Abstract
Most non-tetrapod vertebrates develop mineralized extra-oral elements within the integument. Known collectively as the integumentary skeleton, these elements represent the structurally diverse skin-bound contribution to the dermal skeleton. In this review we begin by summarizing what is known about the histological diversity of the four main groups of integumentary skeletal tissues: hypermineralized (capping) tissues; dentine; plywood-like tissues; and bone. For most modern taxa, the integumentary skeleton has undergone widespread reduction and modification often rendering the homology and relationships of these elements confused and uncertain. Fundamentally, however, all integumentary skeletal elements are derived (alone or in combination) from only two types of cell condensations: odontogenic and osteogenic condensations. We review the origin and diversification of the integumentary skeleton in aquatic non-tetrapods (including stem gnathostomes), focusing on tissues derived from odontogenic (hypermineralized tissues, dentines and elasmodine) and osteogenic (bone tissues) cell condensations. The novelty of our new scenario of integumentary skeletal evolution resides in the demonstration that elasmodine, the main component of elasmoid scales, is odontogenic in origin. Based on available data we propose that elasmodine is a form of lamellar dentine. Given its widespread distribution in non-tetrapod lineages we further propose that elasmodine is a very ancient tissue in vertebrates and predict that it will be found in ancestral rhombic scales and cosmoid scales.
Keywords: evolution, integumentary skeleton, non-tetrapods, origin
Introduction
The ability to mineralize a skeleton is one of the major innovations of vertebrates, providing protection and enhancing locomotion. The earliest evidence of vertebrate skeletal mineralization is encountered in the tooth-like feeding apparatus of conodonts (Sansom et al. 1992; Donoghue, 1998). However, the majority of early skeletonizing vertebrates instead possessed a mineralized integument that included a wide variety of surface covering and/or embedded mineralized elements, demonstrating a diversity of structural organizations and functional properties (Donoghue & Sansom, 2002; Donoghue et al. 2006). These elements constitute the so-called dermal skeleton (= dermoskeleton) (Ørvig, 1965; Francillon-Vieillot et al. 1990; Zylberberg et al. 1992; Donoghue & Sansom, 2002). For most extant lineages, the dermal skeleton has undergone widespread reduction and is routinely partitioned into the dermatocranium, skeletal components of the pectoral apparatus, teeth and tooth-like elements of the oral and pharyngeal cavities, and extra-oral or integumentary investments – hereafter the integumentary skeleton (Moss, 1972; Kresja, 1979; Zylberberg et al. 1992). The integumentary skeleton has long been a source of interest for comparative morphologists, palaeontologists and evolutionary developmental biologists (see review by Goodrich, 1907; Ørvig, 1951, 1967, 1968; Denison, 1963; Moss, 1964; Peyer, 1968; Halstead, 1969, 1974, 1987; Schmidt & Keil, 1971; Reif, 1982a; Maisey, 1988; Smith & Hall, 1990, 1993; Janvier, 1996; Huysseune & Sire, 1998; Donoghue et al. 2000, 2006; Donoghue & Aldridge, 2001; Donoghue & Sansom, 2002; Sire & Huysseune, 2003). With few exceptions, however, the emphasis has largely been directed towards understanding early integumentary skeletal evolution in non-tetrapod vertebrates.
In this review we (1) summarize what is known about the histological structure and development of integumentary skeleton of non-tetrapod vertebrates (the integumentary skeleton in tetrapods is reviewed in a companion paper; Vickaryous & Sire, this Issue); (2) re-address the homology of various skeletal tissues associated with the integumentary skeleton, with particular focus on the hypermineralized covering tissues and the typical condition of elasmoid scales and their tissues; and (3) revise the scenario of integumentary skeletal evolution.
Of particular importance to this scenario is the analysis of tissue structure and formation at the level of cell population condensations and an integration of data from both the fossil record and modern taxa; skeletal elements being extensively mineralized, they are available from the fossil record, which allow us to trace the evolutionary changes throughout geological times. However, the interpretation of the subjacent biological processes depends to a large extent on a precise knowledge obtained from living species.
The phylogenetic framework for this review (Fig. 1) is summarized from Janvier (1996), Donoghue et al. (2000) and Donoghue & Smith (2001).
Fig. 1.
Simplified phylogenetic tree of the vertebrates illustrating the interrelationships of the lineages discussed in the text (after Janvier, 1996, 2007; Donoghue & Smith, 2001; Hill, 2005; Donoghue et al. 2006). The relationships of Euconodonta to or within the vertebrates are still debated; the position of Anaspida and Thelodonti with respect to other stem gnathostomes remains uncertain.
Origin, evolution and diversity of the integumentary skeleton
Regardless of morphology and structure, all elements of the dermal skeleton are primarily housed in the dermis, with the epidermis playing a critical role in regulating their formation (involving various signaling molecules and transcription factors) and/or composition (deposition of material). The tissue composition of each element is governed by the presence and interactions of cell condensations with either odontogenic or osteogenic competence, and the current structural diversity of the integumentary skeleton results from the evolutionary and developmental interplay of these two components. These features are briefly commented on in the following two sections.
Introduction to the integument
Vertebrate integument forms a continuous, heterogeneous covering of the outer body surface consisting of two developmentally and morphologically distinct strata, the epidermis and the dermis (= corium; cutis vera). Early during ontogeny these two layers become separated from one another by a basement membrane, and from the subjacent muscle cells by the hypodermis (Whitear, 1986). In its mature state, the epidermis is stratified and contains a large number of specialized cells, and plays crucial roles as a permeability barrier and in mechanical protection (Bereiter-Hahn et al. 1986). The epidermis is also involved in the formation of non-skeletal epidermal scales and integumentary appendages (feathers, hairs, wristles, claws, beak, nails, etc.), the details of which are provided elsewhere (Maderson, 1972; see also this Issue: Alibardi et al.; Dhouailly; Homberger et al.; Bragulla & Homberger; and Maddin et al.). Although the epidermis does not contain bone or other mineralized tissues, it may become hardened by the accumulation of various keratin proteins and phospholipids with bound calcium (Spearman, 1973). Furthermore, some mineralized integumentary elements, such as teeth and odontodes, may penetrate the epidermis. The epidermis is a derivative of the ectoderm and begins as a single cell layer, which quickly stratifies to form various layers with different properties (e.g. mitotically or synthetically active deep and mid cell layers, or keratinized and protective superficial cells; Lillywhite, 2006). It is also worth noting that the basal layer cells of the epidermis (or of the epithelium when located in the oral or pharyngeal cavities) play a crucial role in regulating morphogenesis and differentiation of integumentary elements through reciprocal interactions with the dermis (see Caton & Tucker, and Dhouailly, this Issue).
The deep counterpart to the epidermis is the dermis, a fibrous connective tissue unique to cephalochordates (Olsson, 1961) and vertebrates (Moss, 1972). The dermis provides both physical and metabolic support to the epidermis (Fox, 1986; Landmann, 1986; Matoltsy, 1986; Whitear, 1986; Lillywhite, 2006), and the structural framework for integumentary elements (Moss, 1969, 1972). Unlike the epidermis, which is derived from ectodermal epithelium, the dermis develops from mesenchyme. Experiments on Gallus gallus (the domestic fowl) have revealed that the dermis has multiple origins across the body: craniofacial dermis develops from cephalic neural crest cells (= ectomesenchyme); the dermis over the occiput and otic regions forms from cephalic mesoderm; and across the postcranium the dermis is derived from the dermatome compartment of paraxial mesoderm (dorsal dermis) and somatopleure (ventral, lateral and limb dermis) (Mauger, 1972a,b; Olivera-Martinez et al. 2001; Dhouailly et al. 2004). Also significant to our discussion is the well-documented migratory abilities of melanoblasts, which are also of neural crest origin, giving rise to all the dermis-bound pigment cells of the body (Le Douarin & Kalcheim, 1999). The presence of tissues otherwise exclusive to teeth (e.g. dentine) in some post-cranial integumentary elements (e.g. tooth-like denticles of armored catfish, the dentine layer of polypterid scales) strongly suggests that they are neural crest in origin, too. This implies that early in ontogeny, ectomesenchymal cells with either the osteogenic and/or odontogenic potential have colonized the dermis in post-cranial regions (see also Vickaryous & Sire, this Issue).
Among cephalochordates (e.g. Branchiostomaspp.) the dermis is a thin, collagen-rich layer that constitutes a dense, regular connective tissue (Olsson, 1961; Moss, 1972; Spearman, 1973; Kemp, 1999). The collagen fibrils are highly organized, forming stacked ply with alternating orientations (often orthogonal). In vertebrates, including myxinoids (hagfish) and petromyzontids (lampreys), the dermis is a thick, collagenous fibrillar framework consisting mostly of type I collagen, and an amorphous ground substance consisting of glycosaminoglycans (e.g. dermatan sulfate), structural glycoproteins, and tissue fluid (Moss, 1972; Junqueira et al. 1998). Except for myxinoids and petromyzontids, the dermis also contains limited amounts of elastin. The most common cells found in the vertebrate dermis are fibroblasts (fibrocytes), followed by mast cells, macrophages, pigment cells (e.g. melanocytes), and scleroblasts, undifferentiated cells ultimately involved in the formation of mineralized integumentary organs. The thickness, structure, and cellular diversity of the dermis varies between taxa, location on the body, and stage of development/ontogenetic age. Unlike the epidermis of most vertebrates, the dermis is also rich in blood and lymphatic vessels, nerves and, with the presence of scleroblasts, retains the ability to develop mineralized organs (Romer, 1956; Moss, 1972).
Structurally (at least amongst crown gnathostomes) the dermis is bilaminar (except for some teleosts; Sire & Huysseune, 2003) with an outermost stratum superficiale (= stratum laxum of aquatic non-tetrapodan osteichthyans; stratum vasculare of chondrichthyans; stratum spongiosum of amphibians; papillary layer of synapsids), and a deeper stratum compactum (= reticular layer of synapsids). The stratum superficiale is typically a loose irregular connective tissue (areolar tissue) containing blood vessels, nerves, and pigment cells. In contrast, the stratum compactum is a more tightly packed feltwork, dominated by large bundles of collagen fibrils with relatively few cells, vessels, and nerves. In most instances, the histological organization of the stratum compactum is densely regular, with the deepest collagen layers forming lamellar and orthogonal arrangements structurally comparable with the plywood-like arrangement of the collagen layers in lamellar bone and in elasmodine of elasmoid scales (Zylberberg et al. 1992; Sire & Akimenko, 2004). The structural configuration of the stratum compactum provides the integument with stress resistance.
Among fossil relatives of modern jawed vertebrates, in which the only portions of the integument preserved are mineralized, it is not unreasonable to postulate that the structural fabric of these (preserved) organs accurately reflects the structural organization of the surrounding unmineralized, and thus unpreserved, connective tissues (Moss, 1972). By extension, the contributing proteins (e.g. type I collagen, proteoglycans, and mineralizing proteins) and molecular mechanisms involved were similar to those observed today. Significantly, these postulates can be tested on lampreys and hagfishes, which are more distant relatives of living jawed vertebrates than the extinct lineage Ostracodermi. Therefore, lampreys, hagfishes and crown-gnathostomes constitute an extant phylogenetic bracket (Witmer, 1995).
Role of papillae and primordia in the development and diversity of the integumentary skeleton
For many modern taxa (particularly teleost fish and tetrapods), the integumentary skeleton has undergone considerable reduction and modification, often rendering the homology and relationships of these elements uncertain. For example, the integument of chondrichthyans (sharks, rays) and some distantly related teleosts (e.g. xiphioids, armoured catfish, the clupeomorph Denticeps, the atheriniform Atherion: Sire & Huysseune, 1996; Sire et al. 1998; Sire & Allizard, 2001) is characterized by the development of numerous tooth-like organs similar to odontodes of early vertebrates. Whereas all these organs appear to share a similar functional (hydrodynamic) role, developmental and phylogenetic evidence clearly indicates that each represents an independent evolutionary derivation (Sire, 2001; Sire & Huysseune, 2003; see also Huysseune et al. this Issue). To address the question of homology, Sire & Huysseune (2003) employed a comparative developmental approach targeting the cellular developmental units known as cell condensations (see also Atchley & Hall, 1991; Hall & Miyake, 1992, 2000; Eames et al. 2003; Vickaryous & Hall, 2006).
Cell condensations are the primary and most basic unit of morphology (Hall & Miyake, 1992) and provide a powerful tool for understanding the evolution of organs and anatomical complexes (Atchley & Hall, 1991; Hall & Miyake, 2000). Unlike the adult phenotype, which may become extensively altered during the course of ontogeny, cell condensations often provide a conserved cornerstone (if only transiently) for the establishment of structural level homology. In the development of the integumentary skeleton, two fundamental types of cell condensations are known to contribute: odontogenic and osteogenic (Smith & Hall, 1990, 1993; Sire & Huysseune, 2003). These cell condensations may act alone or in combination.
An odontogenic condensation is the fundamental developmental unit of odontogenic organs (= teeth), and the initiate of dental tissues such as enamel, enameloid, dentine, and bone of attachment. Structurally, each odontogenic condensation begins as a well-delimited population of densely organized mesenchymal cells, the dental (= odontogenic) papilla. The dental papilla develops close to the inner layer of the epithelium (teeth) or the basal layer of the epidermis (odontodes, mineralized scales). These mineralized tissue-forming mesenchymal cells (scleroblasts) acquire an odontogenic competence. Epithelial cells capping the dental papilla, the dental (= enamel) organ, differentiate into ameloblasts, which deposit the enamel matrix in a polarized fashion towards the mesenchyme. The underlying scleroblasts of the dental papilla differentiate into odontoblasts that deposit the dentine matrix. The histological organization and structural identity of the tissues derived from an odontogenic condensation (e.g. enameloid, dentine, enamel, bone of attachment) depend on the timing of the interaction between the dental papilla mesenchyme and the dental organ epithelium (Osborn, 1981).
An osteogenic condensation (= osteogenic primordium) is the fundamental developmental unit of integumentary elements composed of bone and fibrous connective tissue (e.g. dermal plates, dermal bones). The primordium does not develop primary cartilage, although dermal elements often originate parachondrally (adjacent to a cartilage element; Huysseune & Sire, 1992; Sire & Huysseune, 1993). Unlike an odontogenic condensation, an osteogenic primordium lacks a well-delimited papilla and it initiates deep within the mesenchyme, at a distance from the basal epidermal layer. Cells of the primordium differentiate from scleroblasts with osteogenic competence. Deposition of the extracellular matrix (ECM) by these osteoblasts is not polarized and pre-existing collagen fibrils of the surrounding dermis are often integrated into the developing organ. During the initial, rapid stage of development the pre-existing collagen matrix is often remodeled by the osteoblasts, with new materials (such as various collagens and non-collagenous proteins) being synthesized and directly incorporated into this matrix, thus modifying the physico-chemical environment to favour mineralization. During subsequent stages of development and growth, there may be de novo deposition of bone matrix, mineralization of pre-existing matrix, or some combination of the two.
The terminology associated with various structural categories of integumentary elements is another significant source of confusion. For instance, the term ‘scale’ is routinely employed for both keratinized (epidermal) and mineralized (mesenchymal) organs. Furthermore, ‘mineralized scale’ refers to a broad diversity of integumentary elements with different tissue composition and developmental origins. Therefore we employ adjectives that define their origin, i.e. cosmoid, palaeoniscoid, polypteroid, lepisosteoid and elasmoid scales, or replace the term ‘scale’ altogether, e.g. dermal plate, scute, shield, etc. To minimize any misunderstandings, we have attempted to make clear distinctions when the homology of any structure is uncertain, following Sire & Huysseune (2003).
Tissue diversity of the integumentary skeleton
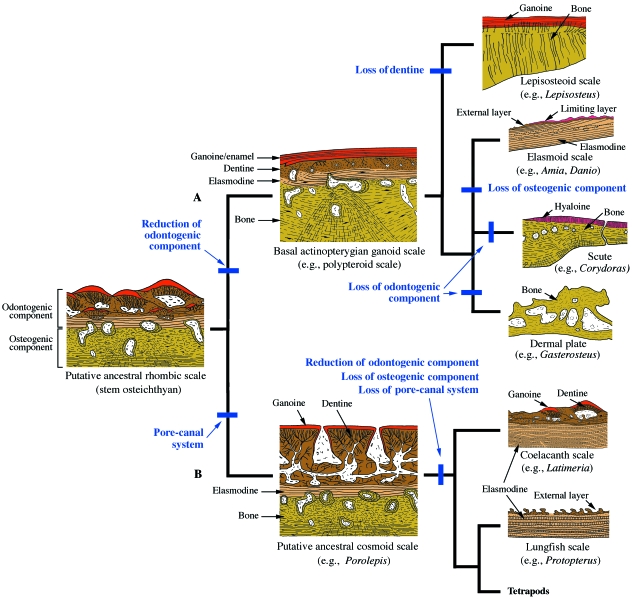
The integumentary skeleton is characterized by a diversity of tissue types categorized as either odontogenic or osteogenic tissues. Both tissue categories are patterned independently, and they can occur either separately (e.g. teeth or dermal plates) or can be combined in a same element (e.g. ganoid scales of bichirs). They may be further subdivided based on the degree of mineralization, relative amounts of collagen and other components of the ECM, mode of development, and associated cell types (Sire & Huysseune, 2003). Whereas the discrimination of skeletal tissues is often subjective, four main groups are widely recognized in the integumentary skeleton: hypermineralized (capping) tissues, dentines, plywood-like tissues, and bony tissues (Table 1).
Table 1.
Summary of the various tissues recognized in the integumentary skeleton of nontetrapodan vertebrates. The taxa with living representatives are underlined
| Taxon | Capping tissue | Dentine | Plywood-like tissue | Bone | Comments |
|---|---|---|---|---|---|
| Pteraspidomorphi | enameloid/none | orthodentine/ mesodentine/none | lamellar bone* | acellular bone† | odontodes |
| Anaspida | ? | ? | lamellar bone* | acellular bone† | ? odontodes |
| Thelodonti | enameloid | orthodentine | none | acellular bone† | odontodes |
| Galeaspida | none | none | lamellar bone* | acellular bone† | – |
| Osteostraci | enameloid/none | mesodentine | lamellar bone* | cellular bone | cosmoid-like organization with putative odontodes |
| Placodermi | none | semidentine/ orthodentine/none | none | cellular bone | odontode-like derivatives |
| Chondrichthyes | enameloid | orthodentine | none | acellular bone | odontodes |
| Acanthodii | ganoine/none | mesodentine/ orthodentine | none | acellular bone/cellular bone | odontode-like squamation |
| Andreolepis | ganoine (monolayered) | orthodentine | none | cellular bone | palaeoniscoid-type ganoid scale |
| Cheirolepis | ganoine (multilayered) | orthodentine | none | cellular bone | palaeoniscoid-type ganoid scale |
| Cladistia | ganoine (multilayered) | orthodentine | elasmodine | cellular bone | polypteroid-type ganoid scale |
| Moythomasia | ganoine (multilayered) | orthodentine | none | cellular bone | palaeoniscoid-type ganoid scale |
| Chondrostei | ganoine/none | none | none | cellular bone | lepisosteoid-type ganoid scale/bony plate |
| Lepisosteiformes | ganoine (multilayered) | none | none | cellular bone | lepisosteoid-type ganoid scale |
| Amiiformes | ganoine/limiting layer | orthodentine/external layer | elasmodine/lamellar bone | cellular bone/none | ganoid to elasmoid scale |
| Teleostei | limiting layer | external layer | elasmodine | acellular bone/cellular bone/none | elasmoid scales, bony plates or scutes |
| Porolepiformes | enamel(oid)/limiting layer | orthodentine/external layer | elasmodine/lamellar bone | cellular bone/none | cosmoid |
| Dipnoi | enamel(oid)/limiting layer | orthodentine/external layer | elasmodine/lamellar bone | cellular bone/none | cosmoid to elasmoid scale |
| Actinistia | enamel(oid)/limiting layer | orthodentine/external layer | elasmodine/lamellar bone | cellular bone/none | cosmoid to elasmoid scale |
In these taxa the lamellar bone of the basal plates has been called ‘isopedine’.
In these early vertebrates the ‘aspidin’ is very similar to acellular bone.
Hypermineralized (capping) tissues
Hypermineralized tissues are a group of hard, protective tissues that form the cap of teeth and the outer layer of some integumentary skeletal elements. In its mature state, a hypermineralized tissue is characterized as crystalline (principally hydroxyapatite crystals, a type of calcium phosphate), acellular (or cell-poor), avascular, non-collagenous (or collagen-poor), and demonstrates extreme hardness. The two best known examples are enamel and enameloid, common to teeth. Additional hypermineralized tissues include: (1) ganoine, the outermost layer of the dermatocranium and integumentary skeleton of basal actinopterygians [e.g. the living bichirs (polypterids) and gars (lepisosteids) (Sire et al. 1987)]; (2) hyaloine, found on the scutes of armored catfish (Sire, 1993); and (3) the limiting layer, on the posterior region of elasmoid scales (Sire, 1985, 1988). An unnamed, hypermineralized tissue superimposed on the osteoderms of some reptiles will be discussed elsewhere (see Vickaryous & Sire, this Issue).
Enamel
Enamel matrix is homogeneous and is highly mineralized (up to 96% inorganic for mammalian teeth; Hall & Witten, 2007), with no collagen or embedded cells. It may be prismatic (mammalian synapsids) or not prismatic (other tetrapods) (Huysseune & Sire, 1998). Tooth enamel formation begins as epithelial cells interact with underlying mesenchymal cells to create a well-defined dental organ. Following a series of inductive interactions with the dental papilla cells (mesenchyme), the inner dental epithelial cells differentiate into pre-ameloblasts, and then ameloblasts. While the collagen-rich dentine matrix is being deposited by underlying odontoblasts, overlying ameloblasts centrifugally deposit an organic enamel matrix, consisting mostly of amelogenin (90%), with a limited amount of enamelin, ameloblastin, tuftelin, and traces of other components. During the mineralization phase, the organic matrix is degraded by metalloproteinases [enamelysine (MMP20), and kallikrein 4 (KLK4)], removed (leaving only 3–4% of the original organic matrix) and replaced by crystalline precipitation (Nanci, 2003).
Enameloid
Enameloid (= durodentine, vitrodentine, bitypic enamel) in its mature state resembles enamel both topologically and functionally. It also derives from inductive epithelial-mesenchymal interactions, which take place between a well-defined dental papilla (mesenchyme) and a dental organ (epithelium). However, unlike enamel, the crystalline arrangement is less ordered and not prismatic due to the presence of a loose network of thin collagen fibrils in the organic phase, prior to maturation.
As currently understood, enameloid is of mixed epithelial-mesenchymal origin: the collagenous matrix is mostly deposited by odontoblasts, but receives a matrix contribution (enamel matrix proteins such as amelogenin and/or collagen) from the fully differentiated adjacent ameloblasts. Ameloblasts are also certainly involved in the degradation of the enameloid matrix by means of the synthesis of MMP20 (a collagenase). Until recently, the ameloblast contribution to enameloid formation and maturation was not demonstrated, and these conclusions were supported by various ultrastructural and immunohistochemical studies performed in chondrichthyans, teleosts and caudate amphibians (Goto, 1978; Prostak & Skobe, 1986, 1988; Herold et al. 1989; Sasagawa, 1989, 2002; Davit-Béal et al. 2007). However, using in situ hybridization the expression of type 1 collagen by the ameloblasts during enameloid formation has been demonstrated unequivocally in a teleost (Huysseune et al. 2008) and in a caudate larva (Sire et al. unpubl. data).
Both enameloid and enamel (see below) have been identified in the integumentary skeleton of the earliest vertebrates, and much has been written on the origin of these two tissues. In living vertebrates, enameloid is present in chondrichthyans, actinopterygians (ray-finned fish) and larval stages of caudate amphibians. Enameloid in these lineages is most likely homologous (Gillis & Donoghue, 2007) and not the product of convergent evolution (Sasagawa, 2002). Of particular importance to understanding the transition between these two hard tissues is the study of enameloid formation in the tetrapod lineage Caudata (= Urodela). A recent structural study of enameloid formation in the larval stages of the salamander Pleurodeles waltl supports the hypothesis that enameloid matrix is synthesized by the odontoblasts before dentine matrix is deposited (with ameloblast participation at least in collagen formation) (Davit-Béal et al. 2007; Sire et al. unpubl. data). In subsequent tooth generations, the amount of enameloid deposited is reduced progressively as odontoblasts reduce their activity. Ultimately, enamel is deposited (by ameloblasts) at the enameloid surface. This finding strongly supports an enameloid to dentine transition instead of the previously proposed enameloid to enamel continuum (Davit-Béal et al. 2007).
Ganoine
Ganoine is a shiny, acellular, non-collagenous, hypermineralized tissue of epidermal origin that covers the ganoid scales in polypterids (bichirs), lepisosteids (gars), and a variety of other osteichthyans (Richter & Smith, 1995). The organic matrix of ganoine is similarly deposited and structurally comparable to enamel, including the incorporation of amelogenin (Zylberberg et al. 1997), and hence the two tissues are considered to be homologous (Sire et al. 1987; Sire, 1995). Uniquely, mature ganoine is arranged in multiple layers (stratified; except for some basal forms – see below) and is always covered by epidermis (i.e. it is never exposed to the external environment, unlike tooth enamel; Goodrich, 1907; Sire, 1990). Ganoid scale formation differs between polypterids and lepisosteids. In polypterids, the scales (polypteroid type) initiate via the odontogenic pathway, beginning with a series of epidermal-dermal interactions leading to the formation of a dental papilla adjacent to the basal epidermal layer cells (Sire, 1989; Sire & Huysseune, 2003). The first tissue to form is dentine, followed by the superficial deposition of ganoine by the basal layer cells of the epidermis, differentiated into ameloblasts. In lepisosteids, the scales (lepisosteoid type) initiate via an osteogenic pathway. A series of epidermal-dermal interactions lead to the formation of an osteogenic primordium deep within the dermis (some distance from the epidermis; Sire, 1995). In lepisosteids ganoine is deposited relatively late during scale development, once its bony surface is close to the basal layer of epidermal cells (differentiated into ameloblasts). Although the initial timing of ganoine deposition differs between the two lineages, the structure of the resulting tissues is virtually identical and both are well-stratified.
Hyaloine
Hyaloine is a poorly understood, non-collagenous, hypermineralized tissue that covers the surface of the post-cranial scutes in armored catfish (Teleostei: Siluriformes) (Sire, 1993). Each scute initiates skeletogenesis via the osteogenic pathway (Sire & Huysseune, 2003): a bone primordium forms deep within the dermis and the presumptive scute grows by centrifugal ossification. Hyaloine matrix is deposited relatively late during development, once the bony scute surface has come into close proximity with the basal surface of the epidermis. Although outwardly similar to ganoine, hyaloine differs in that the superficial-most boundary of this tissue is always separated from the basalmost epidermal layer cells by a narrow (several microns) mesenchyme-filled space. Demineralized hyaloine has the appearance of a thin, stratified fibrillar material, suggestive of periodic deposition. To date the role of the epidermal cells in the formation of hyaloine remains equivocal. However, hyaloine is structurally and spatially comparable with ganoine, and is similarly deposited in close proximity with the well-differentiated basalmost epidermal cells (Sire, 1993; Sire et al. 2002; Sire & Huysseune, 2003).
Limiting layer
The limiting layer is a well-mineralized tissue nearly devoid of collagen (exclusive of Sharpey's fibers), localized superficially on the posterior field of teleost elasmoid scales. Similar to ganoine and hyaloine it develops in close proximity to the basalmost epidermal cells. Elasmoid scales initiate skeletogenesis via the odontogenic pathway (Sire & Huysseune, 2003; Sire & Akimenko, 2004): a scale papilla is formed in the dermis, immediately adjacent to the basal layer cells of the epidermis. Each presumptive elasmoid scale begins as a discrete accumulation of woven-fibered matrix, then is underpinned by multiple lamellae of collagen fibrils organized into a plywood-like arrangement (see Plywood-like tissues below). Elasmoid scales develop obliquely in the dermis. As described above for hyaloine, the limiting layer is superficially separated from basalmost epidermal cells by a narrow mesenchymal space, and deposition is periodic. Although it has been demonstrated that epidermal cells play a role in the formation of the limiting layer (Sire, 1988), as yet there is no clear demonstration of the presence of epidermal products.
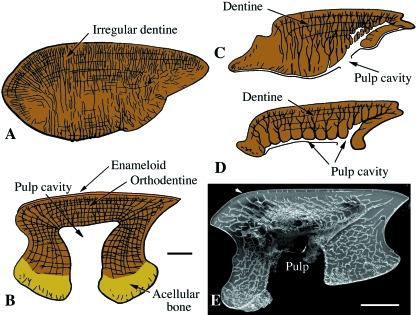
Structural diversity of dentines
Dentine is a collagen-rich, well-mineralized tissue produced by odontoblasts, and characteristic of teeth and tooth-related organs. Dentine is often associated with the hypermineralized tissues enamel or enameloid. Dentine formation consists of two phases, beginning with the synthesis and deposition of an organic, unmineralized matrix – predentine (principally type I collagen and various glycosaminoglycans) – followed by mineralization. Dentine mineralization is mediated by various non-collagenous proteins, including dentine sialophosphoprotein (DSPP) and dentine matrix protein 1 (DMP1) (Hall & Witten, 2007). Mature dentine is 75% inorganic, 20% organic, and 5% liquid (Francillon-Vieillot et al. 1990; Huysseune, 2000; Castanet et al. 2003; Hall & Witten, 2007). In living and extinct vertebrates dentine has a number of structural forms typically characterized by the arrangement of tubules and canaliculi containing cell processes, the presence of embedded cells, and the lamellar organization of the matrix (Ørvig, 1967; Smith & Sansom, 2000).
Orthodentine
Orthodentine is the most common structural form of dentine and is typical of vertebrate teeth (modern and fossil). Orthodentine is acellular (cell bodies of the odontoblasts are localized outside of the tissue matrix) and generally tubular. Each odontoblast has an elongate cell process that extends into the dentine, surrounded by concentric lamellae of matrix forming a series of parallel tubules. Atubular orthodentine was described in first-generation teeth of non-tetrapods, a feature that has been related to the small size of these teeth (Sire et al. 2002).
Mesodentine
Mesodentine (common in fossil Osteostraci) is characterized by odontoblasts (odontocytes) trapped within the matrix, comparable with cellular bone. Also similar to osteocytes these odontocytes demonstrate a reticulate (i.e. non-polarized) branching pattern of cell processes. Like orthodentine, mesodentine is tubular.
Semidentine
Semidentine is unique to the extinct group Placodermi. Similar to mesodentine, semidentine possesses embedded odontocytes within the matrix. However, the odontocyte cell processes are strongly polarized, thus resembling a structural intermediate between the tubular appearance of orthodentine and mesodentine.
In addition to the three main forms of dentine there are a variety of other, less common types including: lamellin, an atubular and acellular dentine (= lamellar-calcospheritic) found in the placoid scales (= odontodes) of early chondrichthyans (Sansom et al. 2000) and scales of heterostracans (Donoghue & Sansom, 2002; Donoghue et al. 2006), sometimes in association with tubular dentines; vasodentine (= vascular dentine), which lacks dentine tubules but is penetrated by blood capillaries, as observed in some teleosts (e.g. Merluccius merluccius), and transiently found in the continuously growing teeth of some rodents (Moss-Salentijn & Moss, 1975); osteodentine, a cellular dentine resembling spongy bone, found in some actinopterygians (e.g. the bowfin Amia calva, some teleosts; see Herold, 1971); trabecular dentine is found in fossil chondrichthyans and fossil dipnoans, and is considered primitive compared to later specialized dentines (Denison, 1974); and plicidentine, histologically similar to orthodentine but with the lamellae organized into elaborate, longitudinally oriented folds, that appear as sinuous lines in transverse section. Plicidentine is found in the labyrinthine teeth of early crossopterygians and stegocephalians (Schultze, 1968), and is also described in the teeth of varanoid squamates (Kearney & Rieppel, 2006).
Ørvig (1967) argued that the three principal structural grades constitute an evolutionary transformation series from mesodentine, in which the cells are entrained in the mineral matrix and the cell processes unpolarized, through semidentine, in which the cell processes are polarized, to orthodentine, when the cells are no longer incorporated in the mineral matrix. However, there is neither stratigraphic nor phylogenetic support for this thesis, although there is a general empirical trend from unordered (spheritic) to increasingly ordered tissue fabrics (Donoghue et al. 2006).
Ganoid scales of polypterids, hereafter polypteroid scales (see section Ganoid scales of basal actinopterygians below) demonstrate a unique form of dentine organization and development that is difficult to categorize. Unlike most dentine-bearing elements that are isolated, simple units (e.g. odontodes and teeth), the superficial-most region of polypteroid scales appears to be derived from the fusion of multiple adjacent odontodes, creating what has been termed an odontocomplex (Ørvig, 1977; Reif, 1982a; Smith & Hall, 1990; Huysseune & Sire, 1998). As a result, morphogenesis and differentiation of the dentine in polypteroid scales differ from that of all other dentine forms. Each polypteroid scale begins within the upper region of the dermis as an accumulation of collagen matrix centrifugally deposited around several regularly spaced blood capillaries. This combination of collagen matrix surrounding vascular canals gives rise to an osteon-like morphology known as a denteon. The cavity of these denteons can be considered homologous with the pulp cavities of odontodes and teeth. Adjacent clusters of dentine fuse, forming a continuous layer of well-mineralized, woven- and parallel-fibered dentine matrix. This vascularized dentine matrix contains numerous polarized cell processes and, in some cases, entrapped odontocytes (Sire et al. 1987; Sire, 1989). Hence, in polypteroid scales the upper dentine region is a tubular, cellular dentine that shares features in common with vasodentine and osteodentine.
Gradually, the dentine matrix thickens on both its upper surface and lateral margins by means of centrifugal deposition. At its deep surface, the dentine region thickens with the deposition of multiple lamellae of collagen fibrils, organized into a plywood-like tissue. This tissue is identifiable as elasmodine, and probably homologous to lamellar dentine, although devoid of cell processes and mineralizing slowly. This skeletally immature polypteroid scale is morphologically recognized as an elasmoid scale (Sire, 1989). Related to this, it seems likely that both the external layer and the basal plate (elasmodine) of the elasmoid scales are derivatives of dentine from an ancestral ganoid (polypteroid-type) scale (see Plywood-like tissues and 1–5-1).
Plywood-like tissues
Several collagen-rich tissues of the integument (dermis, lamellar bone, and the elasmodine of the basal plate of elasmoid scales) exhibit a plywood-like organization composed of multiple collagen lamellae. Within each layer the collagen fibrils are mutually parallel, but between successive layers the fibrillar orientation changes, giving rise to the stacked succession of layers characteristic of plywood. Two main types of plywood orientation are known (Giraud-Guille, 1988): orthogonal (angles of orientation differ by 90° from one lamella to the next), and twisted (various angles of orientation). Orthogonal plywood-like tissues are typical of the stratum compactum of the dermis, whereas twisted plywood is found in bone and fish scales. Significantly, however, plywood-like collagen-rich tissues are relatively common and are not necessarily indicative of a shared origin, even within the integumentary skeleton. For example, when exposed to particular chemical and physical conditions, in vitro acellular type I collagen gels can organize spontaneously into a twisted plywood-like tissue similar to cholesteric liquid crystals (Belamie et al. 2006). When forming in vivo, most plywood-like tissues are acellular and avascular, and may demonstrate varying degrees of mineralization. Whereas lamellar bone is well mineralized, the stratum compactum is not. Elasmodine mineralization is also highly variable, ranging from well-mineralized (polypteroid scales), to poorly mineralized (elasmoid scales of teleosts) and unmineralized (elasmoid scales of coelacanth). As understood at present, these three integumental tissues – stratum compactum, lamellar bone and elasmodine – have different evolutionary origins.
Stratum compactum of the dermis
The formation of the collagenous plywood of the dermis has been described in the teleost Danio rerio by Leguellec et al. (2004). Collagen fibrils (type I collagen) are deposited first by the basal layer cells of the epidermis and accumulate in the space between the epidermis and the differentiating muscle cells. Subsequently, collagen is produced by both epidermal cells and various fibroblast-like cells located at the surface of the developing muscle cells. As the dermis thickens the acellular collagenous matrix begins to organize spontaneously into a plywood-like arrangement. Although it remains to be determined, fibroblast-like cells bordering the deep surface of the dermis may play a role in creating the conditions favouring this arrangement. Gradually, scleroblasts and then pigment cells migrate into the collagen-rich matrix of the dermis. The stratum compactum continues to thicken at its deep surface with the addition of collagen lamellae. At this time, integumentary elements start to form and the superficial-most region of the dermis becomes reorganized into the stratum superficiale.
In some cases, collagen bundles of the dermis are directly incorporated into osteogenic elements of the integumentary skeleton. These collagen bundles – or Sharpey's fibers – may interconnect adjacent integumentary elements and/or anchor each element within the surrounding dermis. Sharpey's fibers are commonly associated with the basal plates of ganoid scales (both polypteroid and lepisosteoid types), the scutes of armored catfish and the dermal plates of some teleosts. In some tetrapods, the elements of the integumentary skeleton (osteoderms) develop via the direct transformation of the pre-existing dermis into bone (metaplastic ossification; see Vickaryous & Sire, this Issue).
Lamellar bone
Lamellar bone is a highly organized ossified tissue characterized by many thin, closely packed collagen fibrils arranged into alternating lamellae that form a plywood-like structure. Sharpey's fibers are often incorporated into the matrix. Compared with other forms of bone (see Bony tissues below) osteogenesis of lamellar bone is relatively slow (Ricqlès et al. 1991). Among non-tetrapods, lamellar bone is typically located along the deepest margins of basal plates of integumentary elements such as ganoid scales, scutes of armoured catfish and dermal plates in some teleosts (e.g. Syngnathiformes)
Isopedine, as defined first by Pander (1856) in the deep region of the basal plates of early vertebrates (jawless heterostracan pteraspidomorphs), corresponds to the description of lamellar bone. Unfortunately, this term was misinterpreted by Goodrich (1907) and some subsequent authors, who employed isopedine to describe any bone-like (including aspidin, one of the oldest vertebrate hard tissues; Donoghue et al. 2006; see Bony tissues below) tissues found within the basal plates of fossilized integumentary elements. Although it remains in current use, in recent years the definition of isopedine has been restricted to a plywood-like arranged lamellar bone. As many tissues previously recognized as isopedine have since been recategorized as either lamellar bone or elasmodine (see below), the future of the term remains in doubt.
Elasmodine
Similar to lamellar bone, elasmodine is a plywood-like tissue characterizing elasmoid scales and composed of multiple layers of collagen fibrils (Bertin, 1944) that may be organized into either an orthogonal or twisted arrangement depending on the species (Meunier & Castanet, 1982). Consequently, elasmodine was long considered homologous to the lamellar bony region (= isopedine) found in the rhombic scales of extinct actinopterygians (Meunier, 1987). By extension, elasmoid scales were initially considered to be derived from the bony regions of rhombic scales (Goodrich, 1907). More recent work based on the study of cell condensations has since demonstrated this interpretation to be incorrect, and shown the observed similarities between elasmodine and isopedine are an example of convergence (Sire & Huysseune, 2003). In addition, unlike lamellar bone, both the collagen fibrils and lamellae of elasmodine are relatively thick, and Sharpey's fibers are rarely incorporated. Furthermore, elasmodine is often deposited during periods of rapid growth.
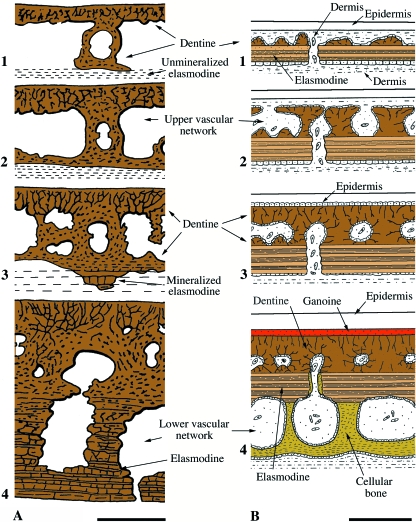
Of particular importance is the observation that elasmoid scale development begins with the formation of a well-defined dermal papilla comparable with that of odontodes and teeth (reviewed in Sire & Huysseune, 2003; Sire & Akimenko, 2004). Based on these data, it is hypothesized that elasmoid scales are composed of odontogenically derived tissues different from the morphologically comparable (but non homologous) osteogenic derivative isopedine (= lamellar bone). Accordingly, Schultze (1996) proposed the term elasmodine (herein considered a form of lamellar dentine) to distinguish it from isopedine. As skeletally mature elements, the homology between elasmodine organization in elasmoid scales and dentine in odontodes/teeth is not readily apparent. The key to resolving this relationship is found in the study of polypteroid scale development. As previously noted, the upper region of the polypteroid scale is odontogenic in origin, whereas its deep basal plate is osteogenic (see section Ganoid scales of basal actinopterygians below). The upper region is the first to form, beginning as a well-defined dermal papilla positioned adjacent to the epidermis (Sire, unpubl. data) that gives rise to an irregular layer composed of patches of woven-fibered dentine in association with nearby capillary blood vessels. Then, thick lamellae of collagen fibrils are deposited at the deepest margin of this first layer, and organized into an orthogonal plywood-like tissue, elasmodine. At this early stage of skeletogenesis the young polypteroid scale is comparable with an elasmoid scale (Sire, 1989, 1990; Sire & Akimenko, 2004). The cells (= odontoblast-like cells) involved in the formation of these tissues have differentiated from the initial dermal papilla cell population. Given the similarities in structure and early development of polypteroid and elasmoid scales, the tissues that compose each are considered to be homologous: the upper layer of polypteroid scale (woven-fibered dentine) is homologous to the woven-fibered external layer of elasmoid scale, and the subjacent plywood-like arrangements, elasmodine, are homologous, and probably dentine-derived, tissues (Sire, 1989).
Bony tissues
Bone
Bony tissues of the integumentary skeleton are highly diverse in structure (e.g. they can be woven-fibered, parallel-fibered or lamellar), morphology, and development, and have differing mechanical properties. Among elements of the integumentary skeleton, bone tissue forms within undifferentiated mesenchyme or the dermis via direct ossification, without the differentiation of a cartilaginous precursor. Most (if not all) bony elements incorporate collagen fibers from the surrounding, pre-existing matrix of the dermis, giving rise to Sharpey's fibers. The most common mode of direct ossification is intramembranous ossification, wherein a condensation of scleroblasts differentiates into osteoblasts and begins to synthesize and deposit osteoid, the unmineralized bone matrix. Similar to dentine, bony tissues are composite tissues with an organic framework of polymerized collagen [type I (primarily) and type V] and various glycosaminoglycans, and mineralized with hydroxyapatite. Bone is also composed of various non-collagenous proteins such as osteocalcin, osteopontin, osteonectin, and bone sialoprotein. Collagens provide tensile strength and mineralization provides hardness. In general, mineralization occurs rapidly after osteoid matrix is deposited. Although bone is most commonly produced by osteoblasts, in certain situations it may also be synthesized by cells that phenotypically resemble fibrobasts (see below). Dermal mineralization has the particularity of being easily inducible under pathological or experimental (localized injections or biomechanical constraints) conditions. With the exception of the bony plate of polypteroid scales that develops secondarily, when the odontogenic (dentine) part is already well developed, all the other bony elements of the integumentary skeleton initiate either from a loosely defined osteogenic primordium, in which differentiated osteoblasts are identified (most of the non-tetrapods dermal bones), or (less common) from metaplasia (= metaplastic ossification, Francillon-Vieillot et al. 1990), wherein the pre-existing collagen matrix of the dermis becomes invested with hydroxyapatite to form bone. Unlike in intramembranous ossification, cells resembling osteoblasts are generally absent during metaplastic ossification.
Most bony tissues of the integumentary skeleton are vascular and cellular (osteocytic), with individual osteoblasts becoming surrounded by, and then entrapped within, the newly synthesized osteoid matrix. These embedded osteoblasts are referred to as osteocytes. In numerous teleosts, the bony tissues, including those of the integumentary skeleton, are acellular (e.g. dermal plates of gasterosteiforms and syngnathiforms). They are devoid of osteocytes (anosteocytic) but may include canaliculi for cell processes of osteoblasts located on the bone surface as, for example, in the dentary bone and spiny rays of the fins in sea bream and bass (Meunier & Sire, unpublished data). This results in a bone tissue that resembles acellular orthodentine. Unlike acellular dentine and cementum (see below), the development of acellular bone is not dependent on the presence of teeth or odontodes. Taken out of the context, the similarities in matrix composition often render the distinction between acellular bone and other acellular tissues equivocal.
Aspidin
Aspidin has been the subject of much debate, much of it focused on whether it represents a form of cellular or acellular bone or, indeed, whether it is in fact a form of dentine (Rohon, 1893, 1901; Gross, 1935; Obruchev, 1941; Bystrow, 1955; Ørvig, 1958, 1965, 1967, 1968; Currey, 1961; Halstead Tarlo, 1963, 1964, 1965; Denison, 1967; Moss, 1968; Halstead, 1969, 1973, 1974, 1987; Smith & Hall, 1990; Smith et al. 1995). The debate is confounded by the almost random designation of aspidin for any number of clearly distinct tissues in very different organisms. Although it is now widely appreciated to be a form of acellular bone, the reasoning underpinning this consensus is not clear. A comprehensive survey of the tissues referred to as aspidin is urgently required but, for the moment, we reserve the concept of aspidin to heterostracans, the group in which it was first codified (Gross, 1930). More specifically, we consider aspidin to be the tissue constituting the so-called ‘spongy’ middle-layer of the heterostracans.
Aspidin is composed of a dense acellular organic matrix, presumably rich in collagen, organized radially within the incremental layers of the osteons that constitute much of the tissue, as well as a woven fabric at the borders between adjacent osteons. The tissues referred to aspidin that make up the bony integumentary skeleton of other ostracoderms such as the arandaspids, astraspids, anaspids and galeaspids, are acellular nevertheless. Thus, it has been argued that acellularity is the primitive condition for bone (Ørvig, 1965). The presence of acellular bone in derived teleosts (as well as in various other vertebrate taxa) appears to have been independently derived on multiple occasions (Moss, 1961; Meunier, 1987; Hall & Witten, 2007).
Galeaspidin is a tissue unique to the extinct jawless galeaspids and it is considered a form of aspidin (Wang et al. 2005). Galeaspidin is structurally similar to lamellar bone [i.e. an acellular, lamellar tissue with orthogonally arranged bundles (= plywood-like) of large, calcified collagen fibrils] except that its ply are composed of coarse (c. 20 µm) fiber-bundles, and these are permeated by a perpendicularly aligned coarse fabric of Sharpey's fibers (Wang et al. 2005). Though the alternating ply fiber-bundles prompt comparison to elasmodine, the structure of galeaspidin is most comparable to stratum compactum and therefore it is likely that the galeaspid integumentary skeleton develops via metaplastic ossification.
Cementum and bone of attachment
The cementum is a thin bone-like supportive tissue that anchors the roots of thecodont teeth (e.g. in mammals and archosaurs) to the alveolar bone of the jaw (Osborn, 1981; Diekwisch, 2001). Although cementum has a similar organic matrix to both bone and dentine (type I collagen, osteopontin, osteocalcin; Hall & Witten, 2007), it differs in that it lacks vascularization and innervation (Osborn, 1981). Cementum may be acellular or cellular. Among non-tetrapod osteichthyans, a cementum-like tissue is sometimes deposited on the surface of bone of attachment. Contrary to its name, bone of attachment as described in a number of teleosts is not composed of true bone. Various developmental studies have demonstrated that the matrix of the attachment bone is deposited by osteoblast-like cells derived from the dental papilla cell (odontogenic) population. Accordingly, these cells are a specialized form of odontoblasts, and bone of attachment is a type of dentine (Sire & Huysseune, 2003).
Origin and diversification of the integumentary skeleton
In this chapter we summarize the current knowledge of the integumentary skeleton diversity in extinct jawless and jawed vertebrates, with our attention focused on tissues that could be identified as deriving from odontogenic or osteogenic cell condensations. For additional details on these early taxa the reader is invited to consult Janvier (1996), Donoghue & Sansom (2002), and Donoghue et al. (2006).
Pteraspidomorphi
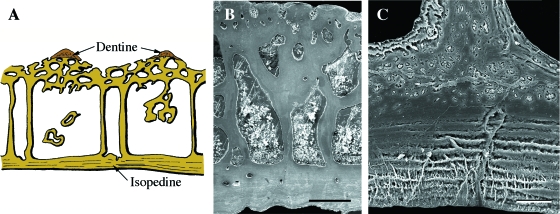
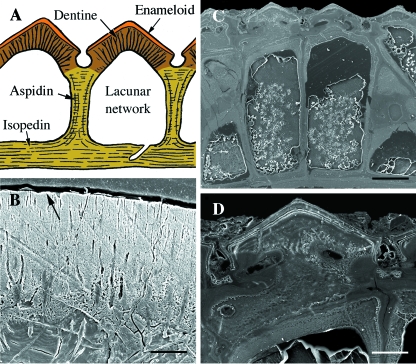
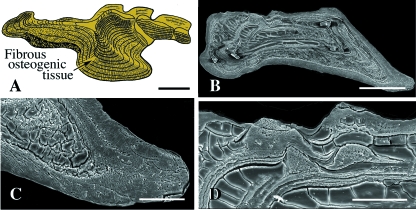
The oldest fossil remains of vertebrates are known from the early Cambrian (c. 520 Ma) (Shu et al. 1999, 2003), with the earliest vertebrates with a mineralized skeleton from the late Cambrian (Donoghue et al. 2000; Sweet & Donoghue, 2001). However, the earliest evidence of vertebrates with a mineralized integumentary skeleton, the pteraspidomorphs, do not appear until the early Ordovician to the late Devonian (c. 460–375 Ma). All pteraspidomorphs, including arandaspids, astraspids, and heterostracans, are characterized by a cephalothoracic capsule composed of two or more large skeletal plates (shields), and a postcranium covered by a large number of smaller overlapping or juxtaposed scales. Shields and scales consist of thick plates of acellular, collagen-rich tissue that are ornamented with tubercles (Donoghue & Sansom, 2002; Sansom et al. 2005; Donoghue et al. 2006) (Figs 2–4). The structure of each tubercle includes a superficial layer of enameloid (monocrystalline in astraspids; fibrous in arandaspids and heterostracans), penetrated by vertical tubules emanating from the subadjacent acellular (= orthodentine-grade) dentine (Figs 3 and 4). The fibrous enameloid suggests that the mineralization was co-ordinated by collagen fibers (Donoghue et al. 2006). The underlying acellular plates have three, structurally distinct regions (Figs 2 and 5): (1) a superficial region underpinning each tubercle; (2) a middle cancellous region with numerous osteonal vascular canals; and (3) a basal region of orthogonally arranged stacked lamellae, similar to the plywood-like organization of lamellar bone (Donoghue & Sansom, 2002; Sansom et al. 2005; Donoghue et al. 2006). The middle layer is known as aspidin (acellular bone) and the basal lamellar layers are interpreted as isopedine (see Plywood-like tissuesabove).
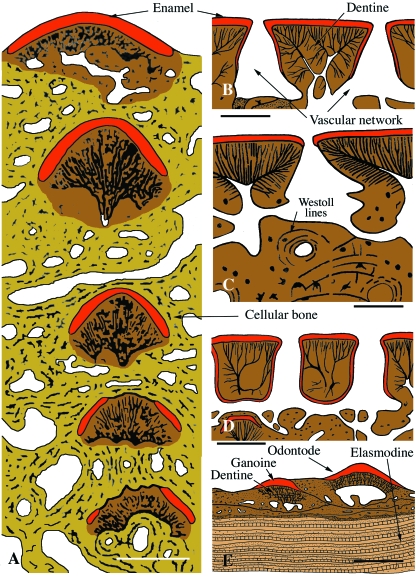
Figs 2–16.
The structure of various integumentary skeletal elements in extinct and extant non-tetrapod vertebrates illustrated using pictures and interpretative drawings. Vertical sections. The various tissues are identified using colours: yellow = bone (various types) and aspidin; brown = dentine (various types); beige = elasmodine; orange = enameloid; red = enamel and ganoine. Most drawings are from Janvier (1996) (with the author's permission), except Figs 9D, 12B, 12C, 13, 14A, 14E and 15B. Scanning electron micrographs (SEM) of etched sections from Donoghue & Sansom (2002) (Figs 4C, 4D, 5B, 5D, 8D), Wang et al. (2005) (Fig. 7B) and Donoghue et al. (2006) (Figs 2B, 2C, 4B, 5C, 6E), and Fig. 8G is new.
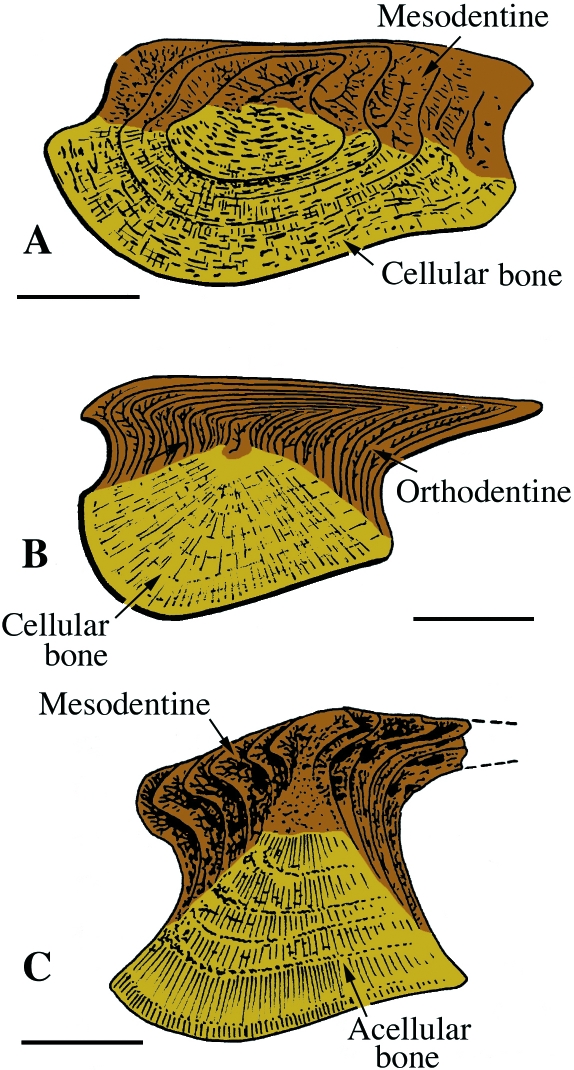
Fig. 2 Pteraspidomorphi. (A) Section through the dorsal shield of Sacabambaspis (Arandaspida from the Ordovician). The main tissue identified is acellular bone, distributed into three layers. The superficial tubercles are odontode-like structures composed of dentine. (B) SEM of a section through the dorsal shield of Corvaspis (Heterostraci, Silurian). (C) SEM of a section of the integumentary skeleton of Loricopteraspis (Arandaspida, Silurian) showing the basal lamellar bone. Scale bars: B = 500 µm; C = 50 µm.
Heterostraci. (A) Schematic illustration of the integumentary skeleton of a generalized early Devonian heterostracan (e.g. Poraspis or Pteraspis). The lamellar, acellular bony plate (isopedin) is surmounted by a layer of cancellous bone and ornamented by odontode-like tubercles composed of dentine capped with enameloid. (B) SEM section of the superficial region of a portion of the integumentary skeleton of Tesseraspis demonstrating the enameloid-dentine junction (arrow). (C,D) SEM sections of the integumentary skeleton of Anglaspis. Scale bars: B = 40 µm; C = 210 µm; D = 80 µm.
Fig. 3.
Pteraspidomorphi (Ordovician). Odontode-like tubercles of various pteraspidomorphs presented as schematics (A–C) and original (D–F) sections. (A,B,D) Astraspis, (C,E,F) Eriptychius, demonstrating the various combinations of enameloid, orthodentine and acellular bone. Scale bars: D = 100 µm; E = 150 µm; F = 50 µm.
Fig. 5.
Anaspida. (A) Schematic illustration of a generalized early Silurian anapsid scale demonstrating the lamellar organization of the acellular (fibrous osteogenic tissue) basal plate. There is no evidence of either dentine or enameloid. (B,C,D) SEM sections of scale from Birkenia (early Silurian) demonstrating the structural organization of the bony tissue. Scale bars: A = 150 µm; B = 200 µm; C = 40 µm; D = 50 µm.
Based on structural similarities with modern tissues, the superficial odontodes are clearly identifiable as odontogenic derivatives. Fossil evidence indicates that the acellular plates grow and pattern independently of the superficial odontodes (Denison, 1964, 1973; Westoll, 1967; White, 1973). Combined with the presence of acellular bone (aspidin), it seems likely that acellular plates are osteogenically derived.
Anaspida
The integumentary skeleton of anaspids (Silurian and Devonian, 443–374 Ma), was either unmineralized or else entirely composed of small scales (Kiaer, 1924; Gross, 1938, 1958; Ritchie, 1980; Arsenault & Janvier, 1991; Blom et al. 2002; Janvier & Arsenault, 2007). Individual scales are elongate, obliquely oriented, and overlap one another to create an imbricated chevron-like pattern. Histologically, anaspid scales are composed of stacked lamellae of acellular collagenous tissue (Fig. 5). This tissue has been identified as acellular bone (aspidin) (Gross, 1938, 1958), although its histological structure is inconsistent with heterostracan-grade aspidin (see Plywood-like tissues above) (Donoghue et al. 2006). These scales demonstrate variable degrees of vascularization, ranging from absent to well-developed. Due to their small size, the histology of anaspid scales is difficult to evaluate and thus it remains unclear whether they are composed exclusively of aspidin (and therefore are derived exclusively from osteogenic cells), or also include odontogenic tissues, as appears to be the case in some taxa (Donoghue & Sansom, 2002; Donoghue et al. 2006) (Fig. 5).
Thelodonti
The integumentary skeleton of the thelodonts (late Ordovician to late Devonian; c. 450–375 Ma) is characterized by numerous minute scales, superficially comparable with chondrichthyan odontodes (Fig. 6, see also Fig. 10). This scale covering begins abruptly around the margin of the oral cavity and continues externally across the body (Märss et al. 2007). Structurally, at least five different thelodont scale-types are known (achanolepid, apalolepid, katoporid, loganiid, and thelodontid; Märss et al. 2007). In general, each thelodont scale is composed of a thick layer of orthodentine (although in loganiid- and katoporid-type scales the branching pattern more closely resembles mesodentine), supported by a growing acellular bony plate that has been interpreted as aspidin (Turner, 1991; Turner & Van der Brugghen, 1993). Superficially, each scale is capped by a thin layer of monocrystalline enameloid (Fig. 6B).
Fig. 6.
Thelodonti. (A–D) Schematic illustrations of four of the five recognized structural forms of thelodont odontode-like scales (Janvier, 1996). (A) Kawalepis (achanolepid type, early Silurian). (B) Thelodus (thelodontid type, Silurian) (note that this form of thelodont scale is very similar to the odontodes of modern chondrichthyans). (C) Loganellia (loganiid type, late Silurian). (D) Phlebolepis (katoporid type, late Silurian). All thelodont scales are composed of a non-growing dentine-rich crown with a variably defined pulp cavity, and an attachment process of acellular bone which may be the only vestige of an osteogenic skeletal derivative. (E) SEM section of a Thelodus scale demonstrating the presence of a thin superficial layer of enameloid (arrowhead) covering the dentine. Scale bars: B = 100 µm; E = 250 µm.
Fig. 10.
Chondrichthyes. Schematic illustrations (A–B,D) and section (C) of chondrichthyan integumentary elements. (A) Integumentary skeleton from the earliest known chondrichthyan, Mongolepis (early Silurian), composed of multiple superimposed layers of odontodes. (B) Elegestolepis (early Silurian) odontode comparable to those of living chondrichthyans. (C) Horizontal section of an integumentary element from an unnamed possible stem-chondrichthyan from the Late Ordovician Harding Sandstone (Sansom et al. 1996). (D) Generalized extant chondrichthyan odontode demonstrating the relationships with the underlying soft tissues of the integument. Scale bars: A = 250 µm; B = 400 µm; C = 100 µm; D = 500 µm.
The presence of dentine, enameloid and bony tissues is consistent with a mode of development involving both odontogenic and osteogenic cell condensations. This conclusion contrasts with the traditional view in which thelodont scales are considered solely odontogenic derivatives (Reif, 1982a; Smith & Hall, 1990; Janvier, 1996; Donoghue & Sansom, 2002; Donoghue et al. 2006).
Galeaspida
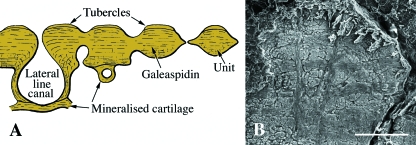
Galeaspids (early Silurian to late Devonian, c. 436–374 Ma) are an extinct lineage characterized by a distinct cephalothoracic integumentary skeleton that is either continuous or composed of a series of interlocking but discrete polygonal-shaped elements known as tesserae. The postcranial skeleton, where it is known, is composed of isolated scale-like elements. The principal tissue of the cephalothorax is a planar plywood-like arrangement of fiber-bundles, aligned orthogonally (Fig. 7). This fabric is penetrated by perpendicularly aligned fiber-bundles or Sharpey's fibers. Uniquely, each of these three axes is orthogonal (Wang et al. 2005), and consequently this arrangement is quite distinct from aspidin (the traditional interpretation of this tissue: Janvier, 1990; Janvier et al. 1993; Zhu & Janvier, 1998). Wang et al. (2005) identified this tissue as a variant of acellular bone they termed galeaspidin. However, the structure of the mineralized integument is strongly similar to stratum compactum and it seems likely that galeaspidin is more plausibly interpreted as metaplastically ossified dermis (see Plywood-like tissues above). Superficially, the shields and scales are ornamented with small tubercles that are composed of a spheritically mineralized tissue of unclear homology, but dentine and enameloid are absent (Wang et al. 2005).
Fig. 7.
Galeaspida (early Devonian). (A) Schematic illustration of the posterior part of the head-shield of Bannhuanaspis. The shield is thought to have formed by the fusion of multiple mineralized units. Each unit is composed of lamellar, acellular bone with many large, perpendicular fiber-like bundles (Sharpey's fibers). This lamellar bone is sometimes ornamented with small tubercles. The dermoskeleton is underlain by mineralized cartilage associated with the neurocranium; there is no evidence of a perichondrium. (B) SEM section of a polybranchiaspid scale-like element demonstrating the unique organization of the matrix. Note the perpendicular Sharpey's fibers. Scale bar: B = 20 µm.
The presence of only bony tissue suggests that the integumentary elements of galeaspids are exclusively derivatives of osteogenic promordia. Interestingly, the superficial, non-collagenous tissue with spheritic mineralization is comparable with the limiting layer of elasmoid scales and the unidentified tissue capping some squamate osteoderms, and suggests some interaction with the overlying epidermis (see Hypermineralized (capping) tissues above).
Osteostraci
The integumentary skeleton of osteostracans (early Silurian to late Devonian, 430–370 Ma) is characterized by a cephalic shield and a postcranium jacketed by scales. The structure of the cephalic shield varies. In the majority of groups it is composed of polygonal tesserae that exhibit marginal accretion. In contrast, the integumentary skeleton of tremataspids is composed of a single continuous layer that shows no evidence of marginal growth. Regardless of morphology, the cephalic shield and postcranial scales are all composed of a comparable stratified assemblage of three tissues (Fig. 8). The superficial region is composed of odontodes demonstrating evidence of either synchronous or superpositional growth. The architecture of the dentine canals varies between taxa and even within individual odontodes. For example, in Hemicyclaspis the architecture varies from orthodentine to mesodentine, from the tip of an odontode to its base. The odontode-like tubercles are restricted to the centre of tesserae and show evidence of a period of superpositional growth in concert with the tesserae margins. In Tremataspis, the superficial layer is a continuous front of mesodentine, which shows evidence only of growth in a single episode (Denison, 1947, 1951) (Fig. 8B). This is consistent with the observation that tremataspids show no size variation, leading to the conclusion that their integumentary skeleton was deposited upon the animal reaching its full size (White & Toombs, 1983). Interestingly, the majority of osteostracan odontodes do not possess a hypermineralized capping tissue layer; there may be evidence for a capping layer of enameloid in some cephalaspid osteostracans, but the skeletal histology of these specimens is too poorly preserved to be definitive (Janvier, 1996).
Fig. 8.
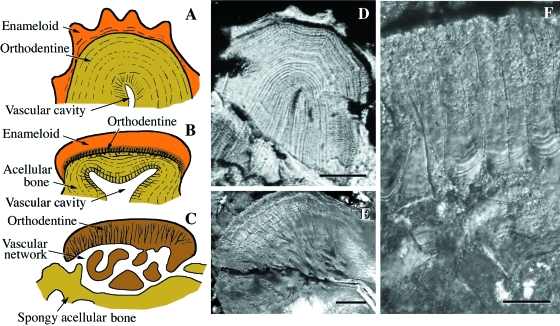
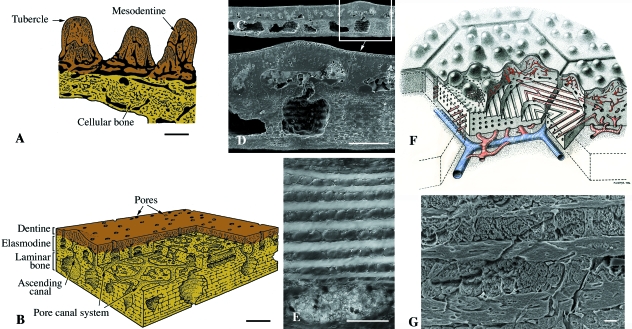
Osteostraci (Silurian). Schematic illustrations (A,B,F) and SEM sections (C–E,G) of osteostracan integumentary elements. (A) Procephalaspis. The basal plate of cellular bone is covered by a layer of tubercles composed of mesodentine, and a thin layer of enameloid. (B) Diagram illustrating the structure of cosmine-like tissue (including the pore-canal system) of the integumentary skeleton of Tremataspis. (C,D) Scale-like element from an unidentified thyestiid. (E) Detail demonstrating the plywood-like tissue (putative elasmodine) of the middle layer of a Tremataspis scale. (F) Reconstruction of the inter-relationships between the polygonal plate-like tesserae of the head-shield and the underlying, richly vascularized region from the osteostracan Alaspis rosamundae (from an unpublished manuscript by Tor Ørvig). Two series of canals are figured in blue and red. (G) Close-up of putative elasmodine in the integumentary skeleton of Hemicyclaspis. Scale bars: A–D: 150 µm; E: 60 µm; G = 10 µm.
Deep to the superficial layer is a bony plate universally recognized as being composed of cellular bone (Powrie & Lankester, 1868–70; Stensiö, 1932; Wängsjö, 1946; Denison, 1947, 1951; Ørvig, 1951, 1968; Gross, 1956, 1961; Reif, 1982a; Maisey, 1988; Janvier, 1996; Donoghue & Sansom, 2002; Donoghue et al. 2006). Significantly, this marks the first phylogenetic appearance of cellular bone in vertebrates and the first evidence of cellular bone resorption (Denison, 1952). The basal plate is divided in three regions: (1) an upper, richly vascularized region (two series of canals oriented parallel to the body wall) (Fig. 8F); (2) an intermediate region, less vascularized, composed of multiple layers of fiber-bundles, with each successive layer aligned orthogonally (Fig. 8E,G); and (3) a deep region, poorly vascularized and composed of parallel-fibered bone. As an alternative to cellular bone, it has been proposed that the putative cell lacunae represent space-like artifacts between the close-packing of the orthogonal arrangement (Wang et al. 2005), and that this tissue represents isopedine (Gross, 1968a; Wang et al. 2005; Donoghue et al. 2006). However, it should be noted that the plywood-like architecture is also characteristic of other tissues, including metaplastic ossification of the stratum compactum and elasmodine. The resemblance of the lamellar tissue layer in osteostracans and elasmodine (as seen in polypteroid scales; Sire, 1989) is striking, and extends beyond the plywood architecture to the fiber-bundle composition and gross architecture of the vascular system that permeates the tissue (Fig. 8E,G, and see also Fig. 13A,C). This comparison holds true for all osteostracans but it is particularly evident in tremataspids, where the odontodes constitute a structural complex similar to the upper region of polypteroid scales. Indeed, the evidence available on the development of the integumentary skeleton in tremataspids suggests a sequence of development comparable with polypteroid scales, wherein the odontodes develop first and the elasmodine layer develops gradually only later (Denison, 1947).
Fig. 13.
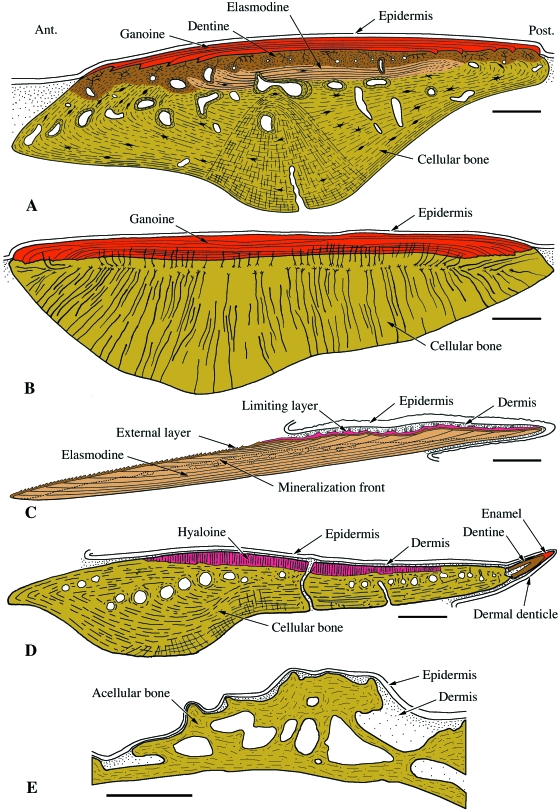
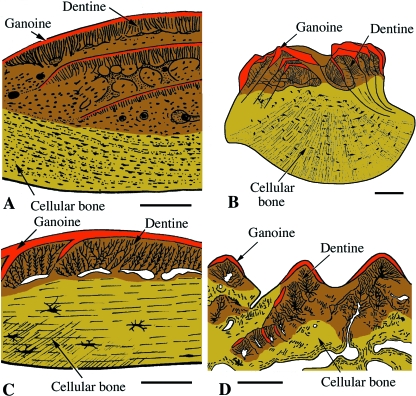
Actinopterygii. Schematic illustrations of integumentary elements from various extant actinopteryians. (A) Polypterus senegalus (polypteroid-type ganoid scale). (B) Lepisosteus ocellatus (lepisosteoid-type ganoid scale). (C) Danio rerio (elasmoid scale). (D) Corydoras aeneus (scute). (E) Gasterosteus aculeatus (dermal plate). Scale bars: A–D: 250 µm; E = 50 µm.
The tissue composition of these elements is consistent with an origin via both odontogenic and osteogenic condensations.
Placodermi
Placodermi (e.g. Arthrodira, Antiarcha) are an extinct clade (or possibly grade) of jawed vertebrates widely recognized as the sister group of crown-gnathostomes (the clade of living jawed vertebrates) (Janvier, 1996). Placoderms (middle Silurian to the late Devonian, c. 425–375 Ma) are characterized by a robust integumentary skeleton composed of large plates encasing the head and cranial portion of the trunk (including the pectoral apparatus). The remainder of the body was covered with smaller overlapping scales, or else lacked mineralized integumentary elements (Denison, 1978). In arthrodirans the integumentary skeleton is composed of a basal region of cellular bone ornamented by odontode-like tubercles made of semidentine (Fig. 9A). Astonishingly, the histology of most placoderms is poorly known, and only the antiarchs Asterolepis and Bothriolepishave been investigated at anything more than a cursory level (Goodrich, 1909; Heintz, 1929; Gross, 1931, 1935; Ørvig, 1968; Burrow, 2005; Donoghue et al. 2006). Those studies have revealed that the integumentary skeleton of antiarchs is composed of three regions. The superficial layer is composed of lamellar cellular bone. The middle layer is more complex, but is typically characterized by a very open meshwork of vascular canals. In some taxa the middle layer is stratified into a superficial compact region (often highly cellular) underpinned by a deeper, more loosely organized region. The boundary between these two middle layers is often quite sharp, except where it has been diminished by secondary osteon activity (Donoghue et al. 2006) (Fig. 9B). In Bothriolepis canadensis, the lower region is spheritically mineralized (Ørvig, 1968; Burrow, 2005; Donoghue et al. 2006). Curiously, this tissue has been identified as perichondral bone (Burrow, 2005). However this interpretation is untenable given its position within the integument, the fact that it is topologically equivalent to cellular bone in the integumentary skeleton of other placoderms, and the observation that the tissue layer is underlain by a basal layer of dermal bone, which itself overlays an unmineralized braincase (Young, 1984; Donoghue et al. 2006). More likely this tissue is a spheritically mineralized bone (Donoghue et al. 2006).
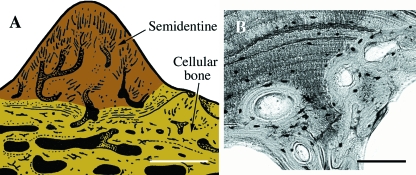
Fig. 9.
Placodermi. (A) Schematic illustration of a section of the integumentary skeleton from a generalized arthrodiran. This tissue composition includes a basal layer of cellular bone ornamented by tubercles made of semidentine (odontocytes embedded in the matrix). To date enameloid has not been reported in placoderms. (B) Bothriolepis (Late Devonian). Section demonstrating bone remodeling. Scale bars: A = 300 µm; B = 160 µm.
It remains unclear to what degree the histology of the integumentary skeleton of antiarchs is representative of placoderms more generally. However, the absence of dentine is certainly a peculiarity of antiarchs as has been documented in superficial tubercles in arthrodires (Ørvig, 1957, 1967; Young, 2003). In arthrodirans, the presence of dentine and bone tissues suggests that the integumentary skeleton of placoderms was derived from both odontogenic and osteogenic condensations.
Chondrichthyes
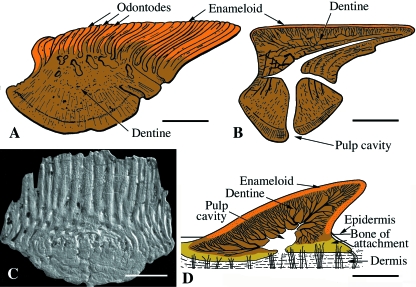
The integument of extant chondrichthyans includes a relatively thin vascularized stratum superficiale and a comparatively thicker, more fibrous stratum compactum. The stratum superficiale is heavily invested with numerous, small, non-overlapping tooth-like elements or odontodes (= placoid scales; see section on Role of papillae and primordiaabove). The basal region of these odontodes rests along the interface with the stratum compactum, whereas the upper region penetrates the epidermis. Similar to the scales of thelodonts, odontodes have a pervasive distribution across the body, and a characteristic anatomy and histology (Fig. 10D). While the squamation of living chondrichthyans is composed of isolated odontodes, many stem-chondrichthyans possessed scales composed of numerous odontodes joined together by marginal accretion, and underpinned by an extensive bony plate (Zangerl, 1968; Reif, 1978, 1982a; Karatajuté-Talimaa et al. 1990; Karatajuté-Talimaa, 1992; Karatajuté-Talimaa & Novitskaya, 1992, 1997; Sansom et al. 2000) (Fig. 10C).
Odontode structure and organization is highly conserved, even among some of the earliest members (although not in the mongolepids, which may yet prove to be stem-gnathostomes rather than stem-chondrichthyans). Superficially each odontode is capped by a layer of acellular, fibrous (mostly collagen) or monocrystalline tissue consistent with enameloid (Fig. 10A,B). Deep to the enameloid is orthodentine, an acellular, collagenous-rich matrix with a characteristic tubule arrangement (oriented perpendicular to the surface). In some species, orthodentine is replaced by a tissue lacking both lacunae and tubules. It remains uncertain if this is an acellular dentine (so-called lamelline) or an acellular bone (Miyake et al. 1999). The collagen-rich tissue surrounds a prominent pulp cavity. Each odontode is firmly embedded within the superficial dermis (stratum superficiale), and is anchored to the stratum compactum by anchoring fibers, permitting the elements to be firmly fixed and yet shed as necessary (Miyake et al. 1999).
Whereas odontodes of living chondrichthyans are derived exclusively from odontogenic papillae (Sire et al. 2002; Sire & Huysseune, 2003), the extensive osteichthyan-like bony base of some stem-chondrichthyan integumentary elements suggests these ‘polyodontodes’ received an osteogenic contribution.
Acanthodii
The post-cranial integumentary skeleton of acanthodians (late Ordovician to early Permian; 445–295 Ma) consists of numerous small, rhombic scales. Each scale is commonly composed of two regions: a superficial region with multiple layers of dentine (either mesodentine or orthodentine), each representing a successive generation of odontodes (Reif, 1982a), and a deeper basal plate composed of bone, typically acellular, less commonly cellular (Gross, 1947, 1957, 1971a; Richter & Smith, 1995; Karatajuté-Talimaa & Smith, 2003) (Fig. 11). Acanthodian scales grow by periodic deposition of dentine at the superficial surface and bone at the deeper and lateral surface. A ganoine-like tissue has been reported (Richter & Smith, 1995) but in general a capping tissue is absent.
Fig. 11.
Acanthodii. Schematic illustrations of acanthodian scales. (A) Nostolepis (Silurian). (B) Gomphonctus (Silurian). (C) Machairacanthus (early Devonian). Each scale grows by accretion, with successive layers of bone (either cellular or acellular) covered by dentine (either mesodentine or orthodentine). It is generally accepted that the combination of bone and dentine is comparable with odontodes. A ganoine-like tissue has been reported but most taxa appear to lack hypermineralized tissues such as enameloid or enamel. Scale bars: A = 150 µm; B = 200 µm; C = 300 µm.
The presence both of dentine and bone suggests that acanthodian scales are derived from odontogenic and osteogenic condensations.
The integumentary skeleton of actinopterygians and the origin of elasmoid scales
Plesiomorphically, the integumentary skeleton of osteichthyans consists of numerous rhombic scales organized into obliquely oriented rows. The scales are thick and localized within the stratum superficiale deep to the epidermis and firmly fixed to the stratum compactum by numerous Sharpey's fibers. Combined, this investiture of scales forms the near continuous scale-jacket (sensu Gemballa & Bartsch, 2002) across the body axis. Rhombic scales have two structural forms, corresponding to the ray-finned/lobe-finned dichotomy: actinopterygians have ganoid scales whereas sarcopterygians have cosmoid scales. Both scale types consist of multiple skeletal tissues (including hypermineralized tissues and a thick bony base), and both demonstrate an evolutionary trend towards reduction. Unlike cosmoid scales, which are entirely extinct, ganoid scales are still found in two lineages of living actinopterygians, polypterids (bichirs and reedfish) and lepisosteids (gars).
Ganoid scales of basal actinopterygians
Ganoid scales are characterized by ganoine (see Hypermineralized (capping) tissues above), overlying a region composed of orthodentine (Goodrich, 1907). True ganoid scales (in the strict sense of having multiple layers of ganoine) are considered apomorphic for actinopterygians, with well-documented examples dating back to the Devonian, 410 million years ago (Goodrich, 1907; Gross, 1968b, 1969, 1971b; Schultze, 1966, 1968, 1977; Märss, 2001; Schultze & Märss, 2004; Benton & Donoghue, 2007). Three types of ganoid scales are currently recognized: palaeoniscoid (in extinct basal actinopterygian lineages), polypteroid (used here for the scales of polypteriforms), and lepidosteoid (in lepisosteiforms).
Among the earliest, most basal actinopterygians (e.g. Andreolepis hedei from the late Silurian, 420 Ma; Gross, 1968b, 1969, 1971b; Janvier, 1971; Schultze, 1977; Märss, 2001; Schultze & Märss, 2004; Botella et al. 2007), individual palaeoniscoid scales are composed of a thick plate of cellular bone capped on the surface by multiple layers of tubular dentine (Fig. 12A). Superimposed on each layer of dentine is a thin monolayer of acellular crystalline tissue, reported to be either ganoine (Richter & Smith, 1995; Märss, 2001) or enameloid (Botella et al. 2007). Similar scales (cellular bone, tubular dentine, and a monolayer of ganoine) are also reported for Dialipina salgueiroensis (Devonian, 370 Ma; Schultze, 1977; Schultze & Cumbaa, 2001). Based in part on the interpretation of these elements as palaeoniscoid-type scales, it has been suggested that Andreolepis and Dialipinaare either basal actinopterygians or more basal osteichthyans, if ganoid scales are plesiomorphic compared to cosmoid (Janvier, 1996; Märss, 2001; Schultze & Cumbaa, 2001; Benton & Donoghue, 2007; Botella et al. 2007).
Fig. 12.
Actinopterygii. Schematic illustrations of palaeoniscoid-type ganoid scales. (A) Andreolepis (late Silurian). (B) Cheirolepis (middle Devonian). (C) Moythomasia (late Devonian). (D) Scanilepis (Triassic). Palaeoniscoid-type ganoid scales grow by deposition of successive layers of dentine and ganoine (= cf. odontocomplexes) (A,C) or apposition of successive odontodes (B,D) onto a deep basal plate made of cellular bone. Scale bars: A,C,D: 100 µm; B: 125 µm.
The earliest undisputed actinopterygians are from the middle to late Devonian (Janvier, 1996). Articulated remains of taxa such as Cheirolepis (Pearson & Westoll, 1979) and Moythomasia (Gross, 1950; Jessen, 1968, 1972; Gardiner, 1984) have palaeoniscoid scales (Fig. 12B,C), consisting of a superficial region of multilayered ganoine covering a region of dentine, and a thick basal plate of vascularized bone (Goodrich, 1907; Ørvig, 1978a,b,c; Sire, 1990). Palaeoniscoid scales are known only from the fossil record, with the most recent examples dating to the Jurassic (200–150 Ma) as in Scanilepis (Fig. 12D). Unlike more derived ganoid scales, most palaeoniscoid scales lack peg-and-socket articulations, likely resulting in a less flexible scale-jacket (Gemballa & Bartsch, 2002).
Polypteroid scales are unique to Polypteriformes (Cladistia) (Fig. 13A). These scales are structurally characterized by an additional tissue layer, elasmodine (see section Plywood-like tissues above), nested between two vascularized regions, dentine above and bone below (Sire et al. 1987). As has been demonstrated, the presence of elasmodine is a key feature in linking polypteroid scales with elasmoid scales (Sire, 1989, 1995; see Evolutionary scenario below).
The third type of ganoid scale, lepisosteoid, is found in Lepisosteiformes (Ginglymodi). Lepisosteoid scales are unique in lacking both elasmodine and dentine (Fig. 13B). Multilayered ganoid is directly apposed to the upper surface of the bony basal plate, which has relatively few blood vessels but numerous nonvascular (and enigmatic) canals of Williamson (Goodrich, 1907; Sire, 1994; Sire & Meunier, 1994). Both polypteroid and lepisosteoid scales date back, at least, to the late Cretaceous, 80–100 Ma (Dutheil, 1999; Janvier, 2007).
Notwithstanding their comparable morphological appearance, polypteroid and lepisosteoid scales demonstrate distinctly dissimilar modes of development. In polypteroid scales, the earliest stages of development are similar to those of elasmoid scales (Sire & Akimenko, 2004; Sire, unpublished data), and scales from young Polypterus senegalus closely resemble elasmoid scales (Sire, 1989). Of particular importance is the recognition that polypteroid scales develop from an odontogenic condensation. Similar to elasmoid scales, immature polypteroid scales are localized superficially within the dermis, adjacent to the interface with the epidermis. They initially form as a patchy layer of well-mineralized woven-fibered tissue. Along the deep margin of this layer, orthogonally arranged collagen lamellae are added, creating the plywood-like elasmodine. At this time, the developing polypteroid scale is framed above and below by capillary networks. In response to the presence of adjacent capillaries, cells along the upper surface continue to deposit woven-fibered matrix, encircling the blood vessels and thus creating vascular canals. These cells do not become trapped within the matrix but their elongate cell processes remain embedded in the matrix, defining the cells as odontoblasts and the tissue as dentine (see Structural diversity of dentines above). With continued deposition, odontoblasts and dentine matrix encroach increasingly towards the interface between the dermis and epidermis. Before contact with the basal surface of the epidermis is established, these superficial odontoblasts disappear. Concurrently, the overlying basal epidermal cells differentiate into ameloblasts. The ameloblasts make contact with the dentine surface and begin to deposit ganoine matrix (Sire et al. 1987; Sire, 1994). At this time the deep margin of developing polypteroid scales are lined by cells (elasmoblasts), which continue to deposit elasmodine. Likely in response to the presence of nearby capillaries, the extracellular matrix accumulating along the deepest margin of the elasmodine surface abruptly changes from highly organized elasmodine to a woven-fibered bone matrix. This alteration of extracellular matrix suggests a change from odontogenic to osteogenic contributions. Once growth of the scale slows down, deposition of the woven-fibered bone gives way to parallel-fibered, and then plywood-like organized lamellar bone. During this phase of ossification the scale incorporates numerous pre-existing collagen fibers of the stratum compactum, giving rise to Sharpey's fibers (Sire et al. 1987; Sire, 1989).
In contrast to polypteriforms, development of lepisosteoid scales demonstrates no evidence (inferred or otherwise) of an odontogenic contribution of the mesenchymal cells (e.g. no dentine deposited). The developing scales have no direct relationship with the epidermis, and do not pass through a transitory elasmoid scale-like phase (Nickerson, 1893; Sire, 1994; Sire & Huysseune, 2003). Lepisosteoid scales develop from an osteogenic primordium composed of numerous, closely packed osteoblast-like cells that deposit collagen and other extracellular matrix components within the stratum compactum of the dermis. Ossification begins within the centre of the osteogenic primordium. Following the appearance of woven-fibered bone, parallel-fibered and then lamellar bone is deposited on the superficial and deep margins, and Sharpey's fibers are incorporated from the surrounding stratum compactum. As growth continues, the superficial scale surface contacts the basalmost cells of the epidermis. Similar to polypteroid scales, the skeletogenic cells located at the superficial surface (in this case osteoblasts) retreat before the contact is established and the bordering basal epidermal cells differentiate into ameloblasts, and ganoine matrix is deposited.
Ganoid scales are also known for a variety of extinct Amiiformes (a lineage close to Teleostei), including sinamiids (early Triassic, 250 Ma), and Caturus furcatus (Jurassic, 200–150 Ma), a species close to extinct and living bowfins. These scales were thin, cycloid in shape and covered with a layer of ganoine (Schultze, 1996; Grande & Bemis, 1998). Amiiformes were long considered the sister group of Lepisosteiformes but this relationship is currently debated (Inoue et al. 2003; Hurley et al. 2007). Consequently, the exact histological structure of the ganoid scales in extinct amiiforms and determining whether elasmodine is present is of considerable interest.
Elasmoid scales
In contrast to their relatives described above, in most amiiforms individual scales are reduced in thickness, lack the bony base, and are of elasmoid type. In the only living species, Amia calva, each scale has a thin, ornamented upper layer, similar to the external layer of teleost elasmoid scales, and a well-developed basal plate made of elasmodine organized into a twisted plywood-like arrangement (Meunier et al. 1978; Meunier, 1981), consistent with their identification as elasmoid scales (discussed below). Although scale development in bowfins remains unknown, the structural similarity of these elements with the teleost elasmoid scale provides strong support for their derivation from odontogenic condensations. Furthermore, the evolution of amiiform scales may parallel that of elasmoid scales, i.e. both derived from an ancestral polypteroid type (see Plywood-like tissues and Ganoid scales of basal actinopterygians above).
Without question, the elasmoid scale is the most common integumentary skeletal element among living vertebrates, including most of the 26 000 species of teleosts, Amia calva (discussed above), extant representatives of non-tetrapodan sarcopterygians (coelacanths and lungfish) and some gymnophionan amphibians (see below and Vickaryous et al. this Issue). Elasmoid scales are thin, imbricated, collagenous plates (Bertin, 1944). Although remarkably diverse in morphology and ornamentation (including both ctenoid and cycloid shapes), all elasmoid scales are fundamentally similar in structure (review in Huysseune & Sire, 1998; Sire & Akimenko, 2004). Each scale is composed of three layers (Fig. 13C). The first to form is the thin, ornamented external layer composed of a well-mineralized, woven-fibered tissue comparable with the dentine layer of polypteroid scales. As the elasmoid scale develops, this external layer expands at its lateral margins. Deep to the external layer is the relatively thick, basal plate composed of elasmodine (see Plywood-like tissues above). Elasmodine mineralizes slowly as the hydroxyapatite crystals do not penetrate deep within the collagen lamellae and for some taxa such as coelacanths, elasmodine remains unmineralized. The last and most superficial layer to form is the limiting layer. The limiting layer is deposited on the external layer surface, but has a restricted distribution in the posterior field of the scale facing the epidermis. Shortly after being deposited the limiting layer becomes hypermineralized (see section Hypermineralized (capping) tissues above). Unlike osseous tissues (see Ganoid scales of basalactinopterygians above, and following section), elasmodine does not become anchored to the deep dermis by Sharpey's fibers.
Scutes and dermal plates
Among some early chondrosteans (e.g. Saurichthys; early Triassic to early Jurassic, 250–200 Ma), elements of the integumentary skeleton resemble lepisosteoid scales: a bony base capped by a superficial region of multilayered ganoine. In most living Acipenseriformes (sturgeons and paddlefish) and related fossil taxa [e.g. the early Triassic form Birgeria (Ørvig, 1978a)], ganoine is lost and thus individual scales (bony plates, scutes) are composed exclusively of cellular, parallel-fibered bone (Bemis et al. 1997). A lepisosteoid-type ganoid scale origin for chondrostean bony plates is supported by developmental data demonstrating a strong parallel between early skeletogenesis of these two elements (Sewertzoff, 1932; Sire, unpubl. data).
Among various teleosts, elasmoid-type scales are replaced by plates of cell-rich bone. In armoured catfish (callichthyids, loricariids and doradids), these bony elements are capped by a layer of hyaloine, creating what are known as scutes (see Hypermineralized (capping) tissues above; Sire, 1993; Sire & Meunier, 1993) (Fig. 13D). These scutes (as well as fin rays and cranial bones) are ornamented with dermal denticles which are tooth-like elements (Sire & Huysseune, 1996; Sire, 2001). For other taxa including gasterosteiforms, tetraodontiforms and syngnathiforms, no capping tissue forms and the resulting dermal plates are composed exclusively of bone (Fig. 13E).
Both dermal plates and scutes begin ossification deep in the dermis, from an osteogenic primordium. As the bony plate begins to deposit osteoid and woven-fibered bone, pre-existing collagen fibers from the surrounded dermis are integrated into the matrix, in addition to various bone matrix components deposited by the osteoblasts. With continued ossification, this primordium becomes progressively surrounded by parallel-fibered bone, and eventually by a region made of plywood-organized lamellar bone, in which are incorporated Sharpey's fibers.
Sarcopterygians and the evolution of cosmoid scales
Cosmoid scales in basal sarcopterygians
Plesiomorphically, the integumentary skeleton of sarcopterygians is characterized by the tissue complex known as cosmine (Ørvig, 1969; Thomson, 1975, 1977; Meinke, 1984, 1986; Borgen, 1989; Bemis & Northcutt, 1992). Overall, the histology of cosmoid scales closely resembles that of the polypteroid scales: a shiny superficial tissue similar to enamel or enameloid, overlying orthodentine and vascular, lamellar bone (Goodrich, 1907; Meinke, 1982, 1984). What make cosmine distinct are the interconnected canals, flask-shaped cavities and superficial pores that course through the dentine and capping tissue (Fig. 14B–D).
Fig. 14.
Sarcopterygii. Schematic illustrations of integumentary elements from various non-tetrapodan aquatic sarcopterygians. (A) Glyptolepis (Dipnomorpha, middle to late Devonian). Scale demonstrating the superposition of multiple odontodes embedded in cellular bone. (B–D) Upper surface of the scales of three species of basal dipnomorphans. (B) Dipterus (late Devonian), (C) Osteolepis (middle Devonian), and (D) Porolepis (early Devonian). Cosmine, consisting of a pore-canal system covered by juxtaposed odontodes composed of dentine and ganoine, is common to many basal dipnomorphans (dipnoans and porolepiforms). (E) Latimeria chalumnae (Actinistia, Extant). As demonstrated by this section through the posterior field, in the coelacanth the scale is of elasmoid type; the upper region is composed of numerous overlapping odontodes, with dentine and ganoine, covering a thick, unmineralized basal plate of elasmodine. Scale bars: A = 250 µm; B = 70 µm; C = 100 µm; D = 140 µm; E = 200 µm.
The oldest known example of a cosmine-like pore-canal system comes from portions of the dermatocranium of the early Devonian (405 Ma) taxon Meemannia eos(Zhu et al. 2006). Pores communicating with the superficial surface are partially lined by tapering layers of enamel, suggesting that the pore-canal system remains static while successive odontogenic contributions are deposited. Other basal sarcopterygians from upper Silurian to lower Devonian (420–410 Ma), including Psarolepis romeri, Achoania jarvikiiand Styloichthys changae, are also characterized by cosmine complete with multiple stacked successions of dentine and enamel (Yu, 1998; Zhu et al. 1999, 2001; Zhu & Yu, 2002). Unlike Meemannia, the cosmine of Psarolepis and Styloichthys reportedly demonstrates some evidence of odontogenic tissue resorption (Zhu et al. 2006).
Although clearly common among fossil forms, cosmine is not known in extant taxa.
Actinistia
Among Actinistia (coelacanths), cosmine is lost from the integumentary skeleton of all but the most basal members. Traces of cosmine have been reported for the middle Devonian (390 Ma) form Miguashaia bureaui, the best known ancient actinistian (Cloutier, 1991; Janvier, 1996). A recent phylogenetic hypothesis placing Styloichthysas a basal actinistian (Friedman et al. 2007) further supports the notion of cosmine as primitive for coelacanths. However, the pore-canal network is highly reduced or entirely absent in more derived actinistians (e.g. Spermatodus pustulosus), although multiple stacked successions of dentine and enamel, situated superficial to a thick basal plate, may be conserved (Meinke, 1982).
In the extant form Latimeria chalumnae, the superficial scale region (corresponding to the region of the pore-canal network in more basal members) is extremely reduced and odontogenic tissues are restricted to the surface of the posterior (overlapping) field (Fig. 14E). The thick bony basal plate of the ancestral cosmoid scale has disappeared, and the basal plate of the scale is composed of unmineralized elasmodine with a twisted plywood-like arrangement (Meunier, 1980; Giraud et al. 1978).
Dipnomorpha
Cosmoid scales are well-known for basal dipnomorphs, including members of both Porolepiformes and Dipnoi (lungfish) (Meinke, 1984, 1986; Janvier, 1996). In porolepiforms such as Porolepis(early Devonian, 410 Ma), each scale is composed of cosmine (Fig. 14D). Among later forms (e.g. Heimenia), development of cosmine is incomplete and instead of a pore-canal system the surface appears to be eroded, yielding isolated tubercles and ridges. By the middle to late Devonian, cosmine can no longer be distinguished in porolepiforms (e.g. Glytolepis and Holoptychius; Janvier, 1996) (Fig. 14A).
Among Devonian Dipnoi (e.g. Dipterus and Gryphognathus), cosmine often demonstrates concentric lines of discontinuity known as Westoll lines (interpreted to be evidence of cyclical remodelling; Janvier, 1996) (Fig. 14C). Among more recent dipnoans, the thickness of the cosmine including the bony basal plate is reduced. In modern taxa (Neoceratodus, Protopterus and Lepidosiren), cosmine is no longer present. The scales are typically elasmoid, with a thick elasmodine basal plate covered with a thin external layer of well-mineralized, woven-fibered tissue (Meunier, 1983). Although details of scale histology for many fossil actinistians and dipnomorphs is poorly known (in particular that portion of the scale deep to the cosmine), we suggest that modern sarcopterygian elasmoid scales share a common ancestry with actinopterygian elasmoid scales dating back to the ancestral rhombic scale (see following section). It is also worth noting that the scales in gymnophionan amphibians also have a structure comparable to elasmoid scales (see Vickaryous & Sire, this Issue).
A revised scenario for the integumentary skeleton evolution in non-tetrapodan vertebrates
To date, efforts towards establishing the evolutionary relationships between the various integumentary skeletal elements of non-tetrapods have been confounded by the heavy reliance on data gleaned from isolated, ontogenetically (and skeletally) mature specimens and a general lack of developmental studies. One serious limitation to ongoing studies is the general lack (or complete omission) of structural details at the tissue level. Structural remodelling during ontogeny can reduce or obscure diagnostic (and synapomorphic) features, resulting in the misinterpretation of tissue/organ relationships.
An important example comes from the study of isopedine. Until recently, all plywood-like skeletal tissues were categorized as isopedine, considered to be a type of bone (or aspidin when acellular), and fundamentally osteogenic in origin. Consequently, the plywood-like arrangement of elasmodine in elasmoid scales was also assumed to be derived from bone. Work by Sire (1989) on the development of the polypteroid scales has since demonstrated that plywood-like tissues in the integumentary skeleton can also be derived from odontogenic cell populations (viz. odontoblasts). Although outwardly similar to lamellar bone, elasmodine (= lamellar dentine) is an excellent example of tissue convergence. The same argument applies to the lamellar plywood-like organization of the stratum compactum of the dermis. This reinterpretation of elasmodine is critical to a deeper understanding of integumentary skeleton evolution.
Polypteroid and elasmoid scales
As noted above, elasmoid-type scales are common to deeply nested members of both Actinopterygii and Sarcopterygii (see Elasmoid scales above). Notwithstanding the other obvious distinctions between these two groups, structural differences of the elasmoid scales are minor. Regardless of origin, all elasmoid scales are composed of a thick basal plate made of elasmodine and a thin external layer of a woven-fibered tissue; depending on both the species and the relationship with the epidermal cover, the external layer may be capped by a well-mineralized tissue with a variable appearance (Fig. 13C).
These similarities in structure and organization are difficult to reconcile as simple convergences between actinopterygians and sarcopterygians. Drawing on the evidence presented above we offer a new hypothesis, considering all elasmoid scales as derivatives of a common ancestral integumentary element. This hypothesis is strongly supported by (1) the presence of elasmodine in adult scales and (2) by the discovery that polypteroid ganoid scales and elasmoid scales share a common mode of early development (Figs 13A, 15B). Polypteroid ganoid scales and elasmoid scales begin development within a well-defined dermal papillae comparable with that of teeth and odontodes. It therefore seems certain that teleost elasmoid scales are paedomorphic polypteroid ganoid scales (Sire, 1989; Sire & Huysseune, 2003) and that elasmodine, and the external and limiting layers, which all are formed within the dermal papilla, are dental tissues (Sire, 1989). The novelty of our scenario is the interpretation of elasmodine as a dental tissue, and more specifically as a form of lamellar dentine. In contrast, most previous research had acknowledged elasmodine remained to be of uncertain origin (e.g. Huysseune & Sire, 1998).
Fig. 15.
Schematic illustrations demonstrating structural similarities in the developing scales of (A) Tremataspis (Osteostraci, Silurian), determined from a rare growth series (Denison, 1947), and (B) Polypterus senegalus (Actinopterygii, Extant); polypteroid-type ganoid scale (after Sire, 1989). Scale bars: A = 250 µm; B = 200 µm.
The deposition of elasmodine ceases early during polypteroid ganoid scale development, while the individual is relatively small (∼60–70 mm body length). Bone deposition then takes place within the adjacent capillary-rich region (see Ganoid scales of basal actinopterygians above). Thus, in larger individuals the presumptive elasmoid scale becomes deeply embedded within a larger element composed of dentine and bone. In addition, it is subjected to ongoing resorption as located in a region housing abundant vasculature. Consequently, the plywood-like arrangement that typically characterizes elasmodine cannot be identified in sections that do not pass through the centre of the scale initium. For example, accurate histological identification of polypteroid- vs. lepisosteoid-type ganoid scales requires careful, targeted sectioning through the central region (Brito et al. 2000; Gayet et al. 2002). Owing to the lamellar organization of the collagen matrix, long interpreted as lamellar bone (i.e. isopedine), the presence of elasmodine appears to have frequently been neglected by previous authors.
Evolutionary scenario: a link between odontocomplexes and elasmodine
In this review, we have focused on categorizing tissues of integumentary skeleton (of non-tetrapodan vertebrates), and establishing their origins as odontogenic and/or osteogenic contributions (see Origin and diversification of the integumentary skeleton above). These data are summarized in Table 1 and a revised scenario is illustrated in Fig. 16. Our hypothesis implies that the oldest vertebrate lineages, particularly stem gnathostomes such as pteraspidomorphs, have both odontogenic (enamel, enameloid, various types of dentine) and osteogenic [cellular and acellular (= aspidin) bone] tissues. There is evidence demonstrating independent loss of one of the contributions on multiple occasions. For example, antiarch placoderms have lost the odontogenic contribution, and thelodonts and chondrichthyans have largely or completely lost the osteogenic contribution. Even when a contribution is retained, its capacity to form tissues may become altered. For example, some placoderms and acanthodians develop dentine but not enamel and enameloid. The current data strongly support the hypothesis that all integumentary skeletal elements arise exclusively from one or both odontogenic and osteogenic contributions. There is also evidence to suggest that the cells responsible for the synthesis and deposition of the extracellular matrix components of the various tissues (i.e. ameloblasts, odontoblasts, and osteoblasts) are able to interact with one another if they receive appropriate signals. Expression of type I collagen by ameloblasts during enameloid formation in teleost teeth is a good illustration of this large range of cell function (Huysseune et al. 2008). This helps to explain how osteogenic-derived elements are able to interact with the basalmost epidermal cells, giving rise to odontogenic-like tissues (e.g. the ganoine of lepisosteoid scales or hyaloine of armoured catfish scutes).
Fig. 16.
A revised scenario depicting the evolution of the integumentary skeleton in non-tetrapods. Although at present uncertain, we hypothesize that elasmodine is present in the ancestral rhombic scales and in cosmoid scales. Furthermore, we propose that similar to modern polypteroid-type ganoid scales, the ancestral rhombic scale was composed of tissues derived from two discrete skeletogenic cell populations: odontogenic and osteogenic. (A) Among actinopterygians, the diversity of scale-type structure can be explained due to the loss and/or modification of these skeletogenic cell components. Loss of the odontogenic component results in the absence of dentine from the lepisosteoid-type ganoid scale. In contrast, loss of the osteogenic component (along with reduction and modification of the odontogenic component) gives rise to the elasmoid scale. Independent losses of the odontogenic component gives rise to the development of dermal plates and to the scutes. Similar to lepisosteoid-type ganoid scales, scutes are characterized by the presence of a well-mineralized layer (hyaloine), similar if not equivalent to ancestral ganoine. (B) Among sarcopterygians, the pore-canal system of the cosmoid scale is lost, followed by reduced and modified expression of the odontogenic-derived components. In actinistians and dipnomorphans these modifications, combined with the loss of the osteogenic component, occur independently (in parallel), giving rise to the sarcopterygian elasmoid scale. Unlike modern dipnoans, extant coelacanths continue to develop superficial odontodes.
In actinopterygians, elasmoid scales are paedomorphic polypteroid scales. It remains unclear why elasmoid scale development stops after elasmodine formation, and why the cells responsible for the osteogenic component are either not activated or not present. Similarly, odontoblasts depositing the superficial dentine layer are arrested in their function prematurely. The important vascular networks located around the polypteroid scale anlage likely play an important role in sustaining cell activity, although this has yet to be demonstrated. The superficial region of the polypteroid scales is an odontocomplex that derives from the superimposition/juxtaposition/fusion of multiple odontodes (Ørvig, 1977). Such odontocomplexes are predicted to be found in the ancestral rhombic scales from which both ganoid (in actinopterygians) and cosmoid (in sarcopterygians) scales have differentiated (Fig. 16). Given the relationships between elasmoid and polypteroid scales, we speculate that: (1) the elasmoid scales in amiids (Amia calva) are derived from polypteroid-type scales and not lepisosteoid-type scales; (2) the integumentary skeleton in the common ancestor of the sarcopterygians (i.e. cosmoid scales) developed first as an elasmoid scale, as identified in polypteroid scales through the presence of elasmodine; and hence (3) the rhombic scales in the last common ancestor (a basal osteichthyan) to both lineages similarly possessed elasmodine (Fig. 16). To date, elasmodine has not been identified in any basal osteichthyan rhombic scales. However, as previously noted the relationship of elasmodine with other tissue types was only recently established. A number of interpretative drawings by Denison and Ørvig (e.g. in Tremataspis) are suggestive of the existence of a lamellar tissue located immediately below the dentine layer. It should be emphasized that only sections passing through the centre of the scale, i.e. at the former location of the scale papilla, will illustrate this feature, and thus future targeted palaeohistological preparations are required.
Additional support for our evolutionary scenario comes from the description of a growth series of the late Silurian (420 Ma) osteostracan Tremataspis (Denison, 1947; Fig. 15A). Remarkably, this staged series is very similar to that described for the modern polypterid Polypterus senegalus (Fig. 15B). Keeping in mind that elasmodine is generally slow to mineralize, the basal, lamellar layer of Tremataspis scales (Denison, 1947) is consistent with progressively mineralizing elasmodine. Provided our hypothesis is confirmed by future histological investigations, the implication is that elasmodine, a type of lamellar dentine, is a very ancient vertebrate tissue (Fig. 16).
The evolution of development of the vertebrate integumentary skeleton
Even before the inception of evolutionary theory, attempts to explain equivalence between the integumentary skeletons of vertebrates focused on the placoid scale as an ideal type, or the fundamental unit of composition (e.g. Williamson, 1849, 1851). This approach continued though the work of Hertwig (1874a,b, 1876, 1879, 1882), Goodrich (1907), and placoid scales were formally the basic elements of the lepidomorial (Jarvik, 1949; Ørvig, 1951; Stensiö, 1958, 1961, 1962, 1964) and odontode (Ørvig, 1967, 1968, 1975, 1977; Reif, 1973, 1974, 1980, 1982a,b) theories (see Donoghue, 2002). The contemporary manifestation, the odontode regulation theory (Reif, 1982a), has been widely adopted as a framework for understanding the evolution of the integumentary skeleton. However, it provides for an incomplete understanding of skeletal developmental evolution in that it addresses only the odontogenic component of the integumentary skeleton (Donoghue, 2002). Implicitly, although effectively, the odontode regulation theory treats the osteogenic component of scales and bony plates as mere evolutionary vestiges of the bone of attachment from odontodes in antecedent vertebrates, as did turn of the century skeletal anatomists (e.g. Hertwig, 1874a,b, 1876, 1879, 1882; Goodrich, 1907). This is unfortunate on two counts. First, it is widely appreciated that the odontogenic and osteogenic cell populations are derived from distinct lineages and that there is at least empirical evidence that their fates are, or can be, independently regulated (Smith & Hall, 1990, 1993; Donoghue, 2002; Donoghue & Sansom, 2002; Sire & Huysseune, 2003; Donoghue et al. 2006). Secondly, while the odontode regulation theory has been utilized to explain the evolution of development of mineralized integumentary skeletal elements in the extinct vertebrates in which this skeletal system was first established (Reif, 1982a; Reif & Richter, 2001; Reif, 2002), in many extant vertebrates the mineralized components of the integumentary skeleton are solely osteogenic derivatives. Thus, the challenge is to derive a universal theory for the evolution and development of the vertebrate integumentary mineralized skeleton, one that is based upon developmental differentiation and patterning (Reif, 1980) in terms of causal molecular mechanisms (Donoghue & Sansom, 2002). The prospectus for this theory has been established (Donoghue & Sansom, 2002) and it must now be tested experimentally, the first steps toward which have already been made (Sire et al. 1997; Sire & Akimenko, 2004).
Acknowledgments
We are particularly indebted to Philippe Janvier for discussions and for giving us the permission to use his published drawings. We express our sincerest thanks to the two anonymous reviewers for their constructive comments that have contributed to improve this article.
References
- Alibardi L, Dalla Valle L, Nardi A, Toni M. Evolution of hard proteins in sauropsid integument in relation to cornification of skin derivatives in amniotes. J Anat. 2009;214:560–586. doi: 10.1111/j.1469-7580.2009.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault M, Janvier P. The anaspid-like craniates of the Escuminac Formation (Upper Devonian) from Miguasha (Québec, Canada), with remarks on anaspid-petromyzontid relationships. In: Chang MM, Liu YH, Zhang GR, editors. Early Vertebrates and Related Problems of Evolutionary Biology. Beijing: Science Press; 1991. pp. 19–40. [Google Scholar]
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Belamie E, Mosser G, Gobeaux F, Giraud-Guille MM. Possible transient liquid crystal phase during the laying out of connective tissues, a chitin and collagen as models. J Phys Condens Matter. 2006;18:115–129. [Google Scholar]
- Bemis WE, Northcutt RG. Skin and blood vessels of the snout of the Australian lungfish, Neoceratodus forsteri, and their significance for interpreting the cosmine of Devonian lungfishes. Acta Zool (Stockh) 1992;73:115–139. [Google Scholar]
- Bemis WE, Findeis EK, Grande L. An overview of Acipenseriformes. Env Biol Fish. 1997;48:25–71. [Google Scholar]
- Benton MJ, Donoghue PCJ. Paleontological evidence to date the Tree of Life. Mol Biol Evol. 2007;24:26–53. doi: 10.1093/molbev/msl150. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J, Matoltsy AG, Sylvia Richards K. Biology of the Integument, Volume 2 Vertebrates. Berlin: Springer-Verlag; 1986. [Google Scholar]
- Bertin L. Modifications proposées dans la nomenclature des écailles et des nageoires. Bull Soc Zool Fr. 1944;69:198–202. [Google Scholar]
- Blom H, Marss T, Miller CG. Silurian and earliest Devonian birkeniid anaspids from the Northern Hemisphere. Trans R Soc Edinb (Earth Sci) 2002;92:263–323. [Google Scholar]
- Borgen UJ. Cosmine resorption structures on three osteolepid jaws and their biological significance. Lethaia. 1989;22:413–424. [Google Scholar]
- Botella H, Blom H, Dorka M, Ahlberg P, Janvier P. Jaws and teeth of the earliest bony fishes. Nature. 2007;448:583–586. doi: 10.1038/nature05989. [DOI] [PubMed] [Google Scholar]
- Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, keratinizing or cornifying epithelia. J Anat. 2009;214:516–559. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito P, Meunier F, Gayet M. The morphology and histology of the scales of the Cretaceous gar Obaichthys(Actinopterygii, Lepisosteidae): phylogenetic implications. C R Acad Sci, Ser IIA- Earth Planet Sci. 2000;331:823–829. [Google Scholar]
- Burrow CJ. Histological structure of the cancellous bone layer in Bothriolepis canadensis(Antiarchi, Placodermi) Lethaia. 2005;38:205–210. [Google Scholar]
- Bystrow AP. The microstructure of the dermal armor of the jawless vertebrates from Silurian and Devonian. In: Berg AS, editor. Memorial Volume. Akademia Nauk SSSR: 1955. pp. 472–523. [in Russian] [Google Scholar]
- Castanet J, Francillon-Vieillot H, Ricqlès A, de Zylberberg L. The skeletal histology of the Amphibia. In: Heatwole H, Davies M, editors. Amphibian Biology, Voume 5 Osteology. Chipping Norton, Australia: Surrey Beatty & Sons; 2003. pp. 1598–1683. [Google Scholar]
- Caton J, Tucker A. Current knowledge in tooth development: a model mineralized system. J Anat. 2009;214:502–515. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier R. Patterns, trends, and rates of evolution within the Actinistia. Env Biol Fish. 1991;32:23–58. [Google Scholar]
- Currey JD. The histology of the scales of Orvikuina(Palaeoniscoidea) Paläontol Z. 1961;35:187–190. [Google Scholar]
- Davit-Béal T, Allizard F, Sire JY. Enameloid/enamel transition through successive tooth replacements in Pleurodeles waltl(Lissamphibia, Caudata) Cell Tissue Res. 2007;328:167–183. doi: 10.1007/s00441-006-0306-1. [DOI] [PubMed] [Google Scholar]
- Denison RH. The exoskeleton of Tremataspis. Am J Sci. 1947;245:337–365. [Google Scholar]
- Denison RH. The exoskeleton of early Osteostraci. Fieldiana Geol. 1951;11:199–218. [Google Scholar]
- Denison RH. Early Devonian fishes from Utah, Part. I, Osteostraci. Fieldiana Geol. 1952;11:265–287. [Google Scholar]
- Denison RH. The early history of the vertebrate calcified skeleton. Clin Orthop Rel Res. 1963;31:141–152. [PubMed] [Google Scholar]
- Denison RH. The Cyathaspididae: a family of Silurian and Devonian jawless vertebrates. Fieldiana Geol. 1964;13:309–473. [Google Scholar]
- Denison RH. Ordovician vertebrates from Western United States. Fieldiana Geol. 1967;16:131–192. [Google Scholar]
- Denison RH. Growth and repair of the shield in Pteraspididae (Agnatha) Palaeontogr Abt A. 1973;143:1–10. [Google Scholar]
- Denison RH. The structure and evolution of teeth in lungfishes. Fieldiana Geol. 1974;33:31–58. [Google Scholar]
- Denison RH. Placodermi. Stuttgart: Gustav Fisher; 1978. [Google Scholar]
- Dhouailly D. Toward feather in birds and hair in mammals: the basic program of the integument. J Anat. 2009;214:587–606. doi: 10.1111/j.1469-7580.2008.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhouailly D, Olivera-Martinez I, Fliniaux I, et al. Skin field formation: morphogenetic events. Int J Dev Biol. 2004;48:85–91. [PubMed] [Google Scholar]
- Diekwisch TGH. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- Donoghue PCJ. Growth and patterning in the conodont skeleton. Philos Trans R Soc Lond. 1998;353:633–666. [Google Scholar]
- Donoghue PCJ. Evolution of development of vertebrate teeth and scales: unravelling concepts, regulatory theories and homologies. Paleobiology. 2002;28:474–507. [Google Scholar]
- Donoghue PCJ, Aldridge RJ. Origin of a mineralised skeleton. In: Ahlberg PE, editor. Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development. London: Taylor & Francis; 2001. pp. 85–105. [Google Scholar]
- Donoghue PCJ, Sansom IJ. Origin and early evolution of vertebrate skeletonization. Microsc Res Techn. 2002;59:185–218. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Smith MP. The anatomy of Turinia pagei(Powrie) and the phylogenetic status of the Thelodonti. Trans R Soc Edinb (Earth Sci) 2001;92:15–37. [Google Scholar]
- Donoghue PCJ, Forey PL, Aldridge RJ. Conodont affinity and chordate phylogeny. Biol Rev. 2000;75:191–251. doi: 10.1017/s0006323199005472. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Sansom IJ, Downs JP. Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J Exp Zool (Mol Dev Evol) 2006;306B:278–294. doi: 10.1002/jez.b.21090. [DOI] [PubMed] [Google Scholar]
- Dutheil D. The first articulated fossil cladistian: Serenoichthys kemkemensisgen. et sp. nov., from the Cretaceous of Morocco. J Vert Paleontol. 1999;19:243–246. [Google Scholar]
- Eames BF, de la Fuente L, Helms JA. Molecular ontogeny of the skeleton. Birth Defects Res (Part C) 2003;69:93–101. doi: 10.1002/bdrc.10016. [DOI] [PubMed] [Google Scholar]
- Fox H. Dermis. In: Bereiter-Hahn J, Matoltsy AG, Sylvia Richards K, editors. Biology of the integument, Volume 2 Vertebrates. Berlin: Springer-Verlag; 1986. pp. 111–115. [Google Scholar]
- Francillon-Vieillot H, de Buffrénil V, Castanet J. Microstructure and mineralization of vertebrate skeletal tissues. In: Carter JG, et al., editors. Skeletal Biomineralization; Patterns, Processes and Evolutionary Trends, Volume 1. New York: Van Nostrand Reinhold; 1990. pp. 471–548. [Google Scholar]
- Friedman M, Coates M, Anderson P. First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs. Evol Dev. 2007;9:329–337. doi: 10.1111/j.1525-142X.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- Gardiner BG. The relationships of the palaeoniscoid fishes, a review based on new specimens of Mimiaand Moythomasiafrom the Upper Devonian of Western Australia. Bull Brit Mus (Nat Hist), Geol. 1984;37:173–428. [Google Scholar]
- Gayet M, Meunier F, Werner C. Diversification in Polypteriformes and special comparison with the Lepisosteiformes. Palaeontology. 2002;45:361–376. [Google Scholar]
- Gemballa S, Bartsch P. Architecture of the integument in lower teleostomes: functional morphology and evolutionary implications. J Morphol. 2002;253:290–309. doi: 10.1002/jmor.10007. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Donoghue PCJ. The homology and phylogeny of chondrichthyan tooth enameloid. J Morphol. 2007;268:33–49. doi: 10.1002/jmor.10501. [DOI] [PubMed] [Google Scholar]
- Giraud MM, Castanet J, Meunier FJ, Bouligand Y. The fibrous structure of coelacanth scales: a twisted plywood. Tissue Cell. 1978;10:671–686. doi: 10.1016/0040-8166(78)90054-x. [DOI] [PubMed] [Google Scholar]
- Giraud-Guille MM. Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif Tissue Int. 1988;42:167–180. doi: 10.1007/BF02556330. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. On the scales of fish, living and extinct, and their importance in classification. Proc Zool Soc, Lond. 1907;77:751–774. [Google Scholar]
- Goodrich ES. Vertebrata Craniata. London: Adam and Charles Black; 1909. [Google Scholar]
- Goto M. Histogenic studies on the teeth of leopard shark (Triakis scyllia. J Stomatol Soc Jpn. 1978;45:527–584. doi: 10.5357/koubyou.45.527. [DOI] [PubMed] [Google Scholar]
- Grande L, Bemis WE. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. Soc Vert Paleontol, Memoir 4. J Vert Paleontol. 1998;18:1–681. [Google Scholar]
- Gross W. Die Fische des mittleren Old Red Süd-Livlands. Geol Palaeontol Abh. 1930;18:121–156. [Google Scholar]
- Gross W. Asterolepis ornataEichwald und das Antiarchi-Problem. Palaeontogr Abt A. 1931;75:1–62. [Google Scholar]
- Gross W. Histologische Studien am Aussenskelett fossiler Agnathen und Fische. Palaeontogr Abt A. 1935;83:1–60. [Google Scholar]
- Gross W. Der histologische Aufbau der Anaspiden-schuppen. Nor Geol Tiddskr. 1938;17:191–195. [Google Scholar]
- Gross W. Die Agnathen und Acanthodier des Obersilurischen Beyrichienkalks. Palaeontogr Abt A. 1947;96:91–158. [Google Scholar]
- Gross W. Umbenennung von AldingeriaGross (Palaeoniscidae; Oberdevon) in Moythomasian. nom. Neues Jahrb Geol Paläontol Mon. 1950;1950:145. [Google Scholar]
- Gross W. Über Crossopterygier und Dipnoer aus dem baltischen Oberdevon im Zusammenhang einer vergleichenden Untersuchung des Porenkanalsystems paläozoischer Agnathen und Fische. K Sven Vetenskapsakad Handl. 1956;5:1–140. [Google Scholar]
- Gross W. Mundzähne und hautzähne der Acanthodier und Arthrodiren. Palaeontogr Abt A. 1957;109:1–40. [Google Scholar]
- Gross W. Anaspidenschuppen aus dem Ludlow des Ostseegebiets. Paläontol Z. 1958;32:24–37. [Google Scholar]
- Gross W. Aufbau des Panzers obersilurischer Heterostraci und Osteostraci Norddeutschlands (Geschiebe) und Oesels. Acta Zool (Stockh) 1961;42:73–150. [Google Scholar]
- Gross W. Beobachtungen mit dem Elektronenraster-Auflichtmikroskop an den Siebplatten und dem Isopedin von Dartmuthia(Osteostraci) Paläontol Z. 1968a;42:73–82. [Google Scholar]
- Gross W. Fragliche Actinopterygier-Schuppen aus dem Silur Gotlands [Problematic actinopterygian scales from the Silurian of Gotland] Lethaia. 1968b;1:184–218. [Google Scholar]
- Gross W. Lophosteus superbusPander, ein Teleostome aus dem Silur Oesels. Lethaia. 1969;2:15–47. [Google Scholar]
- Gross W. Downtonische und dittonische Acanthodier-Reste des Ostseegebietes (Downtonian and Dittonian acanthodian remains from the Baltic Sea area) Palaeontogr Abt A. 1971a;136:1–82. [Google Scholar]
- Gross W. Lophosteus superbusPander: Zähne, Zahnknochen und besondre Schuppenformen. Lethaia. 1971b;4:131–152. [Google Scholar]
- Hall BK, Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hall BK, Witten PE. Plasticity of and transitions between skeletal tissues in vertebrate evolution and development. In: Anderson JS, Sues H-D, editors. Major Transitions in Vertebrate Evolution. Bloomington, IN: Indiana University Press; 2007. pp. 13–56. [Google Scholar]
- Halstead LB. Calcified tissues in the earliest vertebrates. Calcif Tissue Res. 1969;3:107–134. doi: 10.1007/BF02058654. [DOI] [PubMed] [Google Scholar]
- Halstead LB. The heterostracan fishes. Biol Rev. 1973;48:279–332. [Google Scholar]
- Halstead LB. Vertebrate Hard Tissues. London: Wykeham Science Publications Ltd; 1974. [Google Scholar]
- Halstead LB. Evolutionary aspects of neural crest-derived skeletogenic cells in the earliest vertebrates. In: Maderson PFA, editor. Developmental and Evolutionary Aspects of the Neural Crest. New York: John Wiley and Sons; 1987. pp. 339–358. [Google Scholar]
- Halstead Tarlo LB. Aspidin: the precursor of bone. Nature. 1963;199:46–48. doi: 10.1038/199046a0. [DOI] [PubMed] [Google Scholar]
- Halstead Tarlo LB. The origin of bone. In: Blackwood HJJ, editor. Bone and Tooth. Oxford: Pergamon Press; 1964. pp. 3–17. [Google Scholar]
- Halstead Tarlo LB. Psammosteiformes (Agnatha) – a review with descriptions of new material from the Lower Devonian of Poland II – systematic part. Palaeontol Pol. 1965;15:1–168. [Google Scholar]
- Heintz A. Die Downtonischen u. Devonischen Vertebraten von Spitsbergen II. Acanthaspida. Skr Svalb Ishavet. 1929;22:1–81. [Google Scholar]
- Herold RC. Osteodentinogenesis. An ultrastructural study of tooth formation in the pike, Esox lucius. Cell Tissue Res. 1971;112:1–14. [PubMed] [Google Scholar]
- Herold RC, Rosenbloom J, Granovski M. Phylogenetic distribution of enamel proteins: immunohistochemical localization with monoclonal antibodies indicates the evolutionary appearance of enamelins prior to amelogenins. Calcif Tissue Int. 1989;45:88–94. doi: 10.1007/BF02561407. [DOI] [PubMed] [Google Scholar]
- Hertwig O. Ueber das Zähnsystem der Amphibien und seine Bedeutung für die Genese des Skelets der Mundhöhle. Eine vergleichend anatomische, entwickelungsgeschichtliche Untersuchung. Arch Mikrosk Anat Entw-Mech. 1874a;11:1–208. [Google Scholar]
- Hertwig O. Ueber Bau und Entwickelung der Placoidschuppen und der Zähne der Selachier. Jena Z Naturw. 1874b;8:221–404. [Google Scholar]
- Hertwig O. Ueber das Hautskelet der Fische. Morphol Jahrb. 1876;2:328–395. [Google Scholar]
- Hertwig O. Ueber das Hautskelet der Fische. 2: Das Hautskelet der Ganoiden (Lepidosteusund Polypterus. Morphol Jahrb. 1879;5:1–21. [Google Scholar]
- Hertwig O. Ueber das Hautskelet der Fische. 3: Daus Hautskelet der Pediculati, der Discoboli, der Gattung Diana, der Centriscidae, einiger Gattungen aus der Familie der Triglidae und der Plectognathen. Morphol Jahrb. 1882;7:1–42. [Google Scholar]
- Hill RV. Integrative morphological data sets for phylogenetic analysis of Amniota: the importance of integumentary characters and increased taxonomic sampling. Syst Biol. 2005;54:530–547. doi: 10.1080/10635150590950326. [DOI] [PubMed] [Google Scholar]
- Homberger DG, Ham K, Ogunbakin T, et al. The structure of the cornified claw sheath in the domesticated cat (Felis catus): implications for the claw-shedding mechanism and the evolution of cornified digital end organs. J Anat. 2009;214:620–643. doi: 10.1111/j.1469-7580.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley I, Mueller R, Dunn K, et al. A new time-scale for ray-finned fish evolution. Proc R Soc B, Biol Sci. 2007;274:489–498. doi: 10.1098/rspb.2006.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysseune A. Skeletal system. In: Ostrander Gk., editor. Microscopic Functional Anatomy. San Diego: Academic Press; 2000. pp. 307–317. [Google Scholar]
- Huysseune A, Sire J-Y. Bone and cartilage resorption in relation to tooth development in the anterior part of the mandible in cichlid fish: a light and TEM study. Anat Rec. 1992;234:1–14. doi: 10.1002/ar.1092340102. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire J-Y. Evolution of patterns and processes in teeth and tooth-related tissues in non-mammalian vertebrates. Eur J Oral Sci. 1998;106(Suppl. 1):437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire J-Y, Witten PE. Evolutionary and developmental origins of the vertebrate dentition. J Anat. 2009;214:465–476. doi: 10.1111/j.1469-7580.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysseune A, Takle H, Soenens M, Taerwe K, Witten PE. Unique and shared gene expression patterns in Atlantic salmon (Salmo salar) tooth development. Dev Genes Evol. 2008;218:427–437. doi: 10.1007/s00427-008-0237-9. [DOI] [PubMed] [Google Scholar]
- Inoue JG, Miya M, Tsukamoto K, et al. Basal actinopterygian relationships: a mitogenomic perspective on the phylogeny of the ‘ancient fish’. Mol Phyl Evol. 2003;26:110–120. doi: 10.1016/s1055-7903(02)00331-7. [DOI] [PubMed] [Google Scholar]
- Janvier P. Nouveau material d’Andreolepis hedeiGross, actinoptérygien énigmatique du Silurien de Gotland (Suède) C R Acad Sci Paris. 1971;273:2223–2224. [Google Scholar]
- Janvier P. La structure de l’exosquelette des Galeaspida (Vertebrata) C R Acad Sci Paris. 1990;310:655–659. [Google Scholar]
- Janvier P. Early Vertebrates. Oxford: Clarendon Press; 1996. [Google Scholar]
- Janvier P. Living primitive fishes and fishes from deep time. In: Mckenzie DJ, Farrell AP, Brauner CJ, editors. Primitive Fishes, Volume 26 Fish Physiology. New York: Elsevier; 2007. pp. 1–51. [Google Scholar]
- Janvier P, Arsenault M. The anatomy of Euphanerops longaevusWoodward, 1900, an anaspid-like jawless vertebrate from the Upper Devonian of Miguasha, Quebec, Canada. Geodiversitas. 2007;29:143–216. [Google Scholar]
- Janvier P, Thanh T-D, Phuong T-H. A new early Devonian galeaspid from Bac Thai Province, Vietnam. Palaeontology. 1993;36:297–309. [Google Scholar]
- Jarvik E. On the Middle Devonian crossopterygians from the Hornelen Field in Western Norway. Årb Univ Bergen. 1949;1948:1–48. [Google Scholar]
- Jessen H. Moythomasia nitidaGross und M. cf. striataGross, Devonische Palaeonisciden aus dem oberen Plattenkalk der Bergisch-Gladbach – Paffrather Mudle (Rheinisches Schiefergebirge) Palaeontogr Abt A. 1968;128:87–114. [Google Scholar]
- Jessen H. Die Bauchschuppen von Moythomasia nitidaGross (Pisces, Actinopterygii) Paläontol Z. 1972;46:121–132. [Google Scholar]
- Junqueira LC, Carneiro J, Kelley RO. Basic Histology. 9th edn. Norwalk, CT: Appleton & Lange; 1998. [Google Scholar]
- Karatajuté-Talimaa V. The early stages of dermal skeleton formation in chondrichthyans. In: Mark-Kurik E, editor. Fossil Fishes as Living Animals. Tallinn: Institute of Geology; 1992. pp. 223–231. [Google Scholar]
- Karatajuté-Talimaa V, Novitskaya L. Sodolepis, a new representative of Mongolepidida (Chondrichthyes?) from the Lower Silurian of Mongolia. Paleontol Zhurn. 1997;1997:96–103. [Google Scholar]
- Karatajuté-Talimaa V, Smith MM. Early acanthodians from the Lower Silurian of Asia. Trans R Soc Edinb (Earth Sci) 2003;93:277–299. [Google Scholar]
- Karatajuté-Talimaa VN, Novitskaya LI. Teslepis, a new mongolepidid elasmobranchian fish from the Lower Silurian of Mongolia. Paleontol Zhurn. 1992;1992:36–47. [Google Scholar]
- Karatajuté-Talimaa VN, Novitskaya LI, Rozman KS, et al. Mongolepis, a new lower Silurian genus of elasmobranchs from Mongolia. Paleontol Zhurn. 1990;1990:76–86. [Google Scholar]
- Kearney M, Rieppel O. An investigation into the occurrence of plicidentin in the teeth of squamate reptiles. Copeia. 2006;3:337–350. [Google Scholar]
- Kemp TS. Fossil Evolution. New York: Oxford University Press; 1999. [Google Scholar]
- Kiaer J. The Downtonian fauna of Norway. I. Anaspida. With a geological introduction. Videnskapsselskap Skrift I Mat-Naturwidenskap Klasse. 1924;6:1–139. [Google Scholar]
- Kresja R. The comparative anatomy of the integumental skeleton. Hyman's Comp Vert Anat. 1979;3:112–191. [Google Scholar]
- Landmann L. The skin of reptiles: epidermis and dermis. In: Bereiter-Hahn J, Matoltsy AG, Sylvia Richards K, editors. Biology of the Integument, Volume 2 Vertebrates. New York: Springer-Verlag; 1986. pp. 150–187. [Google Scholar]
- Le Douarin N, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Leguellec D, Morvan-Dubois G, Sire J-Y. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio. Int J Dev Biol. 2004;48:217–231. doi: 10.1387/ijdb.15272388. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB. Water relations of tetrapod integument. J Exp Biol. 2006;209:202–226. doi: 10.1242/jeb.02007. [DOI] [PubMed] [Google Scholar]
- Maddin HC, Eckart L, Jaeger K, Russell AP, Ghannadan M. Anatomy and development of the claws of Xenopus laevis(Lissamphibia, Anura) reveal alternative pathways of structural evolution in the integument of tetrapods. J Anat. 2009;214:607–619. doi: 10.1111/j.1469-7580.2009.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderson PFA. When? Why? and How? Some speculations on the evolution of the vertebrate Integument. Am Zool. 1972;12:159–171. [Google Scholar]
- Maisey JG. Phylogeny of early vertebrate skeletal induction and ossification patterns. Evol Biol. 1988;22:1–36. [Google Scholar]
- Märss T. Andreolepis(Actinopterygii) in the Upper Silurian of northern Eurasia. Proc Est Acad Sci, Geol. 2001;50:174–189. [Google Scholar]
- Märss T, Turner S, Karatajute-Talimaa V. Handbook of Paleoichthyology, Vol. 1B: ‘Agnatha’ II Thelodonti. Munich: Verlag Dr Friedrich Pfeil; 2007. [Google Scholar]
- Matoltsy AG. Dermis. In: Bereiter-Hahn J, Matoltsy AG, Sylvia Richards K, editors. Biology of the Integument, Vol. 2 – Vertebrates. New York: Springer-Verlag; 1986. pp. 272–277. [Google Scholar]
- Mauger A. The role of somitic mesoderm in the development of dorsal plumage in chick embryos. I. Origin, regulative capacity and determination of the plumage-forming mesoderm. J Embryol Exp Morphol. 1972a;28:313–341. [PubMed] [Google Scholar]
- Mauger A. The role of somitic mesoderm in the development of dorsal plumage in chick embryos. II. Regionalization of the plumage-forming mesoderm. J Embryol Exp Morphol. 1972b;28:343–366. [PubMed] [Google Scholar]
- Meinke DK. A light and scanning electron microscope study on the dermal skeleton of Spermatodus(Pisces: Coelacanthini) and the evolution of the dermal skeleton in coelacanths. J Paleontol. 1982;56:620–630. [Google Scholar]
- Meinke DK. A review of cosmine: its structure, development, and relationship to other forms of the dermal skeleton of osteichthyans. J Vert Paleontol. 1984;4:457–470. [Google Scholar]
- Meinke DK. Morphology and evolution of the dermal skeleton in lungfishes. J Morphol. 1986;(suppl 1):133–149. [Google Scholar]
- Meunier FJ. Les relations isopédine-tissu osseux dans le post-temporal et les écailles de la ligne latérale de Latimeria chalumnae(Smith) Zool Scr. 1980;9:307–317. [Google Scholar]
- Meunier FJ. ‘Twisted plywood’ structure and mineralization in the scales of a primitive living fish, Amia calva. Tissue Cell. 1981;13:165–171. doi: 10.1016/0040-8166(81)90046-x. [DOI] [PubMed] [Google Scholar]
- Meunier FJ. Les tissus osseux des Ostéichthyens Structure, genèse, croissance et évolution. Université Paris 7: Thèse de Doctorat ès Sciences; 1983. [Google Scholar]
- Meunier FJ. Os cellulaire, os acellulaire et tissus dérivés chez les ostéichthyens: les phénomènes de l’acellularisation et de la perte de minéralisation. Année Biol. 1987;26:201–233. [Google Scholar]
- Meunier FJ, Castanet J. Organisation spatiale des fibres de collagène de la plaque basale des écailles des téléostéens. Zool Scr. 1982;12:141–153. [Google Scholar]
- Meunier FJ, François Y, Castanet J. Etude histologique et microradiographique des écailles de quelques actinoptérygiens primitifs actuels. Bull Soc Zool Fr. 1978;103:309–318. [Google Scholar]
- Miyake T, Vaglia JL, Taylor LH, Hall BK. Development of dermal denticles in skates (Chondrichthyes, Batoidea): patterning and cellular differentiation. J Morphol. 1999;241:61–81. doi: 10.1002/(SICI)1097-4687(199907)241:1<61::AID-JMOR4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Moss ML. Studies on the acellular bone of teleost fish. I. Morphological and systematic variations. Acta Anat. 1961;46:343–362. [PubMed] [Google Scholar]
- Moss ML. The phylogeny of mineralized tissues. Int Rev Gen Exp Zool. 1964;1:298–331. [Google Scholar]
- Moss ML. The origin of vertebrate calcified tissues. In: Ørvig T, editor. Current Problems of Lower Vertebrate Phylogeny. Stockholm: Almquist & Wiksell; 1968. pp. 359–371. [Google Scholar]
- Moss ML. Comparative histology of dermal sclerifications in reptiles. Acta Anat. 1969;73:510–533. doi: 10.1159/000143315. [DOI] [PubMed] [Google Scholar]
- Moss ML. The vertebrate dermis and the integumental skeleton. Am Zool. 1972;12:27–34. [Google Scholar]
- Moss-Salentijn L, Moss ML. Studies on dentin. 2. Transient vasodentin in the incisor teeth of a rodent (Perognathus longimembris. Acta Anat (Basel) 1975;91:386–404. [PubMed] [Google Scholar]
- Nanci A. Enamel: Composition, formation and structure. In: Nanci A), editor. Ten Cate's Oral Histology: Development, Structure, and Function. St. Louis: Harcourt Health Sciences; 2003. pp. 145–191. [Google Scholar]
- Nickerson WS. The development of the scales of Lepidosteus. Bull Mus Comp Zool Harv Coll. 1893;24:115–139. [Google Scholar]
- Obruchev DV. Devonian fishes from the Minusinsk Basin [in Russian] Trans Paleontol Inst. 1941;8:23–48. [Google Scholar]
- Olivera-Martinez I, Thélu J, Teillet M, Dhouailly D. Dorsal dermis development depends on a signal from the dorsal neural tube, which can be substituted by Wnt-1. Mech Dev. 2001;100:233–244. doi: 10.1016/s0925-4773(00)00540-2. [DOI] [PubMed] [Google Scholar]
- Olsson R. The skin of Amphioxus. Z Zellforsch. 1961;54:90–104. doi: 10.1007/BF00384200. [DOI] [PubMed] [Google Scholar]
- Ørvig T. Histologic studies of ostracoderms, placoderms and fossil elasmobranchs 1. The endoskeleton, with remarks on the hard tissues of lower vertebrates in general. Ark Zool. 1951;2:321–454. [Google Scholar]
- Ørvig T. Remarks on the vertebrate fauna of the lower upper Devonian of Escuminac Bay, P. Q., Canada, with special reference to the Porolepiform Crossopterygians. Ark Zool. 1957;10:367–427. [Google Scholar]
- Ørvig T. Pycnaspis splendens, new genus, new species, a new ostracoderm from the Upper Ordovician of North America. Proc US Nat Mus. 1958;108:1–23. [Google Scholar]
- Ørvig T. Palaeohistological notes. 2. Certain comments on the phylogenetic significance of acellular bone in early lower vertebrates. Ark Zool. 1965;16:551–556. [Google Scholar]
- Ørvig T. Phylogeny of tooth tissues: evolution of some calcified tissues in early vertebrates. In: Miles AEW, editor. Structural and Chemical Organisation of Teeth. New York: Academic Press; 1967. pp. 45–110. [Google Scholar]
- Ørvig T. The dermal skeleton: General considerations. In: Ørvig T, editor. Current Problems of Lower Vertebrate Phylogeny. Stockholm: Almquist & Wiksell; 1968. pp. 374–397. [Google Scholar]
- Ørvig T. Cosmine and cosmine growth. Lethaia. 1969;2:241–260. [Google Scholar]
- Ørvig T. Description, with special reference to the dermal skeleton, of a new radotinid arthrodire from the Gedinnian of Arctic Canada. Problèmes Actuels de Paléontologie (Évolution des Vertébrés) 1975;218:41–71. [Google Scholar]
- Ørvig T. A survey of odontodes (‘dermal teeth’) from developmental, structural, functional, and phyletic points of view. In: Andrews SM, Miles RS, Walker AD, editors. Problems in Vertebrate Evolution. Linnean Society Symposium Series 4: 1977. pp. 53–75. [Google Scholar]
- Ørvig T. Microstructure and growth of the dermal skeleton in fossil actinopterygian fishes: Birgeriaand Scanilepis. Zool Scr. 1978a;7:33–56. [Google Scholar]
- Ørvig T. Microstructure and growth of the dermal skeleton in fossil actinopterygian fishes: Nephrotusand Coloboduswith remarks on the dentition in other forms. Zool Scr. 1978b;7:297–326. [Google Scholar]
- Ørvig T. Microstructure and growth of the dermal skeleton in fossil actinopterygian fishes: Boreosomus, Plegmolepisand Gyrolepis. Zool Scr. 1978c;7:125–144. [Google Scholar]
- Osborn JW. Dental Anatomy and Embryology. London: Blackwell Scientific Publications; 1981. [Google Scholar]
- Pander CH. Monographie der fossilen Fische des silurischen Systemsder russich-baltischen Gouvernements. St. Petersburg: Buchdruckerei der Kaiserlichen Akademie der Wissenschaften; 1856. [Google Scholar]
- Pearson DM, Westoll TS. The Devonian actinopterygian CheirolepisAgassiz. Trans R Soc Edinb (Earth Sci) 1979;70:337–399. [Google Scholar]
- Peyer B. Comparative Odontology. Chicago: University of Chicago Press; 1968. [Google Scholar]
- Powrie J, Lankester ER. A monograph of fishes of the Old Red Sandstone of Britain. Pt. 1 Cephalaspidae. Palaeontol Soc Monogr. 1868:1–62. –70. [Google Scholar]
- Prostak KS, Skobe Z. Ultrastructure of the dental epithelium and odontoblasts during enameloid matrix deposition in cichlid teeth. J Morphol. 1986;187:159–172. doi: 10.1002/jmor.1051870204. [DOI] [PubMed] [Google Scholar]
- Prostak KS, Skobe Z. Ultrastructure of odontogenic cells during enameloid matrix synthesis in tooth buds from an elasmobranch, Raja erinacea. Am J Anat. 1988;182:59–72. doi: 10.1002/aja.1001820106. [DOI] [PubMed] [Google Scholar]
- Reif W-E. Ontogenese des Hautskelettes von Heterodontus falcifer(Selachii) aus dem Untertithon. Stuttg Beitr Natkd, Ser B (Geol Paläontol) 1973;7:1–16. [Google Scholar]
- Reif W-E. Morphogenese und Musterbildung des Hautzähnchen-Skelettes von Heterodontus. Lethaia. 1974;7:25–42. [Google Scholar]
- Reif W-E. Types of morphogenesis of the dermal skeleton in fossil sharks. Paläontol Z. 1978;52:110–128. [Google Scholar]
- Reif W-E. A model of morphogenetic processes in the dermal skeleton of elasmobranchs. Neues Jahrb Geol Paläontol, Abh. 1980;159:339–359. [Google Scholar]
- Reif WE. Evolution of dermal skeleton and dentition in vertebrates. The odontode regulation theory. Evol Biol. 1982a;15:278–386. [Google Scholar]
- Reif W-E. Morphogenesis and function of the squamation in sharks. Neues Jahrb Geol Paläontol, Abh. 1982b;164:172–183. [Google Scholar]
- Reif W-E. Evolution of the dermal skeleton of vertebrates: concepts and methods. Neues Jahrb Geol Paläontol, Abh. 2002;223:53–78. [Google Scholar]
- Reif W-E, Richter M. Revisiting the lepidomorial and odontode regulation theories of dermo-skeletal morphogenesis. Neues Jahrb Geol Paläontol, Abh. 2001;219:285–304. [Google Scholar]
- Richter M, Smith MM. A microstructural study of the ganoine tissue of selected lower vertebrates. Zool J Linn Soc. 1995;114:173–212. [Google Scholar]
- Ricqlès A, de Meunier FJ, Castanet J, Francillon-Vieillot H. Comparative microstructure of bone. In: Hall BK, editor. Bone, Volume 3 Bone Matrix and Bone Specific Products. Boca Raton, FL: CRC Press; 1991. pp. 1–78. [Google Scholar]
- Ritchie A. The Late Silurian anaspid genus Rhyncholepisfrom Oesel, Estonia, and Ringerike, Norway. Am Mus Novit. 1980;2699:1–18. [Google Scholar]
- Rohon JV. Die Obersilurischen fische von Oesel. Mem Acad Sci St-Petersbourg. 1893;41:124. [Google Scholar]
- Rohon JV. Beiträge zur Anatomie und Histologie der Psammosteiden. Sitz ber königl Böhm Gesellschaftswiss, Math-Naturw. 1901;16:1–31. [Google Scholar]
- Romer AS. Osteology of the Reptiles. Chicago: University of Chicago Press; 1956. [Google Scholar]
- Sansom IJ, Smith MP, Armstrong HA, et al. Presence of the earliest vertebrate hard tissues in conodonts. Science. 1992;256:1308–1311. doi: 10.1126/science.1598573. [DOI] [PubMed] [Google Scholar]
- Sansom IJ, Smith MP, Smith MM. Scales of thelodont and shark-like fishes from the Ordovician. Nature. 1996;379:628–630. [Google Scholar]
- Sansom IJ, Aldridge RJ, Smith MM. A microvertebrate fauna from the Llandovery of South China. Trans R Soc Edinb (Earth Sci) 2000;90:255–272. [Google Scholar]
- Sansom IJ, Donoghue PCJ, Albanesi GL. Histology and affinity of the earliest armoured vertebrate. Biol Lett. 2005;2:446–449. doi: 10.1098/rsbl.2005.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa I. The fine structure of initital mineralization during tooth development in the gummy shark, Mustelus manazo, Elasmobranchia. J Anat. 1989;164:175–187. [PMC free article] [PubMed] [Google Scholar]
- Sasagawa I. Fine structural and cytochemical observations of dental epithelial cells during the enameloid formation stages in red stingrays Dasyatis akajei. J Morphol. 2002;252:170–182. doi: 10.1002/jmor.1097. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Keil A. Polarizing Microscopy of Dental Tissues. Oxford: Pergamon Press; 1971. [Google Scholar]
- Schultze H-P. Morphologische und histologische Untersuchungen an Schuppen mesozoischer Actinopterygier (Übergang von Ganoid- zu Rundschuppen) Neues Jahrb Geol Paläontol Abh. 1966;126:232–314. [Google Scholar]
- Schultze H-P. Palaeoniscoidea-Schuppen aus dem Unterdevon <au<straliens und Kanadas ind aus dem Mitteldevon Spitzbergens. Bull Br Mus Geol. 1968;16:343–368. [Google Scholar]
- Schultze H-P. Ausgangsform und Entwicklung der rhombischen Schuppen der Osteichthyes (Pisces) Paläontol Z. 1977;51:152–168. [Google Scholar]
- Schultze H-P. The scales of Mesozoic actinopterygians. In: Arratia G, Viohl G, editors. Mesozoic Fishes – Systematics and Paleoecology. Munich: Verlag Dr. F. Pfeil; 1996. pp. 83–93. [Google Scholar]
- Schultze H-P, Cumbaa SL. Dialipinaand the characters of basal actinopterygians. In: Ahlberg PE, editor. Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development. London: Taylor & Francis; 2001. pp. 315–332. [Google Scholar]
- Schultze H-P, Märss T. Revisting Lophosteus, a primitive osteicthyan. Acta Univ Latv. 2004;679:57–78. [Google Scholar]
- Sewertzoff AN. Die Entwicklungen der Knocherschuppen von Polypterus delhesi. Jena Z Naturw. 1932;67:387–418. [Google Scholar]
- Shu D-G, Luo H-L, Conway Morris S, et al. Lower Cambrian vertebrates from south China. Nature. 1999;402:42–46. [Google Scholar]
- Shu D-G, Conway Morris S, Han J, et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature. 2003;421:526–529. doi: 10.1038/nature01264. [DOI] [PubMed] [Google Scholar]
- Sire J-Y. Fibres d’ancrage et couche limitante externe à la surface des écailles du Cichlidae Hemichromis bimaculatus(Téléostéen, Perciforme): données ultrastructurales. Ann Sci Nat, Zool, Paris. 1985;13:163–180. [Google Scholar]
- Sire J-Y. Evidence that mineralized spherules are involved in the formataion of the superficial layer of the elasmoid scale in the cichlids Hemichromis bimaculatusand Cichlasoma octofasciatum(Pisces, Teleostei): an epidermal active participation? Cell Tissue Res. 1988;253:165–171. doi: 10.1007/BF00221751. [DOI] [PubMed] [Google Scholar]
- Sire J-Y. The scales in young Polypterus senegalusare elasmoid: new phylogenetic implications. Am J Anat. 1989;186:315–323. doi: 10.1002/aja.1001860308. [DOI] [PubMed] [Google Scholar]
- Sire J-Y. From ganoid to elasmoid scales in the actinopterygian fishes. Neth J Zool. 1990;40:75–92. [Google Scholar]
- Sire J-Y. Development and fine structure of the bony scutes in Corydoras arcuatus(Siluriformes, Callichthyidae) J Morphol. 1993;215:225–244. doi: 10.1002/jmor.1052150305. [DOI] [PubMed] [Google Scholar]
- Sire J-Y. A light and TEM study of non-regenerated and experimentally regenerated scales of Lepisosteus oculatus(Holostei) with particular attention to ganoine formation. Anat Rec. 1994;240:189–207. doi: 10.1002/ar.1092400206. [DOI] [PubMed] [Google Scholar]
- Sire J-Y. Ganoine formation in the scales of primitive actinopterygian fishes, lepisosteids and polypterids. Connect Tissue Res. 1995;32:535–544. doi: 10.3109/03008209509017006. [DOI] [PubMed] [Google Scholar]
- Sire J-Y. Teeth outside the mouth in teleost fish. How to benefit from a developmental accident. Evol Dev. 2001;3:104–108. doi: 10.1046/j.1525-142x.2001.003002104.x. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Akimenko MA. Scale development in fish: A review, with description of sonic hedgehog expression in the zebrafish (Danio rerio. Int J Dev Biol. 2004;48:233–247. [PubMed] [Google Scholar]
- Sire J-Y, Allizard F. A fourth teleost lineage possessing extra-oral teeth: the genus Atherion(Teleostei; Atheriniformes) Eur J Morphol. 2001;39:295–305. doi: 10.1076/ejom.39.5.295.7375. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Huysseune A. Fine structure of the developing frontal bones and scales of the cranial vault in the cichlid fish Hemichromis bimaculatus(Teleostei, Perciformes) Cell Tissue Res. 1993;273:511–524. [Google Scholar]
- Sire J-Y, Huysseune A. Structure and development of the odontodes in an armoured catfish, Corydoras aeneus(Callichthyidae) Acta Zool (Stockh. 1996;77:51–72. [Google Scholar]
- Sire J-Y, Huysseune A. Formation of dermal skeletal and dental tissues in fish: a comparative and evolutionary approach. Biol Rev. 2003;78:219–249. doi: 10.1017/s1464793102006073. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Meunier FJ. Ornementation superficielle et structure des plaques osseuses dermiques de quelques Siluriformes cuirassés (Loricariidae, Callichthyidae, Doradidae) Ann Sci Nat Zool. 1993;14:101–123. [Google Scholar]
- Sire J-Y, Meunier FJ. The canaliculi of Williamson in holostean bone (Osteichthyes, Actinopterygii): A structural and ultrastructural study. Acta Zool (Stockh) 1994;75:235–247. [Google Scholar]
- Sire J-Y, Géraudie J, Meunier FJ, Zylberberg L. On the origin of ganoine: histological and ultrastructural data on the experimental regeneration of the scales of Calamoichthys calabaricus(Osteichthyes, Brachyopterygii, Polypteridae) Am J Anat. 1987;180:391–402. doi: 10.1002/aja.1001800409. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Quilhac A, Akimenko M-A. Spatial and temporal expression of sonic hedgehog during scale development and regeneration in zebrafish. J Morphol. 1997;232:324. [Google Scholar]
- Sire J-Y, Marin S, Allizard F. A comparison of teeth and dermal denticles (odontodes) in the teleost Denticeps clupeoides(Clupeomorpha) J Morphol. 1998;237:237–256. doi: 10.1002/(SICI)1097-4687(199809)237:3<237::AID-JMOR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Davit-Béal T, Delgado S, Van der Heyden C, Huysseune A. The first generation teeth in non-mammalian lineages: evidence for a conserved ancestral character? Microsc Res Techn. 2002;59:408–434. doi: 10.1002/jemt.10220. [DOI] [PubMed] [Google Scholar]
- Smith MM, Hall BK. Developmental and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev. 1990;65:277–374. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Smith MM, Hall BK. A developmental model for evolution ofthe vertebrate exoskeleton and teeth: role of cranial and trunk neural crest. Evol Biol. 1993;27:387–448. [Google Scholar]
- Smith MM, Sansom IJ. Evolutionary origins of dentine in the fossil record of early vertebrates: diversity, development and function. In: Teaford MF, Smith MM, Ferguson MWJ, editors. Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press; 2000. pp. 65–81. [Google Scholar]
- Smith MM, Sansom IJ, Smith MP. Diversity of the dermal skeleton in Ordovician to Silurian vertebrate taxa from North America: histology, skeletogenesis and relationships. Geobios. 1995;19:65–70. [Google Scholar]
- Spearman RIC. The Integument: A Textbook of Skin Biology. London: Cambridge University Press; 1973. [Google Scholar]
- Stensiö EA. The Cephalaspids of Great Britain. London: British Museum (Natural History); 1932. [Google Scholar]
- Stensiö EA. Les Cyclostomes fossiles ou Ostracodermes. In: Grassé P-P, editor. Traité de Zoologie. Paris: Masson; 1958. pp. 173–425. [Google Scholar]
- Stensiö EA. Permian vertebrates. In: Raasch GO, editor. Geology of the Arctic. Toronto: University of Toronto; 1961. pp. 231–247. [Google Scholar]
- Stensiö EA. Origine et nature des écailles placoïdes et des dents. Problèmes Actuels de Paléontologie (Évolution des Vertébrés) 1962;104:75–85. [Google Scholar]
- Stensiö EA. Les cyclostomes fossiles ou ostracodermes. In: Piveteau J, editor. Traité de Paléontologie. Paris: Masson & Cie; 1964. pp. 96–382. [Google Scholar]
- Sweet WC, Donoghue PCJ. Conodonts: past, present and future. J Paleontol. 2001;75:1174–1184. [Google Scholar]
- Thomson KS. On the biology of cosmine. Bull Peabody Mus Nat Hist. 1975;40:1–59. [Google Scholar]
- Thomson KS. On the individual history of cosmine and a possible electroreceptive function of the pore-canal system in fossil fishes. In. In: Andrews SM, Miles RS, Walker AD, editors. Problems in vertebrate evolution. London: Academic Press; 1977. pp. 247–271. [Google Scholar]
- Turner S. Monophyly and interrelationships of the Thelodonti. In: Chang M-M, Liu Y-H, Zhang G-R, editors. Early Vertebrates and Related Problems of Evolutionary Biology. Beijing: Science Press; 1991. pp. 87–119. [Google Scholar]
- Turner S, Van der Brugghen W. The Thelodonti, an important but enigmatic group of Palaeozoic fishes. Mod Geol. 1993;18:125–140. [Google Scholar]
- Vickaryous M, Sire J-Y. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441–464. doi: 10.1111/j.1469-7580.2008.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickaryous MK, Hall BK. Osteoderm morphology and development in the nine-banded armadillo, Dasypus novemcinctus(Mammalia, Xenarthra, Cingulata) J Morphol. 2006;267:1273–1283. doi: 10.1002/jmor.10475. [DOI] [PubMed] [Google Scholar]
- Wang N-Z, Donoghue PCJ, Smith MM, Sansom IJ. Histology of the galeaspid dermoskeleton and endoskeleton, and the origin and early evolution of the vertebrate cranial endoskeleton. J Vert Paleontol. 2005;25:745–756. [Google Scholar]
- Wängsjö G. On the genus DartmuthiaPatten, with special reference to the minute structure of the exoskeleton. Upps Univ Bull Geol. 1946;31:349–362. [Google Scholar]
- Westoll TS. Radotinaand other tesserate fishes. Zool J Linn Soc. 1967;47:83–98. [Google Scholar]
- White EI. Form and growth in Belgicaspis(Heterostraci) Palaeontogr Abt A. 1973;143:11–24. [Google Scholar]
- White EI, Toombs HA. The cephalaspids from the Dittonian section at Cwm Mill, near Abergavenny, Gwent. Bull Brit Mus (Nat Hist), Geol. 1983;37:149–171. [Google Scholar]
- Whitear M. The skin of fishes including cyclostomes. In: Bereiter-Hahn J, Matoltsy AG, Sylvia Richards K, editors. Biology of the Integument, Volume 2 Vertebrates. Berlin: Springer-Verlag; 1986. pp. 8–64. [Google Scholar]
- Williamson WC. On the microscopic structure of the scales and dermal teeth of some ganoid and placoid fish. Philos Trans R Soc Lond. 1849;139:435–475. [Google Scholar]
- Williamson WC. Investigations into the structure and development of the scales and bones of fishes. Philos Trans R Soc Lond. 1851;141:643–702. [Google Scholar]
- Witmer LM. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason JJ, editor. Functional Morphology in Vertebrate Paleontology. New York: Cambridge University Press; 1995. pp. 19–33. [Google Scholar]
- Young GC. Reconstruction of the jaws and braincase in the Devonian placoderm fish Bothriolepis. Palaeontology. 1984;27:635–661. [Google Scholar]
- Young GC. Did placoderm fish have teeth? J Vert Paleontol. 2003;23:987–990. [Google Scholar]
- Yu X. A new porolepiform-like fish, Psarolepis romerigen. and sp. nov. (Sarcopterygii, Osteichthyes) from the Lower Devonian of Yunnan, China. J Vert Paleontol. 1998;18:261–274. [Google Scholar]
- Zangerl R. The morphology and the developmental history of the scales of the Paleozoic sharks Holmesella? sp. and Orodus. In: Ørvig T, editor. Current Problems of Lower Vertebrate Phylogeny. Stockholm: Almquist & Wiksell; 1968. pp. 399–412. [Google Scholar]
- Zhu M, Janvier P. The histological structure of the endoskeleton in galeaspids (Galeaspida, Vertebrata) J Vert Paleontol. 1998;18:650–654. [Google Scholar]
- Zhu M, Yu X. A primitive fish close to the common ancestor of tetrapods and lungfish. Nature. 2002;418:767–770. doi: 10.1038/nature00871. [DOI] [PubMed] [Google Scholar]
- Zhu M, Yu X, Ahlberg PE. A primitive sarcopterygian fish with an eyestalk. Nature. 2001;410:81–84. doi: 10.1038/35065078. [DOI] [PubMed] [Google Scholar]
- Zhu M, Yu X, Janvier P. A primitive fossil fish sheds light on the origin of bony fishes. Nature. 1999;397:607–610. [Google Scholar]
- Zhu M, Yu X, Wang W, Zhao W, Jia L. A primitive fish provides key characters bearing on deep osteichthyan phylogeny. Nature. 2006;441:77–80. doi: 10.1038/nature04563. [DOI] [PubMed] [Google Scholar]
- Zylberberg L, Géraudie J, Meunier F, Sire J-Y. Biomineralization in the integumental skeleton of the living lower vertebrates. In: Hall BK, editor. Bone, Vol. 4: Bone Metabolism and Mineralization. Boca Raton, FL: CRC Press; 1992. pp. 171–224. [Google Scholar]
- Zylberberg L, Sire J, Nanci A. Immunodetection of amelogenin-like proteins in the ganoine of experimentally regenerating scales of Calamoichthys calabaricus, a primitive actinopterygian fish. Anat Rec. 1997;249:86–95. doi: 10.1002/(SICI)1097-0185(199709)249:1<86::AID-AR11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]