Summary
Mitochondrial outer membrane permeabilisation (MOMP) is the point of no return in many forms of apoptotic cell death. The killing effect of MOMP is twofold; it both initiates a proteolytic cascade of pro-apoptotic enzymes and damages mitochondrial function. Accordingly, prevention of MOMP can rescue cells from death. It is clear that either Bak or Bax, which are Bcl-2 family members, are required for MOMP to occur; however, the pore complexes that are formed by Bak and Bax remain poorly defined in terms of their composition, size, number and structure, as well as the mechanism by which they are regulated by other Bcl-2 family members. We recently reported that a key step leading to Bak homo-oligomerisation following an apoptotic stimulus involves transient exposure of the Bak BH3 domain before it binds to the hydrophobic groove of another activated Bak molecule to form a novel symmetric dimer. To form the higher-order oligomers that probably constitute the apoptotic pore complex, Bak dimers then interact via regions away from the BH3 domain and groove. The BH3:groove interaction within Bak homodimers supports a general model to explain the associations between Bcl-2 family members. In this Commentary, we discuss the implications of these findings for the regulation of apoptosis by Bcl-2 family proteins.
Keywords: Apoptosis, Bak, Bax, Bcl-2 proteins, Conformation change, Mitochondria, Mitochondrial outer membrane permeabilisation
Introduction
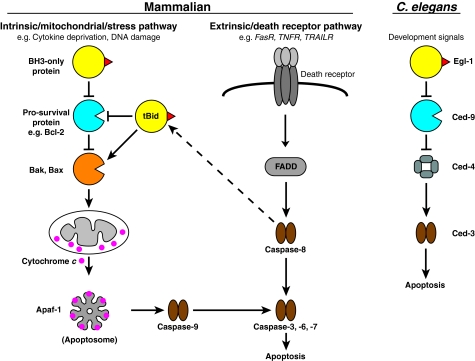
Apoptosis is essential for normal development and tissue homeostasis, and perturbations in its regulation contribute to numerous pathological conditions, including cancer and autoimmune and degenerative diseases (Adams and Cory, 2007; Meier and Vousden, 2007). There are two main pathways that lead to apoptosis: the extrinsic pathway, which is triggered following the activation of cell-surface-expressed death receptors such as CD95 (also known as Fas receptor) and tumour necrosis factor receptor; and the intrinsic pathway, which is activated by cellular stress and is regulated primarily at the level of mitochondria by the Bcl-2 family of proteins (Fig. 1). The intrinsic apoptotic pathway is initiated in response to a variety of stress signals (Willis and Adams, 2005), and a complex interplay of Bcl-2 proteins relays this signal to the mitochondrial outer membrane (OM) to initiate Bak and Bax activation, oligomerisation and OM damage (Fig. 1). Breaching the mitochondrial OM releases apoptogenic factors, including cytochrome c and DIABLO (also known as Smac), which activate a group of aspartate-specific proteases known as caspases (Youle and Strasser, 2008). Caspases, in turn, cleave several hundred cellular proteins to coordinate the destruction of the cell (Dix et al., 2008; Luthi and Martin, 2007). By contrast, the extrinsic apoptotic pathway can activate caspases without the participation of mitochondria. However, in certain cell types, the extrinsic pathway also induces mitochondrial damage by cleaving the pro-apoptotic Bcl-2 family protein Bid to its activated truncated form (tBid), which leads to Bak and Bax activation. For example, hepatocytes from bid–/– mice are resistant to apoptosis induced by Fas ligand and tumour necrosis factor (Kaufmann et al., 2009; Yin et al., 1999).
Fig. 1.
The Bcl-2 protein family controls the mitochondrial pathway of apoptosis. Mammalian apoptosis occurs via the intrinsic and extrinsic pathways, whereas a single pathway operates during the development of the nematode C. elegans. Each pathway culminates in the activation of the proteolytic caspases, but only the intrinsic pathway involves MOMP regulation by Bcl-2 proteins. Several proteins homologous to those involved in the intrinsic pathway are important for development in C. elegans, although there is no true Bak or Bax homologue and no role for MOMP. FADD, Fas-associated death domain; FASR, Fas receptor; TNFR, tumour necrosis factor receptor; TRAILR, TNF-related apoptosis-inducing ligand receptor.
The current development of anti-cancer agents that target the Bcl-2 protein family is showing considerable promise, although how this targeting triggers Bak and Bax to perform their crucial function of mitochondrial outer membrane permeabilisation (MOMP) is not clear. Recent findings indicate that MOMP requires transient exposure of the Bak or Bax BH3 domain, which then binds to the hydrophobic groove in another activated molecule, leading to oligomerisation and pore formation. In this Commentary, we discuss the implications of BH3:groove interactions for Bak and Bax pro-apoptotic function, and for how Bak and Bax are regulated by other members of the Bcl-2 protein family.
Bak or Bax is required for mitochondrial permeabilisation during apoptosis
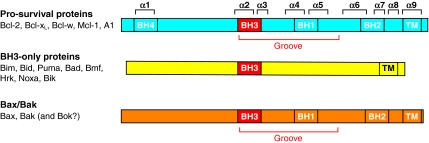
The mammalian Bcl-2 homologues share amino acid sequence homology within four Bcl-2 homology (BH) domains (BH1-BH4), and are categorised into three subclasses according to their function (Fig. 2). The proteins that make up the first subclass are the pro-survival proteins that each contain up to four BH domains. Proteins of the second subclass are pro-apoptotic BH3-only proteins that initiate the intrinsic pathway (Willis and Adams, 2005; Youle and Strasser, 2008). Finally, proteins of the pro-apoptotic Bak and Bax (and perhaps Bok, also known as Mtd) subclass also have multiple BH domains and act downstream of both the BH3-only and pro-survival Bcl-2 members to induce MOMP.
Fig. 2.
Sequence and structural homology of Bcl-2 family proteins. Mammalian Bcl-2 proteins are categorised into three subclasses based on their function and the number of Bcl-2 homology (BH) domains: pro-survival proteins, BH3-only proteins and Bax/Bak proteins. Many members also possess a C-terminal hydrophobic transmembrane (TM) domain that can anchor proteins in the mitochondrial OM. Bak, Bax and the pro-survival proteins each adopt similar α-helical structures (Bcl-2 α-helices 1-9 are indicated). Interactions between different family members can occur via binding of the BH3 domain to the hydrophobic surface groove.
A relevant point to this discussion is evidence that either Bak or Bax is essential for this major checkpoint (MOMP), because mice deficient for both of these proteins, but not either one alone, exhibit severely impaired apoptosis during development (Lindsten et al., 2000). Furthermore, analysis of fibroblasts derived from the mutant mice showed that apoptosis is blocked at the mitochondrial permeabilisation step (Wei et al., 2001). The precise role of Bok in this process is less clear and might not be analogous to that of Bak and Bax, as its expression fails to re-sensitise bak–/–bax–/– fibroblasts to apoptotic stimuli (our unpublished data).
Loss of mitochondrial OM integrity ensures cell death
As discussed above, the `point of no return' in stress-induced apoptosis is irreparable damage to the mitochondrial OM. Breaching the OM releases proteins from the mitochondrial intermembrane space into the cytosol to rapidly activate caspases. The major weapon in the mitochondrial arsenal is cytochrome c, which, when introduced into the cytosol, binds to Apaf-1 and instigates the assembly of the apoptosome and the activation of caspase-9, caspase-3, caspase-6 and caspase-7. Activated caspases rapidly cleave multiple substrates (Dix et al., 2008; Luthi and Martin, 2007) and cause the cell to become packaged into `bite-sized' pieces that display `eat-me' signals for resident phagocytes, which take up and degrade the remnants of apoptotic cells (Ravichandran and Lorenz, 2007). Notably, these caspases act downstream of the point of no return and, therefore, blocking their activation by genetic ablation of either apaf1 or caspase9 delays cell death but does not prevent a loss of clonogenicity or eventual cell death (Ekert et al., 2004). This caspase-independent cell death is generally slower than apoptosis and resembles necrosis in that the cell dies due to loss of mitochondrial function (Chautan et al., 1999). From a functional perspective, this bifurcated attack on cell viability – shutting down mitochondrial function while activating deadly caspases – makes sense, as it guarantees the death of unwanted cells.
It is noteworthy that a role for MOMP is not universally conserved in Bcl-2-regulated apoptosis (Fig. 1). For example, although the apoptosis that occurs during the development of the nematode Caenorhabditis elegans is exquisitely controlled by Bcl-2 homologues, it does not appear to involve cytochrome c (Yuan, 2006). C. elegans expresses three Bcl-2 homologues (the BH3-only proteins Egl-1 and Ced-13, and the pro-survival protein Ced-9) but no bona fide Bak-like or Bax-like protein that induces MOMP. Even a pro-apoptotic mutant form of Ced-9 does not cause mitochondrial disruption (Hengartner and Horvitz, 1994). Likewise, MOMP does not have a confirmed role in cell death in Drosophila melanogaster (Dorstyn et al., 2002; Zimmermann et al., 2002). Although MOMP might not be needed in the cell death programs of the small, short-lived nematode and fly, mitochondria are still involved because several of their Bcl-2 homologues localise to the OM. Furthermore, Ced-9 has been shown to regulate mitochondrial fission (Delivani et al., 2006), suggesting that Bcl-2 homologues have an important role at mitochondria that is independent of regulating apoptosis. Whether MOMP has been subverted in the apoptotic programs of the worm and fly, or whether it evolved to play a key role in mammalian apoptosis, is unclear (Oberst et al., 2008; Yuan, 2006). However, Bak- or Bax-mediated MOMP is recognised as the point of no return in mammalian apoptosis.
BH3:groove interface in killer complexes
It has been known for some time that MOMP and apoptosis are associated with a major conformational change in the structure of Bak and Bax, and that the activated form of these proteins oligomerises to contribute to the permeabilisation of the mitochondrial OM (Korsmeyer et al., 2000; Wei et al., 2000). Although structures of activated Bak and Bax are not yet available, it is clear that delineating each step in their activation will help to elucidate not only how Bak and Bax form the pore complex that mediates MOMP, but also how the function of these proteins is regulated.
Early activation of Bax and Bak
An early step of Bax activation is its translocation from the cytosol to the mitochondrial OM (Fig. 3) (Wolter et al., 1997). This involves the C-terminal transmembrane (TM) domain of Bax losing its attachment to the hydrophobic surface groove, and the insertion of the TM domain into the OM (Nechushtan et al., 1999; Suzuki et al., 2000). Translocation is not required for Bak, as it is already inserted into the OM in healthy cells (Fig. 3). Another conformational change that occurs during activation is at the N-terminus – epitopes in this region of both Bak and Bax become exposed during apoptosis (Griffiths et al., 1999; Nechushtan et al., 1999). The epitope that is recognised by the active-conformation-specific antibody, clone 6A7, maps to the beginning of α-helix 1 of Bax (Hsu and Youle, 1998), which indicates that this helix may reposition during apoptosis.
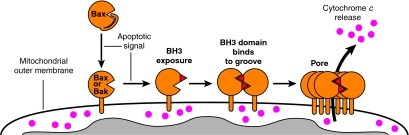
Fig. 3.
The BH3:groove model of Bak and Bax conformational change and oligomerisation during apoptosis. Upon apoptotic signalling, the BH3 domain (red triangle) of Bax or Bak is transiently exposed before binding to the hydrophobic surface groove of another activated molecule. Evidence suggests that reciprocal BH3:groove interactions result in the formation of a symmetric homodimer. The dimers presumably then homo-oligomerise via a secondary interface to form large oligomeric complexes that are responsible for pore formation and release of pro-apoptotic factors such as cytochrome c. Note that, in healthy cells, Bak is already anchored in the OM via the TM domain but a TM:groove interaction restrains Bax primarily to the cytosol. Thus, following apoptosis, the initial step in Bax conformational change is the eversion of the TM domain and its insertion into the OM. Note also that, in this model of pore formation, only the TM domain is shown to be membrane-inserted during apoptosis (i.e. the `TM-only' hypothesis).
Exposure of the BH3 domain and formation of symmetric homodimers
We recently reported that Bak activation involves the exposure of its BH3 domain, which then binds to the hydrophobic surface groove of another activated Bak molecule (Dewson et al., 2008). The resulting BH3:groove interface was found to be required for Bak function because preventing BH3 exposure, or adding antibodies specific for the BH3 domain, blocked Bak oligomerisation and prevented MOMP. An equivalent BH3:groove interaction also forms between Bax molecules during apoptosis (our unpublished data), which is consistent with findings that the region between α-helices 2-5 is important for Bax oligomerisation (George et al., 2007). The BH3 domain is located in α-helix 2, and contains four conserved hydrophobic residues that face towards the hydrophobic core of the protein (Fig. 4). Therefore, the eversion of the BH3 domain during the activation of Bax and Bak is a key step that allows the exposed hydrophobic residues to bind to the hydrophobic groove of partner Bak and Bax molecules (Fig. 3), and probably also to the groove of pro-survival proteins (see below). Whether the exposure of the BH3 domain occurs before or after the exposure of the epitope found on α-helix 1 during Bax and Bak activation is not yet known, as the flexible loop between α-helices 1 and 2 (Fig. 4) could, in theory, allow each conformational change to occur independently.
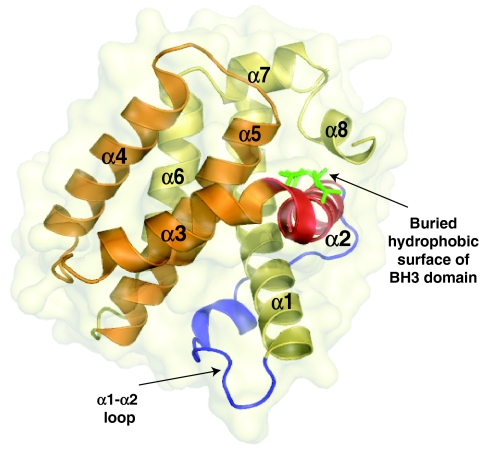
Fig. 4.
Helical structure of non-activated Bak. Ribbon diagram of human Bak (residues 21-183) as shown in the X-ray structure (RCSB Protein Data Bank file 2IMT) (Moldoveanu et al., 2006), which resembles that of non-activated Bax and of Bcl-2 pro-survival proteins. Note that α-helices 1-8 are labelled, but that α-helix 9 (the TM domain) was not present in the X-ray structure. The hydrophobic groove involves α-helices 3-5 (orange) and part of α-helix 2 (red). Note that the conserved hydrophobic amino acids (green) in the BH3 domain face towards the core of the protein. During apoptosis, this region becomes transiently exposed before binding to the hydrophobic groove of another Bak molecule (Dewson et al., 2008). BH3 exposure may be accommodated by the α1-α2 interhelical loop (blue). This figure was generated using PyMol (DeLano, 2002).
The hydrophobic surface groove is a structural feature that is common to Bax and the pro-survival proteins, and involves α-helices 2-5 (Fig. 4). In the case of pro-survival proteins, this groove is the crucial docking site for BH3-only proteins (Hinds and Day, 2005; Petros et al., 2004). The hydrophobic groove in non-activated Bax is occupied by its TM domain. By contrast, in non-activated Bak the groove is unoccupied, but appears occluded by the proximity of α-helices 3 and 4 (Fig. 4) (Moldoveanu et al., 2006), raising the possibility that the groove can bind neither a TM nor a BH3 domain. However, our finding that the Bak groove binds to the exposed Bak BH3 domain during homo-oligomerisation indicates that the Bak groove has considerable plasticity, as observed for the groove in Bcl-xL (Hinds and Day, 2005). It is thus probable that exposure of the BH3 domain and groove opening occur simultaneously during Bak conformational change, thereby coordinating the exposure of the BH3 domain with its binding to the groove of another activated Bak molecule.
We propose that pro-apoptotic Bak (and Bax) dimers are symmetric, and are formed via reciprocal BH3:groove interactions between two activated Bak (or Bax) molecules (Fig. 3). This is structurally feasible because the exposed BH3 domain and the groove would be on the same side of activated Bak (Fig. 4). A symmetric dimer would be expected to strengthen the interaction between the two molecules by increasing the interface surface area. A symmetric homodimer model is novel for the Bcl-2 family proteins, as the reported homodimers of Bcl-xL and its viral homologue F1L are domain-swapped dimers (Jeong et al., 2004; Kvansakul et al., 2008; O'Neill et al., 2006).
Towards a generalised BH3:groove model of Bcl-2 family interactions
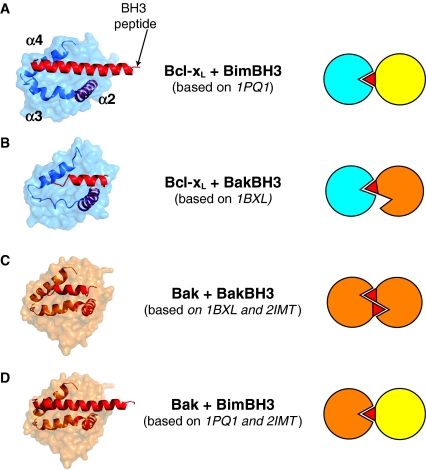
The finding that Bak and Bax homodimers involve a BH3:groove interaction supports mounting evidence that similar interactions are involved in all associations between Bcl-2 family members (Fig. 5). Many binding and functional studies have verified that a BH3:groove interaction is the basis for binding of BH3-only proteins to pro-survival proteins, and that sequence differences in the BH3 domain and in the groove dictate the binding affinity between different members (Certo et al., 2006; Chen et al., 2005; Kuwana et al., 2005). There is also evidence that the sequestration of Bak and Bax by pro-survival proteins involves BH3:groove interactions (Sattler et al., 1997; Willis et al., 2005; Zha et al., 1996), although further analysis is needed to define at what stage of apoptosis these interactions occur. Finally, the binding of BH3-only proteins to the groove of Bak and Bax is a possible mechanism for `direct' activation of Bak and Bax (see below). In summary, the potential for BH3:groove interactions between each of the three Bcl-2 family subclasses, albeit with different affinities, appears to provide a powerful, yet flexible, mechanism by which apoptosis is regulated.
Fig. 5.
BH3:groove interaction as a general mechanism of binding between Bcl-2 proteins. Structural (left) and schematic (right) representations of BH3:groove dimers that may be the basis of all interactions between Bcl-2 members. Structures are represented as space-filled, except for α-helices 2-4 to indicate the location of the hydrophobic surface groove. The bound BH3 peptides (red α-helices) are positioned in the groove, either as shown in the structures based on RCSB Protein Data Bank files (A) 1PQ1 (Liu et al., 2003) and (B) 1BXL (Sattler et al., 1997), or as modelled by aligning structures based on (C) 1BXL and 2IMT (Moldoveanu et al., 2006) or (D) 1PQ1 and 2IMT, using PyMol (DeLano, 2002).
Does the conformational change of Bak and Bax distinguish them from pro-survival proteins?
Might BH3 exposure, and thus the ability to form homo-oligomers, distinguish Bak and Bax from the pro-survival proteins and thereby explain why their functions oppose that of pro-survival proteins? Prior to their activation, the structures of Bak and Bax adopt a fold that is similar to their pro-survival relatives (Moldoveanu et al., 2006; Suzuki et al., 2000); thus, from a structural point of view, the characteristics that distinguish a pro-apoptotic protein from a pro-survival protein are unclear. However, as discussed above, Bak and Bax evert their BH3 domains and oligomerise during apoptosis, whereas this normally does not occur in pro-survival proteins. Indeed, structures of pro-survival proteins bound to their pro-apoptotic targets show that their hydrophobic BH3 residues face the core of the protein (Hinds and Day, 2005; Petros et al., 2004). Furthermore, reports that Bcl-2 and Bcl-xL can be converted into pro-apoptotic proteins by caspase cleavage or the binding of the nuclear receptor, Nur77, may be related to BH3 exposure (Basanez et al., 2001; Cheng et al., 1997; Kolluri et al., 2008). Therefore, the capacity for BH3 exposure and possible homo-oligomerisation, might be what distinguishes the pro-apoptotic Bak and Bax molecules from their pro-survival relatives.
Intriguingly, a conformational change involving α-helices 5 and 6 might occur in both the pro-apoptotic and anti-apoptotic Bcl-2 family members. Andrews and colleagues found that Bax, Bcl-2 and Bcl-xL each insert α-helices 5 and 6 into the OM during apoptosis (Annis et al., 2005; Billen et al., 2008; Kim et al., 2004). This change can be initiated by tBid, and the altered form of Bcl-2 can interact with Bax to inhibit pore formation in liposomes (Peng et al., 2006). Clearly, membrane insertion of the hydrophobic α-helix 5 would remove it from the core of Bcl-2 and thus have a dramatic effect on the overall fold of the protein, presumably destroying the hydrophobic binding groove. Thus, in this case, Bax may not bind to Bcl-2 via a BH3:groove interaction, but via an alternative mechanism, the molecular basis of which is unclear.
Towards a unified model of Bak and Bax activation and regulation
Exactly how Bak and Bax are initially activated has been hotly debated, and has been reviewed in detail elsewhere (Chipuk and Green, 2008; Fletcher and Huang, 2008; Leber et al., 2007). Briefly, according to the direct model, certain activator BH3-only proteins (tBid, Bim and perhaps Puma) directly bind to Bak and Bax to trigger their conformational change and oligomerisation. According to the indirect model, BH3-only proteins bind to pro-survival proteins and cause them to release activated Bak or Bax. In these models, the main role of pro-survival proteins is either to sequester BH3-only proteins (direct model) or to sequester Bak and Bax (indirect model). Efforts to verify either model in physiological settings have been thwarted by the multiplicity of Bcl-2 family members and the selective binding between them. For example, the finding that tBid and Bim are the most potent of the BH3-only proteins has been attributed either to their ability to directly activate Bak and Bax (direct model) or to their ability to bind to all pro-survival proteins (indirect model).
A concern with the direct activation model is that it is difficult to detect the binding of tBid or Bim to Bak or Bax. One explanation for why this is the case is that the interaction may be transient – a `hit-and-run' mechanism. Although the occurrence of such a transient interaction is difficult to prove or disprove, it may be feasible based on a BH3:groove interaction. For example, the activating BH3-only protein might bind to the Bax groove, displacing the TM domain – acting as the initial `hit'. Subsequent Bax BH3 eversion may then disrupt the groove to displace the activator BH3-only protein – acting as the `run'. Another concern with the direct activation model is that apoptosis is not abrogated in bid–/–bim–/– mice and cells, indicating that direct activation of Bak or Bax by tBid or Bim is not essential for apoptosis to occur (Willis et al., 2007). However, these knockout studies do not exclude the possibility that other direct activators (such as p53), post-translational modifications or spontaneous activation of Bak or Bax may compensate for the loss of Bid and Bim (Chipuk et al., 2004; Kim et al., 2006; Linseman et al., 2004). Conversely, a problematic aspect of the indirect activation model is that only minimal Bak and Bax appear to be pre-bound to pro-survival proteins in healthy cells. However, even if a small amount of Bak or Bax was pre-bound to pro-survival proteins, BH3-only proteins might displace Bak and Bax, which could then go on to auto-activate more Bak and Bax (Ruffolo and Shore, 2003; Tan et al., 2006; Willis and Adams, 2005).
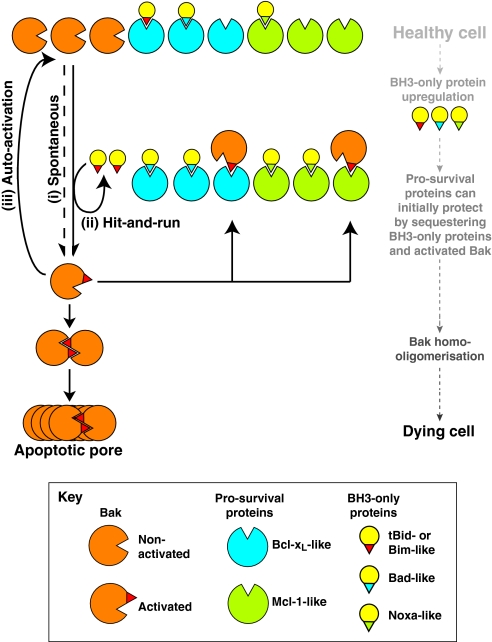
As neither model of Bak and Bax activation fully explains all observations, aspects of both might hold true (Grills et al., 2008; Leber et al., 2007). Fig. 6 illustrates a summary of much of the available data regarding Bak and Bax activation and regulation by other Bcl-2 family members. The central role for Bak and Bax homo-oligomerisation is included beause this step appears to be crucial for apoptosis (Dewson et al., 2008; George et al., 2007). Also included is the possibility that Bak and Bax are metastable and may become activated spontaneously, together with the possibility that activated Bak and Bax may feed back to auto-activate the inactive pool. Clearly, however, further definition of Bak and Bax conformational changes, and the nature of their binding to other Bcl-2 family members, is needed to verify exactly how Bak and Bax are regulated. This is not just a semantic issue; it has important implications for the development of therapeutic compounds that target this pathway. For example, whereas recently developed BH3 mimetics appear to kill cells solely by binding to pro-survival proteins (Oltersdorf et al., 2005; van Delft et al., 2006), it is important to understand how this then leads to activation of Bak and Bax.
Fig. 6.
Towards a unified model of Bak and Bax activation and regulation. Elements of the two models of Bak and Bax activation (direct and indirect) are combined with evidence that these proteins can undergo spontaneous activation and auto-activation. Bak is illustrated, although it is probable that Bax is activated in a similar manner. In this unified model, a low level of spontaneous Bak activation (i) is normally quenched by binding of the activated form to pro-survival proteins. Apoptotic signalling upregulates `activator' BH3-only proteins such as tBid and Bim and/or `sensitiser' BH3-only proteins such as Bad and Noxa. These BH3-only proteins bind to pro-survival proteins, for which they have high affinity. For example, Bad binds strongly to Bcl-xL and Noxa binds strongly to Mcl-1, whereas tBid and Bim bind to all pro-survival proteins. tBid and Bim may also bind and activate Bak in a `hit-and-run' manner (ii), especially if they are not efficiently sequestered by pro-survival proteins. Activated Bak can also auto-activate the inactive pool (iii). As activated Bak accumulates, if not sequestered by pro-survival proteins, it homo-oligomerises to form symmetric dimers, and then higher-order oligomers that can form apoptotic pores in the mitochondrial OM.
Breaching the mitochondrial barrier is a `complex' issue
Activated Bak and Bax form complexes in the mitochondrial OM lipid bilayer that appear responsible for the egress of large folded proteins from mitochondria, including cytochrome c and DIABLO. In synthetic vesicles, the presence of oligomerised Bax can cause the release of 70-250 kDa dextrans (Kuwana et al., 2002; Lovell et al., 2008; Terrones et al., 2008). How Bak and Bax oligomers cause MOMP is not known, although ion channels, proteinaceous pores, lipid pores and lipid destabilisation are proposed mechanisms (Kinnally and Antonsson, 2007; Lucken-Ardjomande and Martinou, 2005).
It is possible that Bak and Bax do not work alone but cooperate with other OM proteins, including voltage-dependent anion channels (VDACs) (Cheng et al., 2003; Shimizu et al., 1999) and components of the mitochondrial fission and fusion machinery (Karbowski et al., 2002). Alternatively, proteins such as the VDACs and the translocase of the outer mitochondrial membrane (TOM) import complex might not contribute directly to the pore, but might assist in mitochondrial targeting of Bak and Bax (Bellot et al., 2007; Setoguchi et al., 2006). However, as permeabilisation of synthetic membranes requires only Bax in concert with tBid (Kuwana et al., 2002; Lovell et al., 2008; Terrones et al., 2008), membrane targeting and disruption might only require activated Bak or Bax, with other proteins having a regulatory role.
To understand how the pore complex forms, the number of Bak or Bax molecules per pore has been estimated. A minimum of four Bax molecules was required for cytochrome c transport across artificial membranes (Korsmeyer et al., 2000), whereas larger complexes (of at least eight molecules) were observed in dying cells (Antonsson et al., 2001; Nechushtan et al., 2001). Recently, innovative fluorescence imaging techniques have been used to estimate that a pore consists of approximately 200 molecules, and that larger complexes develop downstream of MOMP (Zhou and Chang, 2008). Despite these advances, the number of activated Bak or Bax molecules required to form a functional pore is not yet clear.
Importantly, a symmetric dimer model of Bak or Bax oligomerisation (Fig. 3) posits that no free BH3 domain or groove surfaces would be available to link the dimers to form larger multimers. Therefore, dimers must multimerise via alternative interfaces. We have evidence for an α6:α6 interface in Bak oligomers, as cysteines located along the α-helix 6 can crosslink with the same residues in another activated Bak (our unpublished data). Thus, defining how and when interfaces allow activated Bak and Bax to oligomerise will bring us closer to understanding the pore complex, and, importantly, how pore formation might be blocked to prevent apoptosis.
Breaching the OM lipid bilayer via Bak or Bax oligomers
The complexes formed by activated Bak and Bax must ultimately porate the OM lipid bilayer for apoptosis to occur. Thus, if they act independently of other proteins, at least part of the oligomeric Bak or Bax complex must associate with the membrane. The C-terminal region of most Bcl-2 family members contains a TM domain, with the family classified as tail-anchored proteins (Schinzel et al., 2004). Accordingly, the TM domains in Bak and Bax anchor these proteins in the OM, either before (for Bak) or after (for Bax) an apoptotic stimulus (Fig. 3).
As discussed above, the α-helices 5 and 6 of certain Bcl-2 proteins may also insert into the OM as a hairpin loop, according to the long-held `pore-forming domain' theory. This theory stems from the finding that Bcl-2 proteins are structurally homologous to the pore-forming domains of bacterial colicins and diphtheria toxin (Antignani and Youle, 2006; Muchmore et al., 1996). Andrews and colleagues provided experimental evidence that α-helices 5 and 6 of Bax become membrane-inserted following apoptotic signalling, apparently as an intermediate to further conformational change, oligomerisation and pore formation (Annis et al., 2005). Although whether the membrane-inserted α-helices (5, 6 and 9) of Bax (and presumably Bak) associate to form a pore was not delineated, one possibility is that the hydrophobic α-helices 5 and 9 traverse the lipid bilayer, and the amphipathic α-helix 6 lines the solvent-exposed surface of the pore.
It remains unclear whether membrane insertion of the hairpin loop of α-helices 5 and 6 is consistent with our finding of a BH3:groove interaction in oligomerised Bak and Bax, as movement of the α-helix 5 from the core of Bak or Bax may disrupt the hydrophobic groove (Fig. 4) and thereby prevent the binding of an exposed Bak or Bax BH3 domain. Thus, an alternative `TM-only' hypothesis warrants consideration (Fig. 3). In this model, only the C-terminal TM domains may be membrane-inserted, and Bak or Bax oligomerisation would force the TM domains to reposition in a manner that porates the OM. Ideally, the structures of the relevant pore complexes within a membrane will clarify these issues.
Conclusions and perspectives
Despite more than a decade of intense investigation, controversy still rages regarding the activation, regulation and function of Bak and Bax during apoptosis. Recent evidence for a BH3:groove interaction during Bak and Bax homo-oligomerisation has a range of implications for how Bak and Bax are regulated, and supports a central role for similar BH3:groove interactions between all Bcl-2 family members. Further work is needed to define the stepwise activation and oligomerisation of Bak and Bax because each step represents a potential therapeutic target for blocking apoptosis. If blockade could be achieved, cells could fully recover their clonogenic potential, as the mitochondria would remain intact. Furthermore, determining the step(s) at which pro-survival proteins can intervene in the action of Bak or Bax in the apoptotic pathway will be important for optimising safe and effective agents, such as BH3 mimetics that target the Bcl-2 family, and would boost their already excellent potential as anti-cancer therapeutics (Adams and Cory, 2007).
We thank colleagues in the Divisions of Molecular Genetics of Cancer and Structural Biology for discussions. The work was supported by the Cancer Council of Victoria, the Wellcome Trust, the NHMRC and the Australian Research Council. Deposited in PMC for release after 6 months.
References
- Adams, J. M. and Cory, S. (2007). The Bcl-2-regulated apoptosis switch: mechanism and therapeutic potential. Cur. Opin. Immunol. 19, 488-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis, M. G., Soucie, E. L., Dlugosz, P. J., Cruz-Aguado, J. A., Penn, L. Z., Leber, B. and Andrews, D. W. (2005). Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 24, 2096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antignani, A. and Youle, R. J. (2006). How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr. Opin. Cell Biol. 18, 685-689. [DOI] [PubMed] [Google Scholar]
- Antonsson, B., Montessuit, S., Sanchez, B. and Martinou, J. C. (2001). Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276, 11615-11623. [DOI] [PubMed] [Google Scholar]
- Basanez, G., Zhang, J., Chau, B. N., Maksaev, G. I., Frolov, V. A., Brandt, T. A., Burch, J., Hardwick, J. M. and Zimmerberg, J. (2001). Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J. Biol. Chem. 276, 31083-31091. [DOI] [PubMed] [Google Scholar]
- Bellot, G., Cartron, P. F., Er, E., Oliver, L., Juin, P., Armstrong, L. C., Bornstein, P., Mihara, K., Manon, S. and Vallette, F. M. (2007). TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 14, 785-794. [DOI] [PubMed] [Google Scholar]
- Billen, L. P., Kokoski, C. L., Lovell, J. F., Leber, B. and Andrews, D. W. (2008). Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 6, e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo, M., Moore Vdel, G., Nishino, M., Wei, G., Korsmeyer, S., Armstrong, S. A. and Letai, A. (2006). Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351-365. [DOI] [PubMed] [Google Scholar]
- Chautan, M., Chazal, G., Cecconi, F., Gruss, P. and Golstein, P. (1999). Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr. Biol. 9, 967-970. [DOI] [PubMed] [Google Scholar]
- Chen, L., Willis, S. N., Wei, A., Smith, B. J., Fletcher, J. I., Hinds, M. G., Colman, P. M., Day, C. L., Adams, J. M. and Huang, D. C. S. (2005). Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17, 393-403. [DOI] [PubMed] [Google Scholar]
- Cheng, E. H. Y., Kirsch, D. G., Clem, R. J., Ravi, R., Kastan, M. B., Bedi, A., Ueno, K. and Hardwick, J. M. (1997). Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278, 1966-1968. [DOI] [PubMed] [Google Scholar]
- Cheng, E. H., Sheiko, T. V., Fisher, J. K., Craigen, W. J. and Korsmeyer, S. J. (2003). VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513-517. [DOI] [PubMed] [Google Scholar]
- Chipuk, J. E. and Green, D. R. (2008). How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 18, 157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk, J. E., Kuwana, T., Bouchier-Hayes, L., Droin, N. M., Newmeyer, D. D., Schuler, M. and Green, D. R. (2004). Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, 1010-1014. [DOI] [PubMed] [Google Scholar]
- DeLano, W. L. (2002). The PYMOL Molecular Graphics System. Palo Alto, CA: DeLano Scientific.
- Delivani, P., Adrain, C., Taylor, R. C., Duriez, P. J. and Martin, S. J. (2006). Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell 21, 761-773. [DOI] [PubMed] [Google Scholar]
- Dewson, G., Kratina, T., Sim, H. W., Puthalakath, H., Adams, J. M., Colman, P. M. and Kluck, R. M. (2008). To trigger apoptosis Bak exposes its BH3 domain and homo-dimerizes via BH3-grooove interactions. Mol. Cell 30, 369-380. [DOI] [PubMed] [Google Scholar]
- Dix, M. M., Simon, G. M. and Cravatt, B. F. (2008). Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell 134, 679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn, L., Read, S., Cakouros, D., Huh, J. R., Hay, B. A. and Kumar, S. (2002). The role of cytochrome c in caspase activation in Drosophila melanogaster cells. J. Cell Biol. 156, 1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert, P. G., Read, S. H., Silke, J., Marsden, V. S., Kaufmann, H., Hawkins, C. J., Gerl, R., Kumar, S. and Vaux, D. L. (2004). Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J. Cell Biol. 165, 835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, J. I. and Huang, D. C. S. (2008). Controlling the cell death mediators Bax and Bak: puzzles and conundrums. Cell Cycle 7, 39-44. [DOI] [PubMed] [Google Scholar]
- George, N. M., Evans, J. J. and Luo, X. (2007). A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 21, 1937-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, G. J., Dubrez, L., Morgan, C. P., Jones, N. A., Whitehouse, J., Corfe, B. M., Dive, C. and Hickman, J. A. (1999). Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144, 903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grills, C., Crawford, N., Chacko, A., Johnston, P. G., O'Rourke, F. and Fennell, D. A. (2008). Dynamical systems analysis of mitochondrial BAK activation kinetics predicts resistance to BH3 domains. PLoS ONE 3, e3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner, M. O. and Horvitz, H. R. (1994). Activation of C. elegans cell death protein CED-9 by an amino-acid substitution in a domain conserved in Bcl-2. Nature 369, 318-320. [DOI] [PubMed] [Google Scholar]
- Hinds, M. G. and Day, C. L. (2005). Regulation of apoptosis: uncovering the binding determinants. Curr. Opin. Struct. Biol. 15, 690-699. [DOI] [PubMed] [Google Scholar]
- Hsu, Y. T. and Youle, R. J. (1998). Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273, 10777-10783. [DOI] [PubMed] [Google Scholar]
- Jeong, S. Y., Gaume, B., Lee, Y. J., Hsu, Y. T., Ryu, S. W., Yoon, S. H. and Youle, R. J. (2004). Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 23, 2146-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski, M., Lee, Y. J., Gaume, B., Jeong, S. Y., Frank, S., Nechushtan, A., Santel, A., Fuller, M., Smith, C. L. and Youle, R. J. (2002). Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, T., Jost, P. J., Pellegrini, M., Puthalakath, H., Gugasyan, R., Gerondakis, S., Cretney, E., Smyth, M. J., Silke, J., Hakem, R. et al. (2009). Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity 30, 56-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. J., Ryu, S. W. and Song, B. J. (2006). JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation, mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 281, 21256-21265. [DOI] [PubMed] [Google Scholar]
- Kim, P. K., Annis, M. G., Dlugosz, P. J., Leber, B. and Andrews, D. W. (2004). During apoptosis bcl-2 changes membrane topology at both the endoplasmic reticulum and mitochondria. Mol. Cell 14, 523-529. [DOI] [PubMed] [Google Scholar]
- Kinnally, K. W. and Antonsson, B. (2007). A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis 12, 857-868. [DOI] [PubMed] [Google Scholar]
- Kolluri, S. K., Zhu, X., Zhou, X., Lin, B., Chen, Y., Sun, K., Tian, X., Town, J., Cao, X., Lin, F. et al. (2008). A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell 14, 285-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer, S. J., Wei, M. C., Saito, M., Weiler, S., Oh, K. J. and Schlesinger, P. H. (2000). Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7, 1166-1173. [DOI] [PubMed] [Google Scholar]
- Kuwana, T., Mackey, M. R., Perkins, G., Ellisman, M. H., Latterich, M., Schneiter, R., Green, D. R. and Newmeyer, D. D. (2002). Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331-342. [DOI] [PubMed] [Google Scholar]
- Kuwana, T., Bouchier-Hayes, L., Chipuk, J. E., Bonzon, C., Sullivan, B. A., Green, D. R. and Newmeyer, D. D. (2005). BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17, 525-535. [DOI] [PubMed] [Google Scholar]
- Kvansakul, M., Yang, H., Fairlie, W. D., Czabotar, P. E., Fischer, S. F., Perugini, M. A., Huang, D. C. and Colman, P. M. (2008). Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 15, 1564-1571. [DOI] [PubMed] [Google Scholar]
- Leber, B., Lin, J. and Andrews, D. W. (2007). Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis 12, 897-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten, T., Ross, A. J., King, A., Zong, W., Rathmell, J. C., Shiels, H. A., Ulrich, E., Waymire, K. G., Mahar, P., Frauwirth, K. et al. (2000). The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linseman, D. A., Butts, B. D., Precht, T. A., Phelps, R. A., Le S. S., Laessig, T. A., Bouchard, R. J., Florez-McClure, M. L. and Heidenreich, K. A. (2004). Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J. Neurosci. 24, 9993-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Dai, S., Zhu, Y., Marrack, P. and Kappler, J. W. (2003). The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity 19, 341-352. [DOI] [PubMed] [Google Scholar]
- Lovell, J. F., Billen, L. P., Bindner, S., Shamas-Din, A., Fradin, C., Leber, B. and Andrews, D. W. (2008). membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135, 1074-1084. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande, S. and Martinou, J. C. (2005). Regulation of Bcl-2 proteins and of the permeability of the outer mitochondrial membrane. CR Biol. 328, 616-631. [DOI] [PubMed] [Google Scholar]
- Luthi, A. U. and Martin, S. J. (2007). The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 14, 641-650. [DOI] [PubMed] [Google Scholar]
- Meier, P. and Vousden, K. H. (2007). Lucifer's labyrinth-ten years of path finding in cell death. Mol. Cell 28, 746-754. [DOI] [PubMed] [Google Scholar]
- Moldoveanu, T., Liu, Q., Tocilj, A., Watson, M. H., Shore, G. and Gehring, K. (2006). The x-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol. Cell 24, 677-688. [DOI] [PubMed] [Google Scholar]
- Muchmore, S. W., Sattler, M., Liang, H., Meadows, R. P., Harlan, J. E., Yoon, H. S., Nettesheim, D., Chang, B. S., Thompson, C. B., Wong, S. L. et al. (1996). X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381, 335-341. [DOI] [PubMed] [Google Scholar]
- Nechushtan, A., Smith, C. L., Hsu, Y. T. and Youle, R. J. (1999). Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18, 2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan, A., Smith, C. L., Lamensdorf, I., Yoon, S. H. and Youle, R. J. (2001). Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 153, 1265-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst, A., Bender, C. and Green, D. R. (2008). Living with death: the evolution of the mitochondrial pathway of apoptosis in animals. Cell Death Differ. 15, 1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf, T., Elmore, S. W., Shoemaker, A. R., Armstrong, R. C., Augeri, D. J., Belli, B. A., Bruncko, M., Deckwerth, T. L., Dinges, J., Hajduk, P. J. et al. (2005). An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677-681. [DOI] [PubMed] [Google Scholar]
- O'Neill, J. W., Manion, M. K., Maguire, B. and Hockenbery, D. M. (2006). BCL-XL dimerization by three-dimensional domain swapping. J. Mol. Biol. 356, 367-381. [DOI] [PubMed] [Google Scholar]
- Peng, J., Tan, C., Roberts, G. J., Nikolaeva, O., Zhang, Z., Lapolla, S. M., Primorac, S., Andrews, D. W. and Lin, J. (2006). tBid elicits a conformational alteration in membrane-bound Bcl-2 such that it inhibits Bax pore formation. J. Biol. Chem. 281, 35802-35811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros, A. M., Olejniczak, E. T. and Fesik, S. W. (2004). Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644, 83-94. [DOI] [PubMed] [Google Scholar]
- Ravichandran, K. S. and Lorenz, U. (2007). Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 7, 964-974. [DOI] [PubMed] [Google Scholar]
- Ruffolo, S. C. and Shore, G. C. (2003). BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J. Biol. Chem. 278, 25039-25045. [DOI] [PubMed] [Google Scholar]
- Sattler, M., Liang, H., Nettesheim, D., Meadows, R. P., Harlan, J. E., Eberstadt, M., Yoon, H. S., Shuker, S. B., Chang, B. S., Minn, A. J. et al. (1997). Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983-986. [DOI] [PubMed] [Google Scholar]
- Schinzel, A., Kaufmann, T. and Borner, C. (2004). Bcl-2 family members: integrators of survival and death signals in physiology and pathology [corrected]. Biochim. Biophys. Acta 1644, 95-105. [DOI] [PubMed] [Google Scholar]
- Setoguchi, K., Otera, H. and Mihara, K. (2006). Cytosolic factor- and TOM-independent import of C-tail-anchored mitochondrial outer membrane proteins. EMBO J. 25, 5635-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, S., Narita, M. and Tsujimoto, Y. (1999). Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483-487. [DOI] [PubMed] [Google Scholar]
- Suzuki, M., Youle, R. J. and Tjandra, N. (2000). Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103, 645-654. [DOI] [PubMed] [Google Scholar]
- Tan, C., Dlugosz, P. J., Peng, J., Zhang, Z., Lapolla, S. M., Plafker, S. M., Andrews, D. W. and Lin, J. (2006). Auto-activation of the apoptosis Protein Bax Increases mitochondrial membrane permeability and Is inhibited by Bcl-2. J. Biol. Chem. 281, 14764-14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrones, O., Etxebarria, A., Landajuela, A., Landeta, O., Antonsson, B. and Basanez, G. (2008). BIM and tBID are not mechanistically equivalent when assisting BAX to permeabilize bilayer membranes. J. Biol. Chem. 283, 7790-7803. [DOI] [PubMed] [Google Scholar]
- van Delft, M. F., Wei, A. H., Mason, K. D., Vandenberg, C. J., Chen, L., Czabotar, P. E., Willis, S. N., Scott, C. L., Day, C. L., Cory, S. et al. (2006). The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10, 389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, M. C., Lindsten, T., Mootha, V. K., Weiler, S., Gross, A., Ashiya, M., Thompson, C. B. and Korsmeyer, S. J. (2000). tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14, 2060-2071. [PMC free article] [PubMed] [Google Scholar]
- Wei, M. C., Zong, W. X., Cheng, E. H., Lindsten, T., Panoutsakopoulou, V., Ross, A. J., Roth, K. A., MacGregor, G. R., Thompson, C. B. and Korsmeyer, S. J. (2001). Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S. N. and Adams, J. M. (2005). Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17, 617-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S. N., Chen, L., Dewson, G., Wei, A., Naik, E., Fletcher, J. I., Adams, J. M. and Huang, D. C. (2005). Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19, 1294-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S. N., Fletcher, J. I., Kaufmann, T., van Delft, M. F., Chen, L., Czabotar, P. E., Ierino, H., Lee, E. F., Fairlie, W. D., Bouillet, P. et al. (2007). Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856-859. [DOI] [PubMed] [Google Scholar]
- Wolter, K. G., Hsu, Y. T., Smith, C. L., Nechushtan, A., Xi, X. G. and Youle, R. J. (1997). Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139, 1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X.-M., Wang, K., Gross, A., Zhao, Y., Zinkel, S., Klocke, B., Roth, K. A. and Korsmeyer, S. J. (1999). Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886-891. [DOI] [PubMed] [Google Scholar]
- Youle, R. J. and Strasser, A. (2008). The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell. Biol. 9, 47-59. [DOI] [PubMed] [Google Scholar]
- Yuan, J. (2006). Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol. Cell 23, 1-12. [DOI] [PubMed] [Google Scholar]
- Zha, H., Aimé-Sempé, C., Sato, T. and Reed, J. C. (1996). Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271, 7440-7444. [DOI] [PubMed] [Google Scholar]
- Zhou, L. and Chang, D. C. (2008). Dynamics and structure of the Bax-Bak complex responsible for releasing mitochondrial proteins during apoptosis. J. Cell Sci. 121, 2186-2196. [DOI] [PubMed] [Google Scholar]
- Zimmermann, K. C., Ricci, J. E., Droin, N. M. and Green, D. R. (2002). The role of ARK in stress-induced apoptosis in Drosophila cells. J. Cell Biol. 156, 1077-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]