 7.GINA 2006 National Heart Lung and Blood Institute; National Institute of Health. Global Strategy for Asthma Management and Prevention: Global Initiative for Asthma (GINA). 2006 Update. URL: http://www.ginasthma.org.
- 8.Stolley PD. Asthma mortality. Why the United States was spared an epidemic of deaths due to asthma. Am Rev Respir Dis. 1972;105:883–890. doi: 10.1164/arrd.1972.105.6.883. [DOI] [PubMed] [Google Scholar]
- 9.Grainger J, Woodman K, Pearce N, et al. Prescribed fenoterol and death from asthma in New Zealand, 1981-7: a further case-control study. Thorax. 1991;46:105–111. doi: 10.1136/thx.46.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce N, Beasley R, Crane J, Burgess C, Jackson R. End of the New Zealand asthma mortality epidemic. Lancet. 1995;345:41–44. doi: 10.1016/s0140-6736(95)91159-6. [DOI] [PubMed] [Google Scholar]
- 11.Pearce N, Burgess C, Crane J, Beasley R. Fenoterol, asthma deaths, and asthma severity. Chest. 1997;112:1148–1150. doi: 10.1378/chest.112.4.1148-b. [DOI] [PubMed] [Google Scholar]
- 12.Sears MR, Taylor DR, Print CG, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–1396. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- 13.Spitzer WO, Buist AS. Case-control study of prescribed fenoterol and death from asthma in New Zealand, 1977-81. Thorax. 1990;45:645–646. doi: 10.1136/thx.45.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suissa S, Ernst P, Boivin JF, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med. 1994;149:604–610. doi: 10.1164/ajrccm.149.3.8118625. [DOI] [PubMed] [Google Scholar]
- 15.Drazen JM, Israel E, Boushey HA, et al. Comparison of regularly scheduled with as-needed use of albuterol in mild asthma. Asthma Clinical Research Network. N Engl J Med. 1996;335:841–847. doi: 10.1056/NEJM199609193351202. [DOI] [PubMed] [Google Scholar]
- 16.Castle W, Fuller R, Hall J, Palmer J. Severent nationwide surveillance study: Comparison of Salmeterol with Salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ. 1993;306:1034–1037. doi: 10.1136/bmj.306.6884.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144:904–912. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 19.Anderson HR, Ayres JG, Sturdy PM, et al. Bronchodilator treatment and deaths from asthma: case-control study. BMJ. 2005;330:117. doi: 10.1136/bmj.38316.729907.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters EH, Walters J. Inhaled short acting beta2-agonist use in chronic asthma: regular versus as needed treatment. Cochrane Database Syst Rev. 2003:CD001285. doi: 10.1002/14651858.CD001285. [DOI] [PubMed] [Google Scholar]
- 21.Ni Chronin M, Greenstone IR, Danish A, Magdolinos H, Masse H, Zhang X, Ducharme FM. Long Acting beta2-agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD005535. Art. No.: CD005535. [DOI] [PubMed] [Google Scholar]
- 22.Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992;327:1420–1425. doi: 10.1056/NEJM199211123272004. [DOI] [PubMed] [Google Scholar]
- 23.Pauwels RA, Lofdahl CG, Postma DS, Tatterfield AE, O’Byrne P, Barnes PJ, Ullman A. Effect of Inhaled Formoterol and Budesonide on Exacerbations of Asthma. NEJM. 1997;337:1405–1412. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 24.Abramson MJ, Walters J, Walters EH. Adverse effects of beta-agonists: are they clinically relevant? Am J Respir Med. 2003;2:287–297. doi: 10.1007/BF03256657. [DOI] [PubMed] [Google Scholar]
- 25.Walters EH, Walters JA, Gibson MD. Inhaled long acting beta agonists for stable chronic asthma. Cochrane Database Syst Rev. 2003:CD001385. doi: 10.1002/14651858.CD001385. [DOI] [PubMed] [Google Scholar]
- 26.O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–1397. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 27.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 28.Kobilka BK, Frielle T, Dohlman HG, et al. Delineation of the intronless nature of the genes for the human and hamster beta 2-adrenergic receptor and their putative promoter regions. J Biol Chem. 1987;262:7321–7327. [PubMed] [Google Scholar]
- 29.Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987;84:46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyers DA, Postma DS, Stine OC, et al. Genome screen for asthma and bronchial hyperresponsiveness: interactions with passive smoke exposure. J Allergy Clin Immunol. 2005;115:1169–1175. doi: 10.1016/j.jaci.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Postma DS, Howard TD, et al. Major genes regulating total serum immunoglobulin E levels in families with asthma. Am J Hum Genet. 2000;67:1163–1173. doi: 10.1086/321190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Meyers DA, Ober C, et al. Genomewide screen and identification of gene-gene interactions for asthma-susceptibility loci in three U.S. populations: collaborative study on the genetics of asthma. Am J Hum Genet. 2001;68:1437–1446. doi: 10.1086/320589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ober C, Cox NJ, Abney M, et al. Genome-wide search for asthma susceptibility loci in a founder population. The Collaborative Study on the Genetics of Asthma. Hum Mol Genet. 1998;7:1393–1398. doi: 10.1093/hmg/7.9.1393. [DOI] [PubMed] [Google Scholar]
- 34.Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993;8:334–339. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins GA, Tantisira K, Meyers DA, et al. Sequence, haplotype, and association analysis of ADRbeta2 in a multiethnic asthma case-control study. Am J Respir Crit Care Med. 2006;174:1101–1109. doi: 10.1164/rccm.200509-1405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 37.Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- 38.Drysdale CM, McGraw DW, Stack CB, et al. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore PE, Laporte JD, Abraham JH, et al. Polymorphism of the beta(2)-adrenergic receptor gene and desensitization in human airway smooth muscle. Am J Respir Crit Care Med. 2000;162:2117–2124. doi: 10.1164/ajrccm.162.6.9909046. [DOI] [PubMed] [Google Scholar]
- 40.Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993;268:23116–23121. [PubMed] [Google Scholar]
- 41.McGraw DW, Forbes SL, Kramer LA, Liggett SB. Polymorphisms of the 5’ leader cistron of the human beta2-adrenergic receptor regulate receptor expression. J Clin Invest. 1998;102:1927–1932. doi: 10.1172/JCI4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Amato M, Vitiani LR, Petrelli G, et al. Association of persistent bronchial hyperresponsiveness with beta2-adrenoceptor (ADRB2) haplotypes. A population study. Am J Respir Crit Care Med. 1998;158:1968–1973. doi: 10.1164/ajrccm.158.6.9804126. [DOI] [PubMed] [Google Scholar]
- 43.Hall IP, Wheatley A, Wilding P, Liggett SB. Association of Glu 27 beta 2-adrenoceptor polymorphism with lower airway reactivity in asthmatic subjects. Lancet. 1995;345:1213–1214. doi: 10.1016/s0140-6736(95)91994-5. [DOI] [PubMed] [Google Scholar]
- 44.Hall IP, Blakey JD, Al Balushi KA, et al. Beta2-adrenoceptor polymorphisms and asthma from childhood to middle age in the British 1958 birth cohort: a genetic association study. Lancet. 2006;368:771–779. doi: 10.1016/S0140-6736(06)69287-8. [DOI] [PubMed] [Google Scholar]
- 45.Hancox RJ, Sears MR, Taylor DR. Polymorphism of the beta2-adrenoceptor and the response to long-term beta2-agonist therapy in asthma. Eur Respir J. 1998;11:589–593. [PubMed] [Google Scholar]
- 46.Holloway JW, Dunbar PR, Riley GA, et al. Association of beta2-adrenergic receptor polymorphisms with severe asthma. Clin Exp Allergy. 2000;30:1097–1103. doi: 10.1046/j.1365-2222.2000.00929.x. [DOI] [PubMed] [Google Scholar]
- 47.Kotani Y, Nishimura Y, Maeda H, Yokoyama M. Beta2-adrenergic receptor polymorphisms affect airway responsiveness to salbutamol in asthmatics. J Asthma. 1999;36:583–590. doi: 10.3109/02770909909087295. [DOI] [PubMed] [Google Scholar]
- 48.Litonjua AA, Silverman EK, Tantisira KG, Sparrow D, Sylvia JS, Weiss ST. Beta 2-adrenergic receptor polymorphisms and haplotypes are associated with airways hyperresponsiveness among nonsmoking men. Chest. 2004;126:66–74. doi: 10.1378/chest.126.1.66. [DOI] [PubMed] [Google Scholar]
- 49.Ramsay CE, Hayden CM, Tiller KJ, Burton PR, Goldblatt J, Lesouef PN. Polymorphisms in the beta2-adrenoreceptor gene are associated with decreased airway responsiveness. Clin Exp Allergy. 1999;29:1195–1203. doi: 10.1046/j.1365-2222.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 50.Turki J, Pak J, Green SA, Martin RJ, Liggett SB. Genetic polymorphisms of the beta 2-adrenergic receptor in nocturnal and nonnocturnal asthma. Evidence that Gly16 correlates with the nocturnal phenotype. J Clin Invest. 1995;95:1635–1641. doi: 10.1172/JCI117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson AM, Gray RD, Hall IP, Lipworth BJ. The effect of beta2-adrenoceptor haplotypes on bronchial hyper-responsiveness in patients with asthma. Allergy. 2006;61:254–259. doi: 10.1111/j.1398-9995.2006.01001.x. [DOI] [PubMed] [Google Scholar]
- 52.Summerhill E, Leavitt SA, Gidley H, Parry R, Solway J, Ober C. beta(2)-adrenergic receptor Arg16/Arg16 genotype is associated with reduced lung function, but not with asthma, in the Hutterites. Am J Respir Crit Care Med. 2000;162:599–602. doi: 10.1164/ajrccm.162.2.9910108. [DOI] [PubMed] [Google Scholar]
- 53.Dewar JC, Wheatley AP, Venn A, Morrison JF, Britton J, Hall IP. Beta2-adrenoceptor polymorphisms are in linkage disequilibrium, but are not associated with asthma in an adult population. Clin Exp Allergy. 1998;28:442–448. doi: 10.1046/j.1365-2222.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- 54.Contopoulos-Ioannidis DG, Manoli EN, Ioannidis JP. Meta-analysis of the association of beta2-adrenergic receptor polymorphisms with asthma phenotypes. J Allergy Clin Immunol. 2005;115:963–972. doi: 10.1016/j.jaci.2004.12.1119. [DOI] [PubMed] [Google Scholar]
- 55.Thakkinstian A, McEvoy M, Minelli C, et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol. 2005;162:201–211. doi: 10.1093/aje/kwi184. [DOI] [PubMed] [Google Scholar]
- 56.Silverman EK, Kwiatkowski DJ, Sylvia JS, et al. Family-based association analysis of beta2-adrenergic receptor polymorphisms in the childhood asthma management program. J Allergy Clin Immunol. 2003;112:870–876. doi: 10.1016/s0091-6749(03)02023-2. [DOI] [PubMed] [Google Scholar]
- 57.Woszczek G, Borowiec M, Ptasinska A, Kosinski S, Pawliczak R, Kowalski ML. Beta2-ADR haplotypes/polymorphisms associate with bronchodilator response and total IgE in grass allergy. Allergy. 2005;60:1412–1417. doi: 10.1111/j.1398-9995.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- 58.Martinez FD, Graves PE, Baldini M, Solomon S, Erickson R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J Clin Invest. 1997;100:3184–3188. doi: 10.1172/JCI119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choudhry S, Ung N, Avila PC, et al. Pharmacogenetic differences in response to albuterol between Puerto Ricans and Mexicans with asthma. Am J Respir Crit Care Med. 2005;171:563–570. doi: 10.1164/rccm.200409-1286OC. [DOI] [PubMed] [Google Scholar]
- 60.Taylor DR, Epton MJ, Kennedy MA, et al. Bronchodilator response in relation to beta2-adrenoceptor haplotype in patients with asthma. Am J Respir Crit Care Med. 2005;172:700–703. doi: 10.1164/rccm.200501-092OC. [DOI] [PubMed] [Google Scholar]
- 61.Cho SH, Oh SY, Bahn JW, et al. Association between bronchodilating response to short-acting beta-agonist and non-synonymous single-nucleotide polymorphisms of beta-adrenoceptor gene. Clin Exp Allergy. 2005;35:1162–1167. doi: 10.1111/j.1365-2222.2005.02319.x. [DOI] [PubMed] [Google Scholar]
- 62.Lima JJ, Thomason DB, Mohamed MH, Eberle LV, Self TH, Johnson JA. Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther. 1999;65:519–525. doi: 10.1016/S0009-9236(99)70071-8. [DOI] [PubMed] [Google Scholar]
- 63.Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between beta 2-adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet. 1997;350:995–999. doi: 10.1016/S0140-6736(97)03211-X. [DOI] [PubMed] [Google Scholar]
- 64.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzgerald AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE for the National Heart Lung Blood Institute’s Severe Asthma Research Program. Characterization of the Severe Asthma Phenotype by the NHLBI Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000;55:762–767. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Israel E, Drazen JM, Liggett SB, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 67.Israel E, Chinchilli VM, Ford JG, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 68.Liggett SB. Pharmacogenetics of beta-1-and beta-2-adrenergic receptors. Pharmacology. 2000;61:167–173. doi: 10.1159/000028397. [DOI] [PubMed] [Google Scholar]
- 69.Wechsler ME, Lehman E, Lazarus SC, et al. beta-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med. 2006;173:519–526. doi: 10.1164/rccm.200509-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–2593. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 71.Lemanske RF, Jr, Sorkness CA, Mauger EA, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–2603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 72.Bleecker ER, Yancey SW, Baitinger LA, et al. Salmeterol response is not affected by beta2-adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol. 2006;118:809–816. doi: 10.1016/j.jaci.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 73.Bleecker ER, Postma D, Lawrance R, Meyers DA, Ambrose H, Goldman M. Effect of polymorphisms in the beta 2-adrenergic receptor gene on response to longacting beta-agonist therapy. 2007. J Allergy Clin Immunol. 2007;119:S523–524. In Press The LANCE. [Google Scholar]
- 74.Bleecker E, Yancey S, Ortega H, Andersen W. Chest. 2007;132 Abstract In Press Sept 2007. [Google Scholar]
- 75.Tantisira KG, Small KM, Litonjua AA, Weiss ST, Liggett SB. Molecular properties and pharmacogenetics of a polymorphism of adenylyl cyclase type 9 in asthma: interaction between beta-agonist and corticosteroid pathways. Hum Mol Genet. 2005;14:1671–1677. doi: 10.1093/hmg/ddi175. [DOI] [PubMed] [Google Scholar]
- 76.McGraw DW, Fogel KM, Kong S, et al. Transcriptional response to persistent beta2-adrenergic receptor signaling reveals regulation of phospholamban, which alters airway contractility. Physiol Genomics. 2006;27:171–177. doi: 10.1152/physiolgenomics.00044.2006. [DOI] [PubMed] [Google Scholar]
- 77.Small KM, Brown KM, Theiss CT, Seman CA, Weiss ST, Liggett SB. An Ile to Met polymorphism in the catalytic domain of adenylyl cyclase type 9 confers reduced beta2-adrenergic receptor stimulation. Pharmacogenetics. 2003;13:535–541. doi: 10.1097/00008571-200309000-00002. [DOI] [PubMed] [Google Scholar]


