Abstract
In this publication, we describe our synthesis of four key building blocks for the total synthesis of psymberin (1) and its C4 epimer (2). Despite early difficulties in processing material to the advanced intermediate stage, we have been successful in developing high-yielding syntheses for the pyran core, natural sidechain, 4-epi sidechain and aryl fragments of the molecule. Our findings from the optimization process are presented herein.
Introduction
In late 2003, our colleagues in the research group of Professor Philip Crews isolated a highly potent cytotoxic marine natural product from “an undescribed and inconspicuous sponge, Psammocinia sp1.” The molecule, psymberin (assigned by Crews et al. as either 1 or 2), was later determined to be identical to iriciniastatin A, a compound isolated and reported by Pettit and coworkers from extracts of Ircinia ramose.2 The dual isolation of this compound from different sponges combined with the reported difficulty in isolating the compound from many sponge extracts adds evidence to the speculation that this molecule, along with structurally similar compounds, may in fact take origin from symbiotic bacteria.3 Over the past six years, a number of publications relating to the synthesis and semisynthesis of psymberin and analogs have been developed, including De Brabander's elegant first total synthesis of 1 and 2 in 2006, which conclusively determined that psymberin is in fact the 4S isomer 1.4,5 To embark in the total synthesis of this remarkably active molecule, we endeavored to develop a rapid and convergent approach to both 1 and 2 as well as libraries of stereoisomer and structural analogs. Our successful efforts to produce several key building blocks are disclosed herein.
Results and Discussion
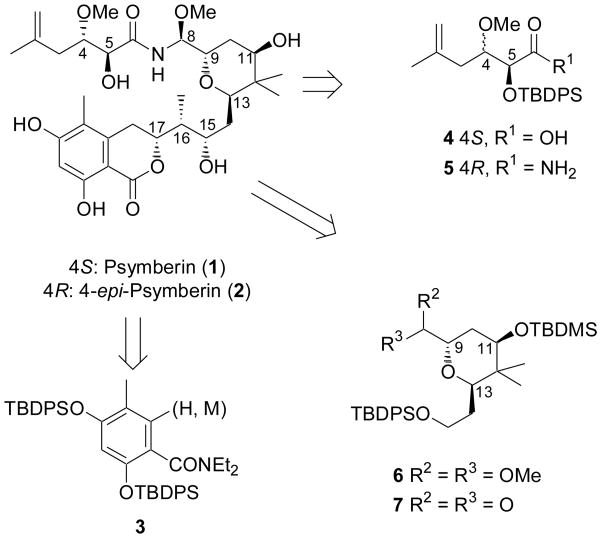
Our retrosynthetic analysis for 1 is depicted in Scheme 1. We envisioned a coupling of aryl fragment 3, through a suitable organometallic agent, to an epoxide (not shown) to be built onto the lower portion of target fragment 6, in which the C8 functionality is suitably protected. Subsequent manipulation of the C8 functionality to a variety of groups was deemed necessary to explore the often challenging formation of the stereochemically defined aminal functionality6 through coupling with fragments such as 4 or 5. Thus, our strategy involved disconnecting the molecule into three main fragments, the aryl fragment 3, the sidechain fragment as 4 or 5, and the pyran core fragment 6, which can be further deconstructed to 7. In addition to allowing for a highly convergent assembly of 1, the modularity of our approach would allow for alteration of stereochemistry and functionality on each of the three subunits so as to rapidly amass a library of analogs.
Scheme 1.
Retrosynthetic Analysis for 1 and 2
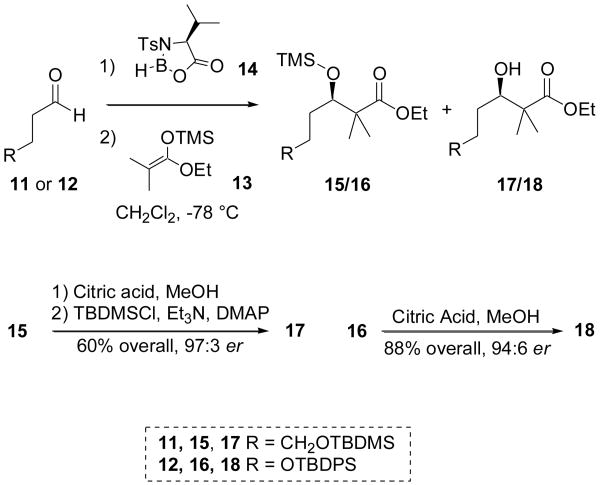
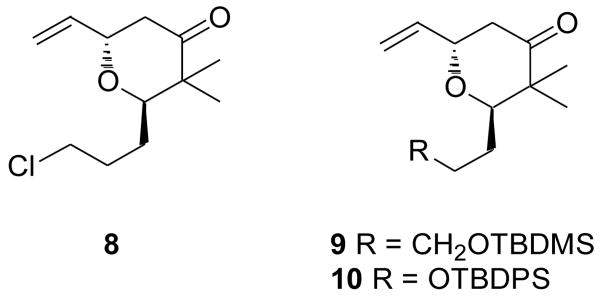
Excited to evaluate the potential for C8 diastereoinduction through several envisioned synthetic approaches, our initial goal was to obtain the advanced intermediate 7 as swiftly as possible. To this end, we relied heavily on the pioneering work of Kocienski and coworkers to synthesize the pyran core. Kocienski's synthesis of pederin, theopederin and the mycalamides all share the common pyran intermediate 8.7 We envisioned that the analogous protected alcohol 9 (and later 10) would dovetail nicely into our synthesis of 1.
Our approach to compounds 9 (1st generation) and 10 (2nd generation) based on Kocienski's template are depicted in Schemes 2 and 3. The hopes of hastily moving multigram quantities of material through Kocienski's process were dashed on the discovery that our first generation substrate 9 suffered from a frustrating instability through many of the transformations. We attributed this decomposition to the loss of the primary tert-butyldimethylsilyl (TBDMS) ether, and thus repeated the synthesis of an analogous compound 10 that was truncated by one carbon and bore the more robust tert-butyldiphenylsilyl (TBDPS) protecting group at the primary alcohol.
Scheme 2.
Valine-mediated asymmetric aldol reaction is more successful with aldehyde 12 than with 11
Scheme 3.
Further advancement of the pyran core fragments
The synthesis began with an asymmetric aldol reaction between silyl enol ether 13 and aldehyde 11 mediated by the oxazaborolidine 14, generated in situ from N-tosyl valine and BH3•THF.8 The enantiomeric ratio in this transformation was estimated to be 97:3 by 1H NMR following conversion to the Mosher ester.9 While our initial yields were far lower than Kocienski's, optimization of the oxazaborolidine formation by increasing the reaction time increased the yield from 20% to 60%. A major challenge in this transformation was the isolation of two products from this transformation: the desired alcohol 17 as well as TMS-ether 15. While treatment of 15 with mild acid could invoke the desired deprotection, it was at this step that we encountered what would be a recurring theme throughout the first generation synthesis: the surprising lability of the TBDMS ether. None of our attempts to remove the TMS ether were successful without concomitant TBDMS loss, thus requiring a re-protection step to convert the diol to 17. Incorporation of the more robust TBDPS protecting group eliminated the need for re-protection and gave 18 in a much higher 88% overall yield with comparable diastereoselectivity, determined to be 94:6 by chiral HPLC analysis.
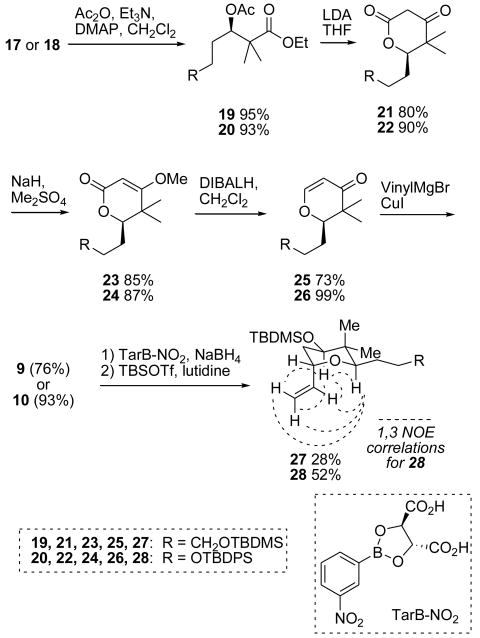
With compounds 17 and 18 in hand, acetylation and Dieckmann condensation proceeded uneventfully to give β-keto lactones 21/22. Recrystallization of both lactones to full enantioenrichment was possible at this stage and confirmed by optical rotation and chiral HPLC analysis. In addition, X-ray crystallographic analysis of an enriched sample of compound 22 gave a Flack parameter of 0.08, indicative of the desired absolute configuration.10 The three remaining steps involved methyl enol ether formation followed by DIBALH reduction to give enones 25 and 26, which then underwent conjugate addition to give β-vinyl ketones 9 and 10, respectively. At this stage, the difference in performance of the two compounds is quite striking: the overall yield of our second-generation substrate 10 (41%) is nearly double that of first generation substrate 9 (23%), and comparable to that for Kocienski's β-vinyl ketone 8 (38%). We attributed the lower comparative yields in the syntheses of 17 and 25 to the inability to perform the requisite acidic workup in each reaction without substantial loss of the TBDMS ether. The TBDMS group can be considered more vulnerable to deprotection under acidic conditions in part because of its decreased size relative to the TBDPS group, and also perhaps due to the increased distance from the bulkier region of the molecule that is imparted by the additional methylene on the pendant chain. However, the cause for the decreased yield in other transformations such as the conversion of 19 to 21 and the remarkable instability of 23 were less clear.
From this point on our synthesis diverges from Kocienski's template. The first desired transformation was an asymmetric ketone reduction on 9 or 10 to give the desired chirality at C11. We had hoped for substrate-induced diastereoselectivity in this transformation, but unfortunately we saw little selectivity with the use of common reducing agents such as LAH, DIBALH, NaBH4, superhydride and Luche conditions. Fortunately, the first asymmetric reducing agent we employed, TarB-NO2 in tandem with NaBH4,11 gave us the desired stereochemistry (confirmed by 1,3 diaxial NOE interactions among the pendant vinyl protons and those at C11 and C13 for compound 28) at C11 with a 19:1 diastereomeric ratio for both the first- and second-generation substrates. This transformation was not as high-yielding as desired, however, due to a competing hydroboration reaction between the pendant alkene and the borane generated in the course of the reaction. Nonetheless, optimization of the dismal 10-33% yields to 55% on the second-generation substrate was eventually achieved. Silyl protection gave compounds 27 and 28.
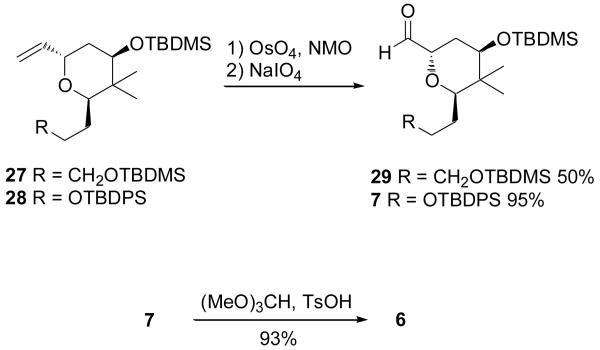
In anticipation of the impending coupling studies at the C8 center, the substrate was subjected to oxidative cleavage of the vinyl group to give aldehydes 29 and 7 (Scheme 4). The optimal yield for the second-generation product 7 was obtained by a two-stage, one-pot treatment with OsO4/NMO followed by NaIO4, in contrast to the lower-yielding first-generation procedure converting 27 to 29, in which the intermediate 1,2 diol was isolated by chromatography prior to periodate cleavage. The relatively unstable aldehyde 7 could be easily protected as the dimethoxy acetal 6 for further manipulations at the lower portion of the molecule. However, installation of C16-C18 was deferred at this stage in favor of first performing model studies on the aminal formation reaction with the sidechain C1-N7 fragments.
Scheme 4.
Completion of the synthesis of aldehydes 29 and 7, for use in model studies for the coupling with 4/5, or transformed to 6 for further functionalization of the pyran core (Scheme 1)
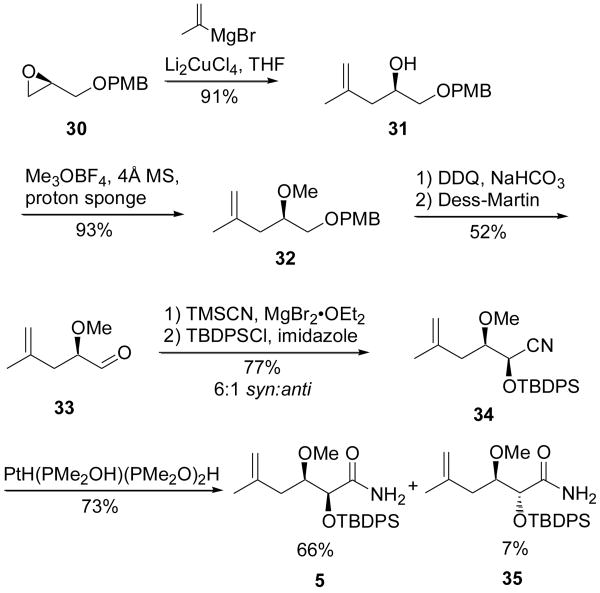
In the early planning stages of our psymberin synthesis, the assembly of sidechain fragments, in both the natural and C4-epimer forms, were also synthetic sequences for which we had anticipated speedy completion. Our synthesis of the 4-epi sidechain 5 is depicted in Scheme 5. Beginning from known PMB ether 30 (obtained from (S)-glycidol) copper-catalyzed epoxide opening with isopropylidene Grignard reagent was followed by methylation to give methyl ether 32. Deprotection and oxidation gave aldehyde 33. Compound 33 was then subjected to syn-selective cyanide induction using the method of Ward, et al. to afford cyanohydrin 34 in a 6:1 syn:anti diastereomeric ratio.12 The unstable cyanohydrin was immediately protected and subjected to nitrile hydrolysis using Parkins and Ghaffar's catalyst13 giving separable amides 5 and 35, which is enantiomeric to the desired “natural” sidechain.
Scheme 5.
Synthesis of the 4-epi sidechain in amide form (5)
With the 4-epi sidechain in hand, our initial inclination was to perform a similar route from (R)-glycidol to obtain the natural sidechain fragment ent-35, with an additional Mitsunobu-like inversion performed on the cyanohydrin formed from ent-33 to afford the desired anti stereochemistry as seen in the natural product. This inversion is somewhat challenging due to the known tendency of aliphatic cyanohydrins to racemize under traditional Mitsunobu conditions.14 Unpublished experiments performed on similar compounds in our laboratory indicated this racemization can be avoided by employing a method similar to Effenberger and Stelzel,15 in which the cyanohydrin hydroxyl is first converted to a sulfonate leaving group and then displaced by an acetate to give the desired inversion of stereochemistry. While our studies indicated good success in this transformation using Shimizu's chlorinated mesylate as a leaving group,16 we ultimately determined that this approach to the natural sidechain was too lengthy and decided to focus our efforts on developing of a more efficient route to the completion of this small fragment.
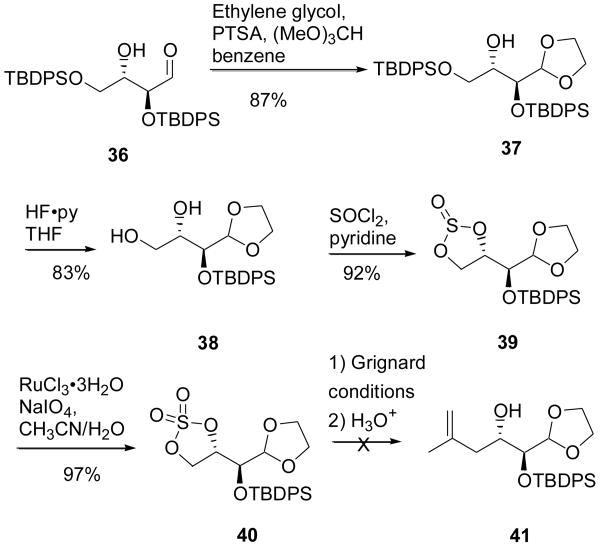
Our more rapid pathway into the natural sidechain fragment focused on synthesizing the compound as the carboxylic acid 4. In order to established the requisite anti stereochemistry at C4-C5, we turned to MacMillan's elegant proline-catalyzed aldol reaction17 that has been shown to give bis-TPS ether 36 (Scheme 6) with a 9:1 diastereomeric ratio and a 93% ee.18 Immediate protection of this aldehyde gave alcohol 37, which was fully resistant to methylation, presumably due to steric bulk about the secondary hydroxyl. Instead, selective deprotection of the primary silyl ether using HF-pyridine gave diol 38.
Scheme 6.
Attempts to synthesize the natural sidechain from MacMillan's aldehyde 36
From diol 38, we first opted to convert the compound to the 1,2 cyclic sulfate ester 40, which has been shown by Sharpless to readily accept various nucleophiles.19 Unfortunately, this compound reacted sluggishly in the presence of isopropylidene Grignard, and we struggled to ascertain appropriate conditions for the cleavage of the resultant sulfate ester to the desired free alcohol.20
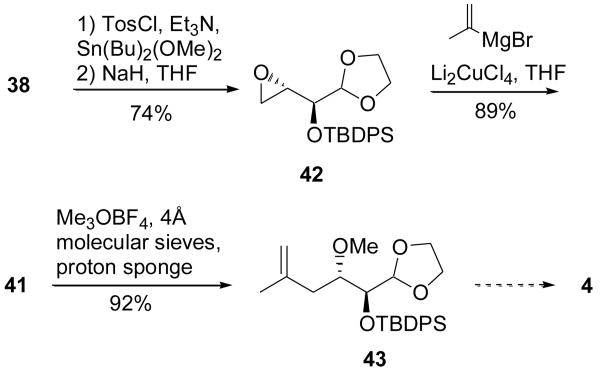
Fortunately, conversion of 38 to epoxide 42 using the method of Martinelli et al. proved far more fruitful (Scheme 7).21 Compound 42 was then opened and methylated to give 43 in good yield with the conditions we had employed in the synthesis of fragment 5. Deprotection of the hemiacetal gives the aldehyde, which is ready for oxidation to either the acid 4 or other functionalities suitable for coupling with the pyran core.
Scheme 7.
Completion of the natural sidechain from diol 38
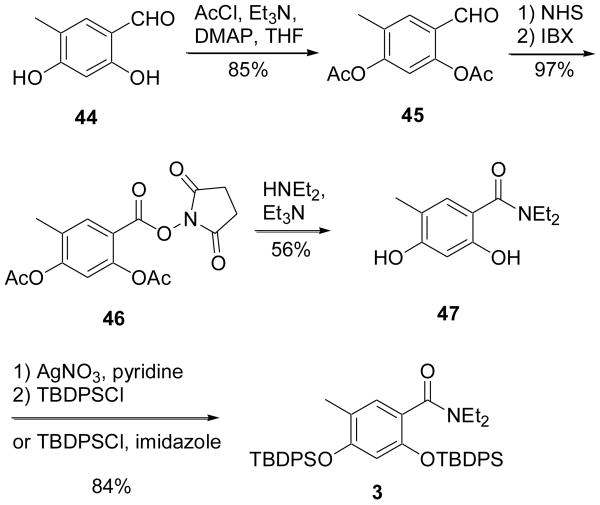
While we were encountering unanticipated difficulties in the synthesis of the sidechain and pyran core fragments, our synthesis of our envisioned aryl fragment proceeded rapidly and in excellent yield. The construction of the desired compound 3 is depicted in Scheme 8, and began from known aldehyde 44.22 Our initial attempts to perform the synthesis on the bis-silylated diphenol (not shown) were met with protection group loss and complex mixtures in the oxidation step. We thus opted for more robust acetate protecting groups, which were then converted to silyl ethers at the penultimate step. The phenols were then protected with acetyl chloride, giving 45 in 85% yield. Oxidation to the desired amide was first performed by treatment with N-hydroxysucccinimde followed by IBX to give activated ester 46.23 Interestingly, the high yield in this transformation was completely dependent on order of addition, requiring premixing of the substrate with NHS prior to oxidation (premixing of IBX with NHS prior to substrate addition gave yields varying from 25-50%). This is indicative that the actual mechanism of this transformation may differ from that reported in the literature,24 which suggests that an intermediate IBX-NHS complex is the active oxidant. The activated ester was then treated with diethylamine to produce the desired aromatic amide with concomitant acyl hydrolysis, affording compound 47 in a 56% yield. The conversion to the desired TBDPS-protected product 3 could be achieved by two routes, which both proved to be similarly successful (84%) on a small scale.
Scheme 8.
Rapid assembly of aryl fragment 3
Our initial unpublished results as well as personal communication with Professor De Brabander indicate the envisioned disconnection of this aryl fragment may suffer from the hydrolytic stability of the diethyl amide, which has thus far been entirely resistant to lactone closure outside of highly acidic refluxing conditions. Thus, some retooling of our dihydroisocoumarin synthesis is apparently necessary. This chemistry, as well as further elaboration of the pyran core and the preliminary results for our envisioned N7-C8 coupling scenarios are currently under investigation in our laboratory. These findings, combined with the eventual completion of the total synthesis of 1, will be reported in due course.
Experimental Section
General Methods
All air and moisture sensitive reactions were carried out under an atmosphere of dry argon or nitrogen using oven-dried or flame-dried glassware and standard syringe techniques.
(2S,4R,6R)-4-(tert-butyldimethylsilanyloxy)-6-[2-(tert-butyldiphenylsilanyloxy)-ethyl]-5,5-dimethyltetrahydropyran-2-carbaldehyde (7)
Compound 28 (60 mg, 0.11 mmol, 1.0 equiv) was dissolved in a mixture of 5 mL t-BuOH, 1 mL THF and 0.5 mL water. N-methylmorpholine-N-oxide (28 mg, 0.24 mmol, 2.2 equiv) was added, followed by one drop of a 4% solution of OsO4 in t-BuOH. The mixture was stirred overnight. The following day, pH 7.0 phosphate buffered saline (10 mL) was added followed by sodium periodate (128 mg, 0.6 mmol, 5.5 equiv). The mixture was stirred at room temperature for two hours and quenched with the addition of 500 mg solid Na2S2O3. After stirring for 30 min, the solution was diluted with water and extracted with ethyl acetate. The combined organics were washed with saturated aqueous Na2S2O3 followed by brine and dried over Na2SO4. Flash column chromatography (5% ethyl acetate in hexanes) gave 57 mg (95%) of aldehyde 7 as a colorless oil. [α]25D = +36.8 (c 1.0, CH2Cl2); 1H NMR (500 MHz, CDCl3) δ = 9.73 (d, J = 1.5 Hz, 1H), 7.70-7.65 (m, 4H), 7.45-7.37 (m, 6H), 4.12 (dd, J = 2.0, 6.5 Hz, 1H), 3.92 (dt, J = 5.0, 10.0 Hz, 1H), 3.83 (ddd, J = 3.5, 6.5, 10.0 Hz, 1H), 3.35 (dd, J = 1.5, 10.0 Hz, 1H), 3.22 (dd, J = 4.5, 11.0 Hz, 1H), 2.10 (ddd, J = 2.0, 4.5, 13.5 Hz, 1H), 1.81-1.72 (m, 2H), 1.63 (dddd, J = 5.0, 5.0, 10.0, 14.0 Hz, 1H), 1.05 (s, 9H), 0.90 (s, 9H), 0.84 (s, 3H), 0.81 (s, 3H), 0.09 (s, 3H), 0.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ = 205.8, 135.6, 133.9, 129.7, 127.7, 78.1, 72.7, 60.8, 38.7, 32.2, 29.3, 26.8, 25.7, 23.0, 19.1, 17.9, 12.7, -4.2, -5.2; IR (thin film) = 2958, 1735, 1472, 1258, 1112, 959, 837, 702 cm-1; HRMS [M+H+] for C32H51O4Si2, calcd. 555.3320, found, 555.3337.
(2R,4R,6S)-4-(tert-butyldimethylsilanyloxy)-2-[2-(tert-butyldiphenylsilanyloxy)-ethyl]-6-dimethoxymethyl-3,3-dimethyltetrahydropyran (6)
Aldehyde 7 (44 mg, 0.08 mmol, 1.0 equiv) was dissolved in 2 mL trimethylorthoformate in an oven-dried flask under an atmosphere of nitrogen. Catalytic PTSA (1 mg) was added and the mixture was stirred at room temperature for 45 minutes. The reaction was quenched with the addition of 1 mL saturated aqueous sodium bicarbonate. The mixture was extracted with ethyl acetate, and the combined organics were washed with brine, dried over Na2SO4, and filtered to give a crude oil that was subjected to flash column chromatography. Following purification, compound 6 (43 mg, 93%) was isolated as a clear, colorless oil. [α]26D = +19.8 (c 1.2, CH2Cl2); 1H NMR (500 MHz, CDCl3) δ = 7.71-7.67 (dt, J = 1.5, 8.0 Hz, 4H), 7.44-7.36 (m, 6H), 4.40 (d, J = 7.0 Hz, 1H), 3.84-3.75 (m, 3H), 3.54 (dd, J = 4.0, 8.0 Hz, 1H), 3.45 (dd, J = 2.0, 11.0 Hz, 1H), 3.36 (s, 3H), 3.22 (s, 3H), 1.95 (bs, 1H), 1.80-1.70 (m, 2H), 1.66 (ddd, J = 5.5, 8.5, 13.5 Hz, 1H), 1.06 (s, 9H), 0.92 (s, 3H), 0.91 (s, 9H), 0.84 (s, 3H), 0.06 (s, 3H), 0.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ = 135.6, 134.4, 134.2, 129.5, 127.6, 103.3, 73.1, 62.0, 54.8, 52.6, 38.1, 32.0, 30.1, 26.8, 25.7, 24.6, 19.1, 17.9, -4.4, -5.1; IR (thin film) = 2956, 1471, 1256, 1091, 975, 835, 701 cm-1; HRMS [M+H+] for C34H57O5Si2, calcd. 601.3739, found, 601.37089.
Supplementary Material
Figure 1.
Kocienski's synthesis of compound 8 served as our template for rapid assembly of pyran core fragments 9 and 10
Acknowledgments
We thank the NIH (CA98878) for support of this work. Y. R. L. thanks MBRS (GM58903-05) for additional support. K. A. M. and J. S. C. thank NSF-REU (CHE-0552641) for funding. We thank J. Kim and J. Loo for technical assistance. Purchase of the 600 MHz NMR used in these studies was supported by funds from the National Institutes of Health (S10RR019918) and National Science Foundation (CHE-0342912). The single crystal X-ray diffraction data for 22 were recorded on an instrument supported by the National Science Foundation, Major Research Instrumentation (MRI) Program under Grant No. CHE-0521569.
Footnotes
Supporting Information Available: Synthetic procedures, complete spectroscopic data, 1H and 13C NMR spectra for all new compounds, chiral HPLC data for 18 and 24 and CIF file for 22. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Lauren E. Brown, Department of Chemistry and Biochemistry, University of California Santa Cruz, Santa Cruz, CA 95064
Yakira R. Landaverry, Department of Chemistry and Biochemistry, University of California Santa Cruz, Santa Cruz, CA 95064
James R. Davies, Selcia Ltd., Fyfield Business & Research Park, Fyfield Road, Ongar, Essex, CM5 0GS, United Kingdom
Kristin A. Milinkevich, Department of Chemistry, One Shields Avenue, University of California, Davis, California 95616 NSF-REU Summer Undergraduate Research Fellowship Program.
Sandra Ast, Universität Potsdam Mathematisch-Naturwissenschaftliche Fakultät, Institut für Chemie, Komplex II, Karl-Liebknecht-Straβe 24-25, 14476 Golm, Germany.
Joseph S. Carlson, NSF-REU Summer Undergraduate Research Fellowship Program
Allen G. Oliver, Department of Chemistry and Biochemistry, University of California Santa Cruz, Santa Cruz, CA 95064
Joseph P. Konopelski, Department of Chemistry and Biochemistry, University of California Santa Cruz, Santa Cruz, CA 95064
References
- 1.Cichewicz RH, Valeriote FA, Crews P. Org Lett. 2004;6:1951–1954. doi: 10.1021/ol049503q. [DOI] [PubMed] [Google Scholar]
- 2.Pettit GR, Xu JP, Chapuis JC, Pettit RK, Tackett LP, Doubek DL, Hooper JNA, Schmidt JM. J Med Chem. 2004;47:1149–1152. doi: 10.1021/jm030207d. [DOI] [PubMed] [Google Scholar]
- 3.See Piel J. Nat Prod Rep. 2009;26:338–362. doi: 10.1039/b703499g. and the references therein.
- 4.For the total synthesis of 1, see Jiang X, García-Fortanet J, De Brabander JK. J Am Chem Soc. 2005;127:11254–11255. doi: 10.1021/ja0537068.Shangguan N, Kiren S, Williams LJ. Org Lett. 2007;9:1093–1096. doi: 10.1021/ol063143k.Huang X, Shao N, Palani A, Aslanian R, Buevich A. Org Lett. 2007;9:2597–2600. doi: 10.1021/ol071068n.Smith AB, Jurica JA, Walsh SP. Org Lett. 2008;10:5625–5628. doi: 10.1021/ol802466t.
- 5.For other articles related to the synthesis and stereochemical analysis of psymberin fragments and analogs, see Kiren S, Williams LJ. Org Lett. 2005;7:2905–2908. doi: 10.1021/ol0508375.Green ME, Rech JC, Floreancig PE. Org Lett. 2005;7:4117–4120. doi: 10.1021/ol051396s.Rech JC, Floreancig PE. Org Lett. 2005;7:5175–5178. doi: 10.1021/ol0520267.Henβen B, Kasparyan E, Marten G, Pietruszka J. Heterocycles. 2007;74:245.Huang X, Shao N, Palani A, Aslanian R, Buevich A, Seidel-Dugan C, Huryk R. Tetrahedron Lett. 2008;49:3592–3595.Jiang X, Williams N, De Brabander JK. Org Lett. 2007;9:227–230. doi: 10.1021/ol062656o.Lachance H, Marion O, Hall DG. Tetrahedron Lett. 2008;49:6061–6064.Huang X, Shao N, Huryk R, Palani A, Aslanian R, Seidel-Dugan C. Org Lett. 2009;11:867–870. doi: 10.1021/ol802772s.
- 6.For a recent example of successful entry into this challenging functionality via asymmetric catalysis, see Li G, Fronczek FR, Antilla JC. J Am Chem Soc. 2008;130:12216–12217. doi: 10.1021/ja8033334.
- 7.(a) Kocienski PJ, Narquizian R, Raubo P, Smith C, Boyle FT. Synlett. 1998:1432–1434. [Google Scholar]; (b) Kocienski P, Narquizian R, Raubo P, Smith C, Farrugia LJ, Muir K, Boyle FT. J Chem Soc Perkin Trans. 2000;1:2357–2384. [Google Scholar]
- 8.(a) Kiyooka Si, Kaneko Y, Kume Ki. Tetrahedron Lett. 1992;33:4927–4930. [Google Scholar]; (b) Kiyooka Si, Kira H, Hena MA. Tetrahedron Lett. 1996;37:2597–2600. [Google Scholar]; (c) Kiyooka Si, Kaneko Y, Komura M, Matsuo H, Nakano M. J Org Chem. 1991;56:2276–2278. [Google Scholar]
- 9.Dale JA, Mosher HS. J Am Chem Soc. 1973;95:512–519. [Google Scholar]
- 10.Flack HD, Bernardinelli G. Acta Crystallogr, Sect A: Found Crystallogr. 1999;55:908–915. doi: 10.1107/s0108767399004262. [DOI] [PubMed] [Google Scholar]
- 11.(a) Suri JT, Vu T, Hernandez A, Congdon J, Singaram B. Tetrahedron Lett. 2002;43:3649–3652. [Google Scholar]; (b) Cordes DB, Nguyen TM, Kwong TJ, Suri JT, Luibrand RT, Singaram B. Eur J Org Chem. 2005:5289–5285. [Google Scholar]; (c) Kim J, Suri JT, Cordes DB, Singaram B. Org Process Res Dev. 2006;10:949–958. [Google Scholar]
- 12.(a) Ward DE, Hrapchak MJ, Sales M. Org Lett. 2000;2:57–60. doi: 10.1021/ol991198z. [DOI] [PubMed] [Google Scholar]; (b) Ward DE, Sales M, Hrapchak MJ. Can J Chem. 2001;79:1775–1785. [Google Scholar]
- 13.Ghaffar T, Parkins AW. Tetrahedron Lett. 1995;36:8657–8660. [Google Scholar]
- 14.Warmerdam EGJC, Brussee J, Kruse CG, van der Gen A. Tetrahedron. 1993;49:1063–1070. [Google Scholar]
- 15.Effenberger F, Stelzer U. Angew Chemie Int Ed Engl. 1991;30:873–874. [Google Scholar]
- 16.Shimizu T, Hiranuma S, Nakata T. Tetrahedron Lett. 1996;37:6145–6148. [Google Scholar]
- 17.(a) Northrup AB, Mangion IK, Hettche F, MacMillan DWC. Angew Chem Int Ed. 2004;43:2152–2154. doi: 10.1002/anie.200453716. [DOI] [PubMed] [Google Scholar]; (b) Northrup AB, MacMillan DWC. Science. 2004;305:1752–1755. doi: 10.1126/science.1101710. [DOI] [PubMed] [Google Scholar]
- 18.In our hands, the diastereomeric ratio for this transformation ranged from 6:1 to 8:1
- 19.Gao Y, Sharpless KB. J Am Chem Soc. 1988;110:7538–7539. [Google Scholar]
- 20.Kim BM, Sharpless KB. Tetrahedron Lett. 1989;30:655–658. [Google Scholar]
- 21.Martinelli MJ, Nayyar NK, Moher ED, Dhokte UP, Pawlak JM, Vaidyanathan R. Org Lett. 1999;1:447–450. [Google Scholar]
- 22.Xie L, Takeuchi Y, Cosentino LM, Lee KH. J Med Chem. 1999;42:2662–2672. doi: 10.1021/jm9900624. [DOI] [PubMed] [Google Scholar]
- 23.Mazitschek R, Mülbaier M, Giannis A. Angew Chem Int Ed. 2002;41:4059–4061. doi: 10.1002/1521-3773(20021104)41:21<4059::AID-ANIE4059>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Schulze A, Giannis A. Adv Synth Catal. 2004;346:252–256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.