Abstract
Ischemic postconditioning initially referred to a stuttering reperfusion performed immediately after reperfusion, for preventing ischemia/reperfusion injury in both myocardial and cerebral infarction. It has evolved into a concept that can be induced by a broad range of stimuli or triggers, and may even be performed as late as 6 h after focal ischemia and 2 days after transient global ischemia. The concept is thought to be derived from ischemic preconditioning or partial/gradual reperfusion, but in fact the first experiment for postconditioning was carried out much earlier than that of preconditioning or partial/gradual reperfusion, in the research on myocardial ischemia. This review first examines the protective effects and parameters of postconditioning in various cerebral ischemic models. Thereafter, it provides insights into the protective mechanisms of postconditioning associated with reperfusion injury and the Akt, mitogen-activated protein kinase (MAPK), protein kinase C (PKC), and ATP-sensitive K+ (KATP) channel cell signaling pathways. Finally, some open issues and future challenges regarding clinical translation of postconditioning are discussed.
Keywords: cerebral ischemia, focal ischemia, neuroprotection, preconditioning, postconditioning, stroke
Introduction
Although extensive studies have shown that many neuroprotectants reduce infarction and improve neurologic functions in animal models of stroke, translational studies ranging from basic research to clinical applications suggest that few neuroprotectants can offer protective effects (Hoyte et al, 2004). This necessitates the exploration of novel therapeutic approaches, such as ischemic postconditioning, a strategy that has emerged recently.
Ischemic postconditioning was initially defined in the field of myocardial ischemia research as a series of brief mechanical occlusions and reperfusions (Na et al, 1996; Zhao et al, 2003b), which has also recently proved to be effective against cerebral ischemia (Pignataro et al, 2008; Zhao et al, 2006). Its protective effect has been shown to be comparable with that of ischemic preconditioning, which refers to a brief, sublethal ischemia that prevents ischemic injury caused by a subsequent prolonged, lethal ischemia (Murry et al, 1986; Perez-Pinzon, 2004).
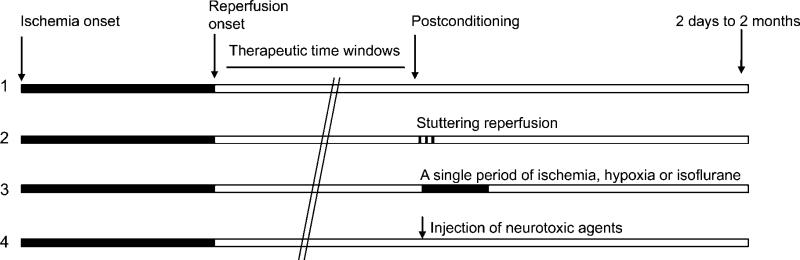
The initial concept of ischemic postconditioning, like that of ischemic preconditioning, has evolved into a concept that includes a broad range of stimuli or triggers (Figure 1). As have reviewed earlier, any sublethal stimulus can serve as a trigger for preconditioning. These stimuli include focal or global cerebral ischemia, inflammation, epilepsy, hyperbaric oxygenation, metabolic inhibition, oxidative stress, cortical spreading depression, hypothermia, and hyperthermia (Dirnagl et al, 2003). In addition, it is widely accepted that some anesthetics can serve as triggers for preconditioning (Kitano et al, 2007; Wei et al, 2007). Ischemic postconditioning is defined to contrast with ischemic preconditioning, implying that a stimulus for ischemic preconditioning is moved temporally from before ischemia to after reperfusion (Vinten-Johansen et al, 2005). Therefore, consistent with the evolved concept of preconditioning, postconditioning, in this review, also refers to a broad range of sublethal stimuli or insults performed immediately or up to 2 days after cerebral ischemia (Burda et al, 2006b), including a series of interruptions of reperfusion (Zhao et al, 2006), a single, brief ischemia or hypoxia (Pignataro et al, 2008), and the application of a pharmacological, neurotoxic agent (Burda et al, 2006b). In this article, postconditioning performed immediately or within 30 min after reperfusion is defined arbitrarily as rapid postconditioning, whereas that performed hours or days after reperfusion is defined as delayed postconditioning (Ren et al, 2008). Last but not the least, isoflurane postconditioning is included in this review not only because it is a widely accepted concept, but also because its implementation was triggered by the concept of ischemic postconditioning (Lee et al, 2008). However, unlike preconditioning, the postconditioning category does not include some well-known traditional strategies for neuroprotection, such as postischemic hypothermia (Busto et al, 1989) and hyperbaric oxygenation (Singhal et al, 2002), because these postischemic treatments have formed their unique research fields, and may have distinct protective mechanisms from ischemic postconditioning.
Figure 1.
Typical protocols for postconditioning. (1) Control ischemia. (2) Postconditioning with a series of interruption of reperfusion (stuttering reperfusion), which consists of 3 or more cycles of brief reperfusion and occlusion. (3) Postconditioning with a single period of ischemia, hypoxia or anesthesia. (4) Postconditioning was induced by injection of neurotoxic agents. Solid bar, ischemia; open bar, reperfusion. The therapeutic time windows for postconditioning vary from a few seconds to 2 days after reperfusion.
In this article, I will briefly examine the history of ischemic postconditioning studies, and then review the postconditioning models used in stroke research and the potential protective mechanisms of postconditioning. Finally, some potential issues, open questions, and hypotheses related to ischemic postconditioning will be discussed.
The historical view on ischemic postconditioning: from the heart to the brain
Ischemic postconditioning was believed to be a brand-new concept when it appeared within a 5- to 6-year span in research on myocardial ischemia (Tsang et al, 2005; Zhao, 2007). It was also believed to be derived from the concept of ischemic preconditioning (Vinten-Johansen et al, 2005), or from partial or controlled reperfusion (Tsang et al, 2005). However, the first report of ischemic postconditioning was published more than five decades ago (Sewell et al, 1955), which is much earlier than the findings of preconditioning in 1964 (reviewed by Dirnagl et al, 2003), and of partial or controlled reperfusion in the 1960s (reviewed by Vinten-Johansen et al, 2005). Nevertheless, the formal concept of postconditioning was conceived of only 13 years ago (Na et al, 1996), which is indeed much later than that of preconditioning.
In the 1950s, it was well-established that a sudden restoration of arterial flow after heart ischemia results in ventricular fibrillation (Sewell et al, 1955), a medical emergency in which uncoordinated contraction (arrhythmia) of the cardiac muscle of the ventricles occurs, leading to the cessation of circulation and death. This hazard is related to the current concept of reperfusion injury. Inspired by the idea of attenuating reperfusion injury caused by the abrupt release of the occluded coronary artery, Sewell et al (1955) studied the protective effect of intermittent reperfusion in dogs with temporary coronary arterial occlusion, during which ventricular fibrillation occurred within a few to 20 secs after sudden reperfusion. They found that intermittent reperfusion, which equates to the current concept of ischemic postconditioning, abolishes fibrillation (Sewell et al, 1955). Interestingly, such protection was once observed in a clinical case reported in 1994, in which the patient was subjected to acute myocardial ischemia (Grech and Ramsdale, 1994). This protective phenomenon was repeated by Na et al (1996), who first coined the term ‘postconditioning’, and found that postconditioning was as effective as preconditioning in preventing ventricular fibrillations in cats.
In the above-mentioned three studies, the outcome of myocardial injury was evaluated by the occurrence of fibrillation, which differs from current studies of ischemic postconditioning, in which infarct size is measured (Zhao et al, 2003b). Although ventricular fibrillation does not equate to infarct size, ventricular fibrillation arrhythmias are well-known complications of acute myocardial infarction (Piccini et al, 2008); they occur before myocardial infarction, or develop during the early phase of myocardial infarction (Wong et al, 2003). Thus, the above three reports (Grech and Ramsdale, 1994; Na et al, 1996; Sewell et al, 1955) are considered pioneering studies of ischemic postconditioning.
However, postconditioning studies did not thrive until Zhao et al (2003b) published their first study on ischemic postconditioning in a myocardial ischemic model. This report served as a springboard for subsequent studies. In it, the authors found for the first time that postconditioning reduced the infarct size by ∼44%, which was comparable with the protective effects of preconditioning (Zhao et al, 2003b). The protective effects of postconditioning in myocardial ischemia have since been confirmed by numerous studies (Zhao and Vinten-Johansen, 2006), including models in rats (Obal et al, 2005), mice (Kin et al, 2005), rabbits (Krolikowski et al, 2006), and pigs (Iliodromitis et al, 2006), as well as in in vitro settings (Dosenko et al, 2006), leading to human clinical trials (Staat et al, 2005).
A subsequent question is whether ischemic postconditioning can also apply to ischemic brains. The rationale of extrapolating postconditioning from treating heart ischemia to brain ischemia largely depends on whether the principle for stroke treatment is similar to that for heart ischemia therapy. Earlier studies have revealed that the mechanisms of ischemic brain injury have many aspects similar to those of myocardial ischemic injury, including ischemia/reperfusion injury (Kinouchi et al, 1991), calpain-mediated necrotic pathways (Yamashima, 2000), cytochrome c/caspase-mediated apoptotic pathways (Chen et al, 1998; Zhao et al, 2003a), MAPK pathways (Noshita et al, 2002), the protein kinase C (PKC) pathway (Shimohata et al, 2007a; Shimohata et al, 2007b), and the Akt survival pathways (Zhao et al, 2005). In addition, preconditioning robustly reduces ischemic damage in both the brain and the heart (Murry et al, 1986; Perez-Pinzon, 2004). On the basis of these similarities, it is a logical and intriguing idea to test whether postconditioning also protects against cerebral ischemia. As a result, a few laboratories, including ours, have proved that ischemic postconditioning protects against brain injury after stroke.
Models and protective parameters for postconditioning against stroke
The protection afforded by any given neuroprotectant against stroke is characterized by its dosage (for pharmacological agents), therapeutic time windows, ischemic models, and its duration of sustainability, among other factors. Postconditioning is no exception. The parameters defining the protective effect of postconditioning against various models are reviewed below (summarized in Table 1).
Table 1.
Summary of postconditioning studies in cerebral ischemia
| Reference | S | Model |
Postconditioning |
Infarct reduction | Mechanisms and behavioral test | ||||
|---|---|---|---|---|---|---|---|---|---|
| Onset | Ocl | Rep | Cycle | ||||||
| (Zhao et al, 2006) | R | pMCAo, CCAo, 15 min | 30 secs | 10 secs | 30 secs | 3 | 80% | Inhibits generation of free radical and apoptosis (TUNEL staining) | |
| 30 min | 30 secs | 10 secs | 30 secs | 3 | 51% | ||||
| 60 min | 30 secs | 10 secs | 30 secs | 3 | 17% | ||||
| (Pignataro et al, 2008) | R | Focal, 100 min | 2 min | 2 min | 2 min | 5 | 23% (not significant) | Akt, but not ERK and p38 activity, contributes to the protection of postconditioning. All the above pathways contribute to the protection of preconditioning. Combination of postconditioning with preconditioning offers no synergistic effect | |
| 5 min | 5 min | 5 min | 3 | 38% | |||||
| 10 min | 10 min | N/A | 1 | 65% | |||||
| 30 min | 10 min | N/A | 1 | NP | |||||
| 120 min OGD, cell culture | 60 min | 30 min | N/A | 1 | NP | ||||
| 30 min | 10 min | N/A | 1 | NP | |||||
| 10 min | 30 min | N/A | 1 | NP | |||||
| 10 min | 10 min | N/A | 1 | ∼24% | |||||
| (Xing et al, 2008b) | R | MCAo, 60 min | 30 secs | 30 secs | 30 secs | 6 | ∼14% at 1 day | Inhibits cytochrome c release, caspase-3 activity and DNA fragmentation; enhances SOD and Bcl-2 expression; improves neurological scores | |
| ∼12% at 3 days | |||||||||
| (Gao et al, 2008a) | R | pMCAo, 30 min CCAo | 30 secs | 30 secs | 10 secs | 3 | 33% at 2 days | Improved CBF at 30 min after CCA release. Combination of preconditioning with postconditioning offered no additional protection | |
| 30 secs | 30 secs | 10 secs | 10 | NP | |||||
| 10 secs | 10 secs | 10 secs | 3 | NP | |||||
| 10 secs | 10 secs | 10 secs | 10 | 56% | |||||
| 3 min | 10 secs | 10 secs | 10 | NP | |||||
| (Gao et al, 2008b) | R | pMCAo, 30 min CCAo | 30 secs | 30 secs | 10 secs | 3 | 39% at 1 month after stroke | Akt pathway contributes to the protection of postconditioning; both MAPK and PKC pathways are involved in the protective mechanisms | |
| (Wang et al, 2008) | R | Global 4 VO, 10 min | 15 secs | 15 secs | 15 secs | 3 | Hipp | ∼70%, 7days | Improves CBF; attenuates cytochrome c release; improves spatial learning and memory |
| ∼77%, 14 days | |||||||||
| Cortex | ∼98%, 7 days | ||||||||
| ∼98%, 14 days | |||||||||
| 30 secs | 30 secs | 30 secs | 3 | Hipp | ∼51%, 7 days | ||||
| ∼67%, 14 days | |||||||||
| Cortex | ∼76%, 7 days | ||||||||
| ∼83%, 14 days | |||||||||
| 60 secs | 15 secs | 60 secs | 3 | Hipp | NP, 7 days | ||||
| NP, 14 days | |||||||||
| Cortex | NP, 7 days | ||||||||
| NP, 14 days | |||||||||
| 45 secs | 15 secs | 15 secs | 3 | Hipp | ∼94%, 7 days | ||||
| ∼94%, 14 days | |||||||||
| Cortex | ∼96%, 7 days | ||||||||
| ∼93%, 14 days | |||||||||
| (Rehni and Singh, 2007) | M | Global, 2 VO, 10 min | 10 secs | 10 secs | 10 secs | 3 | No morphological study | Akt inhibition contributes to postconditioning. Postconditioning improves behavioral tests | |
| (Burda et al, 2006a) | R | 4 VO 8 min | 2 days | 5 min | N/A | 1 | ∼94%, 7days | Cycloheximide, inhibitor of protein synthesis, blocks postconditioning's protection | |
| NE | N/A | 1 | ∼100%, 7days | ||||||
| 10 min | 2 days | 6 min | N/A | 1 | ∼100%, 7days | ||||
| 15 min | 3-NP | N/A | 1 | ∼75%, 7days | |||||
| (Danielisova et al, 2006) | R | 10 min | 2 days | 5 min | N/A | 1 | ∼90%, 7 days | Increased SOD activity | |
| (Danielisova et al, 2008) | R | 8 min | 2 days | Bradykinin | N/A | 1 | ∼91%, 3days, by Fluoro Jade B staining | Postconditioning inhibits changes in MnSOD and CuZnSOD activity | |
| (Scartabelli et al, 2008) | R | 30 min OGD, slice culture | 5 min | 3 min, OGD | N/A | 1 | ∼33% | mGlu1 and mGlu5 antagonists and PI3K–Akt inhibitor abolish protection of postconditioning Combination of preconditionong and postconditioning has no additive protection | |
| 3 min, DHPG | N/A | 1 | ∼56% | ||||||
| 15 min | N/A | 1 | ∼44% | ||||||
| 30 min | N/A | 1 | NP | ||||||
| (Lee et al, 2008) | R | 15 min OGD, Slice culture | Immediately or 10 min later | 1%, 1.5%, 2%, 2.5%, 3% isoflu, for 10, 30, 60 or 80 min | N/A | 1 | 2% isoflu for 30 min applied immediately after OGD reduced ∼36%; 2% isoflu for 30 min applied 10 min also protected | KATP channel blockers inhibit the protection of postconditioning | |
| 90 min MCAo | Immediately | 60 min 2% isoflu | N/A | 1 | ∼48% | ||||
| (Ren et al, 2008) | R | pMCAo, 30 min CCAo* | 10 secs | 10 secs | 10 secs | 10 | ∼50% | Delayed postconditioning improves metabolism, attenuates BBB leakage and edema formation, and inhibits t-PA exacerbated-brain injury. It mitigates behavioral deficits up to 2 months after stroke | |
| 3 h | 10 secs | 10 secs | 10 | NP | |||||
| 30 secs | 30 secs | 30 secs | 3 | ∼39% | |||||
| 3 h | 30 secs | 30 secs | 3 | ∼44% | |||||
| # | 3 h | 15 min | 15 min | 6 | ∼46% | ||||
| 6 h | 15 min | 15 min | 6 | ∼64% | |||||
The typical protocol for performing postconditioning is shown in Figure 1. The onset time, occlusion (Ocl), and reperfusion (Rep) time are documented. The percentage of infarct size reduced by postconditioning is also summarized; when the exact number of infarct size was not reported in the cited literatures, infarct reduction was approximately estimated from the bar graphs. The potential protective mechanisms and the outcomes of behavioral tests are also summarized. S, species; R. rat; M, mouse; PMCAo, CCAo, permanent middle cerebral artery occlusion combined with bilateral common carotid artery occlusion; Focal, focal ischemia; Global, global ischemia; OGD, oxygen glucose deprivation; 2 or 4 VO, 2 or 4 vessel occlusion; Ocl, occlusion; Rep, reperfusion; N/A, non applicable; NP, no protection.
Postconditioning was induced by bilateral CCA occlusion and release.
postconditioning was performed by the ipsilateral CCA occlusion and release.
Postconditioning Protects Against Focal Ischemia
In our first study, we have reported that rapid postconditioning reduces the infarct size as a function of ischemic severity—meaning, it is less effective with longer periods of ischemia (Zhao, 2007; Zhao et al, 2006). We found that rapid postconditioning reduced the infarct size by ∼80%, ∼51%, and ∼17%, respectively, in 15, 30, or 60-min common carotid artery (CCA) occlusion combined with the permanent distal middle cerebral artery (dMCA) occlusion. In another study, we then compared the impact of cycle numbers and duration of reperfusion/occlusion on the protective effect of rapid postconditioning using the ischemic model of 30-min bilateral CCA (bCCA) occlusion combined with permanent dMCA occlusion (Gao et al, 2008a). Our results showed that rapid postconditioning conducted 10 to 30 secs after reperfusion reduced the infarct size, whereas it did not reduce infarction when it was initiated at 3 min after reperfusion. Taken together, this study suggests that the protection afforded by rapid postconditioning depends on the number of cycles and duration of each cycle of reperfusion and occlusion, and on the onset time of postconditioning (Gao et al, 2008a).
Nevertheless, we did not exclude the possibility of delayed postconditioning with other settings performed at later time points protecting against brain injury based on two hypotheses. First, postconditioning may protect against cerebral ischemia at multiple time windows (as does preconditioning). For example, rapid preconditioning performed 1 to 3 h and delayed preconditioning at 1 to 7 days before ischemia offers protection, whereas preconditioning conducted at 12 h before ischemia does not reduce brain injury (Kitagawa et al, 1990; Kuzuya et al, 1993; Perez-Pinzon, 2004). Second, postconditioning with different paradigms has varying therapeutic time windows. We recently tested the hypothesis that delayed postconditioning with different parameters reduces the infarct size, and indeed found that delayed postconditioning with some parameters performed at 3 and 6 h after stroke robustly reduced the infarct size (Table 1), with the strongest protection achieved by delayed postconditioning with 6 cycles of 15-min occlusion or 15-min release of the ipsilateral CCA executed from 6 h (Ren et al, 2008).
It is essential to test whether postconditioning provides lasting protection and preserves brain function, because some neuroprotectants, such as postischemic hypothermia (Dietrich et al, 1993) and rapid ischemic preconditioning (Perez-Pinzon, 2004), have been shown to provide protection for only a few days after ischemia. In addition, reducing injured brain tissue may not translate into the preservation of neurologic function (Dumas and Sapolsky, 2001). In a long-term study, we found that rapid postconditioning performed immediately after reperfusion reduced the lesion size to ∼40% in rats subjected to ischemia when measured 30 days after ischemia, and that rapid postconditioning improves neurologic function, as measured by the vibrissae test detecting an asymmetric forelimb usage (Gao et al, 2008b). In addition, we also found that delayed postconditioning conducted from 6 h after stroke attenuated brain injury and improved the outcomes of behavioral tests up to 2 months using four standard methods, including the vibrissae test, postural reflex test, tail hang test, and home cage test (Ren et al, 2008).
Unlike the case of myocardial ischemia, in which rapid postconditioning has been ubiquitously found to offer remarkable protection (Vinten-Johansen et al, 2005), in our experiments, robust protection by rapid postconditioning is observed only in moderate or mild brain ischemia in which the bilateral CCA occlusion time was 15 or 30 min (Zhao et al, 2006), whereas the protection is mild in more severe ischemia (60 min of CCA occlusion). Xing et al (2008a) have also found that rapid postconditioning reduced infarction by only 16% and 12% 1 and 3 days after stroke, respectively, in a middle cerebral artery (MCA) suture occlusion model. Therefore, in our studies, rapid postconditioning does not seem to generate the same level of protection in the ischemic brain as has been shown in the ischemic heart (Zhao et al, 2003b). However, we have not tested whether the optimized conditions, which were defined in our recent study (Gao et al, 2008a), afford better protection in a longer period of ischemia, and Xing et al did not compare the protective effect with different postconditioning parameters. Therefore, we cannot exclude the possibility that the relatively weak protection is because of the usage of suboptimal parameters of rapid postconditioning.
In contrast with our finding, Pignataro et al (2008) have shown a very strong protection with postconditioning in a severe focal ischemic model, in which the MCA was occluded for 100 min. Their results showed that postconditioning with 3 cycles of 5-min reperfusion or 5-min occlusion reduced infarction by 38%, and that one cycle of 10-min occlusion initiated after 10 min of reperfusion reduced the infarct size by ∼70%, compared with rats subjected to control ischemia. However, postconditioning with 10 min of occlusion started at 30 min of reperfusion offered no protection. Again, this study suggests that the onset time of postconditioning is critical for its neuroprotective effect.
The protective effect of postconditioning can be achieved not only by the mechanical interruption of reperfusion but also by the application of isoflurane (Lee et al, 2008). In a suture MCA occlusion model, postconditioning was conducted by maintaining 2% isoflurane for 60 min, starting at the time the MCA occluding suture was removed; no isoflurane was used during MCA occlusion. For rats receiving control ischemia, only 1 min of isoflurane was used for MCA suture removal (Lee et al, 2008). The results showed that isoflurane postconditioning robustly reduced brain infarction and attenuated neurologic deficits.
Postconditioning Reduces Brain Injury after Global Ischemia
A few groups have studied the protective effects of rapid postconditioning on transient global cerebral ischemia. Wang et al (2008) showed that, as assayed 7 days after reperfusion, rapid postconditioning applied immediately after reperfusion attenuated neuronal death in both the hippocampus and the parietal cortex after a 10 min transient global ischemia. Consistent with its protective effects on neuronal survival, rapid postconditioning improves subject performance on spatial learning and memory in a water-maze test 3 weeks after reperfusion (Wang et al, 2008), suggesting that rapid postconditioning provides long-term protection in global ischemia. Rehni and Singh (2007) have also shown that rapid postconditioning attenuates behavioral deficits after global ischemia in mice. However, they did not report how rapid postconditioning affects neuronal loss.
In all the studies discussed above, rapid postconditioning was conducted during early reperfusion after focal or global ischemia. However, delayed postconditioning can even be conducted at 2 days after reperfusion in rat global ischemia models (Burda et al, 2006b). In these studies, global ischemia was induced by four-vessel occlusion, and postconditioning was conducted using four techniques, namely, short ischemia, injection of 3-nitropropionic acid, norepinephrine, or bradykinin (Burda et al, 2006a; Danielisova et al, 2006). Delayed postconditioning with 5- to 6-min ischemia, or with intraperitoneal injection of norepinephrine or 3-nitropropionic acid at 2 days, resulted in neuronal survival of 80% to 100% after global ischemia (Burda et al, 2006b). Although the authors also defined treatment with norepinephrine and bradykinin as a means of postconditioning that reduces cornu ammonis (CA) 1 neuronal injury (Danielisova et al, 2008), this definition may be a matter of debate, because these two agents are more similar to regular pharmacological treatments.
Postconditioning Protects Against In Vitro Ischemia
Pignataro et al (2008) also found that postconditioning with oxygen glucose deprivation (OGD) reduced neuronal death in cortical culture. Postconditioning with 30 min of OGD conducted at 10, 30, or 60 min after reperfusion did not reduce cell death caused by a 120-min OGD; however, with a 10-min OGD initiated at 10 min of reperfusion, postconditioning robustly blocked cell death (Pignataro et al, 2008). This study suggests that the onset time and duration of postconditioning are critical for generating neuroprotection.
Rapid postconditioning with brief OGD also blocks ischemic injury in rat organotypic hippocampal slice culture (Scartabelli et al, 2008). Results showed that postconditioning with 3 min of OGD started at 5 min after reperfusion reduced cell injury by ∼40%. In the same study, the protection of postconditioning was also induced by adding a low dose of a pharmacological agent, 3,5-dihydroxyphenylglycine (a group 1 mGlu receptor agonist), 5 min after reperfusion and incubating for 30 min. It has been reported that high dosages of 3,5-dihydroxyphenylglycine exacerbate, whereas low dosages inhibit neuronal injury (Scartabelli et al, 2008).
Lee et al (2008) also found that rapid isoflurane postconditioning protects against ischemic injury in slice organ culture, in which OGD was maintained for 15 min, and postconditioning was instituted by application of isoflurane after OGD. They found that the protective effect of isoflurane postconditioning is dependent on the duration and concentration of isoflurane exposure. Finally, isoflurane postconditioning started at 0 or 10 min, but not > 30 min after reperfusion, reduced cell damage, suggesting a similar therapeutic time window with ischemic postconditioning (Lee et al, 2008).
Protective mechanisms of postconditioning against stroke
Protective mechanisms of postconditioning are largely unknown. Unlike preconditioning, which enables the brain to adapt to a subsequent severe ischemia, postconditioning cannot be studied separately from the stroke event. To study the protective mechanisms of postconditioning, usually the brain tissues from animals receiving stroke and postconditioning are harvested and studied, and the results are compared with tissues from control animals. Any findings about changes in pathophysiologic, cellular, and molecular levels in the brain after postconditioning are the outcomes of intermingled effects of postconditioning with stroke, rather than the sole effect of postconditioning. Thus, it is a challenge to dissect the protective mechanisms of postconditioning from the consequences of its protection.
Nevertheless, as ischemic postconditioning first interrupts reperfusion, the very first effect it generates must be closely associated with changes in cerebral blood flow (CBF), and with associated subsequent events, such as free radical production, endothelial function, changes in blood–brain barrier integrity, and inflammation, which occur because of interrupted CBF. The seemingly beneficial changes resulting from ischemic postconditioning may lead to an improved interaction in the neurovascular units between endothelial cells, pericytes, and astrocytes (Lo et al, 2004), which would have been disrupted without ischemic postconditioning. Although no reports have studied the impact of ischemic postconditioning on the neurovascular units, I assume that such an effect is the first protective mechanism of postconditioning. It then translates into protective effects on other downstream cell signaling events, including inflammation, cytochrome c/caspase-mediated apoptotic pathways, Akt, MAPK, PKC, and KATP pathways (Figure 2). Therefore, in this article, the protective mechanisms of postconditioning are reviewed roughly in this order.
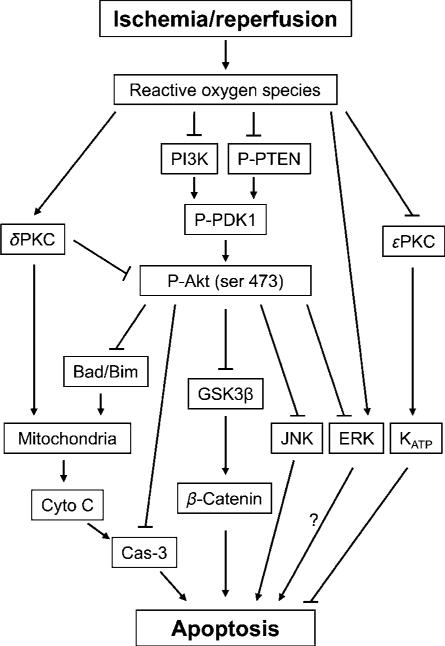
Figure 2.
The diagram shows the cell signaling pathways, which were involved in postconditioning. Reperfusion after stroke leads to ROS eruption, which causes dysfunction of the Akt cell signaling pathway, increases δ PKC activity, although decreases ε PKC activity; in addition, ROS also activates JNK and ERK activity. Furthermore, the Akt pathway is associated with the JNK and ERK pathways. The PI3K–Akt inhibition directly results in dephosphorylation of GSK3β and β-catenin degradation, and indirectly causes cytochrome c release and caspase-s activity. ε PKC may activate KATP channel resulting in neuroprotection. ROS, reactive oxygen species; Cyto C, cytochrome c; Cas-3, caspase-3; GSK 3 β, glycogen synthase kinase 3β; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; P-Akt, phosphorylated Akt; P-PTEN, phosphorylated phosphatase and tensin homolog deleted on chromosome 10; P-PDK1, phosphorylated phosphoinositide-dependent protein kinase-1; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinases; KATP channels, ATP-sensitive potassium channels.
Postconditioning Primarily Targets Early Reperfusion, Reduces Free Radical Generation and Inflammation, Attenuates Edema Formation and Blood–Brain Barrier Leakage, and Blocks Apoptosis
A primary therapeutic strategy for ischemic stroke is to recanalize the occluded blood vessels to allow early reperfusion, which is made possible by administering the thrombolytic agent, tissue plasminogen activator (t-PA) (Fisher and Brott, 2003), or by inserting mechanical devices to remove the occluding clot (Smith et al, 2005). However, reperfusion is a double-edged sword, in that it also generates an overproduction of reactive oxygen species (ROS) or free radicals, leading to reperfusion injury in the ischemic brain (Chan, 1996). Reperfusion injury has been confirmed repeatedly in many studies (Yang and Betz, 1994; Aronowski et al, 1997).
As discussed before (Zhao, 2007), partial reperfusion leads to a reduction in the infarct size. Moreover, our most recent study further showed that controlled reperfusion, in which the CBF was allowed to recover gradually, also attenuates brain injury after stroke (Gao et al, 2008a). Similarly, an in vitro study has shown that gradual (as opposed to abrupt) reoxygenation after in vitro ischemia (OGD) produces less neuronal deaths in cell culture (Burda et al, 1995). In contrast to the protective effect of partial reperfusion, hyperemic response has been suspected to be detrimental to the ischemic brain. Hyperemic response is often followed by hypotension or by a no-reflow phenomenon (Frerichs et al, 1992), which further damages the ischemic brain.
As rapid postconditioning is instituted primarily to disrupt reperfusion, it is important to confirm whether it attenuates the hyperemic response, and whether it mitigates hypotension thereafter. Therefore, we initially measured the CBF in the penumbra in rats subjected to 15 or 30 min of bilateral CCA occlusion combined with permanent MCA occlusion (Gao et al, 2008a; Zhao et al, 2006). A clear hyperemic response was detected after reperfusion in rats subjected to 15 min of occlusion, and the CBF was recovered to preischemic levels in rats with 30 min of occlusion(Gao et al, 2008a; Zhao et al, 2006). Thus, we showed that rapid postconditioning interrupts reperfusion in these two models. Most importantly, rapid postconditioning improves CBF at 30 min after reperfusion (Gao et al, 2008a), which was confirmed by another study using a global ischemia model (Wang et al, 2008). Taken together, rapid postconditioning reduces cerebral ischemic injury by disrupting the early reperfusion and by improving reperfusion thereafter. Sudden, abrupt reperfusion results in overproduction of ROS, which leads to apoptosis (Chan, 1996). In addition, the inflammatory response also exacerbates ischemic injury (Bowen et al, 2006). Therefore, it is reasonable to examine whether rapid postconditioning attenuates ROS production and apoptosis, and whether it inhibits the inflammatory response. Indeed, we found that rapid postconditioning profoundly attenuated the amount of superoxide at 30 min after reperfusion in the model of 30-min CCA occlusion plus permanent MCA occlusion (Zhao et al, 2006). Consistent with our findings, rapid postconditioning has been shown to attenuate lipid peroxidate levels in a focal ischemia model (Xing et al, 2008b). Moreover, other reports showed that rapid postconditioning performed 2 days after global ischemia increased the activities of antioxidant enzymes, including that of superoxide dismutase and catalase (Danielisova et al, 2006).
Furthermore, we have shown that rapid postconditioning blocked TUNEL (terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling) positive staining, a marker of apoptosis, in the penumbra 2 days after stroke (Zhao et al, 2006). In more recent findings, Wang et al (2008) further showed that rapid postconditioning reduced cytochrome c release from the mitochondria to the cytosol, a critical cascade for apoptosis induction. Taken together, these data suggest postconditioning may reduce ischemic injury by blocking apoptosis.
Rapid postconditioning may also inhibit inflammation after stroke. During the inflammatory response, leukocytes extravasate into the brain tissue, releasing ROS, thus attacking lipid membranes, DNA, and proteins (Chan, 1996). Inflammation is mediated by cytokines, such as IL-1β and TNF-α, and adhesion molecules, such as ICAM-1 (Kriz, 2006). A recent study showed that rapid postconditioning inhibits myeloperoxidase activity, an indicator of leukocyte accumulation, in the cortex 24 h after stroke (Xing et al, 2008a). In addition, rapid postconditioning attenuates the expressions of IL-1β and TNF-α mRNA, and the ICAM-1 protein expression in the ischemic cortex at 24 h after ischemia (Xing et al, 2008a). These results suggest that rapid postconditioning may produce an antiinflammatory effect.
Consistent with rapid postconditioning's improvement of CBF, delayed postconditioning enhances glucose uptake or metabolism as detected by micro PET imaging (Ren et al, 2008). In addition, delayed postconditioning attenuates edema formation and the blood–brain barrier leakage. Reperfusion in ischemic stroke patients is usually achieved by the t-PA application. However, the t-PA's side effect that increases hemorrhage may worsen ischemic injury (Kaur et al, 2004). Thus, we tested whether delayed postconditioning counteracts the exacerbating effect of the t-PA, and found that delayed postconditioning mitigated the worsening effect of the t-PA on infarction (Ren et al, 2008). However, in this study, reperfusion was restored by bilateral CCA release. Further study is required to test whether postconditioning reduces infarct size in an ischemia/reperfusion model, in which reperfusion is achieved by the t-PA.
The Akt Pathway Contributes to the Protective Effect of Postconditioning
Consistent with earlier studies showing that the Akt pathway contributes to neuronal survival after stroke (Zhao et al, 2006), rapid postconditioning increases Akt phosphorylation (as measured by western blot) (Gao et al, 2008b; Pignataro et al, 2008), and Akt activity (assayed by an in vitro kinase assay) (Gao et al, 2008b). Furthermore, Akt inhibition partially blocks the protective effect of rapid postconditioning (Gao et al, 2008b; Pignataro et al, 2008). However, rapid postconditioning does not affect the PTEN (phosphatase and tensin homolog deleted on chromosome 10) or the PDK1 (phosphoinositide-dependent protein kinase-1) phosphorylation, but it does inhibit an increase in the GSK3β (glycogen synthase kinase 3β) phosphorylation. In the Akt pathway, Akt activity is increased when phosphorylation of PTEN and PDK1 is improved, and GSK3β phosphorylation supports cell survival (Zhao et al, 2006, review). Dephosphorylation of GSK3β leads to its activation and to the phosphorylation of β-catenin, thus resulting in β-catenin degradation and apoptosis (Zhao et al, 2006). We found that rapid postconditioning blocks β-catenin phosphorylation, but has no effect on the total or nonphosphorylated β-catenin protein levels (Gao et al, 2008b). We concluded that the Akt pathway plays a critical role in the protection of postconditioning. This conclusion is further supported by a recent in vitro experiment showing that Akt inhibition abolished the protective effect of OGD and 3,5-dihydroxyphenylglycine postconditioning in hippocampal slice culture, a model which has been earlier discussed in this article (Scartabelli et al, 2008).
MAPK Pathways are Involved in Postconditioning's Protection
The MAPK pathways, including extracellular signal regulated kinase 1/2 (ERK1/2), p38, and c-Jun N-terminal kinase (JNK) pathways, are also closely related to ischemic injury and neuronal survival (Sawe et al, 2008). Jun N-terminal kinase and p38 appear to be clearly detrimental after stroke, and their inhibition blocks apoptosis in many neuronal death paradigms (Sawe et al, 2008). However, ERK1/2's activity is involved in both neuroprotection, as well as injury exacerbation (Sawe et al, 2008). In general, ERK1/2 phosphorylation (P-ERK1/2) is transiently increased after cerebral ischemia/reperfusion. This increase is apparently involved in the beneficial effects of growth factors, estrogen, preconditioning, and hypothermia on the ischemic brain, but it also promotes inflammation and oxidative stress, and its inhibition reduces ischemic damage (Sawe et al, 2008). Therefore, studying the changes in ERK1/2 may facilitate our understanding of its protective effects in postconditioning.
Our study confirmed that P-ERK1/2 was increased from 1 to 24 h after stroke, and found that rapid postconditioning reduced its level in the penumbra (Gao et al, 2008b). Our results imply a detrimental role for P-ERK1/2 after ischemia, so its inhibition may contribute to the protection of rapid postconditioning. This observation differs with the findings from Pignataro et al (2008), in which rapid postconditioning enhanced P-ERK1/2. However, in their study, increases in P-ERK1/2 may be unrelated to the protective effect of rapid postconditioning, as U0126, the antagonist of ERK1/2, did not block the protection of rapid postconditioning. The discrepancy between our result and that of Pignataro et al is probably because of differing ischemic models and postconditioning parameters used in these two studies.
Therefore, the puzzle regarding the role of ERK1/2 in rapid postconditioning has not been resolved. Earlier studies, including our own, have some limitations. First, the activity of ERK1/2 was evaluated by its phosphorylation alone (Gao et al, 2008b; Sawe et al, 2008). As we saw in the case of Akt, the in vitro Akt kinase assay data suggested that Akt phosphorylation alone does not represent Akt activity (Zhao et al, 2005). We deem that the same kinase activity assay for ERK should be performed. Second, the subcellular localization of ERK1/2 after stroke has not been studied. However, the ERK1/2 sub-cellular translocation may play a critical role in its actual neuroprotective and detrimental effects (Sawe et al, 2008). Third, its spatial distribution in different cell types has not been analyzed. Fourth, in our own study, the ERK1/2 antagonists have not been tested in rapid postconditioning (Gao et al, 2008b). Therefore, further study on the role of ERK1/2 in ischemic rapid postconditioning is warranted.
Postconditioning Inhibits δPKC Cleavage and Improves εPKC Phosphorylation
Stroke-induced apoptosis is also mediated by PKC pathways. There are at least 11 isozymes of the PKC family, including δPKC and εPKC (Casabona, 1997). The PKC isozymes differ according to their intracellular location and function, and their activities are regulated by their subcellular location, cleavage form, and phosphorylation. Although the δPKC activity usually leads to cell death (Shimohata et al, 2007b), εPKC promotes neuronal survival (Shimohata et al, 2007a).
We found that rapid postconditioning had no effect on the protein levels of total δPKC, but blocked the increase in levels of the cleaved form of δPKC, indicative of δPKC activity, at 1 h after stroke in the penumbra (Gao et al, 2008b). Although rapid postconditioning had no effect on phosphorylated δPKC (thr 505) levels, which were decreased by 24 h after stroke onset, it strongly inhibited decreases in phosphorylated εPKC after stroke. Our results suggest that rapid postconditioning may reduce ischemic damage by inhibiting the worsening effect of δPKC, while promoting a beneficial effect of εPKC activity (Gao et al, 2008b).
The Role of KATP Channels
The KATP channels open when ATP is depleted after ischemia, and their opening is critical for the induction of the protective effect of ischemic preconditioning, as well as postconditioning in the heart. Its role in preconditioning against brain ischemia has also been reported. The KATP channels consist of two types that vary by location, namely, sarcolemmal and mitochondrial. The mitochondrial KATP channels have been studied the most, because their opening generates an outward current stabilizing the mitochondrial membrane, therefore, blocking cell death. In the same vein, Lee et al (2008) reported that both a general channel blocker, glibenclamide, and a mitochondrial channel blocker, 5-HD, abolished the protective effect of isoflurane postconditioning, suggesting that the KATP channels may be involved in the protective mechanisms of postconditioning.
Open questions, hypotheses, and future directions
Before postconditioning can be translated clinically, numerous obstacles between the laboratory and the clinic must be cleared, and the protective mechanisms should be studied thoroughly. For instance, stroke occurs mostly in the aged, male and female populations. However, all current studies were carried out in young adult, male rodent animals. Therefore, whether ischemic postconditioning protects against stroke in animals of different genders and ages, and in higher vertebrates, should be tested. In addition, the protective mechanisms remain unknown. Does postconditioning boost endogenous protective mechanisms as does preconditioning? Moreover, does pharmacological postconditioning share similar protective mechanisms with ischemic postconditioning?
Ischemic postconditioning may possibly be applied to many clinical settings for patients subjected to surgery and to endovascular therapy associated with blood vessel occlusion and revascularization. However, before applying postconditioning to stroke patients, at least three prerequisites must be satisfied based on current postconditioning models. First, CBF must be restored promptly after stroke. Second, therapeutic time windows must be well defined. Third, the recanalized cerebral artery must be accessible for performing ischemic postconditioning.
Prerequisite 1
Currently, reperfusion is usually restored using mechanical devices or t-PA. In the case of reperfusion achieved by mechanical devices, ischemic postconditioning may be performed directly through the reopened blood vessels. In the case of t-PA application, although we most recently showed that delayed postconditioning attenuated the worsening effect of t-PA administration in a focal ischemia model with mechanical reperfusion (Ren et al, 2008), no reports have studied the protective effect of ischemic postconditioning in a t-PA reperfusion model, which, therefore, should be pursued. However, the reperfusion pattern induced by t-PA treatment differs from that of mechanical reperfusion models, which may remain a major challenge for postconditioning, as t-PA may require a few hours to resolve a blood clot. When and how ischemic postconditioning can be applied in this case must be explored.
Prerequisite 2
Regarding therapeutic time windows, in the most current studies, postconditioning was conducted from a few seconds to a few minutes after reperfusion. In reality, such a narrow therapeutic time window will constitute a major obstacle to clinical translation. Nevertheless, as discussed earlier in this article, Burda and colleagues have shown repeatedly that postconditioning performed 2 days after reper-fusion in global ischemia still robustly reduces hippocampal injury, and we have shown that delayed postconditioning performed as late as 6 h after stroke reduced infarct size (Ren et al, 2008). These open a wide window for postconditioning application in both focal and global ischemia. However, the protective effects of delayed postconditioning need to be confirmed by other laboratories.
Prerequisite 3
For cases in which cerebral blood vessels are not available for performing ischemic postconditioning, one could use: (1) blood vessels outside the brain; (2) pharmacological mimics; and (3) remote postconditioning.
In most current (animal) studies, ischemic postconditioning was performed directly through the reopened MCA. However, the accessibility of the MCA is a major challenge in clinical settings. Our laboratory performed postconditioning through the CCAs, which may be more easily accessible on stroke patients. In fact, physicians often manually compress the carotid artery for the insertion of guiding catheters into the carotid artery (Yoshimura et al, 2006), or to occlude the carotid artery using an endoluminal balloon for controlling a sudden blow out of the carotid artery because of gross infection, and for resection of the carotid artery (Sakakibara et al, 1998). In addition, carotid endarterectomy is widely used for stroke prevention; briefly occluding the carotid artery has been proven safe (Deriu et al, 1994). Therefore, ischemic postconditioning through manipulation of the CCA may be an alternative method.
In addition, pharmacological mimics have appeared to be an effective means of postconditioning. However, as pharmacological agents used for postconditioning are neurotoxic, it will be critical to define the precise dosage and temporal characteristics of these pharmacological postconditioning to avoid their side effects.
The third alternative for resolving the issue of accessibility of cerebral blood vessel is to use remote ischemic postconditioning. In the research field of myocardial infarction, remote ischemic postconditioning refers to ischemic postconditioning performed in a remote, nonvital organ that protects against myocardial ischemia (Andreka et al, 2007; Penna et al, 2008). It has not been reported whether remote ischemic postconditioning protects against brain ischemia. Pilot results in our laboratory have shown that remote postconditioning performed in the ipsilateral hind limb reduced brain injury after focal ischemia (unpublished results), which, however, is subjected to verification from other laboratories. All the aforementioned hypotheses about performing postconditioning are based on the prerequisites of reperfusion. However, one may even boldly imagine whether reperfusion is necessary for some form of postconditioning. In this case, however, postconditioning would be redefined as an insult applied after stroke. Earlier studies have shown that the ischemic penumbra is salvageable after permanent MCA occlusion, and reperfusion is not a prerequisite for many kinds of neuroprotectants (Clark et al, 2008; Min et al, 2008). Therefore, it is intriguing to test whether some patterns of postconditioning rescue ischemic penumbra without reperfusion.
Concluding remarks
A number of studies have shown that postconditioning reduces infarction in the immediate aftermath of stroke, but more studies are needed to confirm whether it offers long-term protection, and improves animals’ neurobehavioral functions. Postconditioning seems to reduce ischemic injury by blocking the overproduction of ROS and lipid peroxidation, and by inhibiting apoptosis. The initial inhibiting effect on ROS may lead to an improved activity of the Akt and KATP channels, which contributes to the protection of postconditioning. In addition, the changes in the MAPK pathways and in the δPKC and εPKC activities are also associated with the protection of postconditioning. Despite this impressive progress, many obstacles remain for translating postconditoning into the clinic. Future studies are required to address the fundamental protective mechanisms, blood vessel accessibility, and therapeutic time windows in multiple animal models with different genders and ages.
Acknowledgements
The author wishes to thank Ms Felicia F Beppu and Dr Angela Lee for editorial assistance. This work was partially supported by the AHA Grant SDG 0730113N and the NIH Grant 1R21NS057750-01A2.
References
- Andreka G, Vertesaljai M, Szantho G, Font G, Piroth Z, Fontos G, Juhasz ED, Szekely L, Szelid Z, Turner MS, Ashrafian H, Frenneaux MP, Andreka P. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–52. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–56. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Bowen KK, Naylor M, Vemuganti R. Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochem Int. 2006;49:127–35. doi: 10.1016/j.neuint.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Burda J, Danielisova V, Nemethova M, Gottlieb M, Matiasova M, Domorakova I, Mechirova E, Ferikova M, Salinas M, Burda R. Delayed postconditionig initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006a;26:1139–49. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda J, Danielisova V, Nemethova M, Gottlieb M, Matiasova M, Domorakova I, Mechirova E, Ferikova M, Salinas M, Burda R. Delayed postconditionig initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006b;26:1141–51. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda J, Gottlieb M, Vanicky I, Chavko M, Marsala J. Short-term postischemic hypoperfusion improves recovery of protein synthesis in the rat brain cortex. Mol Chem Neuropathol. 1995;25:189–98. doi: 10.1007/BF02960912. [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett. 1989;101:299–304. doi: 10.1016/0304-3940(89)90549-1. [DOI] [PubMed] [Google Scholar]
- Casabona G. Intracellular signal modulation: a pivotal role for protein kinase C. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:407–25. doi: 10.1016/s0278-5846(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–9. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–28. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Penner M, Orellana-Jordan IM, Colbourne F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol. 2008;212:386–92. doi: 10.1016/j.expneurol.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Danielisova V, Gottlieb M, Nemethova M, Burda J. Effects of bradykinin postconditioning on endogenous antioxidant enzyme activity after transient forebrain ischemia in rat. Neurochem Res. 2008;33:1057–64. doi: 10.1007/s11064-007-9550-3. [DOI] [PubMed] [Google Scholar]
- Danielisova V, Nemethova M, Gottlieb M, Burda J. The changes in endogenous antioxidant enzyme activity after postconditioning. Cell Mol Neurobiol. 2006;26:1181–91. doi: 10.1007/s10571-006-9034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu GP, Franceschi L, Milite D, Calabro A, Saia A, Grego F, Cognolato D, Frigatti P, Diana M. Carotid artery endarterectomy in patients with contralateral carotid artery occlusion: perioperative hazards and late results. Ann Vasc Surg. 1994;8:337–42. doi: 10.1007/BF02132994. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–9. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–54. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Dosenko VE, Nagibin VS, Tumanovskaya LV, Zagoriy VY, Moibenko AA, Vaage J. Proteasome inhibitors eliminate protective effect of postconditioning in cultured neonatal cardiomyocytes. Fiziol Zh. 2006;52:15–24. [PubMed] [Google Scholar]
- Dumas TC, Sapolsky RM. Gene therapy against neurological insults: sparing neurons versus sparing function. Trends Neurosci. 2001;24:695–700. doi: 10.1016/s0166-2236(00)01956-1. [DOI] [PubMed] [Google Scholar]
- Fisher M, Brott TG. Emerging therapies for acute ischemic stroke: new therapies on trial. Stroke. 2003;34:359–61. doi: 10.1161/01.str.0000054627.69159.c2. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Siren AL, Feuerstein GZ, Hallenbeck JM. The onset of postischemic hypoperfusion in rats is precipitous and may be controlled by local neurons. Stroke. 1992;23:399–406. doi: 10.1161/01.str.23.3.399. [DOI] [PubMed] [Google Scholar]
- Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008a;86:2505–11. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008b;105:943–55. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech ED, Ramsdale DR. Termination of reperfusion arrhythmia by coronary artery occlusion. Br Heart J. 1994;72:94–5. doi: 10.1136/hrt.72.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyte L, Kaur J, Buchan AM. Lost in translation: taking neuroprotection from animal models to clinical trials. Exp Neurol. 2004;188:200–4. doi: 10.1016/j.expneurol.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Iliodromitis EK, Georgiadis M, Cohen MV, Downey JM, Bofilis E, Kremastinos DT. Protection from postconditioning depends on the number of short ischemic insults in anesthetized pigs. Basic Res Cardiol. 2006;101:502–7. doi: 10.1007/s00395-006-0606-3. [DOI] [PubMed] [Google Scholar]
- Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–63. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–33. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–62. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–4. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1377–86. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–57. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- Krolikowski JG, Weihrauch D, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Role of Erk1/2, p70s6K, and eNOS in isoflurane-induced cardioprotection during early reperfusion in vivo. Can J Anaesth. 2006;53:174–82. doi: 10.1007/BF03021824. [DOI] [PubMed] [Google Scholar]
- Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–9. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–62. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–6. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- Min J, Senut MC, Rajanikant K, Greenberg E, Bandagi R, Zemke D, Mousa A, Kassab M, Farooq MU, Gupta R, Majid A. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res. 2008;86:2984–91. doi: 10.1002/jnr.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Na HS, Kim YI, Yoon YW, Han HC, Nahm SH, Hong SK. Ventricular premature beat-driven intermittent restoration of coronary blood flow reduces the incidenceof reperfusion-induced ventricular fibrillation in a cat model of regional ischemia. Am Heart J. 1996;132:78–83. doi: 10.1016/s0002-8703(96)90393-2. [DOI] [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Hayashi T, Lewen A, Omar G, Chan PH. Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci. 2002;22:7923–30. doi: 10.1523/JNEUROSCI.22-18-07923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal D, Dettwiler S, Favoccia C, Scharbatke H, Preckel B, Schlack W. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesth Analg. 2005;101:1252–60. doi: 10.1213/01.ANE.0000181336.96511.32. [DOI] [PubMed] [Google Scholar]
- Penna C, Mancardi D, Raimondo S, Geuna S, Pagliaro P. The paradigm of postconditioning to protect the heart. J Cell Mol Med. 2008;12:435–58. doi: 10.1111/j.1582-4934.2007.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr. 2004;36:323–7. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- Piccini JP, Berger JS, Brown DL. Early sustained ventricular arrhythmias complicating acute myocardial infarction. Am J Med. 2008;121:797–804. doi: 10.1016/j.amjmed.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–41. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- Rehni AK, Singh N. Role of phosphoinositide 3-kinase in ischemic postconditioning-induced attenuation of cerebral ischemia-evoked behavioral deficits in mice. Pharmacol Rep. 2007;59:192–8. [PubMed] [Google Scholar]
- Ren C, Gao X, Niu N, Yan Z, Chen X, Zhao H. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS ONE. 2008;3:e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y, Kuramoto K, Jikuya T, Sato F, Nakamura K, Abe M, Mitsui T. An approach for acute disruption of large arteries in patients with advanced cervical cancer: endoluminal balloon occlusion technique. Ann Surg. 1998;227:134–7. doi: 10.1097/00000658-199801000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–69. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- Scartabelli T, Gerace E, Landucci E, Moroni F, Pellegrini-Giampietro DE. Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K-Akt signaling pathway: a novel postconditioning strategy? Neuropharmacology. 2008;55:509–16. doi: 10.1016/j.neuropharm.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Sewell WH, Koth DR, Huggins CE. Ventricular fibrillation in dogs after sudden return of flow to the coronary artery. Surgery. 1955;38:1050–3. [PubMed] [Google Scholar]
- Shimohata T, Zhao H, Steinberg GK. Epsilon PKC may contribute to the protective effect of hypothermia in a rat focal cerebral ischemia model. Stroke. 2007a;38:375–80. doi: 10.1161/01.STR.0000254616.78387.ee. [DOI] [PubMed] [Google Scholar]
- Shimohata T, Zhao H, Sung JH, Sun G, Mochly-Rosen D, Steinberg GK. Suppression of deltaPKC activation after focal cerebral ischemia contributes to the protective effect of hypothermia. J Cereb Blood Flow Metab. 2007b;27:1463–75. doi: 10.1038/sj.jcbfm.9600450. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002;58:945–52. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–8. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- Tsang A, Hausenloy DJ, Yellon DM. Myocardial postconditioning: reperfusion injury revisited. Am J Physiol Heart Circ Physiol. 2005;289:H2–7. doi: 10.1152/ajpheart.00091.2005. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin H, Halkos ME, Kerendi F. Postconditioning—A new link in nature's armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2005;100:295–310. doi: 10.1007/s00395-005-0523-x. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, Bruce IC, Luo BY, Xia Q. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39:983–90. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett. 2007;425:59–62. doi: 10.1016/j.neulet.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, Ohman EM. Significance of atrial fibrillation during acute myocardial infarction, and its current management: insights from the GUSTO-3 trial. Card Electrophysiol Rev. 2003;7:201–7. doi: 10.1023/B:CEPR.0000012382.81986.47. [DOI] [PubMed] [Google Scholar]
- Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S. Ischemic post-conditioning protects brain and reduces inflammation in a rat model of focal cerebral ischemia/reperfusion. J Neurochem. 2008a;105:1737–45. doi: 10.1111/j.1471-4159.2008.05276.x. [DOI] [PubMed] [Google Scholar]
- Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008b;39:2362–9. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- Yamashima T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog Neurobiol. 2000;62:273–95. doi: 10.1016/s0301-0082(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–64. doi: 10.1161/01.str.25.8.1658. discussion 1664−1655. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Enomoto Y, Kitajima H, Yamada J, Kaku Y, Iwama T. Carotid-compression technique for the insertion of guiding catheters. AJNR Am J Neuroradiol. 2006;27:1710–1. [PMC free article] [PubMed] [Google Scholar]
- Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. Journal of Neuroimmune Pharmacology. 2007;2:313–8. doi: 10.1007/s11481-007-9089-8. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–21. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003a;85:1026–36. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003b;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–11. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]