Abstract
Background
There are few data on rate of progression in essential tremor (ET).
Objectives
To quantify rate of tremor progression in a cross-sectional sample of 348 ET cases in an epidemiological study; characterize the relationship between age of tremor onset and rate of tremor progression in that sample; and characterize the relationship between age of tremor onset, rate of tremor progression, and severity of underlying brain changes in 9 cases from a brain repository.
Methods
Rate of tremor progression was defined as tremor severity ÷ duration. The degeneration index = number of torpedoes per section ÷ Purkinje cell linear density.
Results
In the epidemiological study, older age of tremor onset was associated with faster rate of tremor progression (p<0.001). In the brain repository, older age of tremor onset was associated with higher degeneration index (p=0.037), and higher degeneration index was associated with faster rate of tremor progression (p=0.018).
Conclusions
In a large clinical sample, older age of onset was associated with more rapid tremor progression. In a brain bank, older age of onset was associated with more degenerative pathology in the cerebellum. As in several neurodegenerative disorders, in older onset cases, it is possible that the disease advances more rapidly.
Keywords: essential tremor, pathology, degeneration, cerebellum, Purkinje cell, torpedo, rate of progression
Introduction
Essential tremor (ET) is a heterogeneous condition, with patients demonstrating differences in tremor location,1, 2 responsiveness to pharmacotherapies,3, 4 putative underlying etiologies (genetic vs. non-genetic), 5, 6 and types of underlying neuropathological changes.7–11 Tremor may worsen in an accelerated manner during later life;12, 13 however, there are few data on rate of progression of ET.1, 14 Data on clinical progression can inform investigators about the evolution and perhaps the nature of underlying pathological changes. Furthermore, an understanding of disease progression is the basis for prognostic discussions with patients. We aimed to: (1) quantify rate of tremor progression in a large cross-sectional sample of ET cases enrolled in an epidemiological study, (2) characterize the relationship between age of tremor onset and rate of tremor progression in that sample, (3) characterize the relationship between age of tremor onset, rate of tremor progression, and severity of underlying brain changes in a different sample of ET cases ascertained through a brain repository.
Methods
Epidemiological Study
348 ET cases were enrolled in a cross-sectional epidemiological study of ET at Columbia-University Medical Center (CUMC). Cases were enrolled from two main sources in the New York area: (1) a computerized billing database at the Neurological Institute of New York, CUMC (N = 200), (2) advertisements to members of the International Essential Tremor Foundation (IETF)(N = 110) who lived in the New York area. The small number (N = 38) of remaining cases came from several sources (e.g., Weill Medical College of Cornell University). Prior to enrollment, each case received a diagnosis of ET from their treating neurologist. The Columbia and Cornell Institutional Review Boards approved of study procedures and written informed consent was obtained upon enrollment.
After enrollment, cases were evaluated in person by a trained tester who administered structured clinical questionnaires (demographic, clinical and family history information). Cases were classified as having a family history of tremor if they reported ≥1 first-degree relative with ET or tremor. Age of tremor onset was by self-report; we have previously demonstrated that this is reported reliably by patients.15
The tester videotaped a tremor examination,16, 17 including postural arm tremor and five tests of kinetic tremor in each arm (12 tests total). Videotapes were reviewed by a neurologist specializing in movement disorders (E.D.L.) who was blinded to clinical data, and tremor was rated (0 – 3) during each test. Thereby, each participant was assigned a tremor score for each arm (range = 0 – 18) and a total tremor score (range = 0 – 36). Inter- and intra-rater reliability of this videotape-based rating have been demonstrated.18 and the scores correlate with other objective measures of tremor severity, demonstrating the validity of these ratings.19 The diagnosis of ET was confirmed by E.D.L. using published diagnostic criteria (moderate or greater amplitude tremor during three activities or a head tremor in the absence of PD, dystonia or another neurological disorder).16, 17, 20 Of 411 enrollees, the ET diagnosis was confirmed in 348 who were included in these analyses.
Brain Repository
The postmortem study was conducted at the Essential Tremor Centralized Brain Repository (ETCBR), Columbia University. 21 Purkinje cells may be estimated by simple counts in selected regions 11 or, more rigorously, by taking into account the length of the Purkinje cell layer (i.e., Purkinje cell linear density). 22 For these analyses, Purkinje cell linear density was systematically assessed in each case. Cases were selected so that none had brainstem or cortical Lewy bodies on routine immunohistochemical staining for alpha-synuclein. This is because previous analyses demonstrated that ET cases with Lewy bodies had normal-appearing cerebella whereas those without Lewy bodies had abnormal cerebella.9, 11, 22 Postmortem tissue for these analyses was available on nine ET cases, reported previously. 22 None had had surgery for tremor.
The ET cases included eight prospectively-collected cases and one archival case. Their ascertainment has been described previously;11 none had participated in the epidemiological study. ET diagnoses were assigned during life by their treating neurologist and confirmed after death using ETCBR diagnostic criteria, as documented previously. 8, 11, 23 ETCBR neurologists obtained clinical records to acquire additional demographic and clinical data on all nine ET cases. No cases were heavy ethanol users (as defined previously) 24 or were exposed to medications known to cause cerebellar damage (e.g., lithium, diphenylhydantoin). The most consistently-available measure of tremor severity was a hand-drawn spiral, available on 8 of 9 cases, and rated by a blinded ETCBR neurologist (range = 0 – 4 in each arm, with 4 indicating inability to complete the spiral due to severe tremor), resulting in a total spiral score (range = 0 – 8).
All brains were well characterized by the New York Brain Bank, including complete neuropathological assessment and determination of any pathological findings. 8, 11, 21, 23, 25 Brains received ratings of Braak stage 26, 27 and CERAD 28 for Alzheimer’s tangle and plaque pathologies. Postmortem interval (PMI) was the number of hours between death and placement of the brain in a cold room or upon ice. For the current analyses, the cerebellum was our primary region of interest because many studies have implicated its involvement in ET.8, 11, 23 A 3 × 20 × 25 mm parasagittal tissue block from the same region of the neocerebellum, including the cerebellar cortex, white matter and dentate nucleus, was harvested from each brain and then immersion-fixed in 10% buffered formalin. Paraffin sections (7 μm) were stained with Luxol Fast Blue and Hematoxylin and Eosin (LH&E) for quantification of torpedoes (Purkinje cell axonal swellings).8, 11, 23 The number of torpedoes in the entire LH&E section was counted; we have previously demonstrated a 6 – 7-fold increase in number of torpedoes in ET cases vs. controls.11
For immunohistochemistry, paraffin sections, 7 μm thick and 20 × 25 mm were placed on subbed glass slides. 22 Calbindin immunoreactivity was evaluated using a primary mouse monoclonal antibody to calbindin D-28K (clone CG-955, Sigma, St. Louis, MO) as documented previously. 22 These were analyzed to quantify Purkinje cell linear density (i.e., Purkinje cells per millimeter of Purkinje cell layer) by a single trained technician who was blinded to clinical information, as described.22
Statistical Analyses
Analyses were cross-sectional and were performed in SPSS, Version 15.0. In the epidemiological study, rate of tremor progression was the total tremor score (range = 0 – 36) divided by tremor duration in years. The tremor asymmetry index was the absolute value of the difference between the tremor scores in the right and left arms. Because rate of tremor progression, age of tremor onset, tremor duration, and tremor asymmetry index were not normally distributed, nonparametric tests were used and, in linear regression analyses, rate of tremor progression (dependent variable) was log transformed (log10). We began with an adjusted model and then added covariates when they were associated with the dependent or independent variable in univariate analyses or when there was strong prior biological support for an association. In some analyses, age of tremor onset was stratified by decade and, in a linear regression analysis, a test for trend was performed (log rate of tremor progression = dependent variable and age of tremor onset stratum = independent variable).
In the Brain Repository, because of the small sample size, Spearman’s correlation coefficients were used. The degeneration index, designed for these analyses, reflected the two main types of pathological changes observed in ET to date (i.e., increased number of torpedoes and Purkinje cell loss).11 It was the number of torpedoes per section divided by the Purkinje cell linear density; high values indicated large numbers of torpedoes and low Purkinje cell linear density (i.e., Purkinje cell loss). The rate of tremor progression was defined as total spiral score divided by tremor duration in years. In a linear regression analysis, rate of tremor progression, which was the dependent variable, was log transformed (log10). In linear regression analyses, we began with an unadjusted model. One by one, we then considered a number of covariates (age, gender, ethnicity, currently taking daily medication for ET, family history of tremor, CERAD plaque score, Braak stage for Alzheimer’s disease, and PMI); if the covariate changed the beta coefficient of the independent variable of interest, it was included in the final adjusted linear regression analysis.
Results
Epidemiological Study
There were 348 ET cases (Table 1). Rate of tremor progression was not associated with current age in years, gender, ethnicity or currently taking daily medication for ET. It was, however, slower in ET cases with vs. without a family history of tremor (rate of tremor progression = 1.37 ± 1.88 vs. 2.95 ± 3.42, Mann Whitney test, z = 6.16, p <0.001). Rate of tremor progression did not differ significantly between ET cases with vs. without head tremor (1.92 ± 2.99 vs. 2.00 ± 2.47, Mann Whitney test, z = 1.17, p = 0.24) nor did it correlate with the tremor asymmetry index (r = 0.009, p = 0.87).
Table 1.
Characteristics of ET cases
| Characteristic | 348 ET cases in epidemiological study | 9 ET cases in brain repository |
|---|---|---|
| Age in years | 67.5 ± 15.1 (18 – 95) | 87.0 ± 9.2 (90, 68 – 98) |
|
| ||
| Women | 170 (50.9%) | 5 (55.6%) |
|
| ||
| Ethnicity | ||
| Non-Hispanic White | 327 (94.0%) | 8 (88.9%) |
| Non-Hispanic African-American | 6 (1.7%) | 1 (11.1%) |
| Hispanic | 10 (2.9%) | 0 (0%) |
| Other | 5 (1.4%) | 0 (0%) |
|
| ||
| Daily medication for ET | 168 (48.3%) | 8 (88.9%) |
|
| ||
| Family history of tremor | 213 (61.2%) | 5 (55.6%) |
|
| ||
| Age of tremor onset in years | 45.0 ± 22.5 (3 – 90) | 55.4 ± 25.4 (60, 14 – 88) |
|
| ||
| Tremor duration in years | 22.6 ± 18.7 (1 – 81) | 31.6 ± 30.3 (30, 9 – 60) |
|
| ||
| Total tremor score (range = 0 – 36) | 18.8 ± 7.2 (1 – 36) | Not applicable |
|
| ||
| Total spiral score (range = 0 – 8) | Not applicable | 4.6 ± 1.2 (4.5, 2.5 – 6) |
|
| ||
| Rate of tremor progression | 1.97 ± 2.68 (0.03 – 20.00)1 | 0.21 ± 0.20 (0.13, 0.08 – 0.67)3 |
|
| ||
| Tremor asymmetry index | 3.0 ± 2.7 (0 – 14) 2 | Not applicable |
|
| ||
| PMI in hours | Not applicable | 5.5 ± 3.1(4.3, 2.9 – 11.5) |
|
| ||
| Braak stage for Alzheimer’s disease | Not applicable | 1.9 ± 1.4 (2.0, 0 – 4) |
|
| ||
| CERAD plaque score | Not applicable | 0.2 ± 0.4 (0, 0 – 1) |
|
| ||
| Torpedoes per section4 | Not applicable | 9.6 ± 6.9 (9.0, 0 –22) |
|
| ||
| Purkinje cell linear density(cells/mm) | Not applicable | 0.85 ± 0.30 (0.9, 0.44 –1.46) |
|
| ||
| Degeneration index5 | Not applicable | 14.4 ± 14.3 (9.47, 0.0 – 44.0) |
Mean ± standard deviation (median, minimum - maximum)
In the epidemiological study, rate of tremor progression was defined as the total tremor score (range = 0 – 36) divided by tremor duration in years.
The tremor asymmetry index was defined as the absolute value of the difference between the tremor score on the right (range = 0 – 18) and the tremor score on the left (range = 0 – 18).
In the brain repository, rate of tremor progression was defined as total spiral score (range = 0 – 8) divided by tremor duration in years. Complete spiral data were present in eight of nine ET cases.
Using a standard 20 × 25 mm LH&E-stained cerebellar section that included portions of the cerebellar cortex, white matter, and dentate nucleus, torpedoes in the entire section were counted.
The degeneration index was defined as the number of torpedoes per section divided by the Purkinje cell linear density; high values indicated large numbers of torpedoes and low Purkinje cell linear density (i.e., Purkinje cell loss).
Older age of tremor onset was associated with more rapid rate of tremor progression (r = 0.74, p <0.001)(Figure 1). We also considered potential medication effects. ET cases on anti-tremor medications may have lower tremor scores because of the effectiveness of these medications or, alternatively, they may have elected to take medications in the first place because of higher tremor scores and these medications might be relatively ineffective. We therefore conducted an analyses in which we excluded all 168 cases who were taking tremor medications, and the results were similar (r = 0.71, p <0.001). In a linear regression analysis that adjusted for current age in years, gender, ethnicity, currently taking daily medication for ET, and family history of tremor, older age of tremor onset was associated with more rapid rate of tremor progression (betaage of onset = 0.018, p <0.001). Duration of tremor is another obvious potential confounder; yet in a linear regression analysis that adjusted for tremor duration, older age of onset was independently associated with faster rate of tremor progression (betaage of onset = 0.005, p <0.001).
Figure 1.
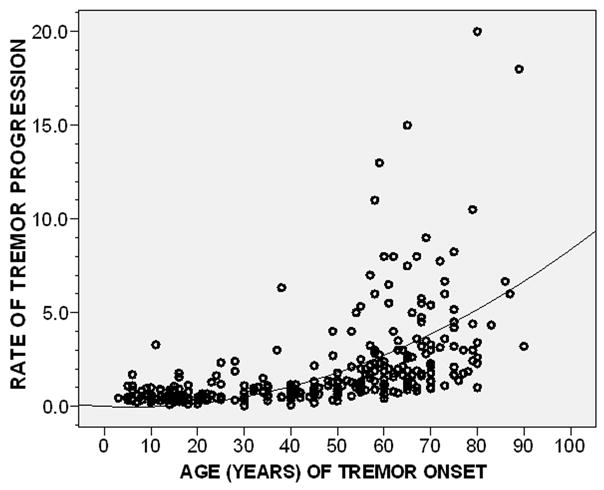
Rate of tremor progression by age of tremor onset (r = 0.74, p < 0.001). Fit line is shown; a logarithmic relationship is seen.
We explored the possibility of ceiling effects by stratifying the sample based on mean total tremor score (≤18.8 vs. >18.8); we found a similar correlation between age of onset and rate of progression in each stratum (r = 0.70, p < 0.001 and r = 0.77, p < 0.001).
Age of tremor onset was stratified by decade; rate of tremor progression was associated with age of onset stratum (p <0.001), with very rapid rates of progression in older age groups (<20 years = 0.60 total tremor score points/year, 21–30 years = 0.79, 31–40 years = 0.97, 41–50 years = 0.98, 51–60 years = 2.41, 61–70 years = 3.20, ≥71 years = 5.28). The association between older age of tremor onset and more rapid rate of tremor progression was found in ET cases from each of the two case sources: CUMC r = 0.71 (p <0.001) and IETF r = 0.72 (p <0.001).
It is conceivable that we systematically excluded cases with tremor onset in their 70s or 80s, which then progressed slowly (i.e., they might not yet have come to medical attention). We performed several analyses to explore this possibility. First, we restricted our analyses to subjects, regardless of their age of onset, with short tremor duration. Thus, in the 122 ET cases with tremor duration ≤ 10 years, older age of tremor onset was associated with more rapid rate of tremor progression (r = 0.29, p = 0.001). Second, if we even further restricted our analyses to the 49 ET cases with tremor duration < 5 years, the correlation remained (r = 0.28, p = 0.05). Third, when we restricted analyses to ET cases who were (1) < 70 years of age or (2) both ≥70 years of age and had disease duration ≥ 10 years (i.e., we systematically excluded older onset ET cases with shorter disease duration), rate of tremor progression was correlated with age of tremor onset (r = 0.68, p <0.001).
Recognizing that the association between age of tremor onset and rate of tremor progression might not be linear, we also squared, cubed, and log transformed the rate of tremor progression variable. In each instance, p<0.001. Visual inspection of each scatter plot indicated that the relationship conformed best with a logarithmic curve (Figure 1).
Brain Repository
There were nine ET cases (Table 1). Older age of tremor onset was associated with a higher degeneration index (r = 0.67, p = 0.05)(Figure 2). In a linear regression analysis adjusted for age, older age of tremor onset was associated with a higher degeneration index (beta = 0.57, p = 0.037).
Figure 2.
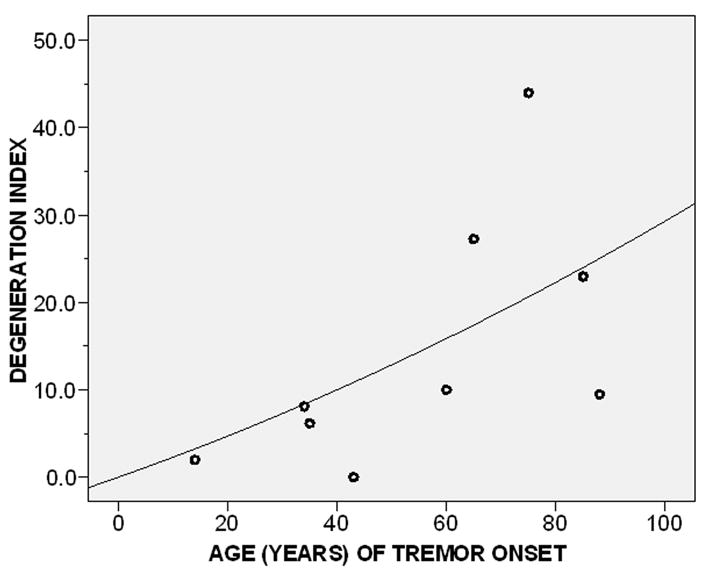
Degeneration index by age of tremor onset (r = 0.67, p = 0.05). The degeneration index was defined as the number of torpedoes per section divided by the Purkinje cell linear density.
Higher degeneration index was associated with a faster rate of tremor progression (r = 0.76, p = 0.03)(Figure 3). In a linear regression analysis, adjusting for PMI, higher degeneration index was associated with faster log rate of tremor progression (beta = 0.039, p = 0.018).
Figure 3.
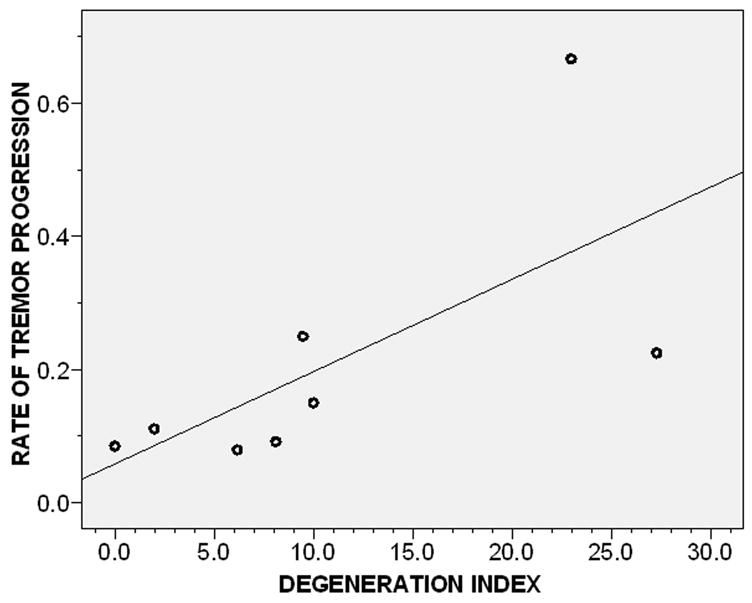
Rate of tremor progression by degeneration index (Pearson’s r = 0.76, p = 0.03). The degeneration index was defined as the number of torpedoes per section divided by the Purkinje cell linear density. Complete spiral data were present in eight of nine ET cases. Fit line is shown.
Older age of onset was associated with a faster rate of tremor progression (r = 0.76, p = 0.03)(Figure 4) and, in a linear regression analysis, with faster log rate of tremor progression (beta = 0.10, p = 0.01).
Figure 4.
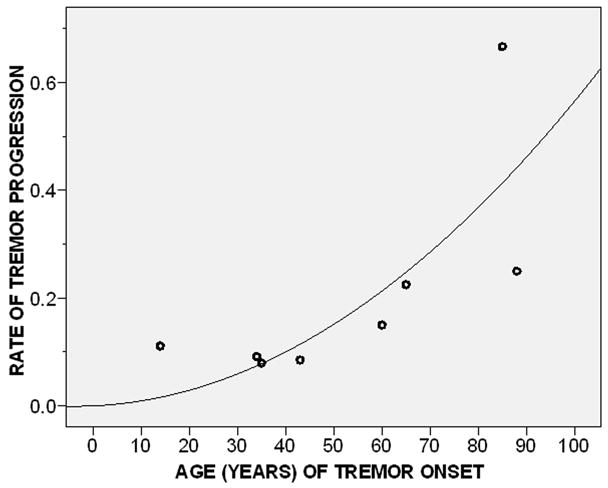
Age of tremor onset by rate of tremor progression (Pearson’s r = 0.76, p = 0.03). Complete spiral data were present in eight of nine ET cases.
Fit line is shown.
Six of nine ET cases were taking propranolol or mysoline at the time of the spiral drawing. Neither the dose of propranolol nor mysoline was correlated with age of tremor onset, degeneration index, or rate of tremor progression, indicating that medications were not a confounding factor.
Discussion
In a large, cross-sectional sample of ET cases, we demonstrated that older age of tremor onset was associated with more rapid tremor progression. Very rapid rates of progression were seen in the older age groups. This finding was confirmed in a sample of ET cases ascertained through a brain bank. Furthermore, older age of onset was associated with more degenerative pathology.
One limitation is that rate of tremor progression was estimated based on cross-sectional data. Nevertheless, a similar cross-sectional approach has been used in assessing rate of motor progression and functional decline in other degenerative disorders (Parkinson’s disease [PD] and Alzheimer’s disease).29, 30 These cross-sectional data are the best available data at present; we recognize that our observations should be followed by a second, confirmatory study with a prospective, longitudinal design.
Rates of clinical progression have received surprisingly little attention in ET. In one prior study,1 we demonstrated a preliminary association between older age of tremor onset and more rapid rate of tremor progression. However, the sample used in that study was one-tenth of the current sample. One other study, of 45 patients who were evaluated twice, noted that shorter disease duration was marginally associated with more rapid progression; however, there was no detailed analysis of the association between age of onset and rate of tremor progression.14 There have been no prior studies correlating postmortem changes with either age of tremor onset or rate of tremor progression.
The slower rate of tremor progression in younger onset ET is similar to that reported in patients with several neurodegenerative disorders. In PD patients, age at disease onset was the main predictor of motor decline, indicating a slower and more restricted pathologic disease process in patients with younger onset PD.31, 32 Postmortem studies in PD also support the notionthat in advanced age, brain pathology advances more rapidly.33 Similar findings have been reported in patients with multiple system atrophy34 and motor neuron disease.35
Medication effects could have been important, yet in the epidemiological study, there was no association between rate of tremor progression and use of tremor medication. Also, the association between older age of tremor onset and more rapid rate of tremor progression persisted even after we excluded all cases who were taking tremor medications. In the brain repository, most cases were taking tremor medications so we could not simply exclude these cases. However, we demonstrated that the dose of medication was not correlated with any of our measures (e.g., rate of tremor progression) and therefore could not have accounted for these results.
This study had limitations. First, although the results of the postmortem study were in agreement with the results of the epidemiological study, the sample size for these analyses was small. Hence, it would be useful to repeat these types of analyses within the framework of a larger study. Second, although we demonstrated that patient’s estimates of age of onset are highly reliable,15 their validity is not known. While it is possible that older individuals could preferentially under-estimate tremor duration (i.e., falsely reporting older ages of onset, thereby resulting in inflated estimates of rate of progression), we are not aware of published data that support this scenario. Third, rate of progression was based on arm rather than head tremor severity; however, all cases had arm tremor. Fourth, by design, we restricted analyses to ET cases without Lewy bodies. Finally, the measure of tremor severity used in the brain bank study (total spiral score) was different from that used in the epidemiological study (total tremor score). The total spiral score is one component of the total tremor score. The correlation between the two measures is very high (e.g., in the epidemiological study, Pearson’s r = 0.85, p < 0.001), indicating that the use of either one of these overlapping measures was likely to have yielded similar results. Indeed, using the total spiral score rather than the total tremor score in the epidemiological study yielded similar results: older age of tremor onset was associated with more rapid rate of tremor progression (r = 0.71, p <0.001). This study had several strengths. It is the most detailed effort to examine the association between age of onset and rate of tremor progression in ET. Furthermore, it is unique in its inclusion of complementary data from both a clinical study and a postmortem study.
Acknowledgments
R01 NS42859 and P50 AG08702 from the National Institutes of Health (Bethesda, MD); the Parkinson’s Disease Foundation (New York, NY); the Arlene Bronstein Essential Tremor Research Fund (Columbia University); and the Claire O’Neil Essential Tremor Research Fund (Columbia University).
Footnotes
Disclosure: The authors report no conflicts of interest.
Statistical Analysis: The statistical analysis were conducted by Dr. Louis.
Author Contributions: Elan D. Louis: Research project conception, organization and execution; statistical analyses design and execution; manuscript writing (writing the first draft and making subsequent revisions).
Phyllis L. Faust, Jean-Paul G. Vonsattel, Lawrence S. Honig, Claire Henchcliffe, Rajesh Pahwa, Kelly E Lyons, Eileen Rios, Cordelia Erickson-Davis, Carol B. Moskowitz, and Arlene Lawton: Research project execution; manuscript writing (making subsequent revisions).
References
- 1.Louis ED, Ford B, Barnes LF. Clinical subtypes of essential tremor. Arch Neurol. 2000;57(8):1194–1198. doi: 10.1001/archneur.57.8.1194. [DOI] [PubMed] [Google Scholar]
- 2.Dogu O, Louis ED, Sevim S, Kaleagasi H, Aral M. Clinical characteristics of essential tremor in Mersin, Turkey--a population-based door-to-door study. J Neurol. 2005;252(5):570–574. doi: 10.1007/s00415-005-0700-8. [DOI] [PubMed] [Google Scholar]
- 3.Benito-Leon J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet. 2007;369(9568):1152–1154. doi: 10.1016/S0140-6736(07)60544-3. [DOI] [PubMed] [Google Scholar]
- 4.Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64(12):2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- 5.Louis ED. Etiology of essential tremor: should we be searching for environmental causes? Mov Disord. 2001;16(5):822–829. doi: 10.1002/mds.1183. [DOI] [PubMed] [Google Scholar]
- 6.Deng H, Le W, Jankovic J. Genetics of essential tremor. Brain. 2007;130(Pt 6):1456–1464. doi: 10.1093/brain/awm018. [DOI] [PubMed] [Google Scholar]
- 7.Tolosa E. Movement disorders: advances on many fronts. Lancet Neurol. 2007;6(1):7–8. doi: 10.1016/S1474-4422(06)70661-5. [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66(11):1756–1759. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- 9.Louis ED, Vonsattel JP. The emerging neuropathology of essential tremor. Mov Disord. 2007;23(2):174–182. doi: 10.1002/mds.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross GWDD, Cerosimo M, et al. Pathological investigation of essential tremor. Neurology. 2004;62(Suppl 5):A537–A538. [Google Scholar]
- 11.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 12.Ashenhurst EM. The nature of essential tremor. Can Med Assoc J. 1973;109(9):876–878. [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley M. Observations of essential (heredofamilial) tremor. Brain. 1949;72:113–139. doi: 10.1093/brain/72.2.113. [DOI] [PubMed] [Google Scholar]
- 14.Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: predictors of disease progression in a clinical cohort. J Neurol Neurosurg Psychiatry. 2006;77(11):1235–1237. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis ED, Schonberger RB, Parides M, Ford B, Barnes LF. Test-retest reliability of patient information on age of onset in essential tremor. Mov Disord. 2000;15(4):738–741. doi: 10.1002/1531-8257(200007)15:4<738::aid-mds1024>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Zheng W, Jurewicz EC, et al. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology. 2002;59(12):1940–1944. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis ED, Ford B, Bismuth B. Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord. 1998;13(2):287–293. doi: 10.1002/mds.870130215. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Wendt KJ, Albert SM, Pullman SL, Yu Q, Andrews H. Validity of a performance-based test of function in essential tremor. Arch Neurol. 1999;56(7):841–846. doi: 10.1001/archneur.56.7.841. [DOI] [PubMed] [Google Scholar]
- 20.Louis ED, Ford B, Lee H, Andrews H. Does a screening questionnaire for essential tremor agree with the physician’s examination? Neurology. 1998;50(5):1351–1357. doi: 10.1212/wnl.50.5.1351. [DOI] [PubMed] [Google Scholar]
- 21.Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20(10):1361–1365. doi: 10.1002/mds.20583. [DOI] [PubMed] [Google Scholar]
- 22.Axelrad JE, Louis ED, Honig LS, et al. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65(1):101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis ED, Vonsattel JP, Honig LS, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol. 2006;63(8):1189–1193. doi: 10.1001/archneur.63.8.1189. [DOI] [PubMed] [Google Scholar]
- 24.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25(2):228–235. [PubMed] [Google Scholar]
- 25.Louis ED, Honig LS, Vonsattel JP, Maraganore DM, Borden S, Moskowitz CB. Essential tremor associated with focal nonnigral Lewy bodies: a clinicopathologic study. Arch Neurol. 2005;62(6):1004–1007. doi: 10.1001/archneur.62.6.1004. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 Suppl):S85–88. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18(4 Suppl):S91–94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 29.Lee CS, Schulzer M, Mak EK, et al. Clinical observations on the rate of progression of idiopathic parkinsonism. Brain. 1994;117 (Pt 3):501–507. doi: 10.1093/brain/117.3.501. [DOI] [PubMed] [Google Scholar]
- 30.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. The rate of decline in function in Alzheimer’s disease and other dementias. J Gerontol A Biol Sci Med Sci. 1999;54(2):M65–69. doi: 10.1093/gerona/54.2.m65. [DOI] [PubMed] [Google Scholar]
- 31.Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology. 2005;65(9):1436–1441. doi: 10.1212/01.wnl.0000183359.50822.f2. [DOI] [PubMed] [Google Scholar]
- 32.Gasparoli E, Delibori D, Polesello G, et al. Clinical predictors in Parkinson’s disease. Neurol Sci. 2002;23 (Suppl 2):S77–78. doi: 10.1007/s100720200078. [DOI] [PubMed] [Google Scholar]
- 33.Kempster PA, Williams DR, Selikhova M, Holton J, Revesz T, Lees AJ. Patterns of levodopa response in Parkinson’s disease: a clinico-pathological study. Brain. 2007;130(Pt 8):2123–2128. doi: 10.1093/brain/awm142. [DOI] [PubMed] [Google Scholar]
- 34.Klockgether T, Ludtke R, Kramer B, et al. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain. 1998;121 (Pt 4):589–600. doi: 10.1093/brain/121.4.589. [DOI] [PubMed] [Google Scholar]
- 35.Norris F, Shepherd R, Denys E, et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci. 1993;118(1):48–55. doi: 10.1016/0022-510x(93)90245-t. [DOI] [PubMed] [Google Scholar]


