Abstract
Background
Hepatic ischemia/reperfusion (I/R) injury is a principal consideration of trauma, resectional liver surgery and transplantation. Despite improvements in supportive care hepatic I/R injury continues to negatively impact patient outcomes due to significant tissue damage and organ dysfunction. CXC chemokines have been implicated as key mediators in the deleterious inflammatory cascade following hepatic I/R and also as important, beneficial regulators of liver recovery and regeneration. As such, their potential to mediate both beneficial and detrimental effects on hepatocytes makes them a key target for therapy. Herein, we provide a review of the inflammatory mechanisms of hepatic I/R injury, with a focus on the divergent functions of CXC chemokines in this response compared to other liver insults, and offer an explanation of this apparent paradox.
Data sources
MEDLINE and PubMed
Conclusions
CXC chemokines are key mediators of both the inflammatory response to hepatic I/R as well as the recovery from this injury. Their contrasting functions in the regeneration of liver mass after an ischemic insult indicates that therapeutic manipulation of these mediator pathways should differ depending on the surgical milieu.
Keywords: Ischemia/reperfusion, chemokines, inflammation, liver regeneration
INTRODUCTION
Hepatic ischemia/reperfusion (I/R) injury is a major complication of major trauma, liver resection, and transplantation.1–3 It occurs when blood flow to liver parenchyma is interrupted for a period of time and subsequently reperfused. This results in the induction of an acute inflammatory response that can lead to significant tissue damage and organ dysfunction both locally and remotely.3–5 The mechanisms of acute inflammation in hepatic ischemia/reperfusion injury has been widely investigated in animal models and has increased our understanding of the pathogenesis of this inflammatory cascade and guided clinical investigations aimed at preventing or minimizing hepatic ischemia/reperfusion injury. Additionally, these experimental models have discovered new functions of soluble mediators elaborated during I/R injury that may govern the ability of the hepatic parenchyma to recover and regenerate after significant injury. This review will summarize our current knowledge of the inflammatory response that contributes to the pathophysiology of hepatic I/R injury. More specifically, we will focus on an important class of small proteins, called CXC chemokines, that regulate both the injury and recovery from I/R and offer intriguing new ideas regarding the relevance of experimental discoveries to the clinical management of transplantation and resection surgery.
MECHANISMS OF HEPATIC I/R INJURY: EARLY PHASE
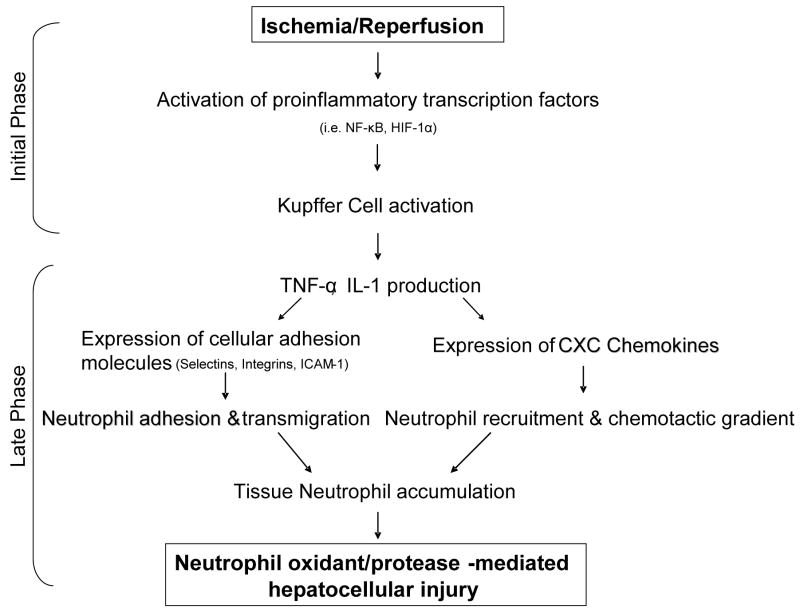
Jaeschke et al. first described and characterized two distinct phases of liver injury after hepatic ischemia/reperfusion injury. The first phase occurs during the initial few hours after reperfusion and is a Kupffer cell-mediated response augmented by complement activation.6,7 These liver-resident macrophages produce reactive oxygen species which cause oxidant induced stress and cell damage to the surrounding hepatocytes.7,8 This phase is associated with modest hepatocellular injury marked by moderate increases in serum transaminase levels but preserved hepatic architecture. Despite the limited degree of injury during this phase, the oxidant stress results in the release of a number of proinflammatory cytokines that serve to initiate and propagate an intense secondary inflammatory response.4,5 The most proximal of these cytokines is interleukin-12 (IL-12), which is increased during the period of ischemia and early reperfusion.9 While the precise cellular source of IL-12 remains unknown, neutralization of IL-12 using antibodies or IL-12 knockout mice results in impaired TNF-α production and diminished subsequent inflammation.9 TNF-α is thought to be the primary factor that propagates the inflammatory response throughout the liver,10,11,12 while another major early-response cytokine, IL-1, is expressed after TNF-α and likely serves an accessory role in neutrophil recruitment.13 Expression of these proinflammatory cytokines is mediated by activation of transcription factors, including HIF-1α and NF-κB, as studies have shown associations between HIF-1α and Kupffer cell cytokine production.14,15 In this manner, the initial injury phase gives rise to a later, inflammation-mediated phase of injury (Figure 1).
Figure 1.
Hepatic ischemia/reperfusion injury inflammatory pathway: the initial phase mediated by Kupffer cells and TNF-α production gives rise to an inflammation-mediated late phase of injury highlighted by neutrophil accumulation and CXC chemokine production.
MECHANISMS OF HEPATIC I/R INJURY: LATE PHASE
The second phase of liver injury after ischemia/reperfusion is characterized by the recruitment of activated neutrophils into the liver parenchyma.16 As discussed above, TNF-α plays a principal role in the induction of mechanisms leading to neutrophil accumulation 11,12,13. TNF-α induces the production and release of neutrophil chemoattractants, particularly CXC chemokines, from Kupffer cells and hepatocytes.17,18 In addition, it stimulates hepatic microvascular endothelial cells to increase their expression of adhesion molecules.11 There are three main classes of adhesion molecules. The selectin family of adhesion molecules is expressed on both leukocytes and endothelial cells and function in the initial capture and transient adhesion of neutrophils to the endothelium.19,22 By bringing neutrophils in close proximity to the endothelial cell surface and reducing their velocity, selectins allow other adhesion molecules expressed on neutrophils and endothelial cells to interact. Specifically, integrins (e.g. MAC-1) expressed on neutrophil surfaces and immunoglobulin molecules (e.g. ICAM-1 and VCAM-1) on endothelial cells mediate firm adhesion and diapedesis of the neutrophil from the vascular space to the interstitium.23–25
Neutrophils that are adherent to the vascular endothelium are activated by CXC chemokines expressed at the site of injury.26,27 (Figure 2). Furthermore, the chemotaxis of these activated neutrophils to the liver parenchyma is directed by a gradient of CXC chemokines.17,18 Accumulated neutrophils damage hepatocytes via elaboration of oxidants and proteases. Neutrophil release of reactive oxygen radicals such as superoxide anion (O2•) and hydroxyl radical (HO•) lead to hepatocyte cell death.28,29 Additionally, release of proteases (e.g. collagenase, elastase, cathepsin G, heparanase) from neutrophil granules also directly damage hepatocytes.30,31 The resulting injury is severe and is characterized by marked hepatocyte necrosis histologically as well as highly elevated levels of ALT in the circulation.
ANTI-INFLAMATORY REGULATORS OF HEPATIC I/R INJURY
In order to prevent an overwhelming and possible lethal inflammatory response to hepatic I/R, endogenous anti-inflammatory mediators are expressed that serve to maintain a homeostatic balance. Several key players have been identified including IL-6, IL-13 and secretory leukocyte protease inhibitor (SLPI). IL-6 has been shown to be protective in hepatic I/R by reducing expression of TNF-α and c-reactive protein, thereby limiting hepatocellular injury and promoting hepatocyte regeneration.32 The role of IL-13 is less clearly understood. Administration of exogenous IL-13 protects against hepatic I/R injury by STAT6, a transcription factor that inhibits the transcription of TNF-α and MIP-2.33 However, IL-13 knockout mice show a greater degree of cellular injury after hepatic I/R with no significant change in TNF-α expression and with decreased tissue neutrophil accumulation due to less VCAM-1 hepatic expression.34 SLPI is a protease inhibitor produced by a various cells in response to I/R.35–37 In hepatic I/R, endogenous SLPI suppresses the expression of proinflammatory cytokines and reduces local and remote organ injury.37
HEPATIC RECOVERY FROM I/R INJURY
Hepatic I/R is commonly encountered in trauma surgery as well as liver transplantation and resection. This directly affects patient outcomes by impacting liver function and viability as well as remote organ injury from inflammation. Experimental models of liver I/R injury have shown that peak hepatocellular injury occurs within 12 hours after reperfusion.38,39 By 48 hours after reperfusion the liver enters a proliferative phase with nearly complete recovery of hepatocellular architecture within 96 hours of reperfusion.38,39
STAT3 and NF-κB are transcription factors known to be critical to liver regeneration.40–42 In experimental models of hepatectomy they have been shown to be upregulated in response to IL-6 and other cytokines to induce liver regeneration.40 STAT3 and NF-κB are also thought to play essential roles in the protective mechanisms of ischemic preconditioning and ischemic hypothermia in hepatocytes.43,44 Both transcription factors are also activated during the recovery from I/R injury and are important for the proliferative/reparative response observed after 48 hours of reperfusion.15,39 Other cell cycle regulators have also been implicated in the recovery phase, including p53, p21, CDK.38
IMPORTANCE OF CXC CHEMOKINES IN LIVER RECOVERY AND REGENERATION
CXC chemokines are critical mediators involved in the recruitment of neutrophils to the liver after I/R.4,17,18 CXC chemokines represent one of four branches of the chemokine superfamily. Chemokine nomenclature is based on a conserved, cysteine-containing amino acid sequence at the amino terminus of each molecule: C, CC, CXC, and CX3C (where X is any amino acid). Very little is known about the C and CX3C branches however they are believed to mediate chemotaxis of precursor T cell and natural killer cells respectively 45. The CC family has been widely studied and at least 27 distinct ligands have been identified which serve as potent chemoattractants for monocytes.45
CXC chemokines are further subdivided into two subsets based on the presence or absence of a Glu-Leu-Arg (ELR) amino acid motif at the amino terminus of the peptide. CXC chemokines possessing the ELR motif bind to the receptors CXCR1 and/or CXCR2, while ELR-negative chemokines bind to CXCR3, CXCR4, CXCR5 and CXCR6.46,47 Table I lists CXC chemokines, their receptors and target cells expressing the different receptors. The ELR-positive CXC chemokines are relevant to liver injury, with CXCR1 and CXCR2 being expressed by neutrophils, endothelial cells and hepatocytes.46,47,48 The effects of CXC chemokines on neutrophils are well-known and include stimulation of chemotaxis as well as induction of respiratory burst activity.49 In endothelial cells, the binding of CXC chemokines to CXCR2 results in proliferation and chemotaxis in a manner facilitating angiogenesis.50,51 CXC chemokines have also been shown to induce proliferation in hepatocytes.52,53 These findings led to the examination of the role of CXC chemokines during liver regeneration after partial hepatectomy.
Table I.
CXC chemokine ligands, corresponding receptors and target cells. Murine CXC chemokine common names and are italicized next to their human equivalents.
| Chemokines | Chemokine Receptor | ELR Status Target Cells | |
|---|---|---|---|
| Old Nomenclature | New Nomenclature | ||
| GROα/KC | CXCL1 | CXCR1, CXCR2 | ELR + Neutrophils |
| GROβ/MIP-2 | CXCL2 | CXCR1, CXCR2 | |
| GROγ | CXCL3 | CXCR1, CXCR2 | |
| ENA-78/LIX | CXCL5 | CXCR2 | |
| GCP-2 | CXCL6 | CXCR1, CXCR2 | |
| NAP-2 | CXCL7 | CXCR1, CXCR2 | |
| IL-8 | CXCL8 | CXCR1, CXCR2 | |
| PF-4 | CXCL4 | CXCR3b | ELR − Lymphocytes |
| MIG | CXCL9 | CXCR3a, CXCR3b | |
| IP-10 | CXCL10 | CXCR3a, CXCR3b | |
| ITAC | CXCL11 | CXCR3a, CXCR3b | |
| SDF-1 | CXCL12 | CXCR4 | |
| BCA-1 | CXCL13 | CXCR5 | |
| BRAK | CXCL14 | Unknown | |
| - | CXCL16 | CXCR6 | |
Colletti et al were the first to define the effects of CXC chemokines on hepatocytes in vitro. 52 They noted hepatocyte proliferation in response to increasing concentrations of ERL-positive CXC chemokines. Subsequently, they investigated the function of CXC chemokines in vivo during liver regeneration using a murine model of 70% hepatectomy. They reported that expression of CXC chemokines was elevated after hepatectomy and that when these chemokines were neutralized using antibodies, there was a significant reduction in liver mass.52 Conversely, treatment of mice with the CXC chemokine, macrophage inflammatory protein-2 (MIP-2), increased hepatocyte proliferation and liver regeneration after partial hepatectomy.53
However, we have recently found that the role of CXC chemokines in liver recovery after I/R is far different from their role in regeneration after partial hepatectomy. We found that genetic deletion or pharmacological antagonism of CXCR2 after I/R injury resulted in augmented hepatocyte proliferation and accelerated recovery from injury.39 While the precise mechanism of the divergent effects of CXC chemokines on liver regeneration between I/R and partial hepatectomy models is unclear, we have preliminary data that suggests that the differences are related to the amount of CXC chemokines produced during these insults. We have found that levels of CXC chemokines are increased 3 to 5-fold after 70% hepatectomy. Similar expression levels were reported by others in this model.52 In contrast, after I/R, levels of CXC chemokines increase 25 to 50-fold.39 We postulate that moderate increases in CXCR2 ligands, as occurs after partial hepatectomy, may promote liver regeneration, whereas much larger increases in expression of CXCR2 ligands, as occurs after I/R injury, may be hepatotoxic and/or oppose hepatocyte proliferation and regeneration (Figure 3). This concept was supported by in vitro studies in which hepatocytes were treated with varying concentrations of MIP-2. Low concentrations of MIP-2 had hepatoprotective effects, whereas high concentrations induced significant cytotoxicity.39 When hepatocytes isolated from CXCR2-knockout mice were used for the same studies, there was no effect of any dose of chemokine, suggesting that CXCR2 may mediate both protective and cytotoxic signaling.
While these studies suggest dynamic and contrasting functions for signaling through CXCR2 in liver recovery after hepatectomy or I/R, they do not define the mechanism(s) by which CXCR2 functions in hepatocytes. Similarly, they have not investigated the potential role of CXCR1, the other receptor that binds ELR-positive CXC chemokines. The signaling pathways utilized by CXCR1 and CXCR2 have been well-studied in neutrophils. However, nothing is known regarding the signaling pathways used by these receptors in hepatocytes. Given the potential clinical impact of these receptors and their ligands, this represents an important gap in our knowledge that warrants further investigation.
SUMMARY
Hepatic ischemia/reperfusion injury continues to impact patient mortality and morbidity despite advances in supportive care and strategies aimed at minimizing tissue injury such as ischemic pre-conditioning and pharmacologic administration of N-acetylcysteine, prostaglandins or prostacyclin.54–57 Consequently, there is still much to gain from therapeutic modalities aimed at suppressing the acute inflammatory response and subsequent organ injury seen after I/R. Several targets have been identified in pre-clinical studies including TNFα, adhesion molecules, and protease inhibitors. Others have identified transcription factors that regulate hepatic I/R injury, such as NF- κB and STAT-6.58–61 CXC chemokines and their receptors, CXCR1 and CXCR2, now appear to also be important mediators that regulate both the inflammatory response and the recovery and regeneration of liver parenchyma after I/R. Our recent work suggests there is a divergent hepatic response to CXC chemokines that is directly related to the level of expression. Since pharmacological antagonists to CXCR1/CXCR2 are in clinical trials for treatment of other inflammatory diseases, the role a greater understanding of the function of these chemokines in the liver may have significant impact on potential therapeutic modulation of liver trauma, transplantation or surgical oncology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delva E, Camus Y, Nordlinger B, et al. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg. 1989;209:211. doi: 10.1097/00000658-198902000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huguet C, Addario-Chieco P, Gavelli A, et al. Technique of hepatic vascular exclusion for extensive liver resection. Am J Surg. 1992;163:602. doi: 10.1016/0002-9610(92)90567-b. [DOI] [PubMed] [Google Scholar]
- 3.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia/reperfusion injury. Am J Surg. 2001;181:160. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 4.Lentsch A, Kato A, Yoshidome H, McMasters K, Edwards M. Inflammatory Mechanisms and Therapeutic Strategies for Warm Hepatic Ischemia/Reperfusion Injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 6.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264:801–809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 7.Jaeschke H, Farhood A. Neutrophil and Kupffer Cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:355–362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion of after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 9.Lentsch A, Yoshidome H, Kato A, et al. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30:1448–1453. doi: 10.1002/hep.510300615. [DOI] [PubMed] [Google Scholar]
- 10.Colletti LM, Remick DG, Burtch GD, et al. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter R. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Colletti LM, Burtch GD, Remick DG, et al. The production of tumor necrosis factor alpha and the development of a pulmonary capillary injury following hepatic ischemia and reperfusion. Transplantation. 1990;49:268–272. doi: 10.1097/00007890-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kato A, Gabay C, Okaya T, Lentsch A. Specific role of Interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Path. 2002;161(5):1797–1803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kan WH, Hsieh CH, Schwacha MG, Choudhry MA, Raju R, Bland KI, Chaudry IH. Flutamide protects against trauma-hemorrhage-induced liver injury via attenuation of the inflammatory response, oxidative stress, and apopotosis. J Appl Physiol. 2008;105(2):595–602. doi: 10.1152/japplphysiol.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin T, Kuboki S, Lentsch AB. Roles of nuclear factor-kappaB in postischemic liver. Hepatol Res. 2008;38(5):429–440. doi: 10.1111/j.1872-034X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaeschke H, Farhood A, Smith C. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 17.Colletti LM, Kunkel SL, Walz A, et al. The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology. 1996;23:506–514. doi: 10.1002/hep.510230315. [DOI] [PubMed] [Google Scholar]
- 18.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice; roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 19.Singh I, Zibari GB, Brown MF, et al. Role of P-selectin expression in hepatic ischemia reperfusion injury. Clin Transplant. 1999;13:76–82. doi: 10.1034/j.1399-0012.1999.130103.x. [DOI] [PubMed] [Google Scholar]
- 20.Burke J, Zibari GB, Brown MF, et al. Hepatic ischemia-reperfusion injury causes E-selectin upregulation. Transplant Proc. 1998;30:2321–2323. doi: 10.1016/s0041-1345(98)00639-3. [DOI] [PubMed] [Google Scholar]
- 21.Sawaya DE, Jr, Zibari GB, Minardi A, et al. P-selectin contributes to the initial recruitment of rolling and adherent leukocytes in hepatic venules after ischemia/reperfusion. Shock. 1999;12:227–232. doi: 10.1097/00024382-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Yada SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494–1502. doi: 10.1002/hep.510290505. [DOI] [PubMed] [Google Scholar]
- 23.Yaday SS, Howell DN, Gao W, Steeber DA, Harland RC, Clavien PA. L-selectin and ICAM-1 mediate reperfusion injury and neutrophil adhesion in the warm ischemic mouse liver. Am J Physiol. 1998;275(6):G1341–1352. doi: 10.1152/ajpgi.1998.275.6.G1341. [DOI] [PubMed] [Google Scholar]
- 24.Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intracellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. L Leukoc Biol. 1995;57:368–374. [PubMed] [Google Scholar]
- 25.Kato A, Okaya T, Lentsch AB. Endogenous IL-13 protects hepatocytes and vascular endothelial cells during ischemia/reperfusion injury. Hepatology. 2003;37(2):304–312. doi: 10.1053/jhep.2003.50075. [DOI] [PubMed] [Google Scholar]
- 26.Huber AR, Kunkel SL, Todd RF, 3rd, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Suematsu M, Miura S, et al. Rat CINC/gro: a novel mediator for locomotive and secretagogue activation of neutrophils in vivo. J Leukoc Biol. 1994;55:652–657. doi: 10.1002/jlb.55.5.652. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interactions. 1991;79:115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 29.Wu TW, Hashimoto, Au JX, et al. Trilox protects rat hepatocytes against oxyradical damage and the ischemic rat liver from reperfusion injury. Hepatology. 1991;13:575–580. [PubMed] [Google Scholar]
- 30.Mavier P, Preaux AM, Guigui B, Lescs MC, Zafrani ES, Dhumeaux D. In vitro toxicity of polymorphonuclear neutrophils to rat hepatocytes: evidence for a protease mediated mechanism. Hepatology. 1988;8:254–258. doi: 10.1002/hep.1840080211. [DOI] [PubMed] [Google Scholar]
- 31.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47(1):186–198. doi: 10.1002/hep.21922. [DOI] [PubMed] [Google Scholar]
- 32.Camargo CA, Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocye proliferation in the rodent. Hepatology. 1997;26:1513–1520. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 33.Yoshidome H, Kato A, Miyazaki M, Edwards MJ, Lentsch AB. IL-13 activates STAT6 and inhibits liver injury induced by ischemia/reperfusion. Am J Pathol. 1999;155:1059–1064. doi: 10.1016/S0002-9440(10)65208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato A, Okaya T, Lentsch AB. Endogenous IL-13 protects hepatocytes and vascular endothelial cells during ischemia/reperfusion injury. Hepatology. 2003;37(2):304–312. doi: 10.1053/jhep.2003.50075. [DOI] [PubMed] [Google Scholar]
- 35.Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 36.Lentsch AB, Jordan JA, Czermak BJ, Diehl KM, Younkin EM, Sarma V, Ward PA. Inhibition of NF-Bκ activation and augmentation of IκBβ by secretory leukocyte protease inhibitor during lung inflammation. Am J Pathol. 1999;154:239–247. doi: 10.1016/s0002-9440(10)65270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lentsch AB, Yoshidome H, Warner Rl, Ward PA, Edwards MJ. Secretory leukocyte protease inhibitor in mice regulates local and remote organ inflammatory injury induced by hepatic ischemia/reperfusion. Gastroenterology. 1999;117:953–961. doi: 10.1016/s0016-5085(99)70355-0. [DOI] [PubMed] [Google Scholar]
- 38.Barone S, Okaya T, Rudich S, et al. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;289(4):C826–835. doi: 10.1152/ajpcell.00629.2004. [DOI] [PubMed] [Google Scholar]
- 39.Kuboki S, Shin T, Huber N, et al. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology. 2008;48(4):1213–1223. doi: 10.1002/hep.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 41.Luedde T, Trautwein C. Intracellular survival pathways in the liver. Liver Int. 2006;26(10):1163–1174. doi: 10.1111/j.1478-3231.2006.01366.x. [DOI] [PubMed] [Google Scholar]
- 42.Terui K, Ozaki M. The role of STAT3 in liver regeneration. Drugs Today (Barc) 2005;41(7):461–469. doi: 10.1358/dot.2005.41.7.893622. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto T, O’Malley K, Efron PA, et al. Interleukin-6 and STAT3 protect the liver from hepatic ischemia and reperfusion injury during ischemic preconditioning. Surgery. 2006;140(5):793–802. doi: 10.1016/j.surg.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Kuboki S, Okaya T, Schuster R, et al. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G201–207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 45.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 46.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6(12):907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 47.Murphy PM. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol Rev. 2002;54(2):227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- 48.Cao X, Zhang W, Wan T, et al. Molecular cloning and characterization of a novel CXC chemokine macrophage inflammatory protein-2 gamma chemoattractant for human neutrophils and dendritic cells. J Immunol. 2000;165(5):2588–95. doi: 10.4049/jimmunol.165.5.2588. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–24007. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 50.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270(45):27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 52.Colletti LM, Green ME, Burdick MD, Kunkel SL, Strieter RM. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock. 1998;10 (4):248–257. doi: 10.1097/00024382-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Ren X, Carpenter A, Hogaboam C, Colletti LM. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Path. 2003;163:563–570. doi: 10.1016/S0002-9440(10)63684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 55.Petrowsky H, McCormack L, Trujillo M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244(6):921–8. doi: 10.1097/01.sla.0000246834.07130.5d. discussion 928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77(8):577–592. doi: 10.1007/s001099900029. [DOI] [PubMed] [Google Scholar]
- 57.Clavien PA, Emond J, Vauthey JN, Belghiti J, Chari RS, Strasberg SM. Protection of the liver during hepatic surgery. J Gastrointest Surg. 2004;8(3):313–327. doi: 10.1016/j.gassur.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Kuboki S, Okaya T, Schuster R, Blanchard J, Denenberg A, Wong HR, Lentsch AB. Hepatocyte NF-κB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol. 2006;292:G201–207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 59.Yoshidome H, Kato A, Miyazaki M, Edwards MJ, Lentsch AB. IL-13 activates STAT-6 and inhibits liver injury induced by ischemia/reperfusion. Am J Pathol. 1999;155:1059–1064. doi: 10.1016/S0002-9440(10)65208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kato A, Yoshidome H, Edwards MJ, Lentsch AB. Reduced hepatic ischemia/reperfusion injury by IL-4: potential anti-inflammatory role of STAT-6. Inflamm Res. 2000;49(6):275–279. doi: 10.1007/PL00000207. [DOI] [PubMed] [Google Scholar]
- 61.Kato A, Yoshidome H, Edwards MJ, Lentsch AB. Regulation of inflammatory liver injury by STAT-6. Am J Pathol. 2000;157(1):297–302. doi: 10.1016/S0002-9440(10)64540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]