Abstract
Background & Aims
Cigarette smoking is an established risk factor for pancreatic cancer, but there is conflicting evidence regarding the effects of alcohol consumption. The effects of cigarettes and alcohol on age of sporadic pancreatic cancer diagnosis have not been examined; we evaluated the independent and synergistic effects of lifetime cigarette smoking and alcohol consumption on age at pancreatic cancer diagnosis in the United States.
Methods
We analyzed data on cigarette smoking and alcohol consumption from the IMPAC Medical Registry Services, Cancer Information Resource File (CIRF), collected from June 1, 1993 to December 31, 2003 for 29,239 reported, histologically confirmed cases of pancreatic adenocarcinoma. We also analyzed data on cigarette smoking and alcohol consumption for 820 histologically confirmed cases of pancreatic adenocarcinoma from the University of Michigan Pancreatic Cancer Registry (UMPCR), collected from January 2004 to October 2007.
Results
Current cigarette smokers were diagnosed at significantly younger ages than never smokers, according to data from the CIRF and UMPCR (8.3 years and 6.3 years, respectively); the UMPCR data indicated dose effects. Past and current alcohol consumption were associated with younger age at diagnosis age in both databases. Current smokers who were current drinkers were diagnosed significantly earlier (CIRF 10.2 years, UMPCR 8.6 years) than abstainers. Past cigarette smoking was modestly associated with younger diagnosis age.
Conclusions
Cigarette smoking and alcohol consumption were associated with pancreatic cancer presentation at a younger age among the general population and have a combined effect on diagnosis age. Past cigarette smoking is less influential. Smoking cessation programs could help prevent pancreatic cancer.
Pancreatic adenocarcinoma is the fourth leading cause of cancer death in the United States.1 The overall 5-year survival rate for pancreatic cancer is slightly lower than 5%, although survival for patients with resectable disease ranges from 20–50%.2–4 The development of prevention and early detection strategies is crucial to increase survival rates of this highly lethal malignancy. Cigarette smoking is the most consistently implicated environmental risk factor for pancreatic cancer, increasing risk 2 to 3-fold5–9 and decreasing the age at pancreatic cancer presentation in individuals with genetic susceptibility, such as hereditary pancreatitis and familial pancreatic cancer patients.10,11 Alcohol is a controversial risk factor for pancreatic cancer, with some studies noting increased risk7,8,12–14 particularly among heavy drinkers.8,13,14 To date, the effects of smoking and drinking on age at pancreatic cancer diagnosis in the general U.S. population have not been defined.
Our primary goal was to evaluate independent and synergistic effects of cigarette smoking and alcohol consumption on the age at pancreatic cancer diagnosis in the general population using a large database employed by academic and community hospital cancer registries as well as in prospectively collected data from a single, large tertiary care center.
Methods
Data Sources
The IMPAC Medical Registry Services, Cancer Information Resource File (CIRF) incorporates cancer patient data from over 350 hospitals nationwide. Our initial analysis uses data from CIRF from June 1, 1993 to December 31, 2003 of all reported, histologically-confirmed cases of pancreatic adenocarcinoma. De-identified demographic factors in the registry include patient age, gender, race, and history of cigarette smoking and alcohol consumption. Secondarily, we perform confirmatory and dose-dependent analyses using the University of Michigan Pancreatic Cancer Registry (UMPCR). UMPCR prospectively collects clinical, demographic, surgical and histological data on all patients with histologically-confirmed pancreatic adenocarcinoma who are seen at the University of Michigan hospital systems and is a participating site in IMPAC. The analysis for this manuscript uses patient data of all reported pancreatic adenocarcinoma cases at the University of Michigan from January 2004 to October 2007; these data are independent from CIRF data. Quantitative data on alcohol consumption and cigarette smoking, and years of abstinence from smoking, were not reported in the CIRF database; these factors were assessed using UMPCR. Enrollment in UMPCR was performed in accordance with the institutional review board at the University of Michigan.
Variables and Definitions
Factors initially assessed include patient age, gender, and history of cigarette smoking and alcohol consumption. Within CIRF, smoking and alcohol use categories are classified as ‘current’, ‘past’, or ‘never’, based on self-reported information documented from each participating site. Current users smoked cigarettes or consumed alcohol within the year prior to diagnosis. Past substance users quit at least one year prior to diagnosis. The following histological diagnoses from the CIRF database were felt to represent pancreatic adenocarcinoma and are included in our analysis: adenocarcinoma (72.7%); carcinoma/epithelial tumor (16%); duct carcinoma (4.7%); mucin-producing adenocarcinoma (2.9%); and mucinous adenocarcinoma (2.7%). Adenosquamous carcinoma, anaplastic carcinoma, undifferentiated carcinoma, and cystadenocarcinoma categories each constitute less than 0.5% of the total study population and are also included in the analysis.
Within UMPCR, smoking history is assessed in pack-years of smoking and years since smoking cessation prior to pancreatic cancer diagnosis. Lifetime nonsmokers have no pack-year history of cigarette smoking and active smokers smoke at the time of diagnosis. Past smokers are classified as quitting one to ten years prior to diagnosis (1–10YPTD) or greater than ten years prior to diagnosis (>10YPTD). For comparison to CIRF data, UMPCR data are classified according to CIRF-defined criteria (e.g. individuals who quit smoking at least one year prior to diagnosis were categorized as “past smokers”). Alcohol consumption is classified as none for lifetime nondrinkers, minimum to moderate for individuals who drink fewer than three standard drinks per day (<39gm/day), and heavy for individuals who drink more than three standard drinks per day (≥39gm/day). For comparison to CIRF data, patients who described themselves on prospective intake questionnaires as “past drinkers” are classified as “past drinkers” regardless of the length of time since they stopped consuming alcohol.
Statistical Analysis
To evaluate age at diagnosis, we implemented a generalized linear regression model with age as the outcome variable. There were four potential explanatory variables: alcohol and smoking use history (current vs. previous vs. never), gender, and the interaction between alcohol and cigarette smoking history. Explanatory variables were chosen to enter the multiple models based on P-value <0.250 in univariate regressions. The initial multivariate model included all such explanatory variables. Non-significant variables were eliminated one at a time using a backwards elimination strategy. Confounding among variables was evaluated by comparing estimates and standard errors among univariate and multiple models. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.1 software (SAS Institute, Cary, NC).
Results
Dataset and Patient Demographics
CIRF consisted of 29,239 pancreatic cancer patients with a mean age of 68.8 [standard deviation (SD) =11.9] years; 49.1% were male, 85.5% were white and 11.7% were black. Data for alcohol consumption and smoking history were available for 65.8% and 73.4% of entries, respectively. UMPCR consisted of 922 patients; complete alcohol, smoking and diagnosis age data were available for 820 patients (88.9%) which were included in our analyses. Data for patients for whom smoking and alcohol consumption habits were not known were not analyzed. Since patients were de-identified, it was not feasible to obtain this missing data. Mean age at diagnosis in UMPCR was 63.0 (SD=11.3) years. The majority of patients were male (448, 54.6%), white (665, 92.5%), and non-diabetic (578, 70.6%), with no known family history of pancreatic cancer (732, 92.1%). Approximately 54% were smokers and 51% were drinkers. Summary quantitative patient data on alcohol and cigarette smoking status from both databases are shown in Table 1.
Table 1.
Quantity and percentage of patients in each smoking and drinking category, both databases
| CIRF | UMPCR | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| Current Smoker | 5597 | 26.1 | 208 | 25.4 |
| Past Smoker | 6595 | 30.8 | 237 | 28.9 |
| Never Smoker | 9256 | 43.2 | 375 | 45.7 |
| Total | 21,448 | 820 | ||
| Current Drinker | 5318 | 27.6 | 332 | 40.5 |
| Past Drinker | 2158 | 11.2 | 83 | 10.1 |
| Never Drinker | 11,764 | 61.1 | 405 | 49.4 |
| Total | 19,240 | 820 | ||
Effect of Smoking on Age at Diagnosis
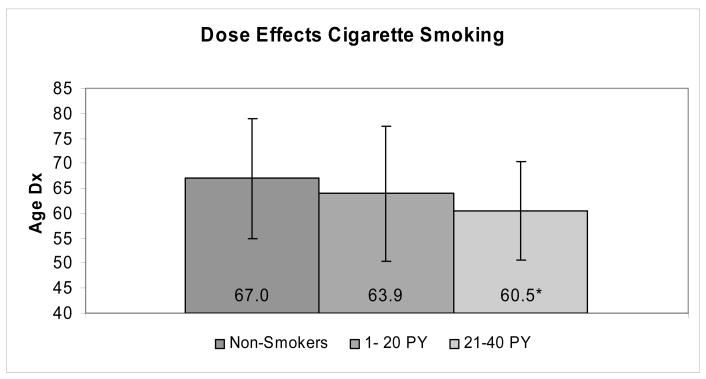
Overall, current smokers were diagnosed at significantly earlier ages than their non-smoking counterparts in CIRF and UMPCR (8.3 years and 6.3 years earlier, respectively; see table 2). In both databases, combining current smokers with past smokers into an ‘ever smokers’ group resulted in an intermediate age at diagnosis that was significantly younger than non-smokers. Dose-dependent effects of cigarette smoking on age of diagnosis were observed in UMPCR. Among nondrinkers, age at pancreatic cancer diagnosis differed by cigarette smoking pack-year history and duration of smoking cessation (Figure 1). Nondrinkers with a 1–20 pack year history and 21–40 pack year history of smoking presented 3.1 years (P = 0.077) and 6.6 years (P = 0.0001) earlier, respectively, than individuals who neither drank nor smoked.
Table 2.
Age at Pancreatic Cancer Diagnosis and Lifetime Cigarette Smoking Habits, Stratified by Gender
| CIRF | |||
|---|---|---|---|
| Males only | Females only | Both genders | |
| Current Smokers | 61.9 ± 11.0a | 64.0 ± 11.6a | 62.8 ± 11.3a |
| Past Smokers | 69.0 ± 10.2 | 69.3 ± 10.6a | 69.1 ± 10.4a |
| Never smokers† | 68.9 ± 12.0 | 72.5 ± 11.6 | 71.2 ± 11.9 |
| Ever Smokers | 65.9 ± 11.2a | 66.7 ± 11.4a | 66.2 ± 11.3a |
| Never smokers† | 68.9 ± 12.0 | 72.5 ± 11.6 | 71.2 ± 11.9 |
| UMPCR | |||
| Males only | Females only | Both genders | |
| Current Smokers | 58.1 ± 8.6a | 58.2 ± 11.6a | 58.1 ± 10.0a |
| Past Smokers | 64.6 ± 9.5 | 65.9 ± 9.0 | 65.0 ± 9.3 |
| Never smokers† | 62.3 ± 12.1 | 66.5 ± 12.2 | 64.4 ± 12.3 |
| Ever Smokers | 61.8 ± 9.7 | 61.8 ± 11.1a | 61.8 ± 10.2b |
| Never smokers† | 62.3 ± 12.1 | 66.5 ± 12.2 | 64.4 ± 12.3 |
reference group is never smokers
P <.001
P <.05
Figure 1.
Dose effects (lifetime pack years of cigarette smoking) vs. non-smoking on age at pancreatic cancer diagnosis for non-drinkers in UMPCR
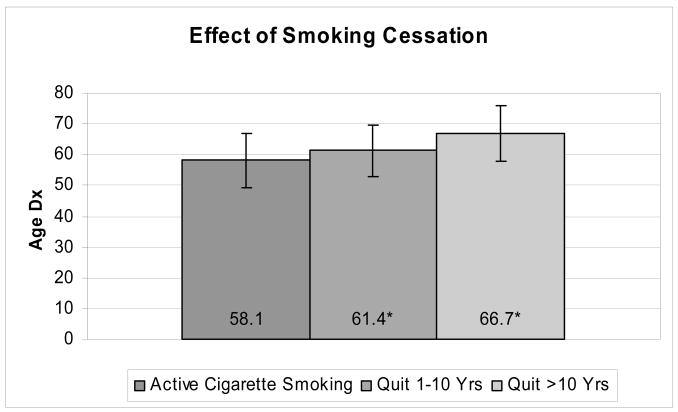
The age of diagnosis for individuals who had stopped smoking differed modestly from non-smokers; in the CIRF database, age at diagnosis for past smokers was 2.1 years younger than age at diagnosis of non-smokers (P <0.001); in UMPCR, there was no significant difference in age at diagnosis (64.4 vs 65.0 years, nonsmokers vs past smokers, P =0.49). The effect of smoking cessation was illustrated in UMPCR; patients who had quit smoking 1–10 YPTD and >10 YPTD had ages at diagnosis 3.3 (P <0.01) and 8.6 (P <0.0001) years later than those of active smokers (Figure 2). Table 2 demonstrates effects of smoking on age at pancreatic cancer diagnosis, stratified by gender. Gender effects in CIRF were isolated to non-smokers and current smokers; female non-smokers were diagnosed with pancreatic cancer on average 3.6 years later than males (P <0.001), and female current smokers were diagnosed on average 2.1 years later than males (P <0.001).
Figure 2.
Comparison of years of smoking cessation vs. active smoking on age at pancreatic cancer diagnosis in UMPCR
Effect of Alcohol consumption on Age at Diagnosis
Overall, current drinkers were diagnosed at significantly younger ages than their never-drinking counterparts in both CIRF and UMPCR (Table 3). In the CIRF database, females were consistently diagnosed at later ages than males in all three alcohol consumption groups. In UMPCR, gender disparity was significant only for never-drinkers (63.4 vs 66.4 years, male vs female, P = 0.01). In both databases, combining current drinkers with past drinkers into a group called ‘ever drinkers’ resulted in an intermediate age at diagnosis that was significantly younger than never-drinkers. Dose-dependent effects of alcohol on diagnosis age were observed in UMPCR (data not shown). Among nonsmokers, age at pancreatic cancer diagnosis differed in a linear and progressive fashion according to the amount of alcohol consumed; patients reporting minimal, moderate and heavy alcohol consumption presented 6.2, 7.5 and 8.7 years earlier than lifelong non-smokers/non-drinkers, respectively (all P <0.01). Past drinkers were diagnosed a non-significant 4.6 years earlier than their non-smoking/non-drinking counterparts.
Table 3.
Age at Pancreatic Cancer Diagnosis and Lifetime Alcohol Consumption Habits, Stratified by Gender
| CIRF | |||
|---|---|---|---|
| Alcohol Use | Males only | Females only | Both genders |
| Current drinkers | 64.0 ± 11.3a | 66.3 ± 11.8a | 65.5 ± 11.5a |
| Past drinkers | 65.3 ± 10.8a | 66.7 ± 11.4a | 65.6 ± 11.0a |
| Never drinkers† | 68.5 ± 11.7 | 71.2 ± 11.7 | 70.2 ± 11.8 |
| Ever drinkers | 65.1 ± 11.1a | 66.4 ± 11.8a | 65.5 ± 11.4a |
| Never drinkers† | 68.5 ± 11.7 | 71.2 ± 11.7 | 70.2 ± 11.8 |
| UMPCR | |||
| Alcohol Use | Males only | Females only | Both genders |
| Current drinkers | 61.1 ± 11.1b | 60.9 ± 11.2a | 61.0 ± 11.1a |
| Past drinkers | 61.3 ± 8.6 | 59.0 ± 9.2b | 60.7 ± 8.8b |
| Never drinkers† | 63.4 ± 10.8 | 66.4 ± 12.0 | 65.1 ± 11.6 |
| Ever drinkers | 61.2 ± 10.6b | 60.6 ± 10.9a | 61.0 ± 10.7b |
| Never drinkers† | 63.4 ± 10.8 | 66.4 ± 12.0 | 65.1 ± 11.6 |
reference group is never drinkers
P <.001
P <.05
Interaction of cigarette smoking and alcohol consumption
The interactive effects of cigarette smoking and alcohol consumption on age difference at pancreatic cancer diagnosis are displayed in table 4. In CIRF, current smokers who were also current drinkers were diagnosed a significant average of 10.2 years earlier than lifetime non-users. The effects of current smoking for non-drinkers on diagnosis age were greater than current drinking for non-smokers (8 years earlier for the former vs. 3.6 years earlier for the latter). In UMPCR, compared to never drinkers/never smokers, current smokers/current drinkers were diagnosed a significant 8.6 years earlier, while past drinkers/current smokers were diagnosed a significant 9.8 years earlier than never users. Similar to CIRF, current smoking in UMPCR had a greater effect on diagnosis age in non-drinkers than current drinking in non-smokers. In CIRF, past smokers who were lifetime non-drinkers were diagnosed 1.5 years earlier than lifetime non-smokers/non-drinkers; in UMPCR, past smokers were not diagnosed significantly earlier in any drinking category than lifetime non-smokers.
Table 4.
Mean Age Difference at Pancreatic Cancer Diagnosis Stratified by Lifetime Smoking and Drinking Habitsa
| CIRF | ||||||
|---|---|---|---|---|---|---|
| smoking | drinking | estimated mean difference | standard error | 95% CI | P valueb | |
| current | −10.2 | 0.29 | −10.8 to | −9.7 | <.0001 | |
| past | −10.1 | 0.45 | −11.0 to | −9.2 | <.0001 | |
| current | never | −8.0 | 0.29 | −8.5 to | −7.4 | <.0001 |
| current | −3.0 | 0.29 | −3.6 to | −2.4 | <.0001 | |
| past | −3.4 | 0.37 | −4.1 to | −2.6 | <.0001 | |
| past | never | −1.5 | 0.26 | −2.0 to | −1.0 | <.0001 |
| current | −3.6 | 0.34 | −4.3 to | −2.9 | <.0001 | |
| past | −3.5 | 0.66 | −4.8 to | −2.2 | <.0001 | |
| never | never† | 0 | N/A | N/A | N/A | N/A |
| UMPCR | ||||||
| smoking | drinking | estimated mean difference | standard error | 95% CI | P valueb | |
| current | −8.6 | 1.37 | −11.3 to | −5.9 | <.0001 | |
| past | −9.8 | 1.88 | −13.4 to | −6.1 | <.0001 | |
| current | never | −8.2 | 1.36 | −10.8 to | −5.5 | <.0001 |
| current | −2.2 | 1.26 | −4.7 to | 0.3 | 0.08 | |
| past | −2.1 | 1.96 | −5.9 to | −1.8 | 0.29 | |
| past | never | −0.7 | 1.32 | −3.3 to | −1.9 | 0.61 |
| current | −6.4 | 1.17 | −8.8 to | −4.1 | <.0001 | |
| past | −4.0 | 3.46 | −10.8 to | 2.8 | 0.25 | |
| never | never† | 0 | N/A | N/A | N/A | N/A |
Abbreviations: CI, confidence interval; N/A, not applicable
reference group is never smokers/never drinkers
All analyses are adjusted for gender
P value is probability > chi square
Discussion
Recent nationally representative Surveillance Epidemiology End Results (SEER) data observed that fewer than 12.5% of pancreatic cancer cases are diagnosed before age 55 and over two-thirds (68.6%) of all cases are diagnosed at or above age 65.15 Our study provides evidence that current cigarette smoking substantially reduces the age at pancreatic cancer diagnosis in the general population, while past cigarette smoking has less substantial effects on diagnosis age. Additionally, consuming alcohol significantly decreases the apparent age of pancreatic cancer presentation regardless of cigarette smoking habits. This information is unequivocally valuable to clinicians who should have increased vigilance for this highly fatal cancer among particular subsets of patients.
Cigarette smoking is a well-established risk factor for pancreatic cancer6,16 although the exact mechanism by which smoking triggers pancreatic tumor development is undefined. Dozens of carcinogens found in cigarette smoke reach the pancreas from the bloodstream and refluxed bile.17–19 Both tobacco-specific nitrosamines nicotine metabolites have been found in the pancreatic juice of smokers.20 Some susceptible individuals may lack functional protective enzyme systems necessary to detoxify tobacco products such as cytochrome P 4501A2 and n-acetyl transferase enzymes, which may differentially affect risk among smokers.21 Smoking also up-regulates pancreatic heterogeneous nuclear ribonucleoprotein, which may play a role in cancer development.22 Additionally, cigarette smoking may trigger chronic inflammation,23 evidenced by its effects on risk and progression of inflammatory-related conditions, such as alcohol-induced chronic pancreatitis24 and Crohn’s disease.25
Parallel trends in age at pancreatic cancer diagnosis have been reported in smokers from high risk pancreatic cancer-prone kindreds and smokers with hereditary pancreatitis.9,10 A recent study of early-onset pancreatic cancer noted that both family history and cigarette smoking influenced the age of diagnosis of pancreatic cancer26; the authors observed that the proportion of early-onset pancreatic cancer worldwide correlated strongly with lung cancer mortality, suggesting that about half of the variation in the proportion of early-onset pancreatic cancer might be explained by smoking.
Our study re-enforced the observation that current, active smoking may have a greater impact than past smoking on pancreatic carcinogenesis. Similarly, a frequently cited study published in 1996 noted a 2.5-fold increased risk of pancreatic cancer among active smokers6; however, risk decreased by 48% within 2 years of smoking cessation and 10 years after cessation, pancreatic cancer risk was almost equivalent to lifetime non-smokers. Other studies have observed similar decreases in risk in as little as 5 years of smoking cessation.27 Our results clarify the continued need for smoking cessation programs on local and national levels.
Some studies demonstrate an association between heavy alcohol consumption and pancreatic cancer development.8,13,14 A recent study of 808 pancreatic cancer patients and 808 healthy, frequency-matched controls who underwent in-person interviews found a 1.6-fold increased risk of pancreatic cancer among heavy drinkers—the same risk level that they noted among ‘ever’ smokers.8 The relationship is plausible, because alcohol’s metabolites, ethanol and acetaldehyde, are known carcinogens.28 Theoretically, heavy alcohol consumption may increase the risk of chronic pancreatitis or diabetes, known risk factors for pancreatic cancer.29–30
Individuals who were smokers and reported minimal to moderate alcohol consumption were diagnosed with pancreatic cancer at a slightly later age than active smokers without a history of drinking. The seemingly protective effect of minimal to moderate alcohol consumption was confined to active smokers. Health effects of alcohol consumption have been characterized as having a J- or U-shaped curve, with non-drinkers and heavy drinkers having increased health risks while moderate drinkers have decreased risks of illnesses such as heart disease and diabetes, and overall mortality.31,32 Two past studies noted decreased pancreatic cancer risk in regular or moderate wine drinkers.33,34 Moderate alcohol consumption may function as a surrogate for a healthy lifestyle and related, preventive behaviors, such as exercising or not smoking, might be responsible for the observed protective health effects.35 An alternative explanation for the association of earlier diagnosed pancreatic cancer in heavy drinkers is that heavy drinking is simply a surrogate marker for chronic pancreatitis.
We noted a difference in age at pancreatic cancer diagnosis between men and women within similar smoking and drinking categories. In the United States, pancreatic cancer incidence is lower for women than for men in all racial groups.35 Theories of why this disparity exists have focused on the protective role of reproductive factors, although the only consistent observation has been that increased parity may be protective for women.36,37 Because only broad categories were available in CIRF, women may have had lower use levels than men, reflective of national trends in cigarette smoking and alcohol consumption.38,39
Smokers may be hypothesized to have an earlier age of pancreatic cancer presentation than non-smokers due to competing risks of mortality.40 Cigarette smoking is recognized as having one of the greatest attributable risks for mortality in the United States, causing a variety of cancers as well as cardiovascular disease and stroke.41,42 The phenomena of competing mortality risks was addressed in a study by Zisman et al43 that examined age at colorectal cancer diagnosis in relation to cigarette smoking and alcohol consumption, similarly using the CIRF database. The authors noted that if competing causes of mortality were responsible for the younger age at colorectal cancer diagnosis observed for smokers, the age of diagnosis of all cancers should be earlier. Thereafter, they examined age of prostate cancer diagnosis in the CIRF database in relation to cigarette smoking, because prostate cancer development is not known to be associated with cigarette smoking.44 The authors found that smoking was not related to age at prostate cancer diagnosis; in fact, prostate cancer patients who were current smokers were diagnosed at later ages than patients who were lifetime non-smokers.
Strengths of our study include study size and histological verification of pancreatic cancer for all cases. These studies were possible because the CIRF database is sufficiently powered to evaluate the impact of cigarette smoking on patient age at diagnosis among the general United States population. Data contained in the CIRF registry derive from a very large number of hospitals and cancer centers and all cancer diagnoses must be recorded in the National Cancer Data Base (NCDB). CIRF data is a valid population sample which accurately reflects national observations; race, gender, stage of disease at diagnosis and tumor location are consistent with those found in the SEER database (data not shown).15
Our study has some notable limitations. Being a case-only study, our research did not evaluate causation, but rather effects on age at diagnosis of pancreatic cancer; the results demonstrate correlation although cause cannot explicitly be implied. Another limitation of this study was that many potentially confounding factors could not be addressed because the CIRF database is not linked to medical records. A recent review of pancreatic cancer epidemiology noted the influence of numerous host and/or environmental factors on risk, including family history, chronic pancreatitis, body mass index, dietary mutagens, exercise habits, diabetes mellitus, fruit and vegetable consumption, and additional factors that we could not evaluate in our study.45
Another study limitation is the broad classification of substance use in the CIRF database. ‘Past users,’ for instance, may include patients with low-level substance who stopped many years earlier as well as individuals who were heavy or very heavy users and quit more recently. There is a well-established correlation between nicotine addiction and alcoholism and use of one substance often predicts use of the other.46 The lack of cigarette and alcohol use levels prevented us from assessing dose-response effects on pancreatic cancer risk in the CIRF database, a limitation we addressed by including UMPCR data in separate analyses. Although approximately one third of patients lacked smoking and/or alcohol data, these substances are not universally recognized pancreatic cancer risk factors (as opposed to lung or liver cancer, respectively), and a great use differential between groups that did or did not have use habits documented is unlikely. Sensitivity analysis demonstrated that given any assumptions regarding smoking status of the non-responders, the results remained statistically significant (data not shown).
In summary, our study is the first to provide compelling evidence that smoking and drinking significantly decrease the age at pancreatic cancer diagnosis among individuals not in high-risk groups. Our results underscore the importance of counseling patients who smoke on the value of quitting. They also reassert the need to focus efforts on preventing smoking initiation as well as the continued institution of smoking cessation programs, to diminish mortality and potential years of life lost due to this lethal disease.
Acknowledgments
Funding for work at the University of Michigan Medical Center was from the University of Michigan Cancer Center Support Grant NIH 5 P30 CA46592-19.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of authors in their work on this manuscript was as follows: Randall E. Brand was responsible for study concept and design, the final analysis and interpretation of data, drafting of the manuscript and critical revision of the manuscript for important intellectual content
Julia B. Greer was responsible for study concept and design, and was the main author responsible for drafting of the manuscript and critical revision of the manuscript for important intellectual content
Eugene Zolotarevsky was responsible for study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content
Rhonda Brand was responsible for study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content
Hongyan Du was responsible for statistical analysis and interpretation of data
Diane Simeone was the main person responsible for data acquisition and also performed critical revision of the manuscript for important intellectual content
Anna Zisman was responsible for study concept and design, analysis of data and drafting of the manuscript
Addi Gorchow was responsible for acquisition of data, analysis and interpretation of data and the drafting of the manuscript
Shih-Yuan (Connie) Lee performed statistical analysis and was responsible for the interpretation of data
Hemant Roy was responsible for study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content
Michelle A. Anderson provided overall study supervision and was responsible for study concept and design, and critical revision of the manuscript for important intellectual content
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56(8):1134–52. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Egawa S, Takeda K, Fukuyama S, et al. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28(3):235–40. doi: 10.1097/00006676-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kalapothaki V, Tzonou A, Hsieh CC, et al. Tobacco, ethanol, coffee, pancreatitis, diabetes mellitus, and cholelithiasis as risk factors for pancreatic carcinoma. Cancer Causes Control. 1993;4(4):375–82. doi: 10.1007/BF00051341. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156(19):2255–60. [PubMed] [Google Scholar]
- 7.Harnack LJ, Anderson KE, Zheng W, et al. Smoking, alcohol, coffee, and tea intake and incidence of cancer of the exocrine pancreas: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 1997;6(12):1081–6. [PubMed] [Google Scholar]
- 8.Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102(12):2696–707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286(2):169–70. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 10.Rulyak SJ, Lowenfels AB, Maisonneuve P, et al. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124(5):1292–9. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- 11.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64(7):2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 12.Olsen GW, Mandel JS, Gibson RW, et al. A case-control study of pancreatic cancer and cigarettes, alcohol, coffee and diet. Am J Public Health. 1989;79(8):1016–9. doi: 10.2105/ajph.79.8.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partanen TJ, Vainio HU, Ojajarvi IA, et al. Pancreas cancer, tobacco smoking and consumption of alcoholic beverages: a case-control study. Cancer Lett. 1997;116(1):27–32. doi: 10.1016/s0304-3835(97)04744-7. [DOI] [PubMed] [Google Scholar]
- 14.Silverman DT, Hoover RN, Brown LM, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology. 2003;14(1):45–54. doi: 10.1097/00001648-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2005. [Google Scholar]
- 16.Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jap J Clin Oncol. 2004;34(5):238–44. doi: 10.1093/jjco/hyh045. [DOI] [PubMed] [Google Scholar]
- 17.Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg. 2006;391(6):603–13. doi: 10.1007/s00423-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 18.Rivenson A, Hoffmann D, Prokopczyk B, et al. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48(23):6912–7. [PubMed] [Google Scholar]
- 19.Schuller HM, Jorquera R, Reichert A, et al. Transplacental induction of pancreas tumors in hamsters by ethanol and the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1993;53(11):2498–501. [PubMed] [Google Scholar]
- 20.Prokopczyk B, Hoffmann D, Bologna M, et al. Identification of tobacco-derived compounds in human pancreatic juice. Chem Res Toxicol. 2002;15(5):677–85. doi: 10.1021/tx0101088. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Jiao L, Li Y, et al. Polymorphisms of cytochrome P4501A2 and N-acetyltransferase genes, smoking, and risk of pancreatic cancer. Carcinogenesis. 2006;27(1):103–11. doi: 10.1093/carcin/bgi171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan-Sanders Y, Hammons GJ, Lyn-Cook BD. Increased expression of heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNP) in pancreatic tissue from smokers and pancreatic tumor cells. Cancer Lett. 2002;183(2):215–20. doi: 10.1016/s0304-3835(02)00168-4. [DOI] [PubMed] [Google Scholar]
- 23.Malfertheiner P, Schutte K. Smoking--a trigger for chronic inflammation and cancer development in the pancreas. Am J Gastroenterol. 2006;101(1):160–2. doi: 10.1111/j.1572-0241.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 24.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54(4):510–4. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahid SS, Minor KS, Stevens PL, et al. The role of smoking in Crohn’s disease as defined by clinical variables. Dig Dis Sci. 2007;52(11):2897–903. doi: 10.1007/s10620-006-9624-0. [DOI] [PubMed] [Google Scholar]
- 26.Raimondi S, Maisonneuve P, Lohr JM, et al. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1894–7. doi: 10.1158/1055-9965.EPI-07-0341. [DOI] [PubMed] [Google Scholar]
- 27.Nilsen TI, Vatten LJ. A prospective study of lifestyle factors and the risk of pancreatic cancer in Nord-Trondelag, Norway. Cancer Causes Control. 2000;11(7):645–52. doi: 10.1023/a:1008916123357. [DOI] [PubMed] [Google Scholar]
- 28.Baan R, Straif K, Grosse Y, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8(4):292–3. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 29.Whitcomb DC. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–G9. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 30.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129(2):504–11. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beulens JWJ, Stolk RP, van der Schouw YT, et al. Alcohol consumption and risk of type 2 diabetes among older women. Diabetes Care. 2005;28(12):2933–8. doi: 10.2337/diacare.28.12.2933. [DOI] [PubMed] [Google Scholar]
- 32.Scherr PA, LaCroix AZ, Wallace RB, et al. Light to moderate alcohol consumption and mortality in the elderly. Journal of the American Geriatrics Society. 1992;40(7):651–7. doi: 10.1111/j.1532-5415.1992.tb01954.x. [DOI] [PubMed] [Google Scholar]
- 33.Bueno de Mesquita HB, Maisonneuve P, Moerman CJ, et al. Lifetime consumption of alcoholic beverages, tea and coffee and exocrine carcinoma of the pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1992;50(4):514–22. doi: 10.1002/ijc.2910500403. [DOI] [PubMed] [Google Scholar]
- 34.Gold EB, Gordis L, Diener MD, et al. Diet and other risk factors for cancer of the pancreas. Cancer. 1985;55:460–7. doi: 10.1002/1097-0142(19850115)55:2<460::aid-cncr2820550229>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 35.Goldfinger TM. Beyond the French paradox: the impact of moderate beverage alcohol and wine consumption in the prevention of cardiovascular disease. Cardiology Clinics. 2003;21(3):449–57. doi: 10.1016/s0733-8651(03)00081-x. [DOI] [PubMed] [Google Scholar]
- 36.Skinner HG, Michaud DS, Colditz GA, et al. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12(5):433–8. [PubMed] [Google Scholar]
- 37.Teras LR, Patel AV, Rodriguez C, et al. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. women (United States) Cancer Causes Control. 2005;16(9):1035–40. doi: 10.1007/s10552-005-0332-4. [DOI] [PubMed] [Google Scholar]
- 38.Department of Health and Human Services (HHS) Substance Abuse and Mental Health Services Administration (SAMHSA) Factsheet. National Household Survey on Drug Abuse. 2001 [Google Scholar]
- 39.American Lung Association. [Accessed July 18, 2008];Women and Smoking Factsheet. 2007 June; Available at: http://www.lungusa.org/site/pp.asp?c=dvLUK9O0E&b=33572.
- 40.Chiang CL. Competing risks in mortality analysis. Annual Review Pub Health. 1991;12:281–307. doi: 10.1146/annurev.pu.12.050191.001433. [DOI] [PubMed] [Google Scholar]
- 41.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–12. [PubMed] [Google Scholar]
- 42.Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction Circulation. 1997;96:3243–7. doi: 10.1161/01.cir.96.9.3243. [DOI] [PubMed] [Google Scholar]
- 43.Zisman AL, Nickolov A, Brand RE, et al. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med. 2006;166(6):629–34. doi: 10.1001/archinte.166.6.629. [DOI] [PubMed] [Google Scholar]
- 44.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 45.Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6(3):275–82. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 46.Falk DE, Yi H-y, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–71. [PMC free article] [PubMed] [Google Scholar]