Abstract
Nitrone spin traps are commonly employed as probes for the identification of transient radicals in chemical and biological systems using electron paramagnetic resonance (EPR) spectroscopy. Nitrones have also found applications as therapeutic agent in the treatment of radical-mediated diseases. Therefore, spin trap that incorporates high reactivity to superoxide radical anion (O2•−), more persistent superoxide adduct, enhanced bioavailability and selective targeting in one molecular design is desirable. In this work, the synthesis of a nitrone spin trap, 4, that is tethered via amide bonds to a β-cyclodextrin (β-CD) and a dodecyl chain was achieved with the expectation that the β-cyclodextrin would lead to persistent spin adduct while the lipophilic chain would impart membrane targeting property. The two constitutional racemic isomers, 4a and 4b, were separated using preparative HPLC and structural analysis as well as self-aggregations properties were carried out using NMR, induced circular dichroism, dynamic light scattering, transmission electron microscopy, and computational approach. EPR spin trapping of 02•− by 4a and 4b was only successful in DMSO and not in aqueous system due most likely to the amphiphilic character of 4 that can favor conformations (or aggregation) hindering radical addition to nitrone. Kinetics of formation and decay of 4a–O2H adduct in polar aprotic solvents show faster reactivity to O2•− and more persistent O2•− adduct compared to nitrones not conjugated to β-CD. Computational analysis of 4a and 4b as well as 4a–OOH and 4b–OOH adducts were carried out and results show that isomerism, both constitutional and stereochemical, affects the orientations of aminoxyl-NO and/or hydroperoxyl groups relative to the β-CD annulus for optimal H-bond interaction and stability.
Introduction
Spin trapping is commonly employed for the identification and detection of free radicals using nitrone spin traps and electron paramagnetic resonance (EPR) spectroscopy.1 The use of spin trapping has been gaining popularity in the investigation of reactive intermediates in the areas of fuel cell research,2 nanotechnology,3 catalysis,4 environmental remediation,5 and photodynamic therapy.6 Moreover, the ability of nitrones to trap radicals make them suitable as antioxidants in the treatment of reactive oxygen species-mediated pathophysiological disorders such as neurodegenerative disease,7 acute stroke,8,9 cancer9 and ischemia-reperfusion injury.10 However, the “antioxidant” property of nitrone spin traps is not clear but goes beyond direct radical scavenging by suppressing pro-apoptotic signal transduction and gene induction processes that can lead to oxidative stress.11
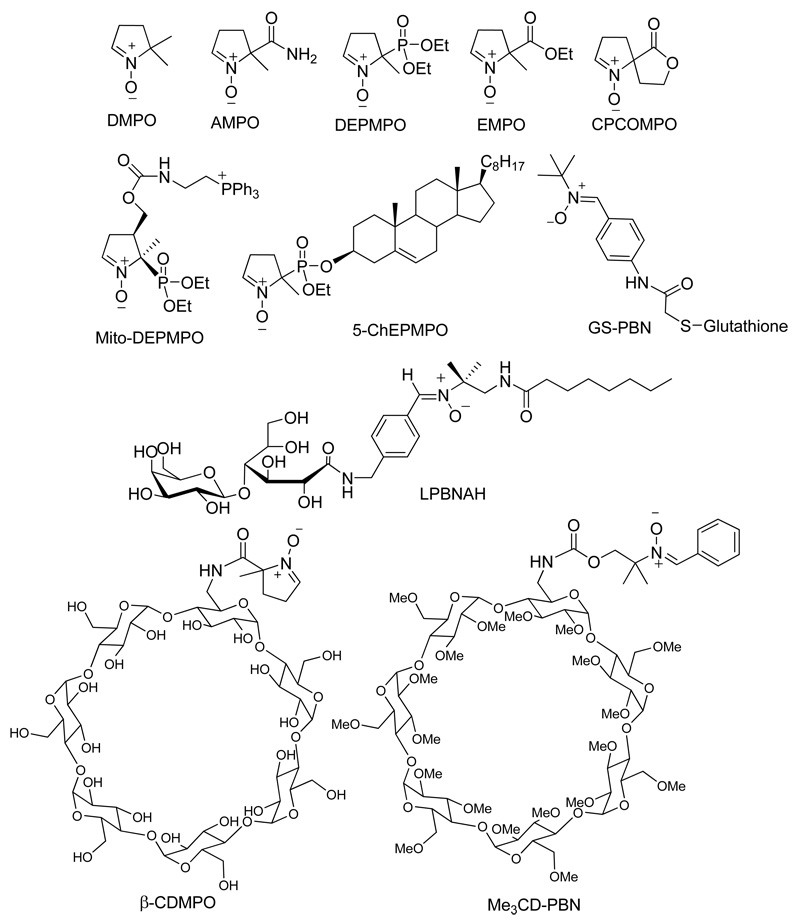
The slow reactivity of nitrones to O2•− and the short half-life of their corresponding spin adducts as well as their passive diffusion through cell membranes limit their application in biological systems as a O2•− probe.12 We previously reported13,14 that cyclic nitrone derivatization at the C-5 position with an amide moiety such as in the case of 5-carbamoyl-5-methyl-l-pyrroline-N-oxide (AMPO) increases electrophilicity of the nitronyl carbon and therefore gives an enhanced reactivity to O2•− compared to 5,5-dimethyl-1 -pyrroline-N-oxide (DMPO),15 5-ethoxycarbonyl-5-methyl-1 -pyrroline-N-oxide (EMPO)16 and 5-diethoxyphosphoryl-5-methyl-l-pyrroline-N-oxide (DEPMPO) (Figure 1).17 Moreover, this increased reactivity of amide-derivatized nitrones to O2•− is facilitated by intramolecular H-bonding interaction between the amide-H and O2•− at the transition state.13 We therefore postulated that amide linker group can provide opportunities for conjugation and still maintains enhanced reactivity to O2•−. Computational and experimental studies showed that intramolecular H-bond interaction contributes to O2•− adduct stability.18 We also showed19 that the presence of flexible H-bond acceptors contribute to O2•− adduct stability compared to rigid H-bond acceptors such as in the case of the spirolactonyl-nitrone, CPCOMPO (Figure 1).
Figure 1.
Examples of functionalized nitrones.
In the early 70’s, Birrell, et al.20 were first to observe the EPR spectra of the inclusion complex from γ-cyclodextrin (γ-CD) and the spin labeled 7-doxylstearic acid showing an anisotropic motion along the z-axis. Several inclusion studies then followed21 and it was reported22 that the rate of reduction of some nitroxides by ascorbic acid was significantly decreased in the presence of β-CD indicating the protective property of β-CD. The synthesis of methylated β-CD,23 Me3CD-PBN (Figure 1), showed improved adduct stability for O2•− with t1/2 ∼ 10 min compared to the PBN-O2•− adduct alone with a half life of < 1 min. We demonstrated that β-CD-cyclic nitrone conjugate (β-CDMPO)24 shows the highest rate for spin trapping of O2•− (58 M−1 s−1) and longest half-life for O2•− adduct (28 min) observed so far compared to the most commonly used spin traps such as DMPO, DEPMPO and EMPO. These improved spin trapping properties for β-CDMPO was made possible by exploiting the advantage of using amide linker13 for improved rate of O2•− trapping and intramolecular H-bond interactions for adduct stability.18 However, although β-CDMPO may provide controlled intracellular delivery of nitrone by making it less susceptible to bioreduction, its target specificity remains a limitation. The main mechanism for intracellular uptake of β-CD is through receptor-mediated endocytosis and this has been shown to be followed by release into the cytosol.25 Therefore, introduction of a target ligand could actively transport the spin trap-β-CD conjugate to its target destination whether it be in the cytosol, cell membrane, or mitochondria. There have been studies made to support the applicability of CD’s as a target delivery vehicle. In fact, β-CD was employed as a non-viral vector for the nuclear delivery of nucleic acids via conjugation to polycations.26
Indeed, natural cyclodextrins, due to their high external hydrophilicity exhibit limited affinity towards biological membranes. To overcome this limitation, several types of amphiphilic cyclodextrins have been developed.27 Among the recently designed amphiphilic cyclodextrins, the monosubstituted cyclodextrins at the C6 position are promising. Of particular interest are the “lollipops”which result from the grafting of an aliphatic chain on a 6-amino-β-cyclodextrin,28 their ammonium derivatives29 as well as peptidolipidyl derivatives in which an L-leucine spacer arm links the saccharide moiety and the alkyl chain.30 This amphiphilic structure could perhaps facilitate delivery of the nitrone across the cell membrane and should exhibit higher intracellular (or membrane) accumulation. Indeed, several amphiphilic nitrones have been previously synthesized showing biological activity in in-vitro,31 ex-vivo32 as well as in-vivo models.33 In the presence of a co-conjugate (i.e., dodecyl group), our expectation is that the dodecyl group could impart membrane affinity and may allow the nitrone to locate very close to the membrane of the cell or organelle. It is well-known that lipophilic antioxidants exhibit membrane affinity as for instance, Vitamin E has been shown to localize within the cellular membranes and lipophilic modification to CD’s have been shown to bestow cell-membrane permeation ability.34
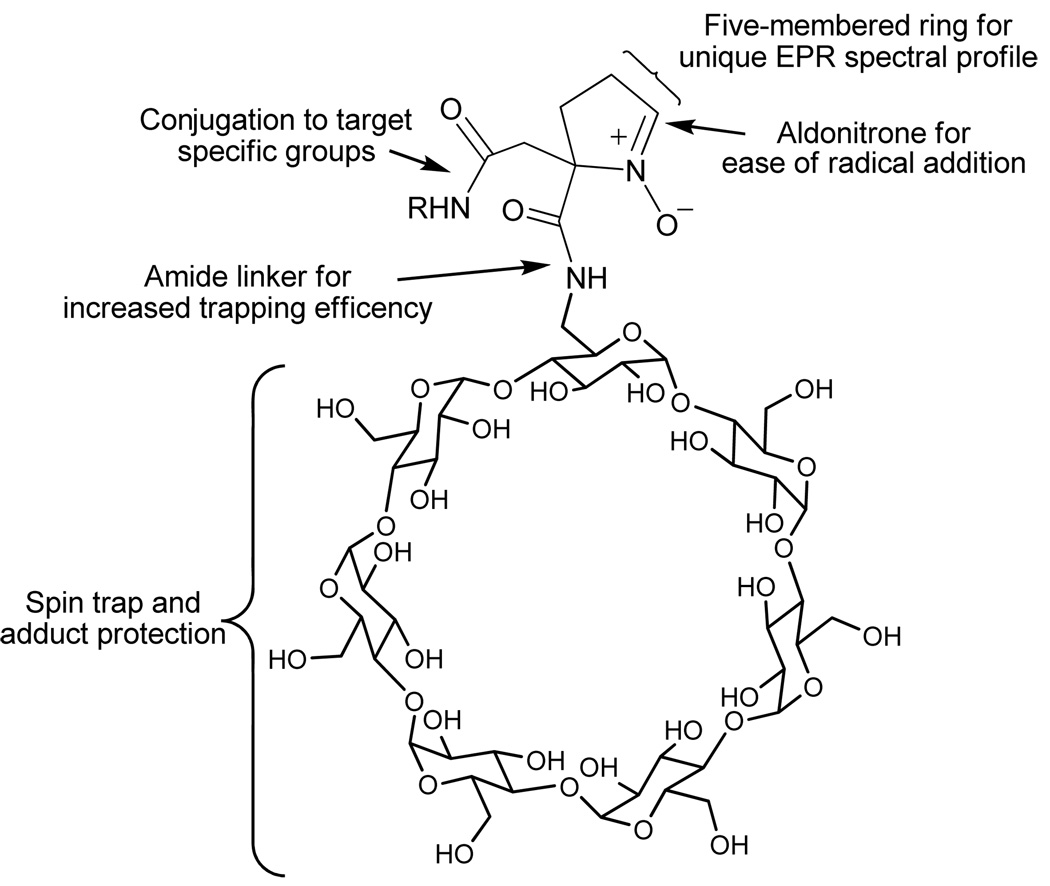
The idea of bifunctionalizing the nitrone to accommodate target-specific groups has become attractive. For example, a variety of functionalized nitrones have been synthesized such as the LPBNAH,31 GS-PBN,35 Mito-DEPMPO,36 and 5-ChEPMPO37 (Figure 1). Although these nitrone-conjugated compounds may exhibit a selective targeting of cellular compartments, the aim to achieve high reactivity to O2•−, longer adduct half-life, enhanced bioavailability and selective targeting in a unified molecular design have been a challenge (Figure 2). These challenges can perhaps be overcome through synthesis of a novel dicarboxylated cyclic nitrone 1 (Figure 3) that can be conjugated via amide bond to various groups for enhanced adduct stability and bioavailability. With the expectation that a β-CD group would lead to persistent spin adduct while a lipophilic chain may impart membrane affinity, we synthesized and demonstrated the spin trapping properties of 1 conjugated to a β-CD and dodecyl chain.
Figure 2.
Representation of a unified design using a bifunctionalized spin trap.
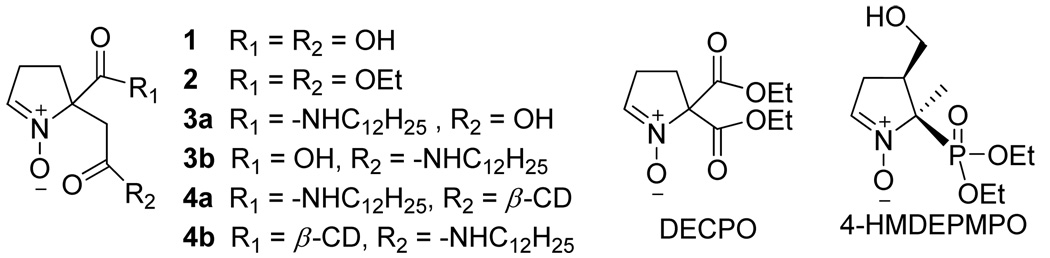
Figure 3.
Structures of bifunctional cyclic nitrones
Results and Discussion
Synthesis
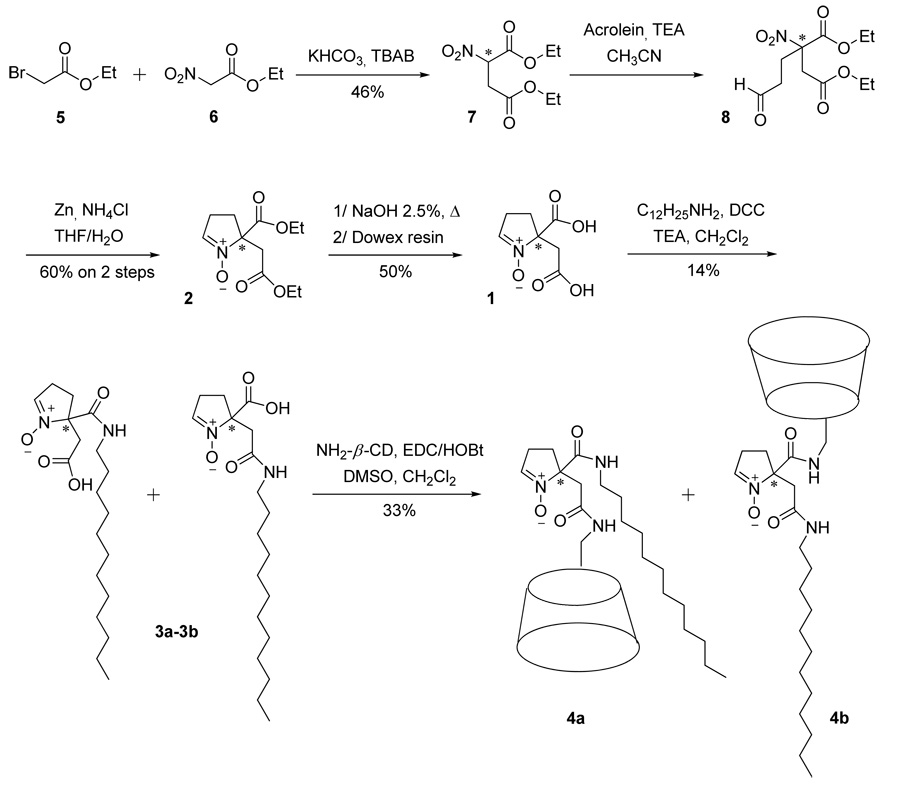
Only few cyclic nitrone analogues with bifunctional groups have been synthesized over the past years such as DECPO,38 and 5-diethoxyphosphoryl-4-hydroxymethyl-5-methyl-1-pyrroline-N-oxide, 4-HMDEPMPO39 (Figure 3). While DECPO may offer opportunities for bi-conjugation via amide bond, we have not been successful in hydrolyzing DECPO to dicarboxylic acid nitrone due to the decarboxylation reaction that occurs during base hydrolysis. In order to resolve this problem, compound 2 (see Figure 3 and Scheme 1) was designed to have an extra methylene group on one of the substituents at the C-5 position which could be hydrolyzed successfully to dicarboxylic acid cyclic nitrone (Scheme 1).
SCHEME 1.
Synthetic scheme for the bifunctional cyclic nitrones 4a and 4b
Nitrodiethyl ester 7 was prepared by alkylation of the nitro ester 6 with bromo ester 5 using KHCO3 and tetrabutylammonium bromide as base and phase transfer catalyst, respectively, according to the published procedures.40 Purification of compound 7 by column chromatography gave 46% yield. Michael addition of acrolein to the nitro-diester 7 led to compound 8 which was immediately used without further purification. Reductive cyclization of 8 using Zn/NH4C141 gave the nitrone 2 after purification. Compound 2 was then subjected to base hydrolysis of the ester bond then followed by ion exchange column chromatography to give 1 as a slightly yellowish solid.
The presence of carboxylic acid functional groups in 1 allows conjugation to a dodecyl group via amide bond however without any expectation of regioselectivity during the grafting. In order to avoid disubstitution of 1 by dodecyl group, equimolar amounts of dodecylamine and 1 were used for the coupling step. As expected, a mixture of the two monosubstituted nitrones 3a and 3b was obtained but evidence of formation of the disubstituted analogue was not investigated as only the polar fractions in the preparative TLC were isolated. However, the low yield of 3a and 3b suggests that biconjugation of the dodecyl group also occurs. Due to similarities in the structure and polarity of 3a and 3b, chromatographic separation of the mixture using preparative TLC was not successful. As shown in Figure S9, the 1H NMR spectrum of the mixture shows the presence of relevant moieties such as the alkyl chain at 0.9–1.5 ppm, the pyrroline group with the amido and carboxy methylenes at 2.5–3.4 ppm and the nitronyl proton at 7.11 ppm. Unfortunately, due to the overlap of the amido and carboxy methylenes peaks, we were unable to determine the ratio between 3a and 3b based on proton integration. Infrared as well as mass spectrometry analyses were also performed confirming the structure of nitrones 3a-3b (see Figure S10 and Sll).
The next step in the synthesis was to graft the β-CD moiety onto the lipophilic nitrones. The coupling of the 3a-3b mixture with the amino-β-CD was performed in the presence of EDC/HOBt yielding a mixture of nitrones 4a and 4b after precipitation in acetone. Purification by reverse-phase preparative HPLC led to pure nitrones 4a and 4b as demonstrated by HPLC chromatograms and mass spectrometry spectra (see S12 and S13–S14).
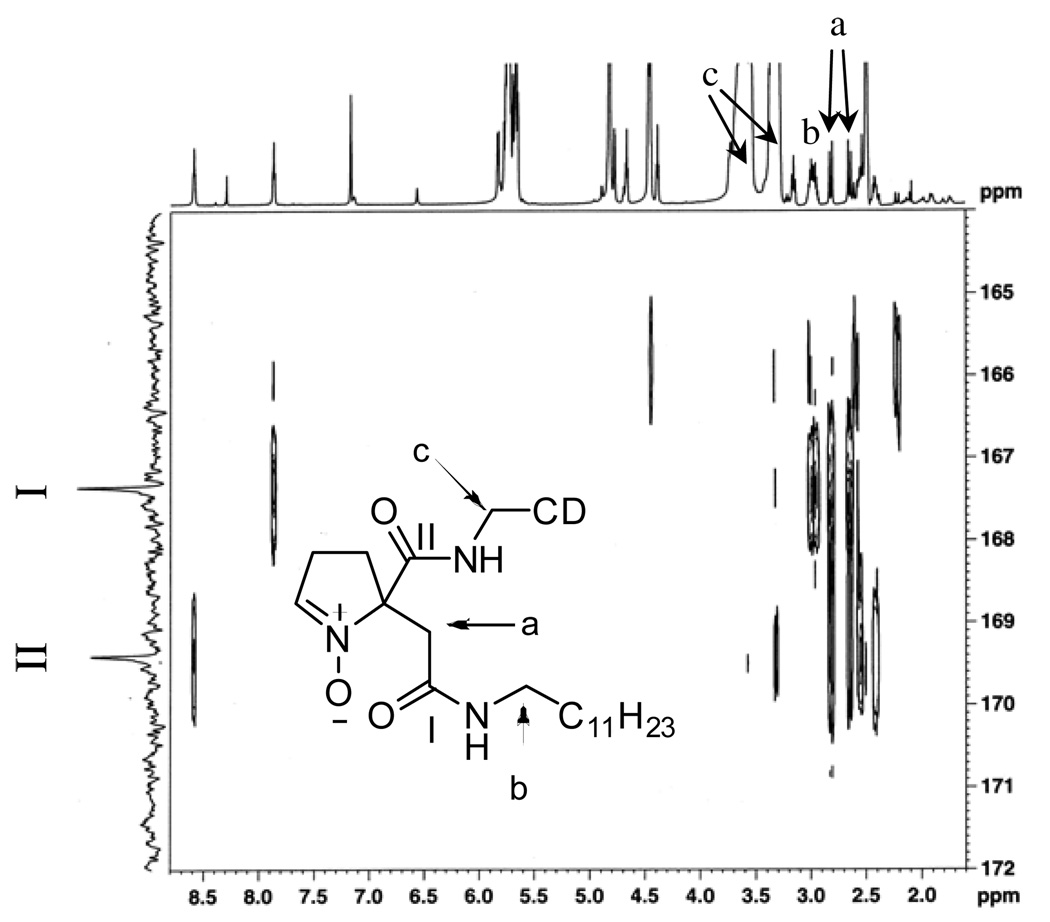
Compounds 4a and 4b have been successfully synthesized and separated, but attempts to spectroscopically differentiate 4a from 4b by 1H and 13C NMR alone was difficult due to the similarities in their spectral profiles. MS/MS analysis of 4a and 4b also gave the same fragmentation patterns showing a consecutive loss of ions with m/z =162 corresponding to a glucopyranoside unit. Also evident from the MS/MS spectra of both 4a and 4b is the loss of a m/z = 140 corresponding to [5-(2-amino-2-oxoethyl)-l-pyrroline N-oxide]•+ fragment (See Figures S15 and S16). Due to the limited quantity of 4a and 4b and their limited solubility in water or methanol, only an extensive NMR analysis of 4b using HSQC, TOCSY, HSQC-TOCSY and HMBC was performed in DMSO-d6 (See Figures S17– S22). The HSQC experiment allowed us to identify the resonance peak of the three methylene-protons of interest not originating from the pyrroline ring: (a) an AB system at 2.60–2.85 ppm; (b) a multiplet at 2.98 ppm; and (c) a doublet at 3.45 ppm that is overlapping with the β-CD peaks (see Figure 4), with corresponding 13C-resonance peaks at 39.5, 38.4 and 40.4 ppm, respectively. Fortunately, TOCSY and HSQC-TOCSY experiments allowed us to assign the 2.98 ppm peaks to the first methylene-protons of the dodecyl group that is attached to the amide-nitrogen (labeled as (b) protons in Figure 4). Since no correlation between the protons of the AB system (i.e., the (a) protons) and any other protons was observed from TOCSY or HSQC-TOCSY, this suggests that the (a) protons are those of the methylene group attached to the pyrroline ring. The next step is to precisely assign which methylene group (i.e., either from the β-CD or the dodecyl) is connected to the (a) protons via the amide bond. As shown in Figure 4, the HMBC experiment provided useful information regarding the connectivity in the molecule. The (a) protons gave long range coupling with the carbon of the dodecyl group that is attached to the amide-nitrogen, (i.e., the (b) protons) as well as with the carbonyl carbon (I) at 167.4 ppm. Although a long range correlation between (a) protons and the other carbonyl carbon (II) at 169.4 ppm was also observed due to their proximity to one another, no correlation was observed between the (b) protons and the other carbonyl carbon (II) at 169.4 ppm. This clearly demonstrates that the (b) protons are closer to the (a) protons than to the (c) protons as shown on Figure 4. Also worth noting are the two amide triplet peaks at 7.86 and 8.58 ppm which are correlated to the carbonyl carbons, I and II, respectively (Figure 4). The amide-hydrogen at 7.86 ppm shows strong correlation with the (b) and (a) protons but only a weak correlation with (c) protons, while the amide-hydrogen at 8.58 ppm shows stronger correlation with (c) and (a) but not with (b) further demonstrating the correct connectivity assignments for 4b.
Figure 4.
1H-13C HMBC spectra (600 MHz) of 4b in DMSO- d6 showing the three methylene groups (a, b and c) of interest and their long range correlation with the two carbonyl carbons (I and II).
NMR and Inclusion Studies
A detailed structural analysis of β-CDMPO was described in our previous paper.24 Figures S23a and S23b show similarities between the ROESY spectra of 4a and 4b in D2O, respectively. Unlike in DMSO, the 1H-NMR spectrum is more complex in D2O, but is due to the presence of extensive H-bonding resulting in the formation of distinctive conformational isomers for 4a and 4b. The presence of various conformational isomers for 4a and 4b was studied computationally and will be discussed in detail in the succeeding section. Only weak correlation of cyclic nitrone with β-CD can be observed. However, a strong long range correlation can be observed from the dodecyl group interaction with the β-CD cavity and this is more pronounced for 4b than in 4a. This difference in the degree of interaction between the dodecyl group with the hydrophobic core of the β-CD in 4b and 4a could be due to the presence of the extra methylene group separating the nitrone and the dodecyl moiety in 4b providing more flexibility for the dodecyl group for interaction with the β-CD.
Furthermore, in order to explore the inclusion properties of 4a and 4b, l-borneol was used as a competitor guest molecule.24 Ordinarily, l-borneol will displace the included moiety in β-CD due to the strong inclusion complex formed by l-borneol with β-CD. As shown in Figures S23c and S23d, the 1H-NMR peaks of 4a and 4b are shifted and broader with the addition of l-borneol. For example, the peaks at ∼5.0 and 3.5–4.0 ppm which are assigned to β-CD-H’s were shifted downfield to −5.3 and 3.8–4.3 ppm, respectively, while the peaks at 1.1–1.5 ppm which are assigned to the dodecyl-H’s were shifted upfield to ∼1.1 ppm. Worth noting is the disappearance of the correlation between β-CD and dodecyl-H’s, but interestingly, the correlation between β-CD and l-borneol was not apparent due perhaps to the fast exchange between l-borneol and the dodecyl group as evidenced by the broadened 1H-NMR spectra of 4a and 4b in the presence of l-borneol. Also, the area of the correlation peaks for β-CD and dodecyl group in 4b is bigger compared to that in 4a indicating that there could be more inclusion isomers in 4b than in 4a. It should be noted, however, that the area of correlation peaks observed between the protons of β-CD and dodecyl group for both 4a and 4b is minor relative to the total peak area observed for the 1H-NMR spectra of the dodecyl protons at the 1–2 ppm region (Figures S23a and S23b) suggesting that inclusion complex may not be the only conformation present in solution.
Induced Circular Dichroism (ICD)
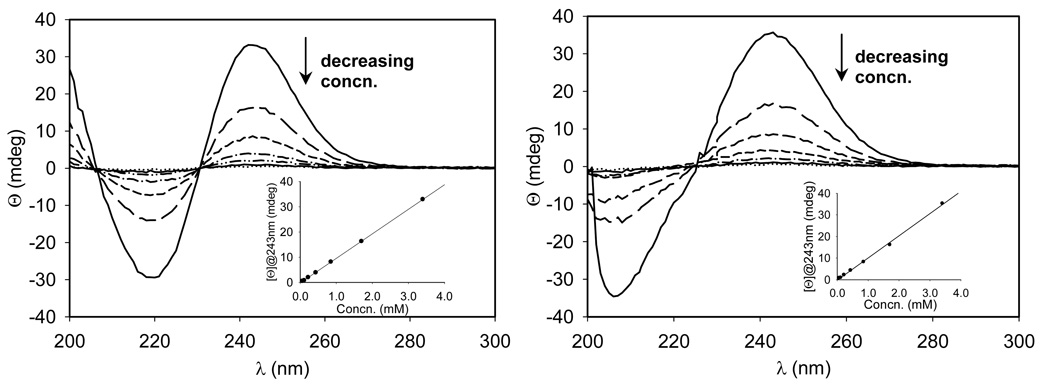
To give more insights into the nature of β-CD and dodecyl group interaction, ICD measurements were carried out. As shown in Figure S24, 1 gave no ICD band as expected for a racemic mixture. However, 4a and 4b show minima at 220 and 205 nm, respectively, while the same maxima at 243 nm can be observed for both isomers (Figure 5). The observed optical activity for 4a and 4b in spite of the presence of racemates could be due to the effect of the intrinsic chirality of β-CD on the orientation or conformation42 of nitrone group thereby exhibiting ICD bands. The high intensity bands suggest strong intramolecular interaction of the nitrone chromophore with the β-CD. The difference in the minima between 4a and 4b suggests that the nitrone moiety in these two isomers may exhibit different spatial orientation in solution consistent with their ROESY spectra and will be further demonstrated by spin trapping studies and particle size analysis in the succeeding sections. Moreover, concentration dependence study of the ICD spectra of 4a and 4b (Figure 5) shows direct correlation of the band intensity at 243 nm with decreasing concentration. This result suggests that the conformations of 4a and 4b is not affected by concentration, otherwise, a nonlinear curve at the same concentration range similar to those previously reported should be observed.43
Figure 5.
Concentration dependence of ICD spectra of 4a (left) and 4b (right). The concentrations of 4a or 4b from bottom to top at 243 nm are 0.06, 0.11, 0.22, 0.43, 0.85, 1.7, 3.4 mM. The inset shows the dependence of the ellipticity at 243 nm on the concentration of 4a or 4b.
Self-Aggregation Properties
The self-aggregation properties in water of pure β-CD’s have been extensively investigated for 15 years. Coleman reported44 the formation of β-CD aggregates with an average diameter of around 200 nm that might be rod-like aggregates, while Gonzáles-Gaitano et al.45 observed the formation of ∼300 nm diameter size clusters. More recently, Baglioni et al.46 demonstrated the formation of aggregates with 190 nm diameter. Cyclodextrins bearing additional groups that are either hydrophilic or hydrophobic have been found to exhibit more pronounced self-aggregation properties than pure CDs leading to well characterized supra-molecular aggregates (e.g., spherical micelles,47,48 or vesicles49).
The self-aggregation properties of compounds 4a and 4b were investigated by dynamic light scattering technique at a concentration range of 1 mM to 0.1 mM, and the results are expressed as volume distribution percentage as shown in Table 1
Table 1.
Particle Size Distribution Parameters by DLS in Aqueous Solution at 25 ºC.
| 4a | 4b | |||||
|---|---|---|---|---|---|---|
| Concn. | DHa | HHW b | PDIc | DHa | HHW b | PDI c |
| 1 mM | 132 ± 27 | 12 ± 3 | 0.761 | 71 ± 12 (20%)d | 11 ± 3 | 0.517 |
| 267 ± 49 (66%)d | 34 ± 7 | |||||
| 0.5 mM | 174 ± 35 | 16 ± 3 | 0.612 | 58 ± 25 (64%)d | 12 ± 3 | 0.478 |
| 166 ± 28 (21%)d | 30 ± 11 | |||||
| 0.1 mM | 128 ± 51 | 11 ± 5 | 0.786 | 171 ± 10 (29%)d | 18 ± 2 | 0.17 |
| 290 ± 9 (71%)d | 32 ± 1 | |||||
DH: hydrodynamic diameter of particles in nanometer. The values reported are average of 14 measurements.
HHW, the width of the peak at half-height, an indication of the degree of polydispersity of the aggregates.
PDI, polydispersity index.
Percentage of volume distribution.
Both 4a and 4b self-organize in water into particles with apparent hydrodynamic diameters that is within the range of ∼100 to 300 nm but is not in agreement with the formation of spherical micelles. This first observation tends to suggest that cyclodextrin derivatives cannot be considered as classical surfactants. Indeed, recent reports showed that C12 surfactants bearing bulky glucose-based polar heads lead to the formation of spherical micelles of 5.2 nm diameter. 50 Smaller particles of ∼60 nm diameter were also observed with compound 4b but not within the whole concentration range mentioned above. These smaller aggregates could be micellar aggregates as demonstrated previously with amphiphilic β-CD compounds.29,48 However, these smaller particles were in equilibrium with two types of aggregates, that is, one at around 170–270 nm and one at 2600 nm (not reported in the table, with percent volume distribution less than 15%). During the course of the experiment with 4a, similar aggregates were also observed but were less persistent. Moreover, the aggregates of 4a exhibit a lower polydispersity than that of 4b, as shown by the Half-Height Width (HHW) and polydispersity index (PDI). This confirms the different behavior of 4a and 4b in aqueous solution as demonstrated above by NMR and circular dichroism. However, the reproducibility of the size distribution of aggregates in independently prepared samples was found to be slightly variable. Hence, the first experimental trial with 4a did not allow us to observe persistent large aggregates but these aggregates were observed in the second trial, however, with a volume distribution below 25% (data not shown) indicating the dynamic nature of this aggregation process.
Transmission Electron Microscopy
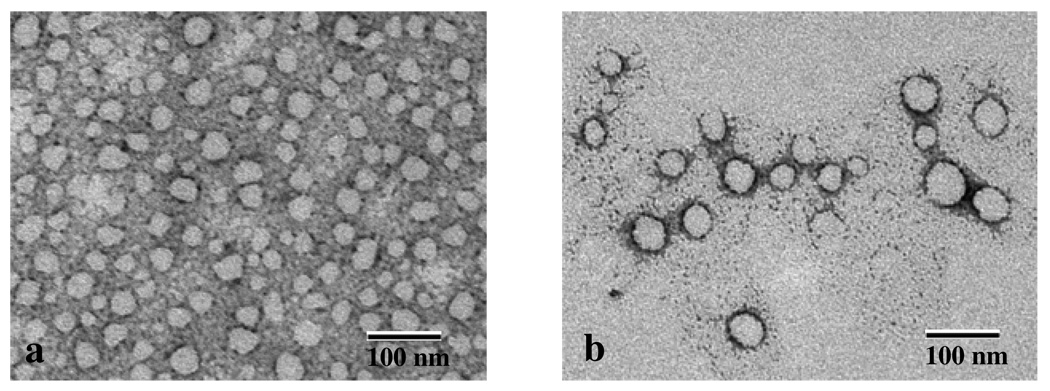
Imaging of the aggregate morphologies formed from 4a was possible and is shown in Figure 6. High density of spherical aggregates with a diameter of 20–50 nm was observed 30 minutes after dissolution of 4a in water. When the solution was allowed to stand for 2 hours, spherical aggregates of bigger diameter ∼100 nm were observed while the density of the small aggregates was decreased. This confirms the occurrence of a dynamic equilibrium as observed by DLS and suggest that the small aggregates formed immediately after the dispersion in water may coalesce to form bigger ones. None of the particles observed showed dark centers that could originate from aqueous core indicating the formation of vesicles. Only disc-like morphology was observed suggesting the formation of nanoparticles but with no precise evidence of their composition.
Figure 6.
Electron micrographs of 4a solution (2 mM) in water (a) 30 minutes and (b) 2 hours after sonication.
EPR Spin Trapping Studies. Compound 1
The spin trapping properties of 1 for HO• and O2•− was investigated. Hydroxyl radical was generated using Fenton reaction while O2•− was produced using xanthine/xanthine oxidase system in phosphate buffer. As shown in Figure S25, 1 gave an EPR signal with HO• but not with O2•− using the same nitrone concentration. This phenomenon is similar to that observed by Rosen, et al,41 although the mechanism for this observation is still unclear but we hypothesize that this could be due to the repulsion between the negatively charged carboxylate anion and O2•−/HO2•, or perhaps the dismutation of O2•− in the presence of exchangeable protons competing with the spin trapping reaction.
Compounds 4a and 4b
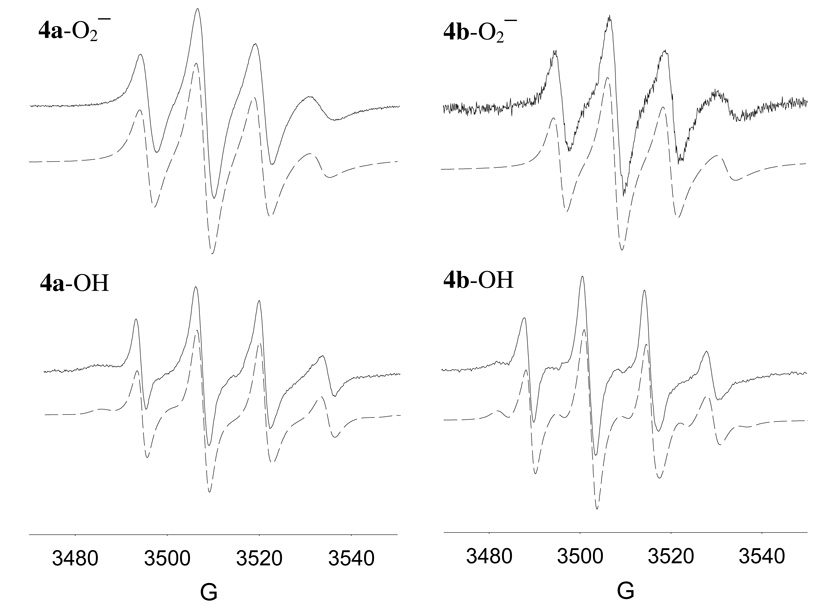
Although the β-CDMPO alone in the absence of hydrophobic dodecyl group gave robust EPR signal upon trapping O2•− in aqueous system,24 generation of O2•− in aqueous system with 4a or 4b (20 mM) only gave a weak EPR signal (data not shown) due probably to the formation of aggregate or formation of a conformation that may shield the nitrone from the radicals. However, EPR signal was stronger when O2•− spin adducts of 4a and 4b were generated in DMSO at the same concentrations (Figure 7). Based on the spectra shown in Figure 7, O2•− adducts exhibited hindered molecular tumbling as characterized by smaller magnitude and broadening of the highest-field line compared to the lowest-field line. These partial anisotropic behaviors are may be due to the relatively large size of the molecules, solvent viscosity and/or dipolar interaction. The EPR parameters for O2•− adducts are shown in Table 2 and gave higher correlation time for 4a–O2•− adduct compared to the 4b–O2•− adduct indicating a more restricted molecular motion for the former. However, due to the significant anisotropy in the spectra observed for the O2•− adducts, the percent population of the isomers cannot be determined. The calculated angle between the slow rotation axis and the magnetic z-axis is around 60˚ for the O2•− adducts.
Figure 7.
X-band EPR spectra of the superoxide radical anion and hydroxyl adducts of 4a and 4b (20 mM) generated using KO2/DMSO and Fe2+/H2O2 in PBS, respectively. Simulated spectra are shown as trace plots. EPR isotropic and anisotropic parameters are shown in Table 2and experimental conditions are described in the text.
Table 2.
EPR parameters for the hydroxyl and superoxide radical anion adducts of 4a and 4b in DMSO.
| Hydroxyl Adducts (in PBS) | ||||||
|---|---|---|---|---|---|---|
| Adducts | g | an (G) | aβ-H (G) | Relaxation Coefficientsa |
||
| α | β | γ | ||||
| 4a-OHa | 2.0054 | 13.8 | 12.7 | 1.82 | 0.41 | 0.46 |
| 4b-OH | 2.0053 | 13.8 | 12.5 | 1.77 | 0.38 | 0.38 |
|
Superoxide Adducts (in DMSO)b | ||||||
| axis | g | an(G) | aβ-H (G) | Correlation time (ns) | ||
| 4a-OO− | x | 2.0089 | 2 | 12 | 1.74 (4a) | |
| or | y | 2.0085 | 3 | 10 | ||
| 4b-OO− | z | 1.992 | 31 | 11 | 1.61 (4b) | |
, where Γ is the linewidth, α, β, and γ are relaxation coefficients and MI is the z component secondary quantum number for the nuclear-spin angular momentum for N atom (MI= 1).
The anisotropic g’s, hfsc’s and correlation times were calculated as follows: From the isotropic signal, the line width variation was interpreted via a relaxation model assuming a rhombic g- and hyperfine tensors, where the anisotropy was partially averaged out by the isotropic rotational tumbling. The spectrum fit was optimized for both the anisotropic tensor elements and the correlation time.
The EPR spectra of the HO• adducts in aqueous solution obtained from Fenton reaction using 20 mM of 4a or 4b are shown in Figure 7. Unlike in the formation of O2•− adducts from 4a and 4b in aqueous solution, the HO• adducts gave relatively more robust signal in water in spite of their tendency to aggregate in aqueous system. As shown in Table 2, there is a difference in relaxation coefficients between 4a–OH and 4b–OH in which the former shows a more hindered molecular tumbling motion than the latter. This observation is consistent with our results from NMR, ICD and DLS studies suggesting a higher equilibrium concentration of the inclusion form for 4b compared to 4a, further confirming the difference in the conformation between 4a and 4b and their respective O2•− and HO• adducts. Due to the higher presence of non-included dodecyl chain in 4a and its adducts, it can be assumed that these may give a more pronounced surfactant-like behavior compared to 4b. The extra methylene group that connects the nitrone moiety and the dodecyl group in 4b could allow formation of an inclusion complex more facile than in 4a. Finally, since 4a and 4b gave similar spectral profile for HO• and O2•−, using a mixture of 4a and 4b for spin trapping applications should not limit spectral interpretation.
Kinetics of O2•− Adduct Formation and Decay
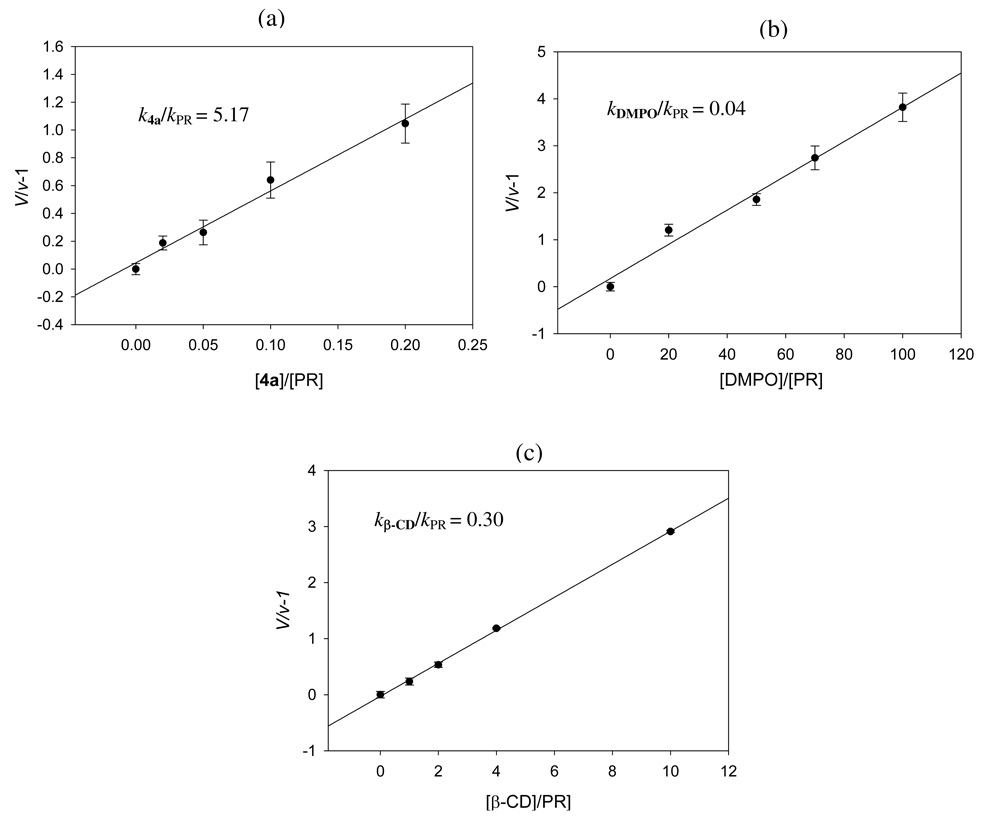
Due to the poor spin trapping ability of 4a for O2•− in aqueous system, kinetic studies were performed in DMF solution using stopped-flow kinetics as previously described.13,51 Figure 8 shows the kinetic plots for the reaction of 4a, DMPO and β-CD with KO2, and gave k5a/kPR, kDMPO/kPR, and kβ-CD/kPRof 5.17, 0.04 (lit. 0.02)51, and 0.30 respectively, indicating that 4a is ∼ 130× more reactive to O2•− than DMPO. Surprisingly, β-CD alone exhibit reactivity towards O2•− and is ∼ 7.5× more reactive than DMPO. The nature of O2•− reactivity to β-CD could be due to the alpha effect similar to those observed for O2•− interaction with amino-acids and non-amino acids bearing H-bond donors.52 Based on the absolute second order rate constant for DMPO of 1.7 M−1 s-1 obtained13 using stopped-flow kinetics, the rate constant for O2•− trapping by 4a can be estimated to be 221 M−1 s−1 which is considerably faster compared to other substituted cyclic nitrones13 such as AMPO (135 M−1 s−1), EMPO (104 M−1 s−1), and DEPMPO (0.65 M−1 s−1) and linear nitrones (0.13–0.80 M−1 s−1) whose rate constants were obtained using the same experimental conditions as 4a.51 Although 4a shows poor reactivity to O2•− in aqueous solution due to the possible formation of aggregates, their improved reactivity to O2•− in non-polar systems can be potentially applied to scavenge O2•− in lipid membranes.
Figure 8.
Stopped-flow kinetic plots of a) 4a; b) DMPO and c) β-CD for their reaction with KO2 in DMF using phenol red (PR) as competitor, where V and v are the initial rates of formation in the absence and presence of various concentrations of the spin trap, respectively. Initial rates were obtained by monitoring the formation of UV-Vis absorption peak at 575 nm. Measurements were done at least in triplicate. See experimental section for details.
The decay kinetics of O2•− adducts of various spin traps were also explored to investigate the effect of the β-CD moiety on the stability of 4a. The various O2•− adducts were generated in DMSO using 2 mM spin trap and KO2 (see Experimental for details) and the half-lives based on first order decay kinetics were calculated as follows (in min): DMPO-O2H (3.6 ± 0.7), DEPMPO-O2H (2.4 ± 0.3); AMPO-O2H (2.6 ± 0.2); β-CDMPO-O2H (5.7 ± 1.1) and 4a-O2H (9.0 ± 2.8). Results show that the half-life for the O2•− adduct of DMPO is longer (3.6 min) compared to 1 min in aqueous solution consistent with that previously reported53 for the decay of DMPO-O2H in aprotic solvents. However, the O2•−adducts of DEPMPO, AMPO, and β-CDMPO in DMSO are considerably shorter compared to that found in aqueous system of 14 min,54 8 min,14 and 28 min,24 respectively. This discrepancy could be due to the annihilation of the intramolecular H-bond interaction in DMSO which is believed to be mostly responsible for adduct stability.14,19,24,55 Nevertheless, this work only aims to give a qualitative trend on the relative stability of the adducts and to demonstrate how conjugation of β-CD group to nitrones can enhance O2•− adduct stability as shown by the higher t1/2 for β-CDMPO-O2H and 4a-O2H compared to the non-conjugated nitrones.
Molecular Modeling of 4a–b and their O2•− Adducts
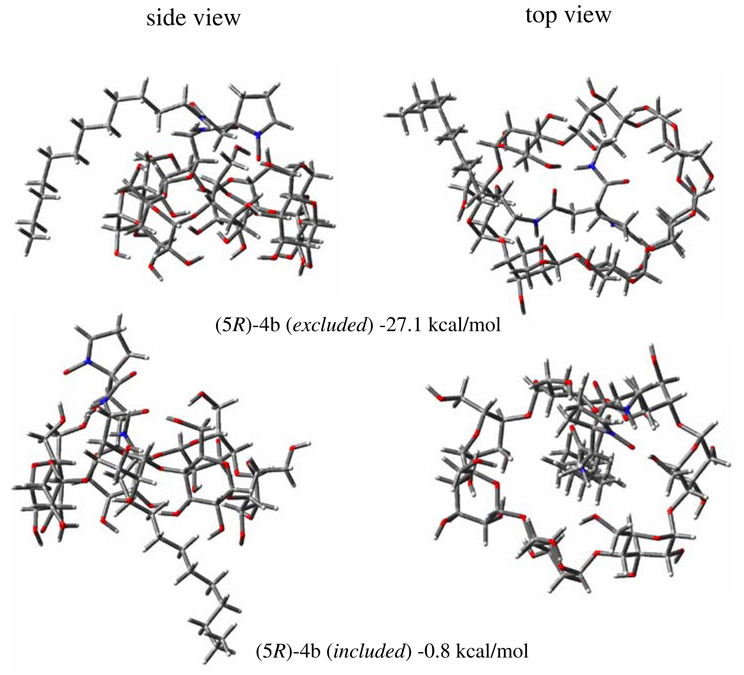
In order to investigate the effects of conformation as well as stereoisomerism on the stability of 4a–b and their corresponding O2•− adducts, computational studies were carried. Table S1 shows the calculated relative bottom-of-the-well energies of the various isomers of 4a and 4b. Two conformational isomers can be seen in Figure 9 for the 5R-4b isomer in which the dodecyl group is outside the β-CD cavity and is more thermodynamic favorable compared to when the dodecyl group is inside. Similar observation can be seen for 5S4a - but only gave ∼6 kcal/mol energy difference between the self-included and excluded conformations (Figure S27). However, the reverse is true for 5R-4a and 5S−4b. These differences in the preferred conformations, i.e., included and excluded, could be due to the orientation of the nitrone moiety relative to the β-CD annulus. Careful examination of the orientation of the nitrone group shows that the N-O points directly towards the β-CD annulus in the lower energy isomers compared to the higher ones in which the N-O points away from the β-CD annulus (see Figure 9 and Figures S27–S28). The preferred conformations show the N-O moiety exhibiting a strong intramolecular H-bonding interaction with the hydroxyl-H of the β-CD. This indicates that the orientation of the nitrone relative to the β-CD annulus is strongly influenced by the stereochemistry around C-5 and the constitutional isomerism of 4. Therefore, the interaction of the nitrone with the β-CD plays a more important role in the conformational stability of 4 than the position of the dodecyl group (i.e., whether included or excluded). The presence of H-bond interaction between the amide-H and β-CD-OH’s might also be a contributing factor to the stability of the conformation but this was also observed in the least stable isomers.
Figure 9.
Side and top views of the optimized geometries of 4b at the HF/3–21G* level of theory showing the dodecyl group outside (top) and inside (bottom) of the β-CD cavity and their relative bottom-of-the-well energies.
The effect of isomerism on the stability of various O2•− adducts of 4a and 4b was also investigated and Table S1 shows the relative bottom-of-the-well energies for these adducts. Similar to that observed for 4a and 4b, the preferred conformation is characterized by intramolecular H-bond interaction of the nitronyl-O and hydroperoxyl-H with the β-CD-OH’s. For example as shown in Figure S29, (2R,5R)-4b–OOH (excluded) gave the most preferred conformation in which intramolecular H-bond interaction of the aminoxyl-O and hydroperoxyl-H with the β-CD-OH’s is evident followed by (2S,5R)-4b–OOH (excluded) in which only the aminoxyl-O shows intramolecular interaction with β-CD-OH and not the hydroperoxyl-H. Both the included isomers, (2S,5R)-4b–OOH and (2R,5R)-4b–OOH are least preferred and that both the N-O and -OOH groups show no direct interaction with the β-CD-OH’s (see Figure S29). In general, for 5S-4b–OOH, 5R-4a–OOH and 5S-4a–OOH isomers, a similar trend was observed in which the most preferred adducts show H-bond interaction of the N-O and -OOH moieties with β-CD-OH’s (see Table S1 and Figures S29–S32). Therefore, the orientations of N-O and -OOH relative to the β-CD annulus is also strongly influenced by the stereochemistry around C-2 and C-5 as well as the constitutional isomerism in 4.
The complex formed between 4a–b and the guest molecule, borneol, was also computationally investigated. Table S1 shows the relative bottom-of-the-well energies for the various conformations of the 4a–b---borneol complex. Results show that the location of the borneol being on the opposite side of the β-CD annulus relative to the nitrone group is more preferred than when the borneol and the nitrone groups are on the same side of the annulus such as in the case of (5R)-4b (see Figure S33). This observation could be due to the repulsive effect between the nitrone group and borneol being on the same side of the annulus. Conversely, the borneol being on the other side of the nitrone group maximizes the intramolecular H-bonding between the nitrone and β-CD as the borneol does not compete for the H-bonding sites. The same trend is observed for (5S)-4b (Figure S34). In the case of (5R)-4a and (5S)-4a (Figures S33 and S34, respectively), the presence of an extra methylene group between the nitrone and β-CD gives the nitrone more freedom to reduce the repulsion with borneol although they are both in the same side of the annulus and therefore does not follow the same observation as in (5R)-4b and (5S)-4b ((Figures S33 and S34, respectively).
Conclusions
Bifunctionalization of cyclic spin trap with β-CD and lipophilic dodecyl chain using amide linkers gave two structural racemic isomers, 4a and 4b which were unequivocally characterized by 1-D, 2-D homo-and hetero-nuclear NMR as well as MS and MS/MS experiments. Circular dichroism and ROESY coupled with competition experiments with borneol suggest that the nitrone and dodecyl group interaction with the β-CD is intramolecular in nature. DLS and TEM show that these compounds self-aggregate in aqueous solution and form particles with sizes ranging from 100–300 nm. Spin trapping of O2•− by 4a and 4b in DMSO is more favored than in aqueous solution suggesting that the self-aggregation play a crucial role in the spin trapping property of the bi-conjugated nitrone. The kinetics of formation and decay of O2•− adducts of 4a in aprotic polar solvents gave increased rate of spin trapping and longer adduct decay compared to the commonly used spin traps and β-CDMPO. Computational analysis of 4a and 4b preferred structures show strong intramolecular H-bond interaction of the nitronyl-NO group with the β-CD-OH’s on the annulus. Moreover, the preferred conformations for the 4a/b-OOH adducts were computationally rationalized and similar to 4a–b alone, the preferred conformations is influenced by both constitutional and stereochemical isomerism that allows optimal intramolecular H-bond interaction between the aminoxyl-NO and the hydroperoxyl-H with the β-CD-OH’s on the annulus. Efforts are now underway to conjugate β-CDMPO to other groups that are target specific to cellular compartments such as the mitochondria, cytosol and extracellular matrix.
Experimental
Synthesis. (a) 5-(2-Ethoxy-2-oxoethyl)-5-(ethoxycarbonyl)-1-pyrroline N-oxide (2)
The synthesis of diethyl 2-nitrosuccinate (7) was carried out according to published procedures40 and its spectral data was in agreement with the literature.40 TEA (0.2 mL) was slowly added to a cooled (10˚C) solution of 7 (1.8 g, 8.2 mmol) and acrolein (0.66 mL, 9.9 mmol) in CH3CN (5mL). The mixture was stirred for 2 h at room temperature and then concentrated in vacuo to give the crude product 8. The crude product 8 was dissolved in THF (90 mL) at −10˚C and a solution of NH4Cl (2.8 g) in 20 mL water was added followed by Zn dust (2 g) which was added portion-wise within 0.5 h at −10˚C. The mixture was stirred for another 2 h at −10˚C and solid NaCl was added and extracted with Et2O. The collected organic phase was dried over anhydrous MgSO4 and filtered. The solvent was removed in vacuo and the residue was purified by flash column chromatography using CH2Cl2/MeOH (97:3 v/v) as eluent to give 2 as yellow oil (1.2 g, 60%). 1H NMR (400 MHz, CDCl3) 1.29 (overlap of two triplets, 6H), 2.71 (m, 1H), 2.76 (m, 3H), 3.05–3.33 (AB system, 2H), 4.13–4.27 (m, 4H), 6.97 (t, 1H). 13C NMR (100 MHz, CDCl3) 8 14.3, 14.5, 26.9, 30.2, 38.0, 61.3, 63.0, 79.9, 136.3, 169.1, 170.0. IR (neat, cm−1) v 3440, 2983, 1733, 1586, 1207, 1183, 1076, 1027. GC-MS calcd for C11H17NO5 m/z 243.1, found 243.0. HRMS calcd for C11H17NO5Na [M + Na]: 266.1004, found 266.0995.
(b) 5-Carboxy-5-(carboxymethyl)-1-pyrroline N-oxide (1)
To compound 2 (1.2 g) was added 2.5% NaOH (10 mL) then the mixture was refluxed under argon for 1.5 h (at this point, the color of the mixture changed to brown). After cooling, the reaction mixture was passed through Dowex (H+ form) ion exchange column using water as eluent. The fractions having pH < 4 were collected and concentrated in vacuo and then purified by flash column chromatography using CH2Cl2/MeOH (8:2 v/v) as eluent to give 1 as white solid (0.46 g, 50%). 1H NMR (400 MHz, D2O) 2.35–2.70 (m, 2H), 2.76 (m, 2H), 2.95–3.25 (m, 2H), 7.37 (s, 1H). 13C NMR (100 MHz, CDCl3) 27.0, 29.7, 37.4, 79.8, 147.2, 171.8, 173.0. IR (neat, cm−1) v 3087, 2934, 2502, 1715, 1604, 1172. HRMS calcd for C7H9NO5Na [M + Na]: 210.0378, found 210.0373.
(c) 5-(Dodecylcarbamoyl)-5-(2-oxo-2((6,6'-deoxy-β-cyclodextrin)methylamino)ethyl)-1-pyrroline N-oxide (4a) and 5-(2-(dodecylamino)-2-oxoethyl)-5-((6,6'-deoxy-β-cyclodextrin) methyl-carbamoyl)-1-pyrroline N-oxide (4b)
To a solution of 1 (40 mg, 0.2 mmol) in CH2Cl2 (2 mL) was added DCC (50 mg, 0.24 mmol), dodecylamine (40 mg, 0.2 mmol) and TEA (2 drops) and stirred for 12 h at room temperature. The product was purified using preparative TLC (thickness, 1.0 mm) and CH2Cl2/MeOH (8:2 v/v) as eluent to give a mixture of 3a and 3b as yellow oil (10 mg, 14%) which was used without any further purification. 1H NMR (400 MHz, CDCl3) 0.89 (t, 3H), 1.33 (m, 18H), 1.45 (m, 2H), 2.62 (m, 2H), 2.76 (m, 1H), 2.92 (m, 1H), 3.10 (m, 2H), 7.12 (s, 1H). IR (neat, cm−1) v 3300, 2923, 2853, 1661, 1548. HRMS calcd for C19H34N2O4Na [M + Na]: 377.2416, found 377.2418. To a solution of 3a-3b (10 mg, 0.03 mmol) in DMSO (2 mL) was added mono-6-deoxy-6-amino-β-cyclodextrin (60 mg, 0.06 mmol), EDC (10 mg, 0.05 mmol), HOBt (3mg, 0.03 mmol), and TEA (1 drop). The mixture was stirred at ambient temperature for 2 days. Acetone was added to the reaction mixture to give the crude product as a white precipitate. The precipitate was further purified using reverse-phase HPLC (C18 5μ, 150mm × 22 mm) with gradient elution from 10% to 40% aqueous MeCN at a flow-rate of 10 mL/min using 254 nm UV detection. Fractions were collected and solvents were removed in vacuo to afford the pure nitrones 4a (7 mg) and 4b (7 mg) as white solids (total yield: 33%). 1H NMR (400 MHz, D2O) 4b 0.88 (t, 3H), 1.16–1.36 (m, 20H), 2.16 (m, 2H), 2.43(m, 1H), 2.56 (m, 2H), 2.63 (s, 1H), 2.81 (m, 4H), 3.00–3.40 (m, 4H), 3.50–3.90 (m, 42H), 4.99 (m, 7H), 7.38 (m, 1H); 4a 0.88 (t, 3H), 1.16–1.36 (m, 20H), 2.16 (m, 2H), 2.43 (m, 1H), 2.56 (m, 2H), 2.63 (s, 1H), 2.81 (m, 4H), 3.00–3.40 (m, 4H), 3.50–3.90 (m, 42H), 4.99 (m, 7H), 7.38 (m, 1H). 1H NMR (600 MHz, DMSO-d6) 4b 0.85 (t, J = 6.9Hz, 3H), 1.23 (broad s, 18H), 1.33 (m, 2H), 2.35–2.58 (m, 4H), 2.60–2.85 (AB system, 2H), 2.97 (m, 2H), 3.15 (t, J = 9 Hz, 1H), 3.25–3.40 (m, 13H), 3.50–3.80 (m, 27H), 4.38 (t, J = 5.6 Hz, 1H), 4.46 (m, 4H), 4.66 (t, J = 5.4 Hz, 1H), 4.78 (s, 1H), 4.82 (s, 6H), 5.55–5.80 (m, 13H), 5.83 (d, J = 6.6 Hz, 1H), 7.16 (s, 1H), 7.86 (t, J = 5.4 Hz, 1H), 8.54 (bs, 1H). 13C NMR (150 MHz, DMSO-d6) 4b 14.0, 22.1, 25.2, 26.3, 27.1, 28.7, 28.9, 29.0, 31.27, 38.4, 39.4, 40.4, 59.8, 72.0, 72.3, 72.9, 79.4, 81.4, 83.6, 101.5, 101.9, 102.6, 138.5, 167.4, 169.4. MALDI calcd for C61H104N3O37 [M + H]: 1470.635, found 1470.674 (4a) and 1470.623 (4b).
Particle size analyses
The hydrodynamic particle size distributions and polydispersity of amphiphilic cyclodextrins at different concentrations were determined using particle size analyzer equipped with a He-Ne laser (λ = 633 nm, 4.0 mW). In a typical experiment, stock solution was prepared by dissolution of the compounds in filtered water (using 0.22 µm filter and water resistivity of 18.2 MΩ.cm) and sonicated for 5 minutes. The stock solutions were allowed to stand for 1 hour, diluted to the appropriate concentrations then transferred to a plastic cuvette for measurements. Solutions were not filtered. The time-dependent correlation function of the scattered light intensity was measured at a scattering angle of 173 relative to the laser source. The hydrodynamic radius (R) of the particles was estimated from their diffusion coefficient (D) using the Stokes-Einstein equation, D = kBT / 6πη/R, where kB is the Boltzmann's constant, T is the absolute temperature, and η is the viscosity of the solvent.
Electron microscopy
A 2 mM solution of 4a was prepared by dissolution of the compound in double distilled and filtered (0.22µm) water then sonicated for 15 minutes. The solution was allowed to stand for 30 minutes and 2h. Solutions of 4a were applied on 200 mesh copper grids coated with resin and a thin carbon layer. A drop of the colloid solution was placed on the grid and allowed to stand for 2 min, and then blotted away. Another drop of 10% w/w phosphotungstic acid solution was also placed on the grid and allowed to stand again for 2 min, and then blotted away. The specimens were examined using digital transmission electron microscope. Electron micrographs were taken at a magnification of 60,000–100,000.
EPR measurements
EPR measurements were carried out on an EPR spectrometer equipped with high sensitivity resonator at room temperature. Unless otherwise indicated, the instrument settings used for general spectral acquisition are: microwave power, 10 mW; modulation amplitude, 1 G; receiver gain, 2.0 × 104; scan time 5.1 s; time constant, 2.6 s; sweep width, 100 G, incremental sweep at 100–300 scans. Time scan spectra were integrated using the WINEPR v.2.11b software. All the spin trapping studies were carried out in DMSO or a phosphate buffer (PBS) (10 mM) at pH 7.0 containing 100 µM diethylene triamine pentaacetic acid (DTPA). Sample cells used were 50 µL glass capillary tubes. The spectrum simulation was carried out by an automatic fitting program.56
Spin trapping. (a) Superoxide radical anion
(i) Xanthine-xanthine oxidase (X-XO). A 50 µL PBS solution contains 100 µM DTPA, 0.4 mM xanthine, 0.5 unit/mL xanthine oxidase and 20 mM 1. (ii) KO2 generating system. To a 20 µL DMSO was added 20 µL of 50 mM stock solution of 4a or 4b in DMSO and 10 µL saturated solution of KO2 in DMSO; (b) Hydroxyl radical. A 50 µL PBS solution contains 0.3% H2O2, 20 mM 4a or 4b, and 0.01% FeCl2 solution.
Stopped-flow kinetics
Stop-flow technique was used to obtain the apparent spin-trapping rate constants of spin traps with superoxide radicals as described previously.51 KO2 was used as a O2•− source and phenol red as a competitor. The growth of the transient absorption at 575 nm from the reaction between O2•− and phenol red was monitored using UV-vis spectroscopy. The plot was linear during the first 7–9 seconds. The data was fitted to a linear equation (t = vx + c), where v is initial rate of product formation in the presence of various concentrations of spin traps. The resulting initial rates were applied to the following equation V/v−1 = (kst[ST])/(kpr[PR]) where V is the initial rate of formation in the absence of spin traps; kST and kPRare rate constants of O2•− reaction with spin trap and phenol red, respectively, while [ST] and [PR] are the concentrations of spin trap and phenol red (500 uM), respectively.
Decay kinetics of various O2•− spin adducts in DMSO
In a typical experiment, 5 µL aqueous solution of a spin trap (20 mM) was added to a 45 µL of KO2 solution in DMSO which was prepared by mixing 5 µL saturated solution of KO2 and 40 µL of DMSO. In the case of 4a, 5 µL of 20 mM 4a in DMSO solution was used and was added to 45 µL of KO2 solution (vide supra). To all of the resulting solutions, 5 µL of water was added to quench the remaining KO2. EPR spectra were recorded and the decay of the superoxide spin adduct was then followed by monitoring the decrease of the lowest field peak of the O2•− adduct. The half-lives were calculated from the first order rate constants obtained between the 5th and 6th min of decay.
Computational studies
All calculations were performed at the Ohio Supercomputer Center. The minimization of initial structures using MMFF9457 coupled with Generalized Born/Surface Area (GB/SA) continuum solvation model using water as the solvent58 was performed with MacroModel 9.659. The MMFF94 minimized structures were further optimized using Hartree-Fock (HF) self- consistent field method60 at the HF/3–21G* level of theory using Gaussian 03.61 The Cartesian coordinates were generated using the GaussView 3.0 Program.
Supplementary Material
Acknowledgement
This publication was made possible by grant number HL 81248 from the NIH National Heart, Lung, and Blood Institute. This work was supported in part by an allocation of computing time from the Ohio Supercomputer Center.
Footnotes
Supporting Information Available: 1H-, 13C-, HSQC, TOCSY, HSQC-TOCSY, HMBC and ROESY NMR, infrared, high resolution mass, MS/MS, circular dichroism and miscellaneous EPR spectra for the compounds as well as Cartesian coordinates for optimized geometries and complete references for. 3, 23, 31, and 59 are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Villamena FA, Zweier JL. Antioxid. Redox Signaling. 2004;6:619–629. doi: 10.1089/152308604773934387. [DOI] [PubMed] [Google Scholar]
- 2.Bosnjakovic A, Kadirov MK, Schlick S. Res. Chem. Intermed. 2007;33:677–687. [Google Scholar]; Danilczuk M, Bosnjakovic A, Kadirov MK, Schlick S. J. Power Sources. 2007;172:78–82. [Google Scholar]; Bosnjakovic A, Schlick S. J. Phys. Chem. B. 2006;110:10720–10728. doi: 10.1021/jp061042y. [DOI] [PubMed] [Google Scholar]
- 3.Ionita P, Conte M, Gilbert BC, Chechik V. Org. Biomol. Chem. 2007;5:3504–3509. doi: 10.1039/b711573c. [DOI] [PubMed] [Google Scholar]; Babu S, Velez A, Wozniak K, Szydlowska J, Seal S. Chem. Phys. Lett. 2007;442:405–408. [Google Scholar]; Kagan VE, et al. Toxicol. Lett. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Fu H, Zhang L, Zhang S, Zhu Y, Zhao J. J. Phys. Chem. B. 2006;110:3061–3065. doi: 10.1021/jp055279q. [DOI] [PubMed] [Google Scholar]
- 5.Xiao G, Wang X, Li D, Fu X. J. Photochem. Photobiol., A. 2008;193:213–221. [Google Scholar]; Chang Q, He H, Zhao J, Yang M; Qu J. Environ. Sci. Tech. 2008;42:1699–1704. doi: 10.1021/es071810e. [DOI] [PubMed] [Google Scholar]; Yu JC, Ho W, Yu J, Yip H, Wong PK; Zhao J. Environ. Sci. Tech. 2005;39:1175–1179. doi: 10.1021/es035374h. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Z, Zhou J, Zhang Y, Qiao R, Xia S, Chen J, Wang X, Zhang B. J. Phys. Chem. B. 2007;111:2688–2696. doi: 10.1021/jp067020t. [DOI] [PubMed] [Google Scholar]; Rajendran M, Inbaraj JJ, Gandhidasan R, Murugesan RJ. Photochem.Photobiol., A. 2006;182:67–74. [Google Scholar]; Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, Sarna T, Hamblin MR. Free Radical Biol. Med. 2007;43:711–719. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas CE. In: Neuroprotection in CNS Diseases. Baer PR, Beal MF, editors. New York: Informa Health Care; 1997. pp. 183–204. [Google Scholar]
- 8.Floyd RA. Aging Cell. 2006;5:51–57. doi: 10.1111/j.1474-9726.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 9.Floyd RA, Kopke RD, Choi C-H, Foster SB, Doblas S, Towner RA. Free Radical Biol. Med. 2008;45:1361–1374. doi: 10.1016/j.freeradbiomed.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradamante S, Jotti A, Paracchini L, Monti E, Morti E. Eur.J. Pharmacol. 1993;234:113–116. doi: 10.1016/0014-2999(93)90713-r. [DOI] [PubMed] [Google Scholar]; Maurelli E, Culcasi M, Delmas-Beauvieux MC, Gallis JL, Miollan M, Tron T, Pietri S. Free Radic. Biol. Med. 1999;27:34–41. doi: 10.1016/s0891-5849(99)00033-7. [DOI] [PubMed] [Google Scholar]; Pietri S, Liebgott T, Frejaville C, Tordo P, Culcasi M. Tosaki A, Braquet P. Eur. J. Biochem. Am. Heart J. 1998;1990;254120:256–265. 819–830. doi: 10.1046/j.1432-1327.1998.2540256.x. [DOI] [PubMed] [Google Scholar]
- 11.Floyd RA, Hensley K. Ann. New York Acad. Sci. 2000;899:222–237. doi: 10.1111/j.1749-6632.2000.tb06189.x. [DOI] [PubMed] [Google Scholar]; Floyd RA, Liu RJ, Wong PK. In: Handbook of Synthetic Antioxidants. Packer L, Cadenas E, editors. New York: Marcel Dekker, Inc; 1997. pp. 339–350. [Google Scholar]
- 12.Khan N, Wilmot CM, Rosen GM, Demidenko E, Sun J, Joseph J, O'Hara J, Kalyanaraman B, Swartz HM. Free Radical Biol. Med. 2003;34:1473–1481. doi: 10.1016/s0891-5849(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 13.Villamena FA, Xia S, Merle JK, Lauricella R, Tuccio B, Hadad CM, Zweier JL. J. Am. Chem. Soc. 2007;129:8177–8191. doi: 10.1021/ja0702622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villamena FA, Rockenbauer A, Gallucci J, Velayutham M, Hadad CM, Zweier JL. J. Org. Chem. 2004;69:7994–8004. doi: 10.1021/jo049244i. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein E, Rosen GM, Rauckman EJ. J. Am. Chem. Soc. 1980;102:4994–4999. [Google Scholar]
- 16.Olive G, Mercier A, Le Moigne F, Rockenbauer A, Tordo P. Free Radical Biol. Med. 2000;28:403–408. doi: 10.1016/s0891-5849(99)00254-3. [DOI] [PubMed] [Google Scholar]
- 17.Frejaville C, Karoui H, Tuccio B, Le Moigne F, Culcasi M, Pietri S, Lauricella R, Tordo P. J. Chem. Soc., Chem. Commun. 1994:1793–1794. doi: 10.1021/jm00002a007. [DOI] [PubMed] [Google Scholar]
- 18.Villamena FA, Hadad CM, Zweier JL. J. Am. Chem. Soc. 2004;126:1816–1829. doi: 10.1021/ja038838k. [DOI] [PubMed] [Google Scholar]; Villamena FA, Merle JK, Hadad CM, Zweier JL. J. Phys. Chem. A. 2005;109:6089–6098. doi: 10.1021/jp0524330. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Tuccio B, Lauricella R, Rockenbauer A, Zweier JL, Villamena FA. Org. Chem. 2008;73:2533–2541. doi: 10.1021/jo702434u. [DOI] [PubMed] [Google Scholar]
- 20.Birrell BG, Griffith HO, French DJ. J. Am. Chem. Soc. 1973;95:8171–8172. [Google Scholar]
- 21.Michon J, Rassat AJ. Am. Chem. Soc. 1979;101:995–996. [Google Scholar]; Martinie J, Michon J, Rassat A. J. Am. Chem. Soc. 1975;97:1818–1823. [Google Scholar]
- 22.Okazaki M, Kuwata KJ. Phys. Chem. 1985;89:4437–4440. [Google Scholar]
- 23.Bardelang D, et al. Chem. Eur. J. 2007;13:9344–9354. doi: 10.1002/chem.200700369. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Tuccio B, Lauricella R, Villamena FA. J. Org. Chem. 2008;73:7108–7117. doi: 10.1021/jo8007176. [DOI] [PubMed] [Google Scholar]
- 25.Ravoo BJ. Cyclodextrin vesicles for drug delivery. Cyclodextrin: From Basic Research to Market, 10th International Cyclodextrin Symposium; Ann Arbor, MI. 2000. pp. 168–172. [Google Scholar]
- 26.Choi HS, Yamashita A, Ooya T, Yui N, Akita H, Kogure K, Ito R, Harashima H. ChemBioChem. 2005;6:1986–1990. doi: 10.1002/cbic.200500242. [DOI] [PubMed] [Google Scholar]; Kulkarni RP, Wu DD, Davis ME, Fraser SE. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7523–7528. doi: 10.1073/pnas.0501950102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kulkarni RP, Mishra S, Fraser SE, Davis ME. Bioconjugate Chem. 2005;16:986–994. doi: 10.1021/bc050081u. [DOI] [PubMed] [Google Scholar]
- 27.Duchêne D, Ponchel G, Wouessidjewe D. Adv. Drug Delivery Rev. 1999;36:29–40. doi: 10.1016/s0169-409x(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 28.Bellanger N, Perly B. J. Mol. Struct. 1992;273:215–226. [Google Scholar]
- 29.Petter RC, Salek JS, Sikorski CT, Kumaravel G, Lin FT. J. Am. Chem. Soc. 1990;112:3860–3868. [Google Scholar]
- 30.Angelova A, Fajolles C, Hocquelet C, Djedaï-Pilard F, Lesieur S, Bonnet V, Perly B, Lebas G, Mauclaire LJ. Colloid Interface Sci. 2008;322:304–314. doi: 10.1016/j.jcis.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Durand G, Poeggeler B, Boeker J, Raynal S, Polidori A, Pappolla MA, Hardeland R, Pucci B. J. Med. Chem. 2007;50:3976–3979. doi: 10.1021/jm0706968. [DOI] [PubMed] [Google Scholar]; Durand G, Polidori A, Ouari O, Tordo P, Geromel V, Rustin P, Pucci B. J. Med. Chem. 2003;46:5230–5237. doi: 10.1021/jm030873e. [DOI] [PubMed] [Google Scholar]; Durand G, Polidori A, Salles J-P, Pucci B. Bioorg. Med. Chem. Lett. 2003;13:859–862. doi: 10.1016/s0960-894x(02)01079-x. [DOI] [PubMed] [Google Scholar]
- 32.Tanguy S, Durand G, Reboul C, Polidori A, Pucci B, Dauzat M, Obert P. Cardiovasc. Drugs Ther. 2006;20:147–149. doi: 10.1007/s10557-006-6754-8. [DOI] [PubMed] [Google Scholar]
- 33.Asanuma T, et al. Chem. Biodivers. 2007;4:2253–2267. doi: 10.1002/cbdv.200790184. [DOI] [PubMed] [Google Scholar]; Poeggeler B, Durand G, Polidori A, Pappolla MA, Vega-Naredo I, Coto-Montes A, Baker J, Hardeland R, Pucci BJ. Neurochem. 2005;95:962–973. doi: 10.1111/j.1471-4159.2005.03425.x. [DOI] [PubMed] [Google Scholar]
- 34.Shao Z, Li Y, Chermak T, Mitra AK. Pharm. Res. 1994;11:1174–1179. doi: 10.1023/a:1018997101542. [DOI] [PubMed] [Google Scholar]
- 35.Liu YP, Ji YQ, Song YG, Liu KJ, Liu B, Tian Q, Liu Y. Chem. Commun. 2005;39:4943–4945. doi: 10.1039/b509903j. [DOI] [PubMed] [Google Scholar]
- 36.Hardy M, Chalier F, Ouari O, Finet J-P, Rockenbauer A, Kalyanaraman B, Tordo P. Chem. Commun. 2007;10:1083–1085. doi: 10.1039/b616076j. [DOI] [PubMed] [Google Scholar]
- 37.Hardy M, Ouari O, Charles L, Finet J-P, Lacazio G, Monnier V, Rockenbauer A, Tordo P. J. Org. Chem. 2005;70:10426–10433. doi: 10.1021/jo0517390. [DOI] [PubMed] [Google Scholar]
- 38.Karoui H, Clement J-L, Rockenbauer A, Siri D, Tordo P. Tetrahedron Lett. 2004;45:149–152. [Google Scholar]
- 39.Chalier F, Hardy M, Ouari O, Rockenbauer A, Tordo PJ. Org. Chem. 2007;72:7886–7892. doi: 10.1021/jo071070s. [DOI] [PubMed] [Google Scholar]
- 40.Diez-Barra E, de la Hoz A, Moreno A. Syn. Commun. 1994;24:1817–1821. [Google Scholar]; Schipchandler MT. Synthesis. 1979;9:666–686. [Google Scholar]
- 41.Tsai P, Elas M, Parasca AD, Barth ED, Mailer C, Halpern HJ, Rosen GM. J. Chem. Soc., Perkin Trans. 2001;2:875–880. [Google Scholar]
- 42.Hamilton JA, Chen L. J. Am. Chem. Soc. 1988;110:5833–5841. [Google Scholar]; Tanaka M, Shono T, Zhu D, Kawaguchi Y. J. Chromatogr. 1989;469:429–433. [Google Scholar]
- 43.Park JW, Song HE, Lee SY. J. Org. Chem. 2003;68:7071–7076. doi: 10.1021/jo034623h. [DOI] [PubMed] [Google Scholar]
- 44.Coleman AW, Nicolis I, Keller N, Dalbiez JP. J. Inclusion Phenom. Macrocyclic Chem. 1992;13:139–143. [Google Scholar]
- 45.Gaitano GG, Brown W, Tardajos GJ. Phys. Chem. B. 2003;101:710–719. [Google Scholar]; González-Gaitano G; Rodrí, guez P, Isasi JR, Fuentes M, Tardajos G, Sánchez MJ. Inclusion Phenom. Macrocyclic Chem. 2003;44:101–105. [Google Scholar]
- 46.Bonini M, Rossi S, Karlsson G, Almgren M, Lo Nostro P, Baglioni P. Langmuir. 2006;22:1478–1484. doi: 10.1021/la052878f. [DOI] [PubMed] [Google Scholar]; Rossi S, Bonini M, Lo Nostro P, Baglioni P. Langmuir. 2007;23:10959–10967. doi: 10.1021/la7011638. [DOI] [PubMed] [Google Scholar]
- 47.Auzely-Velty R, Djedaini-Pilard F, Desert S, Perly B, Zemb T. Langmuir. 2000;16:3727–3734. [Google Scholar]
- 48.Mazzaglia A, Ravoo BJ, Darcy R, Gambadauro P, Mallamace F. Langmuir. 2002;18:1945–1948. [Google Scholar]
- 49.Ravoo BJ, Darcy R. Angew. Chem. Int. Ed. 2000;39:4324–4326. doi: 10.1002/1521-3773(20001201)39:23<4324::AID-ANIE4324>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]; Ravoo BJ, Jacquier J-C, Wenz G. Angew. Chem. Int. Ed. 2003;42:2066–2070. doi: 10.1002/anie.200350923. [DOI] [PubMed] [Google Scholar]
- 50.Abla M, Durand G, Pucci BJ. Org. Chem. 2008;73:8142–8153. doi: 10.1021/jo801379e. [DOI] [PubMed] [Google Scholar]
- 51.Durand G, Choteau F, Pucci B, Villamena FA. J. Phys. Chem. A. 2008;112:12498–12509. doi: 10.1021/jp804929d. [DOI] [PubMed] [Google Scholar]
- 52.Field SM, Villamena FA. Chem. Res. Toxicol. 2008;21:1923–1932. doi: 10.1021/tx8001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roubaud V, Lauricella R, Tuccio B, Bouteiller J-C, Tordo P. Res. Chem. Intermed. 1996;22:405–416. [Google Scholar]
- 54.Tuccio B, Lauricella R, Frejaville C, Bouteiller J-C, Tordo PJ. Chem. Soc. Perkin Trans. 1995;2:295–298. [Google Scholar]
- 55.Villamena FA, Merle JK, Hadad CM, Zweier JL. J. Phys. Chem. A. 2005;109:6083–6088. doi: 10.1021/jp052431f. [DOI] [PubMed] [Google Scholar]
- 56.Rockenbauer A, Korecz L. Appl. Magn. Reson. 1996;10:29–43. [Google Scholar]
- 57.Halgren TA. J. Comput. Chem. 1996;17:490–519. [Google Scholar]
- 58.Still WC, Tempczyk A, Hawley RC, Hendrickson TJ. J. Am. Chem. Soc. 1990;112:6127–6129. [Google Scholar]
- 59.Schrödinger . New York, NY: LLC; 2005. [Google Scholar]
- 60.Pople JA, Nesbet RK. J. Chem. Phys. 1954;22:571–572. [Google Scholar]
- 61.Frisch MJ, et al. Revision B.04 ed. Pittsburgh PA: Gaussian, Inc; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.