Abstract
Vasoactive intestinal peptide (VIP) is a 28 amino acid peptide which belongs to a superfamily of structurally related peptide hormones including pituitary adenylate cyclase-activating polypeptide (PACAP). Although several studies have identified the involvement of PACAP in learning and memory, little work has been done to investigate such a role for VIP. At least three receptors for VIP have been identified including the PACAP receptor (PAC1-R) and the two VIP receptors (VPAC receptors). VIP can activate the PAC1-R only if it is used at relatively high concentrations (e.g. 100 nM); however, at lower concentrations (e.g. 1 nM) it is selective for the VPAC receptors. Our lab has showed that PAC1-R activation signals through PKC/CAKβ/Src pathway to regulate NMDA receptors, however there is little known about the potential regulation of NMDA receptors by VPAC receptors. Our studies demonstrated that application of 1nM VIP enhanced NMDA currents by stimulating VPAC receptors as the effect was blocked by VPAC receptor antagonist [Ac-Tyr1, D-Phe2]GRF (1–29). This enhancement of NMDA currents was blocked by both Rp-cAMPS and PKI14–22 (they are highly specific PKA inhibitors), but not by the specific PKC inhibitor, bisindolylmaleimide I. In addition, the VIP-induced enhancement of NMDA currents was accentuated by inhibition of phosphodiesterase 4, which inhibits the degradation of cAMP. This regulation of NMDA receptors also required the scaffolding protein AKAP. In contrast, the potentiation induced by high concentration of VIP (e.g. 100 nM) was mediated by PAC1-R as well as by Src kinase. Overall, these results show that VIP can regulate NMDA receptors through different receptors and signaling pathways.
Keywords: VIP, NMDA receptor, cAMP/PKA, VPAC receptor, AKAP
Introduction
Vasoactive intestinal peptide (VIP) was initially isolated from porcine intestine as a 28-amino acid peptide (Said and Mutt, 1970). It was identified as a vasodilator in the canine femoral artery. Subsequently, it was shown to serve potentially as a neuromodulator, a neurotrophic factor and/or a potential neurotransmitter (Harmar et al., 1998). Furthermore, it is neuroprotective and enhances cell proliferation in both the peripheral and central nervous systems (Harmar et al., 1998). Although studies showed that the application of VIP agonist could improve memory deficit in a animal model of Alzheimer’s disease (Gozes et al., 1997), the potential role of VIP in the regulation of synaptic plasticity remains unknown.
Vasoactive intestinal peptide belongs to a superfamily of structurally related peptide hormones that stimulate protein kinase A (PKA). This family also includes pituitary adenylate-activating polypeptide (PACAP). PACAP shares 68% sequence similarity to VIP in their N-terminal residues from 1 to 28 (Harmar et al., 1998). At least three receptors for VIP have been identified and they all belong to family B of G-protein-coupled receptors (GPCRs). The PACAP-specific receptor (PAC1-R) exhibits a much higher affinity for PACAP than VIP, whereas VIP receptors, VPAC1-R and VPAC2-R, have similar affinities for PACAP and VIP (Harmar et al., 1998).
Glutamate is the transmitter at the majority of central excitatory synapses and in the hippocampus glutamate receptors are essential for both postsynaptic responses and for the plasticity that underlies some forms of spatial learning and memory (Citri and Malenka, 2008). Long-term potentiation (LTP) and long-term depression (LTD) of the excitatory synapses between CA3 and CA1 hippocampal pyramidal neurons are well characterized forms of plasticity which serve as molecular models of learning and memory (Malenka and Bear, 2004).
At CA3-CA1 synapses the amplitude of baseline excitatory synaptic postsynaptic potentials is determined almost entirely by alpha-amino-3-hydroxy-5-methyl-4 isoxazolepropionic acid receptors (AMPARs). However, synaptic activation of N-methyl-d-aspartate receptors (NMDARs) is required for the induction of either LTP or LTD and cell signals that alter NMDAR activity or expression provide a metaplastic control of plasticity (Abraham, 2008;Abraham and Bear, 1996;Chen and Bear, 2007).
N-methyl-D-aspartate receptor (NMDAR) activity at CA3-CA1 hippocampal synapses is tightly regulated by various GPCRs acting via non-receptor tyrosine kinases such as Pyk2, Src and Fyn (Macdonald et al., 2005;Yaka et al., 2003). For example, we have shown mGluR5, M1 and LPA receptors enhance NMDAR-mediated currents via a calcium-dependent and sequential enzyme signalling cascade that consists of the serine-threonine protein kinase C (PKC), and the non-receptor tyrosine kinases Pyk2 and Src (Kotecha et al., 2003;Lu et al., 1999). Furthermore, LTP at CA3-CA1 synapses requires activation of Pyk2 and Src (Huang et al., 2001). Also PACAP acts via the PAC-1 receptor to enhance NMDA-evoked currents in CA1 hippocampal neurons and it does so by stimulating this sequential Gαq/PKC/Pyk2/Src signal transduction cascade rather than by stimulating the typical Gαs, adenylate cyclase and protein kinase A (PKA) pathway (Macdonald et al., 2005).
Protein kinase A also modulates NMDARs. For example, β-adrenergic receptor agonists increase the amplitude of NMDA excitatory postsynaptic currents (EPSCs) (Raman et al., 1996) and activation of PKA also induces targeting of NMDA receptors to synapses (Crump et al., 2001). Recent studies have shown that the Ca2+ permeability of NMDARs is also under the control of the cyclic AMP (cAMP)-PKA signalling cascade and PKA inhibitors reduce the relative fraction of Ca2+ influx through NMDARs (Skeberdis et al., 2006). The inhibitory molecule, Inhibitor 1 (I-1), which targets the serine/threonine protein phosphatase 1 (PP1) is also a key substrate of PKA. By this means, PKA activation leads to inhibition of PP1 and decreased dephosphorylation (enhanced phosphorylation) of NMDARs (Svenningsson et al., 2004). In addition, the cAMP/PKA signal pathway facilitates the induction of LTP at CA3-CA1 synapses and contributes to the late, protein synthesis-dependent phase of NMDAR-dependent LTP and hippocampal memory formation (Skeberdis et al., 2006).
Using in situ hybridization, autoradiography and immunohistochemistry, PAC1-R, VPAC1-R and VPAC2-R have been identified within the hippocampus (Joo et al., 2004). Although these receptors are best known for their ability to stimulate Gαs, adenylate cyclase, cAMP production and subsequently activate PKA, signalling may also occur in some tissues via Gq/11 and PKC (Harmar et al., 1998). Cunha-Reis et al (2005) reported that VPAC2-R enhanced transmission via the anticipated stimulation of PKA but VPAC1-R did so as a consequence of PKC activation (Cunha-Reis et al., 2005). Given the importance of NMDARs in the induction of LTP we have clarified VIP-dependent signalling to the NMDARs of CA1 pyramidal neurons.
Materials and methods
Cell isolation and whole-cell recordings
CA1 neurons were isolated from postnatal rats (Wistar, 14–22 d) using previously described procedures (Wang and MacDonald, 1995). Following halothane anesthetisation and decapitation, the brain was extracted to cold extracellular fluid (ECF). The extracellular solution consisted of (in mM): 140 NaCl, 1.3 CaCl2, 5 KCl, 25 HEPES, 33 glucose, and 0.0005 tetrodotoxin, pH 7.4 (osmolarity between 315 and 325 mOsm). The hippocampus was rapidly removed and transverse slices were made by hand. After a mild enzymatic digestion, slices were washed three times in fresh ECF and allowed to recover in oxygenated ECF at room temperature (20–22ºC) for two hours before use. Cells were patch clamped using glass recording electrodes (resistances of 3–5 MΩ) constructed from borosilicate glass (1.5 μm diameter, WPI) using a two-stage puller (PP83, Narashige, Tokyo, Japan) and filled with intracellular solution that contained (in mM): 140 CsF, 11 EGTA, 1 CaCl2, 2 MgCl2, 10 HEPES, 2 tetraethylammonium, and 2 K2ATP, pH 7.2 (osmolarity between 290 and 300 mOsm). Drugs or peptides were applied to individual CA1 neurons using a rapid perfusion system (Lu et al., 1999;Macdonald et al., 2005). Upon approaching the cell negative pressure (suction) was applied to the patch pipette to form a seal. After the formation of a tight seal (>1GΩ) negative pressure was then used to rupture the membrane and provide whole cell access. When the whole-cell configuration is formed, the neurons were voltage clamped at −60mV and lifted into a stream of solution supplied by a computer-controlled, multi-barrelled perfusion system. To monitor access resistance, a voltage step of −10 mV was made before each application of NMDA. When series resistance varied by >10%, the cell was discarded. Occasionally drugs were included in the patch pipette. Recordings were conducted at room temperature (20–22°C). Currents were recorded using Axopatch 1D or 200 amplifiers (Axon Instruments, Union City, CA), and data were filtered at 2 kHz and acquired using Clampex (Axon Instruments). To control for variation in response, recordings from control and treated cells were made on the same day. All population data are expressed as mean ± S.E. The Student’s t-test was used to compare between groups and the ANOVA test was used to analyze multiple groups.
Hippocampal slice preparation and recording
Transverse hippocampal slices were prepared from 4- to 6-week-old male Wistar rats using a vibratome (VT100E, Leica) and placed in a holding chamber for at least 1 hr prior to recording in oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (aCSF) (in mM: NaCl, 124.0; KCl, 3.0; MgCl2-6H2O, 1.3; CaCl2, 2.6; NaH2PO4-H2O, 1.25; NaHCO3, 26; D-glucose, 10.0, osmolarity between 300–310 mOsm). A single slice was transferred to the recording chamber continually superfused with oxygenated aCSF at 28–30°C. Synaptic responses were evoked with a bipolar tungsten electrode located about 50 μm from the cell body layer in CA1. Test stimuli were evoked at 0.05 Hz with the stimulus intensity set to 50% of maximal synaptic response. For voltage-clamp experiments the patch pipette (4–6 MΩ) solution contained 132.5 mM Cs-gluconate, 17.5 mM CsCl, 10 mM HEPES, 0.2 mM EGTA, 2 mM Mg-ATP, 0.3 mM GTP, and 5 mM QX 314 (pH 7.25, 290 mOsm). Patch recordings were performed using the “blind” patch method. 10uM bicuculline methiodide and 10uM CNQX was added into ACSF to isolate NMDA receptor mediated EPSCs. Cells were held at −60 mV and series resistance was monitored throughout the recording period. Only recordings with stable holding current and series resistance maintained below 30 MΩ were considered for analysis. Signals were amplified using an Axopatch 1D, sampled at 5 KHz, and analyzed with Pclamp6 software (Axon Instruments, Foster City, CA).
Membrane Adenylyl cyclase assay
Hippocampal tissue from postnatal rats (Wistar, 14–22 d) was dounce-homogenized on ice in 1 ml ice-cold buffer (15 mM HEPES, pH 7.4, 5 mM MgCl2, 2 mM EDTA, 1 mM IBMX, 0.01% ascorbic acid). Approximately 40 μg of tissue homogenate was incubated with drugs (control or 1nM VIP) and reaction mixture (2 mM ATP, 5 μM GTP, 20 mM phosphocreatine, 5 U creatine phosphokinase) at 30 ºC for 15 minutes. This reaction was stopped by 100 μl ice-cold 4% trichloroacetic acid. Then cAMP levels were calculated using the BTI Cyclic AMP radioimmunoassay Kit (Biomedical Technologies, Stoughton, MA, USA) according to manufacturer’s instructions (Macdonald et al., 2005). First samples were incubated overnight with tracer 125I2′-0-succinyl-cAMP and a cyclic AMP binding antibody. The tracer 125I2′-0-succinyl-cAMP is added to each tube at a concentration of 200pmol/nL. After incubation, samples were mixed with 1 ml ice-cold buffer (50 mM sodium acetate, 0.01% sodium azide, pH 7.4) and centrifuged at 2000 × g for 20 minutes. The supernatant was decanted and the radioactivity level bound to the antibody was determined.
Animals
All animal experimentation was conducted in accordance with the Policies on the Use of Animals at the University of Toronto.
Drugs and peptides
The drugs for this study are as follows: NMDA, glycine, bicuculline methiodide and BAPTA from Sigma (St. Louis, MO, USA), VIP, Rp-cAMPS, PKI14–22, bisindolylmaleimide I and phosphodiesterase 4 inhibitor (3,5-Dimethyl-1-(3-nitrophenyl)-1H-pyrazole-4-carboxylic acid ethyl ester) from Calbiochem (San Diego, CA, USA), InCELLect AKAP St-Ht31 inhibitor peptide from Promega (Madison, WI, USA), Bay55-9877, [Ala11, 22, 28]VIP, [Ac-Tyr1, D-Phe2]GRF (1–29) and CNQX from Tocris (Ellisville, MI, USA). Src (40–58) were provided by Dr. M. W. Salter (Hospital for Sick Children, Toronto, Canada). Maxadilan and M65 were a gift from Dr. Ethan A. Lerner (Harvard University, Boston, USA)
Results
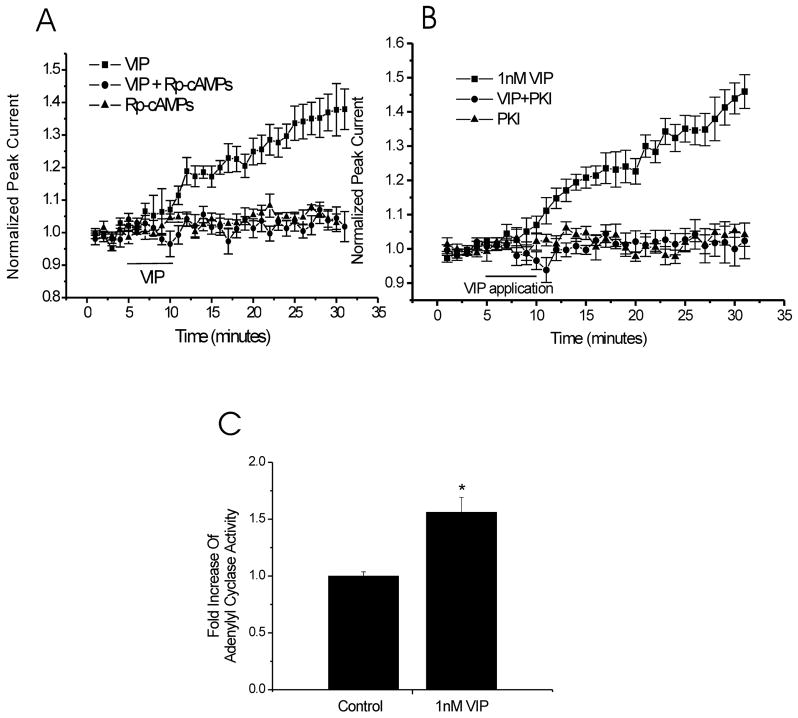
In order to examine the effects of VIP on NMDAR-mediated currents, a concentration of VIP (1nM) was initially chosen to selectively activate VPACRs and not PAC1-R. This concentration was based on the EC50 of VIP for VPAC-Rs (Harmar et al., 1998). Initially individual CA1 pyramidal cells were acutely isolated from the slices cut from young adult rat hippocampus. Using acutely isolated cells, drugs were directly and rapidly applied to an individual cell using a computer driven perfusion system. Unlike the situation of CA1 neurons in situ, the concentrations of applied agents are tightly controlled. NMDA currents were evoked every 60 seconds using a three-second exposure to NMDA (50μM) and glycine (0.5μM). After establishing a stable baseline of peak NMDA-evoked current amplitude, VIP was applied to isolated CA1 hippocampal neurons continuously for five minutes. Applications of VIP (1nM) induced a substantial and long-lasting increase in normalized NMDA evoked peak currents that far outlasted the application of VIP (Fig. 1A). This increase (39 ± 4%, n=6) reached a plateau twenty five minutes after the commencement of the VIP application (20 minutes after terminating its application). To exclude the involvement of receptors other than VPAC1 and VPAC2-Rs in this enhancement of NMDA-evoked currents, [Ac-Tyr1, D-Phe2] GRF (1–29) was co-applied with VIP in a separate series of recordings. Co-applications of [Ac-Tyr1, D-Phe2] GRF (1–29) (0.1μM), a peptide that can selectively block VPAC1/2-Rs (Waelbroeck et al., 1985) together with VIP (1nM) prevented the increase in NMDA-evoked currents induced by VIP (1nM) (4 ± 2%, n= 6) (Fig. 1A). In contrast, similar recordings done in the presence of M65 (0.1μM), a specific PAC1-R antagonist (Moro et al., 1999), failed to alter the VIP (1nM)-induced enhancement of NMDA-evoked currents (39 ± 7%, n= 5) (Fig. 1A).
Figure 1. Low concentration of VIP (1nM) enhances NMDA-evoked peak currents in isolated CA1 pyramidal neurons and this effect is mediated by VPAC receptors.
A. Application of VIP (1nM) to acutely isolated CA1 pyramidal neurons increased NMDA-evoked peak currents (39 ± 4%, n=6, data obtained at 30 min of recording), it lasted throughout the recording period. But in the presence of 0.1μM [Ac-Tyr1, D-Phe2] GRF (1–29), a specific VPAC-R agonist, VIP effect on NMDA-evoked peak currents was inhibited (4 ± 2%, n = 6, data obtained at 30 min of recording). But the addition of 0.1μM M65, a specific PAC1-R antagonist, could not prevent the increase of NMDA-evoked currents (39 ± 7%, n = 5, data obtained at 30 min of recording). B. Sample traces from the same cells with VIP or VIP + [Ac-Tyr1, D-Phe2] GRF (1–29) or VIP + M65 in the bath solution are shown at baseline (t = 3min) and after drug application (t=28min). C. Addition of 10nM [Ala11,22,28]VIP, a VPAC1-selective agonist, caused an enhancement in NMDA-evoked currents (27 ± 2%, n=6, data obtained at 30 min of recording), but the existence of 0.1μM [Ac-Tyr1, D-Phe2] GRF (1–29) blocked the potentiation of NMDA-evoked currents (−7 ± 2%, n=5, data obtained at 30 min of recording) plus 10nM [Ala11,22,28]VIP. D. Application of 1nM Bay55-9837, a specific VPAC2-selective agonist, alone also increased NMDA evoked currents (44 ± 8%, n=6, data obtained at 30 min of recording), but the coapplication of 0.1μM [Ac-Tyr1, D-Phe2] GRF (1–29) with 1n1M Bay55-9837, has no effect on NMDA-evoked currents (4 ± 3%, n=5, data obtained at 30 min of recording).
In order to confirm the involvement of both the VPAC1-R and VPAC2-R in the enhancement of NMDA-evoked currents, the actions of both the VPAC1-selective agonist [Ala11,22,28]VIP (Nicole et al., 2000) and the VPAC2-selective agonist Bay55-9837 (Tsutsumi et al., 2002) were examined. Application of [Ala11,22,28]VIP (10nM) caused an increase in NMDA-evoked currents (27 ± 2%, n=6), and this effect was eliminated in the presence of the VPAC1/2-R antagonist [Ac-Tyr1, D-Phe2] GRF (1–29) (0.1μM), (−7 ± 2%, n=5) (Fig. 1C). Similarly, application of Bay55-9837 (1nM) also resulted in a significant potentiation of NMDA-evoked currents of 44 ± 8% (n=6). In turn, this potentiation was blocked by co-application of Bay55-9837 (1nM) together with [Ac-Tyr1, D-Phe2] GRF (1–29) (0.1μM) (4 ± 3%, n=5) (Fig. 1D).
We also investigated if low concentrations of VIP could enhance the amplitude of NMDAR-mediated excitatory postsynaptic currents (EPSCNMDA) at CA1 synapses in brain slices. We did this by blocking AMPARs and by depolarizing the cells to accentuate the contribution of NMDARs to baseline transmission. Following 7 minutes of a stable baseline recording, 10 nM VIP was added and EPSCNMDA amplitude was continually monitored for the next 30 minutes. Applications of VIP to these slices caused an increase in the peak amplitude of NMDA synaptic currents. This enhancement reached a plateau that was 22 ± 4% (n = 6) above baseline (Fig. 2A). In order to exclude the involvement of PAC1-R in the potentiation of EPSCNMDA induced by 10 nM VIP, we recorded EPSCNMDAs in the presence of [Ac-Tyr1, D-Phe2] GRF (1–29) together with VIP. The inclusion of [Ac-Tyr1, D-Phe2] GRF (1–29) prevented the VIP-induced potentiation of these synaptic currents (0 ± 1%, n = 6), illustrating that VIP increases NMDA synaptic activity via stimulation of VPAC receptors (Fig. 2A).
Figure 2. Low concentration of VIP (10nM) also enhances NMDA currents in hippocampal slices.
A, Application of 10 nM VIP to hippocampal slices increased EPSCNMDAs. Normalized peak amplitude for VIP-treated cells was 22 ± 4% compared with baseline (n = 6). When coapplication of 0.1μM [Ac-Tyr1, D-Phe2] GRF (1–29), VIP failed to increase NMDA currents [normalized peak current, 0 ± 1% (n = 6), data obtained at 35 min of recording]. The black bar indicates time and duration of 10 nM VIP application. B. Sample traces of NMDA EPSC for control and VIP with or without [Ac-Tyr1, D-Phe2] GRF (1–29) -treated cells. Traces represent points immediately before VIP application (t = 5 min) and 23 min after PACAP38 application (t = 30 min).
We then used acutely isolated CA1 neurons to investigate the role of the cAMP/PKA pathway in the potentiation of NMDA-evoked currents based on the observations that VPAC1/2 receptors most often signal through Gαs to cAMP/PKA (Harmar et al., 1998). Rp-cAMPS binds to the regulatory subunit of PKA and inhibits dissociation of the catalytic subunit from the regulatory subunit. Inclusion of this competitive cAMP inhibitor (500μM) in the patch pipette blocked the subsequent effect of VIP (4 ± 3%, n = 6) but itself had no effect on NMDA-evoked currents in isolated CA1 neurons (5 ± 2%, n = 5) (Fig. 3A). Unlike RpCAMPS, PKI14–22 binds to catalytic subunit of PKA to inhibit its kinase activity. Application of this highly selective PKA inhibitory peptide, PKI14–22 (0.3μM), attenuated the VIP-induced potentiation of NMDA-evoked currents (VIP + PKI14–22, 1 ± 4%, n = 6) compared to VIP alone (40 ± 5%, n = 6). In contrast, PKI14–22 alone had no effect on NMDA-evoked currents (1 ± 3%, n = 5) (Fig. 3B). Adenylyl cyclase activity assays were also performed to determine if the application of 1 nM VIP correlated with an increase in AC activity in the hippocampus. Comparing VIP with vehicle-treated tissue, VIP (1nM) induced a 1.56 ± 0.13-fold (n = 4) increase in AC activity (Fig. 3C).
Figure 3. The cAMP/PKA signalling pathway is involved in VIP (1nM) response.
A. Intracellular administration 500μM Rp-cAMPs (a specific cAMP inhibitor) blocked the effect of VIP (4 ± 3%, n = 6, data obtained at 30 min of recording) and was similar to Rp-cAMPs alone (5 ± 2%, n = 5, data obtained at 30 min of recording). B. Addition of 0.3μM PKI14–22 (a specific PKA inhibitor) in all extracellular solutions blocked the potentiation of NMDA-evoked currents induced by VIP (1nM) (PKI14–22 plus VIP, 1 ± 4%, n = 6; VIP alone, 40 ± 5%, n = 6, data obtained at 30 min of recording). C. Adenylyl cyclase activity were compared between vesicle–treated and VIP (1nM)–treated brain tissue, VIP-treated tissue had a 1.56 ± 0.13-fold (n = 4) increase in AC activity (p < 0.05, unpaired t test).
Some VIP-mediated actions in the nervous system have also been associated with an increase in PKC activity (Cunha-Reis et al., 2005). Therefore, we used the PKC inhibitor bisindolylmaleimide I (bis-I) (500nM) to test whether the VIP-induced potentiation of NMDA-evoked currents in the CA1 area of the hippocampus was also PKC-dependent. Application of this inhibitor (500 nM) had no effect on the amplitudes of baseline responses (8 ± 1%, n = 5) and it also failed to alter the VIP-induced potentiation of NMDA-evoked currents (50 ± 10%, n = 6) (Fig. 4A). In addition, one study showed that Ca2+ transients in colonic muscle cells are enhanced by VIP acting via a cAMP/PKA-dependent enhancement of ryanodine receptors (Hagen et al., 2006). In pancreatic acinar cells, VPAC-Rs also evoke a Ca2+ signal by a mechanism involving Gαs (Luo et al., 1999). To test whether the modulation of NMDA-evoked currents by VIP required an elevation of internal Ca2+, high concentrations of the fast Ca2+ chelator BAPTA (20mM) were included in the patch pipette. BAPTA blocked the effect of VIP (1 nM) (5 ± 3%, n = 6). The application of BAPTA by itself caused no time-dependent change in normalized peak NMDA currents (1 ± 4%, n = 7) (Fig. 4B).
Figure 4. PKC is not required for the VIP (1nM) effect while the increase of intracellular Ca2+ is necessary. Moreover VIP (1nM) effect is mediated by AKAP scaffolding protein and can be accentuated by PDE4 inhibitor.
A. Application of the 500nM Bis (a specific PKC inhibitor) in all extracellular solutions can not blocked the VIP-induced potentiation of NMDA-evoked currents (Bis plus VIP, 50 ± 10%, n = 6: Bis alone, 8 ± 1%. n = 5; data obtained at 30 min of recording). B. Intracellular application of 20 mM BAPTA blocked the effect of VIP (1 nM) on the NMDA-evoked currents (BAPTA plus VIP, 5 ± 3%; n = 6; BAPTA alone, 1 ± 4%, n = 7; data obtained at 30 min of recording). C. Inclusion of 100 nM PDE4 inhibitor which prevents the degradation of cAMP in the patch pipette augmented the VIP-induced increase of NMDA-evoked currents (PDE inhibitor plus VIP, 58 ± 3%, n = 6; VIP alone, 32 ± 3%, n = 6; PDE inhibitor alone, 5 ± 2%, n = 6; data obtained at 30 min of recording). D. In the presence of 10μM St-Ht31 inhibitor peptide which disrupts the interaction between AKAP and PKA, 1nM VIP can not induce an increase in NMDA peak currents (St-Ht31 inhibitor peptide plus VIP, 12 ± 3%, n = 6; St-Ht31 inhibitor peptide alone, 6 ± 1%, n = 6; data obtained at 30 min of recording).
Cyclic AMP (cAMP)-specific phosphodiesterase 4 (PDE4), which catalyzes hydrolysis of cAMP, plays a critical role in the control of intracellular cAMP concentrations (Tasken and Aandahl, 2004). Pre-treatment with PDE4-selective inhibitors blocks memory deficits induced by heterozygous deficiency of cAMP-responsive element binding protein (CREB)-binding protein (CBP) (Bourtchouladze et al., 2003), and PDE4 is also involved in the induction of long-term potentiation (LTP) in the CA1 sub region of the hippocampus (Ahmed and Frey, 2003). To investigate if PDE4 is involved in the VIP (1nM) effect on NMDA-evoked currents, we included an inhibitor of PDE4, termed “PDE4 inhibitor” (3,5-Dimethyl-1-(3-nitrophenyl)-1H-pyrazole-4-carboxylic acid ethyl ester), in the patch pipette (100 nM). This compound is a specific inhibitor of phosphodiesterases 4B and 4D (Card et al., 2005). It accentuated the VIP-induced enhancement of NMDA-evoked currents (PDE4 + 1nM VIP, 58 ± 3%, n= 6; 1nM VIP, 32 ± 3%, n= 6). In a separate set of recordings, PDE4 inhibitor (100 nM) on its own had no time-dependent effect on normalized peak NMDA currents (5 ± 2%, n = 6) (Fig. 4C).
Targeting of PKA by the scaffolding protein A-Kinase Anchoring Protein (AKAP) is required for mediation of the biological effects of cAMP (Tasken and Aandahl, 2004). For example, disruption of the PKA-AKAP complex is associated with a reduction of AMPA receptor activity (Snyder et al., 2005). In addition, AKAP/Yotiao targets PKA to NMDARs and interference with this interaction reduces NMDAR currents expressed in HEK293 cells (Westphal et al., 1999). To determine if AKAP was required for VIP (1 nM) modulation of NMDA-evoked currents in hippocampal neurons we included the St-Ht31 inhibitor peptide (10 μM) in the patch pipette. This inhibitor mimics the amphipathic helix that binds the extreme NH2 terminus of the regulatory subunit of PKA and thereby dislodges PKA from AKAP and consequently from its substrates. Because of this property, it has been extensively used to study the functional implications of AKAP in several systems (Vijayaraghavan et al., 1997). Inclusion of St-Ht31 inhibitor peptide (10 μM) blocked the ability of the VIP to increase NMDA-evoked currents (12 ± 3%, n = 6). This peptide (10 μM) alone has no time-dependent effect on NMDA-evoked currents (6 ± 1%, n = 6) (Fig. 4D).
Our lab has showed that low concentrations of PACAP enhance NMDA-evoked currents in CA1 hippocampal neurons via a PKC/Src signal transduction cascade (Macdonald et al., 2005). Therefore, we also studied the involvement of Src in the VIP (1 nM)-mediated increase of NMDA-evoked currents. Intracellular application of the Src inhibitory peptide Src (40–58) did not block the effect of VIP (49 ± 7%, n = 6) (Fig. 5C). By itself, Src (40–58) had no time-dependent effect on the amplitude of NMDA-evoked currents (data not shown).
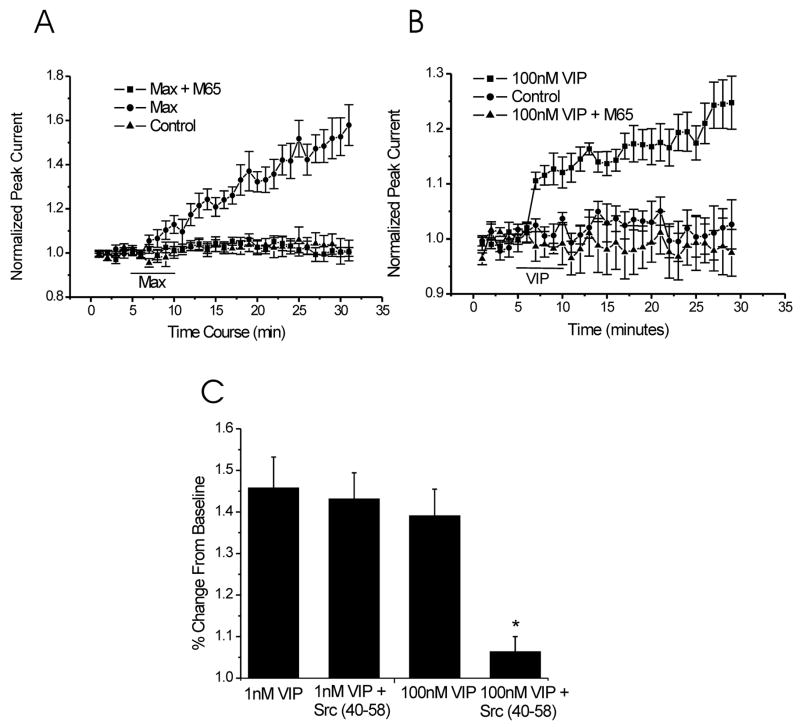
Figure 5. PAC1-R is involved in the potentiation of NMDA-evoked currents induced by VIP (100nM). In addition, Src is required for VIP (100nM) effect but not for VIP (1nM) effect on NMDA-evoked currents.
A. Application of 1nM maxadilan in the extracellular solutions enhanced NMDA-evoked currents (52 ± 7%, n=6, data obtained at 30 min of recording), but the existence of M65 abolished the effect of maxadilan (1nM) (3 ± 4%, n = 6, data obtained at 30 min of recording). B. 100nM VIP also can evoke the increase of NMDA peak currents (VIP, 25 ± 4%, n = 9; control, 2 ± 4%, n = 6; data obtained at 30 min of recording), but coapplication of VIP (100nM) and M65 has no effect on NMDA-evoked current (VIP plus M65, −1 ± 4%, n = 6; data obtained at 30 min of recording). C. Intracellular adminstration of the Src inhibitory peptide Src (40–58) can not inhibit 1nM VIP effect (49 ± 7%, n = 6, data obtained at 30 min of recording), but 100nM VIP effect on NMDA-evoked currents was blocked by Src (40–58) added in the patch pipette (3 ± 4%, n=6, data obtained at 30 min of recording).
In a previous study, we demonstrated that PACAP38 enhances NMDA-evoked currents in CA1 hippocampal neurons via the PAC1-R (Macdonald et al., 2005). PACAP (6–38), a PACAP inhibitor, was used to investigate the involvement of PAC1-R in the PACAP38-mediated potentiation of NMDAR mediated currents. Recent studies have shown that PACAP (6–38) may also inhibit VPAC2-Rs (Dickinson et al., 1997). To confirm the involvement of PAC1-Rs in the PACAP38-induced increase of NMDA-evoked currents we employed the highly selective PAC1-R agonist, maxadilan. Maxadilan is also the only known selective agonist for PAC1-Rs (Moro and Lerner, 1997). Application of maxadilan (1 nM) produced a strong enhancement of NMDA-evoked currents (52 ± 7%, n=6) and this effect was inhibited with the co-application of the PAC1-R antagonist M65 (0.1μM, 3 ± 4%, n = 6) (Fig. 5A).
The affinity of VIP for the PAC1-R is relatively low compared to VPAC1/2-Rs but high concentrations of VIP (100 nM) also activate PAC1-Rs (Harmar et al., 1998). We therefore determined if VIP (100nM) would also increase NMDA-evoked currents via the PAC1-R. VIP (100 nM) was applied for 5 minutes and it enhanced these responses (VIP, 100 nM, 25 ± 4%, n = 9; control, 2 ± 4%, n = 6). We then used the PAC1-R antagonist M65 to probe if the potentiation induced by this higher concentration of VIP was mediated in part by PAC1-R. M65 (0.1 μM) completely blocked the VIP (100 nM)-induced increase in currents (−1 ± 4%, n=6) (Fig. 5B). We also confirmed that this enhancement required activation of Src. Inclusion of the Src inhibitory peptide Src (40–58) in the patch pipette completely abolished the effect of high concentrations of VIP (100 nM) (3 ± 4%, n=6) (Fig. 5C).
Discussion
Using acutely isolated hippocampal CA1 cells, we demonstrated that application of the lower concentration of VIP (1 nM) enhanced NMDA receptor peak currents and it did so by stimulating VPAC1/2-Rs as the effect was blocked by [Ac-Tyr1, D-Phe2]GRF (1–29) (a specific VPAC1/2-R versus PAC1-R antagonist). The enhancement of NMDA currents induced by the low concentration of VIP was also blocked by both the selective cAMP inhibitor Rp-cAMPS and specific PKA inhibitor PKI14–22, but not by the specific PKC inhibitor, bisindolylmaleimide I nor by Src (40–58). Moreover, the VIP-induced enhancement of NMDA-evoked currents was accentuated by application of a phosphodiesterase 4 inhibitor. This regulation of NMDA receptors also required the scaffolding protein, AKAP since St-Ht31 a specific AKAP inhibitor also blocked the VIP-induced potentiation. These results are consistent with signalling via VPAC1/2-Rs and the cAMP/PKA signal cascade. The dependency of this response on Ca2+ buffering indicates that VPAC-Rs signalling depends on the increase in intracellular Ca2+. In contrast, a high concentration of VIP (100 nM) potentiated peak NMDAR-mediated currents; however, this effect was blocked by the highly selective PAC1-R antagonist, M65, as well as by the Src inhibitory peptide, Src(40–58), illustrating that at this concentration the predominant action of VIP is mediated by the PAC1-R targeting Src and not by VPAC1/2-Rs signalling to PKA.
So far the physiological level of VIP in the rat brain remains unknown. Based on the affinity of VIP to VPAC-Rs and PAC1-R, we chosen both 1 nM and 100 nM VIP to investigate its regulation of NMDARs. A low concentration of VIP (1 nM) likely activates both VPAC1 and VPAC2-Rs as an increase is also observed using either the VPAC1-selective agonist [Ala11,22,28]VIP or the VPAC2-selective agonist Bay55-9837. The VPAC antagonist [Ac-Tyr1, D-Phe2] GRF (1–29) (1 μM) inhibited the enhancement of NMDA-evoked currents caused by 1 nM VIP or by either of the VPAC-R selective agonists. This provides evidence for the involvement of both VPAC1 and VPAC2-Rs in the regulation of hippocampal synaptic transmission through modulation of NMDARs.
In a previous study we demonstrated that low concentrations of PACAP (1 nM) enhanced NMDAR activity likely by stimulating the PAC1-R (Macdonald et al., 2005). This was based upon the high potency of the PACAP-induced response and by its sensitivity to the inhibitory peptide PACAP (6–38). We have confirmed this role of the PAC1-R using the highly selective agonist maxadilan and the antagonist M65. This approach also permitted us to demonstrate that a high concentration of VIP (100 nM) acts via the PAC1-R to increase of peak NMDA currents as M65 also abolished the increase of NMDA-evoked current induced by 100 nM VIP.
M65 failed to block the increase of evoked NMDA currents induced by low concentration of VIP (1 nM) implying that at this lower concentration, VIP exerts its effect exclusively via one or both of the VPAC-Rs and not by PAC1-R. This raises the question as to why at least some of the response to the high concentration of VIP was not spared by the PAC1-R antagonist. While a definitive answer to this conundrum will require further investigation, we propose that it is likely attributable to the desensitization and internalization of VPAC-Rs that has been reported to occur in the presence of the high concentrations of VIP (Claing et al., 2000;Gaudin et al., 1996;McDonald et al., 1998). For example, the human VPAC1-R undergoes both desensitization and internalization upon VIP challenge (Gaudin et al., 1996) and the rat VPAC1-R undergoes an agonist-dependent internalization through a dynamin-dependent pathway (Claing et al., 2000). Similarly, VPAC2-R is desensitized and internalized in transfected COS7 or HEK293 cells upon exposure to high concentrations of VIP (McDonald et al., 1998). Whether VPAC-Rs in hippocampal CA1 neurons also show desensitization and internalization after the application of a high concentration of VIP requires further study.
So far no splice variants of VPAC-Rs have been identified, but eight subtypes of the PAC1-R have been characterized (Vaudry et al., 2000). PAC1-R and VPAC1/2-Rs couple strongly to Gαs and stimulate the cAMP/PKA signalling pathway. However, PAC1-R also strongly stimulates the phospholipase C (PLC) pathway, whereas VPAC1-Rs and VPAC2-Rs only weakly activate PLC (McCulloch et al., 2002). Here we show that the activation of VPAC1/2-Rs by low concentration of VIP (1 nM) increases evoked NMDA currents via the cAMP/PKA pathway whereas PAC1-R induces PLC/PKC/Pyk2/Src signalling pathway to enhance NMDA-evoked currents in hippocampal neurons (Macdonald et al., 2005). A potential stimulation of VPAC1/2-Rs by PACAP may account for the previously reported PKA-dependent actions of this agent on NMDAR mediated responses in hippocampal neurons (Yaka et al., 2003).
Vasoactive intestinal peptide applied to hippocampal slices alters a wide variety of neuronal properties and also enhances synaptic transmission (Ciranna and Cavallaro, 2003;Cunha-Reis et al., 2005). Its actions on excitatory synaptic transmission are blocked by the cAMP inhibitor Rp-cAMPS (Ciranna and Cavallaro, 2003) implicating the cAMP/PKA signal pathway. Unlike the case of acutely isolated CA1 neurons, the concentrations of VIP reaching individual cells can not be carefully controlled in this preparation due to the temporal properties of diffusion of peptides into the slice. Furthermore, the VIP-induced increase in excitatory transmission may occur as an indirect consequence of enhancement of the disinhibition of GABAergic interneurons (Cunha-Reis et al., 2004). VIP is also found to increase hippocampal pyramidal cell excitability directly, an action that depends on VPAC2 receptor activation (Cunha-Reis et al., 2006). In addition, the presynaptic effects of VIP in the hippocampus appear to be mediated via a secondary regulation of adenosine receptors (Cunha-Reis et al., 2007).
Administration of either VIP or PACAP provides protection against excitotoxicity as well as ischemia-induced damage of central neurons (Rangon et al., 2005;Said et al., 1998). Given the sensitivity of CA1 neurons to NMDA-induced cell death in global ischemia, and the peptides’ propensity to enhance NMDAR currents, it would appear to be contradictory that they are neuroprotective. However, several studies have shown that activation of synaptically located NMDARs proves neuroprotective while the well known excitotoxic actions of NMDAR are mediated primarily by extrasynaptically located NMDARs (Papadia and Hardingham, 2007). This dichotomy between protection and toxicity might also be related to the subtype of NMDAR expressed at extrasynaptic versus synaptic locations (NR2B versus NR2A containing receptors respectively) (Papadia and Hardingham, 2007). Thus, neuroprotection by VIP could result from an enhancement of the pro-survival actions of NMDARs by preferentially augmenting synaptic over extrasynaptic NMDARs, but this possibility will require further investigation. Alternatively, the neuroprotective effect of VIP may require an indirect stimulation of astrocytes. VIP-stimulated astrocytes secrete neuroprotective proteins, including activity-dependent neurotrophic factor (ADNF) (Dejda et al., 2005). Besides the release of neurotrophic factors, astrocytes actively contribute to neuroprotective processes through the efficient clearance of extracellular glutamate. A recent study showed that activation of VIP/VPAC2-R in astrocytes increased GLAST-mediated glutamate uptake and this effect required both PKA and PKC activation (Goursaud et al., 2008).
Despite the broad substrate specificity of PKA, local pools of cAMP within the cell generate a high degree of specificity in PKA-mediated signalling (Tasken and Aandahl, 2004). cAMP micro domains are controlled by adenylate cyclases that form cAMP as well as PDEs that degrade cAMP (Tasken and Aandahl, 2004). AKAPs target PKA to specific substrates and distinct subcellular compartments, providing spatial and temporal specificity for mediation of biological effects mediated by the cAMP/PKA pathway (Tasken and Aandahl, 2004). Our study shows that a specific phosphodiesterase 4 inhibitor accentuates the VIP-induced enhancement of NMDA-evoked currents and this implies that PDE4 is also involved in the regulation of NMDARs. This result is consistent with reports that the PDE4 inhibitors improve long-term memory consolidation and facilitate LTP in aged mice with memory deficits (Ghavami et al., 2006).
We also showed that an interaction of PKA with AKAP is required for the VPAC1/2-R-induced regulation of NMDARs. Synaptic anchoring of PKA through association with AKAPs plays an important role in the regulation of AMPA receptor surface expression as well as in synaptic plasticity (Snyder et al., 2005). PKA and NMDA receptors have also been closely linked via AKAP/yotiao which binds to PP1 too. At basal condition, constitutive PP1 keeps NMDA channels in a dephosphorylated and low activity state. With high levels of cAMP, PKA is released leading to a shift in the balance of the channel to a phosphorylated and higher activity state (Westphal et al., 1999).
Our results demonstrate that, in contrast to PAC1-Rs, VPAC1/2-Rs couple via cAMP/PKA to regulate the amplitude of NMDARs in CA1 pyramidal neurons. They may also act to increase the Ca2+ permeability of NMDARs (Skeberdis et al., 2006) and by these mechanisms control at the induction of synaptic plasticity CA3-CA1 synapses. Such forms of metaplasticity are implicated in the modulation of learning and behaviour (Abraham, 2008;Skeberdis et al., 2006).
To sum up, our results indicate that VIP can regulate NMDA receptors through different receptors and signaling pathways. High concentration of VIP (100 nM) signals through PAC1-R/PKC/Src signaling pathway, but low concentration of VIP (1 nM) activates VPAC1/2-Rs/cAMP/PKA to regulate the amplitude of NMDARs in CA1 pyramidal neurons. This may explain why VIP agonism is able to partially reverse cognitive deficits in animal models of Alzheimer’s disease (Gozes et al., 1997) and provide an alternate approach to treat memory loss in Alzheimer’s disease.
Acknowledgments
Research described in this article was supported in part by Canadian Institutes of Health Research (15514 & 44008) and EAL from the National Institutes of Health (U01 AI061420). The authors have no financial or other involvement with any of these companies or programs.
This work was supported by grants to JFM from the Canadian Institutes of Health Research (15514 & 44008) and EAL from the National Institutes of Health (U01 AI061420).
Footnotes
Disclosure statement: The authors have nothing to disclose
Reference List
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Frey JU. Expression of the specific type IV phosphodiesterase gene PDE4B3 during different phases of long-term potentiation in single hippocampal slices of rats in vitro. Neuroscience. 2003;117:627–638. doi: 10.1016/s0306-4522(02)00838-2. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card GL, Blasdel L, England BP, Zhang C, Suzuki Y, Gillette S, Fong D, Ibrahim PN, Artis DR, Bollag G, Milburn MV, Kim SH, Schlessinger J, Zhang KY. A family of phosphodiesterase inhibitors discovered by cocrystallography and scaffold-based drug design. Nat Biotechnol. 2005;23:201–207. doi: 10.1038/nbt1059. [DOI] [PubMed] [Google Scholar]
- Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2007;52:200–214. doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Ciranna L, Cavallaro S. Opposing effects by pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide on hippocampal synaptic transmission. Exp Neurol. 2003;184:778–784. doi: 10.1016/S0014-4886(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Claing A, Perry SJ, Achiriloaie M, Walker JK, Albanesi JP, Lefkowitz RJ, Premont RT. Multiple endocytic pathways of G protein-coupled receptors delineated by GIT1 sensitivity. Proc Natl Acad Sci USA. 2000;97:1119–1124. doi: 10.1073/pnas.97.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump FT, Dillman KS, Craig AM. cAMP-dependent protein kinase mediates activity-regulated synaptic targeting of NMDA receptors. J Neurosci. 2001;21:5079–5088. doi: 10.1523/JNEUROSCI.21-14-05079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Reis D, Fontinha BM, Ribeiro JA, Sebastiao AM. Tonic adenosine A1 and A2A receptor activation is required for the excitatory action of VIP on synaptic transmission in the CA1 area of the hippocampus. Neuropharmacology. 2007;52:313–320. doi: 10.1016/j.neuropharm.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Cunha-Reis D, Ribeiro JA, Sebastiao AM. VIP enhances synaptic transmission to hippocampal CA1 pyramidal cells through activation of both VPAC1 and VPAC2 receptors. Brain Res. 2005;1049:52–60. doi: 10.1016/j.brainres.2005.04.077. [DOI] [PubMed] [Google Scholar]
- Cunha-Reis D, Ribeiro JA, Sebastiao AM. VPAC2 receptor activation mediates VIP enhancement of population spikes in the CA1 area of the hippocampus. Ann N Y Acad Sci. 2006;1070:210–214. doi: 10.1196/annals.1317.016. [DOI] [PubMed] [Google Scholar]
- Cunha-Reis D, Sebastiao AM, Wirkner K, Illes P, Ribeiro JA. VIP enhances both pre-and postsynaptic GABAergic transmission to hippocampal interneurones leading to increased excitatory synaptic transmission to CA1 pyramidal cells. Br J Pharmacol. 2004;143:733–744. doi: 10.1038/sj.bjp.0705989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejda A, Sokolowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep. 2005;57:307–320. [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM, Mitchell R, Lutz EM. Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides. 1997;31:175–185. doi: 10.1016/s0143-4179(97)90087-1. [DOI] [PubMed] [Google Scholar]
- Gaudin P, Couvineau A, Maoret JJ, Rouyer-Fessard C, Laburthe M. Stable expression of the recombinant human VIP1 receptor in clonal Chinese hamster ovary cells: pharmacological, functional and molecular properties. Eur J Pharmacol. 1996;302:207–214. doi: 10.1016/0014-2999(96)00096-9. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Hirst WD, Novak TJ. Selective phosphodiesterase (PDE)-4 inhibitors: a novel approach to treating memory deficit? Drugs RD. 2006;7:63–71. doi: 10.2165/00126839-200607020-00001. [DOI] [PubMed] [Google Scholar]
- Goursaud S, Maloteaux JM, Hermans E. Activation of VIP/PACAP type 2 receptor by the peptide histidine isoleucine in astrocytes influences GLAST-mediated glutamate uptake. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05231.x. [DOI] [PubMed] [Google Scholar]
- Gozes I, Bachar M, Bardea A, Davidson A, Rubinraut S, Fridkin M, Giladi E. Protection against developmental retardation in apolipoprotein E-deficient mice by a fatty neuropeptide: implications for early treatment of Alzheimer’s disease. J Neurobiol. 1997;33:329–342. doi: 10.1002/(sici)1097-4695(199709)33:3<329::aid-neu10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Hagen BM, Bayguinov O, Sanders KM. VIP and PACAP regulate localized Ca2+ transients via cAMP-dependent mechanism. Am J Physiol Cell Physiol. 2006;291:C375–C385. doi: 10.1152/ajpcell.00495.2005. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Luo X, Zeng W, Xu X, Popov S, Davignon I, Wilkie TM, Mumby SM, Muallem S. Alternate coupling of receptors to Gs and Gi in pancreatic and submandibular gland cells. J Biol Chem. 1999;274:17684–17690. doi: 10.1074/jbc.274.25.17684. [DOI] [PubMed] [Google Scholar]
- Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J Neurosci. 2005;25:11374–11384. doi: 10.1523/JNEUROSCI.3871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McCulloch DA, MacKenzie CJ, Johnson MS, Robertson DN, Holland PJ, Ronaldson E, Lutz EM, Mitchell R. Additional signals from VPAC/PAC family receptors. Biochem Soc Trans. 2002;30:441–446. doi: 10.1042/bst0300441. [DOI] [PubMed] [Google Scholar]
- McDonald TP, Dinnis DM, Morrison CF, Harmar AJ. Desensitization of the human vasoactive intestinal peptide receptor (hVIP2/PACAP R): evidence for agonist-induced receptor phosphorylation and internalization. Ann N Y Acad Sci. 1998;865:64–72. doi: 10.1111/j.1749-6632.1998.tb11164.x. [DOI] [PubMed] [Google Scholar]
- Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- Moro O, Wakita K, Ohnuma M, Denda S, Lerner EA, Tajima M. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem. 1999;274:23103–23110. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- Nicole P, Rouyer-Fessard C, Couvineau A, Drouot C, Fulcrand P, Martinez J, Laburthe M. Alanine scanning of VIP. Structure-function relationship for binding to human recombinant VPAC1 receptor. Ann N Y Acad Sci. 2000;921:352–356. doi: 10.1111/j.1749-6632.2000.tb06992.x. [DOI] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Tong G, Jahr CE. Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Rangon CM, Goursaud S, Medja F, Lelievre V, Mounien L, Husson I, Brabet P, Jegou S, Janet T, Gressens P. VPAC2 receptors mediate vasoactive intestinal peptide-induced neuroprotection against neonatal excitotoxic brain lesions in mice. J Pharmacol Exp Ther. 2005;314:745–752. doi: 10.1124/jpet.105.086405. [DOI] [PubMed] [Google Scholar]
- Said SI, Dickman K, Dey RD, Bandyopadhyay A, De Stefanis P, Raza S, Pakbaz H, Berisha HI. Glutamate toxicity in the lung and neuronal cells: prevention or attenuation by VIP and PACAP. Ann N Y Acad Sci. 1998;865:226–237. doi: 10.1111/j.1749-6632.1998.tb11182.x. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M, Claus TH, Liang Y, Li Y, Yang L, Zhu J, Dela CF, Peng X, Chen H, Yung SL, Hamren S, Livingston JN, Pan CQ. A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal: a potential therapy for type 2 diabetes. Diabetes. 2002;51:1453–1460. doi: 10.2337/diabetes.51.5.1453. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- Waelbroeck M, Robberecht P, Coy DH, Camus JC, De Neef P, Christophe J. Interaction of growth hormone-releasing factor (GRF) and 14 GRF analogs with vasoactive intestinal peptide (VIP) receptors of rat pancreas. Discovery of (N-Ac-Tyr1, D-Phe2)-GRF(1–29)-NH2 as a VIP antagonist. Endocrinology. 1985;116:2643–2649. doi: 10.1210/endo-116-6-2643. [DOI] [PubMed] [Google Scholar]
- Wang LY, MacDonald JF. Modulation by magnesium of the affinity of NMDA receptors for glycine in murine hippocampal neurones. J Physiol. 1995;486(Pt 1):83–95. doi: 10.1113/jphysiol.1995.sp020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]