Abstract
A stereoselective intramolecular normal demand [4 + 2] cycloaddition of allenamides under thermal conditions without metal assistance is described. This work led to the development of a stereoselective tandem propargyl amide-isomerization–[4 + 2] cycloaddition sequence amenable for rapid assembly of complex nitrogen heterocycles.
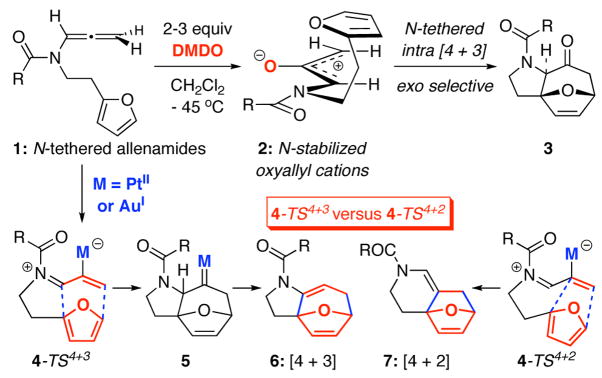
We have been embarking on the chemistry of allenamides in the last ten years.1,2 In particular, allenamides have proven to be an excellent source of nitrogen-stabilized oxyallyl cations3,4 through DMDO-epoxidation, thereby allowing us to develop highly stereoselective [4 + 3] cycloaddition manifolds5–7 including intramolecular8,9 cycloadditions such as using N-tethered allenamide 19 en route to synthetically useful nitrogen heterocycle 3 [Scheme 1]. However, the dependence on DMDO as the key oxidant for the transformation can pose a challenge in terms of scale and operational convenience. Mascareñas’s report10 intrigued us because of their usage of PtCl2/CO in catalyzing a [4 + 3] cycloaddition of allenes. More significantly, they also documented that a different catalyst [AuCl] could effectively direct the reactivity toward the competing [4 + 2] cycloaddition instead of the [4 + 3] cycloaddition. Recently, Toste11 revealed a similar divergence in [4 + 2] versus [4 + 3] cycloaddition when using different ligands along with a Au(I) catalyst. Our own efforts in exploring Mascareñas’s PtCl2 versus AuCl protocol10,12,13 while adopting allenamides led us to an interesting and different direction than the initially anticipated issues regarding competing [4 + 3] and [4 + 2] cycloadditions [see 4-TS4+3→6 vs. 4-TS4+2→7, respectively, in Scheme 1]. We report here a rare normal electron-demand1,14–17 [4 + 2] cycloaddition involving electron-rich heteroatom-substituted allenes under thermal conditions and a stereoselective tandem propargyl amide isomerization–intramolecular [4 + 2] cycloaddition sequence.
Scheme 1.
Cycloadditions of N-Tethered Allenamides.
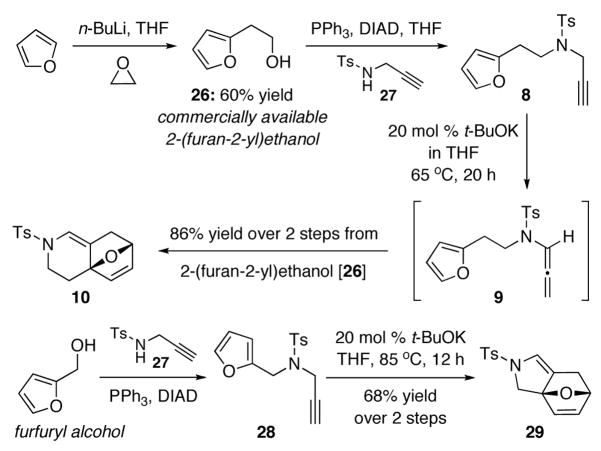
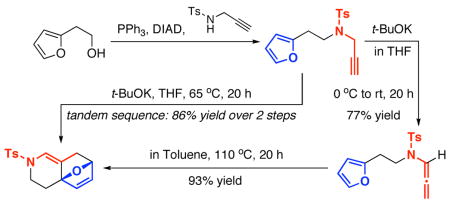
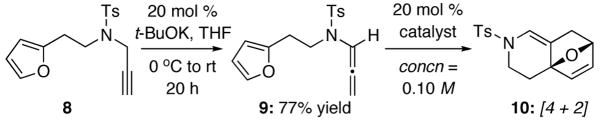
To commence our studies, we initially examined an N-Boc- substituted allenamide, but it was not useful for platinum and gold protocols [see footnote 18 for results]. Consequently, N-sulfonyl-allenamide 919 was prepared from propargyl amide 8 via our base-promoted isomerization protocol using cat t-BuOK.20 We quickly found that with the exception of AuCl [entries 5–7 in Table 1], platinum catalysts [entries 1–4] and Au(III) catalyst [entry 9] were not useful in generating any cycloaddition types of products. Concentrations did not appear to have any impact, as reactions run at 0.04 M led to the same outcome.
Table 1.
Exploring Conditions for the Cycloaddition.
 | ||||||
|---|---|---|---|---|---|---|
| entry | catalysts | 4 Å MS | solvents | temp [°C] | time [h] | yield [%]a |
| 1 | PtCl2 | √ | DCE | 65 | <12 | 0 |
| 2 | PtCl4 | √ | DCE | 65 | 3 | 13c |
| 3 | PtCl4 | √ | THF | 65 | 6 | 15c |
| 4 | PtCl4 | √ | toluene | 23 | 1 | 11c |
| 5 | AuCl | √ | DCEb | 23 | 10min | 66 |
| 6 | AuCl | √ | THF | 65 | 6 | 35c |
| 7 | AuCl | √ | toluene | 65 | <30 min | 42c |
| 8 | AuCl/AgSbF6 | √ | DCE | 23 | 1 | 16c |
| 9 | AuCl3 | √ | DCE | 65 | 10 min | 0 |
| 10 | AgSbF6 | √ | DCE | 65 | 6 | 85c |
| 11 | AgBF4 | √ | DCE | 65 | 6 | 94 |
| 12 | AgBF4 | √ | toluene | 65 | 6 | 80c |
| 13 | AgBF4 | √ | THF | 65 | <12 | 57c |
| 14 | CSAd | √ | DCE | 65 | <12 | 92c |
| 15 | PPTSd | √ | DCE | 65 | 8 | 94c |
| 16 | No | √ | THF | 65 | 30 | 91c |
| 17 | No | No | d8-toluene | 110 | 20 | 93 |
Isolated yields unless otherwise indicated.
DCE: 1,2-Dichloroethane.
NMR yields determined with phenanthrene as the internal standard
10 mol % was used.
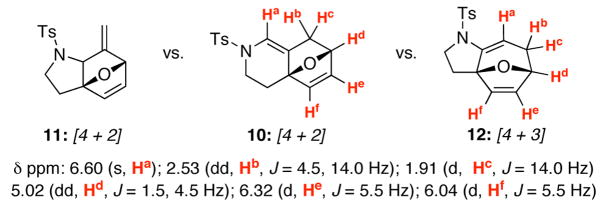
Most intriguingly, the illustration of the corresponding [4 + 2] cycloadduct 10 shown in Table 1 of hindsight after a series of subsequent studies. As shown in Figure 1, although 10 and its regioisomer 11 are readily distinguishable, it is not obvious how to unambiguously distinguish 10 from potential [4 + 3] cycloadduct 12 solely based on the key 1H NMR resonances. However, as we continued our explorations and began to achieve high yielding reactions with silver salts [entries 10–13], Brønsted acids [entries 14 and 15, and then, ultimately simple thermal conditions with [entry 16] or without 4Å MS [entry 17], we recognized that this did not appear to be a simple [4 + 3] cycloaddition process. Instead, it turned out to be exclusively a [4 + 2] cycloaddition pathway under all conditions after attaining an X-ray crystal structure [vide infra].
Figure 1.
[4 + 2] Versus [4 + 3] Cycloadducts.
δppm: 6.60 (s, Ha); 2.53 (dd, Hb, J = 4.5, 14.0 Hz); 1.91 (d, Hc, J = 14.0 Hz) 5.02 (dd, Hd, J = 1.5, 4.5 Hz); 6.32 (d, He, J = 5.5 Hz); 6.04 (d, Hf, J = 5.5 Hz)
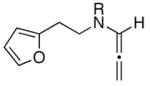
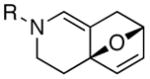
The ability to pursue this cycloaddition thermally represents a unique opportunity for two major reasons. Firstly, as shown in Table 2, this thermally driven allenic-[4 + 2] cycloaddition manifold possesses a much broader synthetic potential than previous work.10,11
Table 2.
Thermal [4 + 2] Cycloaddions of of Allenamides.
| entry | allenamidesa | time [h] | cycloadducts | yield [%]b |
|---|---|---|---|---|
 |
 |
|||
| 1 | 13a: R = p-Ns | 12 | 14a: R = p-Ns | 92 |
| 2 | 13b: R = Boc | 20 | 14b: R = Boc | 65 |
| 3 | 13c: R = (−)-menthyl | 20 | 14c: R = (−)-menthyl | 54c |
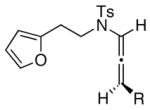 |
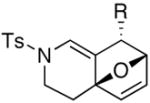 |
|||
| 4 | (±)-15a: R = Ph | 2 | 16a: R = Ph | 77d |
| 5 | (±)-15b: R = Me | 30 | 16b: R = Me | 57d |
| 6 |
17 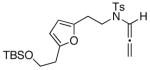
|
<12 |
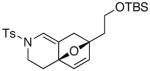 19 19
|
77 |
| 7 |
18 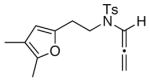
|
4 |
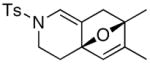 20 20
|
93 |
| 8 |
21 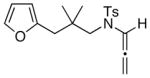
|
24 |
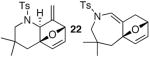 23 23
|
95e |
| 9 |
24 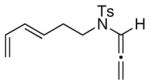
|
20 |
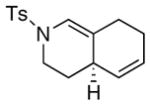 25 25
|
78 |
Unless otherwise noted, all reactions were carried out in THF at 85 °C at concn = 0.10 M. Reactions in entries 3 and 8 were run in toluene. Entries 4 and 8 were run at 45 °C and 110 °C, respectively.
Isolated yields.
Only one isomer by 1H NMR but absolute configuration unassigned.
16a and 16b were found as a ~ 3:1 inseparable isomeric mixture.
Regioisomeric ratio of regioisomers 22 and 23 is ~ 4:1.
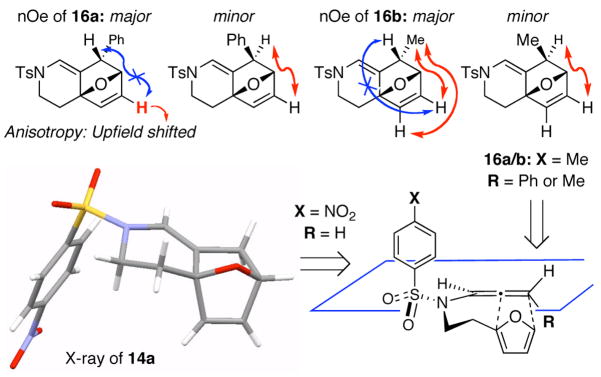
The substrate scope is comprised of: (1) Different N-substituents [entries 1–3] including carbamates; (2) substitutions at the allenic γ-position [(±)-15a and (±)-15b in entries 4 and 5, respectively] that gave the respective cycloadducts 16a and 16b with the major isomers shown as assigned via nOe experiments [Figure 2]; (3) various furan substitutions [entries 6 and 7]; (4) a longer tethering that led to the regiochemical outcome in favor of the internal olefin of the allenic motif [22 in entry 8], which is found as a single diastereomer;21 and also notably in this case, when using 10 mol% of AgBF4 and 4Å MS, 22 was isolated in 58% yield as the only regioisomer after heating in toluene at 110 °C for 36 h;21 and lastly, (5) a simple butadiene [entry 9].
Figure 2.
nOes Experiments and X-Ray Structure of 14a.
The X-ray structure of cycloadduct 14a unambiguously confirms the [4 + 2] cycloaddition pathway [Figure 2], and it provides a general mechanistic picture for this allenic cycloaddition. Based on the nOe assignments of the respective major isomers for 16a and 16b [dr 3:1], the current mechanistic picture also implies that the furan approaches from the more hindered side with R ≠ H. We are not certain of reasons behind this contra-steric approach.
Secondly and more importantly, we recognized the possibility of developing a tandem sequence consisting of propargyl amide isomerization followed by cycloaddition. As shown in Scheme 2, In the presence of 20 mol% t-BuOK at 65 °C, isomerization of propargyl amide 8 and the ensuing cycloaddition led to 10 in 86% yield over three steps furan [or two steps from commercially available 2-(furan-2-yl)ethanol 26]. Likewise, cycloadduct 29 could be obtained in 68% yield in two steps from furfuryl alcohol. We note here that without t-BuOK, this tandem process does not take place even after heating in toluene at 110 °C for 24 h, thereby suggesting that the tandem sequence proceeds through exclusively the respective allenamide intermediate.
Scheme 2.
A Tandem Propargyl Amide-Isomerization–[4 + 2].
In addition, with platinum or gold catalysts, the reaction proceeded through a very different pathway.22,23 Moreover, in a related example from Kanemastu’s account,15 5.0 equiv of t-BuOK was used and the reaction afforded ring-opened and aromatized products instead of furan-cycloadduct 29. The use of catalytic amount of t-BuOK proves to be the key in accessing these structurally more useful cycloadducts.
Finally, this tandem process is general for a range of propargyl amides [Table 3] including those that are terminally substituted [entries 2–4], thereby also representing first examples of successful based-promoted isomerizations of terminally substituted propargyl amides to allenamides.20,24 It is noteworthy that all propargyl amides employed here were prepared from respective furyl alcohols featuring a Mitsunobu reaction using N-sulfonylated propargyl amine [see 27 in Scheme 2], allowing this tandem process amenable for facile constructions of complex nitrogen heterocycles from very simple commercially available material.
Table 3.
Tandem Isomerization–[4 + 2] Cycloadditions.
| entry | propargyl amidesa | time [h] | cycloadducts | yield [%]b |
|---|---|---|---|---|
| 1 |
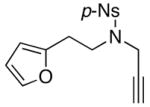 30 30
|
24 |
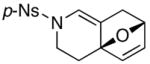 14a 14a
|
84 |
| 2 |
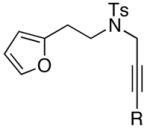 |
24 |
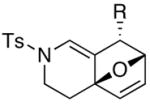 |
79c |
| 3 | 24 | 42c,e | ||
| 4 | 16 | 25d,e | ||
| 5 |
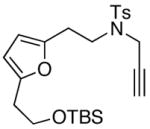 32 32
|
14 |
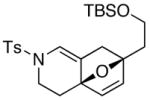 19 19
|
63 |
Unless otherwise noted, all reactions were carried out in THF at concn = 0.10 M with 20 mol % of t-BuOK. For entries 1 and 5: Reaction temp = 65 °C; for entries 3 and 4: temp = 85 °C; and for entry 2: temp = 25 °C.
Isolated yields.
dr = ~3:1.
dr = ~2:1.
The reaction was slower, and also observed was hydrolysis of the starting allenamide.
We have described here a rare normal electron-demand [4 + 2] cycloaddition of N-tethered allenamides under thermal conditions without assistance of any metals. Our efforts also led to the development of an efficient and highly stereoselective tandem propargyl amide-isomerization–[4 + 2] cycloaddition sequence amenable for rapid assembly of highly functionalized nitrogen heterocycles from very simple commercial furyl alcohols. Applications of this method toward constructing isoquinoline, quinoline, or isoindole containing natural products are underway.
Supplementary Material
Acknowledgments
Authors thank NIH-NIGMS [GM066055]. Authors also thank Dr. Vic Young [University of Minnesota] for providing X-ray structural analysis.
Footnotes
Supporting Information Available: Experimental and 1H NMR spectral and characterizations for all new compounds as well as X-ray structrural data available free of charge at http://pubs.acs.org.
References
- 1.For a reviews on the chemistry of allenamides, see: Hsung RP, Wei LL, Xiong H. Acc Chem Res. 2003;36:773. doi: 10.1021/ar030029i.
- 2.For recent reports on allenamide chemistry, see: Hayashi R, Hsung RP, Feltenberger JB, Lohse AG. Org Lett. 2009;11:2125. doi: 10.1021/ol900647s.Skucas E, Zbieg JR, Krische MJ. J Am Chem Soc. 2009;131:5054. doi: 10.1021/ja900827p.Armstrong A, Emmerson DPG. Org Lett. 2009;11:1547. doi: 10.1021/ol900146s.Beccalli EM, Broggini G, Clerici F, Galli S, Kammerer C, Rigamonti M, Sottocornola S. Org Lett. 2009;11:1563. doi: 10.1021/ol900171g.Broggini G, Galli S, Rigamonti M, Sottocornola S, Zecchi G. Tetrahedron Lett. 2009;50:1447.Brummond KM, Yan B. Synlett. 2008:2303.Fuwa H, Tako T, Ebine M, Sasaki M. Chem Lett. 2008;37:904.González-Gómez Á, Añorbe L, Poblador A, Domínguez G, Pérez-Castells J. Eur J Org Chem. 2008:1370.
- 3.For excellent reviews on heteroatom-substituted oxyallyl cations in [4 + 3] cycloadditions, see: Harmata M. Adv Synth Catal. 2006;348:2297.Harmata M. Recent Res Devel In Organic Chem. 1997;1:523.
- 4.For leading examples of nitrogen-stabilized oxyallyl cations in [4 + 3] cycloadditions, see: MaGee DI, Godineau E, Thornton PD, Walters MA, Sponholtz DJ. Eur J Org Chem. 2006:3667.Myers AG, Barbay JK. Org Lett. 2001;3:425. doi: 10.1021/ol006931x.Sung MJ, Lee HI, Chong Y, Cha JK. Org Lett. 1999;1:2017. doi: 10.1021/ol9911932.Dennis N, Ibrahim B, Katritzky AR. J Chem Soc, Perkin Trans. 1976;1:2307.
- 5.For our nitrogen-stabilized oxyallyl cations in intermolecular [4 + 3] cycloadditions with furans and pyrroles, see: Xiong H, Hsung RP, Berry CR, Rameshkumar C. J Am Chem Soc. 2001;123:7174. doi: 10.1021/ja0108638.Antoline JE, Hsung RP, Huang J, Song Z, Li G. Org Lett. 2007;9:1275. doi: 10.1021/ol070103n.Antoline JE, Hsung RP. Synlett. 2008:739.
- 6.For our asymmetric [4 + 3] cycloadditions, see: Huang J, Hsung RP. J Am Chem Soc. 2005;127:50. doi: 10.1021/ja044760b.
- 7.Also see: Harmata M, Ghosh SK, Hong X, Wacharasindu S, Kirchhoefer P. J Am Chem Soc. 2003;125:2058. doi: 10.1021/ja029058z.For an enantioselective formal [4 + 3] cycloaddition, see: Dai X, Davies HML. Adv Synth Catal. 2006;348:2449.
- 8.For intramolecular [4 + 3] of C-tethered allenamides, see: Rameshkumar C, Hsung RP. Angew Chem Int Ed. 2004;43:615. doi: 10.1002/anie.200352632.
- 9.For intramolecular [4 + 3] of N-tethered allenamides, see: Xiong H, Huang J, Ghosh S, Hsung RP. J Am Chem Soc. 2003;125:12694. doi: 10.1021/ja030416n.
- 10.For a recent account on intramolecular [4 + 3] cycloadditions of allenes using PtCl2, see: Trillo B, López F, Gulías M, Castedo L, Mascareñas JL. Angew Chem Int Ed. 2007;47:951. doi: 10.1002/anie.200704566.Trillo B, López F, Montserrat S, Ujaque G, Castedo L, Lledós A, Mascareñas JL. Chem Eur J. 2009;15:3336. doi: 10.1002/chem.200900164.
- 11.Mauleón P, Zeldin RM, González AZ, Toste FD. J Am Chem Soc. 2009;131:6348. doi: 10.1021/ja901649s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For a leading review on this chemistry:, see: Nevado C, Echavarren AM. Synthesis. 2005:167.
- 13.For reviews on platinum and gold chemistry, see: Fürstner A, Davies PW. Angew Chem Int Ed. 2007;46:3410. doi: 10.1002/anie.200604335.Arcadi A. Chem Rev. 2008;108:3266. doi: 10.1021/cr068435d.Shen HC. Tetrahedron. 2008;64:3885.Shen HC. Tetrahedron. 2008;64:7847.
- 14.For a compendium on chemistry of allenes, see: Krause N, Hashmi ASK. Modern Allene Chemistry. 1 and 2 Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004.
- 15.For a leading reference on normal electron-demand Diels-Alder cycloadditions of allenamides generated in situ, see: Lee M, Morimoto H, Kanematsu K. Tetrahedron. 1996;52:8169.
- 16.For an example using N-allenylsulfenimide, see: Bacci JP, Greenman KL, van Vranken DLJ. Org Chem. 2003;68:4955. doi: 10.1021/jo0340410.
- 17.For some examples of normal electron-demand Diels-Alder cycloadditions of allenoethers, see: Hayakawa K, Aso K, Shiro M, Kanematsu K. J Am Chem Soc. 1989;111:5312.Wu HJ, Liu CF, Fang Z, Lin HC. Tetrahedron Lett. 2007;48:6192. and references cited therein.For an example of allenyl sulfides, see: Yeo SK, Shiro M, Kanematsu K. J Org Chem. 1994;59:1621.
-
18.When utilizing a Boc-substituted allenamide [see i], reactions promoted by PtCl2, PtCl4, AuCl, or AuCl3 [in 10–100 mol % at rt-65 °C] led to very low yields of possible cycloadduct [ii] with mostly being hydrolysis of the starting allenamide and decomposition. Only when using AgSbF6, a modest yield was attained for cycloadduct ii.

- 19.See Supporting Information.
- 20.(a) Wei LL, Mulder JA, Xiong H, Zificsak CA, Douglas CJ, Hsung RP. Tetrahedron. 2001;57:459. [Google Scholar]; (b) Xiong H, Hsung RP, Wei LL, Berry CR, Mulder JA, Stockwell B. Org Lett. 2000;2:2869. doi: 10.1021/ol000181+. [DOI] [PubMed] [Google Scholar]
-
21.nOe experiments of cycloadduct 22 and its possible cycloaddition transition state.
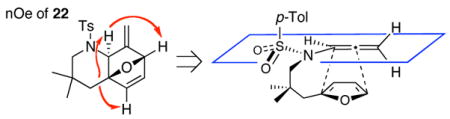 In addition, the regioisomeric cycloadduct 23 was found to equilibrate to 22 via retro-[4 + 2] and [4 + 2] after heating at 110 °C in toluene for 22 h.
In addition, the regioisomeric cycloadduct 23 was found to equilibrate to 22 via retro-[4 + 2] and [4 + 2] after heating at 110 °C in toluene for 22 h.
-
22.When using PtCl4, we were able to isolate some products [iii and iv] that are related to those reported by Hashmi amd Echavarren [see reference 23].
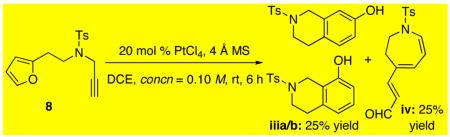
- 23.For a leading reference, see: Hashmi ASK, Salathé R, Frey W. Chem Eur J. 2006;12:6991. doi: 10.1002/chem.200600533.Hashmi ASK, Frost TM, Bats JW. J Am Chem Soc. 2000;122:11553.Martín-Matute B, Cárdenas DJ, Echavarren AM. Angew Chem Int Ed. 2001;40:4754. doi: 10.1002/1521-3773(20011217)40:24<4754::aid-anie4754>3.0.co;2-9.
- 24.For a review, see: Tracey MR, Hsung RP, Antoline J, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb Steve M., editor. Chapter 21.4. Georg Thieme Verlag KG; 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.