Abstract
In zoology it is well known that birds are characterized by the presence of feathers, and mammals by hairs. Another common point of view is that avian scales are directly related to reptilian scales. As a skin embryologist, I have been fascinated by the problem of regionalization of skin appendages in amniotes throughout my scientific life. Here I have collected the arguments that result from classical experimental embryology, from the modern molecular biology era, and from the recent discovery of new fossils. These arguments shape my view that avian ectoderm is primarily programmed toward forming feathers, and mammalian ectoderm toward forming hairs. The other ectoderm derivatives – scales in birds, glands in mammals, or cornea in both classes – can become feathers or hairs through metaplastic process, and appear to have a negative regulatory mechanism over this basic program. How this program is altered remains, in most part, to be determined. However, it is clear that the regulation of the Wnt/beta-catenin pathway is a critical hub. The level of beta-catenin is crucial for feather and hair formation, as its down-regulation appears to be linked with the formation of avian scales in chick, and cutaneous glands in mice. Furthermore, its inhibition leads to the formation of nude skin and is required for that of corneal epithelium. Here I propose a new theory, to be further considered and tested when we have new information from genomic studies. With this theory, I suggest that the alpha-keratinized hairs from living synapsids may have evolved from the hypothetical glandular integument of the first amniotes, which may have presented similarities with common day terrestrial amphibians. Concerning feathers, they may have evolved independently of squamate scales, each originating from the hypothetical roughened beta-keratinized integument of the first sauropsids. The avian overlapping scales, which cover the feet in some bird species, may have developed later in evolution, being secondarily derived from feathers.
Keywords: amniotes, development, evolution, feather, gland, hair, keratin, scale, skin, Wnt
Introduction
The vertebrate integument, that is, the skin and cornea, is composed of a pluristratified epithelium overlying a mesenchyme. It forms the external body envelope, which creates the boundary between the organism and its environment. In all living vertebrates, at least from trout to human, specific types of alpha-keratins, K1-2/K10 and K3/K12, characterize the epidermis and corneal epithelium, respectively, showing a strong homology in the different lineages (O’Guin et al. 1987; Chaloin-Dufau et al. 1993). Only the sauropsids (birds and reptiles) possess an additional capacity for beta-keratin synthesis, an entirely different type of intermediate filament, which appears to result from a phylogenetic innovation that occurred after that of the alpha-keratins (Gregg & Rogers, 1986). For convenience I will use the word ‘reptile’ to distinguish birds from other living sauropsids: crocodiles, turtles and lepidosaurs (snakes and lizards). In all amniotes, the last supra-basal layers of the epidermis are cornified, meaning they are formed of dead cells filled entirely with alpha-keratin filaments coated with specific amorphous proteins and lipids, providing a barrier to water loss. In a variety of living amphibians, such as toads, a ‘stratum corneum-like’ layer exists, but it is only one to three cell depths, and nuclear remnants still persist (Spearman, 2005). In all vertebrates, the corneal epithelium is un-cornified and is protected only by tears. Another main difference between the skin and the cornea, in addition to the transparency of the latter, is the formation by the skin of cutaneous appendages, which are exclusively composed of epidermal cells and depend to a large extent upon dermal influences (Dhouailly, 1977; Chuong, 1998; Millar, 2002). In amniotes, no other anatomical feature differentiates mammals, birds and reptiles from each other so readily as do hairs, feathers and scales. However, each lineage displays various cutaneous appendages. In particular, birds exhibit scaled feet and mammals are not only characterized by hairs, but also by the large number and diversification of their cutaneous glands. The question of the evolutionary origins of amniote cutaneous appendages, as well as their diversity within one species, has long been of interest (among others: Rawles, 1963; Lucas & Stettenheim, 1972; Maderson, 1972, 2003; Bereiter-Hahn et al. 1986; Alibardi, 2003; Sawyer & Knapp, 2003; Prin & Dhouailly, 2004; Wu et al. 2004). The common ancestor of amniotes may have presented both a glandular and a ‘granulated integument’, i.e. an epidermis adorned with a variety of alpha-keratinized bumps, and thus may have presented similarities with the integument of common day terrestrial amphibians. The amphibian skin is often defined by its glabrous and glandular nature. However, it also can exhibit a variety of ‘warts’ or horny cones (Elias & Shappiro, 1957). Whereas the glandular quality of the integument was retained and diversified in the mammalian lineage, it was almost completely lost in the sauropsid lineage. The skin of living mammals, characterized by the mammary gland, also displays sweat, scent and sebaceous glands. In contrast, common day birds possess only one integumentary gland, known as the uropygial gland, which produces an oily secretion used for coating the feathers (Lucas & Stettenheim, 1972). Likewise, living reptiles present a few femoral or pre-cloacal glands, presumed to function in sexual attraction (among others: Maderson, 1972; Antoniazzi MM, Jared C, Junqueira LCU, 2005). Another noticeable difference between mammals and sauropsids is the keratin type of their hard keratinized structures. In mammals, the formation of hairs, claws, nails, hoofs, and some horns involves the production of an additional eight polypeptides of cysteine-rich alpha-keratins (the ‘hair keratins’, which show a helical arrangement) and of their associated amorphous proteins (Langbein et al. 2001). In contrast, the formation of claws, scales and feathers in sauropsids is associated with a totally different type of keratin polypeptides, arranged in pleated sheets, which are the beta-keratins (Dhouailly et al. 1978; Gregg et al. 1984; Gregg & Rogers, 1986; Sawyer et al. 2000; Alibardi & Sawyer, 2002). However, although the claws of squamates are principally made of beta-keratins, recent results show that they can also contain a few cysteine-rich alpha-keratins, and thus some hair-like proteins (Eckhart et al. 2008).
The first cell biology research about the morphogenesis of cutaneous appendages in the 1970s was related to cell interactions between the two skin components, the dermis and the epidermis. Analysing the results of heterospecific recombination between chick, duck and mouse embryos, I was the first to pinpoint (Dhouailly, 1973, 1975, 1977) that a first inductive signal emanating from the dermis (to make an appendage) instructs the epidermis to form a thickening, the placode. As I report ‘this signal had remained unchanged during the amniotes evolution, as it can be understood by an epidermis from a different class’. Thirty years later, I now believe that the same system has been independently re-utilized several times during amniote evolution. I also showed that the placode, once formed, signals back to the dermis to form a dermal condensation, and that the epidermis responds to the proliferation signal originating from the dermal condensation according to its genetic potential (Dhouailly, 1973, 1975). From this stage on, the dialogue that leads to the formation of cutaneous appendage architecture becomes incomprehensible between a dermis and an epidermis belonging to two different lineages (Dhouailly, 1975), but it is still decipherable between two different species from the same lineage (Dhouailly, 1970). What become confused in the dialogue are not the words, i.e. the diffusible proteins that remains similar, but the building of the sentences. Although the integument structures of birds and mammals, such as feet scales, feathers, hairs and glands, are very different in shape, I suggested as early as the 1970s (Dhouailly, 1977; Viallet et al. 1992) that they must speak a common language. Indeed, they share a number of common developmental pathways, such as the Shh, Bmps, Ectodysplasin, Wnts and Notch pathways (for a review see Chuong, 1998; Wu et al. 2004). The best example of this is that humans possessing the hypohydrotic syndrome, display mutations in different components of the Ectodysplasin pathway, revealing defects not only in hairs, but also in sweat glands and teeth. I now include the cornea as a special part of the integument. Its morphogenesis also involves at least the Wnt, BMP and Shh pathways (Gould et al. 2004; Mukhopadhyay et al. 2006).
A point that I did not understand at all in the 1970s, and which I have now a suggestion about, is that while a lizard epidermis is able to respond to part of the first signal that originates from a chick or mouse dermis, forming protruding scale buds, the reverse is not true. The shape and size of the scale buds formed by the lizard epidermis are determined by the regional origin of the chick or mouse dermis, whereas when a chick or a mouse epidermis is recombined with a lizard dermis, it remains flat, i.e. of the ‘inter-follicular type’. Heterotopic homospecific dermal/epidermal recombination showed that the lizard dermis is endowed with regional information, i.e. ventral or dorsal, leading to rounded or rectangular scale shape (Dhouailly, 1975). However, it may lack, or express at a too low level, the main pathway, which appears to have been independently utilized twice during amniote evolution, in the mammalian and avian lineages. This pathway allows the formation of long, protruding cutaneous appendages, hair or feather, and thus appears to be linked, probably secondarily, to the independent acquisition of endothermy in both lineages. This dermal signal, belonging to the Wnt family (among others: Gat et al. 1998; Chodankar et al. 2003; Pearton et al. 2005; Nähri et al. 2008), activates not only the formation of placodes, but also the start of their differentiation. In fact, in chick/mouse dermal/epidermal recombinants, the placodes either give rise to protruding feather buds with aberrant barb ridges, or to downward growths that form the hair pegs, depending on the lineage origin of the epidermis (Dhouailly, 1973).
It should be noted that the fossil record is biased toward large animals, as only a small percentage of small animals are represented. Furthermore, even fewer deposits preserve mammalian hairs (Meng & Wyss, 1997), than avian feathered or scaled integument. This is even true with the fine sandstones of the Liaoning province of China. When the amniote ancestors started to live exclusively on land in the late Carboniferous, they derived from a group of basal amphibiotic tetrapods, and it is plausible that they evolved a skin barrier similar to that of modern toads to prevent desiccation. With time, two amniote lineages became distinct – the synapsids, from which modern mammals are derived, and the sauropsids, which regroup lepidosaurs (lizards and snakes), archosaurs (crocodiles and birds, the only living representatives of a lineage also represented by extinct dinosaurs) and testudines (turtles). Lepidosaurs and archosaurs are diapsids, but the chelonian are anapsids, and their relationships with regard to the other sauropsids are still debated. They could be the most primitive group of sauropsids or, conversely, their absence of temporal fenestra could be secondarily derived.
The important point in what concerns the tetrapod's integument evolution is that synapsids separated from the amniote tree before the innovation of beta-keratin, but, to my knowledge, their first known specimens do not display a preserved integument. A long-held view is that feathers and hairs evolve from the epidermal overlapping scales of a common tetrapod ancestor of sauropsids and mammals (Maderson, 1972; Sharpe, 2001). A correlated view is that avian scales are directly related to reptilian scales (among others: Bornstein, 1911, cited in Lucas & Stettenheim, 1972; Alibardi & Sawyer, 2002). The recent discovery of many intermediate forms of theropods and even of non-theropod dinosaurs exhibiting feather-like appendages in the Liaoning province of China has introduced new insights into the evolution of feathers (Brush, 2000; Prum & Williamson, 2001; Chuong & Homberger, 2003; Hou et al. 2003; Prum, 2005). These specimens show the progressive complexity from tubular proto-feathers to feathers, but they are not proof of a ‘reptilian’ overlapping scale origin. The origin of hair is poorly understood, but if its initial function was sensory, it is likely that vibrissae may have appeared long before an insulating pelage evolved (Maderson, 2003). No intermediate form has ever been found between scales and hairs, resulting in only a few proposals of how mammalian hairs may have evolved from scales. These proposals were based on the development of sensory bristles in the hinge scale region of reptiles (Maderson, 1972), or on the topology of hair-type keratins in different types of tongue papillae (Dhouailly & Sun, 1989). Recently (Eckhart et al. 2008) it was proposed that hair evolved from reptilian claws, based on the finding of alpha hair-like proteins in these mostly beta-keratinized structures. However, these data do not prove that an evolutionary link between hair and reptilian claw exists. Cysteine-rich alpha-keratins are not restricted to mammals, meaning that the evolution of hair involved the co-option of pre-existing proteins, which may have been present in a basal amniote, i.e. a common ancestor of mammals and sauropsids. Less classically, hairs also have been proposed to originate from the innervated conical keratinized structures of basal amphibians (Elias & Bortner, 1957), or from a component of a sebaceous gland apparatus (Stenn et al. 2008).
Here I will defend two views that oppose the classical ones: neither hairs nor feathers derive from reptilian overlapping scales, and feathers are the origin for avian scales, scuta and reticula. My arguments are shaped by several experimental results from different laboratories of developmental biology. Thus they arise from the part of science that I know best: the embryogenesis of the integument of common day amniotes. In what follows, I will first discuss the conditions required by the ectoderm and its underlying mesenchyme to form an integument. Then I will outline how a shared similar stage of placode formation is reached in the formation of avian scales, feathers, and hairs, with the exception of reptilian scales. Finally, I will highlight the parallels between the regulation of the implicated pathways in birds (feathers vs. scales) and in mammals (hair vs. glands). Several experimental results show that avian ectoderm is primarily programmed toward forming feathers, and mammalian ectoderm toward forming hairs. The other ectoderm derivatives, scales in birds, glands in mammals, and cornea in both lineages, appear to require a negative regulatory mechanism of this basic genetic program.
Early morphogenesis of the integument
The early morphogenesis of avian and mammalian integument implicates the formation of a dense dermis, followed by that of placodes overlying dermal condensations, in contrast to squamate skin. The first and most crucial step in integument morphogenesis is the formation of a dense dermis. Two main types of dermis are present in birds and mammals at the onset of skin morphogenesis: a superficial dense dermis (overlying a deep sparse dermis) characteristic of future feather or hair fields, vs. a superficial loose dermis, in future bare skin regions. The origin of the dorsal dermis from the somite dermomyotome has been traced in birds by chick/quail chimerae (Olivera-Martinez et al. 2000) and in mice by mouse/chick chimerae (Houzelstein et al. 2000). A wave of Dermo1 expression correlates with the wave of dense dermis formation: in a medial-lateral wave from day 4.5 of incubation in chicks, and reversely in a lateral-medial wave from 12 days of gestation in mice (Olivera-Martinez et al. 2004a). When the dense dermis formation is prevented, as in the chick Ottawa naked mutant, the embryos develop a few feathers, but for the most part are totally naked (Fig. 1A,A′), in contrast to the wild-type embryo (Fig. 1B,B′). Conversely, the experimental expression of Dermo-1in chick embryos (Hornik et al. 2005) is sufficient to induce a dense dermis formation and the subsequent cutaneous appendage morphogenesis. The next visible step of skin differentiation has been well studied, mostly in birds. It consists firstly of the appearance of placodes within the epidermis, followed by the formation of dermal condensations (Dhouailly, 1984). The ubiquitous dermal signal, which provokes the placode formation, is composed of at least one Wnt, as shown by several studies (among others: Gat et al. 1998; Noramly et al. 1999; Widelitz et al. 2000; Chodankar et al. 2003; Pearton et al. 2005; Nähri et al. 2008). The next step, the placode formation, common to sweat gland, hair, feather, avian scale, and tooth, implicates the ectodysplasin signaling (Mikkola & Thesleff, 2003). The placode individualization is followed by the redistribution of cells of the dense superficial dermis to form a regular array of local condensations under the placodes, which are separated by a loose interfollicular dermis (Michon et al. 2007). Such a redistribution is enhanced by BMP7 and FGF4 from the placodal epidermis and arrested by BMP2 (Michon et al. 2008). In chicks, the formation of rectangular overlapping scales or scuta, which cover the tarsometatarsus and dorsal face of the digits, involves the association of a placode with a dermal condensation (Sawyer, 1972, 1983; Dhouailly, 1984). This is not the case with the morphogenesis of the small tuberculate scales or reticula of the chick foot pads (Sawyer, 1972, 1983; Prin & Dhouailly, 2004). In mammals, the hair placodes are also associated with dermal condensations, and the same signaling pathways have been implicated (for a review see Millar et al. 2002; Botchkarev & Paus, 2003).
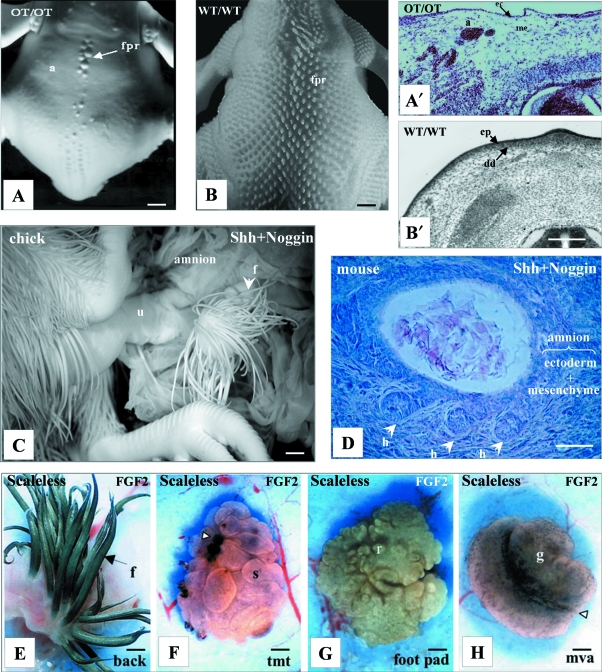
Fig. 1.
The formation and regionalization of the dense dermis. (A,A′) In the chick Ottawa naked mutant (OT/OT), a dorsal view at E10 shows that most part of the skin stays glabrous (A); a transversal section at E7 shows that most parts of the mesenchyme remain loose over the neural tube (A′). Compare with the formation of feather primordia and of a dense dermis formation in a wild type embryo (WT/WT) at the same stages (B,B′). After Olivera-Martinez et al. 2004b. Reproduced with permission of Int J Dev Biol. (C) Production of an ectopic feathered skin in the chick amnion at E14, following the graft of aggregate of Shh- and Noggin-producing cells under the ectoderm at E2 in the extra-embryonic area. After Fliniaux et al. 2004a. Reproduced with permission of the Company of Biologists. (D) Production of a skin with a pluristratified epidermis and three stage 3 hair pegs by a graft under the kidney capsule of mouse amnion associated to a mixed clump of Noggin- and Shh-producing cells. After Fliniaux et al. 2004b. Reproduced with permission of Differentiation. (E–H) The chick scaleless mutant forms a skin that comprises a dense dermis and that has potential regional characteristics which can be revealed by FGF2 treatment (tmt, tarsometatarsus; mva, midventral apterium). The white arrowhead in (F) shows the FGF2 beads, and in (H) the midventral line. After Prin & Dhouailly, 2004. Reproduced with permission of Int J Dev Biol. a, apterium; dd, dense dermis; ec, ectoderm; ep, epidermis; f, feather; fpr, feather primordial; g, glabrous; h, hair bud; me, mesenchyme; r, reticula; s, scuta; u, umbilical chord. Bars: A–C: 1 mm, A′,B′: 400 µm, D: 150 µm, E–H: 200 µm.
The morphogenesis of the scaled integument of reptiles is less well known than that of avian or mammalian skin. In contrast to the latter two, the first steps of reptilian integument morphogenesis are uncommon (Maderson, 1965; Dhouailly, 1975; Maderson & Sawyer, 1979; Dhouailly & Maderson, 1984). Whereas the earliest stages of epidermal differentiation resemble those reported for other amniotes, precocious deposit of dermal collagen fibrils resembles more closely that of anamniotes, such as zebrafish (Le Guellec D, Morvan-Dubois G, Sire JY, 2004). Furthermore, when scale anlagen first appear in a lizard embryo, they consist of symmetrical elevations of the entire skin, with the epidermis exhibiting a uniform thickness. Therefore, there are no distinct epidermal placodes and no dermal condensations in lizard scale formation. Regularly spaced, potentially contractile units appear to join the epidermis to the deep layer of the dermis, and form the frontier between adjacent scales (Dhouailly & Maderson, 1984).
The amnion in birds and mammals: a potential feathered or hairy skin
The amnion, which characterizes the amniotes, has a simple structure: a unistratified ectoderm overlying a unicellular stratum of somatopleural fibroblasts. Almost 40 yr ago, recombination experiments showed that another extra embryonic ectoderm, i.e. the chick chorionic epithelium, is able to undergo complete feather morphogenesis in response to an embryonic chick back dermis (Kato, 1969). Likewise, more recently we showed that mouse amnion ectoderm is able to form hairs under direct dermal influence (Fliniaux et al. 2004b).
More interestingly, we demonstrated an autonomous transformation of the chick (Fliniaux et al. 2004a) or mouse (Fliniaux et al. 2004b) amnion into a typical skin with feathers or hairs, respectively (Fig. 1C,D). In these two species, such a metaplasia requires the same molecular influences: Noggin, which is needed to counter the BMP4 pathway in the ectoderm, and Shh, responsible for stimulating the proliferation of the somatopleural mesoderm. Both effects combine to lead to the creation of a true skin with its respective appendages: feathers or hairs. To obtain these results, we grafted aggregates of cells engineered to produce diffusible Noggin or Shh factors within the amnion. By following sequentially what happens in grafted chick embryos, we showed that host somatopleural mesoderm cells are induced by the grafted cells during a short time window to become a feather-forming dermis. These grafted cells are subsequently dragged distally from the induced skin by the movements and growth of the amnion. Thus, both in birds and in mammals the activation of ectoderm and mesoderm cells leads to the formation of the most complicated skin appendages: feathers and hairs. We never observed the formation of simple scales in the case of chicks, or of simple glands in the case of mice. Thus in my view, avian ectoderm and mammalian ectoderm are genetically programmed to build feathers or hairs, respectively. The formation of other skin appendages, such as foot scales in birds, glands in mammals, or cornea in both lineages may require a negative regulation of this basic program.
Diversity of integument appendages
Feathers vs. scutate scales morphogenesis
Although feathers cover the majority of the body, most common day bird's possess scaled tarsometatarsi and toes, whereas some species, such as the owls, have the upper side of their feet entirely covered with contour and downy feathers. However, in all cases, the plantar face of avian feet is covered with tuberculate scales. Mary Rawles was right (1963): the best model to study regional skin variation during skin morphogenesis is the chick embryo. Not only their shape but also the set of keratins of each appendage type are different (Dhouailly et al. 1978; Sawyer, 1983; Gregg & Rogers, 1986). The feather is the most complex cutaneous appendage yet to be produced during evolution. The teleoptile or adult feathers are of several types, including remiges, rectrices, contour and downy feathers (Lucas & Stettenheim, 1972). A remige, or flight feather, is bilaterally asymmetric and composed of a calamus, bearing a rachis, itself bearing barbs, themselves bearing barbules, the hooks of which interlock, forming a feather vane. Down feathers are radially symmetric, the barbs being directly attached to the calamus. The neoptile (neonatal) feather is downy, but can be radially symmetric or bilaterally asymmetric, possessing or not a rachis, and presenting 10 or 20 barbs, all depending on the dermal species, chick or duck (Dhouailly, 1970).
In the chick embryo, the future feather first appears on the back as a round primordium, composed of a placode overlying a dermal condensation by day 7. Primordia are separated by an interfollicular skin and form a regular hexagonal pattern. After the chick feather bud protrudes by day 7.5, it elongates into a tubular structure, called the feather filament. By day 14 of incubation, the base of the feather filament invaginates into the dermis to form the feather follicle, which houses the epidermal stem cells (Yue et al. 2005), which in turn will give rise to the successive feather generations. The epidermal wall of the feather filament forms a number of barb ridges and is radially or bilaterally symmetric in conformity with the origin of the dermis (Dhouailly, 1970). Recently, two laboratories (Harris et al. 2002; Yu et al. 2002) were able to manipulate the number and size of the feather barbs and rachis by playing with BMP and Shh. They demonstrated that this molecular module is used at different steps of feather morphogenesis, from the placode to barbule morphogenesis, building an increasingly complicated structure: the feather. The epidermis or wall of the feather filament comprises three layers. The intermediate layer gives rise to the feather proper, i.e. calamus, rachis, barbs and barbules, and expresses four main beta-keratin polypeptides of about 14–16 kD. In contrast, the outer and inner layers express only alpha-keratins and disintegrate by hatching, to let the neoptile down feather pop out.
In the chick, three main types of scales can be distinguished: large, oblong overlapping scales, or scuta, cover the dorsal surface of the tarsometatarsus and digits; smaller oblong overlapping scales, or scutella, cover the ventral face of the tarsometatarsus; and small, rounded, non-overlapping scales, or reticula, cover the plantar surface (Lucas & Stettenheim, 1972). The first indication of scuta development is the appearance of three placodes by day 10 at the level of the distal epiphysis of the tarsometatarsus (Sawyer, 1983). The scuta and the scutella correspond only to the outer epidermis, which expresses both alpha- and two major beta-keratins polypeptides of about 18–19 kD, while the hinge or articulate region is an inter-appendage epidermis, similar to the inter-feather alpha-keratinized epidermis (O’Guin & Sawyer, 1982). The tuberculate scales, or reticula, which cover the plantar surface are made only of alpha-keratins, with the exception of their transient embryonic peridermal layer, which is composed of beta-keratins (Sawyer, 1983). The overlapping of the scuta is sustained by a discrete dermal condensation (Sawyer, 1983; Dhouailly, 1984), whereas the non-overlapping reticula do not display such a specialized structure of the dermis (Sawyer, 1983).
Primary experiments in chicks (Saunders et al. 1959) have shown that the regional characteristics within future skin regions of the hindlimb are established early during embryogenesis, between 2 and 4 days of incubation. Recent experiments showed that the over-expression of Dermo-1 induces the formation of a dense dermis, followed by the formation of feathers in apteric regions, of supernumerary feathers in pterylae, and of supernumerary scuta in the hindlimb (Hornik et al. 2005). Consequently, the epidermal potentiality is different in apteric and feathered regions from the epidermal ability in scaled regions. In Scaleless chick mutant embryos, the dense dermis forms (Viallet et al. 1998; Widelitz et al. 2000) and the skin is endowed with regional characteristics, as revealed by the experience with FGF2 beads (Fig. 1E–H) (Dhouailly et al. 1998). While the Hox code may be involved in determining regional specificity in skin (Chuong et al. 1990; Kanzler et al. 1997), by specifying the regional identity of dermal and epidermal progenitors, the following mechanisms, which allow for the competence of the epidermis, appear to be much more complex, especially for the formation of simple scales.
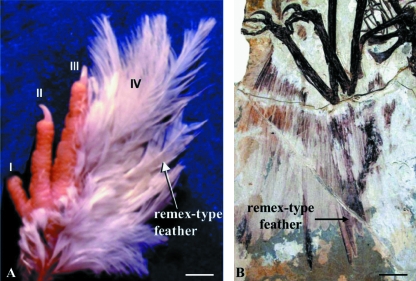
The scuta-feather metaplasia has been easily obtained in several types of experiments (Dhouailly et al. 1980; Tanaka et al. 1987; Zhou & Niswander, 1996; Widelitz et al. 2000). Most of the time, the formation of the feathers, made of feather-type keratins (Dhouailly, unpublished data), do not render the scales unidentifiable, and are formed by continuous growth at the scale tip (Fig. 3B,D). This can occur in nature as shown by several cases of mutation in poultry (Somes, 1990). Ptilopodia, a condition in which one or more rows of feathers replace the scuta, or are carried by them along the fourth tarsometatarsus and digit IV, is characteristic of several breeds of chicken. In the case of the Peking Bantam breed, the first teleoptile feathers appear simultaneously on the wings and the feet, and are similar in morphology (remex-type) and outgrowth, when chickens are raised with care (Fig. 2A). The wonderful discovery of four-winged dinosaurs (Xu et al. 2003) (Fig. 2B) may contribute to the hypothesis that the feet of the ancestors of common day birds were almost entirely covered with feathers.
Fig. 3.
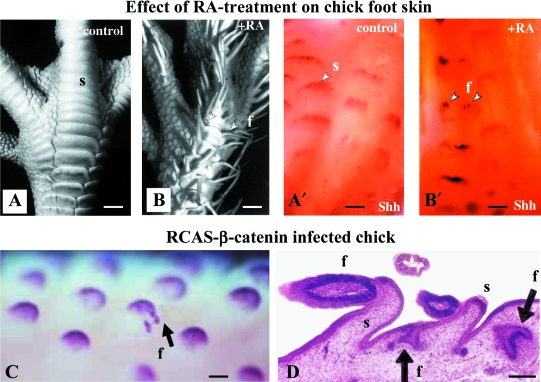
Scale-feather metaplasia. (A,B′) Chick skin morphogenesis at 18 days and Shh expression at 12 days in control (A,A′) and retinoic acid-treated (B,B′) embryos. After retinoic acid treatment, overlapping scutate scales in the control are replaced upon by feathered scuta at 10 and 11 days. The expression of Shh, which is at a low level at the distal tip of the scuta, is up-regulated in two or three spots (arrowheads) of the scuta distal tip. After Prin & Dhouailly, 2004. Reproduced with permission of Int J Dev Biol. (C,D) The over-expression of beta-catenin results not only in the formation of supernumerary small buds in the feather fields (C), but also in scale-feather metaplasia (D). (C) After Noramly et al. 1999. Reproduced with permission of the Company of Biologists. (D) After Widelitz et al. 2000. Reproduced with permission of the Company of Biologists. f, feather; s, scuta. Bars: A,B: 1mm A′,B′,D: 200 µm, C: 250 µm.
Fig. 2.
Four-winged birds, today and yesterday. (A) The Peking Bantam chick epidermis expresses its feather program on the dorsal IV digit and tarsometatarsus of the foot. Téléoptile feathers, which had appeared concomitantly with the wing remiges 7 days after hatching, display at 3 weeks a remex-type and not a simple covert-type. After Prin & Dhouailly 2004. Reproduced with permission of Int J Dev Biol. (B) The well-preserved remex-type feathers at the distal hindlimb position (on tarsometatarsus) of the four-winged dinosaur Microraptor gui, the most interesting discovery among the feathered dinosaurs. After Xu et al. 2003. Reproduced with permission of the Company of Biologists. Bars: A: 5 mm, B: 5 cm.
Taking together different experimental results that led to the scuta-feather metaplasia, I suggest the following scenario concerning the abilities of the anterior chick foot skin to form cutaneous appendages. The ability to form the overlapping oblong scuta is acquired by the mesenchymal cells by day 4 of incubation, at the time of limb bud formation (Saunders et al. 1959). This mesenchyme then provokes in its overlying epidermis a shortening of the basic feather program. By 6–7 days, the treatment with BrdU, which is endowed with mutagenic activity (Maeir P, Weibel B, Zbinden G, 1983), can erase (the mechanism remaining unknown) this restriction and lead to the formation of feathers instead of scuta (Tanaka et al. 1987). By 8.5 days of incubation, the wild-type tarsometatarsal dermis is endowed with a declining ability to inhibit the feather program of its associated epidermis, as shown by the recombination of a wild-type dermis with a Peking Bantam epidermis (Prin & Dhouailly, 2004). By 10 days, the wild-type tarsometatarsal dermis, which has completely lost this ability, is able to induce the formation of feathers in back epidermis (Rawles, 1963). When chick embryos are treated with retinoic acid, at 10 days the competence of the tarsometatarsal epidermis is modified: feathered scales were obtained in the recombinants of treated epidermis and untreated dermis, whereas the reverse recombinants formed only scales (Cadi et al. 1983). By 12 days, the scuta are formed in untreated embryos, and their dermis is endowed with the ‘second message’, which triggers the beta-keratinization of cells (Dhouailly, 1977; Dhouailly et al. 1978; Dhouailly & Sawyer, 1984).
This interpretation creates understanding of why the complex formation of feathers at the tip of scuta is so easy to obtain in different types of experiments. At different steps of hindlimb skin morphogenesis, different pathways may be up-regulated during the experimental conversion of avian scuta into feathers. Such a hypothesis was confirmed by different experiments. Shh expression in the epidermis is known to occur at the time of primordia formation, and at decreasing levels in feather, scuta and reticula morphogenesis. Now, the scuta-feather metaplasia induced by the treatment with retinoic acid appears to result from an enhancement of Shh expression (Fig. 3A,B′) (Prin & Dhouailly, 2004), which by triggering cell proliferation allows the outgrowth of the tips of scutate scales into feathers. The dermal condensation is less developed in scuta than in feather morphogenesis (Sawyer, 1983; Dhouailly, 1984). Recent experiments in my group (Michon et al. 2008) show that the BMP7 and BMP2 play opposite roles, positive and negative, respectively, in the formation of the dermal condensation in chick embryos. Using a dominant negative type I BMP receptor, the growth of feathers on scuta has been obtained (Zhou & Niswander, 1996). As this receptor specifically binds BMP2 and has a low affinity for BMP7, we can presume that the formation of the dermal scale condensation was not restricted. Nevertheless, the most interesting results concern the regulation of the beta-catenin pathway, which is implicated at the first step of cutaneous appendage formation, and leads to the formation of placodes. Forced expression of stabilized beta-catenin, which transduced from the replication competent avian sarcoma virus (RCAS), caused the formation of supplementary feathers in feather fields (Fig. 3C) (Noramly et al. 1999), as well as the scuta-feather metaplasia (Widelitz et al. 2000) (Fig. 3D). Thus, I agree entirely with a previous proposal (Widelitz et al. 2000) that the beta-catenin level in the epidermis may be linked with the different types of epithelial outgrowth, scuta or feather development. Moreover, it may be a link between the Hox code in the hindlimb and the down-regulation of the beta-catenin in this region.
Whereas the scuta-feather metaplasia happens in nature through mutation, and is very easily obtained in various experimental conditions, the reverse, the feather-scuta metaplasia, has never occurred naturally or been obtained experimentally. Only scale-like structures, not true scales, which are made of keratin polypeptides specific to the feather-type, can result from fusion of arrested buds after retinoic acid treatment (Kanzler et al. 1997) or by Shh signaling inhibition (Prin & Dhouailly, 2004).
Feathers and scutate scales of living birds express their unique pattern of beta-keratins, of about 15 and 18 kD, respectively. The classical view on evolution of feather beta-keratins – that they originated by the deletion of a repeat region from avian scale beta-keratins (Gregg et al. 1984) – is challenged by recent results from Alibardi laboratory (Dalla Valle et al. 2008). Comparing juvenile crocodilian beta-keratins with squamate and avian beta-keratins, they propose, in contrast with the classical view, that the beta-keratins of avian scale are in fact derived from the feather beta-keratins.
Finally, the avian scutate (and scutellate scales) which cover the tarsometatarsus and the dorsal face of the foot digit, may have appeared secondarily, after feather innovation, and in only some species lineages. In contrast, all living birds possess small tuberculate scales, or reticula, on their plantar surface. The avian plantar skin bears some resemblance to the hypothetic granulated skin of the sauropsidan ancestors, and their ‘reptilian’ nature has been suspected (Brush, 2000), but what can be presumed from their embryonic morphogenesis and keratin differentiation?
Feathers and scutate scales vs. plantar reticula morphogenesis
The reticula, which cover the avian foot plantar surface, are very different from the scutate scales. Their formation does not involve a placode, or a dermal condensation, and they are only made of alpha-keratins, except for their peridermal layer, which does not persist to adulthood (Sawyer, 1983). They appear first by day 11 in the centre of the chick embryo foot pad as symmetrical roundish bumps (Dhouailly et al. 1980). Reticula-feather metaplasia never occurs in nature, which is easily understood, as it would prevent walking, climbing and perching.
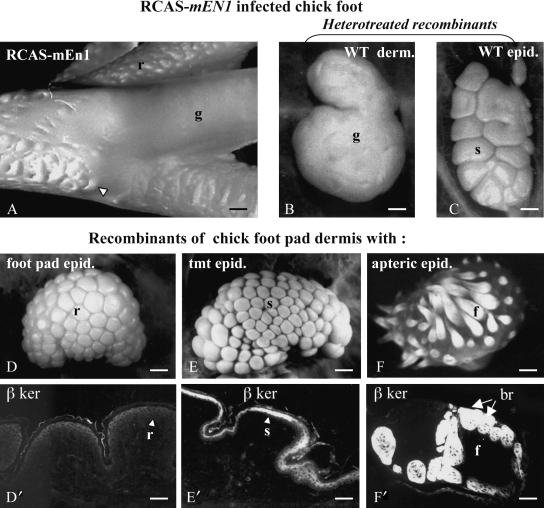
The scuta-reticula metaplasia has been obtained by RCAS-mEngrailed1infection of the dorsal hindlimb ectoderm that converts it to a ventral ectoderm (Prin et al. 2004). This experiment led to the formation of reticula or even of glabrous (nude) skin on the dorsal surface of the feet (Fig. 4A). Hetero-infected dermal/epidermal recombinants showed that only the competence of the epidermis was modified by the expression of En-1 (Fig. 4B,C). The prevention of the outgrowth and bending of scuta appears to be followed by the absence of the expression of beta-keratins in its epidermal layers. Furthermore, dermal/epidermal recombination experiments involving a wild-type plantar dermis (Fig. 4D–F′) showed that this dermis is endowed with the ability to induce reticula, scuta, or feathers, depending on the origin of the epidermis it is associated with: plantar, tarsometatarsal, or apteric, respectively (Prin & Dhouailly, 2004). Whilst the feather program is completely available in the epidermis from feathered or apteric regions as well as in the ectoderm of extra-embryonic area, it appears to be down-regulated in scutate epidermis, and entirely inhibited in plantar epidermis. More precisely, in the case of plantar skin, the inhibition of feather formation is mediated by the epidermal expression of En-1, which down-regulates Shh expression, and prevents that of Wnt7a(Prin et al. 2004). The plantar epidermis does not form placodes and is unable to trigger the formation of dermal condensations, which are required for the next step of cutaneous appendage morphogenesis. In contrast, the dermis of the chick plantar region is able to trigger a complete feather morphogenesis in an apteric epidermis, the later being endowed with an intact cutaneous appendage program (Prin et al. 2004). It should be noted that the same epidermal molecular mechanism prevents hair formation on the mammalian plantar surface (Loomis et al. 1996).
Fig. 4.
Engrailed-1 and the inhibition of cutaneous appendage morphogenesis in the chick foot. Skin differentiation at 18 days on dorsal side of the foot of a RCAS mEn-1-infected chick embryo. The overlapping scuta (compare with the control, Fig. 3A) are replaced by glabrous skin together with convoluted (arrowhead) or rounded reticula. (B,C) Heterotreated recombinants morphogenesis shows that the RCAS-En-1 infection affects only the epidermis and does not change the dorsal tarsometatarsus properties of the dermis. (D–F′) The usual expression of En-1 in the epidermis of the plantar region prevents the formation of beta-keratinized (β-ker) cutaneous appendages (D,D′), while the association of a plantar dermis with a tarsometatarsal epidermis leads to the formation of small scuta (E,E′) and that with a neutral epidermis from the midventral apterium leads to feather morphogenesis (F,F′). Note that the pattern in all cases depends on plantar dermis. After Prin & Dhouailly 2004. Reproduced with permission of Int J Dev Biol. br, barb ridges; derm, dermis; epid, epidermis; f, feather; g, glabrous; r, reticula; s, scuta. Bars: A: 400 µm, B–F′: 150 µm.
Chuong's, as well as Niswander's, group (Zhou & Niswander, 1996; Widelitz et al. 2000) did not obtain reticula-feather metaplasia, only scuta-feather metaplasia. Their experiments, playing with beta-catenin or with the BMP type I receptor, resulted in enhancing respectively the placodal or dermal condensation abilities of the scutate scales to the point of feather level. However, those experiments did not alter the inhibition factor of the plantar epidermis. The reticula-feather metaplasia has been obtained experimentally only once, by retinoic acid treatment during the appearance of reticula buds (Dhouailly et al. 1980). By repeating daily the retinoic acid treatment from day 10 to 12, it is possible to transform all the reticula, which appear sequentially, into feathers. The explanation is that retinoic acid treatment, which enhances Shh expression in the epidermis, leads to the formation of feathers on reticula, bypassing the earlier ectoderm down-regulation of Shh by En-1 (Prin & Dhouailly, 2004). Finally, reticula are not true cutaneous appendages, and appear to be feathers arrested in the initiation step of their morphogenesis: formation of a slight bump, without a placode.
Hair vs. gland morphogenesis
There are various types of hairs, such as sensory hairs or vibrissae, and two major types of hair pelage: primary hairs, or guard hairs, and secondary hairs. The secondary hairs comprise three different types of hairs: auchene, zigzag and awl. The first steps of hair morphogenesis are similar to those of feather morphogenesis: formation of a placode and a dermal condensation, but after this, their morphogenesis differs. The epidermal placode grows downward to form the hair peg, which then circumvallates the dermal condensation, which becomes the dermal papilla. Hair morphogenesis is less complicated than feather morphogenesis. A single fiber, or hair shaft, is produced by the hair follicle, which can be compared to the barb, the basic element of the feather. The bottom portion of the hair peg forms the hair matrix, which is overlaid by the first conical inner root sheath. The concomitant outgrowth of the hair shaft and the inner root sheath push away the apoptotic cells of the center of the upper portion of the hair peg, leaving room for the hair canal (Dhouailly, unpublished data), while the peripheral portion becomes the outer root sheath. The upper portion of the outer root sheath harbors the stem cells (Cotsarelis et al. 1990), which give rise to successive hair generations. The gland morphogenesis starts either by an isolated placode, followed by a growth downwards in the dermis, or by a budding of the lateral wall of the hair peg. The gland lumen forms secondarily. The majority of mammals, such as mice, have glabrous foot pads with well-developed and isolated sweat glands, while a smaller collection, including rabbits, have plantar hairy skin. Sebaceous glands are usually associated with hair follicles, forming a pilo-sebaceous unit, with which a sweat gland is often associated, depending on the species. In monotremes, hair follicles are associated with mammary glands, forming a lactating patch. This association is transiently retained during mammary gland embryonic development in marsupials (Long, 1969): the early development of didelphid mammary gland resembles that of adult monotremes, whereas, during later development, there is a nipple eversion, and degeneration of the associated hair follicles.
Thus, a basic question arises as far as the mammalian integument is concerned: at what point does cutaneous gland development diverge from that of the hair follicle program? Although the same pathway families that are present during skin morphogenesis in birds have been implicated in mammals (for a review see Millar et al. 2002; Botchkarev & Paus, 2003), there are some differences in the pathways, even within the different types of pelage hairs. In Noggin knockout mice, only the primary hair follicles form (among others: Botchkarev et al. 2002; Plikus et al. 2004). Conversely, defects in ectodysplasin (as in Tabby mice) cause a complete lack of the first developing hair follicles (among others: Laurikkala et al. 2002). Human and mice with defects in different components of the ectodysplasin pathway fail not only to develop primary hair follicles, but also sweat glands, and display teeth defects (Mikkola & Thesleff, 2003). Retinoic acid treatment, which provokes feather formation on the tip of chicken scales, also has marked effects on mouse upper-lip morphogenesis, leading to the development of glomerular glands instead of hair vibrissa follicles; however, the treatment does not change the hair pelage developmental program (Hardy & Bellows, 1978). Treatment with a retinoic acid receptor pan agonist induces not only a hair vibrissa-glomerular gland metaplasia, but also a sebaceous gland hypertrophy (Blanchet et al. 1998). Dermal/epidermal heterotopic recombinants between upper-lip and dorsal skin, of which only one component was treated with retinoic acid, showed that the inducing capacities of the dermis to form a dermal condensation are altered. Moreover, only the explants involving an upper-lip epidermis form glands (Viallet & Dhouailly, 1994). Thus, the competence of the snout epidermis is different than that of the remaining body epidermis, but its molecular basis remains totally unknown. Some explanation should come from the knowledge of pathway differences between vibrissae and hair pelage follicles during their morphogenesis.
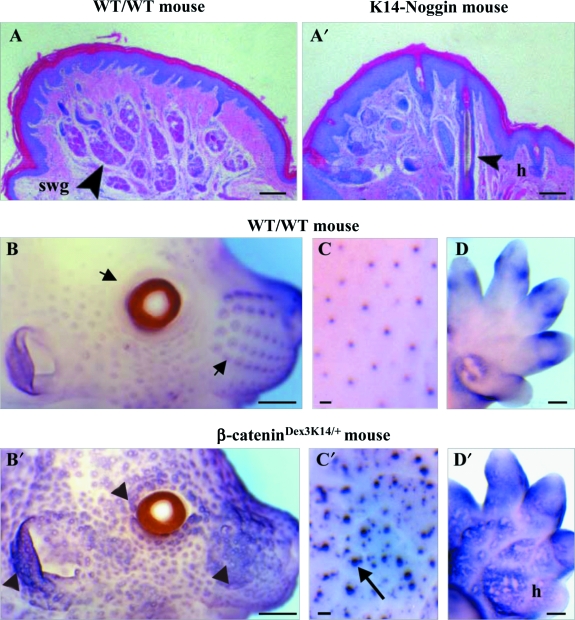
As in birds, the dorsal/ventral orientation of the limb bud depends on Engrailed-1 expression in the ventral ectoderm. In mice, the expression of En-1 in the presumptive palmar/plantar ectoderm is required for eccrine gland development (Loomis et al. 1996): the loss of En-1 expression in the palmar/plantar ectoderm causes a sweat gland-hair metaplasia. This metaplasia occurs in two other experiments: (1) when Noggin, a BMP antagonist, is over-expressed in the plantar epidermis (Fig. 5A,B) (Plikus et al. 2004), and (2) when beta-catenin is over-expressed in mouse embryo epidermis (Fig. 5D,D′) (Nähri et al. 2008). Likewise, recent experiments (Mayer et al. 2008) showed the conversion of the mammary gland nipple into hair-bearing skin by lowering BMP activity. Beta-catenin is the major factor in the initiation of feather morphogenesis (Noramly et al. 1999; Widelitz et al. 2000) and plays a similar role during mammalian skin morphogenesis for both adult (Gat et al. 1998) and embryo (Nähri et al. 2008). The laboratory of I. Thesleff recently showed that the expression of stabilized beta-catenin results in accelerated and supplementary formation of hair placodes in the hairy and glabrous fields in transgenic mice embryo (Fig. 5B,C′), as well as the abutting of hair pegs. Conversely, mice whose Wnt/beta-catenin pathways are inhibited do not form mammary glands, hairs or teeth (Andl et al. 2002; van Genderen et al. 1994; for a review on mammary gland, see Velmaat et al. 2003).
Fig. 5.
Gland-hair metaplasia in mouse plantar region and supernumerary hair follicles. (A,A′) The inhibition of the BMP pathway in K14-Nogginmouse leads to the formation of hair follicles (A′) instead of sweat glands (A). After Plikus et al. 2004. Reprinted with permission from the American Society for Investigative Pathology. (B–D′) The sustained expression of beta-catenin leads not only to the formation of precocious and supplementary hair pelage (compare B and B′, C and C′), but also to the formation of hair buds on the plantar surface (compare D and D′). The arrows and arrowheads attract attention on normal and abnormal skin pattern. After Nähri et al. 2008. Reproduced with permission of the Company of Biologists. swg, sweat gland; h, hair. Bars: A,A′: 100 µm, B,B′: 2 mm, C,C′: 200 µm, D,D′: 4 mm.
Thus, similarly to what happens in birds (Widelitz et al. 2000), the level of beta-catenin in the epidermis may be linked with the different types of epithelial outgrowth, cutaneous glands or hairs, as well as with the different pathways that are involved: for example, Shh for hairs, and Ihh for sebaceous glands (Niemann et al. 2003). In the same way, when Wnt signaling is inhibited by ablation of the beta-catenin gene or by expressing an N-terminally truncated Lef-1 transgene that lacks the beta-catenin binding site, hair follicles are converted into cysts with associated sebocytes (Niemann C, Watt FM, 2002). In reverse, short-term, low level beta-catenin activation stimulates de novo hair follicle formation from sebaceous glands (Silva-Vargas et al. 2005). Furthermore, studies of epidermal stem cells differentiation showed that the level of beta-catenin controls their lineage, i.e. hair keratinocytes or sebocytes (Huelsken et al. 2001; Merrill et al. 2001). It can be postulated that an absence of the beta-catenin pathway activation can lead to the formation of interfollicular skin, i.e. an epidermis deprived of cutaneous appendages. Below, I discuss the question of a possible inhibition of the Wnt/beta-catenin pathway during morphogenesis of cornea, which is deprived of cutaneous appendages.
Hair vs. corneal epithelium morphogenesis
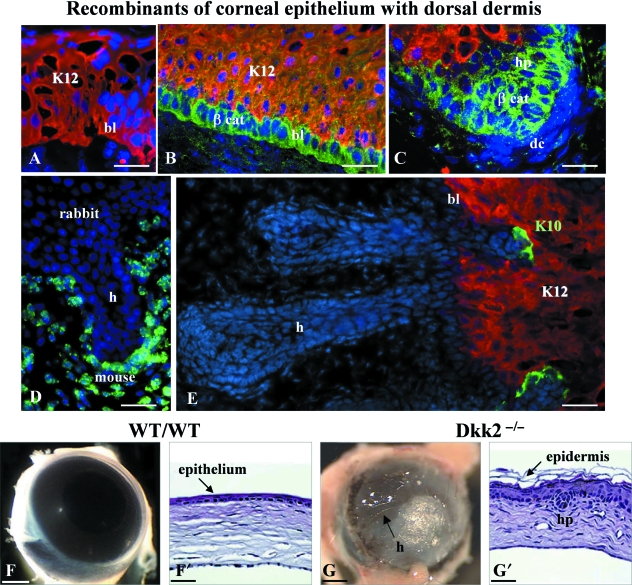
Corneal epithelium specification is unique and still currently under investigation in my group. It depends on an early induction by the neuroderm, followed by a negative regulation (our unpublished data). This epithelium is characterized by the expression of Pax6, the eye master gene, and by a pair of keratins, K12/K3. Our primary results showed that in mammals, the embryonic (Ferraris et al. 1994) and even the adult (Ferraris et al. 2000) corneal epithelium is able to give rise to hairs and interfollicular epidermis under the influence of signals from an embryonic dermis. We then showed that committed basal cells of the adult rabbit corneal epithelium (Fig. 6A) undergo a multistep process of dedifferentiation, followed by a transdifferentiation under the control of Wnt signals from an associated embryonic mouse dorsal dermis (Pearton et al. 2005). After a few days of recombination, there is a strong increase in the level of non-membrane-associated beta-catenin proteins in the cells of the lowest layer of the recombined epithelium (Fig. 6B). Within a week, the first hair pegs are visible as projections into the mouse dermis of basal rabbit cells, which no longer express Pax6 and keratins K12/K3 (Fig. 6C,D). As the hair follicles develop, they start to differentiate their various components. By 2/3 weeks, islands of keratin K10, which is specific to the epidermal program, are detected at the junction of the newly formed hair follicles and the epithelium (Fig. 6E). These cells appear to migrate from the hair follicles and displace what remains of corneal epithelium. The new interfollicular epidermis that forms thus proceeds through the intermediary step of hair follicle formation, with its attendant stem cells (Pearton et al. 2005). In the same way, a complete transformation of the cornea into skin with developed hair follicles (Fig. 6F,G′) has been obtained in Dkk2 knockout mice (Mukhopadhyay et al. 2006). It is well known that the Dickkopf family regulates Wnt pathways by interacting with the Wnt co-receptor LRP5/6. Therefore, one of the requirements for cornea morphogenesis is the expression of Dkk2 to counteract that of Wnt, in order to block the beta-catenin pathway, and the subsequent formation of an epidermis with appendages.
Fig. 6.
Corneal epithelium-hair metaplasia. (A–E) After a few days of recombination of a corneal rabbit adult corneal epithelium with a mouse embryonic dorsal dermis (A), keratin 12 (K12) expression is down-regulated in the basal layer, whereas that of beta-catenin (βcat) is up-regulated (B). After 1 week, the beta-catenin-expressing cells form hair plugs (C). The hair that differentiates, originates from the rabbit cells, as shown by the label of mouse genomic DNA (D). After 2 weeks, islands of epidermal keratin 10 (K10)-expressing cells appear at the junction of hair follicles and what remains of corneal epithelium (E). After Pearton et al. 2005. Reproduced with permission of the Company of Biologists. (F–G′) The corneal phenotype of Dkk2−/− mutant mice is opaque. An epidermis with a few hairs has formed (G,G′) instead of the transparent cornea (F,F′). After Mukhopadhyay et al. 2006. Reproduced with permission of the Company of Biologists. bl, basal layer; dc, dermal condensation; h, hair; hp, hair peg. Bars: A–E: 100 µm, F–G: 1 mm, F′,G′: 50 µm.
A new hypothesis for avian scale, feather and hair origins
A main difference exists during morphogenesis of scales, in lepidosaurs (Dhouailly & Maderson, 1984) and in crocodilians (ALibardi L, Thompson MD, 2001), and of avian scuta, feathers or hairs: only in the latter three is a placode individualized, followed by the formation of a dermal papilla. The appearance of those two structures is linked to the initiation and the long phase of outgrowth especially of feathers and hairs, which both depend on a high expression of the Wnt canonical pathway. This occurrence may have happened independently at least twice during the evolution of tetrapods, using the same molecular pathways: Wnts first, then beta-catenin, and followed by Eda/Edar, Bmps, Shh, and Notch. This has been a re-utilization of a successful system, which may date back to early Ordovician aquatic vertebrates, which possessed tooth-like skin structures, similar to modern chondrichtyans. The latter possess placodes associated with dermal condensations (Sire et al. 2009; Catón & Tucker 2009), as described during tooth morphogenesis (Thesleff, 2003). Moreover, a similar system is utilized in the formation of dermal scales of living actinopterygiens (Sire & Akimenko, 2004). A failure of dermal scale formation is apparent in teleost fish Medaka, which display a mutation of the ectodysplasin receptor (EdaR) in its epidermis (Kondo et al. 2001). This system, which was present in the first synapsids as well as in the first sauropsids, both of which developed teeth, was consequently re-utilized for the skin, both in mammalian and avian lineages.
Experiments which lead to modification of the beta-catenin pathway or of its downstream pathways, such as BMP or Shh, can easily turn avian scuta into perfect feather differentiation (Zhou & Niswander, 1996; Widelitz et al. 2000; Prin & Dhouailly, 2004). Likewise, using the same molecular regulators as those for avian skin, i.e. inhibition of BMP or activation of beta-catenin, can lead to the formation of hair follicles instead of sweat glands (Plikus et al. 2004; Nähri et al. 2008) or of mammary gland nipple (Mayer et al. 2008) in mammals. Conversely, when beta-catenin signaling is down-regulated, hair follicles are converted to sebaceous glands (Niemann et al. 2003), and when it is inhibited, corneal epithelium forms (Mukhopadhyay et al. 2006). Thus, in mammals, as previously suggested for birds (Widelitz et al. 2000), the activation level of the beta-catenin pathway correlates to the regional type of integument differentiation: absent (corneal epithelium or interfollicular epidermis), low (avian scuta or mammalian gland) or high (feather or hair). A special regulation event happens for the plantar face of the feet in both classes (Loomis et al. 1996; Logan et al. 1997; Prin et al. 2004): the Wnt pathway should be at a high level in the dermis, but the En-1 expression intervenes downstream in the epidermis by inhibiting several genes, such as Shh and Wnt7a, which are involved in the outgrowth of hair and feather.
Thus, by manipulating chick or mouse embryos in this manner, parallel effects have been obtained. The different appendages within one class, such as reticula, scuta or feathers in birds, and foot pads, glands or hairs in mammals, can be considered variations of a common theme, by negatively regulating the Wnt/beta-catenin pathway. Another argument is in agreement with this hypothesis, according to which feathers may be the primitive cutaneous appendage for birds, and hairs for mammals: the neutral extra-embryonic ectoderm is genetically programmed toward feather and hair morphogenesis, a morphogenesis that can be achieved by the autonomous transformation of the amnion somatopleure into a dense dermis (Fliniaux et al. 2004a,b). Under the general rule of ontogeny repeating phylogeny, what can be hypothesized for the evolution of the cutaneous appendages of amniotes from these developmental biology results? In the two following sections I will propose my detailed view concerning the scenario of feather, avian scales, hair and mammary glands origins, which is summarized in Fig. 7.
Fig. 7.
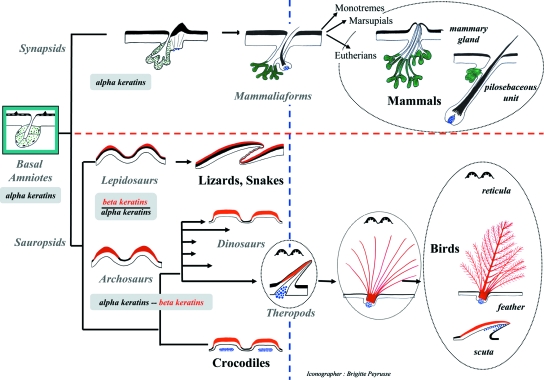
Evolution of amniote cutaneous appendages: a new hypothesis. (The interconnection of the chelonian branch of sauropsids being controversial, their skin evolution is not proposed here).The integument of basal amniotes may have presented both a glandular (green color) and an alpha-keratinized (black color) structure. After the synapsid/sauropsid divergence in the Pennsylvanian, a glandular integument was positively selected in synapsids, which evolved both pilosebaceous units and independent glands, whereas the sauropsids may have almost entirely lost the glandular ability. Two innovations occurred: (1) beta-keratin (red color) formation for the sauropsid branch and (2) a system involving a dermal condensation (blue color), together with an up-regulation of the epidermal beta-catenin. The latter innovation may had happened independently twice during skin evolution, in synapsids and in archosaurs. This system allowed the long outgrowth of both the hair shaft and the feather barbs. In crocodilian lineage, the dermal condensation appears after scale morphogenesis, and is diverted to osteoderm formation. The overlapping scales of the squamates, as well as the avian feather and the crocodilian scutes, may have independently evolved from a primitive granulated integument of first sauropsids. Avian scales, reticula and scuta, made respectively of alpha- and beta-keratins are secondarily derived from feathers (see arguments in text).
Feather and avian scale phylogeny
The feather shows a multiple branched architecture, and is the most complicated cutaneous appendage ever formed during amniote skin evolution. Although the external shape of a feather bud such as that of the avian scuta may show some superficial convergence with a reptilian scale, their morphogenesis is totally different. Unlike with birds, the skin morphogenesis of the lepidosaurs does not involve a placode/dermal condensation system, and probably lacks a corresponding level of the Wnt/beta-catenin pathways.
A few samples of the skin of dinosaurs which have been fossilized, consist of granulated scales, not overlapping scales (Gohlih & Chiappe, 2006). In my view it is not necessary to have overlapping scales to form feathers. More probably, feathers may have evolved from the tuberculate scales of the first archosaurs, possibly made of beta-keratins, and even more precisely of feather-type beta-keratins. Recent results (Dalla Valle et al. 2008) strongly suggest that feather beta-keratins originated deep in archosaur evolution, before the split between birds and crocodiles.
In crocodiles, the non-overlapping beta-keratinized scales (Alibardi & Sawyer, 2002) form long before the osteoderms start to initiate in the dermis (Vickaryous & Sire, 2009). Thus, the dermal condensations associated to the formation of epidermal tuberculate structures may have only developed in the lineage of theropod dinosaurs. Such hypothetic dermal condensations may have re-utilized the Wnt/beta-catenin system, first present in teeth, in skin formation, leading to the formation of long tubular outgrowths, somewhat similar to the long bristles of the tail of Psittacosaurus (Mayr et al. 2002). The discovery of many intermediate forms of feather-like appendages in the northern part of China, brought many new insights in the evolution of feathers (reviewed in Chuong et al. 2000; Chuong & Homberger, 2003; Sawyer & Knapp, 2003; Wu et al. 2004). There are many Mesozoic theropods and even non-theropod dinosaurs that have elongated appendages, which are formed by clumps of elongated fibers, branching structures, or branching structures with a rachis and barbules, like downy feathers (Chiappe, 1995; Chen et al. 1998; Xu et al. 1999, 2001; Brush, 2000). Finally, Caudipteryxevolved different types of feathers such as remiges and rectrices, which have been accepted as vaned feathers (Brush, 2000; Prum, 2005). However, the alleged primitive protofeathers formed of filamentous structures may, at least in some cases, have been misinterpreted, and correspond in fact to degraded collagen fibers, as in the early theropod dinosaur Sinosauropteryx (Lingham-Soliar et al. 2007). Nevertheless, the feather program may have been elaborated relatively rapidly during the course of evolution, first by an increase of the Wnt/beta-catenin, and then by the successive utilization of the same BMP2/Shh module for the building of the different components of the modern feather (Harris et al. 2002; Chuong et al. 2003). Evolutionary changes in the activation level of beta-catenin by the dermis (Widelitz et al. 2000), as well as the inhibition of competence in the plantar epidermis by En-1 (Prin et al. 2004), may be the underlying reasons for the differences in cutaneous appendage morphology in birds. Finally, I propose that avian scales, scuta and reticula have secondarily evolved from feathers, as already suggested as early as 1889 by Davies (cited in Lucas & Stettenheim, 1972). First, birds may have been entirely covered by feathers, except for the plantar face of their feet, of which the feather formation program is blocked at its initiation step by genetic limb regulation. The innovation of plantar reticula may be contemporary with that of the first sketch of proto-feathers. Although the external shape of avian reticula bears some resemblance to the hypothetic granulated integument of first archosaurs, they are made only of alpha-keratins and are therefore not directly related. Likewise, the fact that the feather-scuta metaplasia never occurs naturally or in experiments, and that the beta-keratins of avian scuta appear to be derived from feather beta-keratins (Della Valle et al. 2008) are two main reasons to suggest that scuta (and scutella) may have appeared late, and in some bird lineages only. Mutations, by negatively regulating the local amount of beta-catenin, may have given rise to regional skin variations, depending on bird lineages: avian overlapping scales, as in chick feet, or glabrous skin, as on the neck of vultures.
Hair and mammary gland phylogeny
In living mammals, the main morphological difference between gland and hair embryonic morphogenesis is that only the hair bud is associated to a true dermal condensation, and linked to an increase in the presence of the beta-catenin pathway. The synapsid lineage, which separated from the amniote taxa in the Pennsylvanian about 310 million years ago, may have evolved a glandular rather than a scaled integument, with a thin alpha-keratinized layer adorned with alpha-keratinized bumps. Those bumps may have even presented some cysteine-rich alpha-keratins, precursors of the hair-type keratins. In addition, the first synapsids may have developed both a lipid barrier outside the epidermis, similar to current amphibians living in xeric habitats, and some lipid complex with the alpha-keratins of the stratum corneum as in current mammals (Lillywhite, 2006), as a means to strengthen the barrier against water loss of the integument. For approximately 100 million years, the characters inherited from the synapsid ancestor evolved to the mammaliaform node. The mammaliaform then evolved until the divergence of the first mammalian lineage, the monotremes, followed by the marsupials and the eutherians. The living representatives of these lineages possess a high diversity of cutaneous glands: sebaceous, sweat and mammary glands, which are most commonly associated to hair follicles. For example, in monotremes, even the mammary glands are associated to hair follicles, an association which is transiently retained in marsupial embryos (Long, 1969) but lost in eutherian embryos. In the latter, the mammary glands are not only isolated from hairs, but even the nipple prevents hair from forming around it by the means of the BMP pathway (Mayer et al. 2008). During evolution, this pathway may have been progressively co-opted to suppress hair follicle formation in the nipple vicinity, first in marsupials and then more efficiently in eutherians.
Recently, it was postulated (Wu et al. 2004) that the increased amount of beta-catenin may have led to the protrusion of long hair filaments from reptilian scales. However, based (1) on the metaplasia-gland-hair, which depends on the level of beta-catenin, and (2) on the fact that synapsids, which gave rise to mammals, evolved independently from the sauropsids, I propose that mammalian hairs may have evolved through an increase of beta-catenin from the cutaneous glands of early synapsids, forming pilo-glandular units, in which a large gland was associated to a small horny structure. The subsequent association of this horny structure with a dermal papilla may have led to its outgrowth. A recent complementary hypothesis, based on dermatological observations of the current pilo-sebaceous units (Stenn et al. 2008), proposed that hairs evolved from sebaceous glands, with the hair shaft serving as a wick to draw the product of the gland to the skin surface, strengthening the barrier against water loss. In reverse, the mammary gland apparently derives from an ancestral sweat or sebaceous gland that was associated with hair follicles, an association which is retained in living monotremes, and transiently in living marsupials. The original function of the mammary gland precursor may not have been feeding the young, but as a means to provide moisture to the eggs (Oftedal, 2002). With the mammaliaform node, tooth replacement was diphyodont; suggesting the occurrence of lactation. However, a complete insulator covering of hair may not have existed until the appearance of the earliest true mammals (Rowe, 1992). The discovery in the Liaoning Province of China of a Jurassic mammal Castorcauda lustrasimili with a halo of fur around the skeleton (Ji et al. 2006) shows that by that period a complete pelage was formed. The authors found not only imprints of guard hairs and short dense under-fur, but even a ‘scaly tail’, more exactly epidermal folds interspersed with hairs, as in the tail integument of some living mammals. The skin of common day mammals shows pilo-sebaceous units, or pilo-sebaceous-sweat gland units. In eutherians, the mammary gland became secondarily independent of hair. Some terrestrial species, such as mice, evolved footpads, which are deprived of hairs, but associated with isolated sweat glands. Furthermore, entirely aquatic mammalian species, such as dolphin, lost their hairs. Finally, concerning the evolution of synapsid integument, I suggest that no intermediate forms have ever been found for two simple reasons: glands do not fossilize, and hair has a simple architecture, forming just a long, protruding shaft, in contrast with the complex evolved feather.
Conclusion
The evolution of amniote skin is based on two different events with two separate domains: the re-utilization of the placodal/dermal condensation system, and the innovation, only in sauropsids, of beta-keratins. Finally, the independent co-evolution of the successful Wnt/beta-catenin pathway gave rise to protruding keratinized filaments both in mammals and in the last surviving dinosaurs, the birds.
Consequently, I propose the following scenario for skin evolution (Fig. 7). The ability to form epidermal glands and a first thin, protective layer against desiccation, adorned with alpha-keratinized bumps may have appeared with the early terrestrial tetrapods, a condition that has probably been conserved in some living amphibians, e.g. in toads. The basal amniotes probably presented such an epidermal structure, comprising both glands and alpha-keratinized bumps. After the synapsid /sauropsid divergence in the Pennsylvanian, a glandular integument was positively selected in synapsids, whereas the sauropsids may have almost entirely lost this glandular ability. The hairs may have developed secondarily as an element of the glandular units, by the means of an increase of the Wnt/beta-catenin pathways, and the formation of a dermal papilla. The latter sustained a long outgrowth of the hair shaft. Those hard keratinized structures may have diversified cysteine-rich proteins, which were probably already present in the common ancestor of synapsids and sauropsids. Conversely, the individualization of the sauropsid lineage was characterized by the formation of a totally new type of keratin, beta-keratin. Although the relationships of turtles are still controversial, they probably diverged from the other sauropsids after the innovation of beta-keratins. In the lepidosaur lineage, symmetrical scales probably appeared before the asymmetrical overlapping scales. The first archosaurs may have developed symmetrical tuberculate beta-keratinized scales, then, in theropod dinosaurs, the increase of the Wnt/beta-catenin pathway and the formation of dermal condensations, favored long proto-feather to develop, followed by the formation of branched structures, a rachis, barbules, and finally an asymmetry developed in the vane, as in modern feathers. Thus, hairs and feathers evolved independently, but in parallel. Although the ectoderm cells of living endothermic amniotes, birds and mammals, are primarily programmed to produce pilo-sebaceous units and feathers, some restrictive regulations of these programs led to the formation of isolated glands in mammals and of avian scales. The overlapping avian scuta and scutella which cover the tarsometatarsi and dorsal digits of the feet in some bird lineages thus appear to be secondarily derived from feathers. Finally, the reticulate scales, which cover the plantar surface of all living birds, cannot be the remnants of the ancestral granulated beta-keratinized skin of first sauropsids, and correspond to a secondary, almost complete, inhibition of feather formation.
Acknowledgments
I am indebted to Drs. Jean-Yves Sire, Michael O’Guin and Stefan Nonchev and to Ms Elisabeth Constantz for critical reading of the manuscript. I should like to acknowledge the help of Ms Brigitte Peyrusse and PhD students Sebastien Cadau and Elodie Collomb. I would like to thank previous PhD students and postdoctoral fellows, whose experimental results led to the current hypothesis: Sandrine Blanchet-Rhétore, Rachida Cadi, Catherine Chaloin-Dufau, Pascale Delorme-Tacnet, Corinne Ferraris, Ingrid Fliniaux, Benoit Kanzler, Jean-Jacques Michaille, Frederic Michon, Isabel Olivera-Martinez, Sylvain Missier, David J. Pearton, Fabrice Prin, Jacques Thélu, Jean Viallet, and Ying Yang. I thank all authors for the paper materials used in the figures (referred to in figure legends). I would like to give my great thanks to friends and colleagues, whose wonderful experimental results greatly enhance our knowledge of skin morphogenesis and help my thinking, and particularly among them: Dr. Cheng-Ming Chuong, Dr. Irma Thesleff, Dr. Randall Widelitz, Dr. Bruce Morgan, D, Dr. P. Coulombe, Dr. Cairine Logan, Dr. Cynthia Loomis, Dr. G. Rogers, Dr Paul Maderson, Dr Roger Sawyer, Dr. Lee Niswander, Dr. Beate Brand-Saberi, Dr. Ralf Paus. Finally, I would like to give my sincere thanks to Drs. Jean-Yves Sire and Matt Vickarious who organized the symposium on Vertebrate Integument in Paris.
References
- Alibardi L. Adaptation to the land: the skin of reptiles in comparison to that of amphibians and endothermic amniotes. J Exp Zool B Mol Dev Evol. 2003;298:12–41. doi: 10.1002/jez.b.24. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Thompson MD. Scale morphogenesis and ultrastructure of demis during embryonic development in the alligator (Alligatormississipiensis, Crocodilia, Reptilia) Acta Zoologica. 2001;81:325–338. [Google Scholar]
- Alibardi L, Sawyer RH. Immunocytochemical analysis of beta keratins in the epidermis of chelonians, lepidosaurians and archosaurians. J Exp Zool. 2002;293:27–38. doi: 10.1002/jez.10145. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Antoniazzi MM, Jared C, Junqueira LCU. Epidermal glands in squamate: fine structure of pre-cloacal glands in Amphisbaena alba (Amphisbaenia, Amphis baenidae) J Morphol. 2005;221:101–109. doi: 10.1002/jmor.1052210108. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J, Matolsty AG, Sylvia Richards K. Biology of the Integument Vol 2. Vertebrates. New York: Springer Verlag; 1986. [Google Scholar]
- Blanchet S, Favier B, Chevalier G, et al. Both retinoic acid receptors a (RARa) and g (RARg) are able to initiate mouse upper-lip glandular metaplasia. J Invest Dermatol. 1998;111:206–212. doi: 10.1046/j.1523-1747.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev V, Paus R. Molecular biology of hair morphogenesis: Development and cycling. J Exp Zool. 2003;298B:164–180. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- Botchkarev V, Botchkareva NV, Sharov AA, Funa K, Huber O, Gilchrest BA. Modulation of BMP signaling by Noggin is required for induction of the secondary (non tylotrich) hair follicle. J Invest Dermatol. 2002;118:3–10. doi: 10.1046/j.1523-1747.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- Brush AH. Evolving a protofeather and feather diversity. Am Zool. 2000;40:631–639. [Google Scholar]
- Cadi R, Dhouailly D, Sengel P. Use of retinoic acid for the analysis of dermal-epidermal interactions in the tarsometatarsal skin of the chick embryo. Dev Biol. 1983;100:484–495. doi: 10.1016/0012-1606(83)90241-5. [DOI] [PubMed] [Google Scholar]
- Catón J, Tucker AS. Current knowledge of tooth development: patterning and mineralization of the murine dentition. J Anat. 2009;214:502–515. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloin-Dufau C, Pavitt I, Delorme P, Dhouailly D. Identification of keratins 3 and 12 in corneal epithelium of vertebrates. Epithelial Cell Biol. 1993;2:157–171. [PubMed] [Google Scholar]
- Chen P, Dong Z, Zhen S. An exceptionally well preserved theropod dinosaur from the Yxian formation of China. Nature. 1998;391:147–152. [Google Scholar]
- Chiappe KM. The first 85 million years of avian evolution. Nature. 1995;378:349–355. [Google Scholar]
- Chodankar R, Chang CH, Yue Z, et al. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/beta-catenin pathway. J Invest Dermatol. 2003;120:20–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M. Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: Landes Bioscience; 1998. [Google Scholar]
- Chuong C-M, Homberger DG. Development and evolution of the amniote integument: current landscape and future horizon. J Exp Zool Part B Mol Dev Evol. 2003;298:1–11. doi: 10.1002/jez.b.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Oliver G, Ting SA, Jegalian BG, Chen HM, De Robertis EM. Gradients of homeoproteins in developing feather buds. Development. 1990;110:1021–1030. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- Chuong C-M, Chodankar R, Widelitz RB, Jiang T-X. Evo-Devo of feathers and scales: building complex epithelial appendages. Curr Opin Genet Dev. 2000;10:449–456. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Wu P, Zhang FC, et al. Adaptation to the sky: defining the feather with integument fossils from Mesozoic China and experimental evidence from molecular laboratories. J Exp Zool Part B Mol Dev Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dalla Valle L, Nardi A, Gelmi C, Toni M, Emera D, Alibardi L. β-keratins of the crocodilian epidermis: composition, structure and phylogenetic relationships. J Exp Zool Mol Dev Evol. 2008;310B:1–16. doi: 10.1002/jez.b.21241. [DOI] [PubMed] [Google Scholar]
- Dhouailly D. The determination of specific differentiation of neoptile and teleoptile feathers in the chick and the duck. J Embryol Exp Morphol. 1970;24:73–94. [PubMed] [Google Scholar]
- Dhouailly D. Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. J Embryol Exp Morphol. 1973;30:587–603. [PubMed] [Google Scholar]
- Dhouailly D. Formation of cutaceous appendages in dermo-epidermalrecombinations between reptiles, birds and mammals. Wilhelm Roux’ Arch Entwicklungsmech Org. 1975;177:323–340. doi: 10.1007/BF00848183. [DOI] [PubMed] [Google Scholar]
- Dhouailly D. Dermo-epidermal interactions during morphogenesis of cutaneous appendages in amniotes. Front Matrix Biol. 1977;4:86–121. [Google Scholar]
- Dhouailly D. Specification of feather and scale patterns. In: Malacinski GM, Bryant SW, editors. Pattern Formation. New York: Macmillan Publ; 1984. pp. 581–601. [Google Scholar]
- Dhouailly D, Maderson PFA. Ultrastructural observations on the embryonic development of the integument of Lacerta muralis (Lacertilia, Reptilia) J Morphol. 1984;179:203–208. doi: 10.1002/jmor.1051790302. [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Sawyer R. Avian scale development. XI. Initial appearance of the dermal defect in scaleless skin. Dev Biol. 1984;105:343–350. doi: 10.1016/0012-1606(84)90291-4. [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Sun TT. The mammalian tongue filiform papillae: a theoretical model for primitive hairs. In: van Neste D, LaChapelle J-M, Antoine JL, editors. Trends in Human Hair Growth and Alopecia Research. Boston: Kluwer Academic Publ; 1989. pp. 29–34. [Google Scholar]
- Dhouailly D, Rogers GE, Sengel P. The specification of feather and scale protein synthesis in epidermal-dermal recombinations. Dev Biol. 1978;65:58–68. doi: 10.1016/0012-1606(78)90179-3. [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Hardy MH, Sengel P. Formation of feathers on chick foot scales: a stage-dependant morphogenetic response to retinoic acid. J Embryol Exp Morphol. 1980;58:63–78. [PubMed] [Google Scholar]
- Dhouailly D, Prin F, Kanzler B, Viallet JP. Variation of cutaneous appendages: regional specification and cross-species signals. In: Chuong C-M, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Vol. 1. Georgetown, TX: RG Landes; 1998. pp. 45–56. [Google Scholar]
- Eckhart L, Valle LD, Jaeger K, et al. Identification of reptilian genes encoding hair keratin-like proteins suggests a new scenario for the evolutionary origin of hair. Proc. Natl. Acad. Sci. USA. Nov 10 (epub ahead of print) [DOI] [PMC free article] [PubMed]
- Elias H, Bortner S. On the phylogeny of hair. Am Mus Nat hist Novitates. 1957;1820:1–15. [Google Scholar]
- Elias H, Shapiro J. Histology of the skin of some toads and frogs. Am Mus Novitates. 1957;1819:1–27. [Google Scholar]
- Ferraris C, Chaloin-Dufau C, Dhouailly D. Transdifferentiation of embryonic and postnatal rabbit corneal epithelial cells. Differentiation. 1994;57:89–96. doi: 10.1046/j.1432-0436.1994.5720089.x. [DOI] [PubMed] [Google Scholar]
- Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D. Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development. 2000;127:5487–5495. doi: 10.1242/dev.127.24.5487. [DOI] [PubMed] [Google Scholar]
- Fliniaux I, Viallet JP, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development. 2004a;131:3955–3966. doi: 10.1242/dev.01263. [DOI] [PubMed] [Google Scholar]
- Fliniaux I, Viallet JP, Dhouailly D, Jahoda CA. Transformation of amnion epithelium into skin and hair follicles. Differentiation. 2004b;72:558–565. doi: 10.1111/j.1432-0436.2004.07209009.x. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gohlih UB, Chiappe LM. A new carnivorous dinosaur from the late Jurassic Solnhofen archipelago. Nature. 2006;440:1084–1088. doi: 10.1038/nature04579. [DOI] [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SWM. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- Gregg K, Rogers GE. Feather keratin: composition, structure and biogenesis. In: Bereiter-Hahn J, editor. Biology of the Integument. Vol. 2, Vertebrates. New York: Springer Verlag; 1986. pp. 666–694. [Google Scholar]
- Gregg K, Wilton SD, Parry DAD, Rogers GE. A comparison of genomic and coding sequences for feather and scale keratins: structural and evolutionary implications. EMBO. 1984;3:175–178. doi: 10.1002/j.1460-2075.1984.tb01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH, Bellows CG. The stability of vitamin A-induced metaplasia of mouse vibrissae follicles in vitro. J Invest Dermatol. 1978;71:236–241. doi: 10.1111/1523-1747.ep12515093. [DOI] [PubMed] [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-BMP2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Hornik C, Krishan K, Scaal M, Brand-Saberi B. cDermo-1misexpression induces dense dermis, feathers and scales. Dev Biol. 2005;277:42–50. doi: 10.1016/j.ydbio.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Hou LH, Chuong C-M, Yang A, Zeng CL, Hou JF. Fossil birds of China. China: Yunnan Science and Technology; 2003. [Google Scholar]
- Houzelstein D, Cheraud Y, Auda-boucher G, Fontaine-Perus J, Robert B. The expression of the homeobox gene Msx1 reveals two populations of dermal progenitor cells originating from the somites. Development. 2000;127:2155–2164. doi: 10.1242/dev.127.10.2155. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ji Q, Luo ZX, Yuan CX, Tabrum AR. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science. 2006;311:1123–1127. doi: 10.1126/science.1123026. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Prin F, Thélu J, Dhouailly D. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev Dyn. 1997;210:274–287. doi: 10.1002/(SICI)1097-0177(199711)210:3<274::AID-AJA8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kato Y. Epithelial metaplasia induced on extraembryonic membranes. I. Induction of epidermis from chick chorionic epithelium. J Exp Zool. 1969;170:229–252. doi: 10.1002/jez.1401700209. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kuwahara Y, Kondo M, et al. The Medaka rs-3 locus required for scale development encodes ectodysplasin-A receptor. Curr Biol. 2001;7:1202–1206. doi: 10.1016/s0960-9822(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Winter H, Praetzel S, Schweitzer J. The catalogue of human hair keratins II. Expression of the six Type II members in the hair follicle and the combined catalogue of human Type I and II keratins. J Biol Chem. 2001;276:35123–35132. doi: 10.1074/jbc.M103305200. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Pispa J, Jung HS, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Eda. Development. 2002;129:2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio) Int J Dev Biol. 2004;48:217–31. doi: 10.1387/ijdb.15272388. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB. Water relations of tetrapod integument. J Exp Biol. 2006;209:202–226. doi: 10.1242/jeb.02007. [DOI] [PubMed] [Google Scholar]
- Lingham-Soliar T, Feduccia A, Wang X. A new Chinese specimen indicates that ‘protofeathers’ in the early cretaceous theropod dinosaur Sinosauropteryx are degraded collagen fibres. Proc R Soc B. 2007;274:1823–1829. doi: 10.1098/rspb.2007.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C, Hornbruch A, Campbell I, Lumsden A. The role of Engrailed in establishing the dorsoventral axis of the chick limb. Development. 1997;124:2317–2324. doi: 10.1242/dev.124.12.2317. [DOI] [PubMed] [Google Scholar]
- Long CA. The origin and evolution of mammary glands. Bioscience. 1969;19:519–523. [Google Scholar]
- Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, Joyner AL. The mouse Engrailed-1 gene and ventral limb patterning. Nature. 1996;382:360–363. doi: 10.1038/382360a0. [DOI] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR. Agriculture Handbook 362. Agricultural Research Services. Washington DC: US Department of Agriculture; 1972. Avian anatomy integument. [Google Scholar]
- Maderson PFA. The structure and development of the squamate epidermis. In: Lyne AG, Short BF, editors. The Biology of the Skin and Hair Growth. Sydney: Angus & Robertson; 1965. pp. 129–153. [Google Scholar]
- Maderson PFA. When? Why? And how? Some speculations on the evolution of the vertebrate integument. Am Zool. 1972;12:159–171. [Google Scholar]
- Maderson PFA. Mammalian skin evolution: a re-evaluation. Exp Dermatol. 2003;12:233–236. doi: 10.1034/j.1600-0625.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- Maderson PFA, Sawyer RH. Scale embryogenesis in birds and reptiles. Anat Rec. 1979;193:609. [Google Scholar]
- Maier P, Weibel B, Zbinden G. The mutagenic activity of 5-bromo-2′-deoxyuridine (BrdU) in vivo in rats. Environ Mutagen. 1983;5:695–703. doi: 10.1002/em.2860050508. [DOI] [PubMed] [Google Scholar]
- Mayer JA, Foley J, De La Cruz D, Chuong CM, Widelitz R. Conversion of the nipple to hair-bearing epithelia by lowering bone morphogenetic protein pathway activity at the dermal-epidermal interface. Am J Pathol. 2008;173:1339–1348. doi: 10.2353/ajpath.2008.070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr G, Peters DS, Plodowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturewissenschaften. 2002;89:361–365. doi: 10.1007/s00114-002-0339-6. [DOI] [PubMed] [Google Scholar]
- Meng J, Wyss AR. Multituberculate and other mammal hair recovered from Palaeogene excreta. Nature. 1997;385:712–714. doi: 10.1038/385712a0. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, Das Gupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1205. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F, Charveron M, Dhouailly D. Dermal condensation formation in the chick embryo: requirement for integrin engagement and subsequent stabilization by a possible notch/integrin interaction. Dev Dyn. 2007;236:755–68. doi: 10.1002/dvdy.21080. [DOI] [PubMed] [Google Scholar]
- Michon F, Forest L, Collomb E, Demongeot J, Dhouailly D. BMP-2 and BMP-7 play antagonistic roles in feather induction. Development. 2008;135:2797–2805. doi: 10.1242/dev.018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine Growth Factor Rev. 2003;14:211–224. doi: 10.1016/s1359-6101(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, et al. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- Nähri K, Järvinen E, Birchmeir W, Taketo MM, Mikkola ML, Thesleff I. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development. 2008;135:1019–1028. doi: 10.1242/dev.016550. [DOI] [PubMed] [Google Scholar]
- Niemann C, Watt FM. Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol. 2002;12:185–192. doi: 10.1016/s0962-8924(02)02263-8. [DOI] [PubMed] [Google Scholar]
- Niemann C, Unden AB, Lyle S, Zouboulis ChC, Toftgård R, Watt FM. Indian hedgehog and beta-catenin signaling: Role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003;100S:11873–11880. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noramly S, Freeman A, Morgan BA. Beta-Catenin signaling can initiate feather bud development. Development. 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- O’Guin WM, Galvin S, Schermer A, Sun TT. Patterns of keratin expression define distinct pathways of epithelial development and differentiation. Curr Top Dev Biol. 1987;22:97–125. doi: 10.1016/s0070-2153(08)60100-3. [DOI] [PubMed] [Google Scholar]
- O’Guin WM, Sawyer R. Avian scale development. VII Relationships between morphogenetic and biosynthetic differentiation. Dev Biol. 1982;89:485–492. doi: 10.1016/0012-1606(82)90336-0. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7:225–252. doi: 10.1023/a:1022896515287. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Coltey M, Dhouailly D, Pourquié O. Mediolateral somitic origin of ribs and dermis determined by quail-chick chimeras. Development. 2000;127:4611–4617. doi: 10.1242/dev.127.21.4611. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Missier S, Fraboulet S, Thélu J, Dhouailly D. Differential regulation of the chick dorsal thoracic dermal progenitors from the medial dermomyotome. Development. 2004a;129:4763–4772. doi: 10.1242/dev.129.20.4763. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Viallet JP, Michon F, Pearton DJ, Dhouailly D. The different steps of skin formation in vertebrates. Int J Dev Biol. 2004b;48:107–115. doi: 10.1387/ijdb.15272376. [DOI] [PubMed] [Google Scholar]
- Pearton DJ, Yang Y, Dhouailly D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc Natl Acad Sci U S A. 2005;102:3714–3719. doi: 10.1073/pnas.0500344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang T-X, Chuong C-M. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prin F, Dhouailly D. How and when the regional competence of chick epidermis is established: feather vs scutate and reticulate sales, a problemen routeto a solution. Int J Dev Biol. 2004;48:137–148. doi: 10.1387/ijdb.15272378. [DOI] [PubMed] [Google Scholar]
- Prin F, Logan C, D'Souza D, Ensini M, Dhouailly D. Dorsal versus ventral scales and the dorsoventral patterning of chick foot epidermis. Dev Dyn. 2004;229:564–578. doi: 10.1002/dvdy.20007. [DOI] [PubMed] [Google Scholar]
- Prum RO, Williamson S. Theory of the growth and evolution of feather shape. J Exp Zool. 2001;291:30–57. doi: 10.1002/jez.4. [DOI] [PubMed] [Google Scholar]
- Prum RO. Evolution of the morphological innovation of feathers. J Exp Zool Mol Dev Evol. 2005;304B:570–579. doi: 10.1002/jez.b.21073. [DOI] [PubMed] [Google Scholar]
- Rawles ME. Tissue interactions in the scale and feather development as studied in dermal-epidermal recombinations. J Embryol Exp Morphol. 1963;2:765–789. [PubMed] [Google Scholar]
- Rowe T. Definition, diagnosis and origin of mammalian. J Vert Paleontol. 1992;8:241–264. [Google Scholar]
- Saunders JW, Jr, Gasseling MT, Cairns JM. The differentiation of prospective thigh mesoderm grafted beneath the apical ectodermal ridge of the wing bud in the chick embryo. Dev Biol. 1959;1:281–301. [Google Scholar]
- Sawyer RH. Avian scale development I. Histogenesis and morphogenesis of the epidermis and dermis during formation of the scale ridge. J Exp Zool. 1972;181:365–384. [Google Scholar]
- Sawyer RH. The role of epithelial-mesenchymal interactions in regulating gene expression during avian scale morphogenesis. In: Sawyer RH, Fallon JF, editors. Epithelial-Mesenchymal Interactions in Development. New-York: Praeger; 1983. pp. 115–146. [Google Scholar]
- Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J Exp Zool Part B Mol Dev Evol. 2003;298:57–72. doi: 10.1002/jez.b.26. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Glenn T, French JO, et al. The expression of beta (β) keratins in the epidermal appendages of reptiles and birds. Am Zool. 2000;40:530–539. [Google Scholar]
- Sharpe PT. Fish scale development: hair today, teeth and scales yesterday? Curr Biol. 2001;11:R751–R752. doi: 10.1016/s0960-9822(01)00438-9. [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, et al. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–31. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Sire JY, Akimenko MA. Scale development in fish: a review with description of sonic hedgehog expression in the zebrafish (Danio rerio) Int J Dev Biol. 2004;48:233–248. [PubMed] [Google Scholar]
- Sire JY, Donoghue PCJ, Vickaryous MK. Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates. J Anat. 2009;214:409–440. doi: 10.1111/j.1469-7580.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somes RG., Jr . Mutations and major variants of plumage and skin in chickens. In: Crawford RD, editor. Poultry Breeding and Genetics. Elsevier: Amsterdam; 1990. pp. 169–208. [Google Scholar]
- Spearman RIC. Epidermal keratinization in the salamander and a comparison with other amphibia. J Morphol. 2005;125:129–143. doi: 10.1002/jmor.1051250202. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Zheng Y, Parimoo S. Phylogeny of the hair follicle: the sebogenic hypothesis (Letter to the Editor) J Invest Dermatol. 2008;128:1576–1578. doi: 10.1038/sj.jid.5701200. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Sugihara-Yamamoto H, Kato Y. Epigenesis in developing avian scales. I. Stage-specific alterations of the developmental program caused by 5-bromodeoxyuridine. Dev Biol. 1987;121:467–477. doi: 10.1016/0012-1606(87)90183-7. [DOI] [PubMed] [Google Scholar]
- Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1 deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Velmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- Viallet JP, Dhouailly D. Retinoic acid and mouse skin morphogenesis. II Role of epidermal competence in hair glandular metaplasia. Dev Biol. 1994;166:277–288. doi: 10.1006/dbio.1994.1314. [DOI] [PubMed] [Google Scholar]
- Viallet JP, Ruberte E, Krust A, Zelent A, Dhouailly D. Expression of retinoic acid receptors and dermal-epidermal interactions during mouse skin morphogenesis. In: Morris-Kay G, editor. Retinoids in Normal Development and Teratogenesis. Oxford: Oxford Univ. Press; 1992. pp. 199–210. [Google Scholar]
- Viallet JP, Prin F, Olivera-Martinez I, Hirsinger E, Pourquié O, Dhouailly D. Chick Delta-1 gene expression and the formation of the feather primordia. Mech Dev. 1998;72:159–168. doi: 10.1016/s0925-4773(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Vickaryous MK, Sire JY. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441–464. doi: 10.1111/j.1469-7580.2008.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz RB, Jiang T-X, Lu J, Chuong C-M. BETA-catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated beta-catenin. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- Wu P, Hou L, Plikus M, et al. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:248–267. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang XL, Wu XC. A dromaeosaurid dinosaur with a filamentous integument from the Yixian formation of China. Nature. 1999;401:262–266. [Google Scholar]
- Xu X, Zhou Z, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–204. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Wang X, Kuang X, Zhang F, DU X. Four-winged dinosaurs from China. Nature. 2003;421:335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–1029. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Niswander L. Requirement for BMP signalling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]