Abstract
Cyclic eight-ring pyrrole-imidazole polyamides are sequence-specific DNA-binding small molecules that are cell permeable and can regulate endogenous gene expression. Syntheses of cyclic polyamides have been achieved by solid-phase and solution-phase methods. A rapid solution-phase oligomerization approach to 8-ring symmetrical cyclic polyamides yields 12 and 16 membered macrocycles as well. A preference for DNA binding by the 8 and 16 membered oligomers was observed over the 12-ring macrocycle, which we attributed to a conformational constraint not present in the smaller and larger systems.
Pyrrole-imidazole polyamides are a class of cell-permeable oligomers that target the minor groove of DNA in a sequence specific manner.1,2 Antiparallel arrangements of N-methylpyrrole (Py) and N-methylimidazole (Im) carboxamides (Im/Py) recognize G•C from C•G base pairs, whereas Py/Py specifies for both T•A and A•T.3 Hairpin Py-Im polyamides have been programmed for a broad repertoire of DNA sequences with high affinities.4 These cell permeable5 ligands can influence gene transcription by disrupting protein-DNA interfaces,2 and have been shown to control transcription of genes important in human disease.6 Py-Im polyamides have also been used for a variety of applications ranging from fluorescence-based DNA detection,7 transcriptional activation with artifical transcription factor mimics,5e,8 and self-assembly of DNA nano-architectures.9
We recently reported solution-phase methods for the synthesis of hairpin10a and cyclic polyamides.11a Key to the cyclic polyamide synthesis was a macrocyclization from an acyclic precursor that yielded cyclic polyamide 1z (Figure 1). Activation of the C-terminal Py amino acid of 1z as a pentafluorophenyl ester allowed efficient macrocyclization by the γ-NH2 on the turn moiety under dilute reaction conditions. Our studies of polyamide 1 revealed it possessed very high DNA binding affinities and could modulate gene expression in cell culture from which we infer 8-ring cycles are cell permeable.11a
Figure 1.
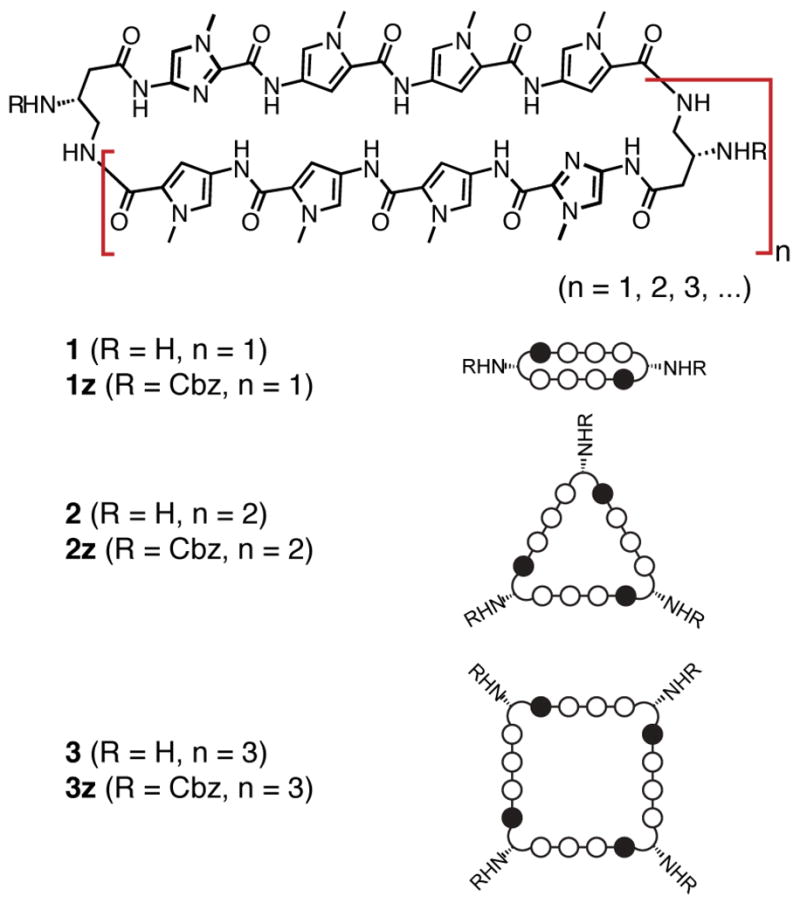
Structures of macrocyclic polyamides 1z–3z and 1–3, and their ball-and-stick models. Polyamide shorthand code: closed circles, Im monomer; open circles, Py monomer.
An orthogonal polymerization/oligomerization strategy for the synthesis of 1 and related polyamides is reported here. This method affords symmetrical Py-Im polyamide macrocycles from simple Py-Im building blocks in a convergent manner (Scheme 1). As serendipitous minor products higher order oligomers such as the 12-membered (2) and 16-membered (3) cyclic polyamides are also produced by this method. In addition to describing the synthetic chemistry to prepare 1–3, we examined the DNA binding properties of such expanded polyamide macrocycles.
Scheme 1.
Synthesis of macrocyclic polyamides 1–3 and higher order cycles by oligomerization of bifunctional intermediate 5.
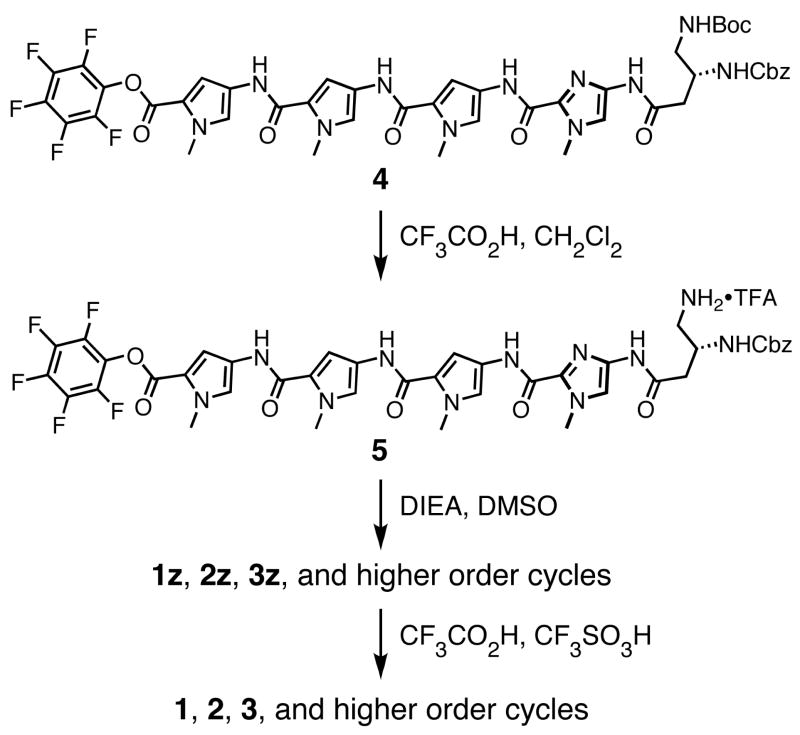
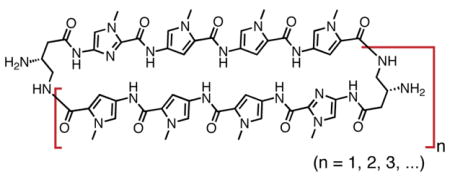
Our strategy for this oligomerization route relied on the palindromic nature of polyamide 1. Disconnection of 1 at both γ-amino turns affords two identical halves of the molecule. Bimolecular coupling between two molecules, followed by intramolecular ring closure delivers cyclic Py-Im polyamides. Bifunctional oligomer 4 contains every atom needed to construct cyclic polyamides 1–3 by this process (Scheme 1).
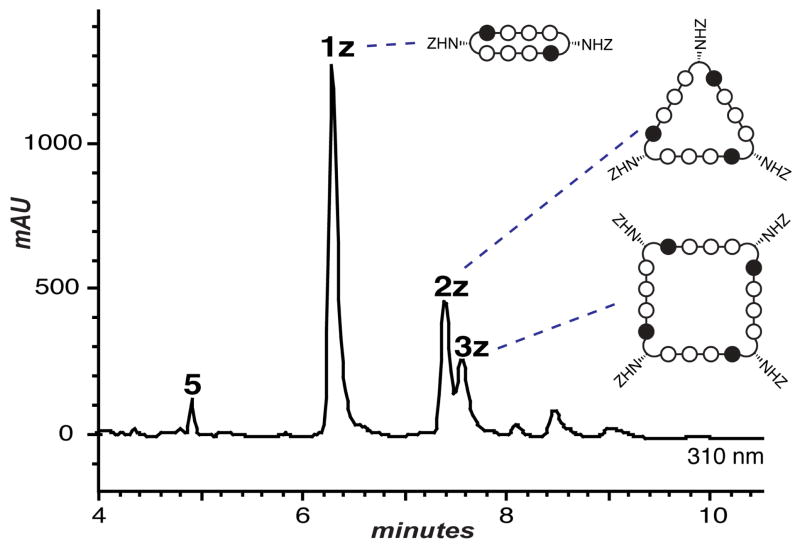
The pentafluorophenyl ester 4 was prepared in one step from the previously reported carboxylic acid of 4.11a Acidic deprotection of the γ-amino functionality of 4 followed by drying in vacuo yields intermediate 5 which is the substrate for the homodimerization/oligomerization reaction. To initiate this sequence, the protected trifluoroacetate salt 5 was diluted with DMSO, then treated with an organic base (DIEA) to unmask the nucleophilic primary γ-amine. The ensuing oligomerization/macrocyclization process provides benzylcarbamate protected cyclic polyamides 1z, 2z, 3z, and trace amounts of unisolated higher-order oligomers. A distribution of uncyclized intermediates corresponding to the dimer (8-ring cycle, 1z), trimer (12-ring cycle, 2z), tetramer (16-ring cycle, 3z), and higher order adducts can be observed at early time points, as evidenced by HPLC analysis at 2 hr (Figure S1). Extended reaction times (20 hours) reveals cyclized polyamides 1z, 2z, and 3z in a ratio of 6.6:2.6:1 almost exclusively (Figure 2). Isolation of 1z (13.9%), 2z (5.5%), and 3z (2.1%) by preprative HPLC, followed by Cbz-deprotection under acidic conditions (solution of CF3CO2H and CF3SO3H) provides polyamide macrocycles 1–3.
Figure 2.
Reverse phase HPLC analysis (20 hr) of the oligomerization reaction revealing products 1z, 2z, and 3z. Peaks were identified by high-resolution mass spectrometry following isolation and Cbz-deprotection.
Quantitative DNase I footprint titrations have historically been utilized to measure polyamide-DNA binding affinities and specificities.12 However, this method is limited to measuring Ka values ≤ 2 × 1010 M−1, which invalidates this technique for quantifying the exceptionally high DNA-binding affinities of cycles 1 and 3. The magnitude of DNA thermal stabilization (ΔTm) of DNA-polyamide complexes has been utilized to rank order polyamides with high DNA binding affinities5g,11a and we have employed melting temperature analysis (ΔTm) for evaluating DNA-binding ability in this study. Polyamide 1, which targets the DNA sequence 5′-WGWWCW-3′ (W = A or T) through pairing of two antiparallel ImPyPyPy strands, increases the melting temperature of 14-mer dsDNA containing one binding site by 23.6 °C.11a With polyamide macrocycles 2 and 3 in hand we evaluated their ability to bind duplex DNA relative to cycle 1. Trimeric macrocycle 2 failed to bind its target double stranded DNA sequence as evidenced by the complete lack of ligand-promoted thermal stabilization of duplex DNA melting (Table 1). This result is presumably due to inherent geometrical constraints of 2, preventing the side-by-side antiparallel alignment of the ImPyPyPy strands, a motif well accomodated by the DNA minor groove. Remarkably, in the case of tetrameric macrocycle 3, dsDNA binding and thermal stabilization was restored to a comparable value to dimer 1. We hypothesize that an even number of ImPyPyPy strands allows 3 to possess a collapsed or folded tetramer geometry, with two adjacent, antiparallel ImPyPyPy strands followed by an idential repeat of this motif linked through two intervening turn units (Fig. S2).
Table 1.
Tm values for cycles 1–3 in the presence of DNA.
| dsDNA sequence = | 5′–TTGC TGTTCT GCAA–3′ 3′–AACG ACAAGA CGTT–5′ |
|
|---|---|---|
| polyamide cycle | Tm/°C | ΔTm/°C |
| – | 60.0 (±0.3) | – |
|
|
83.5 (±0.5) | 23.6 (±0.6) |
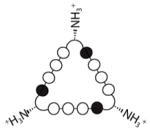 (2) (2) |
60.1 (±0.6) | 0.1 (±0.6) |
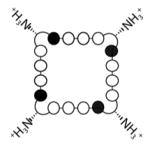 (3) (3) |
83.0 (±0.3) | 23.1 (±0.4) |
All values reported are derived from at least three melting temperature experiments with standard deviations indicated in parentheses. ΔTm values are given as Tm(DNA/polyamide) - Tm(DNA). The propagated error in ΔTm measurements is the square root of the sum of the square of the standard deviations for the Tm values.
In summary, we have demonstrated that macrocyclic polyamides can by synthesized by oligomerization of a bifunctional polyamide to yield a distribution of cyclic polyamide oligomers. Additionally, we show that certain large cyclic polyamide geometries are able to bind dsDNA, a result which could be utilized in the design of highly specific molecules for targeting larger binding site sizes. Due to the size of the 16-ring cycle, the larger series are likely not cell permeable and therefore have limited utility in gene regulation studies. Rather, 16-ring cycles may add to the repetoire of DNA binding motifs for use in DNA nanotechnology.9
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM27681). D.M.C. is grateful for a Caltech Kanel predoctoral fellowship. D.A.H. thanks the California Tobacco-Related Disease Research Program (16FT-0055) for a postdoctoral fellowship.
Footnotes
Supporting Information Available Compound characterization, Figure S1, Figure S2, and experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Dervan PB. Bioorg Med Chem. 2001;9:2215–2235. doi: 10.1016/s0968-0896(01)00262-0. [DOI] [PubMed] [Google Scholar]
- 2.Dervan PB, Edelson BS. Curr Opin Struct Biol. 2003;13:284–299. doi: 10.1016/s0959-440x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 3.(a) Trauger JW, Baird EE, Dervan PB. Nature. 1996;382:559–561. doi: 10.1038/382559a0. [DOI] [PubMed] [Google Scholar]; (b) White S, Szewczyk JW, Turner JM, Baird EE, Dervan PB. Nature. 1998;391:468–470. doi: 10.1038/35106. [DOI] [PubMed] [Google Scholar]; (c) Kielkopf CL, Baird EE, Dervan PB, Rees DC. Nat Struct Biol. 1998;5:104–109. doi: 10.1038/nsb0298-104. [DOI] [PubMed] [Google Scholar]; (d) Kielkopf CL, White S, Szewczyk JW, Turner JM, Baird EE, Dervan PB, Rees DC. Science. 1998;282:111–115. doi: 10.1126/science.282.5386.111. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CF, Phillips JW, Trauger JW, Farkas ME, Belitsky JM, Heckel A, Olenyuk BZ, Puckett JW, Wang CC, Dervan PB. Tetrahedron. 2007;63:6146–6151. doi: 10.1016/j.tet.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Belitsky JM, Leslie SJ, Arora PS, Beerman TA, Dervan PB. Bioorg Med Chem. 2002;10:3313–3318. doi: 10.1016/s0968-0896(02)00204-3. [DOI] [PubMed] [Google Scholar]; (b) Crowley KS, Phillion DP, Woodard SS, Scheitzer BA, Singh M, Shabany H, Burnette B, Hippenmeyer P, Heitmeier M, Bashkin JK. Bioorg Med Chem Lett. 2003;13:1565–1570. doi: 10.1016/s0960-894x(03)00152-5. [DOI] [PubMed] [Google Scholar]; (c) Best TP, Edelson BS, Nickols NG, Dervan PB. Proc Natl Acad Sci U S A. 2003;100:12063–12068. doi: 10.1073/pnas.2035074100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Edelson BS, Best TP, Olenyuk B, Nickols NG, Doss RM, Foister S, Heckel A, Dervan PB. Nucleic Acids Res. 2004;32:2802–2818. doi: 10.1093/nar/gkh609. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xiao X, Yu P, Lim HS, Sikder D, Kodadek T. Angew Chem Int Ed Engl. 2007;46:2865–2868. doi: 10.1002/anie.200604485. [DOI] [PubMed] [Google Scholar]; (f) Nickols NG, Jacobs CS, Farkas ME, Dervan PB. Nucleic Acids Res. 2007;35:363–370. doi: 10.1093/nar/gkl1042. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Dose C, Farkas ME, Chenoweth DM, Dervan PB. J Am Chem Soc. 2008;130:6859–6866. doi: 10.1021/ja800888d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Hsu CF, Dervan PB. Bioorg Med Chem Lett. 2008;18:5851–5855. doi: 10.1016/j.bmcl.2008.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Olenyuk BZ, Zhang GJ, Klco JM, Nickols NG, Kaelin WG, Dervan PB. Proc Natl Acad Sci U S A. 2004;101:16768–16773. doi: 10.1073/pnas.0407617101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kageyama Y, Sugiyama H, Ayame H, Iwai A, Fujii Y, Huang LE, Kizaka-Kondoh S, Hiraoka M, Kihara K. Acta Oncol. 2006;45:317–324. doi: 10.1080/02841860500486648. [DOI] [PubMed] [Google Scholar]; (c) Nickols NG, Jacobs CS, Farkas ME, Dervan PB. ACS Chem Biol. 2007;2:561–571. doi: 10.1021/cb700110z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nickols NG, Dervan PB. Proc Natl Acad Sci U S A. 2007;104:10418–10423. doi: 10.1073/pnas.0704217104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Matsuda H, Fukuda N, Ueno T, Tahira Y, Ayame H, Zhang W, Bando T, Sugiyama H, Saito S, Matsumoto K, et al. J Am Soc Neph. 2006;17:422–432. doi: 10.1681/ASN.2005060650. [DOI] [PubMed] [Google Scholar]; (f) Yao EH, Fukuda N, Ueno T, Matsuda H, Matsumoto K, Nagase H, Matsumoto Y, Takasaka A, Serie K, Sugiyama H, Sawamura T. Hypertension. 2008;52:86–92. doi: 10.1161/HYPERTENSIONAHA.108.112797. [DOI] [PubMed] [Google Scholar]; (g) Takahashi T, Asami Y, Kitamura E, Suzuki T, Wang X, Igarashi J, Morohashi A, Shinojima Y, Kanou H, Saito K, Takasu T, Nagase H, Harada Y, Kuroda K, Watanabe T, Kumamoto S, Aoyama T, Matsumoto Y, Bando T, Sugiyama H, Yoshida-Noro C, Fukuda N, Hayashi N. Chem Biol. 2008;15:829–841. doi: 10.1016/j.chembiol.2008.06.006. [DOI] [PubMed] [Google Scholar]; (h) Hochhauser D, Kotecha M, O’hare C, Morris PJ, Hartley JM, Taherbhai Z, Harris D, Forni C, Mantovani R, Lee M, Hartley JA. Mol Cancer Ther. 2007;6:346–354. doi: 10.1158/1535-7163.MCT-06-0503. [DOI] [PubMed] [Google Scholar]
- 7.(a) Rucker VC, Foister S, Melander C, Dervan PB. J Am Chem Soc. 2003;125:1195–1202. doi: 10.1021/ja021011q. [DOI] [PubMed] [Google Scholar]; (b) Chenoweth DM, Viger A, Dervan PB. J Am Chem Soc. 2007;129:2216–2217. doi: 10.1021/ja0682576. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fujimoto J, Bando T, Minoshima M, Kashiwazaki G, Nishijima S, Shinohara K, Sugiyama H. Bioorg Med Chem. 2008;16:9741–9744. doi: 10.1016/j.bmc.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 8.(a) Arndt HD, Hauschild KE, Sullivan DP, Lake K, Dervan PB, Ansari AZ. J Am Chem Soc. 2003;125:13322–13323. doi: 10.1021/ja0371395. [DOI] [PubMed] [Google Scholar]; (b) Kwonj Y, Arndt HD, Qian M, Choi Y, Kawazoe Y, Dervan PB, Uesugi M. J Am Chem Soc. 2004;126:15940–15941. doi: 10.1021/ja0445140. [DOI] [PubMed] [Google Scholar]; (c) Hauschild KE, Metzler RE, Arndt HD, Moretti R, Raffaelle M, Dervan PB, Ansari AZ. Proc Natl Acad Sci U S A. 2005;102:5008–5013. doi: 10.1073/pnas.0501289102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Stafford RL, Arndt HD, Brezinski ML, Ansari AZ, Dervan PB. J Am Chem Soc. 2007;129:2660–2668. doi: 10.1021/ja067971k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Stafford RL, Dervan PB. J Am Chem Soc. 2007;129:14026–14033. doi: 10.1021/ja075247b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Cohen JD, Sadowski JP, Dervan PB. Angew Chem Int Ed. 2007;46:7956–7959. doi: 10.1002/anie.200702767. [DOI] [PubMed] [Google Scholar]; (b) Schmidt TL, Nandi CK, Rasched G, Parui PP, Brutschy B, Famulok M, Heckel A. Angew Chem Int Ed. 2007;46:4382–4384. doi: 10.1002/anie.200700469. [DOI] [PubMed] [Google Scholar]; (c) Cohen JD, Sadowski JP, Dervan PB. J Am Chem Soc. 2008;130:402–403. doi: 10.1021/ja0772400. [DOI] [PubMed] [Google Scholar]
- 10.Chenoweth DM, Harki DA, Dervan PB. J Am Chem Soc. 2009;131:7175–7181. doi: 10.1021/ja901307m.For additional solution-phase polyamide synthesis studies see: Mrksich M, Parks ME, Dervan PB. J Am Chem Soc. 1994;116:7983–7988.Xiao J, Yuan G, Huang W, Chan AS, Lee KL. J Org Chem. 2000;65:5506–5513. doi: 10.1021/jo000135n.Harris D, Stewart M, Sielaff A, Mulder K, Brown T, Mackay H, Lee M. Heterocycl Commun. 2007;13:17–23.Xiao JH, Huang WQ, Tang FL, Yuan G, Chan ASC, Lee KLD. Chin J Chem. 2000;18:603–607.Boger DL, Fink BE, Hedrick MP. J Am Chem Soc. 2000;122:6382–6394.Mamidyala SK, Firestine SM. Tetrahedron Lett. 2006;47:7431–7434.Sinyakov AN, Feshchenko MV, Ryabinin VA. Russian J Bioorg Chem. 2004;30:98–99.Yuan G, Tang F. ARKIVOC. 2003;ii:32–37.Xiao JH, Yuan G, Huang WQ, Tang FL, Chan ASC, Lee KLD. Chinese Chem Lett. 2000;11:207–208.For additional solid-phase hairpin polyamide synthesis studies see: Baird EE, Dervan PB. J Am Chem Soc. 1996;118:6141–6146.Belitsky JM, Nguyen DH, Wurtz NR, Dervan PB. Bioorg Med Chem. 2002;10:2767–2774. doi: 10.1016/s0968-0896(02)00133-5.Wurtz NR, Turner JM, Baird EE, Dervan PB. Org Lett. 2001;3:1201–1203. doi: 10.1021/ol0156796.Krutzik PO, Chamberlin AR. Bioorg Med Chem Lett. 2002;12:2129–2132. doi: 10.1016/s0960-894x(02)00359-1.Krutzik PO, Chamberlin AR. Methods Mol Biol. 2002;201:77–92. doi: 10.1385/1-59259-285-6:77.Ayame H, Saito T, Bando T, Fukuda N, Sugiyama H. Nucleic Acids Res Suppl. 2003:67–68. doi: 10.1093/nass/3.1.67.Moore MJB, Cuenca F, Searcey M, Neidle S. Org Biomol Chem. 2006;4:3479–3488. doi: 10.1039/b607707b.
- 11.Chenoweth DM, Harki DA, Phillips JW, Dose C, Dervan PB. J Am Chem Soc. 2009;131:7182–7188. doi: 10.1021/ja901309z.For additional solid-phase cyclic polyamide synthesis studies see: Cho J, Parks ME, Dervan PB. Proc Natl Acad Sci USA. 1995;92:10389–10392. doi: 10.1073/pnas.92.22.10389.Zhang Q, Dwyer TJ, Tsui V, Case DA, Cho J, Dervan PB, Wemmer DE. J Am Chem Soc. 2004;126:7958–7966. doi: 10.1021/ja0373622.Herman DM, Turner JM, Baird EE, Dervan PB. J Am Chem Soc. 1999;121:1121–1129.Melander C, Herman DM, Dervan PB. Chem Eur J. 2000;6:4487–4497. doi: 10.1002/1521-3765(20001215)6:24<4487::aid-chem4487>3.0.co;2-c.
- 12.Trauger JW, Dervan PB. Methods Enzymol. 2001;340:450–466. doi: 10.1016/s0076-6879(01)40436-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.