Abstract
Studies leading to a total synthesis of Epothilones B and D are described. The overall synthetic plan was based on late stage fragment assembly of two segments representing C1-C9 and C10-C21 of the structure. The C1-C9 fragment was prepared by elaboration of commercially available (2R)-3-hydroxy-2-methylpropanoate at both ends of the three carbon unit. Introduction of carbons 1–4 containing the gem-dimethyl unit was achieved in a convergent manner using a diastereoselective addition of a stannane equivalent of a β-keto ester dianion. An enantioselective addition of such a stannane equivalent for a β-keto ester dianion was also used to fashion one version of the C10-C21 subunit; however, the fragment assembly (using bimolecular esterification followed by ring-closing metathesis) with this subunit failed. Therefore, fragment assembly was achieved using a Wittig reaction; this was followed by macrolactonization to close the macrocycle. The C10-C21 subunit needed for this approach was prepared in an efficient manner using the Corey-Kim reaction as a key element. Other key reactions in the synthesis include a stereoselective SmI2 reduction of a β-hydroxy ketone and a critical opening of a valerolactone with aniline which required extensive investigation.
Introduction
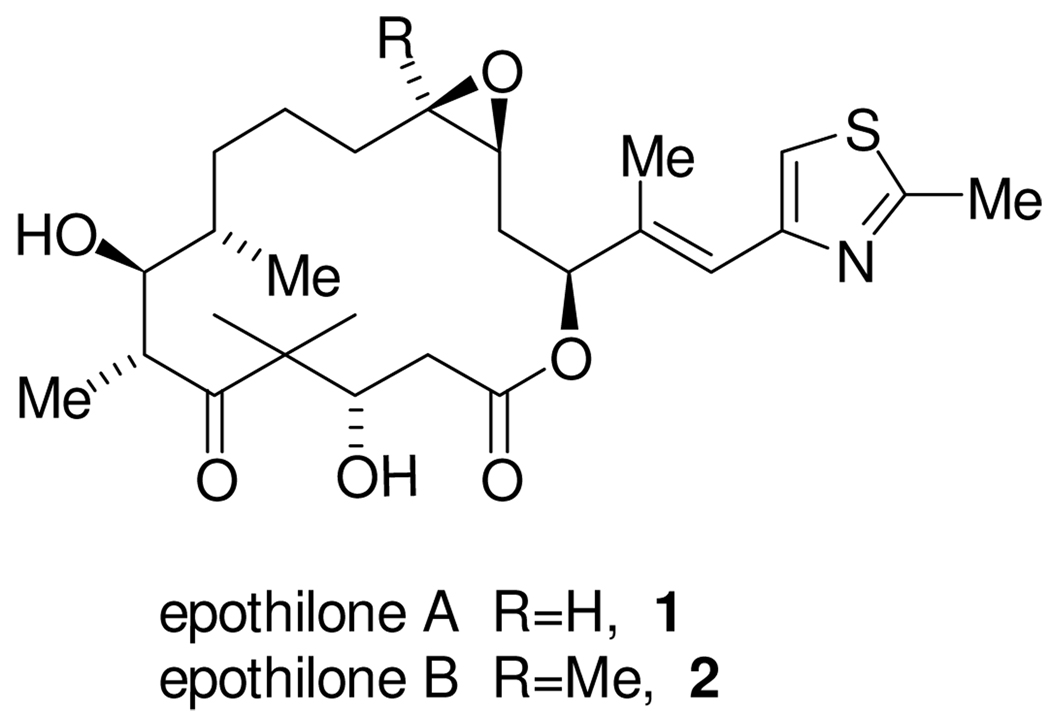
Epothilones A (1) and B (2, Figure 1), were first isolated in 1987 by Höfle and Reichenbach from culture extracts of the myxobacterium Sorangium cellulosum (Myxococcales), first found in soil samples taken from the river banks of the Zambesi River in the Republic of South Africa. In his 1993 patent, Höfle described the gross structure of the epothilones, and reported a very narrow antifungal spectrum against the fungus Mucor hiemalis.1 Practical agricultural use of the epothilones as fungicides and pesticides failed, as they were shown to be phytotoxic. As a result, interest in the epothilones faded.
Figure 1.
Epothilones A and B
In 1995, Bollag and co-workers at Merck2 independently isolated epothilones A and B and found them to be highly cytotoxic to a number of different tumor cell lines. These compounds were then subjected to a filtration-calorimetric assay developed at Merck to detect microtubule-nucleating activity as a screen to search for compounds that could stabilize microtubules. At this time Taxol3 a complex poly-oxygenated diterpene isolated by Wall and Wani4 from the Pacific Yew tree, Taxus brevifolia, was the only known example of a compound that arrested cell growth and brought on apoptosis by the stabilization of microtubules. The efficacy and broad-spectrum cytotoxicity of Taxol had generated enormous interest in finding other microtubule-stabilizing compounds. Of the thousands of compounds that were subjected to the Merck microtubule-nucleating activity screen only epothilones A and B were found to induce the formation of microtubules.
Further research showed that the cytotoxicity of the epothilones resulted from the same microtubule stabilization properties exhibited by Taxol, indicating that these compounds had the potential to become only the second member in this powerful class of anticancer drugs. Epothilones were shown to be competitive inhibitors of [3H]Taxol binding, suggesting that epothilones and Taxol have the same, or a largely overlapping, binding site in the microtubule framework. In general, epothilones A and B are potent antiproliferative compounds with IC50 values in the sub-nM to low nM range. Remarkably, epothilones A and B are active against a number of multidrug-resistant cancer cell lines, which include Taxol-resistant cancer cells.
Because microtubule assembly and disassembly require extremely rapid dynamics, mitosis is acutely vulnerable to microtubule-targeted drugs. Microtubule-stabilizing agents, such as Taxol and the epothilones, can suppress microtubule dynamics and interrupt mitosis, leading to cell death. The success of microtubule-stabilizing agents in the treatment of cancer have led to microtubules being called, arguably, the best molecular target for the development of anticancer treatments that have been identified so far.5 Since the discovery of Taxol and the epothilones, other compounds with microtubule-stabilizing properties have subsequently been identified through natural-product screening including discodermolide,6 sarcodictyin A,7 eleutherobin,8 peloruside A,9 and laulimalide.10
While the gross molecular structures of epothilone A and B were disclosed in Höfle’s original patent, and subsequently confirmed by Bollag, the absolute and relative stereochemistry were not elucidated by Höfle until 1996.11 Since then, the epothilones have received unprecedented attention from the synthetic community, with over 30 total syntheses12,13 of epothilones A and B reported. Danishefsky14 completed the first total synthesis of epothilones A and B in less than 6 months after Höfle assigned the absolute configuration. This was quickly followed by syntheses from Nicolaou15 and Schinzer.16 In comparison to Taxol, the relatively simple structuture of the epothilones has made it quite amenable to the synthesis of analogues, with over 100 reported to date. Given the relatively small size of the epothilones, a more surprising aspect is that the structure has allowed for such a large number of different synthetic approaches.
Ring Closing Metathesis Approach to Epothilone B
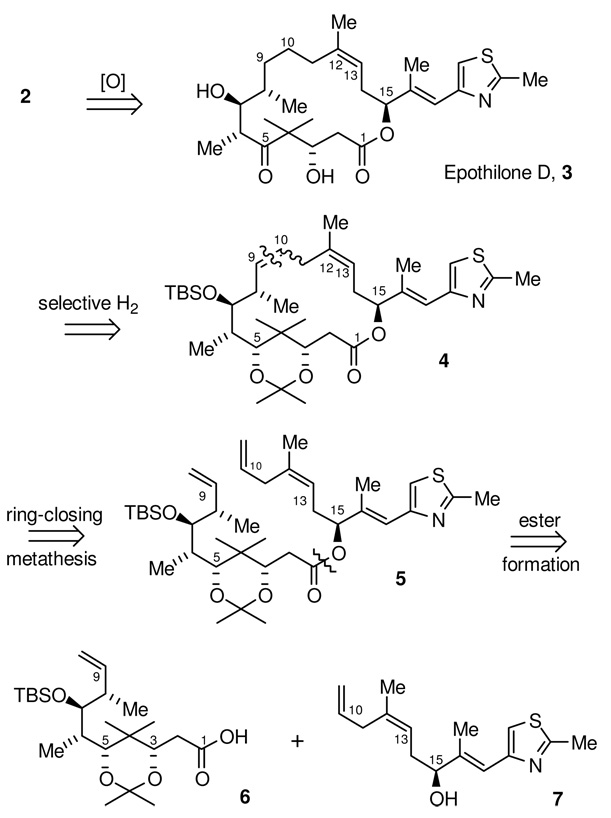
Our original synthetic strategy chosen for epothilone B, 2, is shown in Scheme 1. As with the majority of previously published syntheses, we planned to make epothilone B by a well precedented diastereoselective epoxidation17 of the C12-C13 olefin of epothilone D (3), which would be accessed from the macrolactone 4. To allow for a highly convergent synthesis, we chose to divide the molecule into two fragments of similar size by two sequential disconnections. The first of these was between two of the three contiguous C9-C11 methylene groups, to give 5, followed by disconnection of the ester linkage, giving rise to a C1-C9 fragment 6 and a C10-C21 fragment 7.
Scheme 1.
Retrosynthetic Analysis of RCM Route to Epothilone.
The decision to close the macrolactone through a C9-C10 olefin forming reaction, in our case, ring-closing metathesis (RCM), deserves mention. This approach requires selective hydrogenation of the less sterically encumbered C9-C10 disubstituted olefin in the presence of two additional trisubstituted olefins. While there is precedent for this selective hydrogenation,18 a more obvious choice of an intermediate for RCM might negate this requirement by having a saturated C9-C11 segment and have consummate diastereoselective olefin formation at C12-C13. Unfortunately, this strategy has been tried a number of times, with both epothilone A and B intermediates, with essentially no selectivity19 (~1:1 to 1.5:1) seen for the desired C12-C13 (Z)-olefin geometry. While one might suspect that a C9-C10 RCM would yield the same poor olefin diastereoselectivity, this residual stereochemical information will be lost in the subsequent hydrogenation step which renders any observed diastereoselectivity for the RCM step inconsequential.
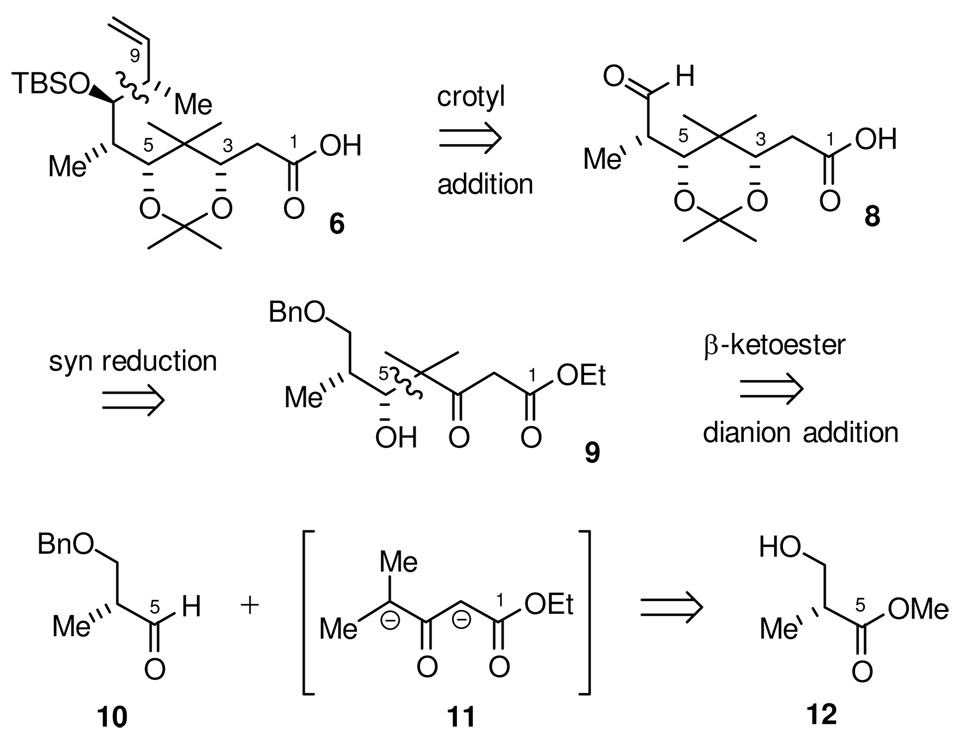
The C1-C9 carboxylic acid, 6, is the more stereochemically-complex coupling partner for the RCM precursor. It is a densely functionalized fragment that contains the majority of the stereocenters found in the natural product. A synthetic strategy was envisioned (Scheme 2) wherein a single stereocenter, the C6-methyl stereocenter derived from commercially available, enantiomerically pure methyl (2R)-3-hydroxy-2-methylpropanoate (12), would be the origin of all the remaining stereocenters in this subunit. Thus, 6 was envisioned to come from a substrate-controlled crotylation reaction that would establish both the C7-hydroxyl and C8-methyl stereocenters. The C3-hydroxyl stereocenter would then be set through a 1,3-syn reduction of the corresponding δ-hydroxy β-keto ester 9. Finally, the C5-hydroxyl stereocenter would be derived from a diastereoselective addition of a β-keto ester dianion synthon 11 to the known aldehyde 10. We saw this “β-keto ester dianion” addition as a unique opportunity to install the C4-gem dimethyl group of epothilone B through a stereoselective connective fragment assembly. This is in contrast to the majority of previous syntheses of the epothilones that carry this gem-dimethyl functional group in as a constituent of a simple precursor.20 In an overall sense, this segment would be assembled in a very convergent manner, by elaboration of aldehyde 10 at both ends.
Scheme 2.
Retrosynthetic Approach to Acid 6
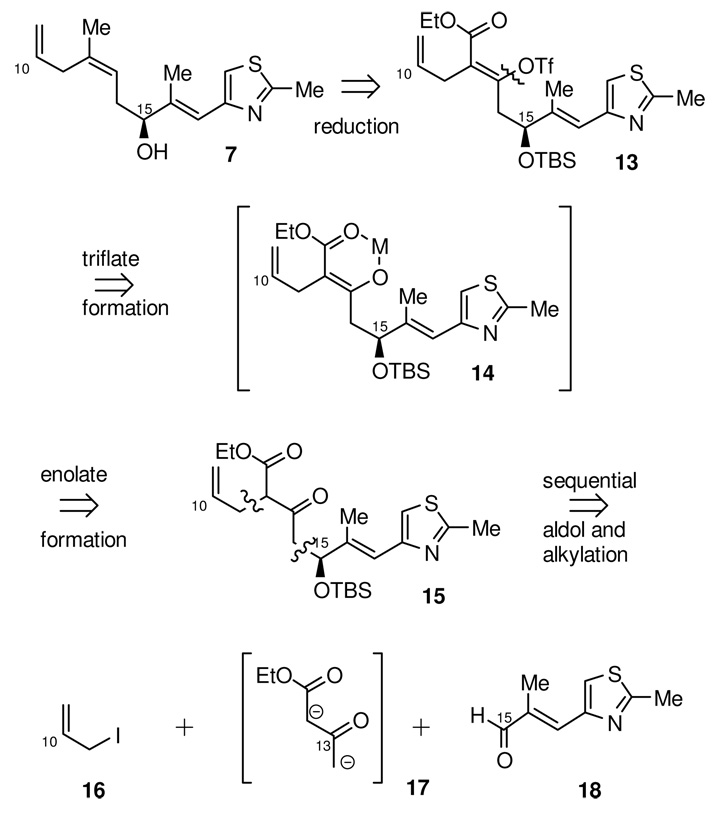
While the C10-C21 fragment 7 appears noticeably less complex than the C1-C9 fragment (Scheme 3), the (Z) C12-C13 trisubstituted olefin presents a significant synthetic challenge. Installation of this acyclic trialkyl-substituted olefin through either a Wittig or metathesis cross coupling reaction inevitably leads to either poor diastereoselectivity for the C12-C13 olefin or low yield. We envisioned that the C12-methyl of 7 could arise from 13 by sequential reduction of the C13-vinyltriflate followed by the full reduction, and then deoxygenation, of the α-allyl α,β-unsaturated ester. This method of indirectly forming the trisubstituted olefin from 13 would rely on the tendency of enolates of β-keto esters to form chelates with their counter ions to selectively form the (Z)-enolate, as depicted in 14. The requisite δ-hydroxy β-keto ester would be prepared via an enantioselective addition of the β-keto ester dianion synthon 17 to the known aldehyde 18. It is worth noting here that while the two subunits 6 and 7 appear to be quite different, a δ-hydroxy β-keto ester is the starting point for the synthesis of each fragment. Thus an exploration of the use of β-keto ester dianion equivalents was to be one focal point of this synthetic plan.
Scheme 3.
Retrosynthetic Analysis of the C10-C21 Subunit
Results and Discussion
Approaches to δ-Hydroxy β-Keto esters
In planning the synthesis of 9 we took note that an effective method for making δ-hydroxy β-keto esters from α-methyl β-alkoxy aldehydes such as 10 might be through the use of the β-keto ester dianion synthon, Chan’s diene 19.21
Although the dimethyl disubstituted reagent 19 was unknown, the parent compound (with each of the γ-methyls replaced with a hydrogen) has been shown to undergo additions to aldehydes with a high degree of diastereoselectivity.22 Initial investigations of this connective approach to directly install the gem-dimethyl group were frustrated by our inability to prepare the requisite γ,γ-disubstituted Chan’s diene 19 from 20.
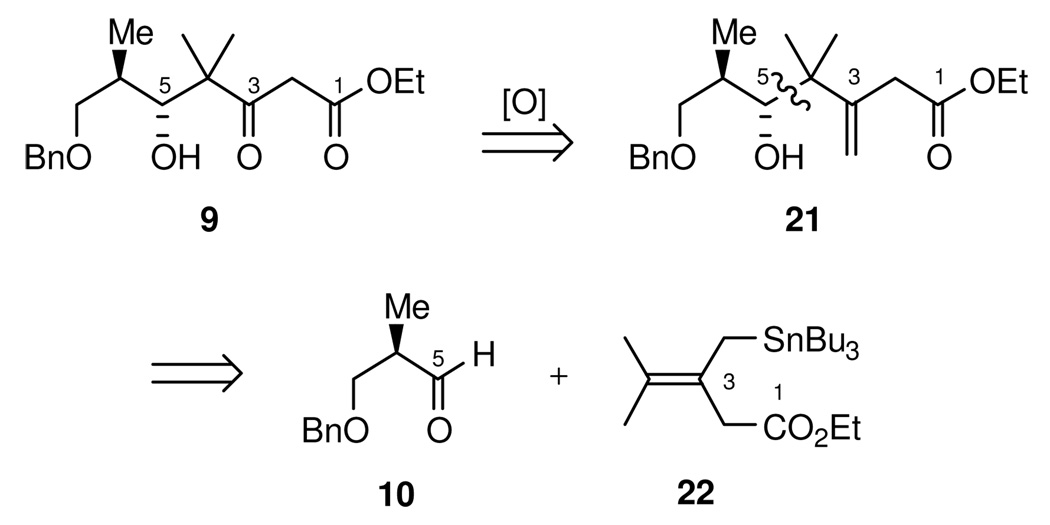
We were thus led to consider the use of an allylstannane equivalent (Scheme 5) as a synthon for the dimethyl substituted Chan’s diene reagent 19. Hence, the desired δ-hydroxy β-keto ester 9 could be envisioned to arise from oxidative cleavage of the olefin in 21, which in turn could arise from the Lewis acid promoted addition of stannane 22 to aldehyde 10.
Scheme 5.
Stannane Approach to δ-hydroxy β-keto esters
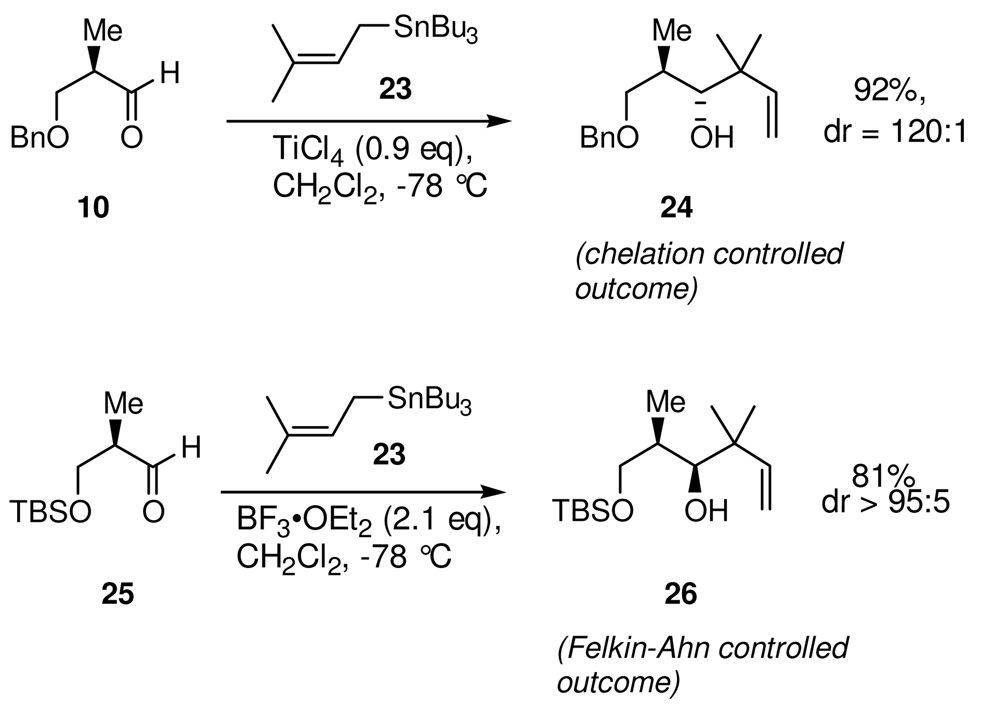
Before embarking upon any attempted preparation of the very highly substituted stannane 22, it seemed advisable to investigate whether this approach to the introduction of the quaternary center could be accomplished in a stereocontrolled manner.23 Model studies with the readily available prenyl-tri-n-butylstannane 23, showed that Lewis acid promoted additions to chiral α-methyl-β-alkoxyaldehydes 10 and 25 proceeded in excellent yield and with high levels of diastereoselectivity (Scheme 6). Moreover, the influence of protecting groups and Lewis acids on reaction diastereoselectivity were found to be in accord with the control elements previously described for additions of allyl- and crotyl-tri-n-butylstannanes24 to these aldehydes and provided access to either the chelation-controlled adduct 24 or the complementary diastereomeric Felkin-Ahn adduct 26 with essentially complete diastereofacial selectivity.
Scheme 6.
Prenyl Stannane Additions
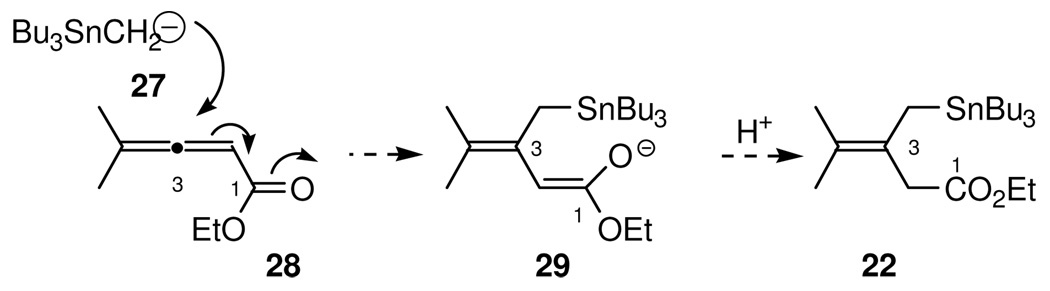
With confidence established for high substrate-controlled aldehyde facial selectivity in the critical stannane addition reaction, the synthesis of the allylstannane 22 was then undertaken. A 1,4-conjugate addition approach to 22 was examined which consisted of a copper mediated addition of tributylstannylmethyllithium 27 to allenic ester 28 followed by protonation of the resulting enolate, as shown in Scheme 7. This approach thus required access to the lithium reagent and the allenic ester 28.
Scheme 7.
Approach to Stannane 22
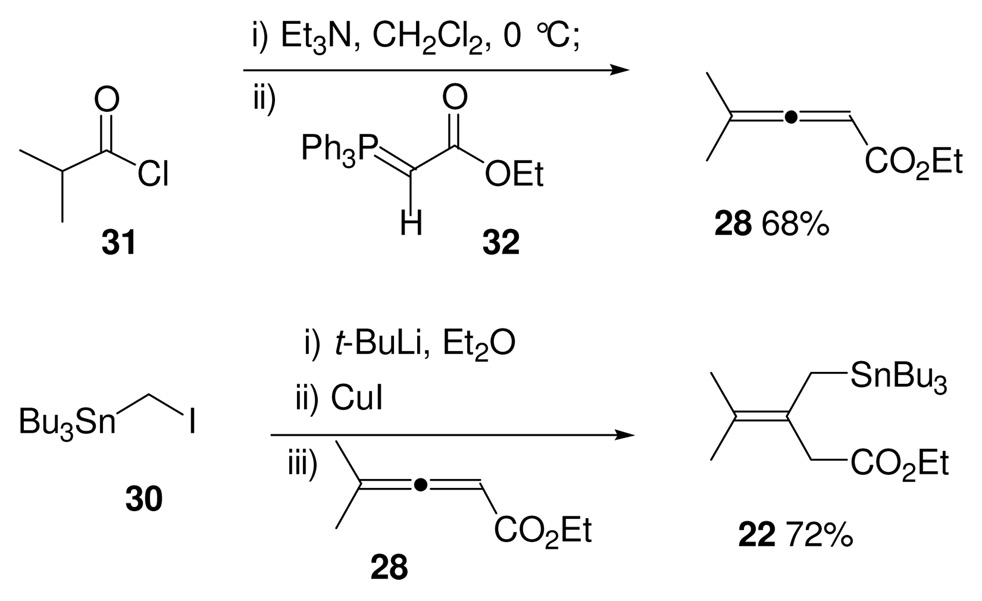
The requisite lithium reagent 27 is known25 and was derived from a lithium-halogen exchange between n-BuLi and iodomethyltributyltin 30. It was found that this known stannane 30 could be prepared from commercially available CH2I2 and Bu3SnCl in >70 gram batches using a modification of the Seyferth procedure.26
For preparation of the allenic ester 28, we took a lead from an Organic Synthesis preparation by Lang and Hansen.27 We found that allenic ester 28 could be made in a single step on multigram scale (Scheme 8), by the Wittig coupling of dimethylketene (derived from reaction of isobutyryl chloride with triethylamine) and the commercially available phosphorane 32. However, initial runs provided the desired allenic ester in low yields of less than 20%. A simple change in the order of addition of the reagents increased the yield to 68% on a >30 gram scale reaction.28
Scheme 8.
Synthesis of Stannane 22
With access to large quantities of iodide 30 and allenic ester 28 secured, the 1,4-conjugate addition was then optimized. This coupling turned out to be more sensitive than we had originally anticipated. The use of n-BuLi to effect the lithium-halogen exchange with iodide 30 led to the formation of the desired stannane 22; however, this cuprate addition gave highly variable yields that ranged from ~20% to >95% yield on up to a 2.5 gram scale. Reproducability and problems upon increasing the scale of this reaction were found to be serious limitations in preparing stannane reagent 22. It appeared that the difficulty was in the initial lithium-halogen exchange. Switching from n-BuLi to the more reactive t-BuLi (Scheme 8) gave much more consistent results. Lithium-halogen exchange of iodide 30 using t-BuLi, followed by formation of the (tri-n-butylstannyl)methyl cuprate and addition into allenic ester 28 gave the desired stannane reagent 22 in a fairly consistent 70% yield on ~7 gram scale.29,30 This β,γ-unsaturated ester proved to be remarkably stable. Not only could it be purified by silica gel chromatography, but it could also be stored, as a neat reagent, for over a year at −20 °C without noticeable decomposition occurring, and without detectable isomerization of the double bond to give a conjugated system.
Synthesis of the C1-C9 Carboxylic Acid Fragment
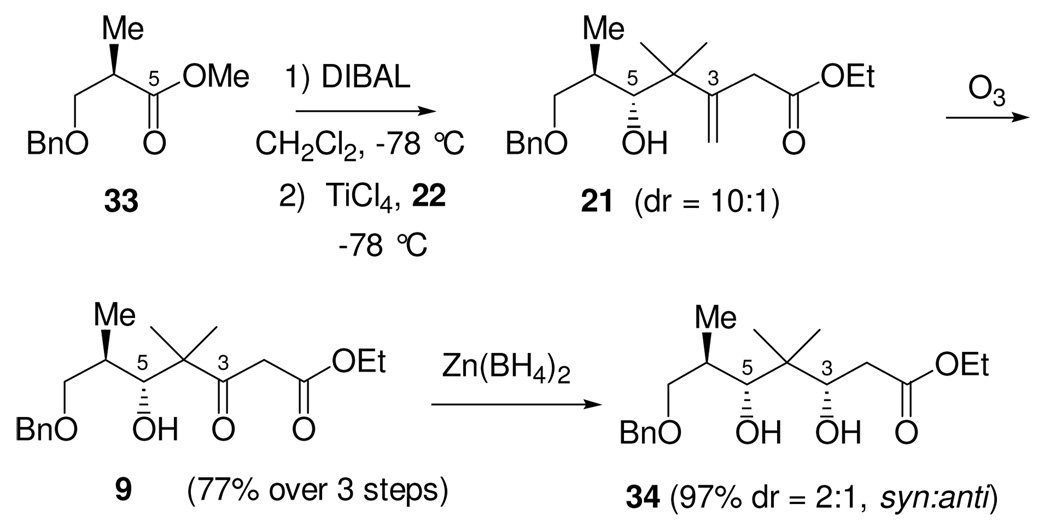
The synthesis towards the C1-C9 fragment (Scheme 9), commenced with the DIBAL half-reduction of α-methyl-β-benzyloxy ester 33 to the corresponding known aldehyde 10. This was followed immediately by Lewis acid mediated addition of stannane 22, using TiCl4 as the Lewis acid, to give the the homoallylic alcohol as a 10:1 mixture of diastereomers in favor of the desired chelation-controlled adduct 21. While analytically pure alcohol 21 could be obtained by silica gel chromatography, it was prone to undergo olefin migration and lactonization. For preparative purposes, it was best to carry it crude into the next step in the sequence. Thus, ozonolysis gave δ-hydroxy β-keto ester 9 in 77% yield over the three steps.
Scheme 9.
Synthesis of C1-C7 Diol 34.
At this point, our intent was to use this newly formed C5-hydroxyl stereocenter to direct the stereoselective reduction of the C3-ketone. Preliminary attempts to establish this C3,C5-syn diol were, unfortunately, poorly selective. For example, while Zn(BH4)2 provided the desired C3,C5-syn diol 34, the ratio of diastereomers was a disappointing 2:1.
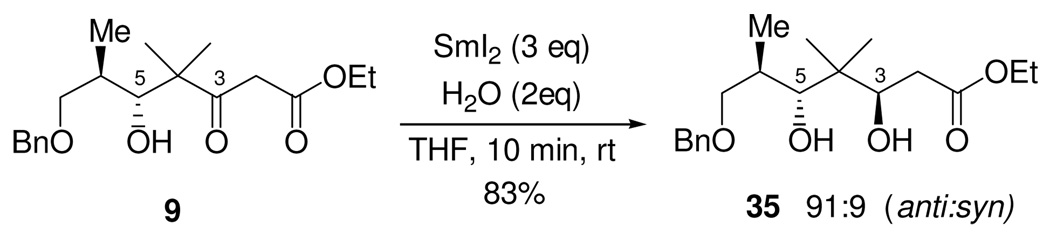
Further investigation revealed similarly low levels of diastereoselectivity for formation of the desired 1,3-syn diol diastereomer through use of the usually powerful, but rather limited number of syn-selective hydride reducing agents. This led us to consider whether the use of single-electron reducing agents, such as SmI2, might provide 1,3-diols stereoselectively from β-hydroxy ketones. A number of features of this reagent suggested that this might be the case. Unlike a number of other single-electron reducing agents, including other low-valent metals and metal complexes, SmI2 is remarkably stable in alcohols and water.31 This permits SmI2 reactions to include a proton source during the reduction, thus allowing immediate protonation of reactive anionic intermediates. Samarium(II) and (III) cations are also very oxophilic and can serve as potential chelating Lewis acids.
Treatment of β-hydroxy ketone 9 in THF, at room temperature, with a solution of SmI2 (3 eq) in THF and water (2 eq) resulted in complete consumption of starting material within 10 minutes. 1H and 13C NMR indicated the formation of essentially a single diol diastereomer (dr > 95:5), which was isolated in 93% yield. The diol was converted to the acetonide and the stereochemistry determined using the methods of Rychnovsky and Evans.32 This revealed that the product of the reaction was the unexpected C3,C5-anti diol 35. The stereochemistry of this diol was further confirmed by conversion of β-hydroxy ketone 9 to the same anti-diol product 35 by independent synthesis using Evans’ Me4NBH(OAc)3 reduction procedure,33 a method which is known to selectively produce 1,3-anti diols with high levels of diastereoselectivity.
While this SmI2 reduction did not furnish the desired 1,3-syn diol necessary for use in the current synthetic strategy for making epothilone B, it was, nevertheless, an excellent empirical result.34 We thus considered the possibilty of incorporating this new, highly selective, and especially convenient 1,3-anti diol reduction into our synthesis of epothilone B.
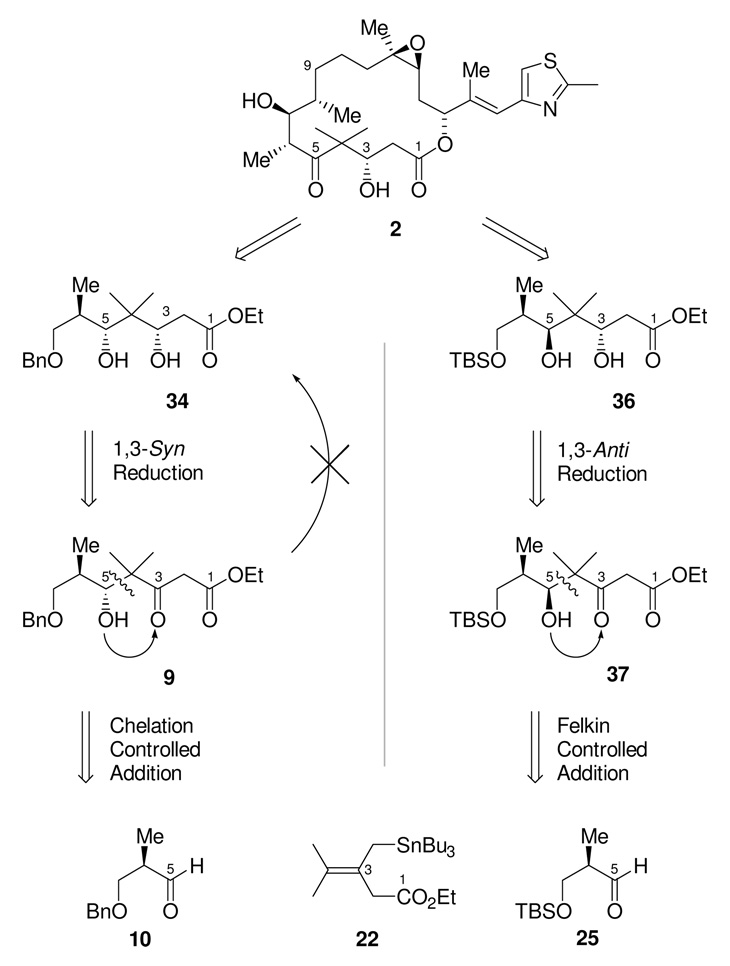
Returning to the structure of epothilone B 2 (Scheme 11) it can be seen that C5 is at the oxidation state of a ketone, which we planned to generate by a late stage oxidation of an existing C5-hydroxyl group. The absolute configuration of the C5-hydroxyl group from either seven-carbon intermediate 34 or 35 is, therefore, inconsequential in terms of the natural product, as this stereochemical information will be lost in the subsequent oxidation. The critical function of the C5-hydroxyl group stereochemistry, rather, is to direct the reduction of a C3-ketone intermediate in order to establish the correct stereochemistry for the C3-hydroxyl group. In essence, there is an overall transfer of oxidation states and chirality between functional groups on C3 and C5. From a retrosynthetic perspective, we are therefore free to arbitrarily choose the absolute stereochemistry of the C5-hydroxyl group. Use of a synthetic plan using the 1,3-anti reduction would ultimately require the δ-hydroxy β-keto ester 37, with opposite stereochemistry at C5 to that of our present intermediate. We anticipated, based on previous work and the earlier model studies with prenyl-tri-n-butylstannane additions, that this diastereomer could be prepared from the Felkin controlled addition of stannane 22 to aldehyde 25, with the β-OH now protected as a TBS ether rather than a benzyl ether. This offered an additional potential advantage in that it seemed likely that the requisite BF3•OEt2 mediated reaction needed to secure the requisite stereoselectivity might be less prone to complications on scale up than would be the reaction using TiCl4.
Scheme 11.
Second Generation C1-C9 Retrosynthetic Analysis
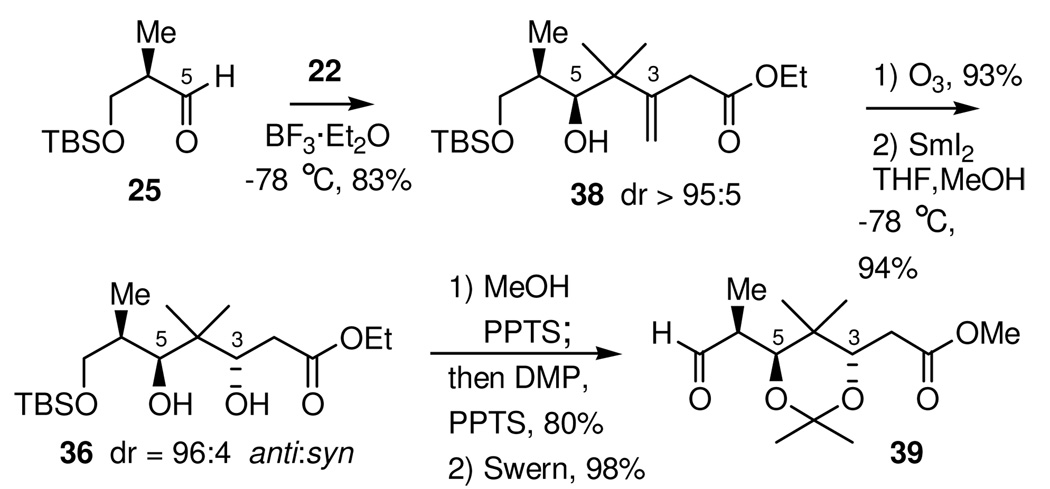
The synthesis of the new C1-C9 fragment (Scheme 12) commenced with the Lewis acid mediated addition of stannane 22 to the β-OTBS aldehyde 25. Using BF3•OEt2 as the Lewis acid did in fact give the desired Felkin controlled adduct 38, with >95:5 diastereoselectivity. Initially, this alcohol was found to be very susceptible to lactonization, with ratios of acyclic to lactonized product varying from 10:1 to 1:6. It was subsequently determined that lactonization was occurring primarily during the quench and workup of the reaction mixture. By pouring the cold (−78 °C) reaction mixture directly into an Erlenmeyer flask that contained a large excess of a vigorously stirring mixture of CH2Cl2, pH 7 buffer, and water, essentially all lactonization of the crude reaction product could be suppressed. On a 2.5 gram scale, this addition gave analytically pure alcohol 38 in 83% yield and with >95:5 diastereoselectivity35 for the Felkin-Ahn adduct. However, for practical reasons on larger scale, this alcohol was consistently carried into the next reaction crude. Following oxidative cleavage of the olefin by ozonolysis, the C3 stereocenter was established by reduction of the resulting β-hydroxy ketone using the SmI2 electron transfer reduction. Under optimal conditions, reduction of the β-hydroxy ketone 37 using SmI2 in a solvent mixture of THF-MeOH gave diol 36 in 94% yield and with a 96:4 level of diastereoselectivity.
Scheme 12.
Synthesis of Aldehyde 39
The next synthetic operation required liberation of the C7-hydroxyl group, which must be oxidized, while protecting those at C3 and C5, which must be retained. It was found that his could be accomplished in a one-pot procedure. Thus, acid catalyzed methanolysis of the primary C7-TBS ether using PPTS in MeOH, at reflux, was followed by concentration and addition of (5:1) dimethoxypropane/MeOH, which resulted in selective formation of the internal acetonide.36 The unpurified mixture was found to contain a small amount of terminal methoxy-isopropyl (MIP) ketal, which was converted to the corresponding primary alcohol by redissolving the crude reaction material in MeOH and immediately removing the MeOH under reduced pressure. Swern oxidation of the primary alcohol then gave aldehyde 39.
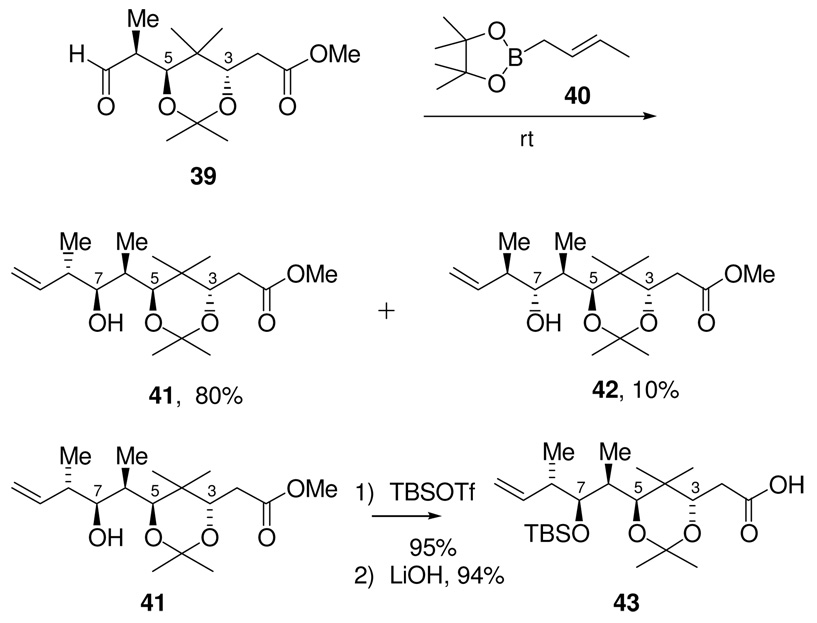
Coupling of aldehyde 39 and crotyl boronate 4037 (Scheme 13) proceeded in high yield and with good diastereoselectivity. The major product was determined by X-ray crystallography to be the desired C6,C7 Felkin-Anh controlled, C7,C8 anti-aldol isomer 41. The minor product 42 was confirmed by Mosher ester analysis and acetonide formation using a C7-C9 1,3-diol derivative to be the C6,C7 anti-Felkin, C7,C8 anti-aldol isomer. This represents an optimum result which was obtained only after extensive experimental investigation.
Scheme 13.
Synthesis of C1-C9 Acid 43
After conversion of the hydroxyl group of 41 to the TBS ether, hydrolysis of the methyl ester gave carboxylic acid 43 (Scheme 13). The synthesis of this fragment was thus completed in 8 steps from the known aldehyde 25, in 40% overall yield.
Synthesis of the C10-C21 Alcohol Fragment
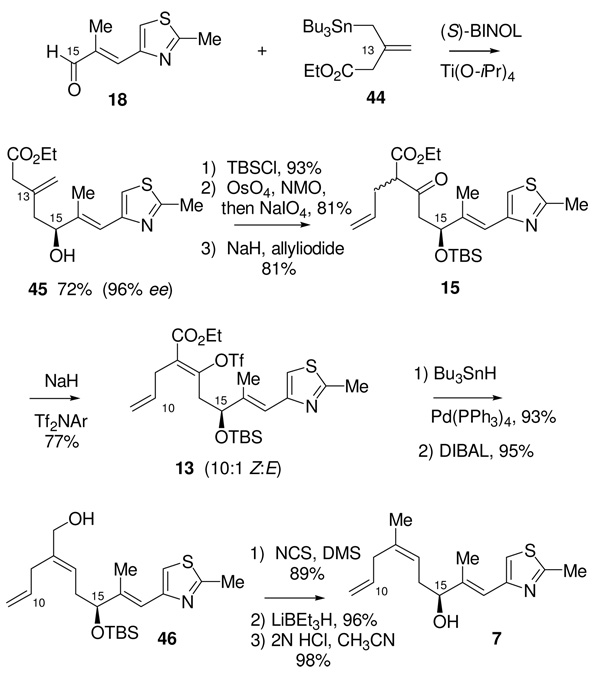
While we were developing our route to acid 43, we had also developed plans to utilize a δ-hydroxy β-keto ester to fashion the requisite trisubstituted olefin in the C10-C21 segment Given the success of the previous stannane addition approach to the formation of δ-hydroxy β-keto esters in the synthesis of the C1-C9 piece, we sought to employ a similar strategy for this fragment. As shown in Scheme 14, the known38 stannane 44 was coupled with the known α,β-unsaturated aldehyde 1839, using our BITIP catalyzed asymmetric allylation reaction.40 Reaction using (S)-BINOL and Ti(OiPr)4 afforded the (S)-homoallylic alcohol 45 in 72% yield and with excellent enantioselectivity (98:2 er). After protection of the resulting alcohol as its TBS ether, selective cleavage of the C13 olefin was initially attempted using ozone; however, these reaction conditions also caused cleavage of the internal C16-C17 trisubstituted olefin. Use of a one-pot, two-step oxidative cleavage procedure, (dihydroxylation with OsO4/NMO followed by oxidative diol cleavage using NaIO4) was much more selective and afforded the corresponding β-keto ester in 80% yield. Alkylation of the β-keto ester with allyl iodide then gave 15 as a 1:1 mixture of diastereomers (and their corresponding enol tautomers).
Scheme 14.
Preparation of the C10-C21 Alcohol 7
With the entire C10-C21 carbon framework assembled, we now turned to setting the (Z) geometry of the trisubstituted C12-C13 olefin, by trapping the sodium enolate of 15 as the vinyl triflate using Comins’ reagent.41 Vinyl triflate 13 was formed with 10:1 selectivity for the desired isomer, and in 77% yield. The vinyl triflate was then reduced under palladium catalyzed tin-hydride reduction conditions42 to afford the requisite (Z)-olefin (94%). The α,β-unsaturated ester was then reduced using DIBAL to give the allylic alcohol 46. This was deoxygenated in two steps by conversion to the allylic chloride using the Corey-Kim procedure,43 followed by hydride reduction to give the requisite C12 methyl substituent. Removal of the silyl ether was then accomplished by acid catalyzed hydrolysis to give alcohol 7. Overall, the synthesis of this fragment was completed in 10 steps from the known aldehyde 18 and in 25% overall yield.
Fragment Coupling and Ring-Closing Metathesis
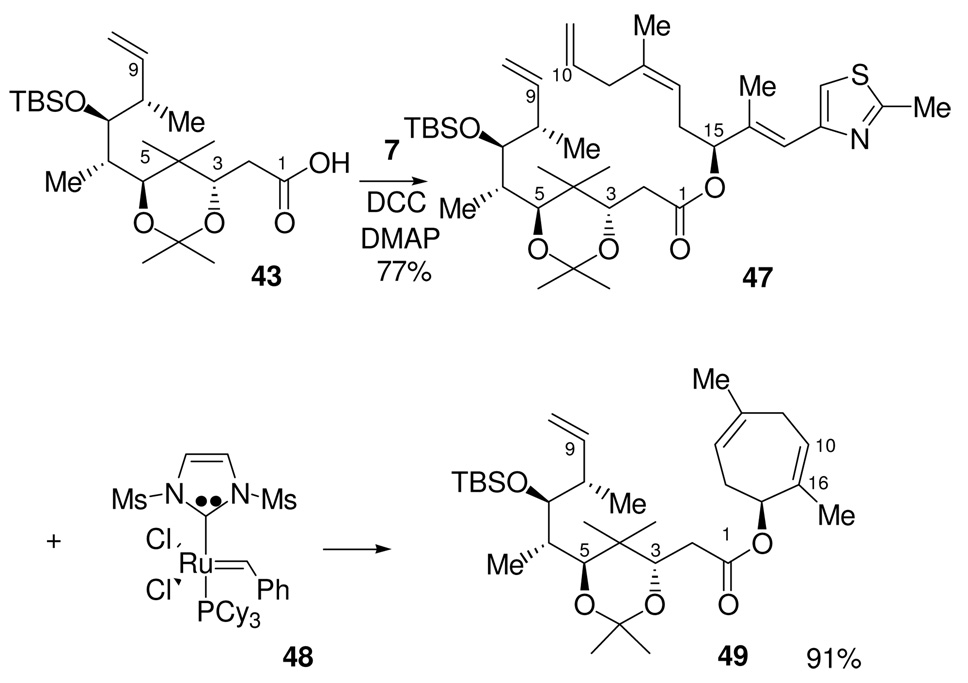
With the C1-C9 and C10-C21 fragments in hand, carboxylic acid 43 and alcohol 7 were then subjected to DCC coupling, which gave the desired ester, 47, in 77% yield. The resulting diene was then exposed to RCM conditions using Grubb’s second-generation ruthenium alkylidene catalyst 48. This yielded a single compound, which unfortunately, turned out to be compound 49, containing a seven-membered ring, rather than the desired macrolactone.
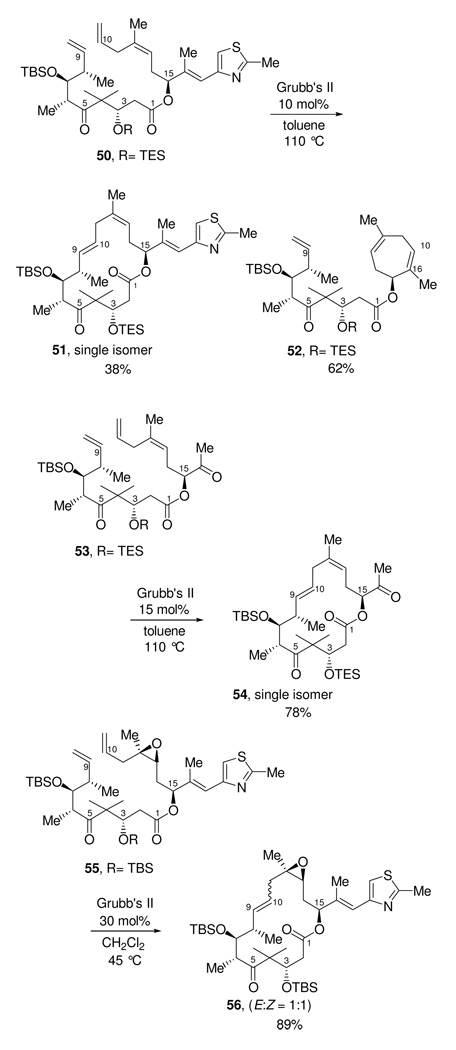
We suspected that the acetonide was the main cause of the complete failure of 47 to form any of the macrolactone via RCM. It is possible that the rigidity imposed by the acetonide did not allow the diene to readily adopt a conformation that allowed the C9 and C10 olefins to become sufficiently close to undergo RCM. With the compound unable to undertake the C9-C10 metathesis, the C10 terminal olefin is then left to undergo RCM with the trisubstituted C16-C17 olefin. Other examples of such RCM reactions in the context of epothilone synthesis have been described. (Figure 1.66). Danishefsky first performed this reaction on a precursor that lacked the C3,C5 acetonide functionality encountered in our synthesis. When compound 50, which has a C3-TES ether and C5 ketone, was subjected to RCM, the desired macrolactone 51 was formed in 38% yield as a single trans isomer, however, the major product of the reaction was the seven-membered ring 52, formed in 62% yield.44 To circumvent this unwanted side-product, Danishefsky synthesized a new precursor, 53, which lacked the C16-C17 trisubstituted olefin. This precludes formation of the seven membered ring. In this case, RCM yielded the macrolactone 54 in 78% yield as a single (E)-isomer. He then installed the thiazole ring in a subsequent step. Sun and Sinha45 performed a RCM reaction on the advanced epothilone precursor 55 containing the C12-C13 epoxide functionality and obtained the macrolactone 56 in 89% yield as a 1:1 mixture of E:Z olefin isomers.
From these reactions, it would appear that the presence of the acetonide functionality is most likely the major cause for the complete failure of our RCM precursor 47 to give the desired macrolactone. Simple removal of the acetonide was not an option due to formation of a six-membered ring lactone under the reaction conditions (vide infra). We therefore decided to abandon the RCM route and complete the synthesis by reversing the order of the two operations of esterification and formation of the C9-C10 alkene.
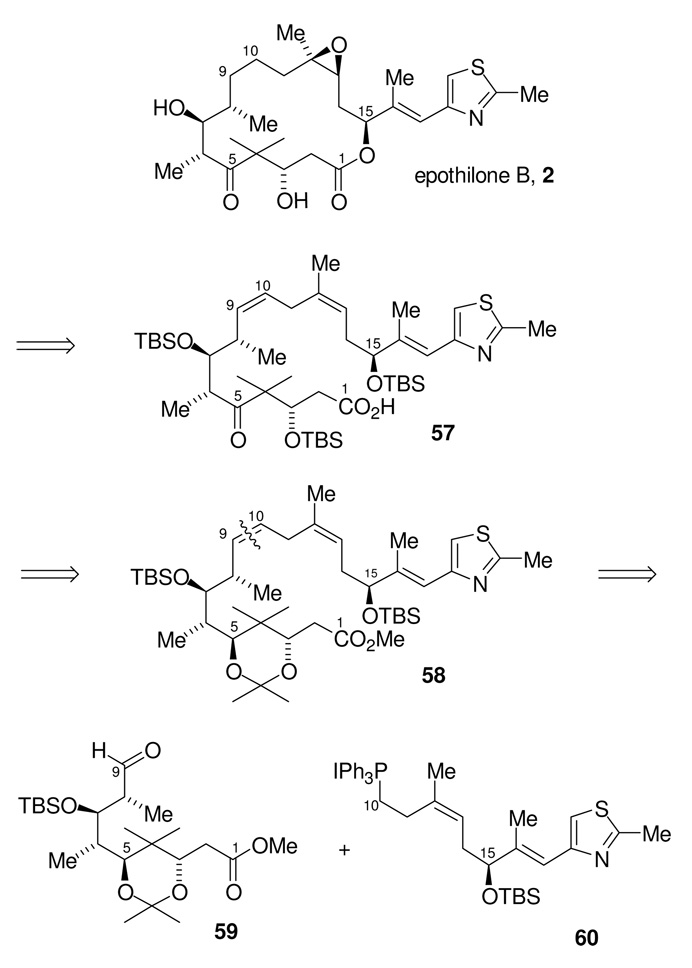
Macrolactonization Route to Epothilone B: Revised Retrosynthetic Analysis of Epothilone B
The new retrosynthetic analysis for epothilone B (2) is shown in Scheme 17. We would now proceed by establishing the C9-C10 olefin before closing the macrolactone. This macrolactonization strategy would intercept a known late stage intermediate 57.18 The C9-C10 olefin of intermediate 58 would result from a Wittig coupling of aldehyde 59 and phosphonium salt 60. Such Wittig reactions have been employed previously for fragment coupling in epothilone synthesis by J. D. White and co-workers.18 As with the previous RCM strategy, this disubstituted olefin would later be selectively reduced to form two of the three contiguous methylene groups found in the natural product.
Scheme 17.
Revised Retrosynthetic Analysis of Epothilone B.
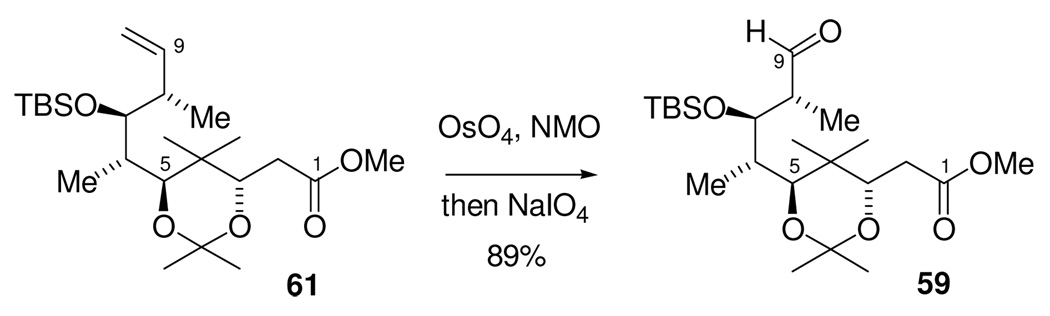
The requisite aldehyde was available trivially from our previous C1-C9 intermediate. Oxidative cleavage of the previously synthesized terminal olefin 61 by the two step procedure of dihydroxylation with OsO4/NMO followed by reaction with NaIO4 gave aldehyde 59 in 89% yield. Therefore, the synthesis of the new C1-C9 fragment was completed in 8 steps from the known aldehyde 25 in 38% overall yield (Scheme 18.)
Scheme 18.
Preparation of Aldehyde 59
Synthesis of the C10-C21 Phosphonium Salt Fragment
Initially we examined a synthetic route to this segment which was based on minor modifications of the existing route. While we were able to prepare the requisite phosphonium salt in this manner (11 steps from the known aldehyde 18 in 14% overall yield) the overall yield was poor and the sequence was much longer than desirable. Thus, we attempted to design a more efficient route to phosphonium salt 60 (Scheme 19).
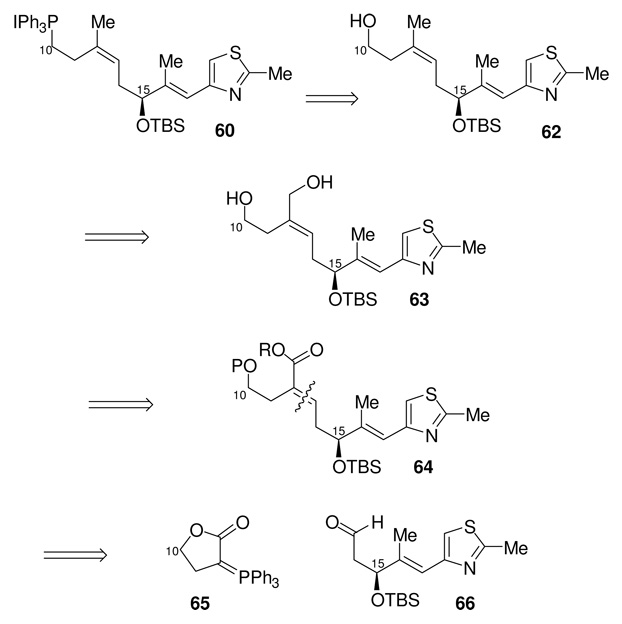
Scheme 19.
Retrosynthetic Approach to Phosphonium Salt 60
Based on our experience to date with this subunit, we believed we could construct the desired C10-C21 fragment 60 from the key α,β-unsaturated ester intermediate 64. The C12-C13 trisubstituted (Z)-olefin geometry should be established with high diastereoselectivity through reaction of aldehyde 66 with a stabilized phosphorane. We decided to try to protect the C10-hydroxyl group by incorporating it into the lactone functionality of phosphorane 65.
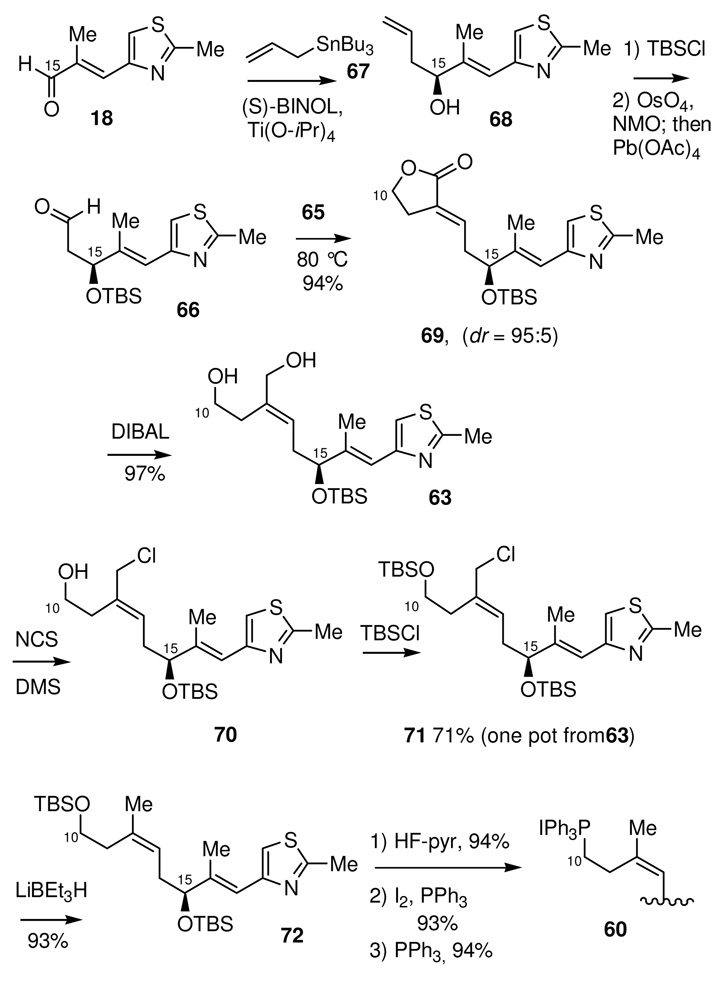
The synthesis (Scheme 20) of the C10-C21 phosphonium salt 60 began with the CAA reaction of aldehyde 18 and allyl-tri-n-butylstannane 67 to give the known CAA adduct 68. After protection of the newly formed hydroxyl group as the corresponding silyl ether, oxidative cleavage of the terminal olefin yielded aldehyde 66.46 Wittig coupling with the known phosphorane 6547 gave lactone 69 in quantitative yield and with excellent (95:5) diastereoselectivity. This transformation not only establishes the necessary geometry for the trisubstituted olefin but also incorporates the remaining carbon framework needed for this C10-C21 segment. Full reduction of the lactone using DIBAL gave diol 63 nearly quantitatively (97%).
Scheme 20.
Preparation of Phosphonium Salt 60
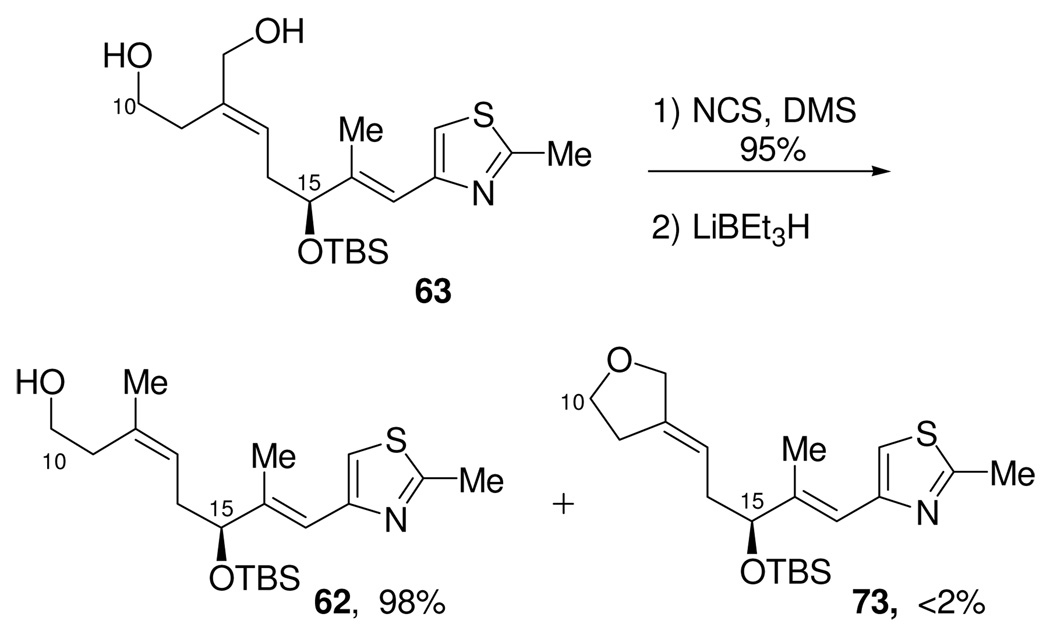
The successful utilization of this route depended upon an efficient differentiation of the two primary hydroxyl moieties in diol 63. We were pleased to find a single-pot procedure48 that allowed for the conversion of diol 63 to the C10-TBS protected allylic chloride 71 by the sequential, selective chlorination of the allylic alcohol by reaction with the Corey-Kim reagent, followed by protection of the remaining primary alcohol as the corresponding TBS-silyl ether to give 71. The resulting C12-allylic chloride was then easily reduced with lithium triethylborohydride (“Super-Hydride®”) to give the trisubstituted (Z)-olefin 72. This was then smoothly converted to the C10-C21 phosphonium salt 60, which was obtained in 28% overall yield from aldehyde 18.
One final improvement remained. Although we were pleased with the success of the one-pot conversion of 63 to 71, in retrospect, it became clear that the TBS ether was being used in only a single step, that being the Super-Hydride® reduction of the allylic chloride 71. Thus, we wondered if it might be possible to conduct this operation on the free alcohol, and thus save the steps of protection and deprotection. The concern here was obviously the formation of a tetrahydrofuran by intramolecular displacement of the allylic chloride by the boron alkoxide anion. To determine the viability of leaving the primary alcohol unprotected, allylic alcohol 63 was first selectively chlorinated (Scheme 21) by reaction with the Corey-Kim reagent to give allylic chloride 70. The resulting chloro-alcohol was then reduced, under carefully optimized conditions, with lithium triethylborohydride (“Super-Hydride®”) to the trisubstituted olefin 62. It was found that this transpired without significant formation of the cyclic ether 73. Alcohol 62 was then converted to phosphonium salt 60 as previously described. This optimization thus provided the C10-C21 fragment from the known aldehyde 18 in just 9 steps and in a much improved 46% overall yield.
Scheme 21.
Selective Allylic Chlorination
Wittig Coupling of the C1-C9 and C10-C21 Fragments
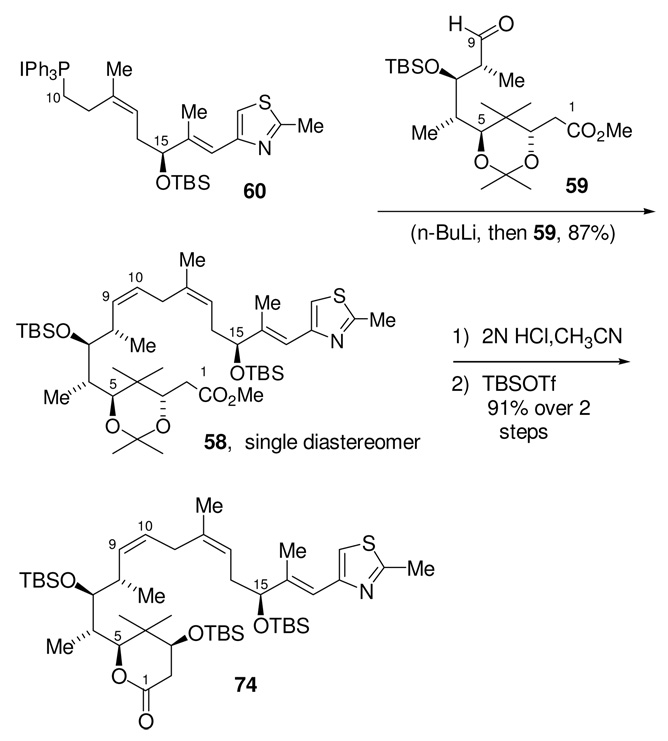
Wittig coupling of aldehyde 59 and phosphonium salt 60, Scheme 22, gave 58 in 87% yield. Differentiation of the C3,C5 diol was initiated by hydrolysis of the acetonide by exposure to 2N HCl in acetonitrile; this was accompanied by spontaneous lactonization. The three free hydroxyl groups were then protected as TBS silyl ethers to give lactone 74. Overall, this two-step sequence served to differentiate the C5-hydroxyl group, which must be oxidized, from the other secondary alcohols. Our plan was to open the lactone and effect the requisite oxidation, ultimately giving the known carboxylic acid intermediate 57, which would complete a formal synthesis of epothilones B and D.18 This sequence, however, proved to be much more difficult than had been anticipated.
Scheme 22.
Synthesis of Lactone 74
Model Studies on the Lactone Opening
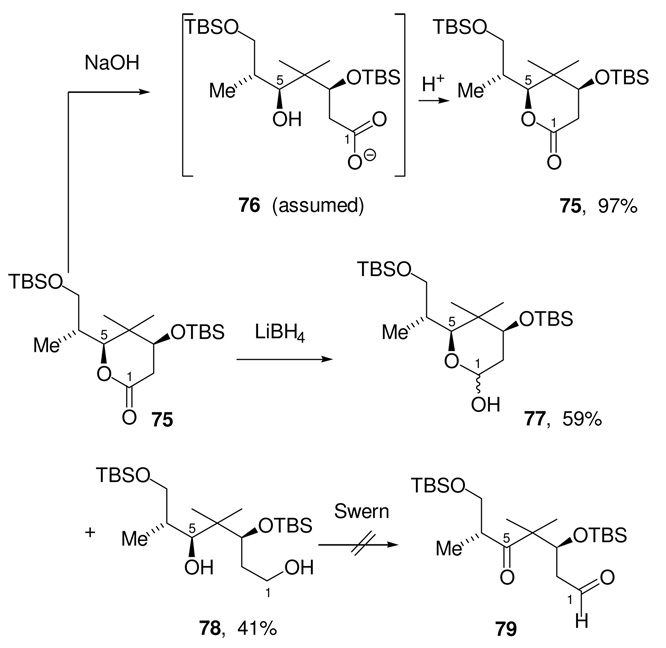
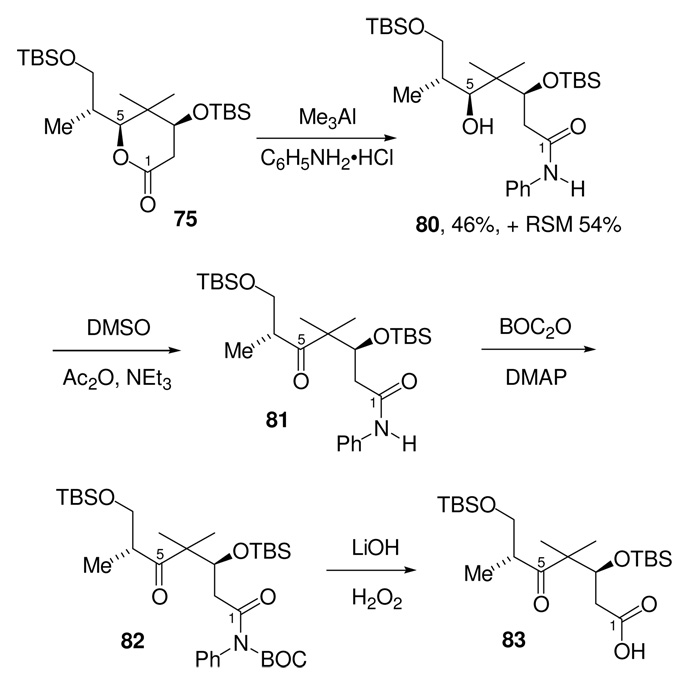
We chose to model the lactone opening with the truncated lactone 75 (Scheme 23). Simple hydrolysis of 75 with LiOH/H2O was attempted first. Monitoring the course of the hydrolysis reaction by TLC showed that after 2 h, all the starting material had been consumed with formation of a very low Rf spot, which was assumed to be the carboxylate 76. Upon mild workup, however, this unstable intermediate reverted to the starting material to give lactone 75, in near quantitative yield, as the sole product of the reaction. We also attempted to open this lactone reductively to give the corresponding diol. After screening a number of hydride reducing agents, only LiBH4 was found to give any significant formation of diol 78. Under these reduction conditions, the majority of the lactone starting material was converted to a 1:1 diastereomeric mixture of lactol 77, which was completely unreactive to any further reduction.
Scheme 23.
Attempted Opening of Model Lactone Substrate 75
In Evans’ synthesis of bryostatin 1,49 an identical lactonization approach was used to differentiate between two secondary hydroxyl groups in a 3,5-dihydroxy ester. In that case, the lactone intermediate was opened using an aluminum amide derivative of aniline (Me3Al/C6H5NH2•HCl). The resulting amide could be subsequently converted to the carboxylic acid in two steps by Boc activation of the amide followed by basic hydrolysis.50 We were interested to see if our C4-gem dimethyl lactone could be converted to the carboxylic acid using the same protocol. In the event, the conversion of the lactone model compound to the desired aniline amide was sluggish and proceeded in a low 46% yield (with 54% recovered starting material), but the amide 80 proved to be stable to both workup and silica gel chromatography.51
With the truncated aniline amide 80 in hand, we thought it prudent to subject this model compound to the same oxidation and hydrolysis conditions we planned to employ to make the desired epothilone intermediate 57. Therefore, the secondary alcohol 80 was oxidized (Scheme 24) using the Albright and Goldman52 modification of the Moffat conditions (Ac2O, DMSO, and Et3N) to provide the desired ketone 81.53 The amide was then activated for hydrolysis by Boc derivatization to give 82. Hydrolysis of this imide using lithium hydroperoxide gave the desired carboxylic acid 83 in 71% yield over the three steps. Thus, we felt that if some improvement in the conversion of the lactone to amide could be realized, that a workable route to convert the fully elaborated lactone 74 to the known intermediate 57 was in hand.
Scheme 24.
Aniline Opening of Model Lactone 75
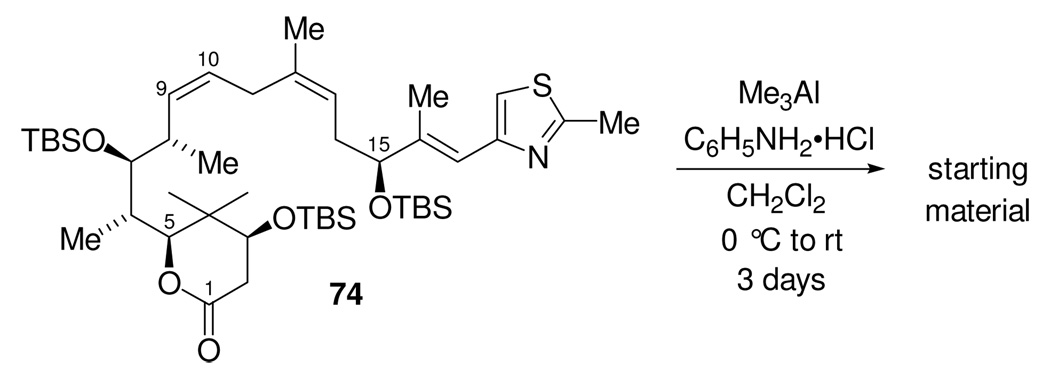
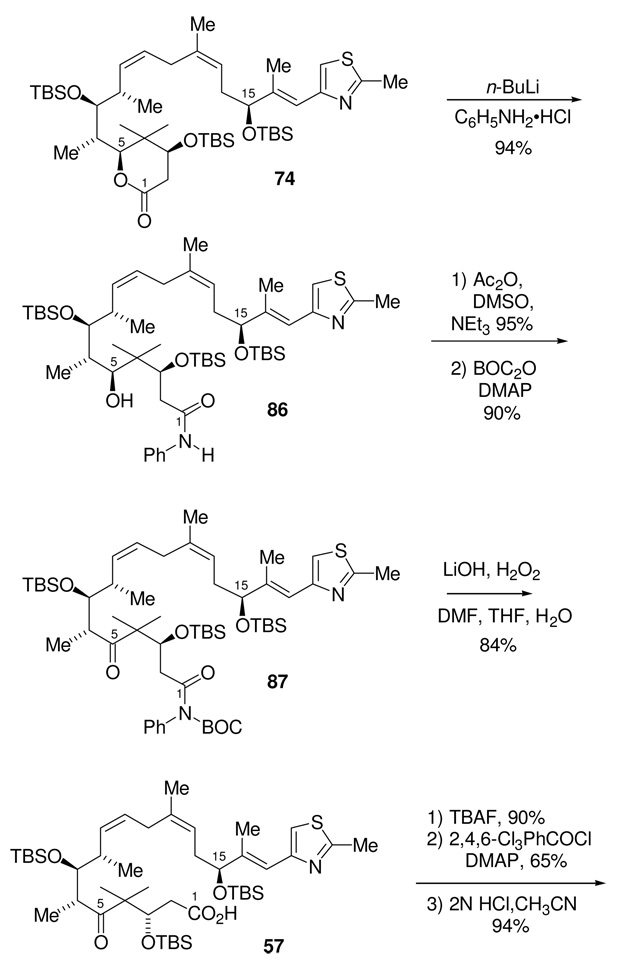
Even though we knew that using the aluminum aniline amide conditions to open the lactone had proceeded in low yield for the model compound, we still decided to try these conditions on the real substrate. Surprisingly, the treatment of lactone 74 with the aluminum aniline amide, Scheme 25, returned only starting material even after 3 days at room temperature.
Scheme 25.
Attempted opening of lactone 74
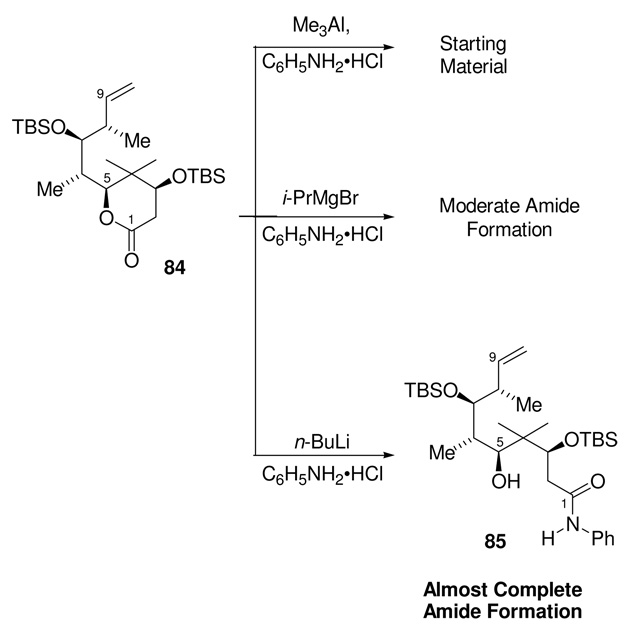
The most logical difference between the model compound 75 and 74 is an increase in steric demand of the fully elaborated lactone. A more sterically encumbered lactone, 84, was therefore employed as a more realistic model for the requisite lactone opening; indeed, this model represents fully half of the real substrate (Scheme 26). Treatment of lactone 84 under the same aluminum amide conditions that had proven unsuccessful for the fully elaborated lactone 74, also gave none of the desired amide 85. This was the expected result of this experiment which was carried out on the basis of logical consistency.
Scheme 26.
Lactone Opening with Advanced Model 84.
Upon searching the literature on related reactions more thoroughly, we found that Williams and co-workers at Merck had reported observations very relevant to the present problem. Specifically, they found that Weinreb amides could easily be made in excellent yield from highly hindered esters using the magnesium salt of the Weinreb amine, in cases where the more standard aluminum-based reagent had failed to yield any amide product.54 In a modification of these Merck conditions, we subjected the model lactone 84 to the magnesium anilide prepared by the reaction of two equivalents of i-PrMgCl with C6H5NH2•HCl. While these conditions yielded moderate amount of the desired aniline amide, the majority of the reaction mixture was still unreacted starting material. Extending this further, we then found that the lithium anilide, formed by the reaction of two equivalents of n-BuLi with C6H5NH2•HCl, gave the desired amide 85 in near quantitative yield.
Completion of the Total Synthesis of Epothilones B and D
Using the optimized lithium aniline amide conditions with the real substrate, 74, we were pleased to find that this lactone was opened to give the aniline amide 86 in 94% yield, Scheme 35. The secondary alcohol was then oxidized under the modified Moffat conditions to provide the corresponding ketone.
Following the precedent provided by Evans, the amide was then activated for hydrolysis by Boc derivatization. Hydrolysis of the derived imide 87 was then affected using the lithium hydroperoxide to afford the known carboxylic acid 57. For this transformation, we initially used the same hydrolysis reaction conditions (LiOH, H2O2, THF, H2O) that we had previously used on the model compound 82 (see Scheme 24). This had given an excellent yield (92%) of the desired carboxylic acid 83. When applied to the Boc protected amide 87, however, the reaction produced low and variable yields of the carboxylic acid 57. In addition, we observed for the first time significant formation of a side product in which the BOC group had been cleaved. Jacobi55 has made note of strong solvent effects for the hydrolysis of similar compounds. When we applied new mixed solvent hydrolysis conditions based on the Jacobi observations (LiOH, H2O2, THF, DMF, H2O) we saw an increase in the yield of carboxylic acid 57, and a subsequent reduction in the amount of BOC cleavage; however, the overall yield of the reaction was still variable. After considerable experimentation, we concluded that the problem we were experiencing was due to adventitious decomposition of H2O2. Use of rigorous precautions (complete avoidance of metal needles and Teflon stirbars, and using glassware prewashed with 30% H2O2 solution) gave much improved results. Despite these precautions, occasionally no conversion of the Boc-protected amide 87 to the carboxylic acid 57 was obtained. In such runs, the reaction simply had to be worked up and started over.
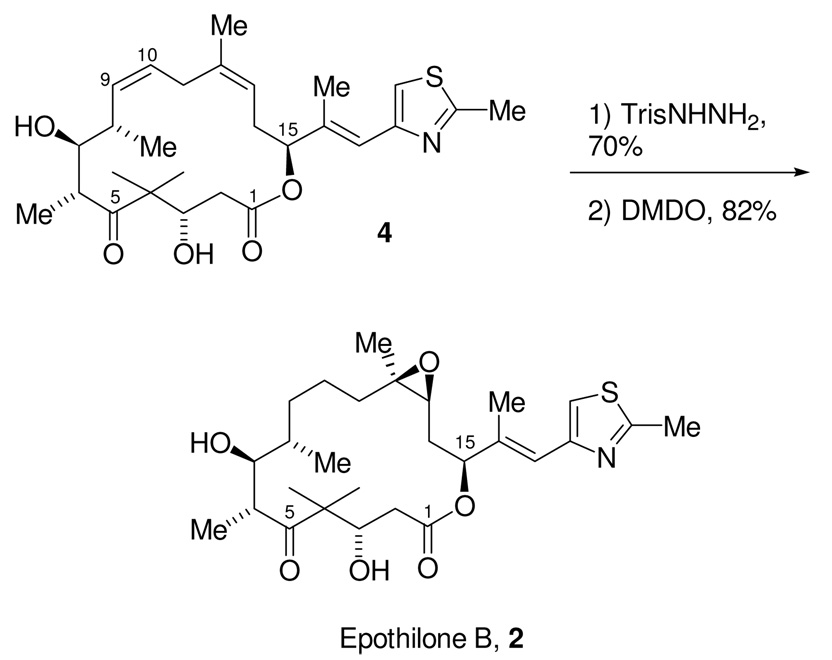
With the synthesis of this carboxylic acid, we had completed a formal synthesis of epothilones B and D.56 The completion of the total synthesis of epothilones B and D began with the selective removal of the secondary allylic TBS ether in 57 using TBAF. The resulting hydroxy acid then underwent macrolactonization under Yamaguchi conditions57 to give the desired macrolactone in 65% yield. Global deprotection was accomplished using 2N HCl in MeCN to give 88. The precedented selective diimide reduction of the disubstituted olefin using triisopropylphenylsulfonylhydrazide58 then provided epothilone D, 3. Finally, the known stereoselective epoxidation of epothilone D using DMDO59 gave epothilone B (2) as an 8:1 mixture in favor of the desired α-epoxide.60
Summary
The total synthesis of epothilone B was completed in 20 steps with an 8.9% total yield. This product became the genesis for three new methodologies in our labs: the anti selective electron transfer reduction of β-hydroxy ketones using SmI2, the CAA addition of stannane 44, as a synthon for Chan’s diene, and the substrate controlled additions of the highly substituted stannane 22.
There are a number of elegant and practical total syntheses of epothilone B published to date. The most notable differences between these and the route described herein are in the formation of the C1-C9 fragment. The C4 gem-dimethyl group was installed in this synthesis through a stereoselective connective fragment assembly rather than as a constituent of a simple precursor. This approach may well find wider application as the introduction of such quaternary centers is often difficult.61,62 In the C10-C21 fragment an efficient approach was developed that installed the C12-C13 trisubsituted (Z)-olefin with high diastereoselectivity, and which culminated in the use of a selective and efficient diol differentiation protocol. Finally, although the lactone opening sequence was very frustrating at the time, eventually we found a solution to this problem which should also prove to be of more general utility.
Experimental Section
4-Methyl-3-tributylstannanylmethyl-pent-3-enoic acid ethyl ester 22
To a stirring solution of iodomethyltributyltin 30 (20.8 g, 48.3 mmol, 2.3 eq) and Et2O (125 mL) in a 500 mL round-bottom flask under an atmosphere of argon, at −78 °C, was slowly added a 1.7 M solution of t-BuLi (100 mL, 85.0 mmol, 4.0 eq) in pentane via cannula directly from a 100 mL commercially available bottle. The resulting cloudy solution was allowed to stir at −78 °C for 10 min. The reaction mixture was then warmed to 0 °C, at which point it became homogeneous and yellow.
In a separate 1 L three-necked, round-bottom flask equipped with a mechanical stirrer, was added purified CuI (4.04 g, 21.2 mmol, 1.0 eq). The flask was sealed and purged with argon. The CuI was covered in Et2O (50 mL) and the resulting light gray slurry cooled to −78 °C. To this stirring slurry was quickly added the tri-n-butylstannyl)methyl lithium solution via cannula. An additional Et2O rinse (10 mL) was used to transfer the remaining organolithium residue into the reaction flask. The resulting dark gray mixture was allowed to warm to −15 °C and stirred for 30 min, at which time the mixture became dark purple. The heterogeneous mixture was cooled to −78 °C and a solution of allenic ester 28 (4.06 g, 28.9 mmol, 1.4 eq) in Et2O (5 ml) was added dropwise via cannula to the reaction mixture. An additional Et2O rinse (5 mL) was used to transfer the remaining allenic ester residue into the reaction flask. The reaction mixture was stirred at −78 °C for 30 min and then warmed to 0 °C. After 30 min at 0 °C, the reaction was quenched by the dropwise addition of EtOH (20 mL) and the mixture was then allowed to warm to rt. To this heterogeneous mixture was added water (100 mL) and stirred for 10 min. The resulting emulsion was filtered through paper using a Buchner funnel and the filtrate collected and added to a separatory funnel containing brine (250 mL). The layers were separated and the aqueous phase was extracted with hexanes (2 × 100 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 7.5 × 33 cm column, eluting with 3% Et2O/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give stannane 22 (6.84 g, 72% yield) as a clear, colorless oil: Rf 0.38 (5% Et2O/hexanes); 500 MHz 1H NMR (CDCl3) δ 4.09 (q, J = 7.3 Hz, 2H), 2.92 (br s with Sn satellites, 2H), 1.77 (s with Sn satellites, 2H), 1.70 (s with Sn satellites, 3H), 1.65 (s with Sn satellites, 3H), 1.56-1.40 (m, 6H), 1.36-1.22 (m, 6H), 1.22 (t, J =6.8 Hz, 3H), 0.89 (t, J =7.3 Hz, 9H), 0.88-0.80 (m, 6H); 125 MHz 13C NMR (CDCl3) δ 172.5, 125.2, 123.0, 60.5, 40.2, 29.3 (with Sn satellites), 27.6 (with Sn satellites), 21.1, 20.8, 17.0, 14.5, 13.9, 10.0 (with Sn satellites); DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 21.1, 20.8, 14.5, 13.9. CH2: 60.5, 40.2, 29.3 (with Sn satellites), 27.6 (with Sn satellites), 17.0, 10.0 (with Sn satellites) ppm; IR (neat) 2854, 1736 cm−1; Anal. Calcd. for C21H42O2Sn: C, 56.65; H, 9.51. Found: C, 56.92; H, 9.61.
Ethyl (5S,6R)-5-hydroxy-4,4,6-trimethyl-3-oxo-7-(1,1,2,2,tetramethyl-1-silapropoxy)heptanoate
To a stirring solution of crude aldehyde 25 (9.36 g, 46.2 mmol, 1.2 eq) and CH2Cl2 (430 mL) in a 1 L round-bottom flask under an atmosphere of nitrogen, at −78 °C, was added a solution of stannane 22 (17.2 g, 38.5 mmol, 1.0 eq) and CH2Cl2 (20 mL) via cannula. An additional CH2Cl2 rinse (10 mL) was used to transfer the remaining aldehyde residue into the reaction flask. After 20 min at −78 °C, a 1.04 M solution of BF3•Et2O (73.9 mL, 77.1 mmol, 2.0 eq) in CH2Cl2 was slowly added via syringe pump (at a rate of 40 mL/h), down the side of the flask. After 2.5 h at −78 °C, the reaction was quenched (cold) by pouring the reaction mixture directly into a 1.5 L Erlenmeyer flask that contained a vigorously stirring mixture of CH2Cl2 (400 mL), pH 7 buffer (250 mL), and water (250 mL) and stirred for 30 min. The layers were separated and the aqueous phase extracted with CH2Cl2 (2 × 100 mL). The combined organic extractions were dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure to give ethyl 3-[(2S,3R)-2-hydroxy-1,1,3-trimethyl-4-(1,1,2,2-tetramethyl-1-silapropoxy)butyl]-but-3-enoate 38, which was used without further purification.
Analytical data for olefin 38: Rf 0.32 (20% acetone/hexanes); [α]22D = −19.2 (c = 1.04, CH2Cl2); 300 MHz 1H NMR (C6D6) δ 5.15 (s, 1H), 5.07 (s, H), 3.93 (q, J = 7.1 Hz, 2H), 3.78 (dd, J = 3.4, 1.0 Hz, 1H), 3.52-3.42 (m, 2H), 3.12-3.11 (m, 2H), 2.69 (d, J = 3.4 Hz, 1H), 1.90-1.79 (m, 1H), 1.15 (s, 3H), 1.06 (s, 3H), 1.03 (d, J = 6.8 Hz, 3H), 0.95 (s, 9H), 0.94 (t, J = 7.1 Hz, 3H), 0.03 (s, 6H); 125 MHz 13C NMR (CDCl3) δ 173.0, 148.2, 115.1, 75.7, 69.4, 60.8, 44.5, 38.4, 35.3, 25.9, 23.4, 23.3, 18.2, 14.2, 11.1, −5.5, −5.5 ppm; IR (neat) 3516, 3096, 2956, 2930, 2885, 2858, 1738, 1635, 1472, 1257, 1177, 1093, 1036 cm−1; Anal. Calcd. for C19H38O4Si C, 63.64; H, 10.68. Found: C, 63.93; H, 10.71.
To a stirring mixture of the crude olefin 38 and EtOAc (800 mL) in a 1 L round-bottom flask, at −78 °C, was bubbled a steady stream of ozone until the reaction solution turned light purple. The solution was then purged with a steady stream of O2 until the purple color subsided. To the −78 °C reaction solution was then added Me2S (15 mL), the bath removed, and the solution allowed to slowly warm to rt over 30 min. The reaction mixture was diluted with pH 7 buffer solution (200 mL) and the layers were separated. The aqueous phase was extracted with CH2Cl2 (2 × 75 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 7 × 33 cm column, eluting with 10% acetone/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give the desired β-hydroxy ketone (9.54 g, 69% yield over 3 steps) as a clear, colorless oil: Rf 0.54 (50% Et2O/hexanes); [α]22D = −19.4 (c = 0.88, CH2Cl2); 500 MHz 1H NMR (CDCl3) δ 4.22-4.15 (m, 2H), 3.89 (s, 1H), 3.74 (d, J = 16.1 Hz, 1H), 3.67 (d, J = 16.1 Hz, 1H), 3.66 (dd, J = 9.5, 3.3 Hz, 1H), 3.58 (dd, J = 9.9, 4.7 Hz, 1H), 3.09 (br s, 1H), 1.87-1.80 (m, 1H), 1.27 (t, J = 7.1 Hz, 3H), 1.22 (s, 3H), 1.16 (s, 3H), 0.90 (s, 9H), 0.86 (d, J = 9.3 Hz, 3H), 0.07 (s, 3H), 0.06 (s, 3H); 125 MHz 13C NMR (CDCl3) δ 209.1, 168.3, 79.0, 70.2, 61.3, 52.5, 46.8, 35.5, 26.1, 23.0, 21.0, 18.4, 14.3, 10.9, −5.3, −5.4; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 26.1, 23.0, 21.0, 14.3, 10.9, −5.3, −5.3. CH2: 70.2, 61.3, 46.8. CH1: 79.1, 35.5 ppm. (A very minor set of signals resulting from the tautomeric form of the β-keto ester were also detected by both 1H and 13C NMR spectroscopy but are not reported here due to the complexity of the spectrum and insufficient signal-to-noise ratio.) IR (neat) 3421, 1736, 1687cm−1; Anal. Calcd. for C18H36O5Si: C, 59.96; H, 10.06. Found: C, 59.76; H, 10.04.
Ethyl (3R,5R,6R)-3,5-dihydroxy-4,4,6-trimethyl-7-(1,1,2,2-tetramethyl-1-silapropoxy)heptanoate (36)
To a suspension of Sm metal (31.8 g, 211 mmol, 7.0 eq) and THF (700 mL) in a 1 L round-bottom flask under an atmosphere of argon, at rt, was added neat CH2I2 (28.3 g, 8.5 mL, 106 mmol, 3.5 eq) via syringe. The mixture became yellow, then green, then dark blue over the course of 1 h. The reaction mixture was allowed to stir for an additional 1 h, after which time a large amount of the suspended Sm still remains.
To the heterogeneous SmI2 solution, cooled to −42 °C (CH3CN/CO2), was added a precooled (0 °C) solution of the β-hydroxy ketone (10.9 g, 30.2 mmol, 1.0 eq), water (6.5 g, 6.5 mL, 362 mmol, 12 eq) and THF (90 mL) via cannula. Two additional THF rinses (5 mL each) were used to transfer the remaining β-hydroxy ketone residue into the reaction flask. The reaction mixture was stirred an additional 20 min at −42 °C, and then transferred to a −20 °C freezer where it was briefly manually agitated every 12 h. After 48 h at −20 °C, the reaction was quenched (cold) by bubbling O2 through the solution until the reaction mixture changed color from blue to yellow-green, the solution was then transferred (cold) directly into a 250 mL Erlenmeyer flask that contained a vigorously stirring mixture of EtOAc (750 mL) and an aqueous pH 7 buffer solution (300 mL). The resulting emulsion was stirred for 1 h, filtered through paper using a Buchner funnel, the precipitate washed with EtOAc, and the filtrate collected. The layers were separated and the aqueous phase extracted with EtOAc (2 × 100 mL). The organic extracts were combined and washed with saturated aqueous Na2S2O3 (200 mL) and brine (200 mL). The aqueous layer was back extracted with CH2Cl2 (2 × 50 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. HPLC analysis of the crude material showed a diastereoselectivity of 96:4 anti:syn (Microsorb, 4% IPA/hexanes, 0.9 mL/min; Tr minor: 5.915 min, Tr major: 6.313 min.). Purification of the material was accomplished by flash column chromatography on a 5 × 33 cm column, eluting with 25% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give diol 36 (10.3 g, 94% yield) as a clear, colorless oil: Rf 0.30 (30% EtOAc/hexanes); [α]22D = −32.1 (c = 1.03, CH2Cl2); 500 MHz 1H NMR (CDCl3) δ 4.19 (q, J = 7.2 Hz, 2H), 4.07 (ddd, J = 7.2, 5.5, 4.3 Hz, 1H), 4.02 (br d, J = 3.9 Hz, 1H), 3.75 (d, J = 3.4 Hz, 1H), 3.64 (dd, J = 9.5, 4.2 Hz, 1H), 3.58 (dd, J = 9.5, 5.1 Hz, 1H), 3.40 (d, J = 3.4 Hz, 1H), 2.49 (d, J = 7.3 Hz, 1H), 2.49 (d, J = 5.9 Hz, 1H), 1.91-1.83 (m, 1H), 1.29 (t, J = 7.3 Hz, 3H), 1.06 (d, J = 7.3 Hz, 3H), 0.96 (s, 3H), 0.95 (s, 3H), 0.90 (s, 9H), 0.07 (s, 6H); 125 MHz 13C NMR (CDCl3) δ 173.6, 80.1, 75.8, 70.5, 60.9, 40.7, 37.5, 35.4, 26.1, 22.1, 21.6, 18.4, 14.4, 11.7, −5.3, −5.3 ppm; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 26.1, 22.2, 21.6, 14.4, 11.7, −5.3, −5.3. CH2: 70.5, 60.9, 37.5. CH1: 80.1, 75.8, 35.4 ppm. IR (neat) 3476, 2956, 2930, 2884, 2858, 1734, 1472, 1302, 1257, 1188, 1084, 1025 cm−1; Anal. Calcd. for C18H38O5Si: C, 59.63; H, 10.56. Found: C, 59.41; H, 10.47.
Methyl 2-[6-((2S,3S,1R)-2-hydroxy-1,3-dimethylpent-4-enyl)(4S,6S)-2,2,5,5-tetramethyl-1,3-dioxan-4-yl]acetate (41)
To crude aldehyde 39 (from Swern oxidation of the corresponding alcohol, 2.85 g, 10.6 mmol) in a 25 mL round-bottom flask, at rt, was added neat crotyl boronate 40 (5.81 g, 31.9 mmol, 3.0 eq) via syringe. A magnetic stir bar was added to the flask, and the flask sealed and purged with argon. After stirring 24 h at rt, the reaction mixture was transferred directly into a 250 mL Erlenmeyer flask that contained a vigorously stirring mixture of CH2Cl2 (50 mL) and water (25 mL). After stirring for 30 min, the layers were separated and the aqueous phase extracted with CH2Cl2 (2 × 25 mL). The combined organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. 1H NMR analysis of the unpurified reaction mixture indicates an 89:11 diastereoselectivity. Purification was accomplished by flash column chromatography on a 5 × 25 cm column, eluting with 1 L of 5%, 4 L of 10%, and 2 L of 20% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give the major homoallylic alcohol isomer 41 (2.80 g, 80% yield) as a light yellow, crystalline solid. Recrystallization of the major isomer 41 from 5% Et2O/hexanes (slow evaporation) provided crystals suitable for X-ray crystallography. Analytical data for the major isomer 41: mp 46–50 °C; Rf 0.33 (5% acetone/CH2Cl2); [α]22D = −14.9 (c = 2.4, CH2Cl2); 500 MHz 1H NMR (C6D6) δ 5.82 (ddd, J = 16.9, 10.5, 7.8 Hz, 1H), 5.02 (dd, J = 9.6, 1.8 Hz, 1H), 5.00 (s, 1H), 4.14 (dd, J = 11.2, 2.1 Hz, 1H), 3.62 (d, J = 4.1 Hz, 1H), 3.38 (s, 3H), 3.34 (ddd, J = 8.0, 2.6, 2.6 Hz, 1H), 2.41 (dd, J = 15.6, 11.0 Hz, 1H), 2.32-2.25 (m, 1H), 2.07 (dd, J = 15.6, 2.3 Hz, 1H), 1.96 (d, J = 2.3 Hz, 1H), 1.86-1.79 (m, 1H), 1.46 (s, 3H), 1.32 (s, 3H), 1.10 (d, J = 6.9 Hz, 3H), 0.86 (d, J = 6.9 Hz, 3H), 0.72 (s, 3H), 0.70 (s, 3H); 125 MHz 13C NMR (C6D6) δ 172.1, 142.3, 115.7, 101.9, 78.8, 78.5, 73.9, 51.5, 42.5, 40.4, 35.0, 34.9, 24.5, 24.1, 21.0, 20.4, 16.8, 8.8; DEPT 125 MHz 13C NMR (C6D6) δ CH3: 51.5, 24.5, 24.1, 21.0, 20.4, 16.8, 8.8. CH2: 115.7, 35.0. CH1: 142.3, 78.7, 78.5, 73.9, 42.5, 34.9 ppm. IR (neat) 3535, 3076,2985, 2938, 2880, 1744, 1636, 1457, 1437, 1381, 1226, 1169, 1123, 1060, 1003 cm−1; Anal. Calcd. for C18H32O5: C, 65.82; H, 9.82. Found: C, 65.79; H, 10.06.
Methyl 2-{6-[(1S,2R,3R)-1,3-dimethyl-4-oxo-2-(1,1,2,2-tetramethyl-1-silapropoxy)butyl]-(4S,6S)-2,2,5,5-tetramethyl-1,3-dioxan-4-yl}acetate (59)
To a stirring solution of olefin 61 (2.36 g, 5.33 mmol, 1.0 eq), NMO (1.25 g, 10.7 mmol, 2.0 eq), and 5:5:1 THF/t-BuOH/H2O (55 mL) in a 250 mL round-bottom flask, at rt, was added a 0.16 M solution of OsO4 (3.4 mL, 0.533 mmol, 0.10 eq) in THF via syringe. After 6 h at rt, NaIO4 (5.70 g, 26.7 mmol, 5.0 eq) was added in one portion followed by H2O (5 mL). After an additional 4 h at rt, the heterogeneous solution was filtered through a fritted funnel, the remaining solids washed with 50% EtOAc/hexanes (100 mL), and the filtrate collected. The filtrate was transferred directly into a 500 mL Erlenmeyer flask that contained a vigorously stirring mixture of 50% EtOAc/hexanes (100 mL) and a saturated aqueous solution of Na2S2O3 (50 mL). After stirring at rt for 30 min, the layers were separated. The organic phase was washed with water (25 mL) and brine (25 mL), dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification was accomplished by Florisil column chromatography on a 5 × 20 cm column, eluting with 10% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give aldehyde 59 (2.10 g, 89% yield) as a clear, colorless oil: Rf 0.22 (15% EtOAc/hexanes); [α]22D = −68.1 (c = 0.48, CH2Cl2); 500 MHz 1H NMR (C6D6) δ 9.75 (d, J = 2.1 Hz, 1H), 4.10 (dd, J = 11.0, 2.1 Hz, 1H), 3.65 (dd, J = 5.5, 4.7 Hz, 1H), 3.61 (d, J = 1.3 Hz, 1H), 3.38 (s, 3H), 2.70-2.62 (m, 1H), 2.35 (dd, J = 15.7, 11.0 Hz, 1H), 2.06 (dd, J = 15.7, 2.1 Hz, 1H), 1.91-1.84 (m, 1H), 1.42 (s, 3H), 1.26 (s, 3H), 1.02 (d, J = 7.2 Hz, 3H), 1.02 (d, J = 7.2 Hz, 3H), 0.91 (s, 9H), 0.68 (s, 3H), 0.65 (s, 3H), 0.01 (s, 3H), 0.00 (s, 3H); 125 MHz 13C NMR (C6D6) δ 203.1, 172.1, 101.8, 78.7, 74.2, 73.6, 51.5, 50.1, 40.7, 38.8, 35.1, 26.6, 24.5, 24.4, 21.1, 21.1, 18.8, 13.5, 12.4, −3.3, −3.9; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 51.5, 26.6, 24.6, 24.5, (21.1 × 2), 13.5, 12.4, −3.3, −3.9. CH2: 35.2. CH1: 203.0, 78.7, 74.3, 73.6, 50.2, 38.9 ppm; IR (neat) 2985, 2956, 2936, 2885, 2858, 2738, 1740, 1653, 1635, 1559, 1506, 1472, 1387, 1296, 1259, 1226, 1170, 1036, 1005 cm−1; HRMS m/z (CI) Calcd. C23H45O6Si (M+H) 445.2985, Obsd. 445.3007.
3-[(4E)(3S)-4-Methyl-5-(2-methyl(1,3-thiazol-4-yl))-3-(1,1,2,2-tetramethyl-1-silapropoxy)pent-4-enylidene]-4,5-dihydrofuran-2-one (69)
To a stirring solution of aldehyde 66 (5.87 g, 18.0 mmol, 1.0 eq) and benzene (180 mL) in a 250 mL round-bottom flask, at rt, was added Wittig reagent 65 (7.49 g, 21.6 mmol, 1.2 eq) in one portion. The flask was fitted with a reflux condenser and nitrogen line and the reaction vessel purged with nitrogen. The reaction mixture was heated at reflux (~80 °C) for 1.5 h. The solution was allowed to cool to rt, the stir bar extracted, and the solvent removed under reduced pressure. To facilitate the removal of triphenylphosphine oxide, the residue was dissolved in CH2Cl2 (100 mL) and silica gel (~25 mL by volume) was added. The crude heterogeneous slurry was concentrated under reduced pressure then dried under high vacuum until the remaining material was a free flowing powder. To a pre-packed flash silica gel column (5 × 15 cm), the above powder was added and topped with sand (5 × 1 cm). The column was eluted with 35% EtOAc/hexane and the crude product-containing fractions were combined and then concentrated under reduced pressure. The diastereoselectivity of the reaction was determined to be 95:5 E/Z by 500 MHz 1H NMR analysis. Purification of the material was accomplished by flash column chromatography on a 5 × 30 cm column, eluting with 10% acetone/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give, as the major isomer, (E)-α,β-unsaturated lactone 69 (6.65 g, 94% yield) as colorless, waxy solid: Rf 0.25 (35% EtOAc/hexanes); [α]22D = +0.2 (c = 1.86, CHCl3); 500 MHz 1H NMR (CDCl3) δ 6.93 (s, 1H), 6.77 (dddd, J = 10.4, 8.0, 2.8, 2.8 Hz, 1H), 6.49 (br s, 1H), 4.38-4.30 (m, 2H), 4.28 (dd, J = 7.6, 5.2 Hz, 1H), 2.94-2.80 (m, 2H), 2.70 (s, 3H), 2.53-2.39 (m, 2H), 2.05 (d, J = 0.9 Hz, 3H), 0.88 (s, 9H), 0.05 (s, 3H), 0.01 (s, 3H); 125 MHz 13C NMR (CDCl3) δ 170.1, 164.8, 153.0, 141.2, 137.4, 127.1, 119.4, 116.0, 77.3, 65.5, 38.2, 25.9, 25.5, 19.4, 18.3, 14.2, −4.5, −4.9; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 25.9, 19.4, 14.2, −4.5, −4.9. CH2: 66.5, 38.2, 25.5. CH1: 137.4, 119.4, 116.0, 77.3 ppm; IR (neat) 3110, 2954, 2929, 2856, 1758, 1682, 1505, 1471, 1440, 1379, 1361, 1254, 1187, 1078, 1030, 949, 837, 811, 778, 756, 667 cm−1; HRMS m/z (CI) Calcd. C20H32NO3SSi (M+H) 394.1872, Obsd. 394.1877.
2-[(4E)(3S)-4-Methyl-5-(2-methyl(1,3-thiazol-4-yl))-3-(1,1,2,2-tetramethyl-1-silapropoxy)pent-4-enylidene]butane-1,4-diol (63)
To a stirring solution of lactone 69 (3.62 g, 9.20 mmol, 1.0 eq) and THF (100 mL) in a 250 mL round-bottom flask under an atmosphere of nitrogen, at −78 °C, was added a 1.5 M solution of DIBAL (15.3 mL, 23.0 mmol, 2.5 eq) in toluene dropwise via cannula from an oven-dried graduated cylinder, down the side of the flask. After 20 min at −78 °C, the reaction mixture was warmed to −42 °C (CH3CN/CO2). After 20 min at −42 °C, the reaction mixture was re-cooled to −78 °C and the reaction quenched by slow dropwise addition of MeOH (10 mL). The reaction mixture was allowed to stir at −78 °C for an additional 10 min and then transferred (cold) directly into a 1.5 L Erlenmeyer flask that contained a vigorously stirring mixture of CH2Cl2 (250 mL) and a saturated aqueous solution of Rochelle’s salts (potassium sodium tartrate) (250 mL). The resulting emulsion was stirred for 3 h, by which time two layers formed. The mixture was filtered through paper using a Buchner funnel and the filtrate collected. The layers were separated and the aqueous phase extracted with CH2Cl2 (3 × 50 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 5 × 14 cm column, eluting with 90% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give diol 63 (3.56 g, 97% yield) as a clear, colorless oil: Rf 0.25 (90% EtOAc/hexanes); [α]22D = −3.2 (c = CHCl3); 500 MHz H NMR (CDCl3) δ 6.92 (s, 1H), 6.44 (br s, 1H), 5.55 (dd, J = 7.6, 7.1 Hz, 1H), 4.19 (dd, J = 7.1, 5.7 Hz, 1H), 4.02 (s, 2H), 3.67 (ddd, J = 16.1, 10.9, 5.7 Hz, 1H), 3.66 (ddd, J = 16.1, 6.6, 5.2 Hz, 1H), 3.55 (br s, 2H), 2.69 (s, 3H), 2.47-2.34 (m, 3H), 2.28 (ddd, J = 14.7, 6.2, 6.2 Hz, 1H), 1.97 (d, J = 1.4 Hz, 3H), 0.88 (s, 9H), 0.06 (s, 3H), 0.02 (s, 3H); 125 MHz 13C NMR (CDCl3) δ 164.9, 152.9, 142.4, 138.2, 127.1, 119.2, 115.4, 78.5, 68.3, 61.6, 35.3, 32.6, 26.0, 19.3, 18.4, 14.2, −4.5, −4.8; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 26.0, 19.3, 14.2, −4.5, −4.8. CH2: 68.3, 61.6, 35.3, 32.6. CH1: 127.1, 119.2, 115.4, 78.5 ppm. IR (neat) 3331, 2928, 2856, 1659, 1507, 1471, 1443, 1388, 1361, 1341, 1254, 1188, 1071, 941, 837, 808, 777, 668 cm−1; Anal. Calcd. for C20H35NO3SSi: C, 60.41; H, 8.87; N, 3.52. Found: C, 60.31; H, 8.95; N, 3.42.
(3E,7E)(6S)-3-(Chloromethyl)-7-methyl-8-(2-methyl(1,3-thiazol-4-yl))-6-(1,1,2,2-tetramethyl-1-silapropoxy)octa-3,7-dien-1-ol (70)
To a stirring heterogeneous mixture of NCS (1.21 g, 9.07 mmol, 2.5 eq) and CH2Cl2 (20 mL) in a 50 mL round-bottom flask under an atmosphere of nitrogen, at 0 °C, was added neat Me2S (620 mg, 730 µL, 9.98 mmol, 2.75 eq) dropwise via syringe. The white, milky solution was allowed to stir for 10 min at which point a precooled solution (0 °C) of diol 63 (1.44 g, 3.63 mmol, 1.0 eq) and CH2Cl2 (2.5 mL) was added via cannula. An additional CH2Cl2 rinse (2.5 ml) was used to transfer the remaining diol residue into the reaction flask. After stirring overnight at 0 °C, the reaction was quenched by the dropwise addition of a solution of saturated aqueous NaHCO3 (2 mL). The reaction mixture was warmed to rt, diluted with CH2Cl2 (25 mL) and water (25 mL), and the layers were separated. The aqueous phase was extracted with CH2Cl2 (2 × 25 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 3 × 15 cm column, eluting with 35% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give allylic chloride 70 (1.43 g, 95% yield) as a clear, colorless oil: Rf 0.27 (35% EtOAc/hexanes); [α]22D = −7.4 (c = 1.12, CHCl3); 500 MHz 1H NMR (CDCl3) δ 6.94 (s, 1H), 6.48 (br s, 1H), 5.74 (dd, J = 8.5, 6.2 Hz, 1H), 4.23 (dd, J = 8.2, 4.1 Hz, 1H), 4.09 (s, 2H), 3.74 (t, J = 5.0 Hz, 1H), 3.73 (t, J = 6.4 Hz, 1H), 2.72 (s, 3H), 2.57 (ddd, J = 14.2, 6.4, 6.4 Hz, 1H), 2.54 (ddd, J = 14.2, 6.4, 6.4 Hz, 1H), 2.44 (ddd, J = 14.2, 5.7, 5.7 Hz, 1H), 2.35-2.32 (m, 1H) 2.30 (ddd, J = 14.2, 5.0, 5.0 Hz, 1H), 2.02 (d, J = 1.4 Hz, 3H), 0.89 (s, 9H), 0.07 (s, 3H), 0.04 (s, 3H); 125 MHz 13C NMR (CDCl3) δ 164.8, 153.0, 142.0, 134.4, 131.1, 119.3, 115.7, 78.5, 61.1, 50.7, 35.8, 32.0, 26.0, 19.4, 18.5, 14.3, −4.4, −4.7 ppm; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 26.0, 19.4, 14.3, −4.4, −4.7. CH2: 61.1, 50.7, 35.8, 32.0. CH1: 131.1, 119.3, 115.7, 78.5 ppm. IR (neat) 3356, 2954, 2929, 2885, 2857, 1703, 1508, 1472, 1441, 1388, 1361, 1255, 1186, 1149, 1074, 945, 837, 807, 777, 690 cm−1; Anal. Calcd. for C20H34ClNO2SSi: C, 57.73; H, 8.24; N, 3.37. Found: C, 57.70; H, 8.26; N, 3.41.
(6S)(3Z,7E)-3,7-Dimethyl-8-(2-methyl(1,3-thiazol-4-yl))-6-(1,1,2,2-tetramethyl-1-silapropoxy)octa-3,7-dien-1-ol (62) from 70
To a stirring solution of allylic chloride 70 (2.40 g, 5.79 mmol, 1.0 eq) and THF (60 mL) in a 250 mL round-bottom flask under an atmosphere of argon, at −78 °C, was slowly added a 1.0 M solution of Super-Hydride® (LiBHEt3) (20 mL, 20.3 mmol, 3.5 eq) in THF via syringe pump (at a rate of 30 mL/min), down the side of the flask. After 30 min at −78 °C, the reaction mixture was warmed to 0 °C. After 16 h at 0 °C, the reaction mixture was warmed to rt. After 3 h at rt, the reaction was quenched by pouring the reaction mixture directly into a 1 L Erlenmeyer flask that contained a vigorously stirring mixture of CH2Cl2 (250 mL) and a solution of saturated aqueous NH4Cl (100 mL), stirred 10 min, the layers were separated, and the aqueous phase extracted with CH2Cl2 (2 × 100 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 5 × 20 cm column, eluting with 35% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give alcohol 62 (2.16 g, 98% yield) as a clear, colorless oil: Rf 0.48 (50% EtOAc/hexanes); [α]22D = −18.5 (c = 0.85, CH2Cl2); 500 MHz 1H NMR (CDCl3) δ 6.93 (s, 1H), 6.46 (s, 1H), 5.33 (dd, J = 7.3, 7.3 Hz, 1H), 4.17 (dd, J = 8.8, 4.4 Hz, 1H), 3.67-3.64 (m, 2H), 2.70 (s, 3H), 2.53-2.41 (m, 2H), 2.29 (br s, 1H), 2.22-2.16 (m, 2H), 2.01, (s, 3H), 1.72 (s, 3H), 0.88 (s, 9H), 0.05 (s, 3H), 0.02 (s, 3H); 125 MHz 13C NMR (CDCl3) δ 164.5, 153.1, 142.5, 133.4, 125.1, 118.9, 115.2, 79.1, 60.6, 35.6, 35.4, 25.9, 23.8, 19.3, 18.4, 14.1, −4.7, −5.0; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 26.0, 24.0, 19.4, 14.2, −4.4, −4.7. CH2: 60.7, 35.8, 35.6. CH1: 125.2, 119.0, 115.4, 79.2 ppm. IR (neat) 3368, 2953, 2927, 2884, 2856, 1653, 1539, 1472, 1255, 1185, 1073 cm−1; Anal. Calcd. for C20H35NO2SSi: C, 62.94; H, 9.24; N, 3.67. Found: C, 61.73; H, 9.04; N, 3.66.
Methyl 2-{6-[(1S,2S,3S,10R)(4Z,7Z,11E)-2,10-bis(1,1,2,2-tetramethyl-1-silapropoxy)-1,3,7,11-tetramethyl-12-(2-methyl(1,3-thiazol-4-yl))dodeca-4,7,11-trienyl](4S,6S)-2,2,5,5-tetramethyl-1,3-dioxan-4-yl}acetate (58)
To a stirring solution of phosphonium salt 60 (1.86 g, 2.47 mmol, 1.05 eq) and THF (22 mL) in a 100 mL round-bottom flask under an atmosphere of argon, at −78 °C, was slowly added a 2.49 M solution of n-BuLi (0.95 mL, 2.49 M in hexanes, 2.35 mmol, 1.0 eq) in hexanes dropwise via syringe, dropwise down the side of the flask. After 2 min at −78 °C, to the deep red-orange solution was slowly added a precooled (−78 °C) solution of aldehyde 59 (1.05 g, 2.35 mmol, 1.0 eq) and THF (2 mL) via cannula. Two additional THF rinses (1 mL each) were used to transfer the remaining aldehyde residue into the reaction flask. After 30 min at −78 °C, the reaction mixture was warmed to rt, during which time the yellow-red reaction mixture became heterogeneous. After 30 min at rt, the reaction was quenched by pouring the reaction mixture directly into a 250 mL Erlenmeyer flask that contained a vigorously stirring mixture of CH2Cl2 (75 mL) and a solution of saturated aqueous NH4Cl (50 mL). The mixture was stirred 10 min, the layers were separated, and the aqueous phase extracted with CH2Cl2 (2 × 25 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 5 × 20 cm column, eluting with 10% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give the Wittig adduct 58 (1.66 g, 89% yield) as a clear, colorless oil: Rf 0.22 (10% EtOAc/hexanes); [α]22D = +13.2 (c = 0.89, CHCl3); 500 MHz 1H NMR (C6D6) δ 6.66 (br s, 1H), 6.58 (s, 1H), 5.76 (dd, J = 10.4, 10.4 Hz, 1H), 5.46 (ddd, J = 10.9, 7.1, 7.1 Hz, 1H), 5.38 (dd, J = 7.3, 7.3 Hz, 1H), 4.22 (dd, J = 6.9, 5.9 Hz, 1H), 4.17 (dd, J = 11.1, 2.1 Hz, 1H), 3.58 (dd, J = 6.7, 2.8 Hz, 1H), 3.46 (d, J = 1.9 Hz, 1H), 3.38 (s, 3H), 3.06-2.88 (m, 2H), 2.88-2.80 (m, 1H), 2.58-2.50 (m, 1H), 2.48-2.40 (m, 1H), 2.40 (dd, J = 15.6, 11.4 Hz, 1H), 2.28 (s, 3H), 2.27 (d, J = 1.0 Hz, 3H), 2.08 (dd, J = 15.4, 2.1 Hz, 1H), 1.94-1.87 (m, 1H), 1.76 (s, 3H), 1.50 (s, 3H), 1.36 (s, 3H), 1.17 (d, J = 6.6 Hz, 3H), 1.17 (d, J = 6.1 Hz, 3H), 1.04 (s, 9H), 1.02 (s, 9H), 0.76 (s, 3H), 0.74 (s, 3H), 0.17 (s, 3H), 0.14 (s, 6H), 0.10 (s, 3H) ppm; 125 MHz 13C NMR (C6D6) δ 172.1, 164.5, 154.5, 142.3, 136.1, 133.1, 128.7, 122.9, 119.7, 116.1, 101.7, 79.8, 79.1, 76.7, 73.8, 51.5, 40.7, 38.0, 37.5, 36.3, 35.1, 31.5, 26.9, 26.5, 24.6, 24.4, 24.3, 21.6, 21.5, 19.5, 19.3, 19.1, 18.9, 14.8, 12.2, −2.9, −3.2, −4.0, −4.4 ppm; DEPT 125 MHz 13C NMR (C6D6) δ CH3: 51.5, 26.9, 26.5, 24.6, 24.4, 24.3, 21.6, 21.5, 19.5, 19.3, 14.8, 12.2, −2.9, −3.2, −4.0, −4.3. CH2: 36.3, 35.1, 31.5. CH1: 133.3, 128.7, 122.9, 119.7, 116.1, 79.8, 79.1, 76.7, 73.8, 38.0, 37.5 ppm. IR (neat) 2956, 2930, 2898, 2856, 1748, 1700, 1653, 1506, 1472, 1457, 1436, 1387, 1362, 1296, 1255, 1227, 1177, 1060, 1005 cm−1; HRMS m/z (CI) Calcd. C43H78NO6SSi2 (M+H) 792.5088, Obsd. 792.5095.
((3S,5S,6S,7S,8S,15R)(9Z,12Z,16E)-5-Hydroxy-4,4,6,8,12,16-hexamethyl-17-(2-methyl(1,3-thiazol-4-yl))-N-phenyl-3,7,15-tris(1,1,2,2-tetramethyl-1-silapropoxy)heptadeca-9,12,16-trienamide (86)
To a stirring suspension of PhNH2•HCl (1.57 g, 12.1 mmol, 7 eq) and THF (10 mL) in a 100 mL round-bottom flask under an atmosphere of argon, at 0 °C, was added a 2.49 M solution of n-BuLi (8.35mL, 20.9 mmol, 12.0 eq) in hexanes via syringe. After 10 min at 0 °C, the gray, cloudy reaction mixture was cooled to −78 °C. To the cold mixture was slowly added a precooled (−78 °C) solution of lactone 74 (1.45 g, 1.73 mmol, 1.0 eq) and THF (4 mL) dropwise via cannula, down the side of the flask. Two additional THF rinses (2 mL each) were used to transfer the remaining lactone residue into the reaction flask. After 45 min at −78 °C, the reaction mixture was warmed to −42 °C (CH3CN/CO2). After 45 min at −42 °C, the reaction was quenched (cold) by pouring the reaction mixture directly into a 250 mL Erlenmeyer flask that contained a vigorously stirring mixture of CH2Cl2 (75 mL) and saturated aqueous NH4Cl (50 mL) and stirred for 10 min. The layers were separated and the aqueous phase extracted with CH2Cl2 (2 × 25 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 5 × 20 cm column eluting with 10% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give hydroxy amide 86 (1.52 g, 94% yield) as a clear, colorless oil: Rf 0.18 (10% EtOAc/hexanes); [α]22D = +3.3 (c = 0.96, CHCl3); 500 MHz 1H NMR (CDCl3) δ 8.06 (s, 1H), 7.50 (d, J = 8.0 Hz, 2H), 7.30 (t, J = 8.0 Hz, 2H), 7.09 (t, J = 7.6 Hz, 1H), 6.95 (s, 1H), 6.93 (br s, 1H), 5.60 (dd, J = 10.2, 10.2 Hz, 1H), 5.27 (ddd, J = 10.9, 8.5, 5.4 Hz, 1H), 5.20 (dd, J = 6.9 Hz, 1H), 4.23 (dd, J = 6.9, 3.1 Hz, 1H), 4.09 (dd, J = 6.4, 6.4 Hz, 1H), 3.68 (s, 1H), 3.54 (dd, J = 4.7, 2.8 Hz, 1H), 3.35 (s, 1H), 2.86-2.62 (m, 4H), 2.70 (s, 3H), 2.48 (dd, J = 15.4, 6.9 Hz, 1H), 2.34-2.22 (m, 2H), 2.00 (d, J = 0.9 Hz, 3H), 1.86-1.78 (m, 1H), 1.68 (s, 3H), 1.03 (s, 3H), 1.01 (d, J = 6.6 Hz, 3H), 0.96 (d, J = 7.1 Hz, 3H), 0.92 (s, 9H), 0.90 (s, 9H), 0.87 (s, 9H), 0.83 (s, 3H), 0.16 (s, 3H), 0.09 (s, 3H), 0.08 (s, 3H), 0.05 (s, 3H), 0.03 (s, 3H), −0.03 (s, 3H); 125 MHz 13C NMR (CDCl3) δ 169.9, 164.7, 153.2, 143.0, 138.3, 135.8, 133.0, 129.2, 127.4, 124.3, 122.1, 119.9, 118.8, 115.3, 80.3, (2 × 79.3), 75.4, 42.5, 41.9, 38.6, (2 × 35.8), 30.9, 26.4, 26.3, 26.0, 23.9, 23.4, 21.5, 19.7, 19.3, 18.6, (2 × 18.4), 14.1, 11.5, −3.5, −3.5, −4.0, −4.5, −4.7, −4.8; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 26.4, 26.3, 26.0, 23.9, 23.4, 21.5, 19.7, 19.3, 14.1, 11.5, −3.5, −3.5, −4.0, −4.5, −4.7, −4.8. CH2: 41.9, 35.8, 30.9. CH1: 133.0, 129.2, 127.4, 124.3, 122.1, 119.9, 118.8, 115.3, 80.3, (2 × 79.3), 75.4, 38.6, 35.8 ppm. IR (neat) 2956, 2929, 2885, 2857, 1667, 1601, 1543, 1499, 1472, 1442, 1388, 1361, 1315, 1253, 1074, 1005, 939, 836, 811, 775, 754 cm−1; HRMS m/z (CI) Calcd. C51H91N2O5SSi3 (M+H) 927.5956, Obsd. 927.5925.
7-[(1E)-1-Methyl-2-(2-methyl(1,3-thiazol-4-yl))vinyl]-(3S,7S,14S,15S,16R)-3,15-bis(1,1,2,2-tetramethyl-1-silapropoxy)-2,2,10,14,16-pentamethyl-6-oxacyclohexadeca-9,12-diene-1,5-dione
To a stirring solution of seco acid (132 mg, 0.179 mmol, 1.0 eq), Et3N (32 mg, 44 µL, 0.313 mmol, 1.75 eq) and THF (4 mL) in a 25 mL round-bottom flask under an atmosphere of argon, at 0 °C, was added neat 2,4,6-trichlorobenzoyl chloride (50 mg, 35 µL, 0.206 mmol, 1.15 eq). After 1 h at 0 °C, the resulting mixed anhydride solution was warmed to rt and diluted with THF (3 mL) and toluene (6 mL). To a stirring solution of DMAP (37 mg, 0.30 mmol, 1.7 eq) and toluene (45 mL) in a 250 mL three-necked, round-bottom flask equipped with a reflux condenser, under an atmosphere of argon, at 75 °C, was slowly added the mixed anhydride solution via syringe pump (at a rate of 7.5 mL/h). An additional 1:1 THF/toluene rinse (2 mL) was used to transfer the remaining mixed anhydride residue into the reaction flask. After complete addition of the mixed anhydride solution, the reaction mixture was stirred an additional 1 h at 75 °C then cooled to rt. The reaction mixture was poured into a separatory funnel that contained CH2Cl2 (75 mL) and saturated aqueous NH4Cl (50 mL), and the layers were separated. The aqueous phase was extracted with CH2Cl2 (2 × 75 mL) and EtOAc (50 mL). The organic extracts were combined, dried over anhydrous Na2SO4, filtered, and then concentrated under reduced pressure. Purification of the material was accomplished by flash column chromatography on a 2.5 × 12 cm column, eluting with 10% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give the desired macrolactone (84 mg, 65% yield) as a clear, colorless oil: Rf 0.64 (20% EtOAc/hexanes); [α]22D = −68.2 (c = 0.96, CHCl3); 500 MHz 1H NMR (CDCl3) δ 6.96 (s, 1H), 6.51 (br s, 1H), 5.66 (d, J = 9.9, 9.9 Hz, 1H), 5.46-5.40 (m, 2H), 5.13 (dd, J = 6.4, 6.4 Hz, 1H), 4.47 (dd, J = 8.3, 2.6 Hz, 1H), 3.77-3.74 (m, 1H), 3.20 (dd, J = 15.8, 9.7 Hz, 1H), 3.04 (qd, J = 7.1, 2.4 Hz, 1H), 2.73 (s, 3H), 2.72-2.52 (m, 3H), 2.58 (dd, J = 14.9, 8.3 Hz, 1H), 2.44 (dd, J = 15.1, 2.8 Hz, 1H), 2.41-2.36 (m, 1H), 2.10 (s, 3H), 1.75 (s, 3H), 1.15 (s, 3H), 1.12 (s, 3H), 1.08 (d, J = 7.1 Hz, 3H), 1.01 (d, J = 7.1 Hz, 3H), 0.94 (s, 9H), 0.88 (s, 9H), 0.15 (s, 3H), 0.13 (s, 3H), 0.09 (s, 3H), 0.07 (s, 3H); 125 MHz 13C NMR (CDCl3) δ 216.0, 169.9, 164.9, 152.9, 137.8, 136.5, 130.8, 126.9, 121.1, 118.9, 116.6, 78.1, 76.5, 73.1, 54.0, 47.4, 41.2, 39.0, 31.1, 31.0, 26.4, 26.1, 24.5, 21.3, 20.5, 19.8, 19.5, 18.8, 18.3, 15.2, 14.7, −3.4, −3.5, −4.5, −4.6; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: 26.4, 26.1, 24.5, 21.3, 20.5, 19.8, 19.5, 15.2, 14.7, −3.4, −3.5, (2 × −4.6). CH2: 41.2, 31.1, 31.0. CH1: 130.8, 126.9, 121.1, 118.9, 116.6, 77.9, 76.2, 72.9, 47.4, 39.0 ppm. IR (neat) 2956, 2930, 2886, 2857, 1738, 1709, 1471, 1253, 1089, 1049, 1022, 868, 837, 775, 758 cm−1; LRMS m/z (CI) Calcd. C39H68NO5SSi3 (M+H) 718.4, Obsd. 718.3.
7-[(1E)-1-Methyl-2-(2-methyl(1,3-thiazol-4-yl))vinyl]-(3S,7S,14S,15S,16R)-3,15-dihydroxy-2,2,10,14,16-pentamethyl-6-oxacyclohexadec-9-ene-1,5-dione (Epothilone D, 3)
To a stirring solution of olefin 4 (20.2 mg, 0.0413 mmol, 1.0 eq), Et3N (580 µL, 420 mg, 4.13 mmol, 100 eq), and 1,2-dichloroethane (5 mL) in a high-pressure vial, at rt, was added TrisNHNH2 (1.23 g, 4.13 mmol, 100 eq) in one portion. The vial was sealed and placed in a preheated oil bath (50 °C). After 6 h an additional 100 eq each of Et3N and TrisNHNH2 was added to the heterogeneous reaction mixture. The vial was sealed and returned to the oil bath. After an additional 6 h, another 100 eq each of Et3N and TrisNHNH2 were added. The vial was sealed and returned to the oil bath. After the reaction mixture stirred an additional 6 h at 50 °C, the reaction mixture was cooled to rt, diluted with EtOAc (25 mL), and filtered through a pad of silica gel (3 × 3 cm) and Celite® (3 × 1 cm). The pad rinsed with EtOAc (100 mL). The filtrate was collected and the solvent removed under reduced pressure. Purification of the material began by flash column chromatography on a 2.5 × 6 cm column, eluting with 100 mL each of 30% and 70% EtOAc/hexanes. The crude product-containing fractions were combined and then concentrated under reduced pressure and re-subjected to flash column chromatography on a 2.5 × 6 cm column, eluting with 50% EtOAc/hexanes. The product-containing fractions were combined and then concentrated under reduced pressure to give Epothilone D, 3 (14.3 mg, 70% yield) as a colorless solid: Rf 0.30 (50% EtOAc/hexanes); [α]22D = −70.6 (c = 0.40, CHCl3); 500 MHz 1H NMR (CDCl3) δ 6.96 (s, 1H), 6.59 (br s, 1H), 5.22 (dd, J = 9.8, 1.5 Hz, 1H), 5.15 (dd, J = 9.8, 4.9 Hz, 1H), 4.32-4.27 (m, 1H), 3.75-3.72 (m, 1H), 3.45 (d, J = 5.4 Hz, 1H), 3.17 (qd, J = 6.8, 2.4 Hz, 1H), 3.04 (s, 1H), 2.70 (s, 3H), 2.69-2.60 (m, 1H), 2.47 (dd, J = 14.6, 11.2 Hz, 1H), 2.36-2.30 (m, 1H), 2.30 (dd, J = 14.6, 2.4 Hz, 1H), 2.27-2.21 (m, 1H), 2.08 (d, J = 1.0 Hz, 3H), 1.92-1.86 (m, 1H), 1.84-1.72 (m, 2H), 1.67 (s, 3H), 1.35 (s, 3H), 1.35-1.25 (m, 3H), 1.20 (d, J = 6.8 Hz, 3H), 1.08 (s, 3H), 1.03 (d, J = 7.3 Hz, 3H); 125 MHz 13C NMR (CDCl3) δ 220.9, 170.6, 165.3, 152.2, 139.5, 138.7, 121.1, 119.5, 115.8, 79.1, 74.3, 72.5, 53.8, 41.9, 39.8, 38.7, 32.8, 32.0, 31.8, 25.6, (2 × 23.1), 19.3, 18.3, 16.1, 16.0, 13.6; DEPT 125 MHz 13C NMR (CDCl3) δ CH3: (2 × 23.1), 19.3, 18.3, 16.1, 16.0, 13.6. CH2: 39.9, 32.8, 32.0, 31.8, 25.6. CH1: 121.1, 119.5, 115.8, 79.1, 74.3, 72.5, 41.9, 38.7 ppm. IR (neat) 3445, 2966, 2935, 2878, 1734, 1686, 1465, 1457, 1449, 1377, 1304, 1294, 1252, 1187, 1145, 978, 732 cm−1; LRMS m/z (CI) Calcd. C27H42NO5S (M+H) 492.3, Obsd. 492.3.
Supplementary Material
Experimental procedures and spectral and analytical data for compounds not given in the Experimental Section, descriptions of studies and proof of stereochemistry for the crotyl addition reactions to 39 and similar intermediates, copies of 1H and 13C NMR spectra. This material is available free of charge via the internet at http://pubs.acs.org.
Scheme 4.
Potential Chan’s Diene Approach
Scheme 10.
SmI2 mediated reduction of of β-hydroxy ketone 9
Scheme 15.
Ester Coupling and Ring-Closing Metathesis
Scheme 16.
Other Epothilone RCM Results
Scheme 27.
Completion of the Synthesis of Epothilone B.
Acknowledgement
Dedicated to the life and memory of our friend Professor A. I. Meyers. Financial support of this research by the National Institutes of Health, through Grant GM28961, is gratefully acknowledged.
References
- 1.(a) Höfle G, Bedorf N, Gerth K, Reichenbach H. (GBF), DE-B 4138042. 1993 [Chem. Abstr, 1993, 119, 180598] [Google Scholar]; (b) Höfle G, Bedorf N, Gerth K, Reichenbach H. (GBF), DE-4138042. 1993 [Chem. Abstr. 1993, 120, 528411] [Google Scholar]
- 2.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Leisch J, Geotz M, Lazarides E, Woods CM. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 3.Taxol® is a registered trademark of Bristol-Myers Squibb. The generic name for Taxol® is paclitaxel. Often, the name Taxol is used when referring to the compound paclitaxel. No infringement on the Bristol-Myers Squibb trademark is implied or intended by this usage.
- 4.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 5.Jordan MA, Wilson L. Nature Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 6.(a) Gunasekera SP, Paul GK, Longley RE, Isbrucker RA, Pomponi SA. J. Nat. Prod. 2002;65:1643–1648. doi: 10.1021/np020219m. [DOI] [PubMed] [Google Scholar]; (b) Gunasekera SP, Pomponi SA, Longley RE. 5,840,750. U. S. Patent. 1998; (c) Gunasekera SP, Gunasekera M, Longley RE, Schulte GK. J. Org. Chem. 1990;55:4912–4915. [Google Scholar]
- 7.Ciomei M, Albanese C, Pastori W, Grandi M, Pietra F, D’Ambrosio M, Guerriero A, Battistini C. Proc. Am. Assoc. Cancer Res. 1997;38:5. [Google Scholar]
- 8.Lindel T, Jensen PR, Fenical W, Long BH, Casazza AM, Carboni J, Fairchild CR. J. Am. Chem. Soc. 1997;119:8744–8745. [Google Scholar]
- 9.(a) West LM, Northcote PT. J. Org. Chem. 2000;65:445–449. doi: 10.1021/jo991296y. [DOI] [PubMed] [Google Scholar]; (b) Hood KA, West LM, Rouwe B, Northcote PT, Berridge MV, Wakefield SJ, Miller JH. Cancer Res. 2002;62:3356–3360. [PubMed] [Google Scholar]
- 10.(a) Corley DG, Herb R, Moore RE, Scheuer PJ, Paul VJ. J. Org. Chem. 1988;53:3644–3646. [Google Scholar]; (b) Quioa E, Kakou Y, Crews P. J. Org. Chem. 1988;53:3642–3644. [Google Scholar]
- 11.(a) Höfle G, Bedorf N, Steinmetz H, Schomburg D, Gerth H, Reichenbach H. Angew. Chem. Int. Ed. Eng. 1996;35:1567–1569. [Google Scholar]; (b) Gerth K, Bedorf N, Höfle G, Irschik H, Reichenback H. J. Antibiot. 1996;49:560–563. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 12.For reviews, see: Mulzer J. Monatshefte Chemie. 2000;131:205–238. Nicolaou KC, Roschanger F, Vourloumis D. Angew. Chem. Int. Ed. Engl. 1998;37:2014–2045. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2. Altmann KH, Bold G, Caravatti G, End N, Florsheimer A, Guagnano V, O’Reilly T, Wartmann M. Chemia. 2000;54:612–621. doi: 10.1016/s0960-894x(00)00555-2. Altmann K-H. Org. Biomol. Chem. 2004;2:2137–2152. doi: 10.1039/b405839a. Nicolaou KC, Ritzén A, Namoto K. Chem. Commun. 2001:1523–1535. doi: 10.1039/b104949f. Watkins EB, Chittiboyina AG, Avery MA. Eur. J. Org. Chem. 2006:4071–4084. Altmann K-H, Pfeiffer B, Arseniyadis S, Pratt BS, Nicolaou KC. ChemMedChem. 2007;2:396–423. doi: 10.1002/cmdc.200600206.
- 13.For recent synthetic work in this area, see: Scheid G, Ruijter E, Konarzycka-Bessler M, Bornscheuer UT, Wessjohann LA. Tetrahedron: Asymmetry. 2004;15:2861–2869. Jung J-J, Kache R, Vines KK, Zheng Y-S, Bijoy P, Valluri M, Avery MA. J. Org. Chem. 2004;69:9269–9284. doi: 10.1021/jo048742o.
- 14.(a) Meng D, Sorenson EJ, Bertinato P, Danishefsky SJ. J. Org. Chem. 1996;61:7998–7999. doi: 10.1021/jo961673w. [DOI] [PubMed] [Google Scholar]; (b) Bertinato P, Sorenson EJ, Meng D, Danishefsky SJ. J. Org. Chem. 1996;61:8000–8001. doi: 10.1021/jo961674o. [DOI] [PubMed] [Google Scholar]; (c) Balog A, Meng D, Kamenecka T, Bertinato P, Su D-S, Sorensen EJ, Danishefsky SJ. Angew. Chem. Int. Ed. Engl. 1996;35:2801–2803. [Google Scholar]; (d) Meng D, Su D-S, Balog A, Bertinato P, Sorensen EJ, Danishefsky SJ, Zheng YH, Chou TC, He L, Horwitz SB. J. Am. Chem. Soc. 1997;119:2733–2734. [Google Scholar]; (f) Su D-S, Meng D, Bertinato P, Balog A, Sorensen EJ, Danishefsky SJ, Zheng Y-H, Chou T-C, He L, Horwitz SB. Angew. Chem. Int. Ed. Engl. 1997;36:757–759. [Google Scholar]; (g) Balog A, Bertinato P, Su D-S, Meng D, Sorensen EJ, Danishefsky SJ, Zheng Y-H, Chou T-C, He L, Horwitz SB. Tetrahedron Lett. 1997;26:4529–4532. [Google Scholar]
- 15.(a) Nicolaou KC, He Y, Vourloumis D, Vallberg H, Yang Z. Angew. Chem. Int. Ed. Engl. 1996;35:2399–2401. [Google Scholar]; (b) Yang Z, He Y, Vourloumis D, Vallberg H, Nicolaou KC. Angew. Chem. Int. Ed. Engl. 1997;36:166–168. [Google Scholar]; (c) Nicolaou KC, Winssinger N, Pastor J, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, Hamel E. Nature. 1997;387:268–272. doi: 10.1038/387268a0. [DOI] [PubMed] [Google Scholar]
- 16.(a) Schinzer D, Limberg A, Bauer A, Böhm OM, Cordes M. Angew. Chem. Int. Ed. Engl. 1997;36:523–527. [Google Scholar]; (b) Schinzer D, Bauer A, Böhm OM, Limberg A, Cordes M. Chem. Eur. J. 1999;5:2483–2491. [Google Scholar]; (c) Schinzer D, Bauer A, Schieber J. Chem. Eur. J. 1999;5:2492–2500. [Google Scholar]
- 17.Stachel SJ, Danishefsky SJ. Tetrahedron Lett. 2001;42:6785–6787. [Google Scholar]
- 18.(a) White JD, Carter RG, Sundermann KF, Wartmann M. J. Am. Chem. Soc. 2001;123:5407–5413. doi: 10.1021/ja010454b. [DOI] [PubMed] [Google Scholar]; (b) White JD, Carter RG, Sundermann KF. J. Org. Chem. 1999;64:684–685. doi: 10.1021/jo982108r. [DOI] [PubMed] [Google Scholar]
- 19.For examples see: May SA, Grieco PA. Chem. Commun. 1998:1597–1598. Nicolaou KC, He Y, Vourloumis D, Vallberg H, Roschangar F, Sarabia F, Ninkovic S, Yang Z, Trujillo JI. J. Am. Chem. Soc. 1997;119:7960–7973.
- 20.For exceptions, see: Balog A, Meng D, Kamenecka T, Bertinato P, Su D, Sorensen EJ, Danishefsky SJ. Angew. Chem. Int. Ed. Engl. 1996;35:2801–2803. Balog A, Bertinato P, Su DS, Meng D, Sorensen EJ, Danishefsky SJ, Zheng YH, Chou TC, He L, Horwitz SB. Tetrahedron Lett. 1997;38:4529–4532. Liu J, Wong CH. Angew. Chem. Int. Ed. Engl. 2002;41:1404–1409. doi: 10.1002/1521-3773(20020415)41:8<1404::aid-anie1404>3.0.co;2-g.
- 21.Chan TH, Brownbridge P. J. Chem. Soc., Chem. Commun. 1979:578–579. [Google Scholar]
- 22.(a) Evans DA, Black WC. J. Am. Chem. Soc. 1993;115:4497–4513. [Google Scholar]; (b) Paterson I, Davies RDM, Heimann AC, Marquez R, Meyer A. Org. Lett. 2003;5:4477–4480. doi: 10.1021/ol0357853. [DOI] [PubMed] [Google Scholar]
- 23.For previous use of prenyl stannanes additions to chiral aldehydes see: Breitfelder S, Schlapbach A, Hoffmann RW. Synthesis. 1998:468–478. Schlapbach A, Hoffmann RW. Tetrahedron Lett. 1993;34:7903–7906. Sohn J-H, Waizumi N, Zhong HM, Rawal VH. J. Am. Chem. Soc. 2005;127:7290–7291. doi: 10.1021/ja050728l.
- 24.(a) Keck GE, Boden EP. Tetrahedron Lett. 1984;25:1879–1882. [Google Scholar]; (b) Keck GE, Abbott DE. Tetrahedron Lett. 1984;25:1883–1886. [Google Scholar]; (c) Keck GE, Abbott DE, Wiley MR. Tetrahedron Lett. 1987;28:139–142. [Google Scholar]; (d) Keck GE, Savin KA, Cressman ENK, Abbott DE. J. Org. Chem. 1994;59:7889–7896. [Google Scholar]
- 25.Kauffmann T, Kriegesmann R, Altepeter B, Steinseifer F. Chem. Ber. 1982;115:1810–1817. [Google Scholar]
- 26.Seyferth D, Andrews SB. J. Organometal. Chem. 1971;30:151–166. [Google Scholar]
- 27.Lang RW, Hansen H-J. Org Synth. Coll. 1990;Vol. 7:232. [Google Scholar]
- 28.It was assumed that this low yield resulted from the difficulty in separating the desired, relatively volatile allenic ester from the large amount of solid reaction byproducts, including triethylammonium chloride and triphenylphosphine oxide. We were surprised to find that an aqueous workup resulted in the formation of a new major byproduct, isobutyric acid. This led us to consider that the ketene intermediate was surprisingly stable and perhaps preformation of the ketene, before adding the Wittig reagent, might minimize some alternative reaction pathways. This simple change in the order of addition of the reagents increased the yield to 68% on a >30 gram scale reaction.
- 29.The limitation of scale here is of practical nature. We limited the reaction scale to the use of a commercially available 100 mL Sure-Seal® bottle of 1.7 M solution of t-BuLi in pentanes, which was transferred directly from the bottle into the reaction solution via cannula.
- 30.The major byproduct of the reaction, though not fully characterized, gave an NMR spectrum that was consistent with the 1,4-addition of a t-butyl anion into the allenic ester, which shows that lithium-halogen exchange was not fully complete even with the use of t-BuLi.
- 31.There is evidence that the use of alcohols and water in SmI2 reductions actually increase its reduction potential. See: Hasegawa E, Curren DP. J. Org. Chem. 1993;58:5008–5010.
- 32.(a) Rychnovsky SD, Yang G, Powers JP. J. Org. Chem. 1993;58:5251–5255. [Google Scholar]; (b) Rychnovsky SD, Rodgers B, Yang G. J. Org. Chem. 1993;58:3511–3515. [Google Scholar]; (c) Evans DA, Rieger DL, Gage JR. Tetrahedron Lett. 1990;31:7099–7100. [Google Scholar]; (d) Rychnovsky SD, Skalitzky DJ. Tetrahedron Lett. 1990;31:945–948. [Google Scholar]
- 33.(a) Evans DA, Chapman TK, Carreira EM. J. Am. Chem. Soc. 1988;110:3560–3578. [Google Scholar]; (b) Evans DA, Chapman TK. Tetrahedron Lett. 1986;27:5939–5942. [Google Scholar]
- 34.This reaction has been studied independently and a model for the observed stereochemistry proposed. See: Keck GE, Wager CA, Sell T, Wager TT. J. Org. Chem. 1999;64:2172–2173. Gary E, Keck GE, Wager CA. Org. Lett. 2000;2:2307–2309. doi: 10.1021/ol006072c.
- 35.Single diastereomer by 500 MHz 1H NMR.
- 36.A number of times this reaction gave yields of approximately 80%; however, some yields were as low as 30% of the desired alcohol. On those occasions where we experienced a low yield, all non-product containing fractions that were collected after the column chromatography were combined and resubjected to the same reaction conditions, which allowed for the further formation of the desired alcohol.
- 37.Hoffmann RW, Zei H. J. Org. Chem. 1981;46:1309–1314. [Google Scholar]
- 38.While this stannane was noticeably less stable to silica gel chromatography than the γ,γ-dimethyl substituted stannane 1.120, it could be purified by quick chromatography on a silica gel column which was slurry packed with 1% Et3N/EtOAc then flushed with hexanes prior to use.
- 39.(a) Meng D, Sorensen EJ, Bertinato P, Danishefsky SJ. J. Org. Chem. 1996;61:7998–7999. doi: 10.1021/jo961673w. [DOI] [PubMed] [Google Scholar]; (b) Shafiee A, Mazloumi A, Cohen VI. J. Heterocycl. Chem. 1979;16:1563. [Google Scholar]; (c) Shafiee A, Shahocini S. J. Heterocycl. Chem. 1989;26:1627. [Google Scholar]
- 40.(a) Keck GE, Tarbet KH, Geraci LS. J. Am. Chem. Soc. 1993;115:8467–8468. [Google Scholar]; (b) Keck GE, Yu T. Org. Lett. 1999;1:289–291. doi: 10.1021/ol990603j. and references therein. [DOI] [PubMed] [Google Scholar]
- 41.Comins DL, Dehghani A. Tetrahedron Lett. 1992;33:6299–6302. [Google Scholar]
- 42.Scott WJ, Stille JK. J. Am. Chem. Soc. 1986;108:3033–3340. [Google Scholar]
- 43.Corey EJ, Kim CU, Takeda M. Tetrahedron Lett. 1972;13:4339–4342. [Google Scholar]
- 44.(a) Rivkin A, Yoshimura F, Gabarda AE, Cho YS, Chou T-C, Dong H, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:10913–10922. doi: 10.1021/ja046992g. [DOI] [PubMed] [Google Scholar]; (b) Rivkin A, Yoshimura F, Gabarda AE, Chou T-C, Dong H, Tong WP, Danishefsky SJ. J. Am. Chem. Soc. 2003;125:2899–2901. doi: 10.1021/ja029695p. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Sinha SC. Angew. Chem. Int. Ed. Engl. 2002;41:1381–1383. doi: 10.1002/1521-3773(20020415)41:8<1381::aid-anie1381>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 46.Nicolaou KC, Ninkovic S, Sarabia F, Vourloumis D, He Y, Vallberg H, Finlay MRV, Yang Z. J. Am. Chem. Soc. 1997;119:7974–7991. [Google Scholar]
- 47.Baldwin JE, Moloney MG, Parsons AF. Tetrahedron. 1992;48:9373–9384. [Google Scholar]
- 48.Hughes RC, Dvorak CA, Meyers AI. J. Org. Chem. 2001;66:5545–5551. doi: 10.1021/jo010439p. [DOI] [PubMed] [Google Scholar]
- 49.(a) Evans DA, Gauchet-Prunet JA, Carreira EM, Charette AB. J. Org. Chem. 1991;56:741–750. [Google Scholar]; (b) Evans DA, Carreira EM. Tetrahedron Lett. 1990;31:4703–4706. [Google Scholar]
- 50.Evans DA, Carter PH, Dinsmore CJ, Barrow JC, Katz JL, Kung DW. Tetrahedron Lett. 1997;38:4535–4538. [Google Scholar]
- 51.No reversion of the amide 1.223 to lactone 1.218 was ever seen, even after prolonged storage at −20 °C.
- 52.Albright JD, Goldman L. J. Am. Chem. Soc. 1967;89:2416–2423. [Google Scholar]
- 53.This oxidation method has been shown to be highly effective for hindered secondary alcohols, and we had previously found it effective for a secondary alcohol vicinal to a gem-dimethyl group: Keck GE, Yu T, McLaws MD. J. Org. Chem. 2005;70:2543–2550. doi: 10.1021/jo048308m.
- 54.Williams JM, Jobson RB, Yasuda N, Marchesini G, Dolling U-H, Grabowski EJJ. Tetrahedron Lett. 1995;36:5461–5464. [Google Scholar]
- 55.Jacobi PA, Murphee S, Rupprecht F, Zheng W. J. Org. Chem. 1996;61:2413–2427. [Google Scholar]
- 56.(a) White JD, Carter RG, Sundermann KF, Wartmann M. J. Am. Chem. Soc. 2001;123:5407–5413. doi: 10.1021/ja010454b. [DOI] [PubMed] [Google Scholar]; (b) White JD, Carter RG, Sundermann KF. J. Org. Chem. 1999;64:684–685. doi: 10.1021/jo982108r. [DOI] [PubMed] [Google Scholar]
- 57.Inanaga J, Hirata K, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]
- 58.(a) Cusack NJ, Reese CB, Risius AC, Roozpeikar B. Tetrahedron. 1996;32:2157–2162. [Google Scholar]; (b) Ermolenko MS, Potier P. Tetrahedron Lett. 2002;43:2895–2898. [Google Scholar]
- 59.Stachel SJ, Danishefsky SJ. Tetrahedron Lett. 2001;42:6785–6787. [Google Scholar]
- 60.When we compared the 13C NMR spectra of our synthetic epothilone B to other published spectra, it was found that there are several different reported values for the carbon spectra of epothilone B. Our 13C NMR spectra data are in excellent agreement with that of Avery.13b Further, we believe that our 13C NMR spectra is correct as our 13C DEPT NMR spectra is consistent with the structure of epothilone B.
- 61.For other examples of the introduction of gem-dialkyl units using this strategy, see: Keck GE, Welch DS, Vivian PK. Org. Lett. 2006;8:3667–3670. doi: 10.1021/ol061173h. Keck GE, Welch DS, Poudel Y. Tetrahedron. Lett. 2006;47:8267–8270. doi: 10.1016/j.tetlet.2006.09.094.
- 62.For the use of a chiral prenyl borane to introduce the epothilone gem-dimethyl substituent, see: Ramachandran PV, Chandra JS, Prabhudas B, Pratihar D, Reddy MVR. Org. Biomol. Chem. 2005;3:3812–3824. doi: 10.1039/b508001k.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and spectral and analytical data for compounds not given in the Experimental Section, descriptions of studies and proof of stereochemistry for the crotyl addition reactions to 39 and similar intermediates, copies of 1H and 13C NMR spectra. This material is available free of charge via the internet at http://pubs.acs.org.