Abstract
We examine how a public health genomics framework can be used to move genomic discoveries into clinical and public health practice for obesity prevention and treatment. There are four phases of translational research: T1: discovery to candidate health application; T2: health application to evidence-based practice guidelines; T3: practice guidelines to health practice; and T4: practice to population health impact. Types of multidisciplinary research and knowledge synthesis needed for each phase, as well as the importance of developing and disseminating evidence-based guidelines, are discussed. Because obesity genomics research is mostly in the discovery phase or in the very early phases of translation (T1), the authors present this framework to illustrate the range of translation activities needed to move genomic discoveries in obesity to actual applications that reduce the burden of obesity at the population level.
The obesity epidemic in the United States is one of grave public health significance. In 2003–2004, ∼66% of the US population was overweight (BMI ≥25 kg/m2) and 32% was obese (BMI ≥30 kg/m2) (1). Because obesity is a risk factor for many chronic diseases, including cancer, type 2 diabetes, and cardiovascular diseases, obesity is considered a major cause of morbidity and mortality (2,3). Moreover, obesity places a heavy burden on our health-care system (4). A recent report estimates that obesity costs the country $118 billion annually, including direct health-care costs for diseases related to obesity and indirect costs, such as loss of productivity at work (5).
Obesity is characterized by etiological heterogeneity and encompasses a number of subtypes with similar clinical presentation. The most common forms of obesity are multifactorial involving many genes and environments, including diet and physical activity patterns. Genes are involved in energy homeostasis and affect energy expenditure, energy intake (through such mechanisms as regulation, reward, executive control), and partitioning of calories, which includes proclivity to store calories ingested in excess of expenditure (6). Genetic variations also influence eating behavior, taste, and satiety (7,8). Moreover, our "obesogenic" environment provides abundant opportunities to increase food intake (e.g., increased availability and access to fast food outlets) and decrease physical activity levels (e.g., fewer opportunities for activity due to lack of sidewalks, walking trails, bike lanes, or parks).
In 1998, the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) developed clinical guidelines for health professionals on defining and treating overweight and obesity (9). In 2003, the US Preventive Services Task Force (USPSTF), an independent panel of experts from multiple disciplines that uses systematic reviews to make evidence-based clinical recommendations, developed screening recommendations for adults (10). Both the USPSTF and the NHLBI obesity guidelines concluded that most effective interventions combine nutrition education and exercise counseling with behavioral strategies to improve a person's ability to modify caloric balance for weight loss. Systematic reviews by the Guide to Community Preventive Services (Community Guide) recommended that worksite programs should combine nutrition and physical activity to prevent or control obesity, rather than use single-component interventions such as diet or physical activity exclusively (11). It is generally accepted that both sides of the energy balance equation—intake and expenditure—are important for weight control (12).
Now is an important time to consider whether these guidelines can be personalized using advances in genomics and related fields, and what additional research may be needed to determine how to personalize obesity interventions. For example, can some individuals be more effective in weight control by concentrating on the energy expenditure side of the equation, while others should concentrate on the energy intake side? Are some macronutrient compositions (like lower fat or lower carbohydrates) more effective in some persons than others? Do some weight-loss medications work better in individuals with certain genotypes?
Since the completion of the Human Genome Project in 2003 (12), there have been rapid advances in human genomics research. The recent successes of genome-wide association studies (GWAS) in locating genes associated with a variety of common diseases are fuelling expectations about the emergence of a new era of personalized health care and disease prevention (13). Personalized medicine is a growing field of study that explores approaches to calculating risk based on genetic, environmental, and other variables, and tailoring patient care/treatment to one's genotype (14,15). An area of active research within pharmacogenomics aims to predict an individual's response to a particular drug therapy by using gene expression profiling (16). This research holds the promise of providing better-targeted medicines. In the meantime, the number of companies offering personal genomic services is on the rise (17). These companies offer consumers information about their own genotypes and provide personalized risk profiles for various common diseases. However, there are serious concerns about the quality of these tests as well as their clinical validity and utility (18).
In the face of rapid developments in genomics and related fields, there is an urgent need for a "population health" approach to assess the net value of this genetic information in disease prevention and health promotion for individuals, families, and populations. As a result, we are now witnessing the emergence of the multidisciplinary field of public health genomics that focuses on how to translate gene discoveries into responsible and effective applications that benefit the health of populations (19).
Public Health Genomics
Public health genomics is devoted to the study and application of knowledge about the human genome and its functions, including interactions with the environment and effects of interventions and therapies, in relation to health and disease in populations (19). The framework of public health genomics can be used in epidemiological studies to identify disease susceptibility and is useful for developing targeted interventions, understanding and quantifying the role of gene–environment interactions, and studying patterns of disease occurrence in populations (20). Information gleaned could be incorporated into targeted health promotion, disease prevention, and intervention strategies, with these strategies then tested in intervention studies to determine whether they are useful in clinical or public health practice. Emerging priorities in public health genomics are 1) the creation and support of population-based research and health genomics databases; 2) the development of the evidence base for genomic applications in health promotion and disease prevention using multidisciplinary observational research and clinical trials; and 3) the assurance of an adequate workforce and training in the field of genomics (21).
Although not ready for practice, potential applications of genomics in obesity prevention and control are several. Genetic testing could be used to predict future obesity, and the most effective interventions could be targeted to those with higher genetic risk, including specific behaviorally based prevention approaches or specific pharmacologic therapies. Perhaps factors could be identified to explain why some obese persons develop subsequent disease, whereas others do not, and interventions to prevent disease onset could be targeted to those at the highest risk. The clinical utility of genetic testing in obesity can only be assessed if there are accompanying interventions (e.g., targeted dietary or physical activity approaches or pharmacotherapy) that are available to prevent disease.
Currently, major challenges exist in the application of personalized approaches to obesity control and prevention. For example, how would the public respond to genetic testing for obesity if widely available? What effect would the test results have on motivation to lose weight? Very little research has been conducted in this area. One study found that parents of overweight children believed genetic testing should be available for their children and that results would be helpful in framing behavior of both parent and child to prevent the development of obesity (22). Research has also shown that a positive obesity gene status did not adversely affect one's confidence to lose weight or control eating behavior (23). Another study examined the potential behavioral consequences of genetic feedback on obesity risk in normal weight individuals. Individuals who were told they were at increased risk for obesity showed higher overall intentions to eat a healthy diet. However, individuals with low external weight locus of control had significantly lower predicted intentions to eat a healthy diet when compared to those with high internal weight locus of control (24). Additional research is needed to understand behavioral responses to genetic information.
Pharmacogenetics/genomics offers the hope of predicting an individual's response to a pharmacologic intervention or treatment, based on his or her genotype. This approach may therefore hold promise for personalizing the prevention and management of obesity through development of new pharmacologic targets. For example, a study that mapped mouse genes influencing the amount of weight lost during caloric restriction found that weight loss exhibited significant genetic variation in response to a calorie-restricted diet (25). These results suggest that those genes could be novel therapeutic targets. Another study found that a variant in solute carrier family 6 (neurotransmitter transporter, serotonin), member 5 (SLC6A4) influenced response to sibutramine during weight reduction. Specifically, treatment effects were enhanced among those whose genotypes contained the short (s) allele in (LS/SS) the gene promoter compared to those homozygous for the long allele (26). These findings should be replicated in a large population-based study before it could be applied with any confidence to developing new public health strategies. Nevertheless, knowledge gained from these types of studies will help identify targets for future drug development that can be used for obesity control.
Public health genomics framework
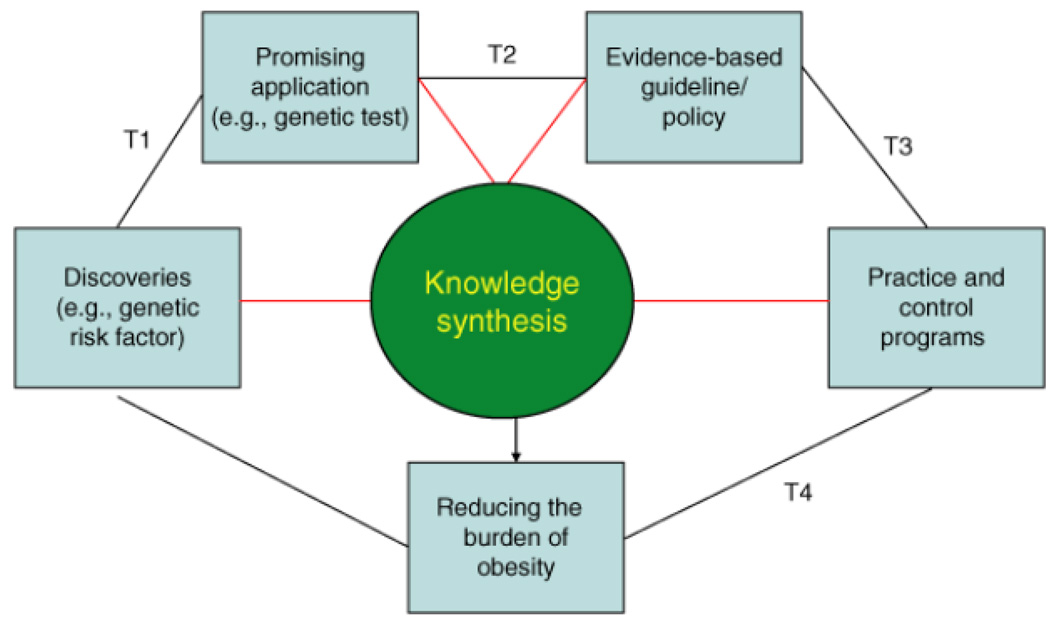
A public health genomics framework can be used to envision a continuum of multidisciplinary translation research in genomic applications (27). The purpose of this framework is to move promising genomic applications to clinical and public health practice for population health benefit. The four phases of translational research in genomic medicine, which may be used in other fields of study are: T1: discovery to candidate health application; T2: health application to evidence-based practice guidelines; T3: practice guidelines to health practice; and T4: practice to population health impact (Table 1 and Figure 1). We present this framework in the context of obesity control and discuss types of research and knowledge synthesis needed for each phase, as well as the importance of developing and disseminating evidence-based guidelines. Although genetic obesity research is still mostly discovery-based, or in very early phases of translation (T1), this framework is presented to illustrate the range of translation phases for genomic applications in population health. The discussion is focused on potential use of genomic tools for risk stratification that can be used to target interventions, including medications and lifestyle approaches. The development of new, genetically based medications for practice would have to go through the regular translation pathway of drug development phase I through IV trials (27).
Table 1.
The continuum of translation research in human genetics: types of research and examples
| Translation research phase | Notation | Typee of research | Examples |
|---|---|---|---|
| T1 | Discovery to candidate health application | Observational studies; Phases I and II clinical trials | Development of an accurate test for the FOA gene, based on the discovery of an association between the FOA gene and obesity; early testing of genetically based interventions for feasibility and safety |
| T2 | Health application to evidence-based practice guidelines | Observational studies; Phase III clinical trials; evidence synthesis and guidelines development | What is the positive predictive value of FOA mutations in at-risk populations? What are the effects of obesity-reduction interventions in persons with and without risk-conferring mutations? |
| T3 | Practice guidelines to health practice | Dissemination research; implementation research; diffusion research; Phase IV cinical trials | Whet proportion of population who met the family history criteria is tested for the FOA gene; are there barriers to testing? What approaches can improve the delivery of effective personalized genetically based approaches in clinical practice settings? |
| T4 | Practice to population health impact | Outcomes research (includes many disciplines); population monitoring of morbidity, mortality, benefits, and risk | Does FOA testing in normal and overweight individuals reduce incidence for obesity or improve outcome? Does delivery of genetically targeted interventions improve public-health outcomes? |
Figure 1.
A population health approach to obesity prevention and control. Modified from Khoury et al. (27)
T1 research: from gene discovery to candidate health applications
T1 research occurs after gene discovery. During this phase, researchers in multiple fields evaluate how genomic discoveries can be used to develop promising health applications for clinical and public health practice. Applications are used either in clinical evaluation (e.g., predictive testing, screening, diagnostic testing, and prognostic testing) or in selection of effective therapeutic options (e.g., pharmacogenomics or lifestyle approaches).
Currently, there are only a few genetic tests available to determine obesity susceptibility; and little is known about their validity, and none have undergone the full gamut of translation research or even replication. Ongoing research efforts include how to: (i) predict increased susceptibility to obesity; (ii) predict variable responses to drug therapy; and (iii) develop prognostic indicators for subsequent morbidity and mortality. To answer these types of questions, clinical and population studies are needed. An integral component of phase I translation in genomics is the ability to synthesize and update information on a timely and ongoing basis. Two systematic knowledge synthesis efforts have been developed for assessing the evidence produced by such studies, which are (1) the Human Genome Epidemiology Network (HuGENet); and (2) a framework for the evaluation of genetic tests (as discussed under T2). HuGENet promotes the integration of observational, population-based research that measures the frequency distributions of alleles and genotypes in human populations, correlates genotypes with phenotypes, estimates disease risk associated with human genetic variants, and assesses gene–gene and gene–environment interactions (27). This research is crucial for determining the clinical validity (clinical sensitivity and specificity) of a diagnostic or predictive genetic test. Because our knowledge in this area is inconclusive due to small studies yielding inconsistent results, collaborative efforts are needed to conduct rigorous, systematic reviews and meta-analyses of findings of genetic associations. This approach can help evaluate the robustness of such associations and help researchers arrive at more precise estimates of genetic risk (27).
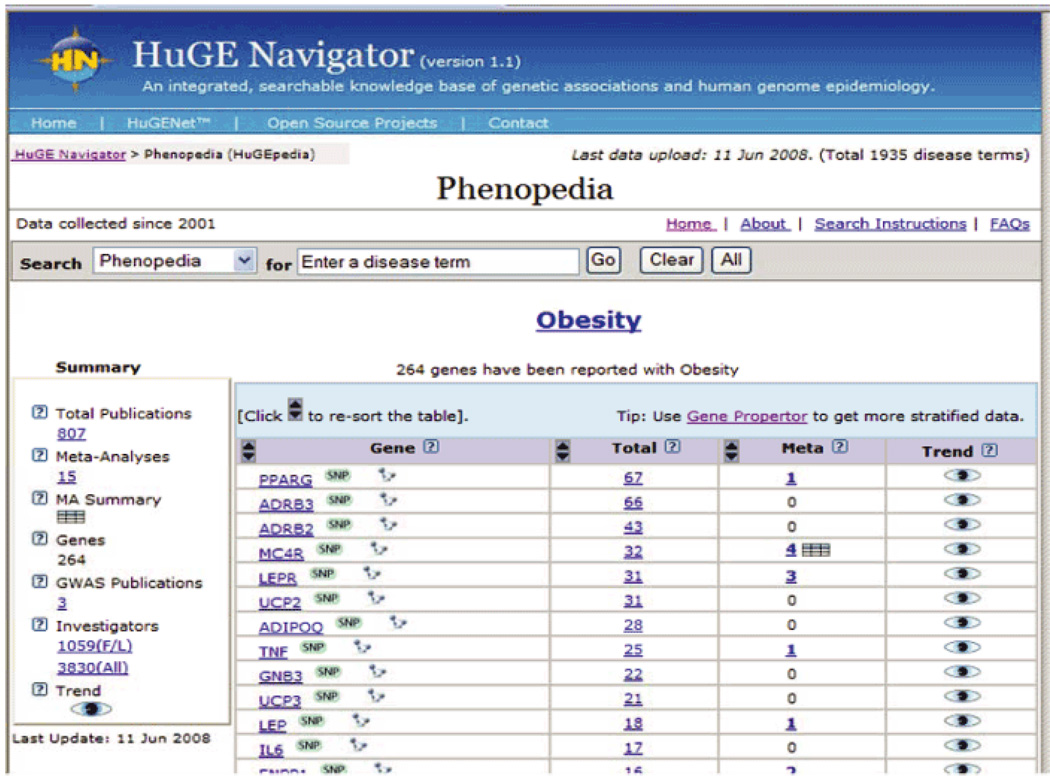
With the expansion of GWAS since 2007, the pace of primary data accumulation and knowledge synthesis has accelerated. HuGENet maintains a continuously updated knowledge base, called the HuGE Navigator (28). As of June 2008, the HuGE Navigator listed 264 genes that have been studied in relation to obesity, with a total of 807 publications, 15 meta-analyses, and 3 GWASs (Figure 2). The 2005 Human Obesity Gene Map reported 11 genes implicated in single-gene disorders and 426 findings of positive associations with obesity phenotypes in 127 candidate genes, of which 23 were supported by at least five positive studies (29). The fact that most of these associations are unreplicated, despite adequately powered attempts to do so, implies that many of these gene–obesity associations are likely false positives, resulting from underpowered studies and selective publication. Furthermore, positive associations do not necessarily constitute causality; thus, replication in other studies is needed. Little is known about the biological significance of these associations, the functions of the genes, their expression, or the regulation of these genes. Although obesity research has progressed since this report, much of the work is still in the discovery phase with few studies focused on health application. An example of T1 research is testing combinations of genetic variants to develop risk profiles for obesity that can potentially guide intervention development.
Figure 2.
Obesity-related genes identified by the HuGE Navigator.
Several commercial entities have prematurely marketed genomic tests as a form of personalized medicine targeted directly to the public; a handful of these companies offer genomic profiles aimed at identifying those with a predisposition to obesity so that this information might be used in prevention and treatment. Specifically, one company offers early-onset obesity testing of melanocortin 4 receptor (MC4R) in children. Another company offers genomic profiles to detect obesity-related genes that include the β3-adrenoceptor gene, UCP1 gene, and the β2-adrenoceptor gene. Individuals receive genetic test results and advice on health management and diet to prevent obesity. It is important to note that much genetic information obtained through these commercial companies was not produced in a laboratory regulated under the Clinical Laboratory Improvement Amendments (CLIA), which ensures quality laboratory testing. Perhaps more importantly, the clinical validity and usefulness of these tests have not been determined.
Population research focusing on diverse groups
Population data that examine the prevalence of obesity in diverse groups are often conducted at the national level. National surveys such as the National Health and Nutrition Examination Survey (NHANES) collect demographic, anthropometric, metabolic and behavioral data to assess the nation's health (30); population cohort studies such as the Framingham Heart Study collect data over time, including data on obesity measures, along with genetic samples (31). Population-based studies are extremely important for discovering and validating gene–obesity associations and estimating their contribution to disease occurrences in other groups (32). Replication of this research in large population-based studies and meta-analyses of these studies have proven vital to advancing our knowledge. For example, mutations in the MC4R gene were shown to be associated with rare forms of severe monogenic obesity (33,34) and thought to be associated with common obesity. However, a meta-analysis of 29,563 subjects from 25 populations found an inverse association between the V103l variant and common obesity risk (35). Evidence for a small protective effect for other MC4R variants has also documented this relationship (36). Because the alleles associated with severe obesity are rare and have little effect on the overall population risk for common obesity, screening for these alleles in individuals with common obesity might not be a cost-effective strategy for assessing genetic risk.
The FTO gene variant is a good example of how population-based studies can document whether a true association exists. The FTO gene region was thought to be associated with type 2 diabetes, but the relationship disappeared when diabetic status was adjusted for BMI (37). Several studies found an association between the single-nucleotide polymorphism (i.e., rs9939609) within the FTO locus and obesity risk in white European adults and children (37,38,39). Specifically, this association was seen in a study of 13 cohorts totaling 38,759 adults and children (37). Studies have also confirmed an association with FTO in several Asian (40,41,42), Hispanic (39) and African American (43) populations. Persons who are homozygous for the risk allele weigh about 3–4 kg more and have a 1.67-fold increased risk of obesity than those without a risk allele (44). The population attributable risk of FTO for obesity is estimated at 20% among Caucasian Americans, which means that if the negative effects of the FTO allele were eliminated, ∼20% of the obese cases could be prevented (44). This reduction in risk could have a significant effect on obesity-related health-care expenditures, morbidity, and mortality. However, additional studies are needed to determine the utility of predicting obesity risk in clinical practice.
Population research: gene–environment interactions
The dramatic and rapid rise in the prevalence of obesity often highlights the importance and emergence of obesogenic environments in obesity development. A gene–environment interaction, whereby sensitivity to environmental influences (e.g., physical, biological, behavioral, and social) is modulated by genotype, most likely plays an important role. Also, gene–environment interactions can occur during critical periods throughout life (e.g., pre-and postnatal development, adolescence, menopause). Population research on gene–environment interactions is particularly important to help elucidate pathways through which obesity development is modulated (45,46,47,48). However, detection of gene–environment interactions is notoriously difficult and requires knowledge of the specific genes and environments involved and when during pathogenesis their interactions are expressed. When such information is not available, studies of gene–environment interactions rely on statistical approaches such as those that provide evidence that putative environmental factors modulate heritability (49,50). For example, findings of moderation of genetic effects on weight by physical activity have been reported in Finnish adult twins (51).
The identification of genes that are implicated in the development of obesity will facilitate more focused research of gene–environment interaction. For example, a study of gene-by-activity interaction conducted in a cohort of 14,716 African Americans and Caucasian Americans reported a significant interaction between the quinine nucleotide binding protein (G protein), beta polypeptide 3 (GNB3) 825 C>T variant and physical activity in predicting obesity status in African Americans (46). Of particular interest was the finding that physically active persons who had the 825T allele had a 20% lower prevalence of obesity for each additional T allele they carried, whereas those with the same genotype who were not physically active had a 23% greater prevalence of obesity (46). The expectation is that population-based gene–environment interaction obesity studies can provide information on polymorphisms that may predict response to diet and physical activity interventions. Also, studies can document how diet and physical activity modulate gene expression (52,53).
Few large population studies of gene–environment interactions and obesity have been conducted because of the difficulty in measuring the effects of phenotype and environmental exposures in quantitative models (44). Advances in measuring environments are needed to move the field forward to provide better assessments of gene–environment interactions. This information will be invaluable in helping to develop environmental interventions targeted to genetic subgroups that can be applied in clinical and public health practice. Meta-analyses from multiple GWAS are needed to replicate and test findings and should take into consideration genetic variation in ancestry among different ethnic and racial groups as it relates to obesity risk. Additionally, the usefulness of genetic findings must be determined through intervention studies testing various genetically informed approaches, such as environmental interventions targeted to subpopulations of various genotypes.
T2 research: from candidate health applications to evidence-based guidelines
This phase of research is largely focused on the translation of candidate health applications, such as new genetic tests, into evidence-based guidelines. T2 research begins once analytic validity has been established, and the early results of clinical validity look promising. Analytic validity is the ability to accurately and reliably measure the genotype of interest (18). A concern raised by the Secretary of Health and Human Services Advisory Committee on Genetics, Health and Society (http://www4.od.nih.gov/oba/sacghs/reports/reports.html) is the need for more oversight of these genetic tests. Genetic tests should have high analytic sensitivity, specificity, and predictive values as well as quality control procedures. Clinical validity of a test measures sensitivity, specificity, and positive and negative predictive value of a test (18). For complex phenotypes such as obesity, which is caused by multiple gene variants and gene–environment interactions, the clinical validity of genetic tests is not clear and is likely to be poor for individual genetic variants. This is due in part to the lack of identification of all susceptibility-associated variants; their modes of interaction with each other and with environmental exposures have also yet to be clearly defined (18). Another concern is clinical utility, which measures the risk and benefits of the genomic test for the management and control of obesity (18). There is insufficient research to determine whether genetic information can predict the occurrence of obesity and how it will impact multiple health endpoints. Perhaps more importantly, there is insufficient research testing as to whether genetically based, or genetically targeted, intervention approaches actually improve patient outcomes. Population and clinical-based studies are required to evaluate the analytic and clinical validity as well as the clinical utility of these tests in diverse populations.
Translation of a genetic test from research into practice starts with identification of the disorder to be tested for, the specific test to be used, and the clinical scenario in which the test will be used (e.g., diagnosis vs. predictive, population to be tested). During this phase, there is a need for clinical trials to study benefits and harms of information provided by the genetic test. Equally important is determining whether genetic tests can provide useful information for targeting commonly used interventions for obesity, compared to interventions delivered without benefit of genetic information. Such data have yet to be collected in the obesity research field.
Selected research areas critical to public health genomics and obesity prevention are discussed. Although these areas apply to all phases of translation, emphasized in this paper is the need to move promising applications to evidence-based guidelines (T2 research).
Nutrigenomics
The field of nutrigenomics is the study of how nutrients and genes interact and how genetic variations can cause people to respond differently to nutrients in food. Without such research, it will not be possible to evaluate the clinical utility of tailoring information for specific groups based on genetic susceptibility. Nutrigenomic research could lead to personalized diets tailored to the genetic make-up of the individual. However, nutrigenomics is a relatively new field of study that is faced with the same challenges as gene–environment studies. These challenges include the need for improved statistical and bioinformatic tools, quality control, standardization, data capture, and storage (54). The European Nutrigenomics Organization (NuGO) has published bioethics guidelines for scientists conducting nutrigenomic research (55). These ethical principles include population-based genomic research and include topics such as informed consent, genotype information (covering criteria for disclosure of genotype results to participants), BayBanks, and use and exchange of data samples. Currently biotechnology companies that market nutrigenomics to the public are focusing on the individual, and their contribution to public health remains to be seen. More importantly, there is no evidence that the products being marketed yield any benefits to consumers. One possible, but underutilized, approach is to use clinical trials to examine effects of nutrition interventions in various genetic subgroups. In the Dietary Approaches to Stop Hypertension (DASH) feeding trial, those participants assigned to the DASH dietary pattern who had the angiotensinogen genotype that conferred higher risk of hypertension was more responsive to the DASH diet, i.e., had greater lowering of blood pressure, than those with the lower risk genotype (56). This genetic analysis was a post hoc analysis and not the main purpose of the DASH trial. Clinical trials focused on these types of questions are needed in order to inform personalized nutrition approaches.
Family history
Family health history is another tool that can be used for obesity control and prevention and can be evaluated to determine analytic validity, clinical validity, and clinical utility. Family history (e.g., number of affected relatives, age at disease onset) is currently used in clinical medicine to assess or predict disease risk. It reflects the consequences of genetic susceptibilities, shared environment, and common behaviors (57). In addition or perhaps as an alternative to genetic testing, family health history could identify persons or populations with increased disease susceptibility by stratifying risk for chronic diseases. Together, knowledge of family history and other known risk factors (e.g., BMI, waist circumference, diet, and physical activity levels, history of obesity consequences such as hypertension, diabetes, or cancer) can be an aid in providing personalized medicine for disease prevention. An advantage of family history assessment is that it is readily available, inexpensive and may not have the same ethical and psychosocial implications of genetic testing. Moreover, family history can be combined with genetic testing to further refine disease risk. Disadvantages include memory recall, reporting bias, and capturing diseases when the prevalence is low. Additional information on the value of family history as a screening tool would be useful, for example its accuracy and reliability for stratifying disease risk, with and without adjunct genetic testing, and the effectiveness of this risk stratification on early detection and prevention efforts (57). Research is needed to determine the potential added value of genetic testing to the simple use of family history in practice. Thus far, the single-nucleotide polymorphisms (SNP) examined do not seem to add much when combined with family history and other risk factors in the prediction of common diseases such as prostate cancer, diabetes, and cardiovascular diseases.
Behavioral research
Behavior approaches are concerned with integrating genetic information and behavior change interventions to control or prevent obesity. The design of these approaches needs to bear in mind that the behaviors targeted as the intervention, and not only the outcome, may also be, in part, genetically influenced. Examples include typical lifestyle behaviors such as smoking, drinking, and exercise. Thus, the ease with which one may change behavior as well as the effect that the specific behavioral change has on the outcome (obesity) will vary because of genetic differences between people. To date, evidence strongly suggests that a small number of genetic polymorphisms interact with environmental factors to increase obesity risk (e.g., apolipoprotein A-V (APOA5), diet, and obesity risk (45)). Studies are underway to determine the effectiveness of behavioral interventions by genotype in obese subjects. One study found that obese study participants with both variants −55CC and −55CT in the uncoupling protein 3 (UCP3) gene had decreases in BMI and weight, but study participants with the variant –55CC had significant decreases in leptin and interleukin-6 in response to a weight loss intervention when compared to obese participants with the –55CT variant (58). This study illustrates the incorporation of genomic data into behavioral research. However, the literature is inconsistent regarding the genetic markers assessed, and additional research is needed before tailored behavioral obesity interventions that use genetic tests can be effective. Regardless of the genetic test, the mainstay for treating obesity is behavioral approaches to improve diet and increase physical activity for weight loss. However it is unknown whether providing obese patients with information about their genetic risks would improve or hinder adherence to behavioral interventions. Behavioral research is needed to address this question in diverse populations.
Health communication
Health communication researchers focus on where and how people receive health-related information and how they act in response to such information. Genetic information can be challenging for the public to understand, especially in the context of a complex health problem such as obesity. It is reported that the public receives most of its genetic information from television, radio, magazines, and newspapers (59). The internet is another source of direct-to-consumer information where one can access various genetic tests from national and international providers (60). There are concerns that these messages may be misleading. The majority of direct-to-consumer companies provided background information on the disease being tested and basic genetics, but the information is not always complete, pertinent, or accurate (61). One study examined the accuracy and nature of media coverage of genetic research and found only a small percent of newspaper and scientific journal articles discussed monetary costs or risks, whereas the majority of articles discussed only the benefits (62). Also, research is needed to explore how the public interprets genetic information. Questions remain on what the public would do with this information (genetic risk communication) upon receiving the test results. Moreover, the clinical utility of tailoring genetic information is yet to be confirmed. Skinner et al. (63) identified information needs of mothers who require assistance when deciding about communicating their breast cancer (BRCA1 and BRCA2) genetic test results to their children. Tailored print materials showed an advantage in increasing knowledge and enhancing accuracy of perceived risk when compared with non-tailored print materials. The field of public health genomics is tasked with the responsibility of educating and providing resources to the public on the accuracy and clinical validity as well as the ethical, legal, and social implications of direct-to-consumer genetic advertisements.
Clinical trials
Clinical trials are essential in providing scientific evidence to determine the benefits and harms of interventions that utilize genetic tests, including screening tests, prognostic tests, and genetically based or genetically targeted interventions or therapies. In the field of pharmacogenomics, clinical trials are used to evaluate treatment effects related to genomic profiles or genomic composite biomarkers (e.g., human-epidermal growth factor receptor 2 (HER2). The benefits and adverse effects of a drug will vary among individuals according to their genotypes (64). Likewise, considerable evidence points to genotype as a determinant of the response to bioactive food constituents (65). The results from clinical trials can provide information for evidence-based guidelines that support or recommend against genetic testing. Furthermore, providing genetic test results may be emotionally harmful for relatively rare mutations that have limited penetrance (66). No clinical trials have evaluated the potential benefits or harms of screening for genetic markers to determine obesity risk. However, the Diabetes Prevention Program trial of persons with pre-diabetes examined the effects of lifestyle intervention, which included weight loss, in various genetic subgroups. Analyses found an interaction between transcription factor-7-like 2 gene (TCF7L2) polymorphisms and the assigned intervention group, in that the elevated risk associated with the risk-conferring genotype was attenuated in the lifestyle intervention group but not in the metformin or placebo group (67), indicating a possible gene–intervention interaction.
Clinical trial methodology is crucial for evaluating outcomes from genetically based screening tests, prognostic tests, and interventions, including both medications and lifestyle approaches. Many more clinical trials are needed that address genetically defined questions in order to inform the evidence base for personalized medicine.
Evidence-based medicine
Evidence-based medicine generally uses multidisciplinary independent panels that conduct systematic assessments of the literature (published and unpublished), critically appraise the research evidence, and develop recommendations on the most appropriate clinical and public health guidelines. Examples of evidence-based guidelines include the Dietary Guidelines for Americans, Evaluation of Genomic Applications in Practice and Prevention (EGAPP) and the USPSTF. In 2003, the USPSTF recommended that clinicians screen all adult patients for obesity and offer intensive counseling and behavior intervention to promote sustained weight loss of obese adults (5,10). These guidelines were the culmination of expert reviews of the literature and grading the strength of the evidence. Several evidence-based guidelines have been developed for obesity prevention and screening (NHLBI, USPSTF), but there is insufficient evidence to advise for genetic testing for obesity. In 2004, the Centers for Disease Control and Prevention (CDC) addressed the need for evidence-based guideline development in genomics. The EGAPP initiative was developed to support evidence reviews and the development of evidence-based recommendations as related to genomic applications for health practice. The EGAPP working group reviews parameters of genetic tests such as analytic validity, clinical validity, and clinical utility; identifies gaps in knowledge; and develops clinical guidelines (68).
T3 research: from guidelines to health practice
This phase of translational research addresses issues such as increasing the spread of knowledge about evidence-based interventions (dissemination research), integrating these interventions into existing programs and structures (implementation and health-services research), and widespread adoption of these interventions by stakeholders (diffusion research). Additional challenges include workforce training, public health literacy, information systems, and public participation. Various organizations such as NIH, CDC, and academic institutions have websites and educational material to educate the public about genetics and genetic testing. Because genetic testing for obesity is in its infancy, little has been done in this area. Also, few genetic and genomic applications are ready for testing, let alone implementation, in clinical practice; obesity genomic testing is no exception. There are currently no evidence-based guidelines for recommending the use of genetic testing in obesity control and prevention. Only two genetic tests (BRCA1 and hereditary hemochromatosis) were addressed by the USPSTF in the past 7 years. The EGAPP working group is currently evaluating several genomic applications in practice, none of which are related to obesity (http://www.egappreviews.org/). Once validity and utility are determined, there is much translational research work yet to be done. T3 research points to the complexities of compliance and education that can ultimately affect the clinical utility of a genetic test in the "real" world (effectiveness) as opposed to the clinical utility of the test done under ideal scenarios of controlled clinical trials (efficacy) (27). An important additional step is to provide equal access to genetic testing, so as not to create or exacerbate existing health disparities. As genetic tests become more commonplace, direct consumer education will be vital. Professional organizations and public health agencies will need to collaborate on tasks such as workforce training, public health literacy, and development of clinical guidelines for practice.
T4 research: from health practice to population health impact
The last phase of translation research assesses how the adoption of evidence-based recommendations and guidelines can make an impact on real-world health outcomes. T4 research is often focused on clinical and public health outcomes and sometimes has been called "outcomes research." The Institute of Medicine considers outcomes research to be a multi-disciplinary field of inquiry, both basic and applied, that examines components of health care such as the use, costs, quality, accessibility, delivery, organization, and financing to increase the knowledge and understanding of the structure, processes, and results of health services for service users and providers (69). The promise of outcomes research aims to help individual patients and their clinicians make informed choices about preventive, diagnostic, therapeutic, and end-of-life care (70). Obesity researchers have traditionally used weight or BMI as commonly accepted health outcomes; however, these measures fail to capture the complexity of the disease and the multidimensional impact of treatment (5). As a result, the Task Force on Developing Obesity Outcomes and Learning Standards (TOOLS) was convened in 1999 to develop outcome measures that are broad in scope and include quality-of-life measures, as well as economic and clinical parameters (71). Outcome measures recommended by TOOLS for overweight and obesity included sleep apnea, quality of life, functional status, and economic data such as medical care cost and morbidity outcomes. These measures can provide additional information for assessing the overall burden of obesity, the effectiveness of the intervention, and help to fully integrate obesity into the medical system. Outcomes research is crucial to understanding the impact of public health genomics on population health.
The USPSTF reviewed the utility of obesity interventions for which the evidence-based guidelines for weight reduction and control are based. As a result of these interventions, BMI screening recommendations for adults and weight screening in children were developed. Currently, BMI screening is conducted at clinical and population levels, but is not a sufficient outcome measure that captures health-related factors such as quality of life, physical functioning and disability, or morbidity and mortality outcomes. Evidence-based interventions that incorporate genomics in obesity research have not been conducted, and thus evidence-based guidelines focused on obesity outcomes have not been established. Ideally, outcomes research would describe, interpret, and predict the impact of various influences, especially interventions on "final" endpoints that weigh on decision makers, patients, and the public. As genomic technologies improve, the integration of genomics into population surveillance for obesity and other health outcomes will become commonplace. However, the challenges ahead for public health genomics and obesity are great.
Conclusion
The goal of public health genomics is to use genomics appropriately to improve population health and eliminate or reduce the burden of disease in all segments of the population. A framework is presented for translation of obesity genomics discovery research to move promising genomics applications to clinical and public health practice. In this framework, multiple clinical and population disciplines are needed to evaluate the real-world benefits and harms of applying genomic advances in practice for the control and prevention of obesity. Components of this multidisciplinary research agenda include: epidemiology, clinical trials, behavioral and social sciences, health services and outcomes research, and economics and communication research. Currently, the focus of public health genomics in the area of obesity research is mostly concentrated in discovery and early phases of T1. For this reason, substantial additional research is needed before genomic applications could be used in obesity control and prevention in clinical and population settings.
Challenges in public health genomics include more reliable and affordable methods and technologies to measure and analyze (i) phenotypes, environments, and behaviors, (ii) their interactions with each other (which likely change over time, from prenatal development through old age), (iii) their relationship to obesity, and (iv) the effects of genetically based or genetically targeted intervention or therapeutic approaches. Some reasons for these challenges include the relatively low magnitudes of the associated increased disease risk, modest frequencies of disease susceptibility alleles in the population, and low penetrance of these alleles (72). Another concern is the difficulty in precise measurement of environmental and behavioral risk factors, and the paucity of intervention research such as clinical trials. Challenges include linking genomic data to environmental, behavioral, social science, and communication datasets to examine interactions and policy and ethical concerns while maintaining confidentiality of genetic information. Ultimately, the impact of advances in genomics on obesity control will depend on the results of observational studies and clinical trials and the reliability of these technologies to provide more benefits than harm over the current approaches to control the global obesity epidemic.
Acknowledgments
T.A.-C. acknowledges Robert Karp of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH, for his review and insightful comments. This publication was sponsored by the National Cancer Institute (NCI). The opinions or assertions contained herein are the views of the authors and are not to be considered as official or reflecting the views of the National Institutes of Health or the Centers for Disease Control and Prevention.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 3.Peeters A, Bonneux L, Nusselder WJ, De Laet C, Barendregt JJ. Adult obesity and the burden of disability throughout life. Obes Res. 2004;12:1145–1151. doi: 10.1038/oby.2004.143. [DOI] [PubMed] [Google Scholar]
- 4.Wee CC, Phillips RS, Legedza AT, et al. Health care expenditures associated with overweight and obesity among US adults: importance of age and race. Am J Public Health. 2005;95:159–165. doi: 10.2105/AJPH.2003.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf AM. Economic outcomes of the obese patient. Obes Res. 2002;10 Suppl 1:58S–62S. doi: 10.1038/oby.2002.191. [DOI] [PubMed] [Google Scholar]
- 6.Chung WKLR. Considerations regarding the genetics of obesity. Obesity. 2008;16:S33–S39. doi: 10.1038/oby.2008.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rankinen T, Bouchard C. Genetics of food intake and eating behavior phenotypes in humans. Annu Rev Nutr. 2006;26:413–434. doi: 10.1146/annurev.nutr.26.061505.111218. [DOI] [PubMed] [Google Scholar]
- 8.Wardle J, Carnell S, Haworth CM, et al. Obesity-associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3643. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 9.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert panel on the identification, evaluation, and treatment of overweight in Adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930–932. doi: 10.7326/0003-4819-139-11-200312020-00012. [DOI] [PubMed] [Google Scholar]
- 11.Katz DL, O'Connell M, Yeh MC, et al. Public health strategies for preventing and controlling overweight and obesity in school and worksite settings: a report on recommendations of the Task Force on Community Preventive Services. MMWR Recomm Rep. 2005;54:1–12. [PubMed] [Google Scholar]
- 12.Kumanyika SK, Obarzanek E, Stettler N, et al. Population-based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science) Circulation. 2008;118:428–464. doi: 10.1161/CIRCULATIONAHA.108.189702. [DOI] [PubMed] [Google Scholar]
- 13.Morton NE. Into the post-HapMap era. Adv Genet. 2008;60:727–742. doi: 10.1016/S0065-2660(07)00425-7. [DOI] [PubMed] [Google Scholar]
- 14.Abrahams E, Ginsburg GS, Silver M. The personalized medicine coalition: goals and strategies. Am J Pharmacogenomics. 2005;5:345–355. doi: 10.2165/00129785-200505060-00002. [DOI] [PubMed] [Google Scholar]
- 15.Glaser J, Henley DE, Downing G, Brinner KM. Health care workgroup of the American health information community. Advancing personalized health care through health information technology: an update from the American Health Information Community's Personalized Health Care Workgroup. J Am Med Inform Assoc. 2008;15:391–396. doi: 10.1197/jamia.M2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van't Veer LJ, Bernards R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature. 2008;452:564–570. doi: 10.1038/nature06915. [DOI] [PubMed] [Google Scholar]
- 17.Haga SB, Khoury MJ, Burke W. Genomic profiling to promote a healthy lifestyle: not ready for prime time. Nat Genet. 2003;34:347–350. doi: 10.1038/ng0803-347. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle—will we get our wish? N Engl J Med. 2008;358:105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 19.Burke W, Khoury MJ, Stewart A, Zimmern RL. The path from genome-based research to population health: development of an international public health genomics network. Genet Med. 2006;8:451–458. doi: 10.1097/01.gim.0000228213.72256.8c. [DOI] [PubMed] [Google Scholar]
- 20.Khoury MJ, Davis R, Gwinn M, Lindegren ML, Yoon P. Do we need genomic research for the prevention of common diseases with environmental causes? Am J Epidemiol. 2005;161:799–805. doi: 10.1093/aje/kwi113. [DOI] [PubMed] [Google Scholar]
- 21.Khoury MJ, Mensah GA. Genomics and the prevention and control of common chronic diseases: emerging priorities for public health action. Prev Chronic Dis. 2005;2:A05. [PMC free article] [PubMed] [Google Scholar]
- 22.Segal ME, Polansky M, Sankar P. Predictors of uptake of obesity genetic testing among affected adults. Hum Genet. 2007;120:641–652. doi: 10.1007/s00439-006-0252-8. [DOI] [PubMed] [Google Scholar]
- 23.Harvey-Berino J, Gold EC, West DS, et al. Does genetic testing for obesity influence confidence in the ability to lose weight? A pilot investigation. J Am Diet Assoc. 2001;101:1351–1353. doi: 10.1016/S0002-8223(01)00323-6. [DOI] [PubMed] [Google Scholar]
- 24.Frosch DL, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1485–1489. doi: 10.1158/1055-9965.EPI-04-0913. [DOI] [PubMed] [Google Scholar]
- 25.Rikke BA, Battaglia ME, Allison DB, Johnson TE. Murine weight loss exhibits significant genetic variation during dietary restriction. Physiol Genomics. 2006;27:122–130. doi: 10.1152/physiolgenomics.00068.2006. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez Roque MI, Camilleri M, Clark MM, et al. Alteration of gastric functions and candidate genes associated with weight reduction in response to sibutramine. Clin Gastroenterol Hepatol. 2007;5:829–837. doi: 10.1016/j.cgh.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9:665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 28.Yu W, Wulf A, Yesupriya A, Clyne M, Khoury MJ, Gwinn M. HuGE Watch: tracking trends and patterns of published studies of genetic association and human genome epidemiology in near-real time. Eur J Hum Genet. 2008;16:1155–1158. doi: 10.1038/ejhg.2008.95. [DOI] [PubMed] [Google Scholar]
- 29.Rankinen T, Zuberi A, Chagnon YC, et al. The human obesity gene map: the 2005 update. Obesity. 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National Center for Health Statistics. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; National Health and Nutrition Examination Survey Data. 2007 < http://www.cdc.gov/nchs/about/major/nhanes/nhanes2007-2008/nhanes07_08.htm>.
- 31.Fox CS, Heard-Costa N, Cupples LA, Dupuis J, Vasan RS, Atwood LD. Genome-wide association to body mass index and waist circumference: the Framingham heart study 100K project. BMC Med Genet. 2007;8 Suppl 1:S18. doi: 10.1186/1471-2350-8-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury MJ, Gwinn M, Burke W, Bowen S, Zimmern R. Will genomics widen or help heal the schism between medicine and public health? Am J Prev Med. 2007;33:310–317. doi: 10.1016/j.amepre.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 2007;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 34.Valli-Jaakola K, Lipsanen-Nyman M, Oksanen L, et al. Identification and characterization of melanocortin-4 receptor gene mutations in morbidly obese Finnish children and adults. J Clin Endocrinol Metab. 2004;89:940–945. doi: 10.1210/jc.2003-031182. [DOI] [PubMed] [Google Scholar]
- 35.Young EH, Wareham NJ, Farooqi S, et al. The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29 563 individuals. Int J Obes (Lond) 2007;31:1437–1441. doi: 10.1038/sj.ijo.0803609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stutzmann F, Vatin V, Cauchi S, et al. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet. 2007;16:1837–1844. doi: 10.1093/hmg/ddm132. [DOI] [PubMed] [Google Scholar]
- 37.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 39.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cha SW, Choi SM, Kim KS, et al. Replication of genetic effects of FTO polymorphisms on BMI in a Korean population. Obesity. 2008;16:2187–2189. doi: 10.1038/oby.2008.314. [DOI] [PubMed] [Google Scholar]
- 41.Chang YC, Liu PH, Lee WJ, et al. Common variation in the FTO gene confers risk of obesity and modulates body mass index in the Chinese population. Diabetes. 2008;57:2245–2252. doi: 10.2337/db08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotta K, Nakata Y, Matsuo T, et al. Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet. 2008;53:546–553. doi: 10.1007/s10038-008-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant SF, Li M, Bradfield JP, et al. Association analysis of the FTO gene with obesity in children of Caucasian and African ancestry reveals a common tagging SNP. PLoS ONE. 2008;3:e1746. doi: 10.1371/journal.pone.0001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loos RJ, Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes Rev. 2008;9:246–250. doi: 10.1111/j.1467-789X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 45.Corella D, Lai CQ, Demissie S, et al. APOA5 gene variation modulates the effects of dietary fat intake on body mass index and obesity risk in the Framingham Heart Study. J Mol Med. 2007;85:119–128. doi: 10.1007/s00109-006-0147-0. [DOI] [PubMed] [Google Scholar]
- 46.Grove ML, Morrison A, Folsom AR, Boerwinkle E, Hoelscher DM, Bray MS. Gene-environment interaction and the GNB3 gene in the Atherosclerosis Risk in Communities study. Int J Obes (Lond) 2007;31:919–926. doi: 10.1038/sj.ijo.0803545. [DOI] [PubMed] [Google Scholar]
- 47.Ordovas JM, Tai ES. Why study gene-environment interactions? Curr Opin Lipidol. 2008;19:158–167. doi: 10.1097/MOL.0b013e3282f6a809. [DOI] [PubMed] [Google Scholar]
- 48.Tholin S, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. Am J Clin Nutr. 2005;81:564–569. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- 49.Purcell S, Sham P. Variance components models for gene-environment interaction in quantitative trait locus linkage analysis. Twin Res. 2002;5:572–576. doi: 10.1375/136905202762342035. [DOI] [PubMed] [Google Scholar]
- 50.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 51.Heitmann BL, Kaprio J, Harris JR, Rissanen A, Korkeila M, Koskenvuo M. Are genetic determinants of weight gain modified by leisure-time physical activity? A prospective study of Finnish twins. Am J Clin Nutr. 1997;66:672–678. doi: 10.1093/ajcn/66.3.672. [DOI] [PubMed] [Google Scholar]
- 52.Kallio P, Kolehmainen M, Laaksonen DE, et al. Dietary carbohydrate modification induces alterations in gene expression in abdominal subcutaneous adipose tissue in persons with the metabolic syndrome: the FUNGENUT Study. Am J Clin Nutr. 2007;85:1417–1427. doi: 10.1093/ajcn/85.5.1417. [DOI] [PubMed] [Google Scholar]
- 53.Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci U S A. 2008;105:8369–8374. doi: 10.1073/pnas.0803080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elliott RM, Johnson IT. Nutrigenomic approaches for obesity research. Obes Rev. 2007;8 Suppl 1:77–81. doi: 10.1111/j.1467-789X.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- 55.Bergmann MM, Bodzioch M, Bonet ML, Defoort C, Lietz G, Mathers JC. Bioethics in human nutrigenomics research: European Nutrigenomics Organisation workshop report. Br J Nutr. 2006;95:1024–1027. doi: 10.1079/bjn20061758. [DOI] [PubMed] [Google Scholar]
- 56.Svetkey LP, Moore TJ, Simons-Morton DG, et al. Angiotensinogen genotype and blood pressure response in the Dietary Approaches to Stop Hypertension (DASH) study. J Hypertens. 2001;19:1949–1956. doi: 10.1097/00004872-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Yoon PW, Scheuner MT, Peterson-Oehlke KL, Gwinn M, Faucett A, Khoury MJ. Can family history be used as a tool for public health and preventive medicine? Genet Med. 2002;4:304–310. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 58.de Luis DA, Aller R, Izaola O, Sagrado MG, Conde R. Modulation of adipocytokines response and weight loss secondary to a hypocaloric diet in obese patients by-55CT polymorphism of UCP3 gene. Horm Metab Res. 2008;40:214–218. doi: 10.1055/s-2008-1046796. [DOI] [PubMed] [Google Scholar]
- 59.Geller G, Bernhardt BA, Holtzman NA. The media and public reaction to genetic research. JAMA. 2002;287:773. [PubMed] [Google Scholar]
- 60.Mykitiuk R. Caveat emptor: direct-to-consumer supply and advertising of genetic testing. Clin Invest Med. 2004;27:23–32. [PubMed] [Google Scholar]
- 61.Geransar R, Einsiedel E. Evaluating online direct-to-consumer marketing of genetic tests: informed choices or buyers beware? Genet Test. 2008;12:13–23. doi: 10.1089/gte.2007.0024. [DOI] [PubMed] [Google Scholar]
- 62.Bubela TM, Caulfield TA. Do the print media “hype” genetic research? A comparison of newspaper stories and peer-reviewed research papers. CMAJ. 2004;170:1399–1407. doi: 10.1503/cmaj.1030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skinner CS, Schildkraut JM, Berry D, et al. Pre-counseling education materials for BRCA testing: does tailoring make a difference? Genet Test. 2002;6:93–105. doi: 10.1089/10906570260199348. [DOI] [PubMed] [Google Scholar]
- 64.Simon R, Wang SJ. Use of genomic signatures in therapeutics development in oncology and other diseases. Pharmacogenomics J. 2006;6:166–173. doi: 10.1038/sj.tpj.6500349. [DOI] [PubMed] [Google Scholar]
- 65.El Sohemy A. Nutrigenetics. Forum Nutr. 2007;60:25–30. doi: 10.1159/000107064. [DOI] [PubMed] [Google Scholar]
- 66.Madlensky L, McLaughlin JR, Carroll JC, Goel V, Frank JW. Risks and benefits of population-based genetic testing for Mendelian subsets of common diseases were examined using the example of colorectal cancer risk. J Clin Epidemiol. 2005;58:934–941. doi: 10.1016/j.jclinepi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355:241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Recommendations from the EGAPP Working Group: testing for cytochrome P450 polymorphisms in adults with nonpsychotic depression treated with selective serotonin reuptake inhibitors. Genet Med. 2007;9:819–825. doi: 10.1097/gim.0b013e31815bf9a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thaul S, Lohr KN, Tranquada RE, editors. Washington, DC: National Academy Press; 1994. Health Services Research: Opportunities for an Expanding Field of Inquiry: An Interim Statement. Institute of Medicine. [PubMed] [Google Scholar]
- 70.Lipscomb J, Donaldson MS. Outcomes research at the National Cancer Institute: measuring, understanding, and improving the outcomes of cancer care. Clin Ther. 2003;25:699–712. doi: 10.1016/s0149-2918(03)80106-6. [DOI] [PubMed] [Google Scholar]
- 71.Task Force on Developing Obesity Outcomes and Learning Standards (TOOLS). Obes Res; Proceedings of a symposium; June 11–13, 1999; Warrenton, Virginia, USA. 2002. pp. 1S–93S. [DOI] [PubMed] [Google Scholar]
- 72.Eaton D. Gene-environment interactions. In: Hernandez LM, editor. Implications of Genomics for Public Health Workshop Summary. Institute of Medicine. Washington, DC: National Academy Press; 2005. [PubMed] [Google Scholar]