Abstract
According to the classical theory, teeth derive from odontodes that invaded the oral cavity in conjunction with the origin of jaws (the ‘outside in’ theory). A recent alternative hypothesis suggests that teeth evolved prior to the origin of jaws as endodermal derivatives (the ‘inside out’ hypothesis). We compare the two theories in the light of current data and propose a third scenario, a revised ‘outside in’ hypothesis. We suggest that teeth may have arisen before the origin of jaws, as a result of competent, odontode-forming ectoderm invading the oropharyngeal cavity through the mouth as well as through the gill slits, interacting with neural crest-derived mesenchyme. This hypothesis revives the homology between skin denticles (odontodes) and teeth. Our hypothesis is based on (1) the assumption that endoderm alone, together with neural crest, cannot form teeth; (2) the observation that pharyngeal teeth are present only in species known to possess gill slits, and disappear from the pharyngeal region in early tetrapods concomitant with the closure of gill slits, and (3) the observation that the dental lamina (sensu Reif, 1982) is not a prerequisite for teeth to form. We next discuss the progress that has been made to understand the spatially restricted loss of teeth from certain arches, and the many questions that remain regarding the ontogenetic loss of teeth in specific taxa. The recent advances that have been made in our knowledge on the molecular control of tooth formation in non-mammalians (mostly in some teleost model species) will undoubtedly contribute to answering these questions in the coming years.
Keywords: dentition, development, evolution, odontodes, teeth, vertebrates
Introduction
Teeth are elements of the dermal skeleton present in a wide range of, typically, jawed vertebrates (Reif, 1982; Smith & Hall, 1990; Donoghue & Sansom, 2002). Owing to their excellent preservation in the fossil record, the important evolutionary information they contain and their paradigmal status in developmental research, many paleontological and neontological disciplines focus on teeth. Therefore it is not surprising that a number of excellent textbooks and review articles have been devoted to the development and evolution of the vertebrate dentition. Hallmarks amongst these are Owen's (1845) and Peyer's (1968) seminal works, and the volumes edited by Miles (1967) and Teaford et al. (2000). Excellent reviews have been published in recent years on the development and evolution of the dentition in some selected lineages [see, for example, Stock, 2007 on the dentition of zebrafish (Danio rerio) and its relatives; Davit-Béal et al. 2007 on the amphibian dentition; and Thenius, 1989 on mammalian dentitions]. However, large gaps still persist in the literature regarding the development and evolution of actinopterygian and squamate dentitions.
Over the last 10 years, and since our previous review on the subject (Huysseune & Sire, 1998), new ideas have been advanced and intriguing new data have been gathered on the evolutionary history of the dentition. The focus of this paper is the evolutionary origin of teeth and the evolutionary modifications in the distribution of teeth, with emphasis on non-mammalian dentitions (developmental aspects of the mammalian dentition being dealt with by Catón & Tucker, 2009).
Theories on the evolutionary origin of teeth
The ‘outside in’ theory
Where and when did teeth arise? According to the classical theory, teeth are derived from skin odontodes (dermal denticles) that came to reside within the oral cavity when competent odontode-forming cells invaded the latter in conjunction with the origin of jaws (e.g. Ørvig, 1967; Peyer, 1968; Kemp, 1999), or even after the mandibular arch evolved (Reif, 1982). Ideas about the homology of odontodes and teeth are based on paleontological evidence, the structural similarities of teeth and odontodes, and on the shared developmental pathways of these elements (Huysseune & Sire, 1998). Both teeth and odontodes are composed of a dentin cone with a base of acellular or cellular bone-like tissue. In most cases the dentin is covered by a hypermineralized layer consisting of enamel or enameloid (Reif, 1982). Both structures have a pulp cavity that contains odontoblasts, connective tissue, nerve fibres and blood vessels. Extant chondrichthyans (sharks and rays) have retained odontodes, called placoid scales (Reif, 1982). Accordingly, shark teeth and shark placoid scales often serve as a textbook example illustrating the homology between these two elements (Huysseune & Sire, 1998; Hall & Witten, 2007).
The ‘inside out’ hypothesis
A recent, alternative hypothesis suggests that teeth evolved prior to the origin of jaws, with oral teeth being co-opted from endodermally derived pharyngeal denticles (the ‘inside out’ hypothesis, to distinguish it from the above described classical, ‘outside in’ theory). This hypothesis, with M.M. Smith and M. Coates as major proponents (Smith & Coates, 1998, 2000, 2001; Smith, 2003; Johanson & Smith, 2005), is based on both a reinterpretation of the fossil record and on embryological studies of extant species. First, Conodonta were accepted by Smith and co-workers to represent the first vertebrate group to show skeletal mineralisation, and conodont elements were seen as the earliest expression of oropharyngeal denticles (Smith & Coates, 1998). Second, the discovery of pharyngeal denticles, which showed a particular spiral arrangement, called tooth whorls, in some thelodonts, agnathans (= jawless vertebrates) found in 425 million year (Ma) old Silurian deposits (Van der Brugghen & Janvier, 1993), was seen as important evidence that teeth were present in the pharynx prior to the establishment of jaws. In the view of Smith & Coates (2001), the arrangement of these whorl-like sets of pharyngeal denticles suggests the presence of a dental lamina, considered by Reif (1982) to be the quintessential character of teeth and the only feature that could distinguish teeth from odontodes. Smith & Coates (2001) proposed that these pharyngeal denticle whorl sets presaged the polarised denticle whorl-like sets spaced around the jaw margin in primitive fossil and extant gnathostomes. Further support for their hypothesis was seen in the idea that the splanchnocranium (to which pharyngeal denticles are attached) should be considered different in origin from the integumentary skeleton (and skin denticles) (cf. Donoghue & Sansom, 2002). It is worth noting however, that in addition to pharyngeal denticles, thelodonts also possess an integumentary skeleton characterized by numerous, minute odontodes similar to chondrichthyan odontodes (Janvier, 1996; Sire et al. 2009).
Additional arguments for the ‘inside out’ hypothesis were also drawn from neontological research. The genetic independence of tooth and jaw (and other dentigerous bone) development, required to support the independent origin of the dentition from skin denticles, is supported by many functional studies, mostly in mice (reviewed by McCollum & Sharpe, 2001). Amongst teleosts, the so-called ‘flathead’ mutants of the zebrafish (Danio rerio), a widely used model species, reveal that teeth can develop in the absence of the underlying branchial arch cartilage (Schilling et al. 1996). Essential for the ‘inside out’ hypothesis is the notion that pharyngeal denticles develop in, and are patterned by, endoderm (in conjunction with odontogenic, neural crest-derived mesenchyme). In zebrafish, the presence and position of teeth on the last branchial arch has led to the widespread acceptance that pharyngeal teeth develop from endodermal epithelium. This was seen as an argument in support of the ‘inside out’ hypothesis.
The evolutionary origin of jaw teeth from prepatterned, endodermally derived pharyngeal denticles, as suggested by Smith & Coates (1998, 2000, 2001) and Smith & Johanson (2003a), has in turn been questioned by various authors on the basis of paleontological data (Purnell, 2001; Miller et al. 2003; Burrow, 2003; Young, 2003). These arguments have reciprocally been addressed by Smith & Johanson (2003b) and by Johanson & Smith (2005). In return, Hall (2005), and Reif (2002, 2006) have again argued in favour of the classical ‘outside in’ theory. Clearly, the debate is ongoing.
A modified ‘outside in’ hypothesis
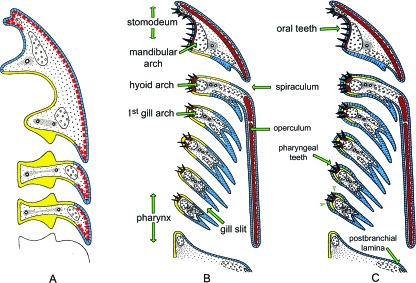
Here, we propose an alternative hypothesis that integrates both paleontological and embryological data, and is consistent with the above described findings. We remove, however, the conodonts from the discussion given that only the Euconodonta are currently recognized as vertebrates, yet that there is still no consensus about the structural homology of their denticles with any kind of vertebrate odontode (Scott, 1934; Morris, 1980; Pridmore et al. 1996; Kemp, 2002a,b; Zhuravlev, 2005; Reif, 2006; Trotter et al. 2007; Janvier, 2007). In accordance with the classic ‘outside in’ theory, we hypothesize that teeth are derived from odontodes, which were originally ectodermal in origin. These ectodermal odontodes developed inside the oropharyngeal cavity as a result of competent ectoderm migrating inwards – not only via the mouth, but also via each of the gill slits (Fig. 1). Our hypothesis agrees with the ‘inside out’ hypothesis in that we acknowledge that teeth likely arose prior to the origin of jaws [but the way jaws originated is itself seriously debated, as excellently summarized by Janvier, 2007]. The odontogenic potential of the ectodermal epithelium may have been subsequently transferred to the endoderm, provided there was an intimate contact between these two germ layers, such as where mouth and gill slits form. Consequently, provided pharyngeal denticles or teeth indeed develop from endodermal epithelium, the adjacent presence or even physical contact of ectoderm with endodermal epithelium would have been a requirement for their development. Yet, unequivocal evidence for an endodermal origin of pharyngeal teeth or denticles in extant primitive gnathostomes, and by inference in extinct thelodonts, still needs to be collected. Although widely accepted based on position in the endodermal lining of the pharynx (see, for example, Piotrowski & Nüsslein-Volhard, 2000; Yelick & Shilling, 2002), unequivocal evidence supporting the endodermal origin of pharyngeal teeth remains wanting as universal, reliable markers of endoderm are at present unavailable. Using clonal analysis, it has been shown that in the zebrafish the pharyngeal epithelium is only partially derived from endoderm (Warga & Nüsslein-Volhard, 1999). To date, strong evidence for endodermal participation in tooth formation has been collected only for urodele amphibians. However, careful examination of the reports of the many experimental studies that have been carried out on salamanders in the last century, indicates that ectoderm and endoderm are both required to form teeth (e.g. Ströer, 1933; Sellman, 1946; Wilde, 1955), or, if teeth develop from supposedly endodermally derived enamel organs, the ectoderm is required (Sellman, 1946) or at the very least it was not removed (e.g. Adams, 1924; de Beer, 1947; Barlow & Northcutt, 1995). Only Cassin & Capuron (1979) report teeth forming in grafts with neural crest and endoderm alone. Working with Pleurodeles waltl, Chibon (1966, 1970), on the other hand, noted that the odontoblasts exert an inductive action upon the epithelium, which can be an ectodermal epithelium (in anterior teeth), an endodermal epithelium (in posterior teeth), or one of mixed origin. A recent study on the axolotl (Ambystoma mexicanum) using GFP transgenes has confirmed Chibon's hypothesis that the epithelial component of the teeth can be both ectodermal and endodermal (Soukup et al. 2008). Nevertheless, the experiments of Soukup et al. (2008) do not represent evidence against the necessity of a close proximity between ectoderm and endoderm. In these experiments, it remains to be clarified whether the participation of endoderm in the enamel organs is not just an expression of a potential not normally displayed during regular development, rather than the normal in vivo capacity. Other not normally odontogenic tissues have indeed been shown to be able to express an odontogenic potential. For example, using the same species (A. mexicanum), Graveson et al. (1997) demonstrated that caudal regions of neural crest cells, not typically involved in tooth formation during normal development, can produce teeth under the appropriate in vitro conditions. Graveson et al. (op. cit.) attributed this unexpressed potential to an apparent lack of exposure of these neural crest cells to the appropriate inductive tissues, i.e. stomodeal ectoderm and oral endoderm (Sellman, 1946; Wilde, 1955; Graveson, 1993). Graveson's experiments were nevertheless not a test of whether teeth form from endodermal or ectodermal epithelium. Among mammals, Imai et al. (1998) used an endodermal cell-tracing system with a recombinant adenovirus, but could only demonstrate that tooth germs in the rat form in ectoderm adjacent to labelled endodermal cells (foregut endoderm); they did not find evidence for an active role of the endoderm itself. Returning to zebrafish teeth, it is rather confusing that, on the one hand, Smith & Coates (1998) use the argument of zebrafish mutants to show that teeth can develop independently from the underlying splanchnoskeleton, and, on the other hand, link teeth and splanchnoskeleton in emphasizing the need for endoderm to pattern both. Johanson & Smith (2005, p. 339) express this as ‘this regulatory mechanism from endoderm can also be utilised for denticles and teeth as part of the splanchnocranial skeleton’.
Fig. 1.
Comparison of the arrangement of the gill slits, the position of the branchial arches and of the gills in agnathans (A) and gnathostomes (B,C). Odontodes and dermal bones are indicated in red. (A,B) Extent of ectoderm (blue) and endoderm (yellow) as usually assumed. In (C) we postulate (contra B) that the ectoderm penetrates further inwards (arrowheads), and possibly covers the endoderm, as observed by Edwards (1929) (cf. Fig. 3) (modified after Jollie, 1968).
The key issue of our revised hypothesis is the assumption that odontogenic competent ectoderm invaded the oropharyngeal cavity through both the mouth and the gill slits. Even if odontogenic competence would have been transferred from ectoderm to endoderm (i.e. the tooth-forming capacity would have come to reside in the endoderm – an assumption that still needs to be tested in representative species of basal actinopterygian lineages, e.g. polypterids, the bichirs), tooth-forming endoderm would still require a close proximity, perhaps even physical contact, with the ectoderm.
Paleontological data can be reconciled with this view as long as it can be shown that the taxa known to possess pharyngeal denticles possessed gill slits, or other structures permitting ectoderm to penetrate into the body, such as the nasopharyngeal duct (including Rathke's pouch) or the spiraculum (corresponding to the opening between mandibular and hyoid arch) (cf. Bjerring, 1977).The denticles in the thelodont Loganellia extend anteriorly onto the wall of the large median nasopharyngeal duct (Van der Brugghen & Janvier, 1993). One may thus assume that they could develop because of an ectodermal contribution through this duct. Most osteostracans have slit-shaped external gill openings, the condition in tremataspidids (with small rounded openings) being derived; extant agnathans (hagfish and lampreys) and most extinct agnathans (anaspids, astrapidids and galeaspids) have small rounded-shaped external gill openings (Janvier, 2007, p. 92). However, even the possession of reiterative slits is not a requirement, as physical contact between ectoderm and endoderm, without the formation of an open gill slit proper, could be sufficient for interactions to occur between the two tissue layers. In the zebrafish, the first tooth anlage appears after a connection has been established between ectoderm and endoderm in the form of an elongate, initially two-cell-thick strand bridging the skin with the pharynx on both sides, well before the actual formation of the gill slit within this strand (Fig. 2). Interestingly, Jackman et al. (2004) found that treatment of 32–78-h zebrafish embryos with SU5402 (a lipophilic reagent, which inhibits signaling through inactivation of FGF receptors) affected both early dental epithelial morphogenesis and 6th pharyngeal pouch morphology. In our view, the altered tooth morphogenesis likely results from an altered signaling from the pouch epithelium. In 1929, Edwards performed a detailed embryological study of mouth and pharynx formation in the carp (Cyprinus carpio), a family member of the zebrafish that also forms pharyngeal teeth. By careful analysis of histological sections, he concluded that in the tooth-forming region, the pharyngeal epithelium was composed of a superficial layer of flattened cells, derived from migration of ectoderm, overlying a layer of endodermal epithelium. Edwards (1929) also reported that the enamel organs were derived from the deep (endodermal) layer, but clearly illustrated the close contact between ectodermal and endodermal layers (Fig. 3).
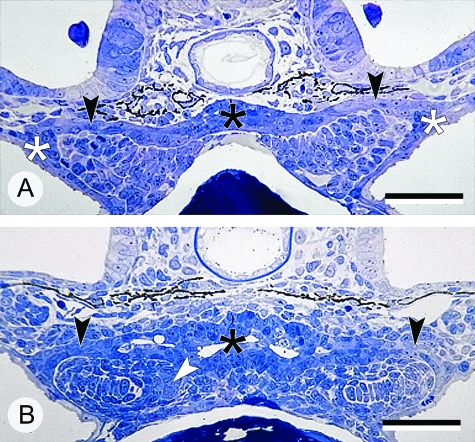
Fig. 2.
Semithin sections of forming pharyngeal pouches in zebrafish (Danio rerio) at 56 h (A) and 72 h (B) post-fertilization. (A) An epithelial connection (black arrowheads) is seen between the epidermis (white asterisks) and the foregut (black asterisk). (B) The first tooth germs (white arrowhead) are forming whilst the gill slits are still closed (black arrowheads), and the pharyngeal lumen opens (black asterisk). Scale bars = 40 µm.
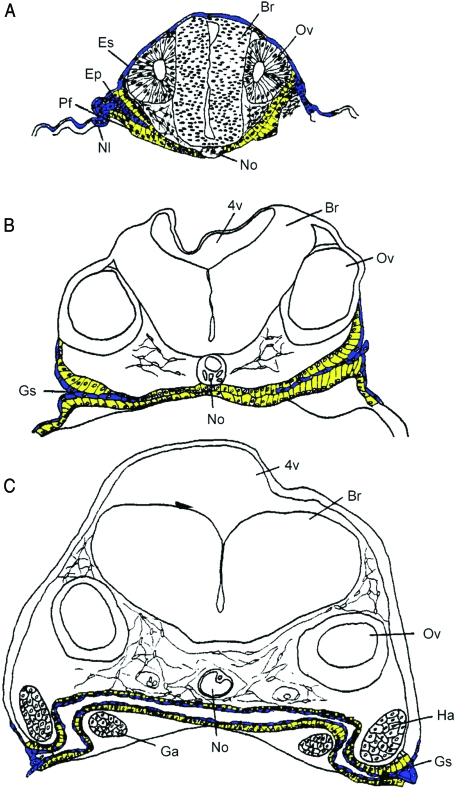
Fig. 3.
Three stages in the development of the gill slits and pharynx of the carp (Cyprinus carpio). (A–C) respectively 36, 56 and 78 h post-fertilization. An ectodermal plug (blue) invaginates the endodermal pharyngeal folds (yellow) (A) and forms a layer of flattened (ectoderm-derived) cells (blue) on top of the columnar (endoderm-derived) epithelial cells (yellow) (B,C). The latter produce the enamel organs of the teeth. 4v, fourth ventricle; Br, brain; Ep, proliferating ectodermal plug; Es, epidermal stratum; Ga, gill arch; Gs, gill slit; Ha, hyoid arch; Nl, inner layer of ectoderm; No, notochord; Ov, otic vesicle; Pf, pharyngeal fold (modified after Edwards, 1929).
According to Smith & Coates (1998), differences in patterning and regulation of pharyngeal denticles (when compared to odontodes), and the putative presence of a dental lamina, provide supporting evidence for their ‘inside out’ hypothesis. Upon closer investigation, we view these interpretations as doubtful based on the following: (1) Teeth (pharyngeal or associated to other parts of the visceral skeleton) often display a highly unordered pattern in extant species [e.g. on the pharyngeal jaws of cichlids (Huysseune, 1995), the oral jaws of gadids (Holmbakken & Fosse, 1973), or during certain life stages of the fish, such as the post-spawning period in wild Atlantic salmon (Witten et al. 2005)]. Also, whereas differential gene expression patterns in oral vs. pharyngeal teeth in rainbow trout have been used to support the suggestion that oral teeth evolved by co-option from pharyngeal teeth (Fraser et al. 2004), gene expression data from medaka (Oryzias latipes) support the notion of serial homology between oral and pharyngeal teeth (Debiais-Thibaud et al. 2007). In our view there is no proof for germ layer-related fundamental developmental differences between teeth and odontodes. (2) Teeth can form without a dental lamina (Fig. 4A). There are many examples of jaw teeth forming without passing through a dental lamina stage (see, for example, Donoghue & Aldridge, 2001, for such examples). In addition, first-generation teeth, widely considered to be representative of an ancestral type of teeth, develop from the superficial epithelium in most non-mammalian osteichthyans examined so far (Sire et al. 2002). Furthermore, not even a discontinuous and non-permanent dental lamina (sensu Reif, 1982) is required for tooth replacement, as demonstrated for salmon by Huysseune & Witten (2008) (compare Fig. 4A with 4B–F). (3) Short whorl-like arrangements of teeth can be produced without a dental lamina. In zebrafish, the first tooth generations in position 4V are co-functional (Van der heyden & Huysseune, 2000). These teeth form a series with a whorl-like arrangement due only to space constraints. A successional lamina is virtually non-existent in these early tooth generations; it only becomes prominent at older developmental stages (Huysseune, 2006). Finally, one should also consider the possibility that patterning of pharyngeal denticles into families (as exemplified in thelodonts) may well be the outcome of a heterochronic shift of patterning of the crowns of skin denticles. Merging of pharyngeal denticles into a single unit would yield a single crown with a herring bone-like morphology comparable to that of skin denticles. It is worth noting that the earliest chondrichthyan teeth are of ‘diplodont’ type (i.e. having a base bearing two large divergent cusps, and one or two smaller cusps in-between) (Janvier, 1996, p. 149). Interestingly, this tooth morphology is similar to that of most pharyngeal denticles in basal chondrichthyans and osteichthyans.
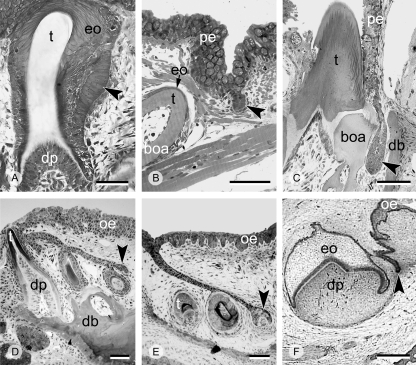
Fig. 4.
Different types of dental lamina (black arrowheads) during tooth replacement. Replacement tooth formation without the presence of a dental lamina (A, Atlantic salmon, Salmo salar); with a transient, successional dental lamina (B, zebrafish, Danio rerio, and C, jewel cichlid, Hemichromis bimaculatus), or with a permanent dental lamina (D, Pleurodeles waltl, a urodele amphibian; E, Chalcides sexlineatus, a scincid lizard; F, human first lower deciduous molar). boa, bone of attachment; db, dentigerous bone; dp, dental papilla; eo, enamel organ; oe, oral epithelium; pe, pharyngeal epithelium; t, tooth. Scale bars (A–E) = 50 µm, (F) = 500 µm. (F), courtesy of Ralf J. Radlanski.
The presence of teeth in the pharyngeal cavity could thus have been an early event in vertebrate evolution whereby ectoderm invaded through the gill slits, possibly interacting with endoderm, thereby being involved in pharyngeal denticle (teeth) development. One obvious question to ask is why pharyngeal teeth did not evolve more often? Denticles cover the median part of the oral roof in some osteostracans, which, together with thelodonts, are the only agnathans with oral or pharyngeal denticles (Janvier, 2007). The relative rarity of pharyngeal denticles in agnathans may be related to differences in the distribution of ectoderm and endoderm at the gill slits, as evidenced by the differing origins of gill filaments in modern lampreys and gnathostomes. Early in the 20th century it was discovered that lamprey gill filaments are endodermal in origin, and, alongside the branchial nerves and blood vessels, reside medial to the skeletal gill arches (Goette, 1901). In contrast, among gnathostomes, gill filaments are ectodermal in origin and, at least in osteichthyans, are positioned lateral to the skeletal gill arches (Goette, 1901; Janvier, 2007). If ectoderm penetrated also less deeply into the gill slits in extinct agnathans, or only late, this could be a reason why pharyngeal denticles are not more widespread in agnathans.
Our hypothesis states that teeth originated prior to jaws, but only when the formation of gill slits allowed for a relatively deep invasion of ectoderm into the oropharyngeal cavity and extensive physical contact between ectoderm and endoderm. Such a scenario may explain why pharyngeal teeth, located deeply within the oropharyngeal cavity, were lost in early tetrapods and maintained only on the margins of the jaws and roof of the oral cavity. We propose that loss of gill slits, and the ability for the ectoderm to invade, leads to the disconnection of the competent ectodermal epithelium and the endodermally lined pharynx. Data gleaned from the fossil record supports this conclusion. For example, among Tetrapodomorph fish, the group of lobe-finned fish (sarcopterygians) that is generally believed to have given rise to tetrapods, with Eusthenopteron (late Devonian, 385 Ma) as an important representative, the internal surfaces of the gill arch elements are covered with tooth plates (Nelson, 1969; Carroll, 1988). The branchial skeleton of Ichthyostega, an upper Devonian (365 Ma) amphibian close to the ancestry of all later terrestrial vertebrates, is poorly known, but the animal possibly had a small gill slit (Janvier, 1996). Acanthostega, an early tetrapod from the upper Devonian, retained a fish-like branchial skeleton, fish-like internal gills and an open opercular chamber for use in aquatic respiration (Coates & Clack, 1991), and it possessed branchial tooth plates (Coates, 1996 in Graveson et al. 1997). Acanthostega falls outside the Neotetrapoda, a group that is characterized by the closure of the gill slit (Janvier, 1996). Branchial tooth plates have also been reported for the basal temnospondyl amphibian Colosteus scutellatus (upper Carboniferous, 310 Ma) (Hook, 1983). Interestingly, Schoch (2002) noted the coincidence between the loss of branchial denticles, considered to be homologous to teeth, and the loss of gill slits in temnospondyls (Fig. 5). Schoch (2002) suggested a functional explanation for the simultaneous loss of both branchial denticles and gill slits: open gill clefts allow for a unidirectional flow of water from the buccal cavity through the branchial chamber, thus enabling branchial denticles to assist in the capture and processing of prey items. Paleontologists indeed tend to use the presence of denticulated pieces of bone (branchial ossicles, sensu Schoch, 2001) in temnospondyl amphibians as evidence for the presence of a (cartilaginous) branchial skeleton (Boy, 1988) and open gill slits (e.g. Berman, 1973; Schoch, 2001). We propose that the simultaneous loss of gill slits and of pharyngeal denticles (teeth) is not just functionally but also developmentally related, as the loss of gill slits could prevent the invagination of odontogenic, or inductively competent, ectoderm. However, it is important to note that the presence of gill slits does not necessarily predict the development of branchial denticles. For example, lungfish (Dipnoi) lack pharyngeal denticles. This is assumed to be a secondary loss, possibly related to the extensive evolution of the dentition during the early history of the group (Ahlberg et al. 2006). The loss could be analogous to the loss of teeth on the different gill arches in teleosts.
Fig. 5.
Branchial denticulated plates (branchial ossicles) in the pre-metamorphosis stage of Onchiodon labyrinthicus, a temnospondyl amphibian from the lower Permian (reproduced from Schoch, 2001, Fig. 3, with permission from the author).
Also significant to our hypothesis is that in no other tetrapods have pharyngeal teeth, or branchial denticles, ever been observed, despite the presence of pharyngeal endoderm, and despite the likely presence of segmentally arranged ectodermal–endodermal contacts. We speculate that such contacts are constituted solely of outpockets of endoderm abutting the ectoderm, and that without ectodermal invagination into the body, tooth development is not initiated. This will be easy to test once a reliable marker for endoderm is available. In the urodele amphibian Ambystoma mexicanum, endodermally derived oral teeth are formed in close proximity to the invaginated ectoderm (Soukup et al. 2008). In our view, the concomitant lack of gill slits and pharyngeal teeth in tetrapods is a strong argument for the ectodermal origin of teeth. Even if the competence to form teeth had been transferred during evolution from ectoderm to endoderm, and the ectoderm would still be required as an inductive tissue, tooth formation would be blocked because of the loss of extensive contact between the two embryonic layers (ectoderm and endoderm).
Given that the spiraculum, similar to other gill slits, could allow ectoderm to migrate inwards, it is interesting to note that Eusthenopteron retains denticles inside the spiracular canal (Jarvik, 1980). Most tetrapod stem group members are now assumed to retain an open spiraculum (Clack, 2007). Possibly, the rise of the tympanic membrane could have been the ultimate event that definitively sealed off this route for ectodermal migration.
The recent discussion in the literature of whether placoderms (e.g. antiarchs, arthrodires), phylogenetically the most basal clade of jawed vertebrates (early Silurian to late Devonian, 435–360 Ma), have pharyngeal denticles, can also be viewed in the light of our proposed hypothesis. The so-called postbranchial lamina, which carries patterned arrays of denticles (Johanson & Smith, 2003, 2005) is again an area where invagination could have carried ectodermal competence deep into the body. Johanson & Smith (2005) saw a difference in arrangement and morphology between these organized denticles and those ornamenting the surface of the head shield. Again, for the reasons given above, we do not consider differences in patterning sufficient to justify an independent, endodermal origin of pharyngeal denticles. In the case of the placoderm postbranchial lamina, the pharyngeal denticles may represent another ‘experiment’ of nature. We consider them ‘experiments’ as much as we, and others, consider extra-oral teeth (see below) a developmental ‘experiment’ the other way around (inwards out, i.e. production of structures outside the mouth by a developmental programme used normally to produce structures inside the oral cavity only). This interpretation implies that the occurrence of pharyngeal denticles in agnathans and early gnathostomes holds no phylogenetic information, and hence avoids discussions and speculations on the multiple origins of teeth through convergent evolution (Smith & Johanson, 2003a).
Our hypothesis revives the view of Nelson (1969, 1970), who assumed that small tooth plates were distributed over the entire surface of the oropharyngeal cavity early in gnathostome evolution, comparable to the distribution of placoid scales in elasmobranchs (cf. Reif, 1982), and that these tooth plates, whether large or small, were freely located in the tissues. Subsequently, enlarged tooth plates may have appeared in areas of particular functional importance, and possibly first on the jaws. Although Nelson did not explicitly state that competent ectoderm entered the oropharyngeal cavity through the gill slits, he does refer to a relation between the presence (or absence) of gill slits and the presence (or absence) of tooth plates.
Evolutionary modifications in the distribution of teeth
A prominent evolutionary trend towards tooth reduction
Looking at the distribution of teeth both in actinopterygian and sarcopterygian lineages, it is clear that the number of tooth-bearing skeletal elements has been reduced during vertebrate evolution. An overview of these trends was presented by Huysseune & Sire (1998). Among sarcopterygians, Latimeria has many dental plates associated with the jaws, palatal bones, hyoid and branchial arches (Millot & Anthony, 1958). The dentition associated with the (post-hyoid) visceral arches was lost in early tetrapods, a loss formerly associated with the loss of respiratory function in the transition from aquatic to terrestrial life. We now believe that loss of the dentition on the visceral arches is only secondarily associated with the loss of their respiratory function and primarily associated with the loss of the gill slits (see above). In caudate lissamphibians, larval teeth are attached to the paired bones of the upper jaw (premaxillaries, maxillaries, prevomers and palatines) and the lower jaw (dentaries and coronoids) (Davit-Béal et al. 2007). During metamorphosis, the palatines disappear from the upper jaw and are replaced by the extension of the vomers. In the lower jaw, the coronoids disappear and only the dentaries remain. In anurans, teeth, when present, are restricted to the upper jaw, with the exception of some hylids, in which teeth are also found on the dentary (see Davit-Béal et al. 2007, for a review). Vomers, as well as the parasphenoid bone, are believed to be evolutionarily derived from the anterior part of the splanchnocranium too (Nelson, 1969; for a historical review and critical comments on the interpretation of the anterior neurocranial skeleton, see Kimmel & Eberhart, 2008). The restriction of teeth to these skeletal elements can be easily explained by the fact that an ectodermal-endodermal contact necessary to allow these teeth to form, is established only through the mouth opening. By extension, this may explain the restricted oral distribution of teeth in sauropsids (Edmund, 1969) and in mammals (Thenius, 1989).
Within the actinopterygians, the teleost fish, with 26 000 species representing approximately one third of all extant vertebrate species, display a prominent evolutionary trend in the loss of teeth from different visceral arches. Representatives of basal teleostean lineages such as elopomorphs have tooth plates associated with all branchial arches. In contrast, advanced teleosts, such as acanthomorphs, usually retain teeth only on the mandibular and the posteriormost branchial arches (Nelson, 1969; Vandewalle et al. 1994). In the ostariophysan lineage on the other hand, oral teeth have been lost in cypriniforms (Stock, 2007). Tooth loss in these teleost taxa can not, of course, be explained by a loss of ectodermal invagination and ectodermal/endodermal interactions as all teleosts retain gill slits. Instead, the cause of tooth loss is more likely to be found in changes in the molecular networks that regulate tooth initiation, under the selective pressure of regionalization of functions with respect to food processing and respiration.
Molecular networks regulating tooth formation in non-mammalians
We are only scratching the surface in our understanding of the molecular networks that are responsible for tooth formation in non-mammalians, and there is an enormous backlog in knowledge compared to what is known for mammals (mostly in the mouse) (see the review by Catón & Tucker, 2009). However, at least one question that has been settled over the past years is whether and how teeth can develop in a Hox-expressing environment, as is the case for the post-mandibular arches in non-mammalians (Prince et al. 1998). Indeed, teeth in mammals develop on the mandibular arch only, which is a non-Hox expressing environment (Rijli et al. 1993). It had previously been demonstrated that, at least in birds, Hox-gene expression and jaw formation are mutually exclusive (Couly et al. 1998; Grammatopoulos et al. 2000). James et al. (2002) experimentally demonstrated that teeth in the mouse can develop in a Hox-expressing environment. This implies that, whereas a loss of Hox gene expression in the first branchial arch might have been required for jaws to form (Rijli et al. 1993; Cohn, 2002), the evolutionary acquisition of teeth on the first arch was independent of a Hox patterning programme.
The small number of papers published so far on expression patterns of developmental genes involved in tooth formation in non-mammalians have revealed both conserved and divergent patterns when compared to mammals (Fraser et al. 2004, 2006a,b; Jackman et al. 2004; Laurenti et al. 2004; Borday-Birraux et al. 2006; Wise & Stock, 2006; Debiais-Thibaud et al. 2007; Huysseune et al. 2008). A profound knowledge of the molecular networks and the genes involved is nevertheless imperative if we want to approach the question of ontogenetic and evolutionary tooth loss. To date, most of what is known about the evolutionary loss of teeth associated with certain branchial arches has been published by Stock and colleagues (Stock et al. 2006; Jackman & Stock, 2006; Wise & Stock, 2006; Stock, 2007). Briefly summarized, Stock and colleagues first discovered that oral tooth loss in cypriniforms is associated with a loss of oral bmp2b (Wise & Stock, 2006) and oraldlx2b expression (Stock et al. 2006), two developmental genes involved in tooth formation (Jackman et al. 2004; Wise & Stock, 2006). Next, using a zebrafish dlx2b:GFP reporter construct, they showed that this construct can drive dlx2b expression in the oral tooth germs in a member of the sister lineage, the Mexican tetra (Astyanax mexicanus, a characiform), indicating that loss of oral dlx2b expression in cypriniforms results from changes in one or more trans-acting regulators rather than in the cis-regulatory regions of this gene (Jackman & Stock, 2006). These authors concluded that preservation of oral enhancer function, unused for more than 50 million years, could be the result of pleiotropic function in the pharyngeal dentition.
The above-mentioned findings raise the question of whether teeth can be re-acquired after having been lost in certain areas of the oropharyngeal cavity, or from certain bones. One example is the reappearance of the second lower molar tooth in extant lynx (Felis lynx) (Kurtén, 1963). The reappearance of a fourth row of pharyngeal teeth in the cyprinid fish Barbus paludinosus (Golubtsov et al. 2005) has been discussed as a possible case of taxic atavism. Similar to the spontaneous reappearance of lost characters in individuals (atavism), lost characters can reappear in entire taxa (taxic atavism) (Stiassny, 1991). Additional examples of taxic atavisms from other taxa and other organ systems (fins, muscles, skull bones) are discussed by Raikow et al. (1979), Meyer (1999), Gatesy et al. (2003) and Hall (2007). Other studies relevant to the evolutionary loss of teeth in tetrapods are reviewed by Davit-Béal et al. (2009) in this volume.
Spatially restricted tooth loss (such as the loss of oral teeth in cyprinids or tooth loss on the lower jaw in most frogs) or ontogenetic tooth loss [such as in sturgeons (Peyer, 1968), in armoured catfish (Huysseune & Sire, 1997), or in Pangasianodon gigas, the giant catfish (Kakizawa & Meenakarn, 2003)], reveals the presence of developmental modules in the dentition, as discussed by Stock (2001). In an excellent review, this author analysed the various levels at which modules (units that develop under semi-autonomous control) can be identified in the vertebrate dentition, and discussed how these could be related to modularity in the genetic control of development.
The case of extra-oral teeth
A final issue regarding the distribution of teeth in the oropharyngeal cavity that needs to be addressed concerns a developmental conundrum, which is the presence of extra-oral teeth. Within recent years, several teleost species have been described with denticles structurally identical to teeth, developed across extra-oral surfaces of the head. These species represent a wide diversity of unrelated lineages including Siluriformes (Sire & Huysseune, 1996), Clupeomorpha (Sire et al. 1998), Atheriniformes (Sire & Allizard, 2001), Xiphioidea (Sire & Allizard, 2001) and Lophiiformes (Pietsch & Orr, 2007). Initially, these extra-oral elements were considered to be dermal denticles reminiscent of ancestral odontodes (e.g. Sire & Huysseune, 1996). However, given the variety of species involved it became clear that these structures should be regarded as a novel activation of an existing tooth developmental programme in extra-oral locations and not merely as the reappearance of an ancestral character (Sire, 2001, see also Stock, 2001). Note that such developmental ‘experiments’ have only been reported for the ectoderm and not for the endoderm, lending support to the idea of an ectodermal primacy in tooth evolutionary history.
Conclusions and perspectives
Based on a reappraisal of available evidence, we challenge the current views of the evolutionary origin of teeth, and propose a revised ‘outside in’ hypothesis. We suggest that teeth may have arisen before the origin of jaws, as a result of the invasion of competent, odontode-forming ectoderm into the oropharyngeal cavity through the mouth and gill slits, to interact with neural crest-derived mesenchyme (Hall, 2000). This hypothesis supports the homology between skin denticles (odontodes) and teeth.
Our hypothesis is based on (1) the assumption that endoderm alone, together with neural crest, cannot form teeth, given that – with one exception – supposedly endodermally derived teeth were never observed to develop without the nearby presence of ectoderm in extant species; (2) the observation that pharyngeal teeth are present only in species known to possess gill slits, and disappear from the pharyngeal region in early tetrapods concomitant with the closure of gill slits, and (3) the assumption that the dental lamina (sensu Reif, 1982) is not a prerequisite for tooth development, although it may have facilitated tooth formation in advance of need. We regard the presence of pharyngeal denticles in extinct thelodonts, and the postbranchial lamina denticles of placoderms as independent ‘experiments’ of nature, involving the same odontogenetically competent tissues, that is, neural crest and ectoderm. Extra-oral teeth observed in various unrelated taxa of teleost fishes, are seen as similar developmental ‘accidents’, but the other way around, carrying the tooth developmental programme to the external surface of the body. Whereas our revised ‘outside in’ hypothesis accounts for the complete loss of teeth from the post-mandibular branchial arches, it cannot explain the spatially restricted loss of teeth from certain arches, as occurs in numerous teleosts and frogs, or the ontogenetic loss of teeth in specific taxa.
One of the primary advantages of our hypothesis is that it can be tested on paleontological data (it predicts a correlation between the presence of pharyngeal teeth and gill slits) and developmental data in extant species (by challenging endoderm alone to make teeth in association with neural crest-derived mesenchyme). In addition, it may serve as a guide for further developmental research, such as a search for an ectodermal signal necessary for pharyngeal tooth development.
The recent advances that have been made in our knowledge on the molecular control of tooth formation in non-mammalians (mostly in some teleost model species) will undoubtedly contribute to answering these questions in the coming years.
Acknowledgments
The authors acknowledge the insightful comments of two anonymous referees. This paper was made within the frame of the European COST ACTION B23-Oral facial development and regeneration. A.H. acknowledges grants from the FWO-Vlaanderen nos 3G0159.05 and KaN 1.5.116.06.
References
- Adams AE. An experimental study of the development of the mouth in the amphibian embryo. J Exp Zool. 1924;40:311–379. [Google Scholar]
- Ahlberg PE, Smith MM, Johanson Z. Developmental plasticity and disparity in early dipnoan (lungfish) dentitions. Evol Dev. 2006;8:331–349. doi: 10.1111/j.1525-142X.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Barlow LA, Northcutt RG. Embryonic origin of amphibian taste buds. Dev Biol. 1995;169:273–285. doi: 10.1006/dbio.1995.1143. [DOI] [PubMed] [Google Scholar]
- Berman DS. A trimerorhachid amphibian from the upper Pennsylvanian of New Mexico. J Paleontol. 1973;47:932–945. [Google Scholar]
- Bjerring HC. A contribution to structural analysis of the head of craniate animals. Zool Scr. 1977;6:127–183. [Google Scholar]
- Borday-Birraux V, Van der heyden C, Debiais-Thibaud M, et al. Expression of dlxgenes in zebrafish tooth development. Evolutionary implications. Evol Dev. 2006;8:130–141. doi: 10.1111/j.1525-142X.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Boy JA. Über einige Vertreter der Eryopoidea (Amphibia: Temnospondyli) aus dem europäischen Rotliegend (?höchstes Karbon-Perm). 1. Sclerocephalus. Paläont Z. 1988;62:107–132. [Google Scholar]
- Burrow CJ. Comment on ‘Separate evolutionary origins of teeth from evidence in fossil jawed vertebrates’. Science. 2003;300:1661b. doi: 10.1126/science.300.5626.1661a. [DOI] [PubMed] [Google Scholar]
- Carroll RL. Vertebrate Paleontology and Evolution. New York: W.H. Freeman & Company; 1988. [Google Scholar]
- Cassin C, Capuron A. Buccal organogenesis in Pleurodeles waltlMichah (urodele amphibian), study by intrablastocelic transplantation and in vitroculture. J Biol Buccale. 1979;7:61–76. [PubMed] [Google Scholar]
- Catón J, Tucker A. Current knowledge of tooth development, a model mineralized element system. J Anat. 2009;214:502–515. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibon P. Analyse expérimentale de la régionalisation et des capacités morphogénétiques de la crête neurale chez l’amphibien urodèle Pleurodeles waltliiMichah. Mém Soc Zool Fr. 1966;36:1–107. [Google Scholar]
- Chibon P. L’origine de l’organe adamantin des dents. Etude au moyen du marquage nucléaire de l’ectoderme stomodéal. Ann Embryol Morphol. 1970;3:203–243. [Google Scholar]
- Clack JA. Devonian climate change, breathing and the origin of the tetrapod stem group. Integr Comp Biol. 2007;47:510–523. doi: 10.1093/icb/icm055. [DOI] [PubMed] [Google Scholar]
- Coates M. The Devonian tetrapod Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships, and patterns of skeletal evolution. Trans R Soc Edin. 1996;87:363–421. [Google Scholar]
- Coates MI, Clack JA. Fish-like gills and breathing in the earliest known tetrapod. Nature. 1991;352:234–236. [Google Scholar]
- Cohn MJ. Lamprey Hoxgenes and the origin of jaws. Nature. 2002;416:386–387. doi: 10.1038/416386a. [DOI] [PubMed] [Google Scholar]
- Couly G, Grapin-Botton A, Coltey P, Ruhin B, Le Douarin NM. Determination of the identity of the derivatives of the cephalic neural crest: incompatibility between Hox gene expression and lower jaw development. Development. 1998;125:3445–3459. doi: 10.1242/dev.125.17.3445. [DOI] [PubMed] [Google Scholar]
- Davit-Béal T, Allizard F, Sire J-Y. Enameloid/enamel transition through successive tooth replacements in Pleurodeles waltl(Lissamphibia, Caudata) Cell Tissue Res. 2007;328:167–183. doi: 10.1007/s00441-006-0306-1. [DOI] [PubMed] [Google Scholar]
- Davit-Béal T, Tucker A, Sire J-Y. Loss of teeth and enamel in tetrapods: Fossil record, genetic data and morphological adaptations. J Anat. 2009;214:477–501. doi: 10.1111/j.1469-7580.2009.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer GR. The differentiation of neural crest cells into visceral cartilages and odontoblasts in Amblystoma, and a re-examination of the germ-layer theory. Proc R Soc Lond B. 1947;134:377–398. [PubMed] [Google Scholar]
- Debiais-Thibaud M, Borday-Birraux V, Germon I, et al. Development of oral and pharyngeal teeth in the medaka (Oryzias latipes): Comparison of morphology and expression of eve1gene. J Exp Zool. 2007;308B:693–708. doi: 10.1002/jez.b.21183. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Aldridge RJ. Ahlberg PE, editor. Origin of a mineralized skeleton. Major Events in Early Vertebrate Evolution. 2001:85–104. Systematics Association Special Volume Series 61. [Google Scholar]
- Donoghue PCJ, Sansom IJ. Origin and evolution of vertebrate skeletonization. Microsc Res Techn. 2002;59:352–372. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Edmund AG. Dentition. In: Gans C, Bellairs Ad’A, Parsons TS, editors. Biology of the Reptilia. Vol. 1. Morphology A. London: Academic Press; 1969. pp. 117–200. [Google Scholar]
- Edwards LF. The origin of the pharyngeal teeth of the carp (Cyprinus carpioLinnaeus) Ohio J Sci. 1929;29:93–130. [Google Scholar]
- Fraser GJ, Berkovitz BK, Graham A, Smith MM. Gene deployment for tooth replacement in the rainbow trout (Oncorhynchus mykiss): a developmental model for evolution of the osteichthyan dentition. Evol Dev. 2006b;8:446–457. doi: 10.1111/j.1525-142X.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- Fraser GJ, Graham A, Smith MM. Conserved deployment of genes during odontogenesis across osteichthyans. Proc R Soc Lond B. 2004;271:2311–2317. doi: 10.1098/rspb.2004.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GJ, Graham A, Smith MM. Developmental and evolutionary origins of the vertebrate dentition: molecular controls for spatio-temporal organisation of tooth sites in osteichthyans. J Exp Zool. 2006a;306B:183–203. doi: 10.1002/jez.b.21097. [DOI] [PubMed] [Google Scholar]
- Gatesy J, Amato G, Norell M, DeSalle R, Hayashi C. Combined support for wholesale taxic atavism in gavialine crocodylians. Syst Biol. 2003;52:403–422. doi: 10.1080/10635150390197037. [DOI] [PubMed] [Google Scholar]
- Goette A. Über die Kiemen der Fische. Z Wissenschaftl Zool. 1901;69:533–577. [Google Scholar]
- Golubtsov AS, Dzerjinskii KF, Prokofiev AM. Four rows of pharyngeal teeth in an aberrant specimen of the small African barb Barbus paludinosus(Cyprinidae): novelty or atavistic alteration? J Fish Biol. 2005;67:286–291. [Google Scholar]
- Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development. 2000;127:5355–5365. doi: 10.1242/dev.127.24.5355. [DOI] [PubMed] [Google Scholar]
- Graveson AC. Neural crest: contributions to the development of the vertebrate head. Am Zool. 1993;33:424–433. [Google Scholar]
- Graveson AC, Smith MM, Hall BK. Neural crest potential for tooth development in a urodele amphibian: developmental and evolutionary significance. Dev Biol. 1997;188:34–42. doi: 10.1006/dbio.1997.8563. [DOI] [PubMed] [Google Scholar]
- Hall BK. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol Dev. 2000;2:3–5. doi: 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- Hall BK. Skeletal biology in an Evo-Devo-Paleo lab. Palaeontol Newsletter. 2005;59:26–34. [Google Scholar]
- Hall BK. Homoplasy and homology: Dichotomy or continuum? J Human Evol. 2007;52:473–479. doi: 10.1016/j.jhevol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Hall BK, Witten PE. The origin and plasticity of skeletal tissues in vertebrate evolution and development. In: Anderson JS, Sues H-D, editors. Major Transitions in Vertebrate Evolution. Bloomington: Indiana University Press; 2007. pp. 13–56. [Google Scholar]
- Holmbakken N, Fosse G. Tooth replacement in Gadus callarius. Z Anat Entwickl-Gesch. 1973;143:65–79. doi: 10.1007/BF00519911. [DOI] [PubMed] [Google Scholar]
- Hook RW. Colosteus scutellatus(Newberry), a primitive temnospondyl amphibian from the Middle Pennsylvanian of Linton, Ohio. Am Mus Novit. 1983;2770:1–41. [Google Scholar]
- Huysseune A. Phenotypic plasticity in the lower pharyngeal jaw dentition of Astatoreochromis alluaudi(Teleostei: Cichlidae) Arch Oral Biol. 1995;40:1005–1014. doi: 10.1016/0003-9969(95)00074-y. [DOI] [PubMed] [Google Scholar]
- Huysseune A. Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation. Int J Dev Biol. 2006;50:637–643. doi: 10.1387/ijdb.052098ah. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire J-Y. Structure and development of teeth in three armoured catfish, Corydoras aeneus, C. arcuatusand Hoplosternum littorale(Siluriformes, Callichthyidae) Acta Zool. 1997;78:69–84. [Google Scholar]
- Huysseune A, Sire J-Y. Evolution of patterns and processes in teeth and tooth-related tissues in non-mammalian vertebrates. Eur J Oral Sci. 1998;106(Suppl. 1):437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Witten PE. An evolutionary view on tooth development and replacement in wild Atlantic salmon (Salmo salarL.) Evol Dev. 2008;10:6–14. doi: 10.1111/j.1525-142X.2007.00209.x. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Takle H, Soenens M, Taerwe K, Witten PE. Unique and shared gene expression patterns in Atlantic salmon (Salmo salar) tooth development. Dev Genes Evol. 2008;218:427–437. doi: 10.1007/s00427-008-0237-9. [DOI] [PubMed] [Google Scholar]
- Imai N, Osumi N, Eto K. Contribution of foregut endoderm to tooth initiation of mandibular incisor in rat embryos. Eur J Oral Sci. 1998;106(Suppl. 1):19–23. doi: 10.1111/j.1600-0722.1998.tb02148.x. [DOI] [PubMed] [Google Scholar]
- Jackman WR, Stock DW. Transgenic analysis of Dlx regulation in fish tooth development reveals evolutionary retention of enhancer function despite organ loss. Proc Natl Acad Sci USA. 2006;103:19390–19395. doi: 10.1073/pnas.0609575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274:139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- James CT, Ohazama A, Tucker AS, Sharpe PT. Tooth development is independent of a Hox patterning programme. Dev Dyn. 2002;225:332–335. doi: 10.1002/dvdy.10168. [DOI] [PubMed] [Google Scholar]
- Janvier P. Early Vertebrates. Oxford: Clarendon Press; 1996. Oxford Monographs on Geology and Geophysics 33. [Google Scholar]
- Janvier P. Homologies and evolutionary transitions in early vertebrate history. In: Anderson JS, Sues H-D, editors. Major Transitions in Vertebrate Evolution. Bloomington: Indiana University Press; 2007. pp. 57–121. [Google Scholar]
- Jarvik E. Basic Structure and Evolution of Vertebrates. Vol. 1. London: Academic Press; 1980. [Google Scholar]
- Johanson Z, Smith MM. Placoderm fishes, pharyngeal denticles, and the vertebrate dentition. J Morphol. 2003;257:289–307. doi: 10.1002/jmor.10124. [DOI] [PubMed] [Google Scholar]
- Johanson Z, Smith MM. Origin and evolution of gnathostome dentitions: a question of teeth and pharyngeal denticles in placoderms. Biol Rev. 2005;80:303–345. doi: 10.1017/s1464793104006682. [DOI] [PubMed] [Google Scholar]
- Jollie M. Some implications of the acceptance of a delamination principle. In: Ørvig T, editor. Current Problems of Lower Vertebrate Phylogeny. Stockholm: Almqvist & Wiksell; 1968. pp. 89–107. [Google Scholar]
- Kakizawa Y, Meenakarn W. Histogenesis and disappearance of the teeth of the Mekong giant catfish, Pangasianodon gigas(Teleostei) J Oral Sci. 2003;45:213–221. doi: 10.2334/josnusd.45.213. [DOI] [PubMed] [Google Scholar]
- Kemp A. Amino acid residues in conodont elements. J Paleontol. 2002a;76:518–528. [Google Scholar]
- Kemp A. Hyaline tissue of thermally unaltered conodont elements and the enamel of vertebrates. Alcheringa. 2002b;26:23–36. [Google Scholar]
- Kemp NE. Chapter 2. Integumentary System and Teeth. In: Hamlett WC, editor. Sharks, Skates, and Rays. The Biology of Elasmobranch Fish. Baltimore: The Johns Hopkins University Press; 1999. pp. 43–68. [Google Scholar]
- Kimmel CB, Eberhart JK. The midline, oral ectoderm, and the arch-0 problem. Integr Comp Biol. 2008;48:668–680. doi: 10.1093/icb/icn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtén B. Return of a lost structure in the evolution of the felid dentition. Soc Scient Fenn Comment Biol B. 1963;26:1–12. [Google Scholar]
- Laurenti P, Thaeron-Antono C, Allizard F, Huysseune A, Sire J-Y. The cellular expression of eve1suggests its requirement for the differentiation of the ameloblasts, and for the initiation and morphogenesis of the first tooth in the zebrafish (Danio rerio. Dev Dyn. 2004;230:727–733. doi: 10.1002/dvdy.20080. [DOI] [PubMed] [Google Scholar]
- McCollum M, Sharpe PT. Evolution and development of teeth. J Anat. 2001;199:153–159. doi: 10.1046/j.1469-7580.2001.19910153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Homology and homoplasy: the retention of genetic programmes. In: Bock GR, Cardew G, editors. Homology. Chichester: John Wiley & Sons; 1999. pp. 141–157. [DOI] [PubMed] [Google Scholar]
- Structural and Chemical Organization of Teeth. New York: Academic Press; 1967. [Google Scholar]
- Miller RF, Cloutier R, Turner S. The oldest articulated chondrichthyan from the early Devonian period. Nature. 2003;425:501–504. doi: 10.1038/nature02001. [DOI] [PubMed] [Google Scholar]
- Millot J, Anthony J. Anatomie de Latimeria chalumnae. Tome I. Squelette, muscles et formation de soutien. Paris: CNRS; 1958. [Google Scholar]
- Morris SC. Conodont function: fallacies of the tooth model. Lethaia. 1980;13:107–108. [Google Scholar]
- Nelson GJ. Gill arches and the phylogeny of fishes, with notes on the classification of vertebrates. Bull Am Mus Nat Hist. 1969;141:475–552. [Google Scholar]
- Nelson GJ. Pharyngeal denticles (placoid scales) of sharks, with notes on the dermal skeleton of vertebrates. Am Mus Novit. 1970;2413:1–26. [Google Scholar]
- Ørvig T. Phylogeny of tooth tissues. Evolution of some calcified tissues in early vertebrates. In: Miles AEW, editor. Structural and Chemical Organization of Teeth. Vol. 1. London: Academic Press; 1967. pp. 45–110. [Google Scholar]
- Owen R. Odontography. London: Hippolyte Balliere; 1840. –1845. [Google Scholar]
- Peyer B. Comparative Odontology. Chicago: University Press; 1968. [Google Scholar]
- Pietsch TW, Orr JW. Phylogenetic relationships of deep-sea anglerfishes of the suborder Ceratioidei (Teleostei: Lophiiformes) based on morphology. Copeia. 2007;2007:1–34. [Google Scholar]
- Piotrowski T, Nüsslein-Volhard C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio. Dev Biol. 2000;225:339–356. doi: 10.1006/dbio.2000.9842. [DOI] [PubMed] [Google Scholar]
- Pridmore PA, Barwick RE, Nicoll RS. Soft anatomy and the affinities of conodonts. Lethaia. 1996;29:317–328. [Google Scholar]
- Prince VE, Joly L, Ekker M, Ho RK. Zebrafish hoxgenes: genomic organization and modified colinear expression patterns in the trunk. Development. 1998;125:407–420. doi: 10.1242/dev.125.3.407. [DOI] [PubMed] [Google Scholar]
- Purnell MA. Feeding in extinct jawless heterostracan fishes and testing scenarios of early vertebrate evolution. Proc R Soc Lond B. 2001;269:83–88. doi: 10.1098/rspb.2001.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikow RJ, Borecky SR, Berman SL. The evolutionary re-establishment of a lost ancestral muscle in the bowerbird assemblage. Condor. 1979;81:203–206. [Google Scholar]
- Reif WE. Evolution of dermal skeleton and dentition in vertebrates. The odontode regulation theory. Evol Biol. 1982;15:287–368. [Google Scholar]
- Reif WE. Evolution of the dermal skeleton of vertebrates: Concepts and methods. Neues Jahrb Geol Paläont Abh. 2002;223:53–78. [Google Scholar]
- Reif WE. Conodonts, odontodes, stem-groups, and the ancestry of enamel genes. Neues Jahrb Geol Paläont Abh. 2006;241:405–439. [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dollé P, Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Piotrowski T, Grandl H, et al. Jaw and branchial arch mutants in zebrafish. I. Branchial arches. Development. 1996;123:329–344. doi: 10.1242/dev.123.1.329. [DOI] [PubMed] [Google Scholar]
- Schoch RR. Can metamorphosis be recognised in Palaeozoic amphibians? Neues Jahrb Geol Paläont Abh. 2001;220:335–367. [Google Scholar]
- Schoch RR. The evolution of metamorphosis in temnospondyls. Lethaia. 2002;35:309–327. [Google Scholar]
- Scott HW. The zoological relationships of the conodonts. J Paleontol. 1934;8:448–455. [Google Scholar]
- Sellman S. Some experiments on the determination of the larval teeth in Ambystoma mexicanum. Odontol Tidskr. 1946;54:1–128. [Google Scholar]
- Sire J-Y. Teeth outside the mouth in teleost fishes: how to benefit from a developmental accident. Evol Dev. 2001;3:104–108. doi: 10.1046/j.1525-142x.2001.003002104.x. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Allizard F. A fourth Teleost lineage possessing extra-oral teeth: The genus Atherion(Teleostei; Atheriniformes) Eur J Morphol. 2001;39:295–305. doi: 10.1076/ejom.39.5.295.7375. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Davit-Béal T, Delgado S, Van der heyden C, Huysseune A. First-generation teeth in nonmammalian lineages: Evidence for a conserved ancestral character? Microsc Res Techn. 2002;59:408–434. doi: 10.1002/jemt.10220. [DOI] [PubMed] [Google Scholar]
- Sire J-Y, Donoghue PCJ, Vickaryous MK. Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates. J Anat. 2009;214:409–440. doi: 10.1111/j.1469-7580.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sire J-Y, Huysseune A. Structure and development of the odontodes in an armoured catfish, Corydoras aeneus(Siluriformes, Callichthyidae) Acta Zool. 1996;77:51–72. [Google Scholar]
- Sire J-Y, Marin S, Allizard F. Comparison of teeth and dermal denticles (odontodes) in the teleost Denticeps clupeoides(Clupeomorpha) J Morphol. 1998;237:237–255. doi: 10.1002/(SICI)1097-4687(199809)237:3<237::AID-JMOR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Smith MM. Vertebrate dentitions at the origin of jaws: when and how pattern evolved. Evol Dev. 2003;5:394–413. doi: 10.1046/j.1525-142x.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Smith MM, Coates MI. Evolutionary origins of the vertebrate dentition: phylogenetic patterns and developmental evolution. Eur J Oral Sci. 1998;106(Suppl. 1):482–500. doi: 10.1111/j.1600-0722.1998.tb02212.x. [DOI] [PubMed] [Google Scholar]
- Smith MM, Coates MI. Evolutionary origins of teeth and jaws: Developmental models and phylogenetic patterns. In: Teaford M, Smith M, Ferguson M, editors. Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press; 2000. pp. 133–151. [Google Scholar]
- Smith MM, Coates MI. Ahlberg PE, editor. The evolution of vertebrate dentitions: phylogenetic pattern and developmental models. Major Events in Early Vertebrate Evolution. 2001;61:223–240. Systematics Association Special Volume Series. [Google Scholar]
- Smith MM, Hall BK. Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Smith MM, Johanson Z. Separate evolutionary origins of teeth from evidence in fossil jawed vertebrates. Science. 2003a;299:1235–1236. doi: 10.1126/science.1079623. [DOI] [PubMed] [Google Scholar]
- Smith MM, Johanson Z. Response to comment on ‘Separate evolutionary origins of teeth from evidence in fossil jawed vertebrates’. Science. 2003b;300:1661c. doi: 10.1126/science.1079623. [DOI] [PubMed] [Google Scholar]
- Soukup V, Epperlein HH, Horacek I, Cerny R. Dual epithelial origin of vertebrate oral teeth. Nature. 2008;455:795–799. doi: 10.1038/nature07304. [DOI] [PubMed] [Google Scholar]
- Stiassny MLJ. Atavisms, phylogenetic character reversals, and the origin of evolutionary novelties. Neth J Zool. 1991;42:260–276. [Google Scholar]
- Stock DW. The genetic basis of modularity in the development and evolution of the vertebrate dentition. Philos Trans R Soc Lond B. 2001;356:1633–1653. doi: 10.1098/rstb.2001.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock DW. Zebrafish dentition in comparative context. J Exp Zool. 2007;308B:523–549. doi: 10.1002/jez.b.21187. [DOI] [PubMed] [Google Scholar]
- Stock DW, Jackman WR, Trapani J. Developmental genetic mechanisms of evolutionary tooth loss in cypriniform fishes. Development. 2006;133:3127–3137. doi: 10.1242/dev.02459. [DOI] [PubMed] [Google Scholar]
- Ströer WFH. Experimentelle Untersuchungen über die Mundentwicklung bei den Urodelen. W Roux’ Arch Entw Mech. 1933;130:131–186. doi: 10.1007/BF01380894. [DOI] [PubMed] [Google Scholar]
- Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Thenius E. Zähne und Gebiss der Säugetiere. In: Niethammer J, Schliemann H, Starck D, editors. Handbuch der Zoologie. Berlin: Walter de Gruyter; 1989. pp. 1–513. [Google Scholar]
- Trotter JA, FitzGerald JD, Kokkonen H, Barnes CR. New insights into the ultrastructure, permeability, and integrity of conodont apatite determined by transmission electron microscopy. Lethaia. 2007;40:97–110. [Google Scholar]
- Van der Brugghen W, Janvier P. Denticles in thelodonts. Nature. 1993;364:107. [Google Scholar]
- Van der heyden C, Huysseune A. Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae) Dev Dyn. 2000;219:486–496. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1069>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Vandewalle P, Huysseune A, Aerts P, Verraes W. Bels V, Chardon M, Vandewalle P, editors. The pharyngeal apparatus in teleost feeding. Biomechanics of Feeding in Vertebrates Adv Comp Env Physiol. 1994;18:59–92. [Google Scholar]
- Warga RM, Nüsslein-Volhard C. Origin and development of the zebrafish endoderm. Development. 1999;126:827–838. doi: 10.1242/dev.126.4.827. [DOI] [PubMed] [Google Scholar]
- Wilde CE. The urodele neuroepithelium. I. The differentiation in vitro of the cranial neural crest. J Exp Zool. 1955;130:573–591. [Google Scholar]
- Wise SB, Stock DW. Conservation and divergence of Bmp2a, Bmp2b, and Bmp4 expression patterns within and between dentitions of teleost fishes. Evol Dev. 2006;8:511–523. doi: 10.1111/j.1525-142X.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- Witten PE, Hall BK, Huysseune A. Are breeding teeth in Atlantic salmon a component of the drastic alterations of the oral facial skeleton? Arch Oral Biol. 2005;50:213–217. doi: 10.1016/j.archoralbio.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Yelick PC, Schilling TF. Molecular dissection of craniofacial development using zebrafish. Crit Rev Oral Biol Med. 2002;13:308–322. doi: 10.1177/154411130201300402. [DOI] [PubMed] [Google Scholar]
- Young GC. Did placoderm fish have teeth? J Vert Paleontol. 2003;23:987–990. [Google Scholar]
- Zhuravlev AV. Specific features of the hard tissues of late paleozoic conodont elements. Paleontol J. 2005;39:289–293. [Google Scholar]