Abstract
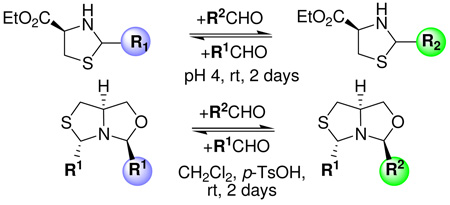
New dynamic combinatorial libraries (DCLs) were generated using the reversible aminothiol exchange reaction of thiazolidines and aromatic aldehydes. The reaction proceeded in aqueous buffered media at pH 4 and room temperature to generate thermodynamically controlled mixtures of heterocycles. The synthesis of an enantiomerically pure thiazolidinyloxazolidine is also reported. The oxazolidine moiety could be exchanged in CH2Cl2 in the presence of catalytic p-TsOH.
Dynamic combinatorial chemistry (DCC) has considerable potential in the discovery of small molecule ligands for artificial receptors and large biomolecules. DCC is largely based on the use of reversible reactions to generate compound mixtures - Dynamic Combinatorial Libraries (DCLs) - that are in thermodynamic equilibrium. The composition of the library is determined by the properties of each of the library members under the particular conditions of the experiment; this equilibrium is likely to respond to the presence of a template or another change in the environment.1
The covalent reversible reactions usable in DCC are relatively rare compared to the irreversible processes favored in traditional synthetic chemistry, and most of the former involve carbonyl compounds, imines and acetals.
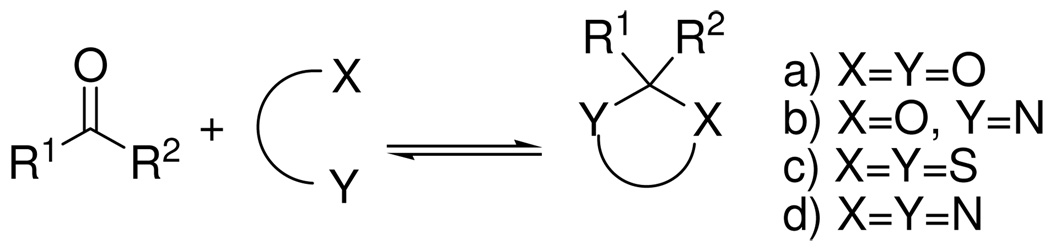
Four different types of substrates have previously been used to generate DCLs from carbonyl compounds by acetal exchange (Figure 1): a) diols in the presence of catalytic TfOH,2 H2SO4,3 or p-TsOH;4 b) aminoalcohols, leading to mixtures of imines, oxazinanes and oxazolines;5 c) thiols in the presence of catalytic Zn(OTf)2,6a or Hf(OTf)4;6b and d) diamines in aqueous buffered media at pH 4, conditions described by our group for the formation of pyrazolotriazinone heterocycles.7
Figure 1.
Cyclic acetal formations suitable for DCL.
The acid-catalyzed transacetylation of thiazolidines and related compounds attracted our closer attention as a possible extension of the latter heterocycle formation process.
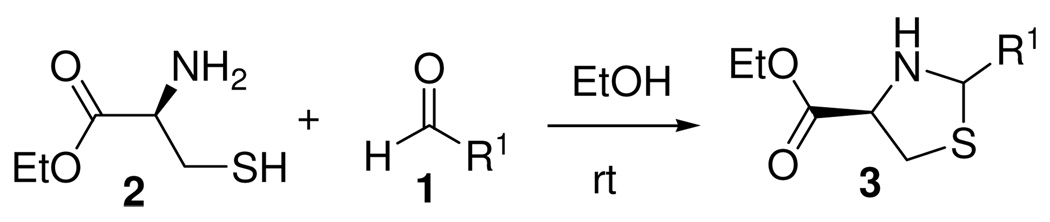
Thiazolidines 3 were selected as simple models in order to identify equilibration conditions for DCL formation. In spite of a literature report on the reversible formation of thiazolidines under basic conditions,8 this transformation has not been previously reported as useful for DCC. 4-Carboxyl ethyl-2-arylthiazolidines can be readily obtained by condensation of aldehydes (1) and cysteine ethyl ester (2) in EtOH at room temperature (Scheme 1).
Scheme 1.
Thiazolidine formation from cysteine
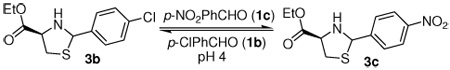
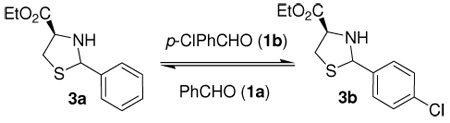
The discovery of new reversible reactions compatible with an aqueous enviroment is an important objective for the use of biomolecules as templates in DCLs. We screened different aqueous conditions, with variations in pH and reaction time, in order to establish optimal thermodynamic exchange conditions for the thiazolidine exchange. The reaction of 3a with equimolar amounts of p-Cl-benzaldehyde (1b) at room temperature was used as a reference (Table 1).
Table 1.
Optimization of thiazolidine exchange reaction of 3a.
 | ||||
|---|---|---|---|---|
| entry | Reaction conditionsa | time (h) | 3a/3b ratiob | 2 (%)c |
| 1 | pH 4, rt | 24 | 45/55 | 3 |
| 2 | pH 4, rt | 48 | 44/56 | 2 |
| 3 | pH 4, rt | 72 | 46/54 | 10 |
| 4 | pH 5, rt | 24 | 71/29 | 5 |
| 5 | pH 5, rt | 48 | 68/32 | 10 |
| 6 | pH 5, rt | 72 | 52/48 | 9 |
| 7 | pH 6, rt | 24 | 96/4 | 0 |
| 8 | pH 6, rt | 48 | 97/3 | 0 |
| 9 | pH 6, rt | 72 | 90/10 | 0 |
| 10 | pH 7, rt | 72 | 98/2 | 0 |
| 11 | pH 4, 35 °C | 24 | 45/55 | 18d |
| 12 | pH 4, 35 °C | 48 | 35/65 | 16e |
The starting concentration of each component was 1 mM; the reaction mixture was stirred in a buffered acetate solution at pH 4 and pH 5, and in a phosphate solution at pH 6 and pH 7.
The ratio was determined by 1H NMR.
The mass balance was quantitative
Total yield 3a + 3b (64%).
Total yield 3a + 3b (49%).
Thermodynamic equilibration of a mixture of 3a and 1b occurred at pH 4 over 24 to 48 h at room temperature.9 After 3 d, heterocycles 3a and 3b were stable in the aqueous environment and thiazolidines (90–98%) and ester 2 (2–10%) were recovered (entries 1, 2 and 3). Equilibration at pH 5 was slower, but after 3 d, the ratio indicated that equilibrium was reached (entries 4, 5 and 6). Equilibration at pH 6 was not complete after 3 d at rt (entries 7, 8 and 9). Equilibration at pH 7 did not proceed during 3 d at rt (entry 10) and the presence of ester 2 was not detected. The method of choice for blocking further equilibration is a simple raise of pH to 7; since we did not observe any further equilibration at this pH, the yields of recovered products were quantitative.
When the temperature was increased to 35 °C at pH 4, the equilibration ocurred faster, but significant amounts of cysteine ester 2 were observed. The total recovered yield for thiazolidines was 64% and 49% after 1 d and 2 d, respectively (Table 1, entries 11 and 12). The mass balance was decreasing, probably due to ester hydrolysis in compound 2.
Thiazolidines 3a and 3b have different stabilities depending on pH; at pH 4–5 thiazolidine hydrolysis occurs to an acceptable extent (2–10%) at rt over 3 d. Temperature seems to play an important role in these systems; higher temperatures accelerate the exchange process but under concomitant hydrolysis of the thiazolidine and the cysteine ethyl ester.
We also probed the reversibility of the system by starting from products 3b and 3c at pH 4 (Table 2). If equilibration was reached, the distribution pattern should be identical. At pH 4, equilibration required 48 h at rt, providing a comparable product ratio as observed for the inverse process (entries 1 and 2, Table 2)
Table 2.
Thiazolidine exchange processes starting with 3b or 3c.
The starting concentration of each component was 1 mM, the reaction mixture was stirred at rt in a buffered acetate solution at pH 4.
The ratio was determined by 1H NMR and confirmed by preparative isolation.
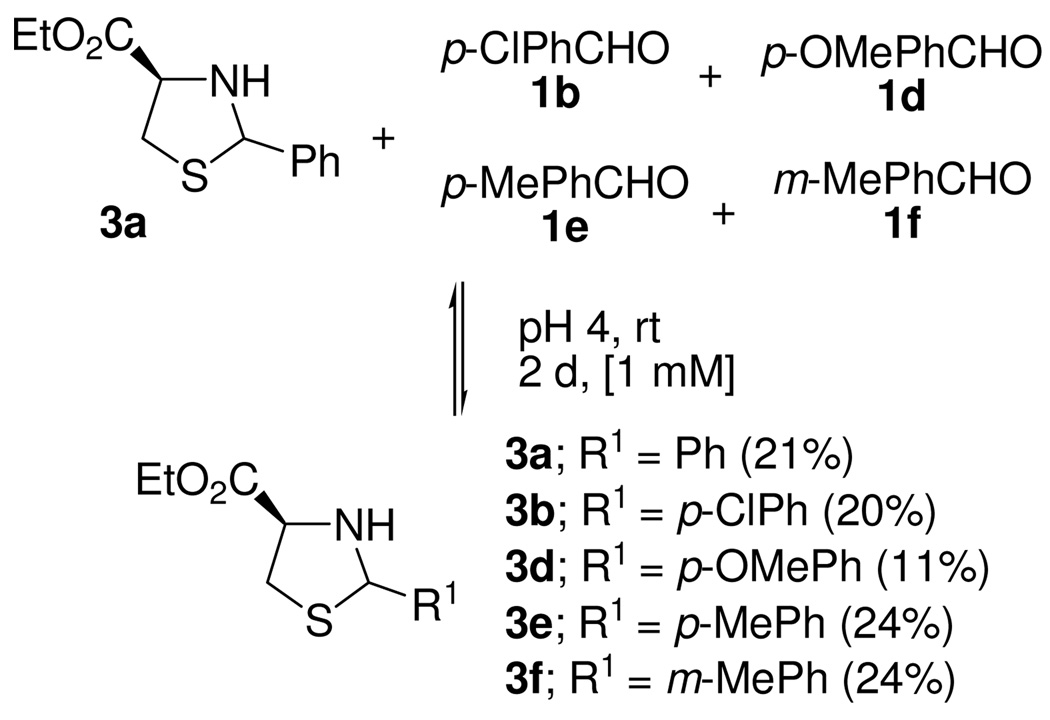
Thiazolidine 3a with aldehydes 1b and 1d–f were allowed to equilibrate, the starting concentrations of the mixture components were kept at 1 mM each. The distribution of the corresponding heterocycles varied slightly for thiazolidines 3a,b,e and f (20–24%) except for 3d (11%) after 2 d. Thiazolidine 3d bearing an EDG on the benzene ring has the lowest stability in the acidic medium. The mass balance indicated a 93% yield of thiazolidines and 7% of ester 2 after 4 d, and the distribution remained unchanged. Even though some formation of compound 2 was observed, thiazolidines are stable in the acid media (pH 4) for 4 d. This stability enables this DCL for the observation of template effects.
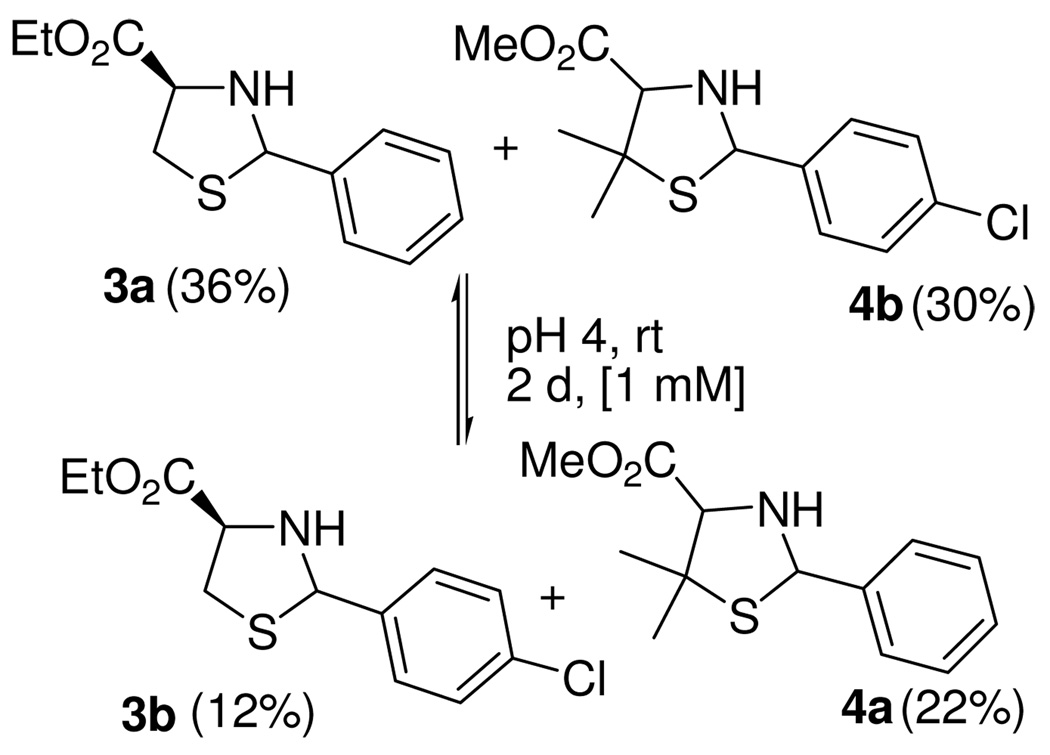
We also studied the possibility for direct side-chain metathesis of thiazolidines. An equimolecular mixture of 3a and 4b, which differ by their substitution at 2, 4 and 5-positions of the heterocycles, was equilibrated during 2 d at pH 4 and rt (Scheme 3). As expected, a mixture of four products (the original starting materials and two crossover derivatives) was formed. Small amounts of penicillamine methyl ester (3%) and cysteine ethyl ester (7%) were also detected, but 90% of the thiazolidine products was recovered after 3 d.
Scheme 3.
Metathesis of thiazolidines.a
a The yields in parentheses reflect the equilibrium distribution. The ratio was determined by 1H NMR and confirmed by preparative isolation.
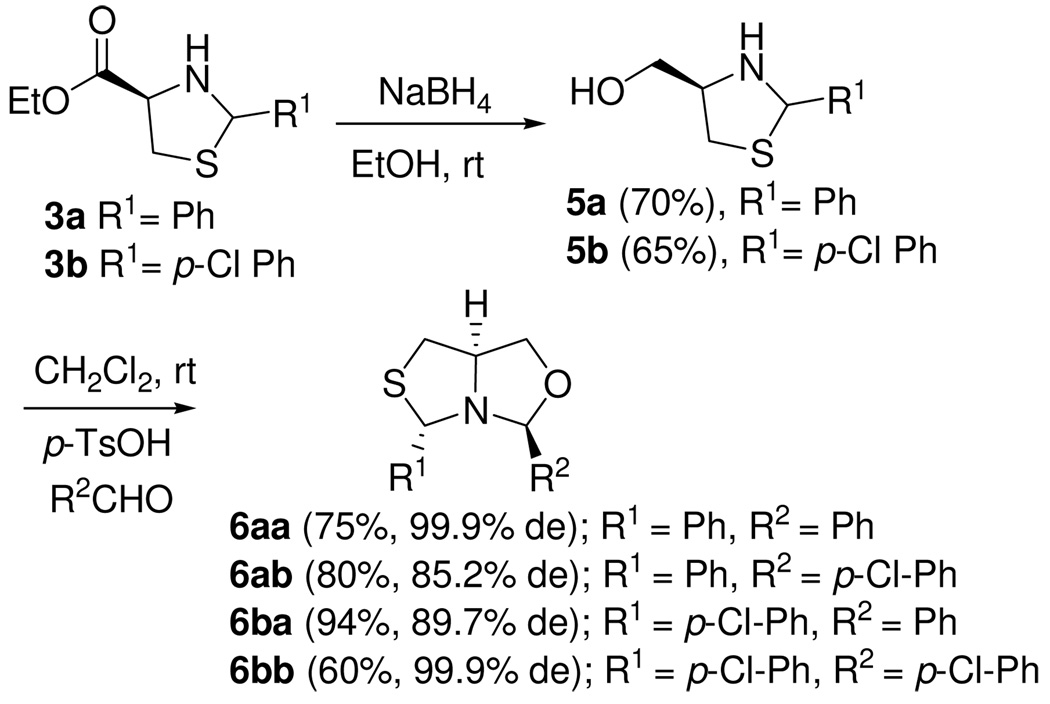
As a means to increase diversity in the side chains at the 4-position of the heterocycles, the ester moiety of thiazolidines 3a–b was converted to the alcohol by reduction with NaBH4. When the aminoalcohols 5a–b were treated with aldehydes 1a–b in acid media (p-TsOH), the fused thiazolidine-oxazolidines 6 were formed. This class of compounds represents a new example of a bicyclic DCC scaffold (Scheme 4).10 Even though aminoalcohols 5 were used as a 1:1 mixture of diastereomers, only anti-6 was formed. The relative configuration was confirmed by NOE experiments. This result is not completely unexpected due to the fact that thiazolidines undergo facile ring opening and closure reactions.11
Scheme 4.
Synthesis of fused thiazolidine-oxazolidine heterocycles.
We hypothesize that this reaction proceeds by a thermodynamic equilibration, with anti-6 being the more stable fused heterocycle.
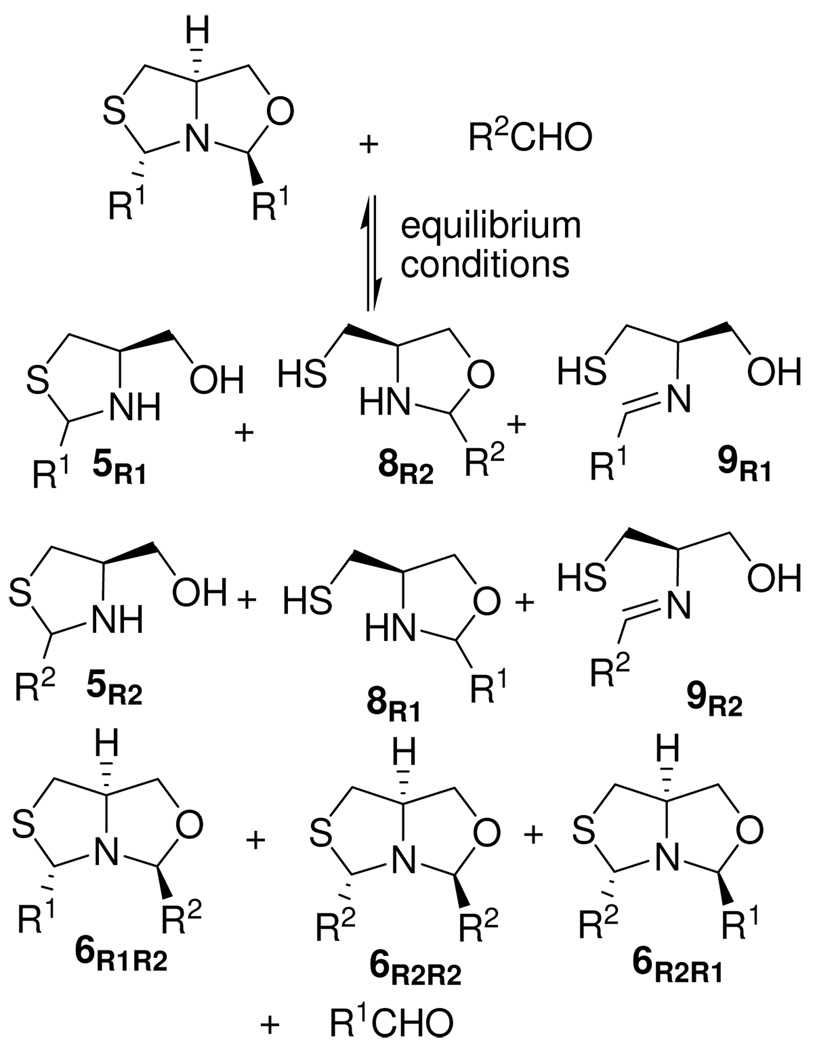
Thiazolidinyloxazolidine heterocycles present interesting opportunities for exchange processes: products include thiazolidine 5R, oxazolidine 8R, the fused heterocycles at the thiazolidine or the oxazolidine site 6RR and also the imine isomers 9R,12 as shown in Scheme 5.
Scheme 5.
Potential library of thiazolidine-oxazolidine heterocycles.
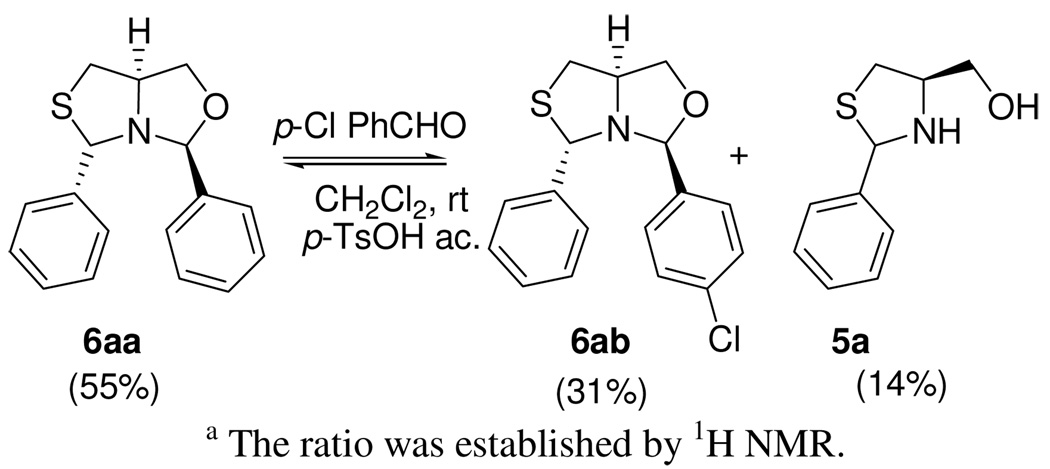
When bicycle 6aa [10 mM] in CH2Cl2 was equilibrated at rt with an equimolar amount of aldehyde 1b in the presence of catalytic p-TsOH, only the mixture of the exchanged products 6aa and 6ab and free aminoalcohol 5a was obtained (Scheme 6).13 In order to identify the products, it was necessary to perform a chiral-HPLC (Chiralcel-OD) analysis of the possible products 6ba and 6ab, because they have almost identical signals in the 1H NMR spectra. The chromatographic analysis indicated that the exchange was limited to the N-C-O linkage, forming 6ab. Alternative species like imines 9a, 9b or oxazolidines 8a, 8b were not detected in the 1H NMR spectra or the HPLC traces.
Scheme 6.
Exchange reaction of bicycle 6aa.a
a The ratio was established by 1H NMR.
We also performed an experiment in absence of p-TsOH in CDCl3 with compound 6aa and aldehyde 1b at a 10 mM concentration, and we found no evidence of exchange or decomposition after 3 d. This observation is indicative that the products are stable and that the exchange requires acid catalysis.
It is important to point out that conditions for the synthesis and exchange of these bicycles are quite similar, i.e. CH2Cl2 and p-TsOH. In the acidic media, the diasteromeric mixture of alcohol 5 is in a fast equilibration by ring opening and closing. During this equilibration only the acetal N-C bond of thiazolidine would be broken and the R1 side chain remains linked to the sulfur atom, probably via a sulfenium cation. This mechanism explains that we did not observe the formation of 6R2R1 in the synthesis of 6R1R2 and also why the exchange occcurred only at the oxazolidine site.
In summary, we explored a new exchange reaction between thiazolidines and carbonyl compounds. The thermodynamic exchange proceeds in an acidic aqueous environment (pH 4) and represents a new reversible reaction useful for DCC methodologies. A structural diversification of the core scaffolds can be accomplished by modifications at the 5- and 4-positions of the heterocycles. The thiazolidines 3a–f are stable in buffered media over 4 d, and these conditions are suitable for the generation of DCLs as well as for the direct screening of these libraries. Moreover, the exchange reaction can be stopped by raising the pH to 7, thus providing a convenient way to analyze the compound distribution patterns. As an important extension of this work, we also present the synthesis of fused thiazolidine-oxazolidine heterocycles such as anti-6, representing a new compound class. These bicycles are stable under neutral or basic conditions but can be equilibrated at the oxazolidine moiety in CH2Cl2 in the presence of catalytic p-TsOH.
Supplementary Material
Scheme 2.
Exchange reaction between thiazolidines 3a and aldehydes 1b, 1d–f.a
a The yields in parentheses reflect the equilibrium distribution. The ratio was determined by 1H NMR and confirmed by preparative isolation.
Acknowledgment
This work was supported by the National Institutes of Health-FIRCA (R03TW007772), and NIH CA078039. C.S. thanks UdelaR (CSIC-N° 349). We would like to thank H. Pezzaroglo of Nuclear Magnetic Resonance Laboratory, UdelaR for NMR spectra.
Footnotes
Supporting Information Available: Experimental procedures, 1H NMR and HPLC of DCLs, and spectral data for compounds 4b, 5b, 6aa, 6ab, 6ba, and 6bb. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews, see: Ladame S. Org. Biomol. Chem. 2008;6:219–226. doi: 10.1039/b714599c. Ludlow RF, Otto S. Chem. Soc. Rev. 2008;37:101–108. doi: 10.1039/b611921m. Lehn J-M. Chem. Soc. Rev. 2007;36:151–160. doi: 10.1039/b616752g. Corbett PT, Leclaire J, Vial L, West KR, Wietor J-L, Sanders JKM, Otto S. Chem. Rev. 2006;106:3652–3711. doi: 10.1021/cr020452p.
- 2.(a) Cacciapaglia R, Di Stefano S, Mandolini L. J. Am. Chem. Soc. 2005;127:13666–13671. doi: 10.1021/ja054362o. [DOI] [PubMed] [Google Scholar]; (b) Fuchs B, Nelson A, Star A, Stoddart JF, Vidal S. Angew. Chem. Int. Ed. Engl. 2003;42:4220–4224. doi: 10.1002/anie.200351558. [DOI] [PubMed] [Google Scholar]; (c) Cacciapaglia R, Di Stefano S, Mandolini L, Mencarelli P, Ugozzoli F. Eur. J. Org. Chem. 2008:186–195. [Google Scholar]
- 3.Berkovich-Berger D, Lemcoff NG. Chem. Commun. 2008:1686–1688. doi: 10.1039/b800384j. [DOI] [PubMed] [Google Scholar]
- 4.Lemcoff NG, Fuchs B. Org. Lett. 2002;4:731–734. doi: 10.1021/ol017230i. [DOI] [PubMed] [Google Scholar]
- 5.(a) Star A, Goldberg I, Fuchs B. Angew. Chem., Int. Ed. 2000;39:2685–2689. [PubMed] [Google Scholar]; (b) Star A, Goldberg I, Fuchs B. J. Organomet. Chem. 2001;630:67–77. [Google Scholar]
- 6.(a) Sutton LR, Donaubauer WA, Hampel F, Hirsch A. Chem. Commun. 2004:1758–1759. doi: 10.1039/b401021c. [DOI] [PubMed] [Google Scholar]; (b) Wu Y-C, Zhu J. J. Org. Chem. 2008;73:9522–9524. doi: 10.1021/jo8021988. [DOI] [PubMed] [Google Scholar]
- 7.Wipf P, Mahler SG, Okumura K. Org. Lett. 2005;7:4483–4486. doi: 10.1021/ol051839s. [DOI] [PubMed] [Google Scholar]
- 8.(a) Woodward GE, Schroeder EF. J. Am. Chem. Soc. 1937;59:1690–1694. [Google Scholar]; (b) Szilagyi L, Gyorgydeak Z. J. Am. Chem. Soc. 1979;101:427–432. [Google Scholar]
- 9.Typical procedure. A solution of compound 3a (20.8 mg, 0.09 mmol) and p-Cl-benzaldehyde (12.3 mg, 0.09 mmol) in a mixture of acetate buffer at pH 4 (63 mL) and MeOH (27 mL) was stirred at rt for 2 d. The pH was raised to 7 by adding a saturated solution of NaHCO3 and the reaction mixture was extracted with CH2Cl2. The combined organic layers were dried (MgSO4), filtered and concentrated in vacuo (temperature should never exceed 22 °C). The residue was analyzed by 1H NMR to determine the ratio and products were confirmed by preparative isolation of 3a+ 3b (26 mg, 98% yield).
- 10.Related fused thiazolidine-oxazolidinones and thiazolidine-oxazolidines were obtained when aminoalcohol 5a was treated with phosgene: González A, Lavilla R, Piniella JF, Alvarez-Larena A. Tetrahedron. 1995;51:3015–3024.
- 11. Deroose FD, De Clercq PJ. J. Org. Chem. 1995;60:321–330. and ref 8b
- 12.In ref 5a, Fuchs and coworkers report traces of imine in a DCL constructed from aminoalcohols.
- 13.Typical procedure. A mixture of compound 6aa (100 mg, 0.36 mmol) and p-Cl-benzaldehyde (50 mg, 0.36 mmol) in CH2Cl2 (35.5 mL) and p-toluenesulfonic acid (10 mg, 0.13 mmol) was stirred at rt for 2 d. The reaction mixture was poured into water, the pH adjusted to pH 7 and the mixture was extracted with CH2Cl2 (3x30 mL). The organic layers were dried, filtered and solvent was removed under reduced pressure. The residue was analyzed by 1H NMR to determine the product ratio and the products were confirmed by preparative isolation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.