Abstract
The immuno-modulatory properties of airway smooth muscle have become of increasing importance in our understanding of the mechanisms underlying chronic inflammation and structural remodeling of the airway wall in asthma and chronic obstructive pulmonary disease (COPD). ASM cells respond to many cytokines, growth factors and lipid mediators to produce a wide array of immuno-modulatory molecules which may in turn orchestrate and perpetuate the disease process in asthma and COPD. Despite numerous studies of the cellular effects of cytokines on cultured ASM, few have identified intracellular signaling pathways by which cytokines modulate or induce these cellular responses. In this review we provide an overview of the transcriptional mechanisms as well as intracellular signaling pathways regulating cytokine functions in ASM cells. The recent discovery of toll-like receptors in ASM cells represents a significant development in our understanding of the immuno-modulatory capabilities of ASM cells. Thus, we also review emerging evidence of the inflammatory response to toll-like receptor activation in ASM cells.
Keywords: Airway smooth muscle, cytokines, asthma, airway inflammation, toll-like receptors (TLR), corticosteroids, nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), janus activated kinase/Signal Transducers and Activators of Transcription (JAK/STAT)
Introduction
Cytokines and chemokines play a central role in regulating inflammatory and immune responses in chronic lung diseases such as asthma and COPD. Indeed, in vivo studies using selective inhibitors as well as neutralizing antibodies against various cytokines and chemokines demonstrate their importance in antigen-induced airway inflammation (leukocyte infiltration) and hyper-responsiveness in animal models of asthma [1-3]. Studies in knock-out or transgenic mice also illustrate the importance of cytokines in the abnormal airway changes induced by allergen challenge in sensitized animals [4]. A potential site for the deleterious action of many cytokines in airways disease is the airway smooth muscle a primary effector tissue historically thought to only regulate bronchomotor tone. In human cultured ASM cells that retain physiological responsiveness, cytokines alter pro-inflammatory gene expression that in turn may play an important role in the pathogenesis of chronic inflammatory airways disease [5]. Despite numerous studies of the cellular effects of cytokines on cultured ASM, few have identified downstream signaling cascades by which cytokines modulate or induce these cellular responses. In this review we discuss the role of three major intracellular signaling pathways: Mitogen-Activated Protein Kinase (MAPK), Nuclear Factor-kappa B (NF-κB), and Janus kinases and Signal Transducers and Activators of Transcription (STATs) in regulating cytokine functions, with a particular focus on inflammatory gene expression, in regulating ASM functions.
The capacity for ASM cells to respond to numerous cytokines has revealed the extensive immune-regulatory potential of these cells. In response to cytokines such as IL-1β, TNF-α and IFN-γ, ASM cells can be induced to express a host of cell-adhesion and co-stimulatory molecules that allow interactions between the ASM and inflammatory cells that infiltrate the airways. Moreover, ligation of ASM cell-surface molecules such as CD40 and OX40L by their respective counter-ligands leads to activation of ASM inflammatory responses. Further advances in understanding the immune-regulatory potential of ASM have come with the discovery that cytokines also up-regulate the expression of multiple toll-like receptors (TLRs) in ASM cells. These latter receptors are pattern-recognition receptors that mediate innate and adaptive immune and inflammatory responses to microbial infection, tissue injury or inflammation. Emerging evidence now suggests a role for TLRs in the development, perpetuation and exacerbation of chronic inflammatory airway disease [6]. Thus, we also discuss the potential role of TLRs in the amplification of ASM inflammatory responses.
1. MAPKs
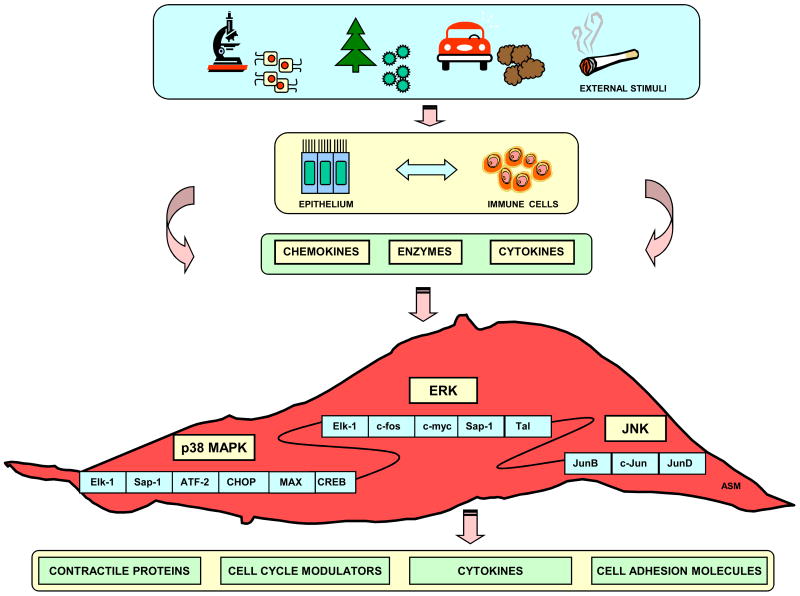
The MAPK signal transduction pathway consists of MAPK, MAPK kinase (MEK, MAPKK, or MKK), and MAPK kinase kinase (MEKK, MAPKKK, or MKKK). The MAPK cascade activation occurs by sequential phosphorylation of Thr-X-Tyr motifs. In mammalian cells, there are five distinct subfamilies including extracellular signal-regulated kinase (ERK), p38 MAPK (p38), c-Jun N-terminal kinase (JNK), ERK3/4 and ERK5. Among the five distinctive MAPK pathways, ERK, p38 MAPK and JNK have been extensively studied in ASM cells [7] (Figure 1).
Figure 1. Schematic overview of MAPK pathways regulating airway smooth muscle functions.
A variety of external stimuli activate immune cells or airway epithelial cells to release a variety of biological mediators. These mediators transduce their effects through ERK, p38 or JNK signaling cascades leading to expression of genes that modulate airway smooth muscle contractile, proliferative and secretory responses.
1.2 MAPK signaling in ASM inflammatory gene expression
(a) p42/44 ERK
ERK signaling induces downstream activation of different intracellular transcription factors such as Elk-1, c-fos, c-myc, Sap-1, and Tal, and consequently modulates DNA synthesis and cell proliferation [8]. In ASM, activation of ERK signaling is elicited by various stimuli including platelet derived growth factor (PDGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), endothelin-1 (ET-1), thrombin, oncostatin M, leukemia inhibitory factor (LIF), insulin-like growth factor I, and 5-hydroxytryptamine [9-14]. Cytokines are also important activators of ERK signaling. Phosphorylation of ERK1/2 by IL-1β leads to production of numerous inflammatory mediators including prostaglandin-E2 (PGE2), eotaxin, RANTES, and GM-CSF [15]. ERK is also involved in mediating ASM eotaxin and IL-8 release in response to Th2 cytokines (IL-4, IL-9, IL-13) and the Th17 cytokine IL-17 [16-18]. The interleukin-17B receptor (IL-17BR) is also up-regulated in ASM cells in an ERK-dependent manner [19].
(b) p38
p38 signaling is activated in response to physical and chemical challenges including oxidative stress, UV irradiation, hypoxia, ischemia as well as various cytokines [20, 21]. The down-stream effectors of this cascade are transcription factors such as Elk-1, Sap-1, ATF-2, CREB, CHOP, and Max. p38 mediates bFGF-induced ASM proliferation [22] and ASM inflammatory gene expression in response to multiple stimuli. Indeed, p38 MAPK mediates IL-17A induced IL-6, IL-8 and eotaxin secretion [23-26] as well as bradykinin induced IL-6 secretion [27]. Although there are no published reports of the MAPKs regulating IL-5 secretion, p38 regulates expression of the IL-5 receptor (IL-5R) in response to IL-1β, TNFα and IFN-γ [28]. p38 MAPK appears to have both positive and negative regulatory effects on cytokine-induced inflammatory responses in ASM; it acts to augment TNF-α-induced IL-6 and RANTES release and IL-1β-induced eotaxin release, but inhibits TNF-α induced ICAM-1 expression and IL-1β induced GM-CSF release [15, 29]. This suggests a gene specific role of p38 MAPK in regulating specific transcriptional outcomes.
We recently made the novel finding that, under basal conditions, p38 negatively regulates IFN-β promoter activity (Damera et al., unpublished data). In line with this, treatment of ASM cells with the p38 inhibitor SB203580 showed a specific reduction in tonic p38 activity and enhanced IFN-β transcription and protein secretion. Functional studies using an IFN-β neutralizing antibody reversed the inhibitory effect of SB203580 on TNF-α-induced IL-8 secretion, indicating an important role of autocrine IFN-β in regulating p38-dependent inflammatory responses.
(c) c-Jun NH2-terminal kinases (JNK)
JNK signaling is activated by environmental stress, pro-inflammatory cytokines and genotoxic agents. Following activation of JNK, three Jun transcription factors (JunB, c-Jun and JunD), which are all members of the AP-1 family, are activated [30]. These transcription factors modulate gene expression responsible for many biological responses, including migration, proliferation, differentiation and cell death [31]. In murine studies, administration of the JNK inhibitor SP600125 after allergen challenge prevents T cell-mediated inflammation and ASM cell proliferation, indicating a role for JNK signaling in allergic airway inflammation and remodeling [32]. Studies using the JNK inhibitor SP600125 implicated JNK in the regulation of IL-1β- and TNF-α-induced RANTES, GM-CSF, and IL-8 secretion in ASM cells [33]. IFN-γ and TNF-α induced fractalkine expression also occurs through JNK dependent mechanisms [34].
1.2 Implications of MAPK cross-talk in ASM cells
While unique stimuli initiate the majority of cellular responses by specific signaling cascades, it is not uncommon to derive such responses by multiple and parallel signaling cascades. Exogenous addition of TGF-β1 to ASM cultures increases [3H]-thymidine incorporation and ASM cell proliferation via ERK, p38 and JNK-dependent pathways [35]. Similarly, TNF-α-mediated induction of CD38, a potent modulator of calcium homeostasis and ASM tone, involves all MAPK cascade components [36]. The induction of matrix metalloproteinase-9 (MMP-9) expression by cytokines also involves active participation of several MAPK pathways [37, 38]. Interestingly, in some instances, the induction of one MAPK pathway may antagonize another. Indeed, LPS-induced activation of p38 MAPK down-regulates changes in ASM responsiveness and IL-6 secretion associated with ERK1/2 activation [39].
2. NF-κB
Nuclear factor-kappa B (NF-κB) is a ubiquitously expressed transcription factor that mediates the expression of many inflammatory mediators, including cytokines, adhesion molecules, chemokines, and growth factors [40]. NF-κB-dependent pro-inflammatory genes are believed to play a central role in a variety of inflammatory diseases including chronic inflammatory airway diseases such as asthma. Increased markers of NF-κB pathway activity have been demonstrated in the airways of, or samples from, asthma patients [41-45] as well as in rodent models of asthma [46-49]. For this reason, the NF-κB signalling pathway is an attractive target for novel asthma therapies. Indeed, studies have shown that targeting NF-κB, using various molecular methodologies, inhibits aspects of the allergic response in rodent models of asthma [50-56].
2.1 NF-κB signaling cascade
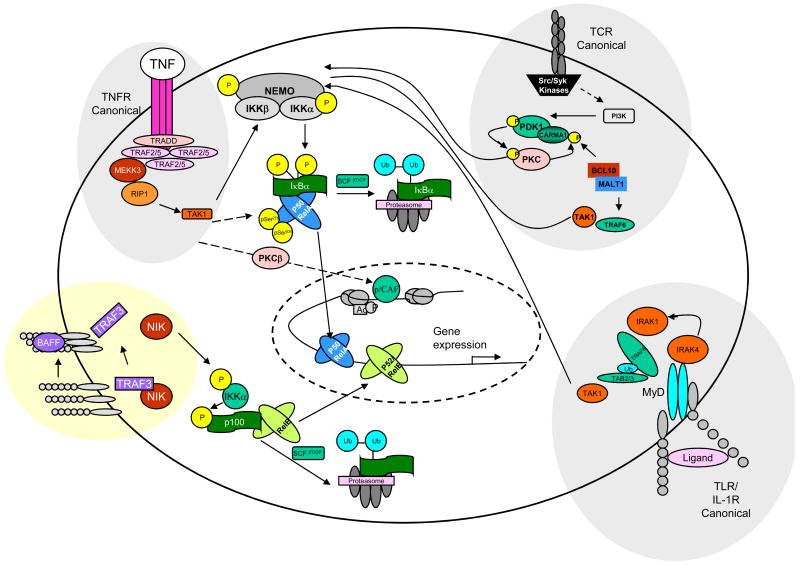
NF-κB is activated in response to a number of stimuli, including physical and chemical stress, lipopolysaccharide (LPS), double-stranded RNA, T- and B-cell mitogens and proinflammatory cytokines [57]. NF-κB induced gene expression is controlled by a complex series of enzymatic signalling events at multiple levels. An overview of the NF-κB activation cascade is depicted in Figure 2.
Figure 2. NF-κB signal transduction pathways.
In resting cells, the majority of NF-κB is bound to I-κB inhibitory protein, often IκBκ, which masks the nuclear localisation sequence (NLS) and holds the complex in the cytoplasm. In the ‘conical’ or ‘classical’ NF-κB activation pathway, ligand binding to a cell surface receptor (e.g. tumor necrosis factor-receptor (TNFR) or Toll-like receptor) recruits adaptors (e.g., TRAFs and RIP) leading to the recruitment of an IKK complex directly onto the cytoplasmic adaptors, activating the IKK complex. IKK then phosphorylates IκB at two serine residues, which leads to its ubiquitination and degradation by the proteasome. NF-κB then enters the nucleus to turn on target genes. TNFR activation can also lead to the phosphorylation of p65 at Ser 276 and 536, and recruitment of cofactors such as p/CAF (via PKCβ) to heighten transcription. TCR engagement leads to recruitment and activation of receptor-associated tyrosine kinases of the Src and Syk families. The latter phosphorylate phospholipase C and phosphatidylinositol 3-kinase (PI3K). Phosphorylation of phosphoinositides by PI3K leads to membrane recruitment and activation of PDK1, which may directly phosphorylate and activate PKCθ to control further recruitment of CARMA1 into the signaling complex. Assembly of these molecules into lipid rafts and PKCθ-dependent phosphorylation of CARMA1 initiate recruitment of BCL10 and MALT1 and possibly TRAF6 and TAK1, leading to IKK activation. The general model shown here for TCR signaling can also be applied to BCR signaling, although a role of PDK1 in this pathway needs to be demonstrated and instead of PKCθ, it involves PKCβ. The non-canonical or non-classical pathway differs from the canonical pathway in that only certain receptor signals (e.g., Lymphotoxin B (LTb), B-cell activating factor (BAFF), CD40) activate this pathway and because it proceeds through an IKK complex that contains two IKKα subunits (but not NEMO). In the noncanonical pathway, receptor binding leads to activation of the NF-κB-inducing kinase NIK, which phosphorylates and activates an IKKα complex, which in turn phosphorylates two serine residues adjacent to the ankyrin repeat C-terminal IκB domain of p100, leading to its partial proteolysis and liberation of the p52/RelB complex. This complex then enters the nucleus to turn on target genes. Figure adapted from Edwards et al, 2008 [66].
NF-κB is made up of a hetero- or homodimer of members of the DNA-binding Rel family of proteins which contains five known mammalian members: p50 (NF-κB1, precursor of which is p105), p65 (Rel A, NF-κB3), p52 (NF-κB2, precursor of which is p100), c-Rel and Rel B. The p65 and p50 subunits are ubiquitously expressed, whereas p52, c-Rel and Rel B are restricted to specific differentiated cell types [58]. In resting cells, the majority of NF-κB is bound to I-κB inhibitory protein, which holds the complex in the cytoplasm. Upon cellular stimulation, the I-κB protein is phosphorylated, ubiquinated, and degraded by the proteosomal pathway. With the I-κB removed, NF-κB translocates to the nucleus and mediates gene transcription [59]
I-κB phosphorylation and activation of Rel proteins can occur via the classical (canonical) or non-classical (non-canonical) pathway. In the classical pathway, a critical phosphorylation of the I-κB protein is performed by the I-κB kinase (IKK) complex, which consists of at least three subunits, including two catalytic subunits IKK-α and -β, also known as IKK-1 and -2, and one regulatory subunit IKK-γ (also known as NEMO) [57]. Of the two catalytic subunits, IKK-β is 20 fold more active than IKK-α in the phosphorylation of I-κB [60]. It is also thought that IKK-β, not IKK-α, is critical for NF-κB activation [61-64] and hence attempts to target this pathway for therapeutic intervention have focused on inhibitors of this subunit [65, 66]. Stimuli of the classical pathway include the TLR/IL-1R family members, ligation of the T-cell receptor (TCR), and TNFR signalling [59](Figure 2). IKK-2 has been shown to be critical in NF-κB activation in ASM cells [67, 68].
In addition to the classical pathway, an alternative (non-canonical) pathway has been described mainly in B cells. This latter pathway can be activated by different stimuli such as lymphotoxin β, CD40 ligand, and receptor activator of NF-κB ligand [69, 70] The alternative NF-κB pathway is characterised by the inducible phosphorylation and processing of p100 to p52, and subsequent nuclear translocation of the heterodimer p52:Rel B is independent of IKKγ and IKKβ and only requires the IKKα subunit [71]. This pathway is believed to play key roles in adaptive immunity [72].
The NF-κB pathway can be further controlled by post translational modifications, including the modulation of Rel protein interactions with other components of the transcriptional machinery. Altered activation of NF-κB can occur via its phosphorylation status, for example the phosphorylation of p65 enhances transcription, yet phosphorylation of p105 can reduce its processing into p50 and hence reduce activation [73]. Acetylation of the Rel proteins also play a key role [74, 75]. Additionally, covalent modifications of the chromatin environment which regulates the access of transcription factors to gene promoters alter NF-κB-dependent transcription. This control is achieved by recruitment of protein complexes that alter chromatin structure via enzymatic modifications of histone tails and/or nucleosome remodelling. NF-κB activation requires several cofactor histone acetyltransferases, including CBP, p300, p/CAF, and SRC-1, of which p/CAF appeared to be relatively more important [74, 76].
2.2 NF-κB signaling in ASM inflammatory gene expression
A multitude of studies in ASM cells implicate a role for NF-κB in the regulation of inflammatory chemokines, cytokines, and adhesion molecules. Indeed, NF-κB is involved in IL-17-induced IL-8 release [23, 77]; IL-1β and TNF-α-induced GRO-α release [78]; neutrophil-derived elastase-induced TGF-β expression [79]; in the expression of cell adhesion molecules such as ICAM-1 and VCAM-1 induced by TNF-α, IL-1β and LPS [80-82]. As stated above, IKK-2 plays a crucial role in the classical NF-κB pathway and for this reason there has been considerable interest in studying and developing ways to manipulate this kinase in order to identify new therapeutics for the treatment of asthma. Data from ASM cells demonstrate that inhibition of IKK2 using the small molecule inhibitors TPCA-1, PS-1145 and ML120B, or molecular intervention using adenoviral approaches to knock down IKK2, demonstrate a role for this kinase in the expression of ICAM-1, cyclooxygenase-2, IL-6, IL-8, GM-CSF, RANTES, monocyte chemotactic protein-1 (MCP-1), GRO-α, neutrophil-activating protein-2 (NAP-2), and epithelial neutrophil activating peptide 78 (ENA-78), some of which are upregulated and play a role in asthma pathogenesis [67, 68]. Similarly, in rodent models of asthma, modulation of IKK-2 using parallel molecular techniques, have shown positive disease modifying data [83-86]. These data suggest that inhibition of IKK2 and hence the NF-κB pathway may have therapeutic implications for asthma treatment.
Of interest, TNF-α but not IL-1β activation of NF-κB signaling involves recruitment of the downstream transducer protein TRAF2 by TNF-α receptor 1 (TNFR1) via the receptor-associated death domain protein, TRADD [87, 88]. Similar findings were also reported in ASM cells from guinea pigs where TNFR1 activation with agonistic antibodies also induced NF-κB activation [89].
Recent work investigating pro-inflammatory stimuli on NF-κB activity with regard to phosphorylation and chromatin remodeling in ASM cells has emerged. TNF-α has been reported to phosphorylate both IKK-β [90] and the p65 subunit at Ser276 and Ser536 in ASM cells [91]. In the latter study, the authors also demonstrated that TNF-α recruits the histone acetyl-transferase p/CAF to the CCL-11 (eotaxin) promoter to increase NF-κB mediated transactivation of this gene [91]. p300/CBP acetylation is also required for NF-κB mediated TNF-α-induced VCAM-1 and ICAM-1 induction in ASM cells [80, 92, 93].
3. JAK/STATs
The classical components of the IFN signaling cascade include the Janus tyrosine kinases and signal transducers and activators of transcription (STATs) factors. Activation of each IFN receptor complex stimulates different receptor-associated tyrosine kinases, namely, JAK1 and Tyk2 by IFN-α/β (type I), or JAK1 and JAK2 by IFN-γ (type II) [94]. JAKs-mediated phosphorylation of STAT proteins results in STAT assembly in dimeric or oligomeric forms, which translocate to the nucleus, where they can regulate gene expression via DNA binding motifs called either γ-activated sequence (GAS) elements (recognized by STAT1 homodimers) or IFN-stimulated response element (ISRE, recognized by STAT1-STAT2 heterodimers) [95, 96]. Up-regulation of STAT1 and STAT1-dependent genes such as ICAM-1 and IFN regulatory Factor-1 (IRF-1) are observed in asthmatic airways suggesting the potential contribution of IFN-associated JAK/STATs in the regulation of immuno-modulatory genes associated with asthma [97].
3.1 Modulation of ASM synthetic functions by IFNs
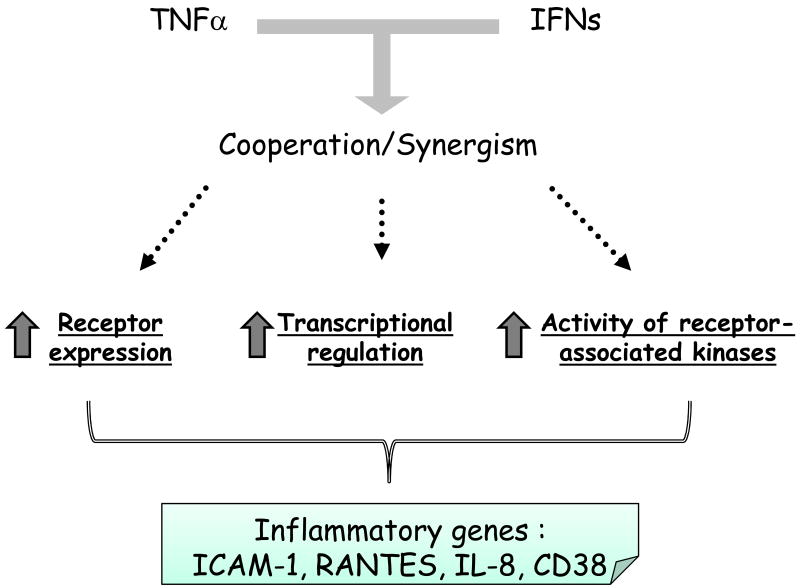
IFNs regulate many cellular responses in human ASM cells: IFN-γ induces the expression of ICAM-1 and VCAM-1 [98], the CysLT1 receptor [99] and the secretion of nerve growth factor in ASM cells [100]. IFN-γ also synergizes with TNF-α to augment expression of CD38 [101] and several chemokines including RANTES, IP-10 and fractalkine [34, 102, 103]. Most studies that used a combination of IFN-γ and TNF-α showed that the synergistic action involves several molecular mechanisms. In some instances, their co-operativity may be explained by the IFN-γ-induced up-regulation of TNF-α receptors [104] or vice-versa [105] (Figure 3). Furthermore, both cytokines may collaborate at the gene level by increasing promoter activation through a synergistic interaction between transcription factors activated by IFN-γ (STATs, IRF-1) and TNF-α (NF-κB) [106, 107] (Figure 3). These amplifying properties of IFN-γ may explain, at least in part, why viral infection, which increases production of IFNs, is an important trigger for asthma and chronic obstructive pulmonary disease exacerbation [108]. Another mechanism of co-operation could be secondary induction of IFN-β, which has been shown to mediate TNF-α induced RANTES and CD38 expression [101, 109] (Figure 3) (see below).
Figure 3. Schematic overview of the mechanism underlying TNF-α and IFN-γ synergism.
IFN-γ and TNF-α synergistically modulate the expression of different inflammatory genes such ICAM-1, RANTES, IL-8 and CD38. Their cooperativity may be explained at the receptor level by the IFNγ-induced up-regulation of TNF-α receptors or vice-versa. Alternatively, both cytokines may collaborate at the gene level by increasing promoter activation through a synergistic interaction between transcription factors activated by IFN-γ (STATs, IRF-1) and TNF-α (NF-κB). Another mechanism underlying such cooperation could be the induction of defined genes by TNF-α via activation of the autocrine action of IFN-β.
In some instances, however, IFNs may antagonize TNF-α inflammatory responses by inhibiting the NF-κB pathway. Indeed, Keslacy and colleagues recently reported that IFN-γ potently inhibits TNF-α-induced NF-κB-dependent genes including IL-6, IL-8 and eotaxin in ASM cells [90]. Multiple mechanisms underlying IFNs inhibitory effect on NF-κB pathways have been proposed including inhibition of NF-κB DNA binding, prevention of IκB degradation, or regulation of TNF-α receptor 1 via STAT interaction [110]. Specifically, in ASM cells, IFN-γ inhibits the transcriptional activity of NF-κB by reducing the acetylation level of p65 [90].
3.2 Autocrine IFN-β regulates pro-asthmatic gene expression in ASM cells
In ASM cells, TNF-α is able to activate JAK1 and Tyk2, and STAT1- and STAT2-dependent gene expression via the autocrine action of IFN-β [109]. Indeed, autocrine IFN-β regulates i) TNF-α-induced inflammatory gene expression, by suppressing IL-6 and promoting RANTES secretion and ii) TNF-α-associated airway hyper-responsiveness, by potentiating the ability for TNF-α to enhance GPCR-dependent contractile responses [111, 112].
The putative implication of IFN-β in lung diseases is supported by the heightened expression of IFN-β in the airways in mouse models of allergic asthma [111]. We therefore propose that the functional cross talk between type I and II IFNs and TNF-α in lung structural cells, particularly the ASM, is a novel axis in the pathogenesis of lung diseases, although a similar phenomenon could also occur in other cell types (such as hemopoietic cells). A recent study by Ivashkiv and colleagues recently confirmed the inflammatory potential of IFN-β/TNF-α interaction in macrophages [113]. This elegant study showed that IFN-β-mediated autocrine loops were essential for maintaining TNF-α-induced inflammatory genes that prime macrophages for augmented responses to additional stimulation by cytokines and toll-like receptors agonists. In previous studies performed in 3T3-L1 adipocytes, TNF-α was shown to induce phosphorylation of STAT1 by directly interacting with both JAK1 and JAK2 [114], whereas in Hela cells, STAT1 was shown to physically interact with TNFR1 and the adaptor proteins TNF receptor-associated death domain (TRADD), but not TRAF-2 [115]. TNF-α also induces STAT1 phosphorylation at serine 727 in macrophages.
In Summary, ASM-derived IFN-β is a novel signaling component of TNF-α inducible genes involved in airway inflammation (Figure 3) and regulation of airway hyper-responsiveness [111].
3.3 IFNs interference with ASM steroid responsiveness
Most anti-inflammatory effects of steroids are mediated via the glucocorticoid receptor alpha isoform (GRα), which suppresses expression of inflammatory genes through mechanisms known as transactivation or transrepression [116]. As a result of alternative splicing mechanisms, another glucocorticoid receptor isoform, namely GRβ, has been described [117]. We and others recently showed that treatment of ASM cells with the specific combination of IFNs with TNF-α impairs the ability of steroids to inhibit the expression of various proinflammatory genes such as CD38, RANTES and ICAM-1 by a mechanism involving the up-regulation of GRβ isoform [118]. Interestingly, steroids augment IFN-γ/TNF-α induced fractalkine and TLR2 expression in ASM [34, 119]: whether this involves similar mechanisms involved in the attenuation of corticosteroid activity by IFN-γ/TNF-α remains to be established. Although the pathological role of the GRβ isoform is not well understood, previous reports demonstrate a strong correlation between steroid resistance in individuals with asthma and the expression levels of GRβ [120]. More importantly, increased GRβ in the airways has been detected in patients who died of asthma [121]. Indeed, by its ability to act as a dominant-negative inhibitor of steroid action in other cell types [122], GRβ has been associated with steroid resistance in different inflammatory diseases [123]. GRβ over-expression in ASM cells also prevents the capacity for steroids to induce transactivation activity and inhibit cytokine-induced pro-inflammatory gene expression [118].
Interestingly, short-term treatment of ASM cells with IFNs and TNF-α inhibits, in a GRβ-independent manner, the capacity for steroids to induce transactivation partially through the cellular accumulation of IRF-1 [124]. IRF-1 is an early response gene involved in diverse transcriptional regulatory processes [125]. Interestingly, a strong association was found between IRF-1 polymorphism and childhood atopic asthma [126]. Early steroid dysfunction seen after short incubation with IFNs and TNFα could be reproduced by enhancing IRF-1 cellular levels using constitutively active IRF-1 which dose-dependently inhibited glucocorticoid response element (GRE)-dependent gene transcription [124]. Consistently, reducing IRF-1 cellular levels using siRNA approach in TNF/IFN-treated ASM cells significantly restored steroid transactivation activities. These findings demonstrate for the first time that IRF-1 is a novel alternative GRβ-independent mechanism mediating steroid dysfunction induced by proinflammatory cytokines. The fact that different studies showed that the expression of IRF-1 was largely increased after viral infections [127] combined with the suppressive effect of IRF-1 on steroid signaling in ASM cells [124], may explain the reduced steroid responsiveness seen in asthmatic patients experiencing viral infections [128].
4. TLRs in chronic inflammatory airways disease
TLRs may be considered as a ‘sensing’ system that protects the host from infectious and non-infectious tissue injury and inflammation. TLRs also serve a homeostatic role to maintain tissue integrity and regeneration. TLRs ‘sense’ diverse molecules including microbial products and endogenous ligands generated in response to cell stress or injury. Currently, there are 10 known human TLRs named TLR1 through TLR10. TLR2 and TLR4, which primarily mediate recognition of bacterial cell wall components (eg LPS – the major ligand for TLR4) and endogenous ‘danger signals’ (eg heat shock proteins, extracellular matrix fragments) are the best studied of this receptor family. TLR3, TLR7 and TLR8 mediate recognition of viral RNA whilst TLR9 mediates recognition of bacterial DNA containing CpG motifs. Activation of TLRs triggers the activation of immune and inflammatory responses through NF-κB, IRF3/7 and MAP kinase dependent signaling pathways [6].
Epidemiological studies suggest that genetic polymorphisms in TLR genes, together with early-life exposure to environmental TLR stimulants (e.g. LPS in house dust, microbial exposure associated with certain farming activities, respiratory viral infections) are likely to be important, but also very complex, determinants of asthma incidence and severity. On the converse, emerging evidence shows that allergic airway inflammation impairs innate host-defense mechanisms, including TLR function, which results in impaired bacterial clearance [129, 130]. This may offer some explanation for increased bacterial colonization in asthmatic lungs and also provides some basis for infective exacerbations of asthma. Emerging evidence also indicates a role for TLR4 in the airway inflammatory response to cigarette smoke exposure, the primary causative factor of COPD [131-133].
The demonstration of functional TLR expression in human ASM cells over the past few years adds to the growing body of evidence of the immuno-modulatory capabilities of ASM cells. This has wide-ranging implications for the disease process in asthma and COPD, as activation of TLRs in ASM may exacerbate airway inflammatory responses by inducing expression of cell adhesion molecules and release of cytokines and chemokines, and may also amplify ASM-inflammatory cell interactions.
4.1 TLR expression in ASM cells
Human ASM cells in culture express TLR1 through TLR10 mRNA under basal conditions. TLR2, TLR3 and TLR6 are the most highly expressed, whilst TLR4 is the least expressed [119]. Interestingly, in one study, constitutive expression of TLR7 or TLR8 was not demonstrated [134]. Whether this was due to cell donor differences, type of ASM cells used (eg tracheal vs bronchial; distal vs proximal) or methodological issues remains to be resolved. In addition to evidence of TLR gene expression, cell surface and intracellular protein expression for both TLR2 and TLR3 has also been demonstrated [119, 135]. TLR2, TLR3 and TLR4 expression in ASM cells is up-regulated in response to inflammatory cytokines including IL-1β, TNF-α and IFN-γ, and microbial products including LPS and dsRNA. Combined stimulation with IFN-γ and TNF-α has synergistic and additive effects on TLR2 and TLR4 mRNA expression, respectively [119, 135].
4.2 TLR activation and ASM inflammatory gene expression
Evidence of TLR expression in ASM has fuelled recent interest in ASM cell inflammatory responses to TLR ligands. Stimulation of ASM cells with synthetic TLR2 ligands, LPS or poly IC induces the production of various cytokines and chemokines [39, 119, 134-136]; Pam3CSK4 (a synthetic bacterial lipopeptide) and FSL-1 (S-(2,3-bispalmitoyloxypropyl)-Cys-Gly-Asp-Pro-Lys-His-Pro-Ser-Phe), which activate TLR2/TLR1 and TLR2/TLR6 heterodimers, respectively, induce IL-8 release; LPS induces expression of IL-6, IL-8 and eotaxin; and polyriboinosinc-polyribocytidylic acid (poly IC) induces expression of IL-6, IL-8, eotaxin, RANTES and IP-10. Stimulation of ASM cells with poly IC together with IL-1β or TNF-α has synergistic effects on IL-6, IL-8, IP-10 and RANTES release. Interestingly, poly IC induced eotaxin expression is inhibited in the presence of IL-1β or TNF-α, but is augmented by the Th2 cytokine IL-4.
The specific activation of TLR2 in mediating IL-8 release has not been confirmed in ASM cells, although anti-TLR2 or transfection with a dominant negative mutant form of TLR2 inhibits ERK1/2 signaling in response to the microbial derived TLR2 ligand lipoteichoic acid (LTA), thus providing functional evidence of TLR2 activation in ASM cells [137]. Although ASM cells express TLR3 on the cell surface and in intracellular endosomes, specific activation of endosomal rather than surface TLR3 was shown to be responsible for poly IC mediated eotaxin release [135]. The specific activation of TLR4 in mediating LPS-induced cytokine and chemokine release in ASM cells remains to be established.
In addition to inducing ASM cell cytokine and chemokine release, activation of TLRs in ASM cells may also amplify airway inflammatory responses by facilitating ASM-inflammatory cell interactions. This is demonstrated by studies showing that addition of TLR2, TLR4, TLR7 or TLR8 ligands to ASM cells in co-culture with peripheral blood mononuclear cells (PBMCs) leads to greater release of IL-6, IL-8 and CCL2 compared to TLR-activation of either cell type alone [134, 138]. IL-1β produced by LPS-activated monocytes was shown to be responsible, to some extent, for amplification of ASM-PBMC inflammatory responses [138]. Poly IC and LPS may also promote ASM-inflammatory cell interactions via inducing the expression of cell adhesion molecules such as ICAM-1 and VCAM-1, respectively [134]. Indeed, LPS has been shown to mediate VCAM-1-induced neutrophil adhesion in ASM cells [81].
In vitro infection of human ASM cells with respiratory viruses such as rhinovirus or respiratory syncytial virus leads to production of several cytokines and chemokines including IL-1β, IL-6, IL-8 and IL-11 [139-141]. The role of TLRs in mediating these responses has not as yet been addressed, although it is likely that viral-sensing TLRs as well as other intracellular viral recognition proteins such as protein kinase R, and cell-surface molecules such as ICAM-1 (which is a receptor for rhinovirus) are involved. Whether infection of ASM cells with respiratory viruses, or indeed other microbial pathogens that colonize the lungs in asthma and COPD, occurs in vivo is an important area of further investigation; especially given the potential impact of microbial-TLR interactions on ASM inflammatory responses.
Activation of TLRs in ASM occurs not only in response to microbial-derived products but may also occur in response to endogenous molecules present within the inflammatory milieu. Recently, it was shown that neutrophil-derived elastase (NE) activates ASM cells to synthesize TGF-β via a mechanism involving TLR4 and its associated down-stream signaling cascade. However, stimulation of TGF-β synthesis by NE was only partially inhibited by a TLR4-blocking antibody indicating that other mechanisms or perhaps TLRs may be involved. Interestingly, TLR4 protein expression on ASM was reduced following treatment with NE, indicating that NE-dependent TLR4 responses may require internalization of the receptor [79].
Although our understanding of the role of TLRs in the pathogenesis of asthma and COPD is only just evolving, evidence of their pro-inflammatory functions in ASM further extends the role of ASM as a critical mediator of the airway inflammatory response, potentially having the capacity to respond to environmental as well as endogenous molecules involved in the perpetuation and exacerbation of airway inflammatory disease. Studies of the expression and function of TLRs in ASM cells in vivo is an important area of future research.
Conclusions
Cytokines play a principal role in modulating inflammatory as well as immune responses in chronic inflammatory diseases such as asthma and COPD. Pro-inflammatory and immunomodulatory cytokines activate multiple signaling cascades in ASM cells that lead to amplification of ASM inflammatory responses. Research over the past decade has taken us forward in our understanding of MAPK, NF-κB and JAK/STAT signaling mechanisms involved in regulating ASM inflammatory gene expression and studies in animal models show that specific targeting of these pathways offer therapeutic potential for the treatment of chronic inflammatory airways disease [32, 68, 142-144]. Whilst there is some advantage in targeting these signaling pathways in isolation, further understanding of the cross-talk mechanisms and pathway interactions that exacerbate inflammatory responses or impair steroid responsiveness in ASM cells may provide novel targets or approaches for the future therapy of chronic inflammatory airways disease.
TLR ligands represent potentially exciting new therapeutic approaches for the treatment of asthma. Indeed, several studies published in the last five years demonstrate protective effects of TLR2, TLR3, TLR4, TLR7/8 and TLR9 ligands against allergic airway inflammation, airway hyperreactivity and airway remodeling in animal models of asthma [145-152]. The mechanisms that underlie protection against asthma in these models are slowly being unraveled and studies so far have focused on delineating immuno-modulatory pathways. However, evidence that the synthetic TLR7/8 ligand R-848 imparts some of its protection against airway remodeling by inhibiting ASM proliferation [152] indicates that the ASM is a potential target of immuno-modulatory therapy. An understanding of the signaling pathways regulating TLR-dependent inflammatory responses in ASM is an important area of further investigation.
Acknowledgments
Supported by National Institutes of Health grant 1 K99 HL089409-01 (to Dr. Tliba), American Lung Association grant RG-49342-N (to Dr. Tliba.). Dr Tliba is a Parker B. Francis Fellow in Pulmonary Research.
List of Abbreviations
- ASM
ASM, Airway Smooth Muscle
- ATF-2
Activating Transcription Factor-2
- bFGF
Basic fibroblast growth factor
- CBP
CREB binding protein
- COPD
Chronic obstructive pulmonary disease
- CREB
cAMP response element-binding protein
- DsRNA
Double-stranded RNA
- EGF
Epidermal growth factor receptor
- ERK
Extracellular signal-regulated kinase
- ENA-78
Epithelial Neutrophil Activating Peptide-78
- ET-1
Endothelin-1
- FSL-1
S-(2,3-bispalmitoyloxypropyl)-Cys-Gly-Asp-Pro-Lys-His-Pro-Ser-Phe, TLR2 ligand
- GAS
Gamma-activated sequence
- GM-CSF
Granulocyte colony-stimulating factor
- GRE
Glucocorticoid response element
- GROα
Growth-related oncogene protein-alpha
- ICAM-1
Intercellular Adhesion Molecule-1
- IFN
Interferon
- IKK
IκB kinase
- IL
Interleukin
- IL-5R
Interleukin 5 receptor
- IL-17BR
Interleukin 17B receptor
- IRF
IFN-regulatory factor
- ISRE
IFN-stimulated response element
- JAK
Janus kinase
- JNK
C-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- LTA
Lipoteichoic acid
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemotactic protein-1
- NE
Neutrophil-derived elastase
- NEMO
NF-kappaB Essential Modulator
- NF-κB
Nuclear factor-kappa B
- ML120B
N-(6-chloro-7-methoxy-9H-beta-carbolin-8-yl)-2-methylnicotinamide, a potent and selective small molecule inhibitor of IKK2.NAF-2 Neutrophil Activating Protein-2
- Pam3CSK4
Synthetic bacterial lipopeptide
- p/CAF
p300-CBP coactivated factor
- PDGF
Platelet-derived growth factor
- Poly IC
Polyriboinosinic polyribocytidylic acid
- PS1145
N-(6-Chloro-9H-pyrido[3,4-b]indol-8-yl)-3-pyridinecarboxamide dihydrochloride, selective inhibitor of IκB kinase
- RANTES
Regulated on Activation, Normal T Expressed and Secreted
- RIP
Fas/TNFα related receptor interacting protein
- siRNA
Small interfering RNA
- SRC
Steroid receptor coactivator
- STAT
Signal Transducers and Activators of Transcription
- TAK1
TGFbeta1-activated kinase 1
- TCR
T cell receptor
- TLR
Toll-like receptor
- TNFR1
TNF receptor 1
- TPCA-1
IKK2 inhibitor: 2-[aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide)
- TRADD
TNFR1-associated death domain
- TRAF2
TNF receptor-associated factor 2
- VCAM-1
Vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lukacs NW, Strieter RM, Kunkel SL. Leukocyte infiltration in allergic airway inflammation. Am J Respir Cell Mol Biol. 1995;13:1–6. doi: 10.1165/ajrcmb.13.1.7598934. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TNC, Proudfoot A, et al. The Coordinated Action of CC Chemokines in the Lung Orchestrates Allergic Inflammation and Airway Hyperresponsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wegmann M, Goggel R, Sel S, Sel S, Erb KJ, Kalkbrenner F, et al. Effects of a Low-Molecular-Weight CCR-3 Antagonist on Chronic Experimental Asthma. Am J Respir Cell Mol Biol. 2007;36:61–67. doi: 10.1165/rcmb.2006-0188OC. [DOI] [PubMed] [Google Scholar]
- 4.Kanehiro A, Lahn M, Makela MJ, Dakhama A, Joetham A, Rha YH, et al. Requirement for the p75 TNF-α Receptor 2 in the Regulation of Airway Hyperresponsiveness by γδ T Cells. J Immunol. 2002;169:4190–4197. doi: 10.4049/jimmunol.169.8.4190. [DOI] [PubMed] [Google Scholar]
- 5.Howarth PH, Knox AJ, Amrani Y, Tliba O, Panettieri RA, Johnson M. Synthetic responses in airway smooth muscle. J Allergy Clin Immunol. 2004;114:S32–S50. doi: 10.1016/j.jaci.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Sukkar MB, Chung KF. Expression and function of toll-like receptors in smooth muscle cells. In: Savineau J, editor. New Frontiers in Smooth Muscle Biology and Physiology. Transworld Research Network; 2007. pp. 411–430. [Google Scholar]
- 7.Gerthoffer WT, Singer CA. MAPK regulation of gene expression in airway smooth muscle. Respir Physiol Neurobiol. 2003;137:237–250. doi: 10.1016/s1569-9048(03)00150-2. [DOI] [PubMed] [Google Scholar]
- 8.Shaul YD, Seger R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochimica et Biophysica Acta (BBA) - Mol Cell Res. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Faffe DS, Flynt L, Mellema M, Moore PE, Silverman ES, Subramaniam V, et al. Oncostatin M causes eotaxin-1 release from airway smooth muscle: Synergy with IL-4 and IL-13. J Allergy Clin Immunol. 2005;115:514–520. doi: 10.1016/j.jaci.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Ravenhall C, Guida E, Harris T, Koutsoubos V, Stewart A. The importance of ERK activity in the regulation of cyclin D1 levels and DNA synthesis in human cultured airway smooth muscle. Br J Pharmacol. 2000;131:17–28. doi: 10.1038/sj.bjp.0703454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faffe DS, Flynt L, Mellema M, Whitehead TR, Bourgeois K, Panettieri RA, Jr, et al. Oncostatin M causes VEGF release from human airway smooth muscle: synergy with IL-1β. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1040–1048. doi: 10.1152/ajplung.00333.2004. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Johnson PRA, Roth M, Hunt NH, Black JL. ERK activation and mitogenesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1019–1029. doi: 10.1152/ajplung.2001.280.5.L1019. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher MD, Abe MK, Chao TS, Jain M, Green JM, Solway J, et al. Role of MAP kinase activation in bovine tracheal smooth muscle mitogenesis. Am J Physiol Lung Cell Mol Physiol. 1995;268:L894–901. doi: 10.1152/ajplung.1995.268.6.L894. [DOI] [PubMed] [Google Scholar]
- 14.Orsini MJ, Krymskaya VP, Eszterhas AJ, Benovic JL, Panettieri RA, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol. 1999;277:L479–L488. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]
- 15.Hallsworth MP, Moir LM, Lai D, Hirst SJ. Inhibitors of mitogen-activated protein kinases differentially regulate eosinophil-activating cytokine release from human airway smooth muscle. Am J Respir Crit Care Med. 2001;164:688–697. doi: 10.1164/ajrccm.164.4.2011004. [DOI] [PubMed] [Google Scholar]
- 16.Gounni AS, Hamid Q, Rahman SM, Hoeck J, Yang J, Shan L. IL-9-Mediated Induction of Eotaxin1/CCL11 in Human Airway Smooth Muscle Cells. J Immunol. 2004;173:2771–2779. doi: 10.4049/jimmunol.173.4.2771. [DOI] [PubMed] [Google Scholar]
- 17.Moore PE, Church TL, Chism DD, Panettieri RA, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol. 2002;282:L847–L853. doi: 10.1152/ajplung.00245.2001. [DOI] [PubMed] [Google Scholar]
- 18.Peng Q, Matsuda T, Hirst SJ. Signaling Pathways Regulating Interleukin-13-stimulated Chemokine Release from Airway Smooth Muscle. Am J Respir Crit Care Med. 2004;169:596–603. doi: 10.1164/rccm.200307-888OC. [DOI] [PubMed] [Google Scholar]
- 19.Lajoie-Kadoch S, Joubert P, Letuve S, Halayko AJ, Martin JG, Soussi-Gounni A, et al. TNF-α and IFN-γ inversely modulate expression of the IL-17E receptor in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1238–1246. doi: 10.1152/ajplung.00301.2005. [DOI] [PubMed] [Google Scholar]
- 20.Mittelstadt PR, Salvador JM, Fornace AJ, Ashwell JD. Activating p38 MAPK: new tricks for an old kinase. Cell Cycle. 2005;4:1189–1192. doi: 10.4161/cc.4.9.2043. [DOI] [PubMed] [Google Scholar]
- 21.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes DJ, Ravenhall CE, Harris T, Tran T, Vlahos R, Stewart AG. Contribution of the p38MAPK signalling pathway to proliferation in human cultured airway smooth muscle cells is mitogen-specific. Br J Pharmacol. 2004;142:1182–1190. doi: 10.1038/sj.bjp.0705809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1beta-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1023–1029. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 24.Henness S, Johnson CK, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A augments TNF-α-induced IL-6 expression in airway smooth muscle by enhancing mRNA stability. J Allergy Clin Immunol. 2004;114:958–964. doi: 10.1016/j.jaci.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Henness S, van Thoor E, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A acts via p38 MAPK to increase stability of TNF-α-induced IL-8 mRNA in human ASM. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1283–1290. doi: 10.1152/ajplung.00367.2005. [DOI] [PubMed] [Google Scholar]
- 26.Rahman MS, Yamasaki A, Yang J, Shan L, Halayko AJ, Gounni AS. IL-17A Induces Eotaxin-1/CC Chemokine Ligand 11 Expression in Human Airway Smooth Muscle Cells: Role of MAPK (Erk1/2, JNK, and p38) Pathways. J Immunol. 2006;177:4064–4071. doi: 10.4049/jimmunol.177.6.4064. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Tliba O, Panettieri RA, Amrani Y. Bradykinin induces interleukin-6 production in human airway smooth muscle cells. Modulation by Th2 cytokines and dexamethasone Am J Respira Cell Mol Biol. 2003;28:330–338. doi: 10.1165/rcmb.2002-0040OC. [DOI] [PubMed] [Google Scholar]
- 28.Hedges JC, Singer CA, Gerthoffer WT. Mitogen-Activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol. 2000;23:86–94. doi: 10.1165/ajrcmb.23.1.4014. [DOI] [PubMed] [Google Scholar]
- 29.Amrani Y, Ammit AJ, Panettieri RA. Tumor necrosis factor receptor (TNFR) 1, but not TNFR2, mediates Tumor necrosis factor-a-induced interleukin-6 and RANTES in human airway smooth muscle cells: Role of p38 and p42/44 mitogen activated protein kinases. Mol Pharmacol. 2001;60:646–655. [PubMed] [Google Scholar]
- 30.Salh B. c-Jun N-terminal kinases as potential therapeutic targets. Expert Opin Ther Targets. 2007;11:1339–1353. doi: 10.1517/14728222.11.10.1339. [DOI] [PubMed] [Google Scholar]
- 31.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Eynott PR, Nath P, Leung SY, Adcock IM, Bennett BL, Chung KF. Allergen-induced inflammation and airway epithelial and smooth muscle cell proliferation: role of Jun N-terminal kinase. Br J Pharmacol. 2003;140:1373–1380. doi: 10.1038/sj.bjp.0705569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oltmanns U, Issa R, Sukkar MB, John M, Chung KF. Role of c-jun N-terminal kinase in the induced release of GM-CSF, RANTES and IL-8 from human airway smooth muscle cells. Bri J Pharmacol. 2003;139:1228–1234. doi: 10.1038/sj.bjp.0705345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukkar MB, Issa R, Xie S, Oltmanns U, Newton R, Chung KF. Fractalkine/CX3CL1 production by human airway smooth muscle cells: induction by IFN-γ and TNF-α and regulation by TGF-β and corticosteroids. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1230–1240. doi: 10.1152/ajplung.00014.2004. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Khalil N. TGF-β1 increases proliferation of airway smooth muscle cells by phosphorylation of map kinases. Respir Res. 2006;7:2. doi: 10.1186/1465-9921-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirumurugaan KG, Jude JA, Kang BN, Panettieri RA, Walseth TF, Kannan MS. TNF-α induced CD38 expression in Human Airway Smooth Muscle Cells: Role of MAP kinases and Transcription Factors NF-κB and AP-1. Am J Physiol Lung Cell Mol Physiol. 2007:00472–02006. doi: 10.1152/ajplung.00472.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kelly EA, Busse WW, Jarjour NN. Increased Matrix Metalloproteinase-9 in the Airway after Allergen Challenge. Am J Respir Crit Care Med. 2000;162:1157–1161. doi: 10.1164/ajrccm.162.3.9908016. [DOI] [PubMed] [Google Scholar]
- 38.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, et al. Interleukin-1β induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-κB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol. 2007;211:759–770. doi: 10.1002/jcp.20992. [DOI] [PubMed] [Google Scholar]
- 39.Shan X, Hu A, Veler H, Fatma S, Grunstein JS, Chuang S, et al. Regulation of Toll-like receptor 4-induced proasthmatic changes in airway smooth muscle function by opposing actions of ERK1/2 and p38 MAPK signaling. Am J Physiol Lung Cell Mol Physiol. 2006;291:L324–333. doi: 10.1152/ajplung.00056.2006. [DOI] [PubMed] [Google Scholar]
- 40.Baldwin AS. Series introduction: the transcription factor NF-κB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hart Lorraine A, Krishnan Vijaya L, Adcock Ian M, Barnes Peter J, Chung KF. Activation and Localization of Transcription Factor, Nuclear Factor-kappa B, in Asthma. Am J Respir Crit Care Med. 1998;158:1585–1592. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 42.Wilson SJ, Wallin A, Della-Cioppa G, Sandstrom T, Holgate ST. Effects of Budesonide and Formoterol on NF-kappa B, Adhesion Molecules, and Cytokines in Asthma. Am J Respir Crit Care Med. 2001;164:1047–1052. doi: 10.1164/ajrccm.164.6.2010045. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, Qi Y, Liu X, Jiang Q, Liu S, Jiang Y, et al. Activation of NF-κB in bronchial epithelial cells from children with asthma. Chin Med J. 2001;114:909–911. [PubMed] [Google Scholar]
- 44.Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The Allergen Der p1 Induces NF-kB Activation through Interference with IκBα Function in Asthmatic Bronchial Epithelial Cells. Biochem Biophys Res Commun. 1997;236:522–526. doi: 10.1006/bbrc.1997.6997. [DOI] [PubMed] [Google Scholar]
- 45.Gagliardo R, Chanez P, Mathieu M, Bruno A, Costanzo G, Gougat C, et al. Persistent Activation of Nuclear Factor-κB Signaling Pathway in Severe Uncontrolled Asthma. Am J Respir Crit Care Med. 2003;168:1190–1198. doi: 10.1164/rccm.200205-479OC. [DOI] [PubMed] [Google Scholar]
- 46.Bureau F, Delhalle S, Bonizzi G, Fievez L, Dogne S, Kirschvink N, et al. Mechanisms of Persistent NF-κB Activity in the Bronchi of an Animal Model of Asthma. J Immunol. 2000;165:5822–5830. doi: 10.4049/jimmunol.165.10.5822. [DOI] [PubMed] [Google Scholar]
- 47.Lin CC, Lin CY, Ma HY. Pulmonary function changes and increased Th-2 cytokine expression and nuclear factor κB activation in the lung after sensitization and allergen challenge in brown Norway rats. Immunol Lett. 2000;73:57–64. doi: 10.1016/s0165-2478(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 48.Poynter ME, Irvin CG, Janssen-Heininger YMW. Rapid Activation of Nuclear Factor-κB in Airway Epithelium in a Murine Model of Allergic Airway Inflammation. Am J Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Zhang Z, Xu Y. Effects of interleukin-18 on asthmatic airway inflammation and nuclear factor kappa-B in murine models. Chin Med J. 2003;116:323–327. [PubMed] [Google Scholar]
- 50.Donovan CE, Mark DA, He HZ, Liou HC, Kobzik L, Wang Y, et al. NF-κB/Rel Transcription Factors: c-Rel Promotes Airway Hyperresponsiveness and Allergic Pulmonary Inflammation. J Immunol. 1999;163:6827–6833. [PubMed] [Google Scholar]
- 51.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential Role of Nuclear Factor kappa B in the Induction of Eosinophilia in Allergic Airway Inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 53.Jeong Dw, Yoo MH, Kim TS, Kim JH, Kim IY. Protection of Mice from Allergen-induced Asthma by Selenite. prevention of eosinophil infiltration by inhibition of NF-κB activation J Biol Chem. 2002;277:17871–17876. doi: 10.1074/jbc.M200808200. [DOI] [PubMed] [Google Scholar]
- 54.Huang TJ, Adcock IM, Chung KF. A novel transcription factor inhibitor, SP100030, inhibits cytokine gene expression, but not airway eosinophilia or hyperresponsiveness in sensitized and allergen-exposed rat. Br J Pharmacol. 2001;134:1029–1036. doi: 10.1038/sj.bjp.0704344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson WR, Jr, Chi EY, Teo JL, Nguyen C, Kahn M. A Small Molecule Inhibitor of Redox-Regulated NF-κB and Activator Protein-1 Transcription Blocks Allergic Airway Inflammation in a Mouse Asthma Model. J Immunol. 2002;169:5294–5299. doi: 10.4049/jimmunol.169.9.5294. [DOI] [PubMed] [Google Scholar]
- 56.Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-κB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004;4:1817–1828. doi: 10.1016/j.intimp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 57.Hayden MS, Ghosh S. Shared Principles in NF-κB Signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Siebenlist U, Franzoso G, Brown K. Structure, Regulation and Function of NF-κB. Ann Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 59.Hacker H, Karin M. Regulation and Function of IKK and IKK-Related Kinases. Sci STKE 2006. 2006 doi: 10.1126/stke.3572006re13. re13. [DOI] [PubMed] [Google Scholar]
- 60.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li Jw, et al. IKK-1 and IKK-2: Cytokine-Activated IκB Kinases Essential for NF-B Activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 61.Hu MCT, Wang YP, Qiu WR, Mikhail A, Meyer CF, Tan TH. Hematopoietic progenitor kinase-1 (HPK1) stress response signaling pathway activates IkappaB kinases (IKK-alpha/beta) and IKK-beta is a developmentally regulated protein kinase. Oncogene. 1999;18:5514–5524. doi: 10.1038/sj.onc.1202740. [DOI] [PubMed] [Google Scholar]
- 62.Li Q, Antwerp DV, Mercurio F, Lee KF, Verma IM. Severe Liver Degeneration in Mice Lacking the IκB Kinase 2 Gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 63.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, et al. The IKKbeta Subunit of IκB Kinase (IKK) is Essential for Nuclear Factor kappa B Activation and Prevention of Apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, et al. Limb and Skin Abnormalities in Mice Lacking IKK. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 65.Adcock IM, Chung KF, Caramori G, Ito K. Kinase inhibitors and airway inflammation. Eur J Pharmacol. 2006;533:118–132. doi: 10.1016/j.ejphar.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 66.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-κB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Catley MC, Sukkar MB, Chung KF, Jaffee B, Liao SM, Coyle AJ, et al. Validation of the Anti-Inflammatory Properties of Small-Molecule IκB Kinase (IKK)-2 Inhibitors by Comparison with Adenoviral-Mediated Delivery of Dominant-Negative IKK1 and IKK2 in Human Airways Smooth Muscle. Mol Pharmacol. 2006;70:697–705. doi: 10.1124/mol.106.023150. [DOI] [PubMed] [Google Scholar]
- 68.Birrell MA, Hardaker E, Wong S, McCluskie K, Catley M, De Alba J, et al. Iκ-B Kinase-2 Inhibitor Blocks Inflammation in Human Airway Smooth Muscle and a Rat Model of Asthma. Am J Respir Crit Care Med. 2005;172:962–971. doi: 10.1164/rccm.200412-1647OC. [DOI] [PubMed] [Google Scholar]
- 69.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, et al. The Lymphotoxin-β Receptor Induces Different Patterns of Gene Expression via Two NF-κB Pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 70.Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, et al. The IκB Function of NF-κB2 p100 Controls Stimulated Osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKα of a Second, Evolutionary Conserved, NF-κB Signaling Pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 72.Lawrence T, Bebien M. IKK alpha in the regulation of inflammation and adaptive immunity. BiochemSoc Transac. 2007;35:270–272. doi: 10.1042/BST0350270. [DOI] [PubMed] [Google Scholar]
- 73.Naumann M, Scheidereit C. Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ito K, Charron CE, Adcock IM. Impact of protein acetylation in inflammatory lung diseases. Pharmacol Ther. 2007;116:249–265. doi: 10.1016/j.pharmthera.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, et al. Transcriptional Activation by NF-κB Requires Multiple Coactivators. Mol Cell Biol. 1999;19:6367–6378. doi: 10.1128/mcb.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wuyts WA, Vanaudenaerde BM, Dupont LJ, Van Raemdonck DE, Demedts MG, Verleden GM. Interleukin-17-Induced Interleukin-8 Release in Human Airway Smooth Muscle Cells: Role for Mitogen-Activated Kinases and Nuclear Factor-κB. J Heart Lung Transplant. 2005;24:875–881. doi: 10.1016/j.healun.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Issa R, Xie S, Lee KY, Stanbridge RD, Bhavsar P, Sukkar MB, et al. GRO-α regulation in airway smooth muscle by IL-1beta and TNF-α: role of NF-κB and MAP kinases. Am J Physiol Lung Cell Mol Physiol. 2006;291:L66–74. doi: 10.1152/ajplung.00384.2005. [DOI] [PubMed] [Google Scholar]
- 79.Lee KY, Ho SC, Lin HC, Lin SM, Liu CY, Huang CD, et al. Neutrophil-Derived Elastase Induces TGF-beta1 Secretion in Human Airway Smooth Muscle via NF-κB Pathway. Am J Respir Cell Mol Biol. 2006;35:407–414. doi: 10.1165/rcmb.2006-0012OC. [DOI] [PubMed] [Google Scholar]
- 80.Lee CW, Lin WN, Lin CC, Luo SF, Wang JS, Pouyssegur J, et al. Transcriptional regulation of VCAM-1 expression by tumor necrosis factor-α in human tracheal smooth muscle cells: Involvement of MAPKs, NF-κB, p300, and histone acetylation. J Cell Physiol. 2006;207:174–186. doi: 10.1002/jcp.20549. [DOI] [PubMed] [Google Scholar]
- 81.Lin WN, Luo SF, Lee CW, Wang CC, Wang JS, Yang CM. Involvement of MAPKs and NF-κB in LPS-induced VCAM-1 expression in human tracheal smooth muscle cells. Cell Signal. 2007;19:1258–1267. doi: 10.1016/j.cellsig.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Wang CC, Lin WN, Lee CW, Lin CC, Luo SF, Wang JS, et al. Involvement of p42/p44 MAPK, p38 MAPK, JNK, and NF-κB in IL-1β-induced VCAM-1 expression in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L227–237. doi: 10.1152/ajplung.00224.2004. [DOI] [PubMed] [Google Scholar]
- 83.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, et al. NF-κB Activation in Airways Modulates Allergic Inflammation but Not Hyperresponsiveness. J Immunol. 2004;173:7003–7009. doi: 10.4049/jimmunol.173.11.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, et al. Allergen-induced peribronchial fibrosis and mucus production mediated by IκB kinase β -dependent genes in airway epithelium. PNAS. 2005;102:17723–17728. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chapoval SP, Al-Garawi A, Lora JM, Strickland I, Ma B, Lee PJ, et al. Inhibition of NF-κB Activation Reduces the Tissue Effects of Transgenic IL-13. J Immunol. 2007;179:7030–7041. doi: 10.4049/jimmunol.179.10.7030. [DOI] [PubMed] [Google Scholar]
- 86.Inayama M, Nishioka Y, Azuma M, Muto S, Aono Y, Makino H, et al. A Novel IκB Kinase-beta Inhibitor Ameliorates Bleomycin-induced Pulmonary Fibrosis in Mice. Am J Respir Crit Care Med. 2006;173:1016–1022. doi: 10.1164/rccm.200506-947OC. [DOI] [PubMed] [Google Scholar]
- 87.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signalling mechanisms. Ann Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 88.Hunter I, Nixon GF. Spatial Compartmentalization of Tumor Necrosis Factor (TNF) Receptor 1-dependent Signaling Pathways in Human Airway Smooth Muscle Cells: Lipid rafts are essential for TNF-α-mediated activation of RhoA but dispensible for the activation of the NF-κB AND MAPK pathways. J Biol Chem. 2006;281:34705–34715. doi: 10.1074/jbc.M605738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McFarlane SM, Jupp OJ, Cobban HJ, Hunter I, Anderson HM, Vandenabeele P, et al. Stimulation of stress-activated but not mitogen-activated protein kinases by tumour necrosis factor receptor subtypes in airway smooth muscle. Biochem Pharmacol. 2001;61:749–759. doi: 10.1016/s0006-2952(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 90.Keslacy S, Tliba O, Baidouri H, Amrani Y. Inhibition of Tumor Necrosis Factor-α-Inducible Inflammatory Genes by Interferon-γ Is Associated with Altered Nuclear Factor-κB Transactivation and Enhanced Histone Deacetylase Activity. Mol Pharmacol. 2007;71:609–618. doi: 10.1124/mol.106.030171. [DOI] [PubMed] [Google Scholar]
- 91.Clarke DL, Sutcliffe A, Deacon K, Bradbury D, Corbett L, Knox AJ. PKCβII Augments NF-κB-Dependent Transcription at the CCL11 Promoter via p300/CBP-Associated Factor Recruitment and Histone H4 Acetylation. J Immunol. 2008;181:3503–3514. doi: 10.4049/jimmunol.181.5.3503. [DOI] [PubMed] [Google Scholar]
- 92.Zerfaoui M, Suzuki Y, Naura AS, Hans CP, Nichols C, Boulares AH. Nuclear translocation of p65 NF-κB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: Differential requirement for PARP-1 expression and interaction. Cell Signal. 2008;20:186–194. doi: 10.1016/j.cellsig.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee CW, Lin CC, Luo SF, Lee HC, Lee IT, Aird WC, et al. Tumor Necrosis Factor-α Enhances Neutrophil Adhesiveness: Induction of Vascular Cell Adhesion Molecule-1 via Activation of Akt and CaM Kinase II and Modifications of Histone Acetyltransferase and Histone Deacetylase 4 in Human Tracheal Smooth Muscle Cells. Mol Pharmacol. 2008;73:1454–1464. doi: 10.1124/mol.107.038091. [DOI] [PubMed] [Google Scholar]
- 94.Darnell JE., Jr STATs and Gene Regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 95.Hoey T, Schindler U. STAT structure and function in signaling. Curr Opin Genet Dev. 1998;8:582–587. doi: 10.1016/s0959-437x(98)80015-4. [DOI] [PubMed] [Google Scholar]
- 96.Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends in Biochem Sci. 2000;25:496–502. doi: 10.1016/s0968-0004(00)01624-8. [DOI] [PubMed] [Google Scholar]
- 97.Sampath D, Castro M, Look DC, Holtzman MJ. Constitutive activation of an epithelial signal transducer and activator of transcription (STAT) pathway in asthma. J Clin Invest. 1999;103:1353–1361. doi: 10.1172/JCI6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lazaar AL, Albelda SM, Pilewski JM, Brennan B, Pure E, Panettieri RA. T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J Exp Med. 1994;180:807–816. doi: 10.1084/jem.180.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amrani Y, Moore PE, Hoffman R, Shore SA, Panettieri RAJ. Interferon-γ Modulates Cysteinyl Leukotriene Receptor-1 Expression and Function in Human Airway Myocytes. Am J Respir Crit Care Med. 2001;164:2098–2101. doi: 10.1164/ajrccm.164.11.2108005. [DOI] [PubMed] [Google Scholar]
- 100.Kemi C, Grunewald J, Eklund A, Olgart Hoglund C. Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respir Res. 2006;7:18. doi: 10.1186/1465-9921-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tliba O, Panettieri RA, Jr, Tliba S, Walseth TF, Amrani Y. Tumor Necrosis Factor-α Differentially Regulates the Expression of Proinflammatory Genes in Human Airway Smooth Muscle Cells by Activation of Interferon-β-Dependent CD38 Pathway. Mol Pharmacol. 2004;66:322–329. doi: 10.1124/mol.104.001040. [DOI] [PubMed] [Google Scholar]
- 102.John M, Hirst SJ, Jose PJ, Robichaud A, Berkman N, Witt C, et al. Human airway smooth muscle cells express and release RANTES in response to T helper 1 cytokines. J Immunol. 1997;158:1841–1847. [PubMed] [Google Scholar]
- 103.Hardaker EL, Bacon AM, Carlson K, Roshak AK, Foley JJ, Schmidt DB, et al. Regulation of TNF-a and IFN-g induced CXCL10 expression: participation of the airway smooth muscle in the pulmonary inflammatory response in chronic obstructive pulmonary disease. FASEB J. 2004;18:191–193. doi: 10.1096/fj.03-0170fje. [DOI] [PubMed] [Google Scholar]
- 104.Kost ER, Mutch DG, Herzog TJ. Interferon-γ and Tumor Necrosis Factor-α Induce Synergistic Cytolytic Effects in Ovarian Cancer Cell Lines--Roles of the TR60 and TR80 Tumor Necrosis Factor Receptors. Gynecol Oncol. 1999;72:392–401. doi: 10.1006/gyno.1998.5257. [DOI] [PubMed] [Google Scholar]
- 105.Krakauer T, Oppenheim JJ. IL-1 and tumor necrosis factor-alpha each up-regulate both the expression of IFN-gamma receptors and enhance IFN-gamma-induced HLA-DR expression on human monocytes and a human monocytic cell line (THP-1) J Immunol. 1993;150:1205–1211. [PubMed] [Google Scholar]
- 106.Ohmori Y, Schreiber RD, Hamilton TA. Synergy between Interferon-gamma and Tumor Necrosis Factor-alpha in Transcriptional Activation Is Mediated by Cooperation between Signal Transducer and Activator of Transcription 1 and Nuclear Factor kappa B. J Biol Chem. 1997;272:14899–14907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 107.Suk K, Kim YH, Chang I, Kim JY, Choi YH, Lee KY, et al. IFN-α sensitizes ME-180 human cervical cancer cells to TNF-α-induced apoptosis by inhibiting cytoprotective NF- κB activation. FEBS Letters. 2001;495:66–70. doi: 10.1016/s0014-5793(01)02335-3. [DOI] [PubMed] [Google Scholar]
- 108.Gern JE, Busse WW. Association of Rhinovirus Infections with Asthma. Clin Microbiol Rev. 1999;12:9–18. doi: 10.1128/cmr.12.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tliba O, Tliba S, Da Huang C, Hoffman RK, DeLong P, Panettieri RA, Jr, et al. Tumor Necrosis Factor α Modulates Airway Smooth Muscle Function via the Autocrine Action of Interferon β. J Biol Chem. 2003;278:50615–50623. doi: 10.1074/jbc.M303680200. [DOI] [PubMed] [Google Scholar]
- 110.Tliba O, Amrani Y. Airway Smooth Muscle Cell as an Inflammatory Cell: Lessons Learned from Interferon Signaling Pathways. Proc Am Thorac Soc. 2008;5:106–112. doi: 10.1513/pats.200705-060VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jain D, Keslacy S, Tliba O, Cao Y, Kierstein S, Amin K, et al. Essential role of IFN-β and CD38 in TNF-α-induced airway smooth muscle hyper-responsiveness. Immunobiol. 2008;213:499–509. doi: 10.1016/j.imbio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kovarik P, Stoiber D, Eyers PA, Menghini R, Neininger A, Gaestel M, et al. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. PNAS. 1999;96:13956–13961. doi: 10.1073/pnas.96.24.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L, Tassiulas I, Park-Min KH, Reid AC, Gil-Henn H, Schlessinger J, et al. ‘Tuning’ of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat Immunol. 2008;9:186–193. doi: 10.1038/ni1548. [DOI] [PubMed] [Google Scholar]
- 114.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB. Induction of Jak/STAT Signaling by Activation of the Type 1 TNF Receptor. J Immunol. 1998;160:2742–2750. [PubMed] [Google Scholar]
- 115.Wang Y, Wu TR, Cai S, Welte T, Chin YE. Stat1 as a Component of Tumor Necrosis Factor Alpha Receptor 1-TRADD Signaling Complex To Inhibit NF-kappa B Activation. Mol Cell Biol. 2000;20:4505–4512. doi: 10.1128/mcb.20.13.4505-4512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leung DYM, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111:3–22. doi: 10.1067/mai.2003.97. [DOI] [PubMed] [Google Scholar]
- 117.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nat. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tliba O, Cidlowski JA, Amrani Y. CD38 Expression Is Insensitive to Steroid Action in Cells Treated with Tumor Necrosis Factor-α and Interferon-γ by a Mechanism Involving the Up-Regulation of the Glucocorticoid Receptor β Isoform. Mol Pharmacol. 2006;69:588–596. doi: 10.1124/mol.105.019679. [DOI] [PubMed] [Google Scholar]
- 119.Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, et al. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol. 2006;118:641–648. doi: 10.1016/j.jaci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 120.Leung DYM. Immunologic basis of chronic allergic diseases: clinical messages from the laboratory bench. Pediat Res. 1997;42:559–568. doi: 10.1203/00006450-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 121.Christodoulopoulos P, Leung DYM, Elliott MW, Hogg JC, Muro S, Toda M, et al. Increased number of glucocorticoid receptor-β-expressing cells in the airways in fatal asthma. J Allergy Clin Immunol. 2000;106:479–484. doi: 10.1067/mai.2000.109054. [DOI] [PubMed] [Google Scholar]
- 122.Oakley RH, Sar M, Cidlowski JA. The Human Glucocorticoid Receptor β Isoform. J Biol Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- 123.Pujols L, Mullol J, Torrego A, Picado C. Glucocorticoid receptors in human airways. Allergy. 2004;59:1042–1052. doi: 10.1111/j.1398-9995.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 124.Tliba O, Damera G, Banerjee A, Gu S, Baidouri H, Keslacy S, et al. Cytokines Induce an Early Steroid Resistance in Airway Smooth Muscle Cells: Novel Role of Interferon Regulatory Factor-1. Am J Respir Cell Mol Biol. 2008;38:463–472. doi: 10.1165/rcmb.2007-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Review: Activities of IRF-1. J Interferon Cytokine Res. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 126.Nakao F, Ihara K, Kusuhara K, Sasaki Y, Kinukawa N, Takabayashi A, et al. Association of IFN-γ and IFN regulatory factor 1 polymorphisms with childhood atopic asthma. J Allergy Clin Immunol. 2001;107:499–504. doi: 10.1067/mai.2001.113051. [DOI] [PubMed] [Google Scholar]
- 127.Mamane Y, Heylbroeck C, Génin P, Algarté M, Servant MJ, LePage C, et al. Interferon regulatory factors: the next generation. Gene. 1999;237:1–14. doi: 10.1016/s0378-1119(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 128.Yamada K, Elliott WM, Hayashi S, Brattsand R, Roberts C, Vitalis TZ, et al. Latent adenoviral infection modifies the steroid response in allergic lung inflammation. J Allergy Clin Immunol. 2000;106:844–851. doi: 10.1067/mai.2000.110473. [DOI] [PubMed] [Google Scholar]
- 129.Beisswenger C, Kandler K, Hess C. Allergic airway inflammation inhibits pulmonary antibacterial host defense. J Immunol. 2006;177:1833–1837. doi: 10.4049/jimmunol.177.3.1833. [DOI] [PubMed] [Google Scholar]
- 130.Wu Q, Martin RJ, LaFasto S, Efaw BJ, Rino JG, Harbeck RJ, et al. Toll-like Receptor 2 Down-regulation in Established Mouse Allergic Lungs Contributes to Decreased Mycoplasma Clearance. Am J Respir Crit Care Med. 2008;177:720–729. doi: 10.1164/rccm.200709-1387OC. [DOI] [PubMed] [Google Scholar]
- 131.Karimi K, Sarir H, Mortaz E, Smit J, Hosseini H, De Kimpe S, et al. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respir Res. 2006;7:66. doi: 10.1186/1465-9921-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maes T, Bracke KR, Vermaelen KY, Demedts IK, Joos GF, Pauwels RA, et al. Murine TLR4 is implicated in cigarette smoke-induced pulmonary inflammation. Int Arch Allergy Immunol. 2006;141:354–368. doi: 10.1159/000095462. [DOI] [PubMed] [Google Scholar]
- 133.Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, et al. Cigarette Smoke-Induced Pulmonary Inflammation Is TLR4/MyD88 and IL-1R1/MyD88 Signaling Dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 134.Morris GE, Parker LC, Ward JR, Jones EC, Whyte MK, Brightling CE, et al. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J. 2006;20:2153–2155. doi: 10.1096/fj.06-5910fje. [DOI] [PubMed] [Google Scholar]
- 135.Niimi K, Asano K, Shiraishi Y, Nakajima T, Wakaki M, Kagyo J, et al. TLR3-Mediated Synthesis and Release of Eotaxin-1/CCL11 from Human Bronchial Smooth Muscle Cells Stimulated with Double-Stranded RNA. J Immunol. 2007;178:489–495. doi: 10.4049/jimmunol.178.1.489. [DOI] [PubMed] [Google Scholar]
- 136.Issa R, Sorrentino R, Sukkar M, Sriskandan S, Chung KF, Mitchell J. Differential regulation of CCL-11/eotaxin-1 and CXCL-8/IL-8 by Gram-positive and Gram-negative bacteria in human airway smooth muscle cells. Respir Res. 2008;9:30. doi: 10.1186/1465-9921-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee CW, Chien CS, Yang CM. Lipoteichoic acid-stimulated p42/p44 MAPK activation via Toll-like receptor 2 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L921–930. doi: 10.1152/ajplung.00124.2003. [DOI] [PubMed] [Google Scholar]
- 138.Morris GE, Whyte MKB, Martin GF, Jose PJ, Dower SK, Sabroe I. Agonists of Toll-like Receptors 2 and 4 Activate Airway Smooth Muscle via Mononuclear Leukocytes. Am J Respir Crit Care Med. 2005;171:814–822. doi: 10.1164/rccm.200403-406OC. [DOI] [PubMed] [Google Scholar]
- 139.Elias JA, Wu Y, Zheng T, Panettiere R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am J Physiol - Lung Cell Mol Physiol. 1997;273:L648–L655. doi: 10.1152/ajplung.1997.273.3.L648. [DOI] [PubMed] [Google Scholar]
- 140.Oliver B, Johnston S, Baraket M, Burgess J, King N, Roth M, et al. Increased proinflammatory responses from asthmatic human airway smooth muscle cells in response to rhinovirus infection. Respir Res. 2006;7:71. doi: 10.1186/1465-9921-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hakonarson H, Carter C, Maskeri N, Hodinka R, Grunstein MM. Rhinovirus-mediated changes in airway smooth muscle responsiveness: induced autocrine role of interleukin-1β. Am J Physiol - Lung Cell Mol Physiol. 1999;277:L13–L21. doi: 10.1152/ajplung.1999.277.1.L13. [DOI] [PubMed] [Google Scholar]
- 142.Nath P, Eynott P, Leung SY, Adcock IM, Bennett BL, Chung KF. Potential role of c-Jun NH2-terminal kinase in allergic airway inflammation and remodelling: effects of SP600125. Eur J Pharmacol. 2005;506:273–283. doi: 10.1016/j.ejphar.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 143.Nath P, Leung SY, Williams A, Noble A, Chakravarty SDS, Luedtke GR, et al. Importance of p38 mitogen-activated protein kinase pathway in allergic airway remodelling and bronchial hyperresponsiveness. Eur J Pharmacol. 2006;544:160–167. doi: 10.1016/j.ejphar.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 144.Williams AS, Issa R, Leung SY, Nath P, Ferguson GD, Bennett BL, et al. Attenuation of Ozone-Induced Airway Inflammation and Hyper-Responsiveness by c-Jun NH2 Terminal Kinase Inhibitor SP600125. J Pharmacol Exp Ther. 2007;322:351–359. doi: 10.1124/jpet.107.121624. [DOI] [PubMed] [Google Scholar]
- 145.Akdis CA, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, et al. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003;33:2717–2726. doi: 10.1002/eji.200323329. [DOI] [PubMed] [Google Scholar]
- 146.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, et al. TLR2 Agonist Ameliorates Established Allergic Airway Inflammation by Promoting Th1 Response and Not via Regulatory T Cells. J Immunol. 2005;174:7558–7563. doi: 10.4049/jimmunol.174.12.7558. [DOI] [PubMed] [Google Scholar]
- 147.Revets H, Pynaert G, Grooten J, De Baetselier P. Lipoprotein I, a TLR2/4 Ligand Modulates Th2-Driven Allergic Immune Responses. J Immunol. 2005;174:1097–1103. doi: 10.4049/jimmunol.174.2.1097. [DOI] [PubMed] [Google Scholar]
- 148.Velasco G, Campo M, Manrique OJ, Bellou A, He H, Arestides RSS, et al. Toll-Like Receptor 4 or 2 Agonists Decrease Allergic Inflammation. Am J Respir Cell Mol Biol. 2005;32:218–224. doi: 10.1165/rcmb.2003-0435OC. [DOI] [PubMed] [Google Scholar]
- 149.Quarcoo D, Weixler S, Joachim RA, Stock P, Kallinich T, Ahrens B, et al. Resiquimod, a new immune response modifier from the family of imidazoquinolinamines, inhibits allergen-induced Th2 responses, airway inflammation and airway hyper-reactivity in mice. Clin Exp Allergy. 2004;34:1314–1320. doi: 10.1111/j.1365-2222.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- 150.Moisan J, Camateros P, Thuraisingam T, Marion D, Koohsari H, Martin P, et al. TLR7 ligand prevents allergen-induced airway hyperresponsiveness and eosinophilia in allergic asthma by a MYD88-dependent and MK2-independent pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L987–995. doi: 10.1152/ajplung.00440.2005. [DOI] [PubMed] [Google Scholar]
- 151.Sel S, Wegmann M, Sel S, Bauer S, Garn H, Alber G, et al. Immunomodulatory Effects of Viral TLR Ligands on Experimental Asthma Depend on the Additive Effects of IL-12 and IL-10. J Immunol. 2007;178:7805–7813. doi: 10.4049/jimmunol.178.12.7805. [DOI] [PubMed] [Google Scholar]
- 152.Camateros P, Tamaoka M, Hassan M, Marino R, Moisan J, Marion D, et al. Chronic Asthma-induced Airway Remodeling Is Prevented by Toll-like Receptor-7/8 Ligand S28463. Am J Respir Crit Care Med. 2007;175:1241–1249. doi: 10.1164/rccm.200701-054OC. [DOI] [PubMed] [Google Scholar]